Abstract
Peptides, functional nutrients with a size between those of large proteins and small amino acids, are easily absorbed by the human body. Therefore, they are seeing increasing use in clinical medicine and have revealed immunomodulatory and anti-inflammatory properties which could make them effective in healing skin wounds. This review sorted and summarized the relevant literature about peptides during the past decade. Recent works on the extraction, modification and synthesis of peptides were reviewed. Importantly, the unique beneficial effects of peptides on the skin were extensively explored, providing ideas for the development and innovation of peptides and laying a knowledge foundation for the clinical application of peptides.
1. Introduction
With the development and renewal of science and technology, researchers eventually discovered a class of organic compounds whose molecular weight lies between proteins and amino acids. These compounds are easily absorbed, require low energy consumption to produce, and demonstrate high affinity, specificity, and low toxicity [1]. These compounds are known as peptides, and have been revealed as new components of therapeutic drugs. An increasing number of studies have proved that peptides have unique efficacy in antibacterial, anti-inflammatory, and anti-tumor aspects [2,3]. Given their attractive pharmacological and intrinsic properties, peptides are considered an excellent starting point for the design of new therapies, with good safety, tolerability, and efficacy in clinical application [4]. This provides huge advantages over traditional small molecules. In addition, peptide-based therapies typically have a lower production complexity than protein-based biopharmaceuticals [5], which significantly reduces production costs. Therefore, in this regard, peptides are optimally positioned between small molecules and biopharmaceuticals, and given their increased use, suitable methods for efficiently extracting them from natural sources have become the focus of attention [6]. However, many studies have shown that naturally occurring peptides are generally not suitable for direct clinical application because of their inherent weaknesses [7], including poor chemical and physical stability, and short circulating plasma half-life [8]. To address these issues, researchers must conduct studies to improve the application of peptides derived from modification and synthesis.
Although it is not fatal, skin damage often increases pain and affects the self-image of the patient; regeneration and wound healing are also essential for tissue homeostasis and the survival of organisms [9]. The causes of skin wounding are diverse, and the underlying mechanisms of wound healing are equally complex, such as inflammation [10] and oxidative stress [11]. It is well known that increasing numbers of scholars are interested in the exploration of skin diseases. Peptides have revealed many biological functions, most notably as signaling/regulatory molecules in a variety of physiological processes, including anti-inflammatory, defense, immunity, and homeostasis. These have been identified as good choices for skin healing agents [12]. This review integrated recent studies, introduced the extraction, modification, and synthesis of peptides, and focused on exploring the research progress of peptides in the field of skin wounding, providing a good knowledge foundation for the future application and development of peptides.
2. Extraction of Peptides
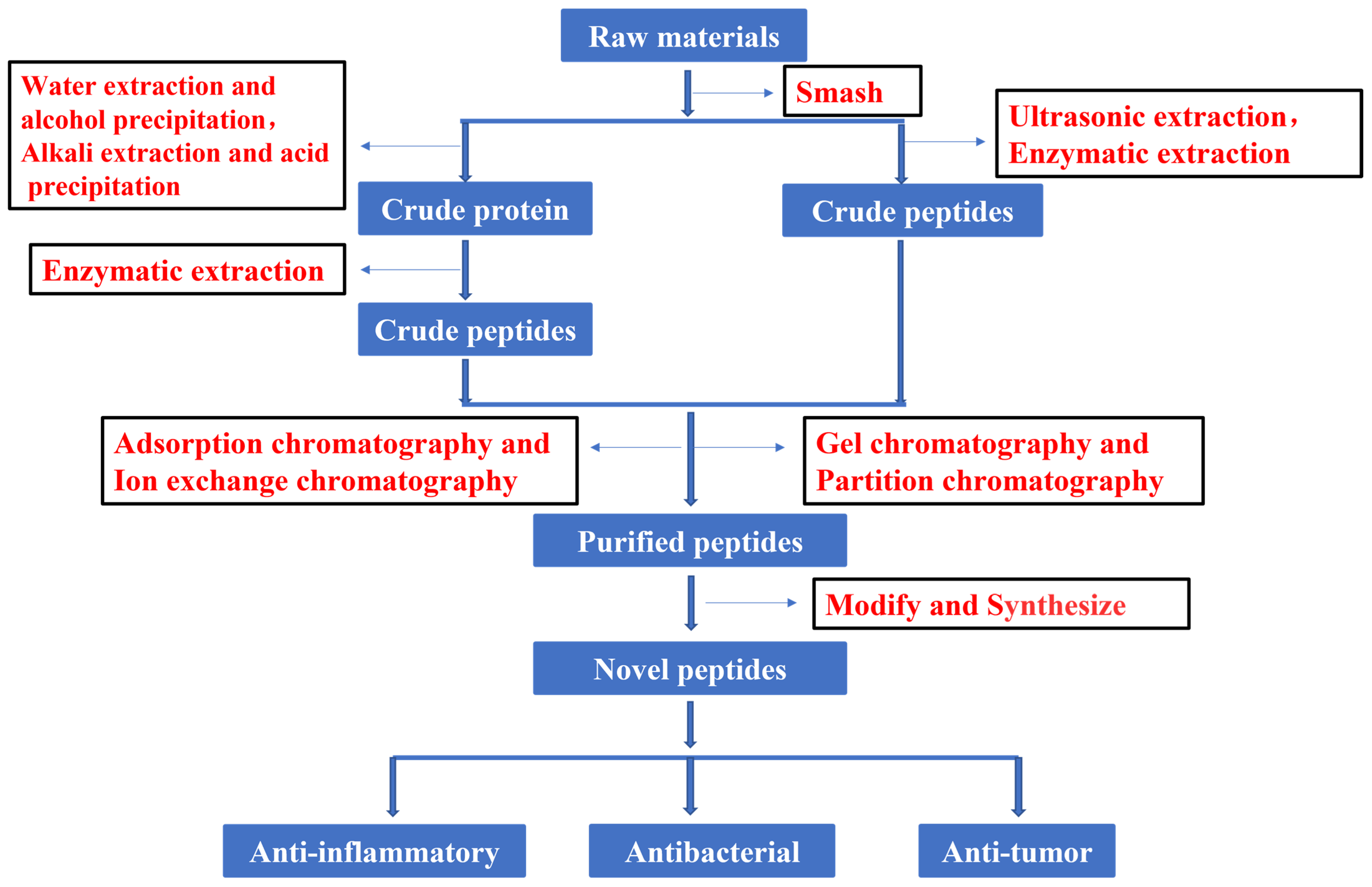
In recent years, much attention has focused on the extraction and purification of peptides. Figure 1 shows the current basic process for obtaining peptides. The development and utilization of peptides also provide new ideas for the innovation of therapeutic drugs. To increase the peptide extraction rate, enzymolysis and pretreatment are often used before extraction and separation. There are many types of proteases in nature. Proteases can be divided into three categories according to their origin: proteases of plant origin, proteases of animal origin, and proteases of microbial origin. Papain is a highly active endo-cysteine protease from papaya. It is one of the widely used proteases of plant origin. Trypsin is an important endoprotease in human and animal intestines. In the pancreas, trypsin is produced by activating trypsinogen [13]. Flavourzyme is sold as an industrial peptide enzyme preparation derived from Aspergillus oryzae [14]. Proteases can also be classified according to their pH value as alkaline proteases, neutral proteases, or acidic proteases. Although all three of these proteases are found in plants and animals, microbial populations are their most widespread source [15]. Researchers have generally applied five kinds of hydrolase (Flavourzyme, trypsin, acid protease, neutral protease, and alkaline protease) to extract antioxidant peptides from the mackerel (Scomberomorus niphoniusis) defatted visceral powder. The diphenyl bitter hydrazine radical scavenging rate, hydroxyl radical scavenging rate, and hydrolysis degree are used as indicators for the selection and optimization of hydrolytic enzymes to optimize the best hydrolysis solution [16]. This was the case with apricot kernel (Semen Armeniacae Amarum) hydrolysate that was obtained by hydrolysis and degreasing with the compound protease of alkaline protease and Flavourzyme [17]. Some studies have used trypsin, Flavourzyme, and neutral and alkaline protease to extract antioxidant proteins from frog breast oil (Ranae Oviductus) [18].
