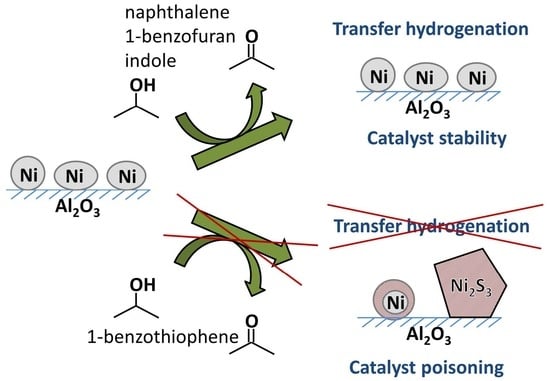
Reductive Transformation of O-, N-, S-Containing Aromatic Compounds under Hydrogen Transfer Conditions: Effect of the Process on the Ni-Based Catalyst
Abstract
:1. Introduction
2. Results and Discussion
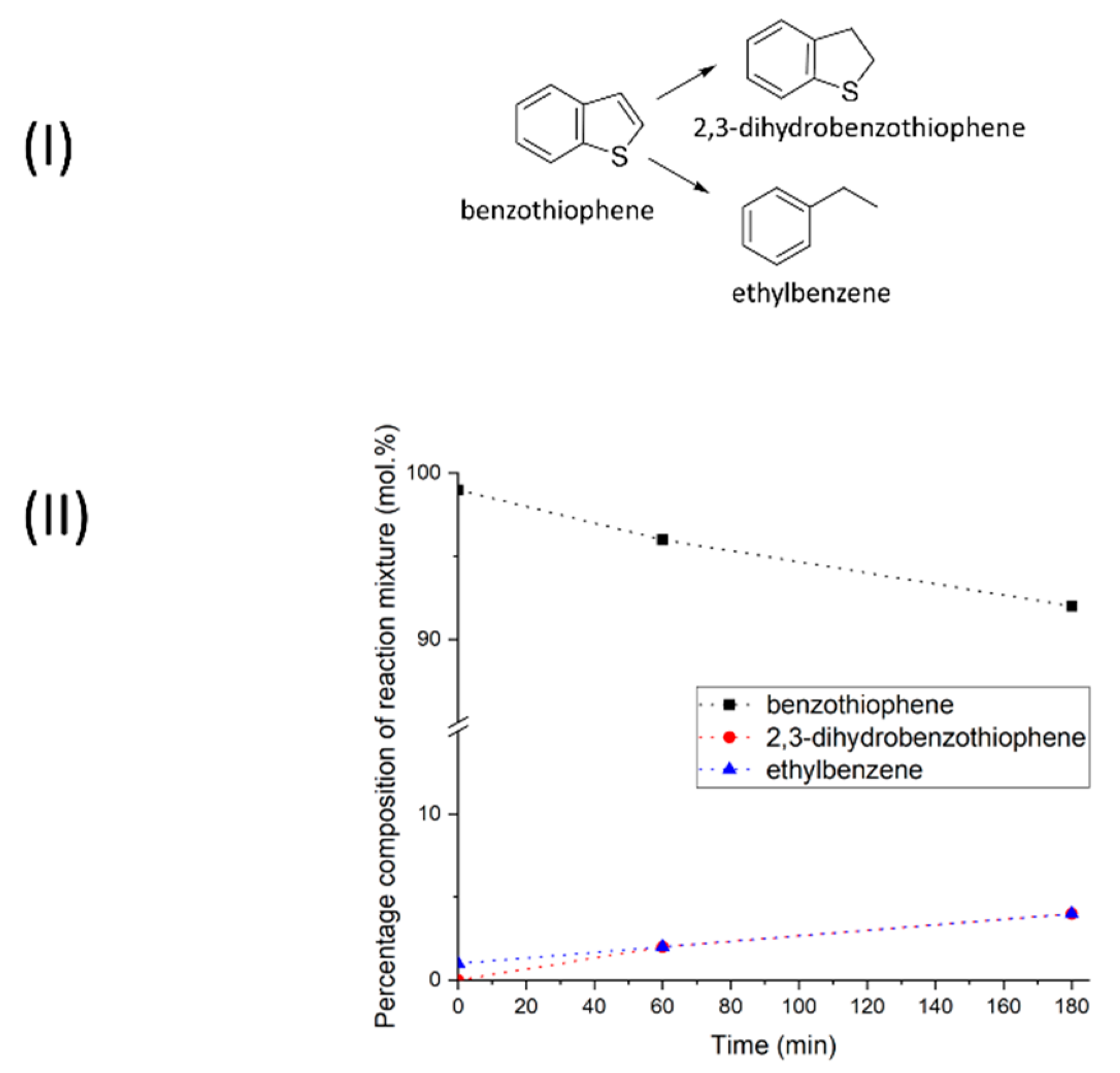
2.1. Reductive Transformations of O-, N-, S-Containing Aromatic Compounds under Hydrogen Transfer Conditions
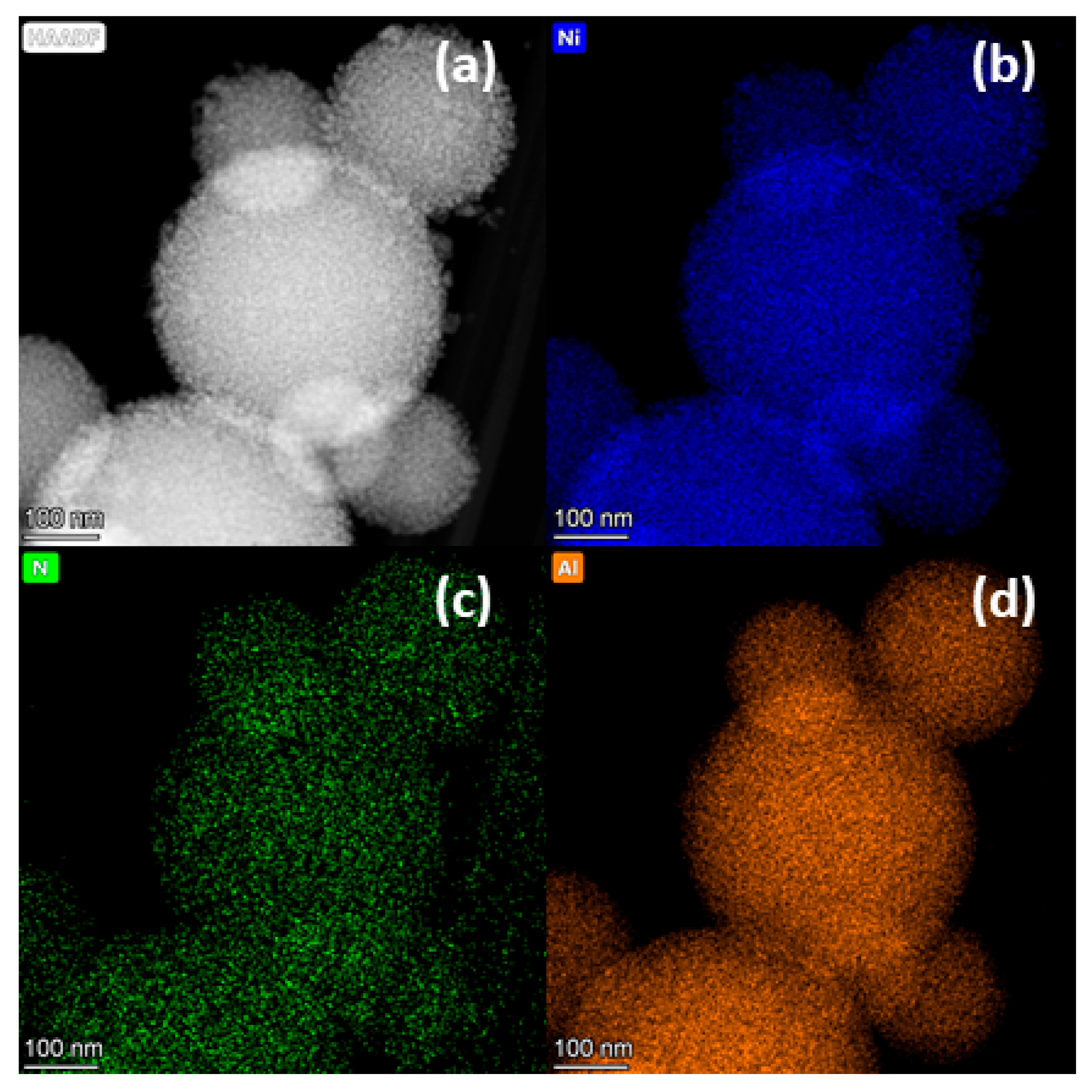
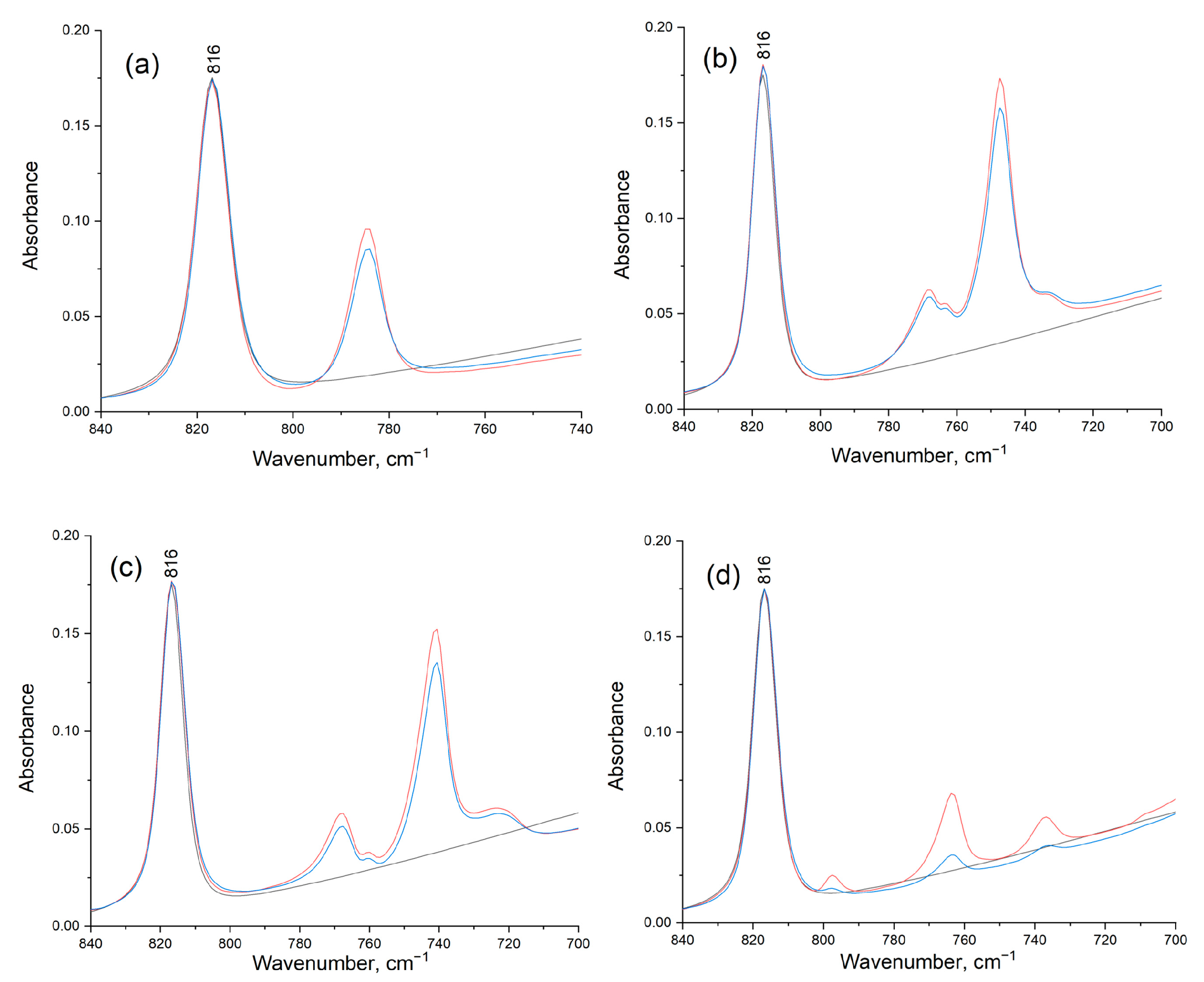
2.2. Effect of Catalytic Transformations on the Structure and Composition of the Ni-Containing Catalyst
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the Ni–Alum Catalyst
3.3. Description of the Characteristics of the Ni–Alum Catalyst
3.4. Catalytic Tests
3.5. Product Analysis
3.6. Calculations of Kinetic Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Speight, J. A Review of: “Hydrotreating Catalysis Science and Technology” Topsoe, H., Clausen, B.S., and Massoth, F.E., Springer-Verlag New York, 1996. ISBN No. 3-540-60380-8 Price Not Available at Time of Review. Fuel Sci. Technol. Int. 1996, 14, 1465. [Google Scholar] [CrossRef]
- Tanimu, A.; Alhooshani, K. Advanced Hydrodesulfurization Catalysts: A Review of Design and Synthesis. Energy Fuels 2019, 33, 2810–2838. [Google Scholar] [CrossRef]
- Nadeina, K.A.; Budukva, S.V.; Vatutina, Y.V.; Mukhacheva, P.P.; Gerasimov, E.Y.; Pakharukova, V.P.; Klimov, O.V.; Noskov, A.S. Unsupported Ni—Mo—W Hydrotreating Catalyst: Influence of the Atomic Ratio of Active Metals on the HDS and HDN Activity. Catalysts 2022, 12, 1671. [Google Scholar] [CrossRef]
- Furimsky, E. Catalytic Hydrodeoxygenation. Appl. Catal. A Gen. 2000, 199, 147–190. [Google Scholar] [CrossRef]
- Stratiev, D.; Ivanov, M.; Chavdarov, I.; Argirov, G.; Strovegli, G. Revamping Fluid Catalytic Cracking Unit, and Optimizing Catalyst to Process Heavier Feeds. Appl. Sci. 2023, 13, 2017. [Google Scholar] [CrossRef]
- Salomatina, A.A.; Nadeina, K.A.; Klimov, O.V.; Danilova, I.G.; Gerasimov, E.Y.; Prosvirin, I.P.; Pakharukova, V.P.; Chesalov, Y.A.; Noskov, A.S. Influence of Ni/Mo Ratio on Structure Formation of Ni–Mo Complex Compounds in NiMo/Al2O3 Catalysts for Selective Diene Hydrogenation. Energy Fuels 2022, 36, 15088–15099. [Google Scholar] [CrossRef]
- Straka, P.; Blažek, J.; Toullis, D.; Ihnát, T.; Šimáček, P. The Effect of the Reaction Conditions on the Properties of Products from Co-Hydrotreating of Rapeseed Oil and Petroleum Middle Distillates. Catalysts 2021, 11, 442. [Google Scholar] [CrossRef]
