Multi-Omics Elucidates Difference in Accumulation of Bioactive Constituents in Licorice (Glycyrrhiza uralensis) under Drought Stress
Abstract
:1. Introduction
2. Results
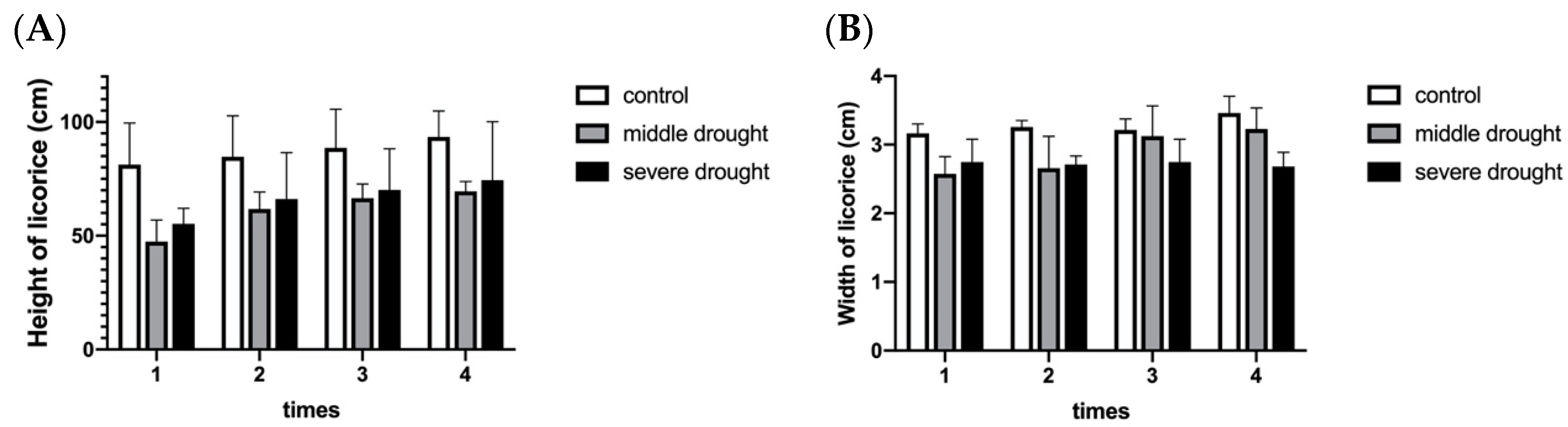
2.1. Morphological Observation and Antioxidant Enzyme Activities of Licorice under Drought Stress
2.2. GO and KEGG Analysis of DEPs and DEGs
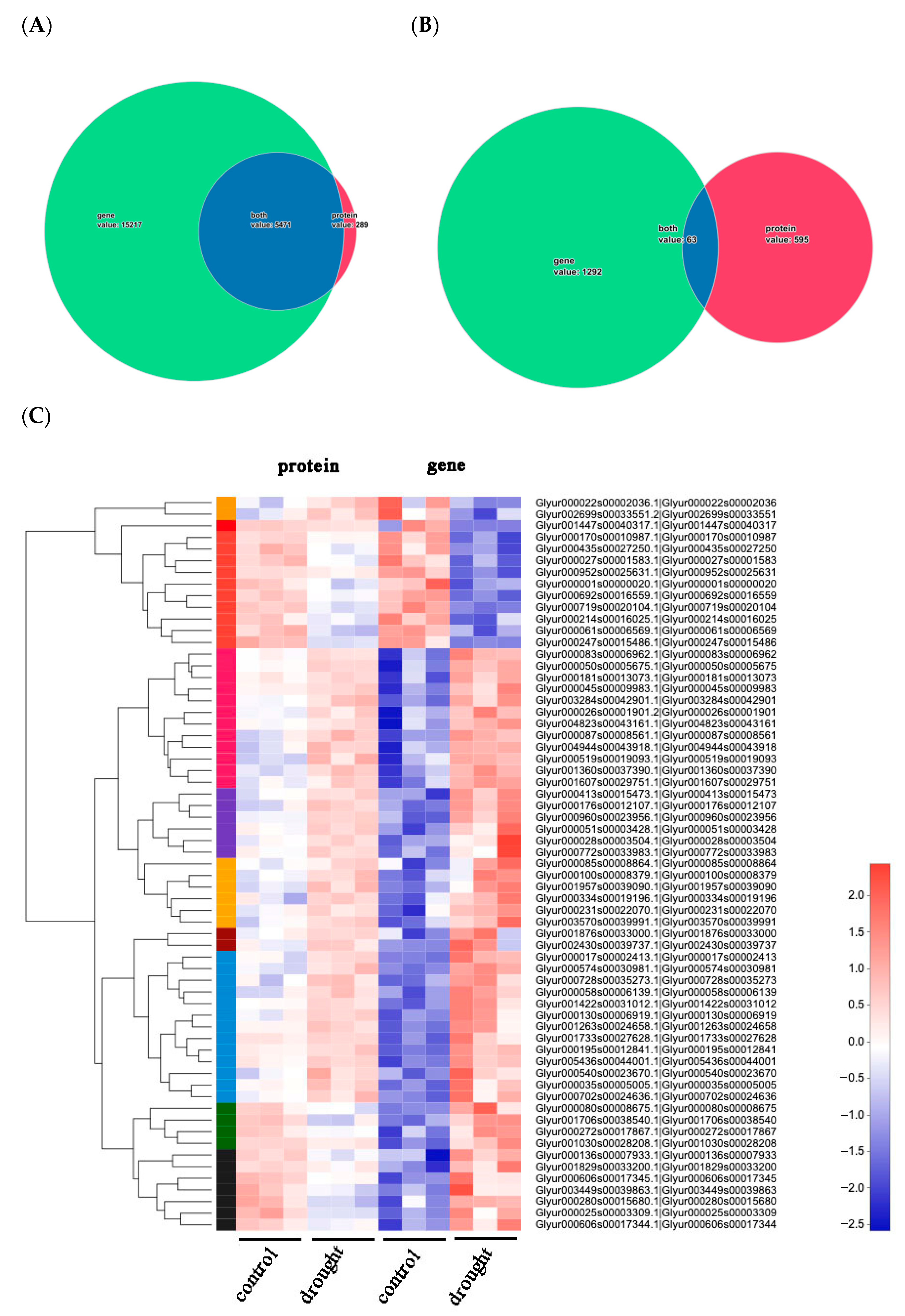
2.2.1. Global Analysis of Protein and Gene Data
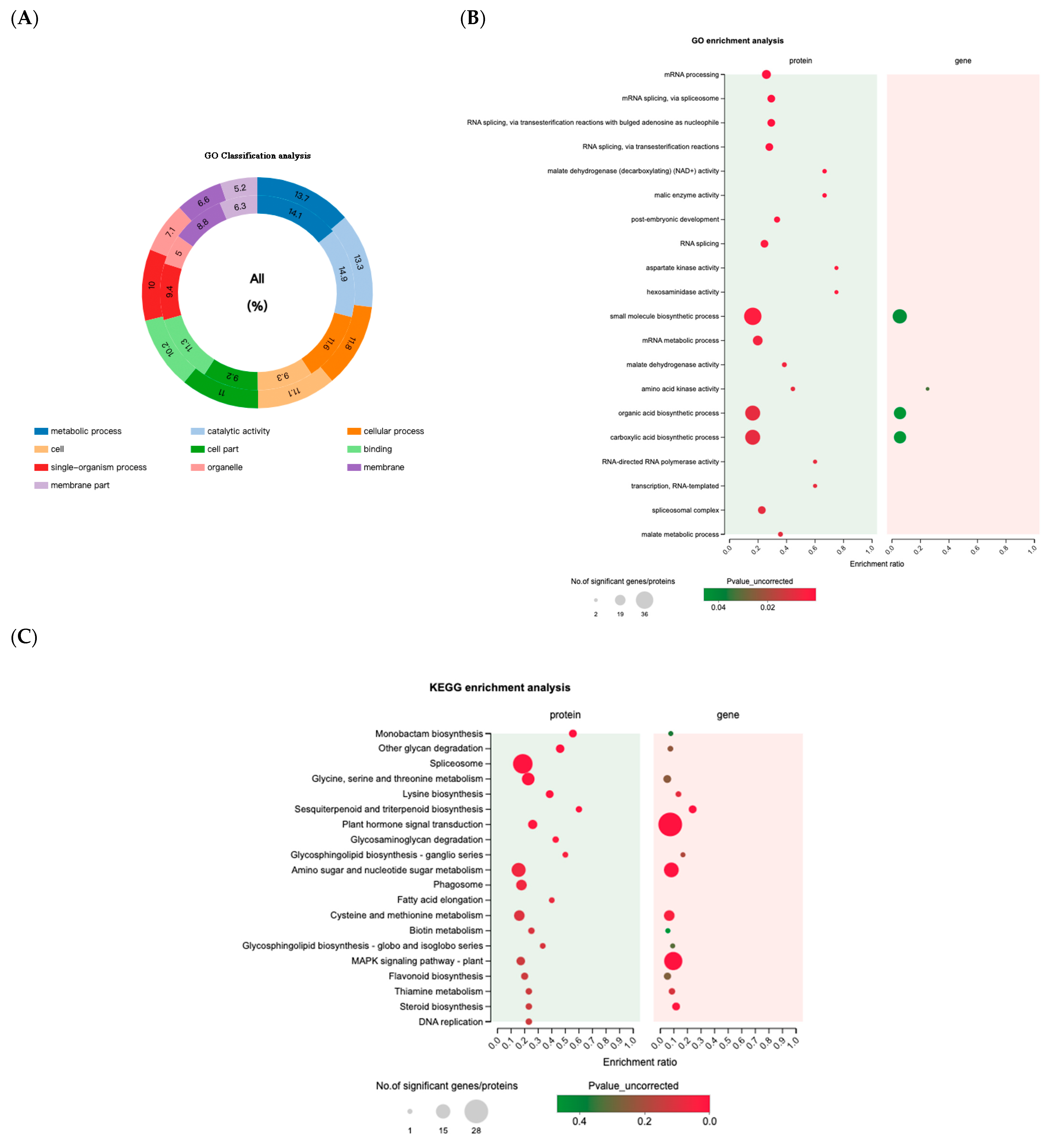
2.2.2. Functional Classification and Enrichment Analysis of DEPs and DEGs
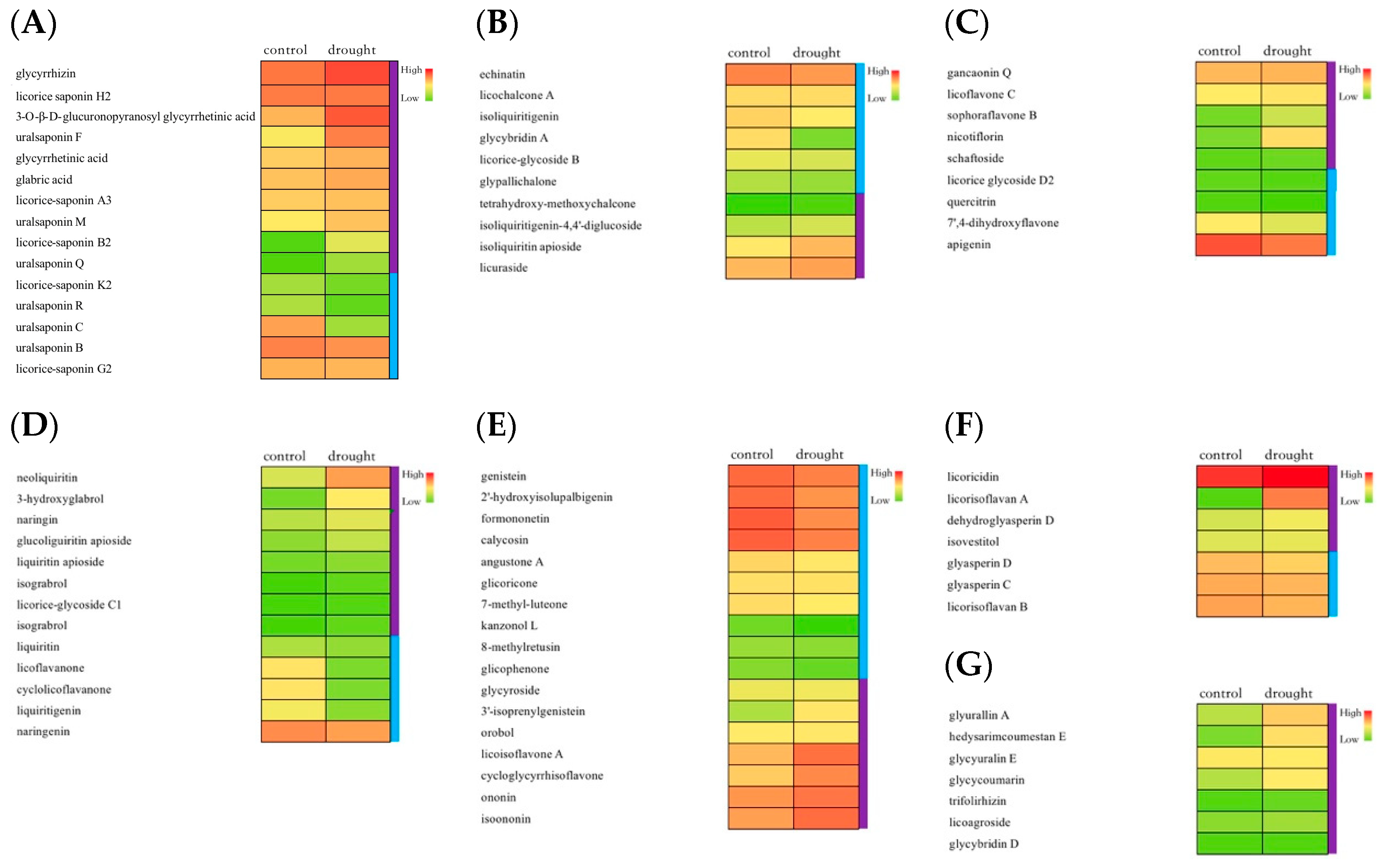
2.3. Determination of Multiple Bioactive Constituents
2.4. Advanced Analysis of Transcriptome and Proteome
2.4.1. Cluster Analysis of Basic Information
2.4.2. Annotation and Enrichment Analysis of GO and KEGG in Common DEPs and DEGs
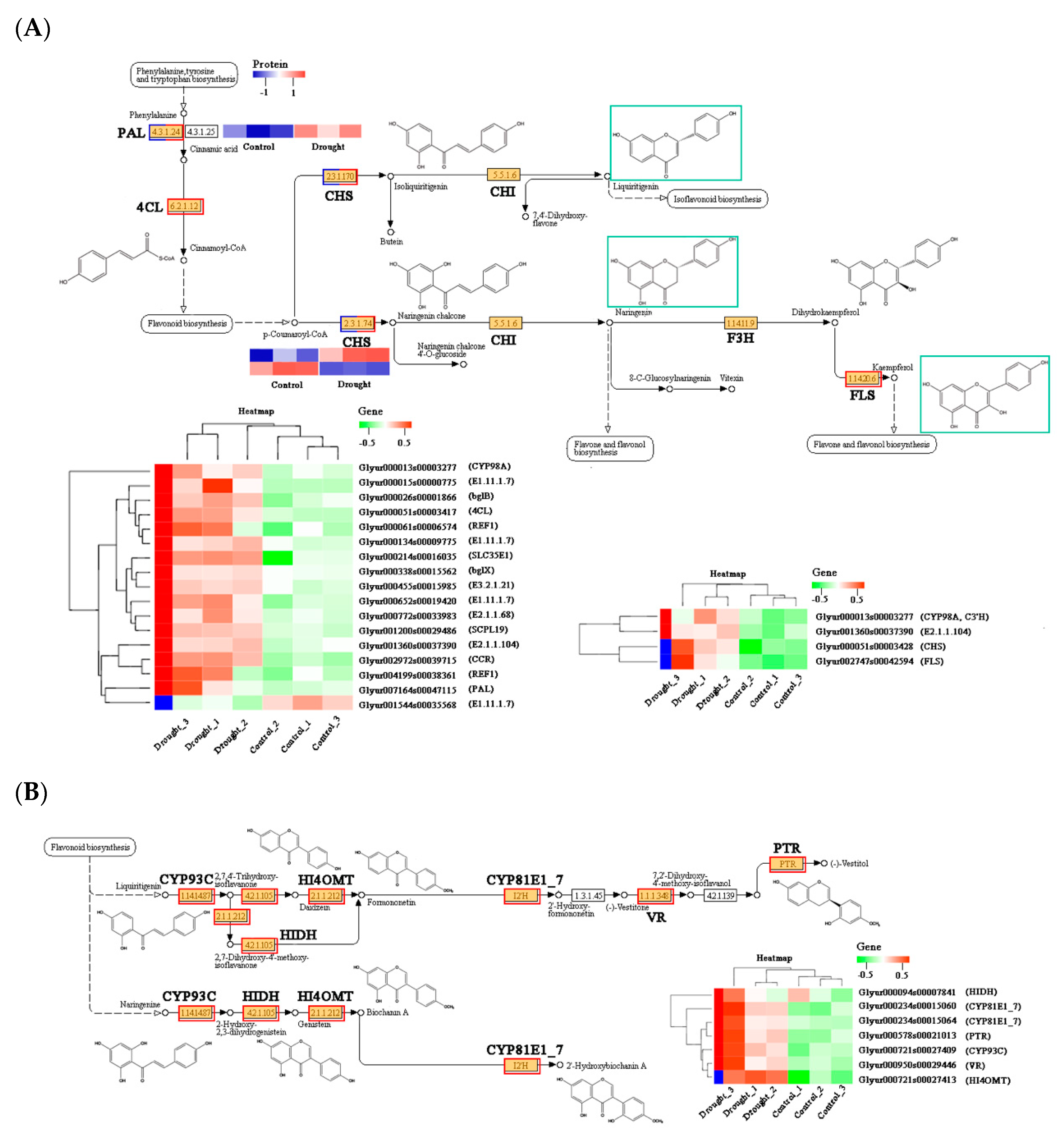
2.5. Combined Analysis of Key DEPs and DEGs in Triterpenoid and Flavonoid Pathways
2.5.1. Correlation Analysis among Transcripts, Proteins and Triterpenoid Derivatives
2.5.2. Correlation Analysis among Transcripts, Proteins, and Flavonoid Derivatives
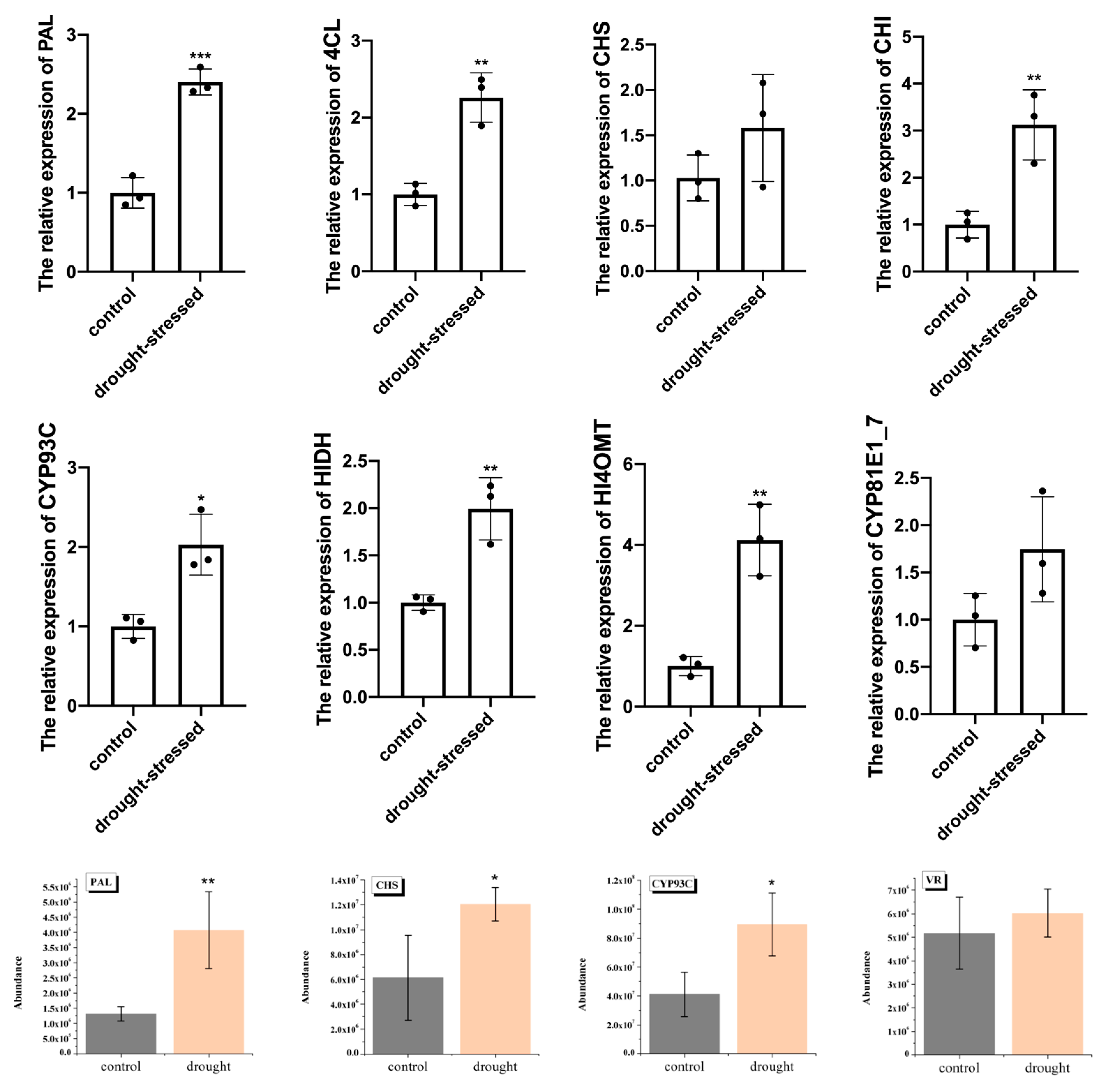
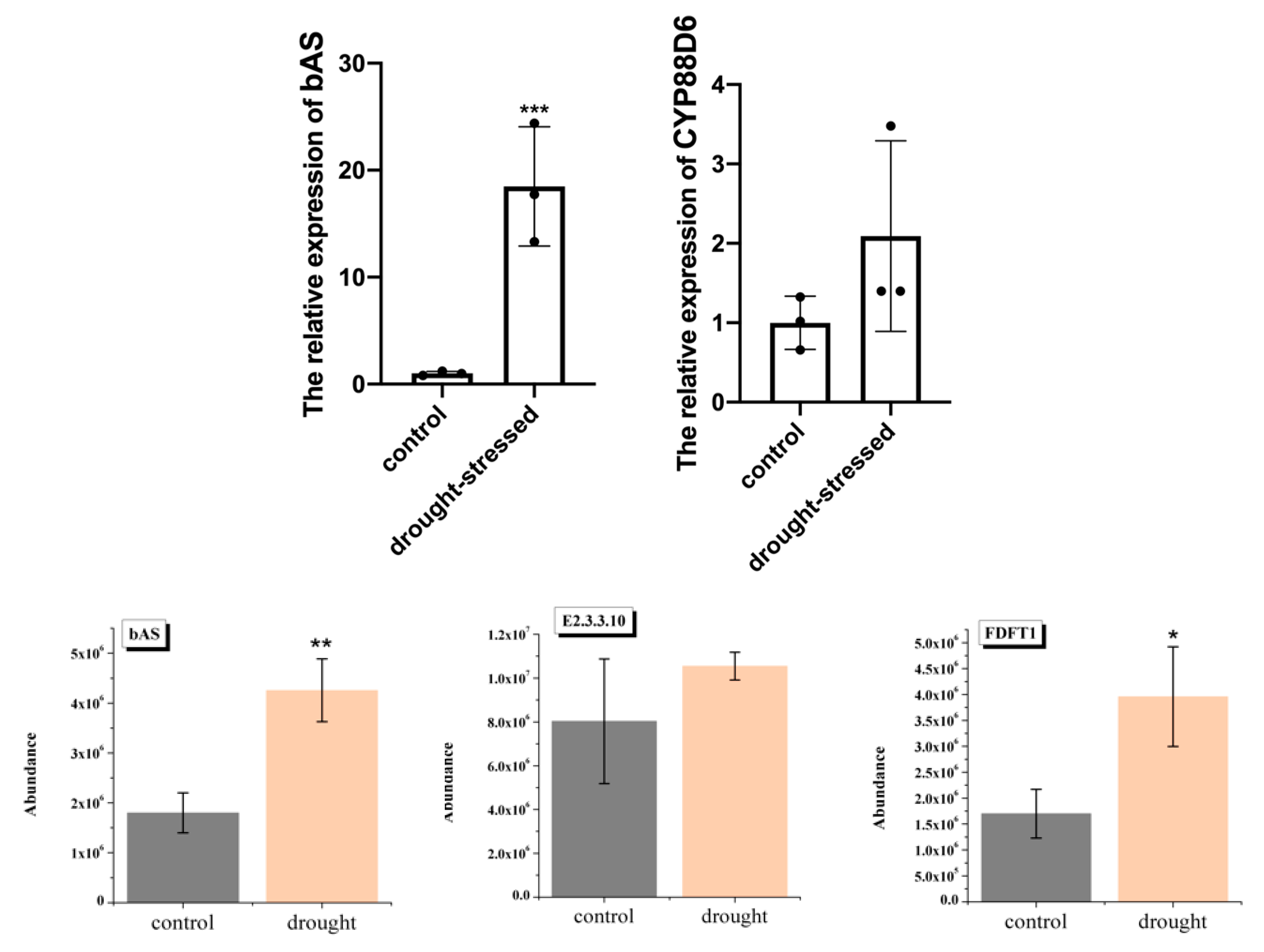
2.6. Quantitative Analysis of Selected Genes and Proteins Involved in Flavonoid and Triterpenoid Biosynthesis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Drought Treatments
4.2. Physiological Experiment
4.3. Proteome Experiment
4.3.1. Protein Extraction, Digestion, and TMT Labeling
4.3.2. High-pH Separation and LC–MS/MS Analysis
4.3.3. Parallel Reaction Monitoring Analysis
4.4. Transcriptome Experiment
4.4.1. RNA Isolation and cDNA Library Construction
4.4.2. Transcriptome Sequencing and Functional Annotation
4.4.3. Quantitative Real-Time Polymerase Chain Reaction Analysis
4.5. Metabolite Profiles of Licorice Bioactive Constituents