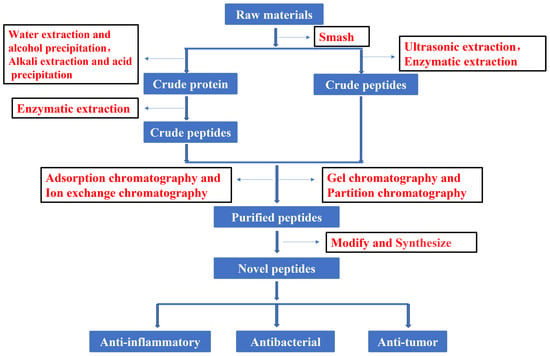
Figure 1.
Flow chart of peptide process.
After obtaining the crude extract, chromatography is often applied to separate the desired peptides. According to the separation principle, chromatography can be divided into adsorption chromatography, ion-exchange chromatography, gel chromatography, and distribution chromatography. Experiments have demonstrated that the cation exchange column has been widely used in the separation and purification of peptides. This is the case for the separation and analysis of active antioxidant peptides from mackerel, and it was found to be the most suitable chromatographic method in [16]. Reportedly, the cation exchange column was also developed to enrich protein N-terminal peptides. Briefly, N-terminal peptides with or without n-acetylation can be separated from internal peptides by strong cation-exchange chromatography according to the charge/orientation retention type based on the peptides [19]. Surprisingly, the separation of responsible peptides from egg white hydrolysates [20] and antioxidant peptides from feather hydrolysates [21] was optimized by cation exchange chromatography and reverse-phase chromatography. Furthermore, it was reported that purified hirudin peptides were obtained from leeches (Hirudo) by strong base anion-exchange column chromatography and G10 gel column chromatography [22].
Gel filtration has also been the preferred option for obtaining the desired active ingredients from the crude extract. This was the case with the hydrolysates of pearl millet (Pennisetum glaucum) that were separated by gel filtration chromatography to obtain antioxidant peptides [23]. The active peptides of apricot kernel (Semen Armeniacae Amarum) hydrolysates were further isolated by gel filtration chromatography on Sephadex G-25 and G-15, and their antioxidant potential was further evaluated and proved [17]. In order to understand the taste of Philippine clams (Ruditapes philippinarum), 14 novel umami peptides were isolated and identified by gel chromatography, HPLC, and UPLC-ESI-QTOF-MS/MS [24]. A peptide was also found in the foot of green mussel (Perna canaliculus), which was purified by size-exclusion chromatography (SEC); its sequence was identified by LC-MS/MS and its anti-inflammatory effect was investigated by in vitro experiment [25].
For the separation of peptides, HPLC has gained the highest application value because of its high speed, high efficiency, and high sensitivity [26,27]. This was evident in peptides isolated from fermented milk (yogurt) [28] and rapeseed (Brassica campestris L.) [29] by preparative reversed-phase HPLC, then purified and analyzed by ESI-MS/MS. Notably, a novel peptide with antiplatelet activity was isolated and identified from silver carp (Hypophthalmichthys molitrix) skin by the same method [30], as was a novel peptide purified by solid-phase extraction and HPLC from the interleaf of banana (Musa paradisiaca) plants, with its primary structure determined by MS and amino acid analysis [31].
3. Modification of Peptides
Natural active peptides are known to play an irreplaceable role in immune regulation [31,32], immune hormones [33], enzyme inhibition [34], and antiviral properties [35,36]. Despite their potential use as therapeutic agents, there are many potential problems with natural peptides due to their low stability and proteolysis, resulting in short activity duration and low bioavailability in vivo. One way to overcome these shortcomings is to use modified peptides, known as peptides mimics [37]. For example, natural peptides found in venoms could be used directly in routine therapy, but many of these peptides might need to be truncated or stabilized to improve their therapeutic properties. Thus, a complementary strategy is the generation of peptides mimics by displaying key residues forming the pharmacophore of the peptide toxin on a non-peptide scaffold [38]. Some studies have proposed a chemical modification box for peptides, which was used for the modification of peptides’ skeleton, amino acid side chain, and higher-order structure. This method was used to overcome the main issues encountered during the transition from natural peptides to peptide therapeutic agents, therefore promoting the synthesis and development of solid-phase peptides [39]. To improve the activity and increase the function of peptides, the NMEGylation-covalent binding of oligo-N-methoxyethylglycine (NMEG) chains was evaluated as a novel form of peptide/protein modification, especially for the stability and solubility of C20 peptides [40]. In addition, a new type of peptide was designed by a modified method, which greatly broadened the application space of peptides in different fields. To form a novel peptide, a six-membered carbon ring with an amino group on the ring binds substituted amino acids to arginine-rich peptides. Further studies found the value of this peptide in the development of cell-penetrating peptides [41]. The physicochemical properties of peptides are generally regulated by introducing one or more methyl groups into peptidyl amide bonds, while the pharmacokinetic properties of peptides are endowed with unprecedented characteristics [42].
4. Synthesis of Peptides
The applications of different modification methods have significantly improved the inherent shortcomings of natural peptides, such as stability and cell penetration. In addition to designing new peptides by modification, it was possible to understand the synthesis of new compounds that do not exist in Nature by using different methods and means.