- Timko, M.T.; Ghoniem, A.F.; Green, W.H. Upgrading and Desulfurization of Heavy Oils by Supercritical Water. J. Supercrit. Fluids 2015, 96, 114–123. [Google Scholar] [CrossRef]
- Jafarian, M.; Haseli, P.; Saxena, S.; Dally, B. Emerging Technologies for Catalytic Gasification of Petroleum Residue Derived Fuels for Sustainable and Cleaner Fuel Production—An Overview. Energy Rep. 2023, 9, 3248–3272. [Google Scholar] [CrossRef]
- Fedyaeva, O.N.; Antipenko, V.R.; Vostrikov, A.A. Heavy Oil Upgrading at Oxidation of Activated Carbon by Supercritical Water-Oxygen Fluid. J. Supercrit. Fluids 2017, 126, 55–64. [Google Scholar] [CrossRef]
- Djimasbe, R.; Ilyasov, I.; Kwofie, M.; Khelkhal, M.; Emelianov, D.; Al-Muntaser, A.; Suwaid, M.; Varfolomeev, M. Direct Hydrogen Production from Extra-Heavy Crude Oil under Supercritical Water Conditions Using a Catalytic (Ni-Co/Al2O3) Upgrading Process. Catalysts 2022, 12, 1183. [Google Scholar] [CrossRef]
- Pavlova, P.L.; Minakov, A.V.; Platonov, D.V.; Zhigarev, V.A.; Guzei, D.V. Supercritical Fluid Application in the Oil and Gas Industry: A Comprehensive Review. Sustainability 2022, 14, 698. [Google Scholar] [CrossRef]
- Sim, S.; Kim, J.; Kong, W.B.; Kang, J.; Lee, Y.-W. Kinetic Study of Extra Heavy Oil Upgrading in Supercritical Methanol with and without Zinc Nitrate. J. Supercrit. Fluids 2019, 146, 144–151. [Google Scholar] [CrossRef]
- Jia, W.; Song, S.; Li, C.; Wu, X.; Yang, F.; Zhang, Y.; Wang, B. Simulation of Multiphase Behavior in Supercritical CO2–Heavy Oil–Water Sludge Extraction Systems Using Cubic-Plus-Association Equation of State. J. Supercrit. Fluids 2023, 200, 106001. [Google Scholar] [CrossRef]
- Tan, J.; Wang, Z.; Chen, S.; Hu, H. Progress and Outlook of Supercritical CO 2 –Heavy Oil Viscosity Reduction Technology: A Minireview. Energy & Fuels 2023, 37, 11567–11583. [Google Scholar] [CrossRef]
- Kwek, W.; Khan, M.K.; Sarkar, B.; Insyani, R.; Yi, M.; Kim, J. A Non-Catalytic, Supercritical Methanol Route for Producing High-Yield Saturated and Aromatic Compounds from de-Oiled Asphaltenes. J. Supercrit. Fluids 2017, 120, 140–150. [Google Scholar] [CrossRef]
- Khan, M.K.; Sarkar, B.; Zeb, H.; Yi, M.; Kim, J. Simultaneous Breaking and Conversion of Petroleum Emulsions into Synthetic Crude Oil with Low Impurities. Fuel 2017, 199, 135–144. [Google Scholar] [CrossRef]