4.6. Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xiang, C.; Qiao, X.; Ye, M.; Guo, D.A. Classification and distribution analysis of components in Glycyrrhiza using licorice compounds database. Acta Pharm. Sin. 2012, 47, 1023–1030. [Google Scholar]
- Song, W.; Si, L.; Ji, S.; Wang, H.; Fang, X.M.; Yu, L.Y.; Li, R.Y.; Liang, L.N.; Zhou, D.; Ye, M. Uralsaponins M-Y, antiviral triterpenoid saponins from the roots of Glycyrrhiza uralensis. J. Nat. Prod. 2014, 77, 1632–1643. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, L.; Shan, L.; Fan, G.; Gao, X. Liquorice, aunique “guide drug” of traditional Chinese medicine: A review of its role in drug interactions. J. Ethnopharmacol. 2013, 150, 781–790. [Google Scholar] [CrossRef]
- Bailly, C.; Vergote, G. Glycyrrhizin: An alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol. Ther. 2020, 214, 107618. [Google Scholar] [CrossRef]
- Sikander, M.; Malik, S.; Rodriguez, A.; Yallapu, M.M.; Narula, A.S.; Satapathy, S.K.; Dhevan, V.; Chauhan, S.C.; Jaggi, M. Role of Nutraceuticals in COVID-19 Mediated Liver Dysfunction. Molecules 2020, 25, 5905. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, L.H.; Cai, Z.C.; Chen, C.H.; Liu, Z.X.; Liu, S.J.; Zou, L.S.; Tan, M.X.; Chen, J.L.; Liu, X.H.; et al. Metabolite Profiling and Transcriptome Analysis Explains Difference in Accumulation of Bioactive Constituents in Licorice (Glycyrrhiza uralensis) Under Salt Stress. Front. Plant Sci. 2021, 12, 727882. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Chen, L.H.; Cai, Z.C.; Chen, C.H.; Liu, Z.X.; Liu, X.H.; Zou, L.S.; Chen, J.L.; Tan, M.X.; Wei, L.F.; et al. Comparative Proteomic Analysis Reveals the Molecular Mechanisms Underlying the Accumulation Difference of Bioactive Constituents in Glycyrrhiza uralensis Fisch under Salt Stress. J. Agric. Food. Chem. 2020, 68, 1480–1493. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, L.H.; Cai, Z.C.; Chen, C.H.; Liu, Z.X.; Liu, X.H.; Zou, L.S.; Chen, J.L.; Tan, M.X.; Wei, L.F.; et al. Dynamic Variations in Multiple Bioactive Constituents under Salt Stress Provide Insight into Quality Formation of Licorice. Molecules 2020, 24, 3670. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, Z.; Fan, X.; Zhou, Q.; Cao, J.; Ji, G.; Jing, S.; Feng, B.; Wang, T. Transcriptome Analysis Reveals Complex Molecular Mechanisms Underlying UV Tolerance of Wheat (Triticum aestivum L.). J. Agric. Food Chem. 2019, 67, 563–577. [Google Scholar] [CrossRef]
- Zhou, Y.; Yao, L.; Huang, X.; Li, Y.; Wang, C.; Huang, Q.; Yu, L.; Pan, C. Transcriptomics and metabolomics association analysis revealed the responses of Gynostemma pentaphyllum to cadmium. Front. Plant Sci. 2023, 14, 1265971. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Q.; Wei, S.; Wang, D.; Fang, Y.; Liu, F.; Zhao, Z.; Hou, J.; Wang, W. Effect of genotype and environment on five bioactive constituents of cultivated licorice (Glycyrrhiza uralensis) populations in northern China. Biol. Pharm. Bull. 2015, 38, 75–81. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Samsampour, D.; Ebrahimi, M.; Abadía, J.; Khanahmadi, M. Effect of drought stress on growth parameters, osmolyte contents, antioxidant enzymes and glycyrrhizin synthesis in licorice (Glycyrrhiza glabra L.) grown in the field. Phytochemistry 2018, 156, 124–134. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, D.; Liu, X.Y.; Li, M.; Tang, Y.F.; Mi, J.; Ren, G.X.; Liu, C.S. Multi-Omics Analysis Provides Crucial Insights into the Drought Adaptation of Glycyrrhiza uralensis Fisch. J. Agric. Food Chem. 2023, 71, 5391–5402. [Google Scholar] [CrossRef]
- Nasrollahi, V.; Mirzaie-Asl, A.; Piri, K.; Nazeri, S.; Mehrabi, R. The effect of drought stress on expression of key genes involved in biosynthesis of triterpenoid saponins in licorice (Glycyrrhiza glabra). Phytochemistry 2014, 103, 32–37. [Google Scholar] [CrossRef]