Previous studies provided new ideas for the development and utilization of peptides, as well as new therapeutic directions for clinical application. In one work it was reported that a peptide was synthesized based on a known chemical formula. The basic peptide components of the Lactobacillus casei peptidoglycan complex were used as a reference to compose this chemical formula, which has potential as an effective anti-tumor agent [43]. A new method has been developed in which lysine residues are linked to the C-terminal of the desired peptides by a standard peptide bond during synthesis. The immobilized carboxypeptidase B (CPB) is then used to remove these lysine residues after purification, thus improving the total synthesis and purification yield of the peptides [44]. Similarly, there is a method in which the heterozygous organic peptides’ macrocyclic compounds are synthesized by cyclizing ribosomal-derived peptide sequences with non-peptide organic connectors [45]. Furthermore, cyclic RGD peptides could be efficiently synthesized based on microflow triphosgene-mediated peptide chain extension and microflow photochemical macrocyclic lactamization [46]. A novel strategy was also described for the generation of bicyclic peptides containing non-peptide skeleton elements, starting from recombinant peptide precursors. These compounds were produced by a ‘one-pot and two-step’ sequence in which the peptides were macrocycled via bifunctional oxyamine/1,3-amino-thiol synthetic precursors, and then the intramolecular disulfide was formed between the synthetic precursor mercaptan and a cysteine embedded in the peptide sequence [47]. In another one-pot method, goadsporin (GS) was synthesized using recombinant enzymes in a flexible in vitro translation system (called the FIT-GS system) [48].
5. Beneficial Effects of Peptides on Skin
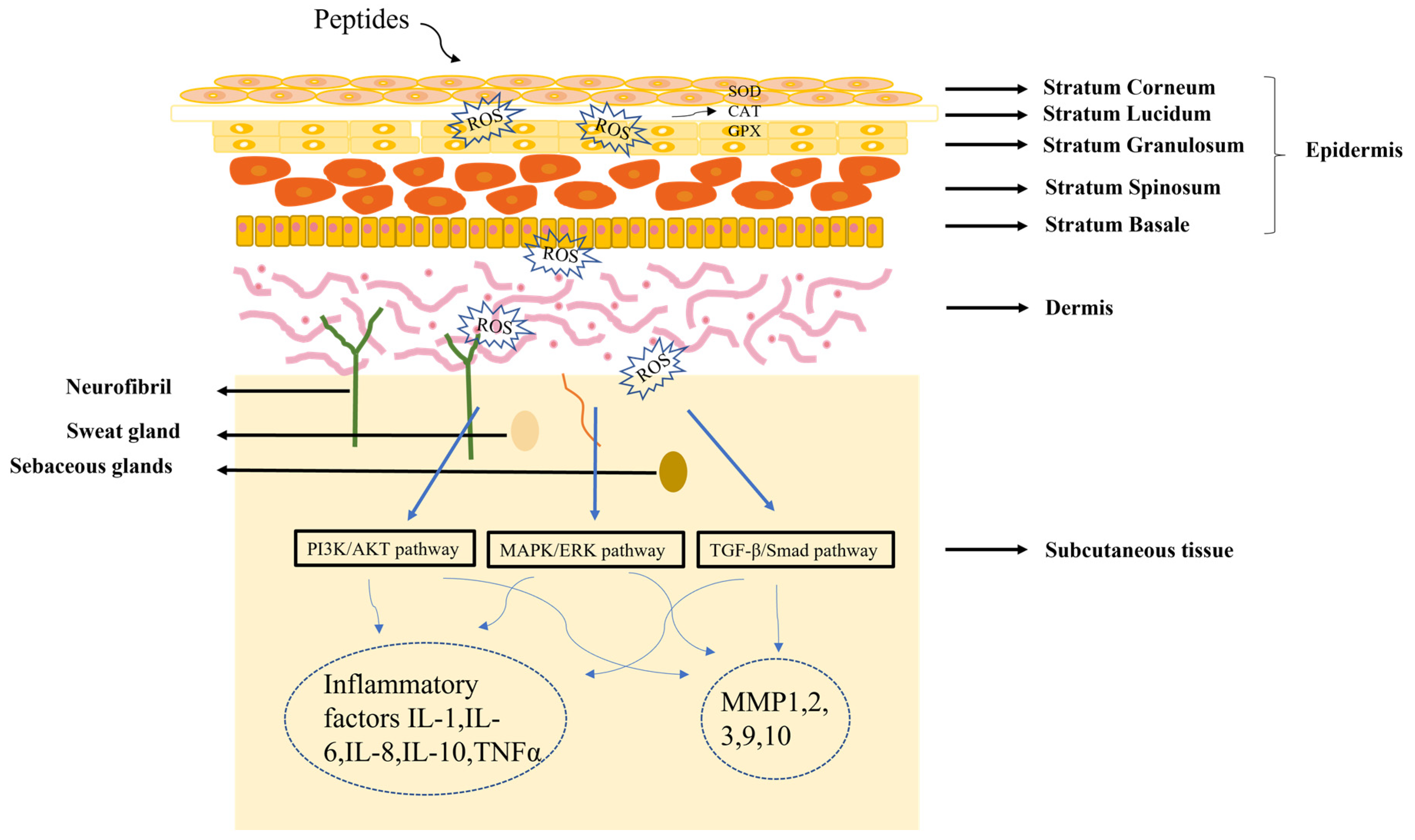
The skin is composed of epidermis, dermis and subcutaneous tissue. Understanding skin structure is fundamental for the treatment of all skin conditions. The healing of skin wounds is an important biological process which can regenerate new skin after a wound. Skin injuries can be divided into skin trauma and burns, skin disease, and skin cancer. Among them, chronic wounds caused by skin injuries and burns are the most common skin diseases due to the slow healing of hypoxia, abnormal peripheral sensory nerve function, and insufficient blood tissue supply. The most significant sign of chronic wounds is severe abnormal immune skin function [49]. The active components of peptides could serve as first-line innate immune defense against exogenous microorganisms in the skin, in addition to coordinating adaptive immune responses to perform various immunomodulatory functions. Different authors found that peptides repair skin damage through a variety of mechanisms (Figure 2) [50,51]. Many skin diseases and injuries have been reported to involve the production of ROS radicals [52], and a dramatic increase in ROS levels can cause oxidative stress. Peptides acting on the skin can have a therapeutic effect by inhibiting the production of ROS. In addition, the skin has a vast antioxidant system, including superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase (CAT) [53], and the therapeutic process of peptides on the skin involves the regulation of these factors. When skin pathology occurs, it is often regulated by the PI3K/AKT [54], MAPK/ERK [55], and TGFβ/Smad pathways [56]. Further studies have shown that peptides can regulate inflammatory factors (IL-1, IL-6, IL-8) or matrix metalloproteinases (MMP1, MMP2, MMP3) by PI3K/AKT, MAPK/ERK, and TGFβ/Smad pathways, thereby reducing the inflammatory response of the skin [57,58].
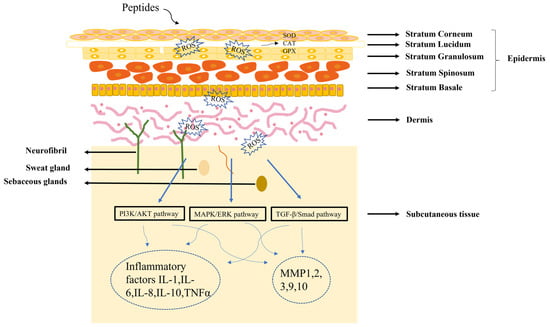
Figure 2.