- Sarkar, B.; Kwek, W.; Verma, D.; Kim, J. Effective Vacuum Residue Upgrading Using Sacrificial Nickel(II) Dimethylglyoxime Complex in Supercritical Methanol. Appl. Catal. A Gen. 2017, 545, 148–158. [Google Scholar] [CrossRef]
- Khan, M.K.; Kwek, W.; Kim, J. Conversion of Petroleum Emulsion into Light Fraction-Rich Upgraded Oil in Supercritical Methanol. Fuel 2018, 218, 78–88. [Google Scholar] [CrossRef]
- Yan, T.; Chen, K.; Wang, L.; Liu, Y.; Zhang, Y.; Jiang, Z.; Fang, T. Experimental Investigation of Upgrading Heavy Oil with Supercritical Methanol. Energy Fuels 2017, 31, 5882–5890. [Google Scholar] [CrossRef]
- Kwek, W.; Khan, M.K.; Sarkar, B.; Kim, J. Supercritical Methanol as an Effective Medium for Producing Asphaltenes-Free Light Fraction Oil from Vacuum Residue. J. Supercrit. Fluids 2018, 133, 184–194. [Google Scholar] [CrossRef]
- Khan, M.K.; Kwek, W.; Kim, J. Upgrading Heavy Crude Oils and Extra Heavy Fractions in Supercritical Methanol. Energy Fuels 2017, 31, 12054–12063. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; ISBN 9780198506980. [Google Scholar]
- Wang, Y.; Prinsen, P.; Triantafyllidis, K.S.; Karakoulia, S.A.; Yepez, A.; Len, C.; Luque, R. Batch versus Continuous Flow Performance of Supported Mono- and Bimetallic Nickel Catalysts for Catalytic Transfer Hydrogenation of Furfural in Isopropanol. ChemCatChem 2018, 10, 3459–3468. [Google Scholar] [CrossRef]
- Chen, W.; Peng, Q.; Fan, G.; Cheng, Q.; Tu, M.; Song, G. Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol over Al-Containing Ferrihydrite. J. Ind. Eng. Chem. 2023, 119, 574–585. [Google Scholar] [CrossRef]
- Wang, F.-F.; Guo, R.; Jian, C.; Zhang, W.; Xue, R.; Chen, D.-L.; Zhang, F.; Zhu, W. Mechanism of Catalytic Transfer Hydrogenation for Furfural Using Single Ni Atom Catalysts Anchored to Nitrogen-Doped Graphene Sheets. Inorg. Chem. 2022, 61, 9138–9146. [Google Scholar] [CrossRef]
- Achour, A.; Ojagh, H.; Ho, P.H.; Creaser, D.; Pajalic, O.; Holmberg, J.; Olsson, L. Towards Stable Nickel Catalysts for Selective Hydrogenation of Biomass-Based BHMF into THFDM. J. Environ. Chem. Eng. 2023, 11, 109461. [Google Scholar] [CrossRef]