- Mochida, K.; Sakurai, T.; Seki, H.; Yoshida, T.; Takahagi, K.; Sawai, S.; Uchiyama, H.; Muranaka, T.; Saito, K. Draft genome assembly and annotation of Glycyrrhiza uralensis, a medicinal legume. Plant J. 2017, 89, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Seki, H.; Ohyama, K.; Sawai, S.; Mizutani, M.; Ohnishi, T.; Sudo, H.; Akashi, T.; Aoki, T.; Saito, K.; Muranaka, T. Licorice beta-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proc. Natl. Acad. Sci. USA 2008, 105, 14204–14209. [Google Scholar] [CrossRef] [PubMed]
- Seki, H.; Sawai, S.; Ohyama, K.; Mizutani, M.; Ohnishi, T.; Sudo, H.; Fukushima, E.O.; Akashi, T.; Aoki, T.; Saito, K.; et al. Triterpene Functional Genomics in Licorice for Identification of CYP72A154 Involved in the Biosynthesis of Glycyrrhizin. Plant Cell 2011, 23, 4112–4123. [Google Scholar] [CrossRef]
- Nomura, Y.; Seki, H.; Suzuki, T.; Ohyama, K.; Mizutani, M.; Kaku, T.; Tamura, K.; Ono, E.; Horikawa, M.; Sudo, H.; et al. Functional specialization of UDP-glycosyltransferase 73P12 in licorice to produce a sweet triterpenoid saponin, glycyrrhizin. Plant J. 2019, 99, 1127–1143. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.Y.; Seki, H.; Fujisawa, Y.; Shimoda, Y.; Hiraga, S.; Nomura, Y.; Saito, K.; Ishimoto, M.; Muranaka, T. A cellulose synthase-derived enzyme catalyses 3-O-glucuronosylation in saponin biosynthesis. Nat. Commun. 2020, 11, 5664. [Google Scholar] [CrossRef]
- Xu, G.J.; Cai, W.; Gao, W.; Liu, C.S. A novel glucuronosyl transferase has an unprecedented ability to catalyse continuous two step glucuronosylation of glycyrrhetinic acid to yield glycyrrhizin. New Phytol. 2016, 212, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, Y.; Ren, C.H.; Cheng, X.; Chen, J.H.; Ge, Z.Y.; Sun, Z.P.; Zhuo, X.; Sun, F.F.; Chen, Y.L.; et al. Identification of proteomic markers for ram spermatozoa motility using a tandem mass tag (TMT) approach. J. Proteom. 2020, 210, 103438. [Google Scholar] [CrossRef]