Diagram of the mechanism of peptide treatment of skin damage.
5.1. Skin Burns and Trauma
There are many kinds of chronic skin wound disorders, such as the commonly observed skin burns [59,60] and trauma [61]. Researchers have discovered many medicines [62] and methods [63,64] that can treat chronic wounds. According to relevant data, effective peptides in skin injury have been widely extracted and found in Nature [65]. With the innovation and development of science and technology, scientists have gradually explored the specific mechanism of those peptides for skin therapy. Traditional herbal products are often applied for skin healing, and plant therapeutic agents such as honeysuckle (Lonicera japonica Thunb) [66], patchouli (Pogostemon cablin (Blanco) Benth) [67], and aloe (Aloe vera (L.) Burm.) [68] often act to promote wound healing through their various bioactive ingredients. However, researchers speculate that the content of plant peptides is low, and it is difficult to achieve effective treatment with peptides from plants alone. Therefore, new studies are seeking other species to broaden the range of skin treatments.
Animal peptides have attracted extensive attention from scholars as new molecular platforms for skin therapeutics [69]. For example, gecko duct analogs (GJ-CATH3) have been found to exhibit significant wound-healing properties in mouse models with full-thickness skin wounds. These peptides have the potential to stimulate HaCaT cell proliferation while also preventing a decrease in SOD activity and an increase in MDA concentration in damaged skin tissues [70]. Multifunctional peptides were found in salamander (Cynops orientalis) skin, and might play an important role in the host’s immune response to bacterial infection and skin wound repair [71,72].
Previous studies have shown that many aquatic animal peptides also have skin-healing properties. The relationship between wound healing and wound microbiome colonization was investigated by using skin collagen peptides of salmon (Oncorhynchus keta) and tilapia (Oreochromis mossambicus). Several studies revealed that collagen peptides were related to the regulation of microbial community colonization in wound tissue. They were also found to promote wound healing by controlling inflammatory response and increasing wound angiogenesis and collagen deposition [73,74]. Peptides extracted from the enzymatic digestion of perch (Lateolabrax japonicus) could also accelerate wound healing by enhancing the formation of microvessels at the wound site [75]. The active peptides (Aps) of pearl oyster (Pinctada Martensii) increased collagen synthesis and type III collagen content in wounds via the TGF-/Smad signaling pathway, inhibiting scar formation and promoting skin wound healing in [76].
It has been reported that amphibian skin has an excellent ability to enhance wound healing [77]. These active ingredients, especially polypeptide extracts, have been proven to effectively promote skin wound healing. Thus, polypeptide extracted from amphibian skin has great potential for skin repair [78]. According to Table 1, reporting on skin treatments with amphibian-sourced peptides, a new 24-residue peptide belonging to the ducting family was identified from the skin of the plateau frog (Nanorana pleskei) and has been shown to promote wound contraction and repair in in vivo and in vitro experiments [79]. Ot-WHP, as a wound-healing-promoting peptide from the Chinese concave-eared torrent frog (Odorrana tormota), has the same effect on skin treatment, according to another study [80]. There was also a new short peptide (named RL-QN15) in the skin secretions of Rana serrata that could regulate cytokines secreted by macrophages and accelerate re-epithelization and granulation tissue formation [81]. Researchers evaluated two peptides, called CW49 and pseudin-2 (Pse-T2), from frog (Odorrana graham) skin, showing that the former could treat skin injury by promoting angiogenesis [82], and the latter by destroying the membrane integrity to kill bacterial cells [83]. Peptides extracted from other frogs also had skin-healing properties [84,85,86,87,88,89,90,91,92,93].

Table 1.
Amphibians for skin treatment.
5.2. Infectious and Inflammatory Skin Disease
Some peptides have been revealed to induce cell proliferation, migration, and differentiation. These peptides could also regulate inflammatory response and control the production of various cytokines/chemokines. These capabilities enable them to promote wound healing and improve skin barrier function [95]. An increasing body of evidence proves that peptides play an important role in skin defense, and some have therapeutic effects on viral resistance in atopic eczema (AE) [96]. A homing peptide CRKDKC (CRK) was found to be widely used in wound recovery and angiogenesis in tumors, and the removal of cysteine from CRK produces a skin-homing therapeutic molecule (DCN-tCRK). Experiments in vivo have proved that this molecule could inhibit TGF in skin-β signal transduction, thereby improving recessive dystrophic epidermolysis bullosa [97]. There is also an emollient containing oat plant extract that has revealed anti-inflammatory and barrier repair properties to treat specific dermatitis [98]. Other antimicrobial peptides had similar dermatitis repair effects [99].
5.3. Neoplastic Skin Disease
Emerging evidence suggested that peptide components not only treated some inflammatory skin diseases but played a role in the healing of skin tumors [100]. An example of this was three antimicrobial peptides which were identified from frog skin secretions by ‘shotgun’ cloning and MS/MS fragmentation. By testing the antimicrobial and biofilm activity of microorganisms, they were found to induce bacterial death by destroying cell membranes and binding to bacterial DNA, thereby alleviating skin cancer [101]. Antimicrobial peptides obtained from insects were shown to have antibacterial and anti-inflammatory effects on the skin, but also anti-cancer effects [102]. Another study found that the insect-derived peptide poecilocorisin-1 had a potential therapeutic effect on malignant melanoma skin cancer [103]. The combined therapy of antimicrobial peptides and chemotherapeutic drugs was also developed as a new method for the treatment of skin cancer, and revealed a synergistic therapeutic effect on skin cancer in mice [104].
6. Clinical Application and Prospect of Peptides in Skin Healing
Skin wound healing, especially chronic wound healing, is a common and challenging clinical problem. The development and utilization of clinical therapeutic agents have attracted extensive attention [105,106,107]. There is thus an urgent need to develop new interventions to promote skin repair. Recent studies showed that both peptides and nanoparticles might be potential therapies for skin wounds [108,109]. Researchers synthesized antibacterial photodynamic gold nanoparticles (AP-AuNPs), which combined an antibacterial peptide and nanoparticles. AP-AuNPs are used as a wound-dressing nanomaterial in skin infections to promote wound healing [110]. In addition, ZnO nanoparticles of different sizes were also functionalized with an amphipathic peptide to improve their photoprotection capability in skin [111]. For example, marine peptides extracted from tilapia (Oreochromis mossambicus) were combined with a biological material called chitosan (CS) and used as a therapeutic agent for skin wound healing [112]. In many studies, researchers designed new peptide scaffolds to obtain peptides that could be stable in the wound environment, and their efficacy in promoting wound healing was demonstrated in vitro and in vivo [113]. The fusion of peptides and mediators could also form potential new drugs for the treatment of skin wounds and inflammation. One study was assessed to analyze the molecular design characteristics of peptide-based hydrogels for improving wound healing [114]. Transglutaminase (TG) was thus identified from the transcriptomes of Spirulina, and its free-radical-scavenging potential was evaluated. With the help of an electrostatically spun chitosan/polyvinyl alcohol nanofiber pad, this TG could promote wound healing in vitro [115]. Therefore, we expect to see the design of a carrier that could carry peptides, maximize the efficacy of peptides, expand the application range of peptides, and develop more drugs for skin treatment [1].