- Richel, A.; Maireles-Torres, P.; Len, C. Recent Advances in Continuous Reduction of Furfural to Added Value Chemicals. Curr. Opin. Green Sustain. Chem. 2022, 37, 100655. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, P.; El Azab, I.H.; Bin Xu, B.; Guo, Z.; Elnaggar, A.Y.; Mersal, G.A.M.; Liu, X.; Zhi, Y.; Lin, Z.; et al. An Efficient Bifunctional Ni-Nb2O5 Nanocatalysts for the Hydrodeoxygenation of Anisole. Chin. J. Chem. Eng. 2022, 49, 187–197. [Google Scholar] [CrossRef]
- Feng, J.; Yang, Z.; Hse, C.Y.; Su, Q.; Wang, K.; Jiang, J.; Xu, J. In Situ Catalytic Hydrogenation of Model Compounds and Biomass-Derived Phenolic Compounds for Bio-Oil Upgrading. Renew. Energy 2017, 105, 140–148. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Wu, J.; Zhu, L.; Wang, S. Hydrodeoxygenation of Lignin-Derived Phenolics to Cycloalkanes over Ni–Co Alloy Coupled with Oxophilic NbOx. Appl. Energy 2022, 328, 120199. [Google Scholar] [CrossRef]
- Philippov, A.A.; Nesterov, N.N.; Pakharukova, V.P.; Martyanov, O.N. High-Loaded Ni-Based Catalysts Obtained via Supercritical Antisolvent Coprecipitation in Transfer Hydrogenation of Anisole: Influence of the Support. Appl. Catal. A Gen. 2022, 643, 118792. [Google Scholar] [CrossRef]
- Hutchings, G.J. Catalyst Synthesis Using Supercritical Carbon Dioxide: A Green Route to High Activity Materials. Top. Catal. 2009, 52, 982–987. [Google Scholar] [CrossRef]
- Nesterov, N.S.; Simentsova, I.I.; Yudanov, V.F.; Martyanov, O.N. A Comparative FMR Study of the Reduction of Co-Containing Catalysts for the Fischer-Tropsch Process in Hydrogen and Supercritical Isopropanol. J. Struct. Chem. 2016, 57, 90–96. [Google Scholar] [CrossRef]
- Gubin, S.P.; Buslaeva, E.Y. Supercritical Isopropanol as a Reducing Agent for Inorganic Oxides. Russ. J. Phys. Chem. B 2009, 3, 1172–1186. [Google Scholar] [CrossRef]
- Gavrikov, A.V.; Loktev, A.S.; Ilyukhin, A.; Mukhin, I.E.; Bykov, M.A.; Maslakov, K.I.; Vorobei, A.M.; Parenago, O.O.; Sadovnikov, A.; Dedov, A.G. Supercritical Fluid Assisted Modification Combined with the Resynthesis of SmCoO3 as Effective Tool to Enhance Long-Term Performance of SmCoO3-Derived Catalysts for Dry Reforming of Methane to Syngas. Dalt. Trans. 2022, 51, 18446–18461. [Google Scholar] [CrossRef] [PubMed]
- Gavrikov, A.V.; Loktev, A.S.; Ilyukhin, A.B.; Mukhin, I.E.; Bykov, M.A.; Vorobei, A.M.; Parenago, O.O.; Cherednichenko, K.A.; Sadovnikov, A.A.; Dedov, A.G. Partial Oxidation of Methane to Syngas over SmCoO3-Derived Catalysts: The Effect of the Supercritical Fluid Assisted Modification of the Perovskite Precursor. Int. J. Hydrogen Energy 2023, 48, 2998–3012. [Google Scholar] [CrossRef]