- Du, W.; Xiong, C.W.; Ding, J.; Nybom, H.; Ruan, C.J.; Guo, H. Tandem Mass Tag Based Quantitative Proteomics of Developing Sea Buckthorn Berries Reveals Candidate Proteins Related to Lipid Metabolism. J. Proteome Res. 2019, 18, 1958–1969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yao, X.; Ren, H.; Chang, J.; Wang, K. RNA-Seq Reveals Flavonoid Biosynthesis-Related Genes in Pecan (Carya illinoinensis) Kernels. J. Agric. Food Chem. 2019, 67, 148–158. [Google Scholar] [CrossRef]
- Baker, A.; Lin, C.C.; Lett, C.; Karpinska, B.; Wright, M.H.; Foyer, C.H. Catalase: A critical node in the regulation of cell fate. Free Radic. Biol. Med. 2023, 199, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Saed-Moucheshi, A.; Sohrabi, F.; Fasihfar, E.; Baniasadi, F.; Riasat, M.; Mozafari, A.A. Superoxide dismutase (SOD) as a selection criterion for triticale grain yield under drought stress: A comprehensive study on genomics and expression profiling, bioinformatics, heritability, and phenotypic variability. BMC Plant Biol. 2021, 21, 148. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yi, H. Enhancement of drought tolerance in Arabidopsis plants induced by sulfur dioxide. Ecotoxicology 2022, 31, 637–648. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants under Abiotic Stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Cai, Z.C.; Shi, J.J.; Chen, S.Y.; Tan, M.X.; Chen, J.L.; Chen, L.H.; Zou, L.S.; Chen, C.H.; Liu, Z.X.; et al. Comparative Metabolite Profiling of Wild and Cultivated Licorice Based on Ultra-Fast Liquid Chromatography Coupled with Triple Quadrupole-Time of Flight Tandem Mass Spectrometry. Chem. Pharm. Bull. 2019, 67, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.X.; Li, N.; Wang, T.; Yang, M.Y. Research progress of flavonoids in liquorice. China J. Chin. Materia Medica 2003, 7, 10–14. [Google Scholar]