7. Discussion
There have been many attempts to explore and develop peptide extraction and synthesis methods to advance beyond traditional models and improve peptide availability. Therefore, this review summarized these extraction, modification, and synthesis methods to provide innovative ideas for the efficient utilization of peptides. In addition, it focused on the peptides that have shown protective and therapeutic effects on the skin. Although the potential of peptides for skin therapy was found in many organisms, many peptides with skin therapeutic properties are yet to be discovered. The current literature collection suggests that peptides are relatively scarce in clinical applications. One possible reason is that peptides are not easily stored, due to their facility to break down and deteriorate easily. Therefore, it is necessary to explore new ways to facilitate their clinical application, especially regarding their advantages in the skin field which could provide relief for patients suffering from skin injuries. Based on experimental evidence, it was found that peptides could affect skin mechanisms by regulating inflammation, oxidative stress, apoptosis, aging, and autophagy. These findings suggest that peptides can influence many pathophysiological processes and biochemical signaling pathways. Therefore, peptides have the potential for use as therapeutic agents for other diseases. The findings provide a theoretical reference for broader applications of peptides.
8. Conclusions
This review describes the extraction, modification and synthesis of peptides in recent years, which might provide ideas for the acquisition and development of novel peptides. In addition, this review highlights possible mechanisms of action of peptides in the treatment of skin diseases, and these findings suggest peptides could be candidates for the alleviation and treatment of skin diseases. However, many detailed studies are needed to clarify whether peptides have the same effect in other pathologies.
Author Contributions
Conceptualization, J.A.; methodology, J.A.; software, J.A.; validation, J.A.; formal analysis, J.A.; investigation, J.A.; resources, J.A.; data curation, J.A.; writing—original draft preparation, J.A.; writing—review and editing, J.A., I.S.N.T., Z.W. and W.L.; supervision, Z.W. and W.L.; project administration, Z.W. and W.L.; funding acquisition, Z.W. and W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grants of the Jilin Science & Technology Development Plan (No. 20200301037RQ).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://pubmed.ncbi.nlm.nih.gov/.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
SEC, size-exclusion chromatography; NMEG, N-methoxyethylglycine; CPB, carboxypeptidase B; Pse-T2, pseudin-2; AE, atopic eczema; CRKDKC, CRK; GS, goadsporin; MPs, marine peptides; CS, chitosan; TG, transglutaminase.
References
- Boparai, J.K.; Sharma, P.K. Mini Review on Antimicrobial Peptides, Sources, Mechanism and Recent Applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Yavari, B.; Mahjub, R.; Saidijam, M.; Raigani, M.; Soleimani, M. The Potential Use of Peptides in Cancer Treatment. Curr. Protein Pept. Sci. 2018, 19, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Agyei, D.; Ahmed, I.; Akram, Z.; Iqbal, H.M.N.; Danquah, M.K. Protein and Peptide Biopharmaceuticals: An Overview. Protein Pept. Lett. 2017, 24, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef]
- Borrajo, P.; Pateiro, M.; Barba, F.J.; Mora, L.; Franco, D.; Toldra, F.; Lorenzo, J.M. Antioxidant and Antimicrobial Activity of Peptides Extracted from Meat By-products: A Review. Food Anal. Method 2019, 12, 2401–2415. [Google Scholar] [CrossRef]
- Sarker, A. A review on the application of bioactive peptides as preservatives and functional ingredients in food model systems. J. Food Process. Preserv. 2022, 46, e16800. [Google Scholar] [CrossRef]
- Acquah, C.; Chan, Y.W.; Pan, S.; Agyei, D.; Udenigwe, C.C. Structure-informed separation of bioactive peptides. J. Food Biochem. 2019, 43, e12765. [Google Scholar] [CrossRef]
- Chin, J.S.; Madden, L.; Chew, S.Y.; Becker, D.L. Drug therapies and delivery mechanisms to treat perturbed skin wound healing. Adv. Drug Deliver. Rev. 2019, 149, 2–18. [Google Scholar] [CrossRef]
- Han, Y.P.; Tuan, T.L.; Wu, H.; Hughes, M.; Garner, W.L. TNF-alpha stimulates activation of pro-MMP2 in human skin through NF-(kappa)B mediated induction of MT1-MMP. J. Cell Sci. 2001, 114, 131–139. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, J.Y.; Jo, E.H.; Noh, H.M.; Park, S.; Park, M.C.; Kim, D.K. Chijabyukpi-Tang Inhibits Pro-Inflammatory Cytokines and Chemokines via the Nrf2/HO-1 Signaling Pathway in TNF-alpha/IFN-gamma-Stimulated HaCaT Cells and Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis-Like Skin Lesions in Mice. Front. Pharmacol. 2020, 11, 1018. [Google Scholar] [CrossRef] [PubMed]
- de Souza, G.S.; de Jesus Sonego, L.; Santos Mundim, A.C.; de Miranda Moraes, J.; Sales-Campos, H.; Lorenzon, E.N. Antimicrobial-wound healing peptides: Dual-function molecules for the treatment of skin injuries. Peptides 2022, 148, 170707. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, S.J.; Rudnick, P.A.; Liang, Y.; Dong, Q.; Stein, S.E.; Nesvizhskii, A.I. Comprehensive analysis of protein digestion using six trypsins reveals the origin of trypsin as a significant source of variability in proteomics. J. Proteome Res. 2013, 12, 5666–5680. [Google Scholar] [CrossRef] [PubMed]
- Merz, M.; Eisele, T.; Berends, P.; Appel, D.; Rabe, S.; Blank, I.; Stressler, T.; Fischer, L. Flavourzyme, an Enzyme Preparation with Industrial Relevance: Automated Nine-Step Purification and Partial Characterization of Eight Enzymes. J. Agric Food Chem. 2015, 63, 5682–5693. [Google Scholar] [CrossRef] [PubMed]