- Da Silva, E.P.; Winkler, M.E.G.; Giufrida, W.M.; Cardozo-Filho, L.; Alonso, C.G.; Lopes, J.B.O.; Rubira, A.F.; Silva, R. Effect of Phase Composition on the Photocatalytic Activity of Titanium Dioxide Obtained from Supercritical Antisolvent. J. Colloid Interface Sci. 2019, 535, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Mottola, S.; Mancuso, A.; Sacco, O.; De Marco, I.; Vaiano, V. Production of Hybrid TiO2/β-CD Photocatalysts by Supercritical Antisolvent Micronization for UV Light-Driven Degradation of Azo Dyes. J. Supercrit. Fluids 2022, 188, 105695. [Google Scholar] [CrossRef]
- Franco, P.; Navarra, W.; Sacco, O.; De Marco, I.; Mancuso, A.; Vaiano, V.; Venditto, V. Photocatalytic Degradation of Atrazine under Visible Light Using Gd-Doped ZnO Prepared by Supercritical Antisolvent Precipitation Route. Catal. Today 2022, 397–399, 240–248. [Google Scholar] [CrossRef]
- Franco, P.; Sacco, O.; De Marco, I.; Sannino, D.; Vaiano, V. Photocatalytic Degradation of Eriochrome Black-T Azo Dye Using Eu-Doped ZnO Prepared by Supercritical Antisolvent Precipitation Route: A Preliminary Investigation. Top. Catal. 2020, 63, 1193–1205. [Google Scholar] [CrossRef]
- Nesterov, N.S.; Shalygin, A.S.; Pakharukova, V.P.; Martyanov, O.N. Coprecipitation of Au Clusters and Alumina Sol in Supercritical CO2—The Facile Way to Stabilize Gold Nanoparticles within Oxide Matrix. J. Sol-Gel Sci. Technol. 2019, 92, 523–528. [Google Scholar] [CrossRef]
- Nesterov, N.S.; Pakharukova, V.P.; Filippov, A.A.; Martyanov, O.N. Synthesis of Ni–Cu Solid Solutions by Precipitation in the Supercritical CO2 Medium. J. Struct. Chem. 2023, 64, 1114–1125. [Google Scholar] [CrossRef]
- Li, J.; Shi, L.; Feng, G.; Shi, Z.; Sun, C.; Kong, D. Selective Hydrogenation of Naphthalene over γ-Al2O3-Supported NiCu and NiZn Bimetal Catalysts. Catalysts 2020, 10, 1215. [Google Scholar] [CrossRef]
- Feiner, R.; Schwaiger, N.; Pucher, H.; Ellmaier, L.; Derntl, M.; Pucher, P.; Siebenhofer, M. Chemical Loop Systems for Biochar Liquefaction: Hydrogenation of Naphthalene. RSC Adv. 2014, 4, 34955. [Google Scholar] [CrossRef]
- Chen, X.; Ma, Y.; Wang, L.; Yang, Z.; Jin, S.; Zhang, L.; Liang, C. Nickel-Aluminum Intermetallic Compounds as Highly Selective and Stable Catalysts for the Hydrogenation of Naphthalene to Tetralin. ChemCatChem 2015, 7, 978–983. [Google Scholar] [CrossRef]