- Wu, C.; Wang, Y.; Sun, H. Targeted and untargeted metabolomics reveals deep analysis of drought stress responses in needles and roots of Pinus taeda seedlings. Front. Plant Sci. 2023, 13, 1031466. [Google Scholar] [CrossRef] [PubMed]
- Karlson CK, S.; Mohd Noor, S.N.; Khalid, N.; Tan, B.C. CRISPRi-Mediated Down-Regulation of the Cinnamate-4-Hydroxylase (C4H) Gene Enhances the Flavonoid Biosynthesis in Nicotiana tabacum. Biology 2022, 11, 1127. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, L.H.; Xu, C.Q.; Shi, J.J.; Chen, S.Y.; Tan, M.X.; Chen, J.L.; Zou, L.S.; Chen, C.H.; Liu, Z.X.; et al. A Comprehensive Review for Phytochemical, Pharmacological, and Biosynthesis Studies on Glycyrrhiza spp. Am. J. Chin. Med. 2020, 48, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xie, Z.; Wang, L.; Li, M.; Lang, D.; Zhang, X. Silicon alleviates salt and drought stress of Glycyrrhiza uralensis seedling by altering antioxidant metabolism and osmotic adjustment. J. Plant Res. 2017, 130, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lv, Q.; Ma, C.; Qu, J.; Cai, F.; Deng, J.; Huang, J.; Ran, P.; Shi, T.; Chen, Q. Metabolite Profiling and Transcriptome Analyses Provide Insights into the Flavonoid Biosynthesis in the Developing Seed of Tartary Buckwheat (Fagopyrum tataricum). J. Agric. Food Chem. 2019, 67, 11262–11276. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, H.; Yuan, Y. A Comparison of the Flavonoid Biosynthesis Mechanisms of Dendrobium Species by Analyzing the Transcriptome and Metabolome. Int. J. Mol. Sci. 2022, 23, 11980. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Chen, W.; Wang, W.; Zhang, H.; Liu, X.; Luo, J. Comprehensive profiling and natural variation of flavonoids in rice. J. Integr. Plant Biol. 2014, 56, 876–886. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol. Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef]