- Gurumallesh, P.; Alagu, K.; Ramakrishnan, B.; Muthusamy, S. A systematic reconsideration on proteases. Int. J. Biol. Macromol. 2019, 128, 254–267. [Google Scholar] [CrossRef]
- Xue, Y.; Guo, R.; Zhang, B. [Separation and screening of antioxidant peptides from Scomberomorus niphonius based on nano flow liquid chromatography]. Se Pu 2020, 38, 1431–1439. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, J.; Zhao, H.; Zhao, X.; Xue, H.; Sun, Y.; Xue, W. Isolation and Structural Characterization of Antioxidant Peptides from Degreased Apricot Seed Kernels. J AOAC Int. 2018, 101, 1661–1663. [Google Scholar] [CrossRef]
- Wang, S.; Gan, Y.; Mao, X.; Kan, H.; Li, N.; Zhang, C.; Wang, Z.; Wang, Y. Antioxidant Activity Evaluation of Oviductus Ranae Protein Hydrolyzed by Different Proteases. Molecules 2021, 26, 1625. [Google Scholar] [CrossRef]
- Chang, C.H.; Chang, H.Y.; Rappsilber, J.; Ishihama, Y. Isolation of Acetylated and Unmodified Protein N-Terminal Peptides by Strong Cation Exchange Chromatographic Separation of TrypN-Digested Peptides. Mol. Cell. Proteom. 2021, 20, 100003. [Google Scholar] [CrossRef]
- Jahandideh, F.; Liu, P.; Wu, J. Purification and identification of adipogenic-differentiating peptides from egg white hydrolysate. Food Chem. 2018, 259, 25–30. [Google Scholar] [CrossRef]
- Wan, M.Y.; Dong, G.; Yang, B.Q.; Feng, H. Identification and characterization of a novel antioxidant peptide from feather keratin hydrolysate. Biotechnol. Lett. 2016, 38, 643–649. [Google Scholar] [CrossRef]
- Hu, B.; Xu, L.; Li, Y.; Bai, X.; Xing, M.; Cao, Q.; Liang, H.; Song, S.; Ji, A. A peptide inhibitor of macrophage migration in atherosclerosis purified from the leech Whitmania pigra. J. Ethnopharmacol. 2020, 254, 112723. [Google Scholar] [CrossRef]
- Agrawal, H.; Joshi, R.; Gupta, M. Isolation, purification and characterization of antioxidative peptide of pearl millet (Pennisetum glaucum) protein hydrolysate. Food Chem. 2016, 204, 365–372. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.C.; Pan, D.D.; Zhang, Z.G.; Zhou, T.Q.; Dang, Y.L. Isolation, characterization and molecular docking of novel umami and umami-enhancing peptides from Ruditapes philippinarum. Food Chem. 2021, 343, 128522. [Google Scholar] [CrossRef]
- Joshi, I.; Nazeer, R.A. EGLLGDVF: A Novel Peptide from Green Mussel Perna viridis Foot Exerts Stability and Anti-Inflammatory Effects on LPS-Stimulated RAW264.7 Cells. Protein Pept. Lett. 2020, 27, 851–859. [Google Scholar] [CrossRef]
- Lian, Z.R.; Wang, N.; Tian, Y.W.; Huang, L.H. Characterization of Synthetic Peptide Therapeutics Using Liquid Chromatography-Mass Spectrometry: Challenges, Solutions, Pitfalls, and Future Perspectives. J. Am. Soc. Mass Spectrom. 2021, 32, 1852–1860. [Google Scholar] [CrossRef]
- Esposito, S.; Mele, R.; Ingenito, R.; Bianchi, E.; Bonelli, F.; Monteagudo, E.; Orsatti, L. An efficient liquid chromatography-high resolution mass spectrometry approach for the optimization of the metabolic stability of therapeutic peptides. Anal. Bioanal. Chem. 2017, 409, 2685–2696. [Google Scholar] [CrossRef]
- Plaisancie, P.; Claustre, J.; Estienne, M.; Henry, G.; Boutrou, R.; Paquet, A.; Leonil, J. A novel bioactive peptide from yoghurts modulates expression of the gel-forming MUC2 mucin as well as population of goblet cells and Paneth cells along the small intestine. J. Nutr. Biochem. 2013, 24, 213–221. [Google Scholar] [CrossRef]
- Wang, L.F.; Zhang, J.; Yuan, Q.; Xie, H.H.; Shi, J.Y.; Ju, X.R. Separation and purification of an anti-tumor peptide from rapeseed (Brassica campestris L.) and the effect on cell apoptosis. Food Funct. 2016, 7, 2239–2248. [Google Scholar] [CrossRef]
- Yang, Y.; Song, H.; Wang, B.; Tian, Q.; Li, B. A novel di-peptide Met-Glu from collagen hydrolysates inhibits platelet aggregation and thrombus formation via regulation of Gq-mediated signaling. J. Food Biochem. 2020, 44, e13352. [Google Scholar] [CrossRef]
- Villegas-Escobar, V.; Ceballos, I.; Mira, J.J.; Argel, L.E.; Peralta, S.O.; Romero-Tabarez, M. Fengycin C Produced by Bacillus subtilis EA-CB0015. J. Nat. Prod. 2013, 76, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Schwardt, O.; Lamers, C.; Bechtler, C.; Ricklin, D. Therapeutic Peptides as Emerging Options to Restore Misguided Host Defence and Homeostasis: From Teaching to Concept to Clinic. Chimia 2021, 75, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Pfeil, J.; Simonetti, M.; Lauer, U.; Volkmer, R.; von Thulen, B.; Durek, P.; Krahmer, R.; Leenders, F.; Hamann, A.; Hoffmann, U. Tolerogenic Immunomodulation by PEGylated Antigenic Peptides. Front. Immunol. 2020, 11, 529035. [Google Scholar] [CrossRef] [PubMed]
- Ambadapadi, S.; Munuswamy-Ramanujam, G.; Zheng, D.H.; Sullivan, C.; Dai, E.; Morshed, S.; McFadden, B.; Feldman, E.; Pinard, M.; McKenna, R.; et al. Reactive Center Loop (RCL) Peptides Derived from Serpins Display Independent Coagulation and Immune Modulating Activities. J. Biol. Chem. 2016, 291, 2874–2887. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.L.; Su, K.Y.; Mao, X.L.; Zhang, X.W. Separation and identification of enzyme inhibition peptides from dark tea protein. Bioorganic Chem. 2020, 99, 103772. [Google Scholar] [CrossRef]
- Nyanguile, O. Peptide Antiviral Strategies as an Alternative to Treat Lower Respiratory Viral Infections. Front. Immunol. 2019, 10, 1366. [Google Scholar] [CrossRef]
- Conlon, J.M.; Mechkarska, M.; Lukic, M.L.; Flatt, P.R. Potential therapeutic applications of multifunctional host-defense peptides from frog skin as anti-cancer, anti-viral, immunomodulatory, and anti-diabetic agents. Peptides 2014, 57, 67–77. [Google Scholar] [CrossRef]