- Philippov, A.A.; Chibiryaev, A.M.; Martyanov, O.N. Catalyzed Transfer Hydrogenation by 2-Propanol for Highly Selective PAHs Reduction. Catal. Today 2020, 379, 15–22. [Google Scholar] [CrossRef]
- Huang, K.; Fu, H.; Shi, W.; Wang, H.; Cao, Y.; Yang, G.; Peng, F.; Wang, Q.; Liu, Z.; Zhang, B.; et al. Competitive Adsorption on Single-Atom Catalysts: Mechanistic Insights into the Aerobic Oxidation of Alcohols over Co N C. J. Catal. 2019, 377, 283–292. [Google Scholar] [CrossRef]
- Song, H.; Gong, J.; Song, H.; Li, F. A Novel Surface Modification Approach for Synthesizing Supported Nickel Phosphide Catalysts with High Activity for Hydrodeoxygenation of Benzofuran. Appl. Catal. A Gen. 2015, 505, 267–275. [Google Scholar] [CrossRef]
- Edelman, M.C.; Maholland, M.K.; Baldwin, R.M.; Cowley, S.W. Vapor-Phase Catalytic Hydrodeoxygenation of Benzofuran. J. Catal. 1988, 111, 243–253. [Google Scholar] [CrossRef]
- Du, Y.; Chen, H.; Chen, R.; Xu, N. Poisoning Effect of Some Nitrogen Compounds on Nano-Sized Nickel Catalysts in p-Nitrophenol Hydrogenation. Chem. Eng. J. 2006, 125, 9–14. [Google Scholar] [CrossRef]
- Kumar, V.V.; Naresh, G.; Sudhakar, M.; Anjaneyulu, C.; Bhargava, S.K.; Tardio, J.; Reddy, V.K.; Padmasri, A.H.; Venugopal, A. An Investigation on the Influence of Support Type for Ni Catalysed Vapour Phase Hydrogenation of Aqueous Levulinic Acid to γ-Valerolactone. RSC Adv. 2016, 6, 9872–9879. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of Nickel Catalyst Poisoning; Elsevier Science Publishers, B.V.: Amsterdam, The Netherlands, 1987; Volume 34. [Google Scholar]
- Struis, R.P.W.J.; Schildhauer, T.J.; Czekaj, I.; Janousch, M.; Biollaz, S.M.A.; Ludwig, C. Sulphur Poisoning of Ni Catalysts in the SNG Production from Biomass: A TPO/XPS/XAS Study. Appl. Catal. A Gen. 2009, 362, 121–128. [Google Scholar] [CrossRef]
- Moudler, J.; Stickle, W.; Sobol, P.; Bomben, K. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corp.: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Tan, C.; Rudd, C.; Parsons, A.; Sharmin, N.; Zhang, J.; Chen, W.; Ahmed, I. Chitosan as a Coupling Agent for Phosphate Glass Fibre/Polycaprolactone Composites. Fibers 2018, 6, 97. [Google Scholar] [CrossRef]