- Kubra, G.; Khan, M.; Munir, F.; Gul, A.; Shah, T.; Hussain, A.; Caparrós-Ruiz, D.; Amir, R. Expression Characterization of Flavonoid Biosynthetic Pathway Genes and Transcription Factors in Peanut Under Water Deficit Conditions. Front. Plant Sci. 2021, 12, 680368. [Google Scholar] [CrossRef]
- Kleinwächter, M.; Selmar, D. New insights explain that drought stress enhances the quality of spice and medicinal plants: Potential applications. Agron. Sustain. Dev. 2015, 120, 295–304. [Google Scholar] [CrossRef]
- Gharibi, S.; Sayed Tabatabaei, B.E.; Saeidi, G.; Talebi, M.; Matkowski, A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech.f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind. Crop. Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The flavonoid biosynthetic pathway in Arabidopsis: Structural and genetic diversity. Plant Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Mori, T.; Saito, K. Alternation of flavonoid accumulation under drought stress in Arabidopsis thaliana. Plant Signal Behav. 2014, 9, e29518. [Google Scholar] [CrossRef]
- Bai, Y.C.; Li, C.L.; Zhang, J.W.; Li, S.J.; Luo, X.P.; Yao, H.P.; Chen, H.; Zhao, H.X.; Park, S.U.; Wu, Q. Characterization of two tartary buckwheat R2R3-MYB transcription factors and their regulation of proanthocyanidin biosynthesis. Physiol. Plant 2014, 152, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Savoi, S.; Wong, D.C.; Arapitsas, P.; Miculan, M.; Bucchetti, B.; Peterlunger, E.; Fait, A.; Mattivi, F.; Castellarin, S.D. Transcriptome and metabolite profiling reveals that prolonged drought modulates the phenylpropanoid and terpenoid pathway in white grapes (Vitis vinifera L.). BMC Plant Biol. 2016, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qiao, L.; Su, X.; Ji, B.; Dong, C. Drought Stress Stimulates the Terpenoid Backbone and Triterpenoid Biosynthesis Pathway to Promote the Synthesis of Saikosaponin in Bupleurum chinense DC. Roots. Molecules 2022, 27, 5470. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, N.; Tindjau, R.; Wong, D.C.J.; Matzat, T.; Haslam, T.; Song, C.; Gambetta, G.A.; Kunst, L.; Castellarin, S.D. Drought stress modulates cuticular wax composition of the grape berry. J. Exp. Bot. 2020, 71, 3126–3141. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, Y.; Zhang, Q.; Cheng, L.; Han, M.; Ren, Y.; Yang, L. Effects of drought-re-watering-drought on the photosynthesis physiology and secondary metabolite production of Bupleurum chinense DC. Plant Cell Rep. 2019, 38, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Bourmaud, A.; Gallien, S.; Domon, B. Parallel reaction monitoring using quadrupole-Orbitrap mass spectrometer: Principle and applications. Proteomics 2016, 16, 2146–2215. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wu, D.; Jiang, L.; Liu, X.; Xie, T. Multi-Omics Elucidates Difference in Accumulation of Bioactive Constituents in Licorice (Glycyrrhiza uralensis) under Drought Stress. Molecules 2023, 28, 7042. https://doi.org/10.3390/molecules28207042
Wang C, Wu D, Jiang L, Liu X, Xie T. Multi-Omics Elucidates Difference in Accumulation of Bioactive Constituents in Licorice (Glycyrrhiza uralensis) under Drought Stress. Molecules. 2023; 28(20):7042. https://doi.org/10.3390/molecules28207042
Chicago/Turabian StyleWang, Chengcheng, Dawei Wu, Liying Jiang, Xunhong Liu, and Tiantian Xie. 2023. "Multi-Omics Elucidates Difference in Accumulation of Bioactive Constituents in Licorice (Glycyrrhiza uralensis) under Drought Stress" Molecules 28, no. 20: 7042. https://doi.org/10.3390/molecules28207042
APA StyleWang, C., Wu, D., Jiang, L., Liu, X., & Xie, T. (2023). Multi-Omics Elucidates Difference in Accumulation of Bioactive Constituents in Licorice (Glycyrrhiza uralensis) under Drought Stress. Molecules, 28(20), 7042. https://doi.org/10.3390/molecules28207042