- Gentilucci, L.; De Marco, R.; Cerisoli, L. Chemical Modifications Designed to Improve Peptide Stability: Incorporation of Non-Natural Amino Acids, Pseudo-Peptide Bonds, and Cyclization. Curr Pharm Design 2010, 16, 3185–3203. [Google Scholar] [CrossRef]
- Brady, R.M.; Baell, J.B.; Norton, R.S. Strategies for the Development of Conotoxins as New Therapeutic Leads. Mar Drugs 2013, 11, 2293–2313. [Google Scholar] [CrossRef]
- Erak, M.; Bellmann-Sickert, K.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide chemistry toolbox—Transforming natural peptides into peptide therapeutics. Bioorganic Med. Chem. 2018, 26, 2759–2765. [Google Scholar] [CrossRef]
- Park, M.; Jardetzky, T.S.; Barron, A.E. NMEGylation: A Novel Modification to Enhance the Bioavailability of Therapeutic Peptides. Biopolymers 2011, 96, 688–693. [Google Scholar] [CrossRef]
- Kato, T.; Kita, Y.; Iwanari, K.; Asano, A.; Oba, M.; Tanaka, M.; Doi, M. Synthesis of six-membered carbocyclic ring alpha, alpha-disubstituted amino acids and arginine-rich peptides to investigate the effect of ring size on the properties of the peptide. Bioorganic Med. Chem. 2021, 38, 116111. [Google Scholar] [CrossRef]
- Chatterjee, J.; Laufer, B.; Kessler, H. Synthesis of N-methylated cyclic peptides. Nat. Protoc. 2012, 7, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Fichera, G.A.; Fichera, M.; Milone, G. Antitumoural activity of a cytotoxic peptide of Lactobacillus casei peptidoglycan and its interaction with mitochondrial-bound hexokinase. Anticancer Drugs 2016, 27, 609–619. [Google Scholar] [CrossRef]
- Chemuru, S.; Kodali, R.; Wetzel, R. Improved chemical synthesis of hydrophobic Abeta peptides using addition of C-terminal lysines later removed by carboxypeptidase B. Biopolymers 2014, 102, 206–221. [Google Scholar] [CrossRef]
- Smith, J.M.; Fasan, R. Synthesis of macrocyclic organo-peptide hybrids from ribosomal polypeptide precursors via CuAAC-/hydrazide-mediated cyclization. Methods Mol. Biol. 2015, 1248, 23–38. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Tikhonova, I.G.; Martin, L.; Walker, B. Smac-Derived Aza-Peptide as an Aminopeptidase-Resistant XIAP BIR3 Antagonist. Protein Pept. Lett. 2015, 22, 836–843. [Google Scholar] [CrossRef]
- Smith, J.M.; Hill, N.C.; Krasniak, P.J.; Fasan, R. Synthesis of bicyclic organo-peptide hybrids via oxime/intein-mediated macrocyclization followed by disulfide bond formation. Org. Biomol. Chem. 2014, 12, 1135–1142. [Google Scholar] [CrossRef]
- Ozaki, T.; Yamashita, K.; Goto, Y.; Shimomura, M.; Hayashi, S.; Asamizu, S.; Sugai, Y.; Ikeda, H.; Suga, H.; Onaka, H. Dissection of goadsporin biosynthesis by in vitro reconstitution leading to designer analogues expressed in vivo. Nat. Commun. 2017, 8, 14207. [Google Scholar] [CrossRef] [PubMed]
- Petkovic, M.; Mouritzen, M.V.; Mojsoska, B.; Jenssen, H. Immunomodulatory Properties of Host Defence Peptides in Skin Wound Healing. Biomolecules 2021, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Norlen, L.; Lundborg, M.; Wennberg, C.; Narangifard, A.; Daneholt, B. The Skin’s Barrier: A Cryo-EM Based Overview of its Architecture and Stepwise Formation. J Invest Dermatol 2022, 142, 285–292. [Google Scholar] [CrossRef]
- Mansfield, K.; Naik, S. Unraveling Immune-Epithelial Interactions in Skin Homeostasis and Injury. Yale J. Biol. Med. 2020, 93, 133–143. [Google Scholar]
- Awad, F.; Assrawi, E.; Louvrier, C.; Jumeau, C.; Giurgea, I.; Amselem, S.; Karabina, S.A. Photoaging and skin cancer: Is the inflammasome the missing link? Mech. Ageing Dev. 2018, 172, 131–137. [Google Scholar] [CrossRef]
- Baek, J.; Lee, M.G. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016, 21, 164–169. [Google Scholar] [CrossRef]
- Teng, Y.; Fan, Y.; Ma, J.; Lu, W.; Liu, N.; Chen, Y.; Pan, W.; Tao, X. The PI3K/Akt Pathway: Emerging Roles in Skin Homeostasis and a Group of Non-Malignant Skin Disorders. Cells 2021, 10, 1219. [Google Scholar] [CrossRef]
- Lugović-Mihić, L.; Ćesić, D.; Vuković, P.; Novak Bilić, G.; Šitum, M.; Špoljar, S. Melanoma Development: Current Knowledge on Melanoma Pathogenesis. Acta Dermatovenerol. Croat. 2019, 27, 163–168. [Google Scholar]
- Kasuya, A.; Tokura, Y. Attempts to accelerate wound healing. J. Dermatol. Sci. 2014, 76, 169–172. [Google Scholar] [CrossRef]
- Bang, J.S.; Jin, Y.J.; Choung, S.Y. Low molecular polypeptide from oyster hydrolysate recovers photoaging in SKH-1 hairless mice. Toxicol. Appl. Pharmacol. 2020, 386, 114844. [Google Scholar] [CrossRef]
- Nagae, K.; Uchi, H.; Morino-Koga, S.; Tanaka, Y.; Oda, M.; Furue, M. Glucagon-like peptide-1 analogue liraglutide facilitates wound healing by activating PI3K/Akt pathway in keratinocytes. Diabetes Res. Clin. Pract. 2018, 146, 155–161. [Google Scholar] [CrossRef]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef]
- Frazier, T.; Alarcon, A.; Wu, X.Y.; Mohiuddin, O.A.; Motherwell, J.M.; Carlsson, A.H.; Christy, R.J.; Edwards, J.V.; Mackin, R.T.; Prevost, N.; et al. Clinical Translational Potential in Skin Wound Regeneration for Adipose-Derived, Blood-Derived, and Cellulose Materials: Cells, Exosomes, and Hydrogels. Biomolecules 2020, 10, 1373. [Google Scholar] [CrossRef] [PubMed]
- Virador, G.M.; de Marcos, L.; Virador, V.M. Skin Wound Healing: Refractory Wounds and Novel Solutions. Methods Mol. Biol. 2019, 1879, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Valachova, K.; Svik, K.; Biro, C.; Soltes, L. Skin wound healing with composite biomembranes loaded by tiopronin or captopril. J. Biotechnol. 2020, 310, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar]