- Peñas-Garzón, M.; Sampaio, M.J.; Wang, Y.L.; Bedia, J.; Rodriguez, J.J.; Belver, C.; Silva, C.G.; Faria, J.L. Solar Photocatalytic Degradation of Parabens Using UiO-66-NH2. Sep. Purif. Technol. 2022, 286, 120467. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Huang, W.; Zhang, T.; Hu, X.; Perman, J.A.; Ma, S. A Metal–Organic Framework and Conducting Polymer Based Electrochemical Sensor for High Performance Cadmium Ion Detection. J. Mater. Chem. A 2017, 5, 8385–8393. [Google Scholar] [CrossRef]
- Guo, H.; Ke, Y.; Wang, D.; Lin, K.; Shen, R.; Chen, J.; Weng, W. Efficient Adsorption and Photocatalytic Degradation of Congo Red onto Hydrothermally Synthesized NiS Nanoparticles. J. Nanoparticle Res. 2013, 15, 1475. [Google Scholar] [CrossRef] [PubMed]
- Rahman, G.; Chae, S.Y.; Joo, O. Efficient Hydrogen Evolution Performance of Phase-Pure NiS Electrocatalysts Grown on Fluorine-Doped Tin Oxide-Coated Glass by Facile Chemical Bath Deposition. Int. J. Hydrogen Energy 2018, 43, 13022–13031. [Google Scholar] [CrossRef]
- Jing, F.; Lv, Q.; Xiao, J.; Wang, Q.; Wang, S. Highly Active and Dual-Function Self-Supported Multiphase NiS–NiS 2 –Ni 3 S 2/NF Electrodes for Overall Water Splitting. J. Mater. Chem. A 2018, 6, 14207–14214. [Google Scholar] [CrossRef]
- Nesterov, N.S.; Pakharukova, V.P.; Philippov, A.A.; Gerasimov, E.Y.; Tsybulya, S.V.; Martyanov, O.N. Synthesis of Catalytic Precursors Based on Mixed Ni-Al Oxides by Supercritical Antisolvent Co-Precipitation. Catalysts 2022, 12, 1597. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater Subshell Photoionization Cross-Sections at 1254 and 1487 EV. J. Electron Spectros. Relat. Phenomena 1976, 8, 129–137. [Google Scholar] [CrossRef]
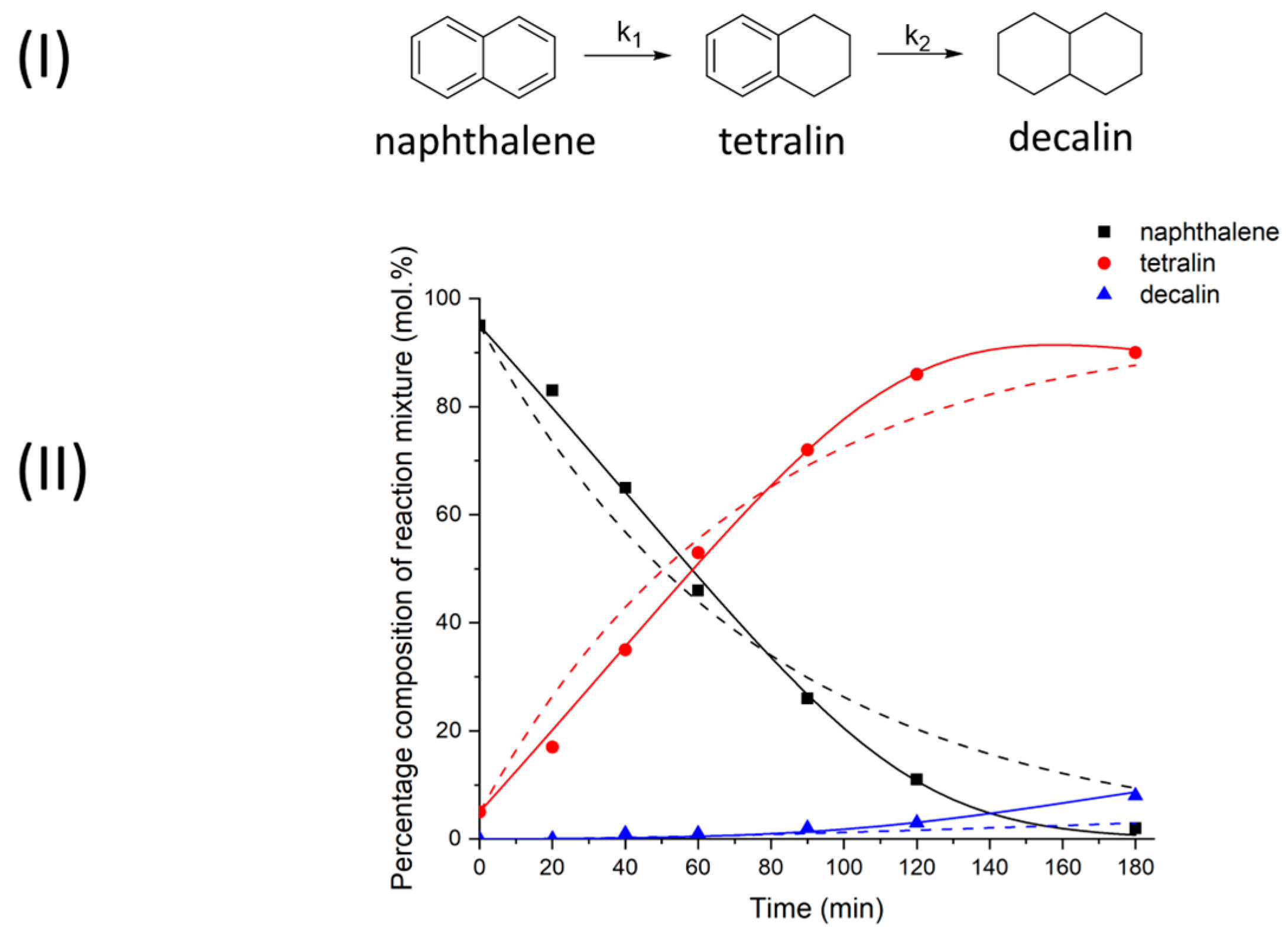
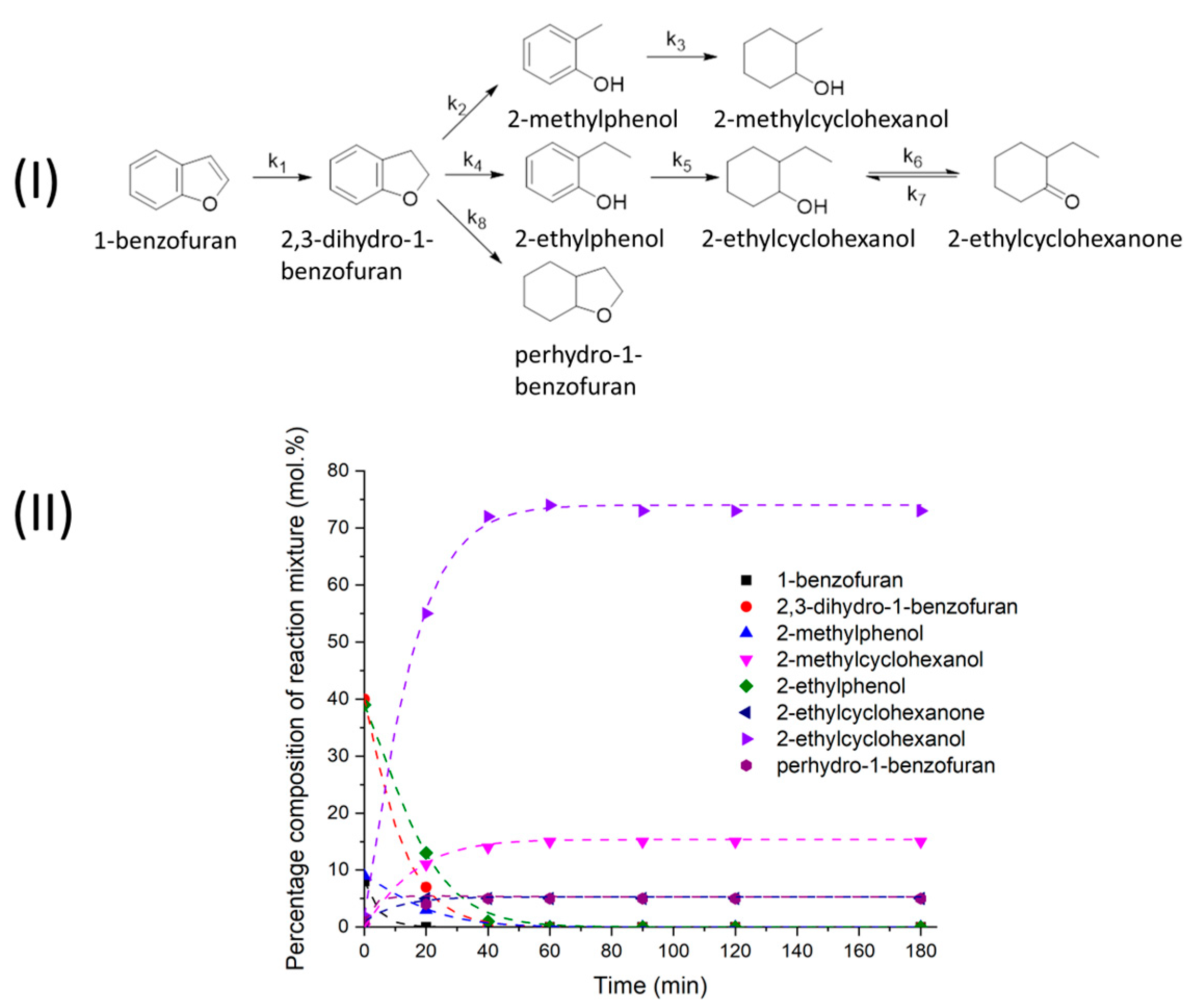
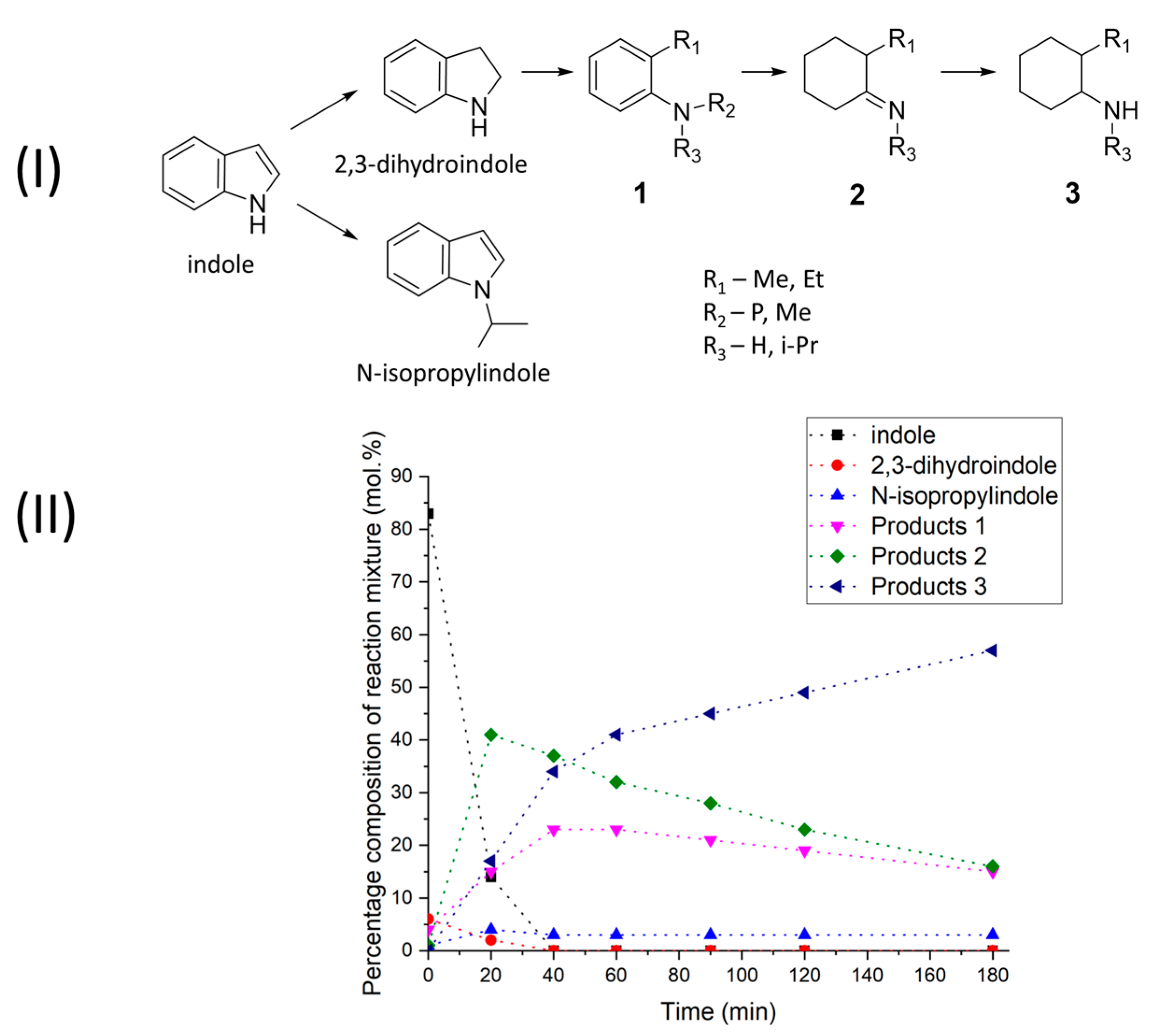

| Kinetic Model | k1 × 104, s−1 | k2 × 104, s−1 | K |
|---|---|---|---|
| Equation (1) | 2.14 ± 0.14 | 0.04 ± 0.03 | - |
| Equation (2) | 8.37 ± 0.83 | 0.20 ± 0.02 | 0.016 ± 0.002 |
| Kinetic Model | k1 × 104, s−1 | k2 × 104, s−1 | k3 × 104, s−1 | k4 × 104, s−1 | k5 × 104, s−1 | k6 × 104, s−1 | k7 × 104, s−1 | k8 × 104, s−1 |
|---|---|---|---|---|---|---|---|---|
| Equation (1) | 34.84 ± 22.60 | 2.24 ± 0.13 | 16.04 ± 1.37 | 13.24 ± 0.61 | 18.84 ± 0.43 | 20.65 ± 1.68 | 275.75 ± 14.86 | 1.51 ± 0.10 |
| Sample | Phase | Lattice Parameter, Å | DXRD, nm |
|---|---|---|---|
| Fresh | Ni0 | a = 3.527 (1) | 5.0 (5) |
| γ-Al2O3 | a = 7.950 (3) | 3.0 (5) | |
| After naphthalene | Ni0 | a = 3.528 (1) | 5.5 (5) |
| γ-Al2O3 | a = 7.950 (3) | 3.0 (5) | |
| After 1-benzofuran | Ni0 | a = 3.527 (1) | 5.5 (5) |
| γ-Al2O3 | a = 7.950 (3) | 3.0 (5) | |
| After indole | Ni0 | a = 3.527 (1) | 5.5 (5) |
| γ-Al2O3 | a = 7.950 (3) | 3.0 (5) | |
| After 1-benzothiophene | Ni0 | a = 3.540 (2) | 4.5 (5) |
| Ni2S3 | a = b = 5.745 (1), c = 7.135 (1) | 18.0 (5) | |
| γ-Al2O3 | a = 7.950 (3) | 3.0 (5) |
| Element | el.% Al | el.% O | el.% Ni | el.% N | el.% S | N/Ni | S/Ni | Al/Ni | |
|---|---|---|---|---|---|---|---|---|---|
| Sample | |||||||||
| After naphthalene | 19.0 | 64.9 | 16.2 | - | - | - | - | 1.2 | |
| After 1-benzofuran | 18.1 | 63.7 | 18.2 | - | - | - | - | 1.0 | |
| After indole | 20.0 | 64.7 | 14.0 | 1.4 | - | 0.10 | - | 1.4 | |
| After 1-benzothiophene | 14.1 | 62.4 | 18.0 | - | 5.5 | - | 0.3 | 0.8 | |
| Element | el.% N | el.% O | el.% Al | el.% Ni | |
|---|---|---|---|---|---|
| Sample | |||||
| Ni–Alum catalyst after indole transformation | 0.2 | 46.4 | 20.8 | 26.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nesterov, N.S.; Pakharukova, V.P.; Philippov, A.A.; Prosvirin, I.P.; Shalygin, A.S.; Martyanov, O.N. Reductive Transformation of O-, N-, S-Containing Aromatic Compounds under Hydrogen Transfer Conditions: Effect of the Process on the Ni-Based Catalyst. Molecules 2023, 28, 7041. https://doi.org/10.3390/molecules28207041
Nesterov NS, Pakharukova VP, Philippov AA, Prosvirin IP, Shalygin AS, Martyanov ON. Reductive Transformation of O-, N-, S-Containing Aromatic Compounds under Hydrogen Transfer Conditions: Effect of the Process on the Ni-Based Catalyst. Molecules. 2023; 28(20):7041. https://doi.org/10.3390/molecules28207041
Chicago/Turabian StyleNesterov, Nikolai S., Vera P. Pakharukova, Alexey A. Philippov, Igor P. Prosvirin, Anton S. Shalygin, and Oleg N. Martyanov. 2023. "Reductive Transformation of O-, N-, S-Containing Aromatic Compounds under Hydrogen Transfer Conditions: Effect of the Process on the Ni-Based Catalyst" Molecules 28, no. 20: 7041. https://doi.org/10.3390/molecules28207041
APA StyleNesterov, N. S., Pakharukova, V. P., Philippov, A. A., Prosvirin, I. P., Shalygin, A. S., & Martyanov, O. N. (2023). Reductive Transformation of O-, N-, S-Containing Aromatic Compounds under Hydrogen Transfer Conditions: Effect of the Process on the Ni-Based Catalyst. Molecules, 28(20), 7041. https://doi.org/10.3390/molecules28207041