- Nour, S.; Imani, R.; Chaudhry, G.R.; Sharifi, A.M. Skin wound healing assisted by angiogenic targeted tissue engineering: A comprehensive review of bioengineered approaches. J. Biomed. Mater. Res. A 2021, 109, 453–478. [Google Scholar] [CrossRef]
- Pickart, L.; Vasquez-Soltero, J.M.; Margolina, A. GHK Peptide as a Natural Modulator of Multiple Cellular Pathways in Skin Regeneration. Biomed. Res. Int. 2015, 2015, 648108. [Google Scholar] [CrossRef]
- Nicolaus, C.; Junghanns, S.; Hartmann, A.; Murillo, R.; Ganzera, M.; Merfort, I. In vitro studies to evaluate the wound healing properties of Calendula officinalis extracts. J. Ethnopharmacol. 2017, 196, 94–103. [Google Scholar] [CrossRef]
- Liang, J.; Lian, L.; Wang, X.; Li, L. Thymoquinone, extract from Nigella sativa seeds, protects human skin keratinocytes against UVA-irradiated oxidative stress, inflammation and mitochondrial dysfunction. Mol. Immunol. 2021, 135, 21–27. [Google Scholar] [CrossRef]
- Alemzadeh, E.; Oryan, A. Effectiveness of a Crocus sativus Extract on Burn Wounds in Rats. Planta Med. 2018, 84, 1191–1200. [Google Scholar] [CrossRef]
- Alves, R.S.; Alves, L.B.; Altoe, L.S.; Sarandy, M.M.; Freitas, M.B.; Silveira, N.J.F.; Novaes, R.D.; Goncalves, R.V. Peptides from Animal Origin: A Systematic Review on Biological Sources and Effects on Skin Wounds. Oxid. Med. Cell. Longev. 2020, 2020, 4352761. [Google Scholar] [CrossRef]
- Cai, S.; Lu, C.; Liu, Z.; Wang, W.; Lu, S.; Sun, Z.; Wang, G. Derivatives of gecko cathelicidin-related antioxidant peptide facilitate skin wound healing. Eur. J. Pharmacol. 2021, 890, 173649. [Google Scholar] [CrossRef]
- Luo, X.; Ouyang, J.; Wang, Y.; Zhang, M.; Fu, L.; Xiao, N.; Gao, L.; Zhang, P.; Zhou, J.; Wang, Y. A novel anionic cathelicidin lacking direct antimicrobial activity but with potent anti-inflammatory and wound healing activities from the salamander Tylototriton kweichowensis. Biochimie 2021, 191, 37–50. [Google Scholar] [CrossRef]
- Mu, L.; Tang, J.; Liu, H.; Shen, C.; Rong, M.; Zhang, Z.; Lai, R. A potential wound-healing-promoting peptide from salamander skin. FASEB J. 2014, 28, 3919–3929. [Google Scholar] [CrossRef]
- Mei, F.; Liu, J.; Wu, J.; Duan, Z.; Chen, M.; Meng, K.; Chen, S.; Shen, X.; Xia, G.; Zhao, M. Collagen Peptides Isolated from Salmo salar and Tilapia nilotica Skin Accelerate Wound Healing by Altering Cutaneous Microbiome Colonization via Upregulated NOD2 and BD14. J. Agric. Food. Chem. 2020, 68, 1621–1633. [Google Scholar] [CrossRef]
- Elbialy, Z.I.; Atiba, A.; Abdelnaby, A.; Al, H., II.; Elsheshtawy, A.; El-Serehy, H.A.; Abdel-Daim, M.M.; Fadl, S.E.; Assar, D.H. Collagen extract obtained from Nile tilapia (Oreochromis niloticus L.) skin accelerates wound healing in rat model via up regulating VEGF, bFGF, and alpha-SMA genes expression. BMC Vet. Res. 2020, 16, 352. [Google Scholar] [CrossRef]
- Lin, Y.A.; Chu, P.Y.; Ma, W.L.; Cheng, W.C.; Chan, S.T.; Yang, J.C.; Wu, Y.C. Enzyme-Digested Peptides Derived from Lates calcarifer Enhance Wound Healing after Surgical Incision in a Murine Model. Mar. Drugs 2021, 19, 154. [Google Scholar] [CrossRef]
- Yang, F.; Qin, X.; Zhang, T.; Zhang, C.; Lin, H. Effect of Oral Administration of Active Peptides of Pinctada Martensii on the Repair of Skin Wounds. Mar. Drugs 2019, 17, 697. [Google Scholar] [CrossRef]
- Fu, Y.; Li, C.; Li, X.; Zeng, L.; Wang, Y.; Fu, Z.; Shu, L.; Liu, Y.; Liu, N.; Yang, Y.; et al. Amphibian-derived peptide homodimer promotes regeneration of skin wounds. Biomed. Pharmacother. 2022, 146, 112539. [Google Scholar] [CrossRef]
- Feng, G.; Wu, J.; Yang, H.L.; Mu, L. Discovery of Antioxidant Peptides from Amphibians: A Review. Protein Pept. Lett. 2021, 28, 1220–1229. [Google Scholar] [CrossRef]
- Wu, J.; Yang, J.; Wang, X.; Wei, L.; Mi, K.; Shen, Y.; Liu, T.; Yang, H.; Mu, L. A frog cathelicidin peptide effectively promotes cutaneous wound healing in mice. Biochem. J. 2018, 475, 2785–2799. [Google Scholar] [CrossRef]
- He, X.; Yang, Y.; Mu, L.; Zhou, Y.; Chen, Y.; Wu, J.; Wang, Y.; Yang, H.; Li, M.; Xu, W.; et al. A Frog-Derived Immunomodulatory Peptide Promotes Cutaneous Wound Healing by Regulating Cellular Response. Front. Immunol. 2019, 10, 2421. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, Z.; Yang, M.; Zeng, L.; Qi, B.; Yin, S.; Li, B.; Li, Y.; Fu, Z.; Shu, L.; et al. Discovery of a novel short peptide with efficacy in accelerating the healing of skin wounds. Pharmacol. Res. 2021, 163, 105296. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Duan, Z.; Tang, J.; Lv, Q.; Rong, M.; Lai, R. A short peptide from frog skin accelerates diabetic wound healing. FEBS J. 2014, 281, 4633–4643. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Seo, C.H.; Luchian, T.; Park, Y. Pse-T2, an Antimicrobial Peptide with High-Level, Broad-Spectrum Antimicrobial Potency and Skin Biocompatibility against Multidrug-Resistant Pseudomonas aeruginosa Infection. Antimicrob. Agents Chemother. 2018, 62, e01493-18. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mu, L.; Tang, J.; Shen, C.; Gao, C.; Rong, M.; Zhang, Z.; Liu, J.; Wu, X.; Yu, H.; et al. A potential wound healing-promoting peptide from frog skin. Int. J. Biochem. Cell Biol. 2014, 49, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, W.; Peng, Y.; Gu, Q.; Luo, J.; Zhou, J.; Wu, J.; Hou, Y.; Cao, J. Amelioration of radiation-induced skin injury by HIV-TAT-mediated protein transduction of RP-1 from Rana pleurade. Int. J. Med. Sci. 2014, 11, 44–51. [Google Scholar] [CrossRef]
- Wang, S.; Feng, C.; Yin, S.; Feng, Z.; Tang, J.; Liu, N.; Yang, F.; Yang, X.; Wang, Y. A novel peptide from the skin of amphibian Rana limnocharis with potency to promote skin wound repair. Nat. Prod. Res. 2021, 35, 3514–3518. [Google Scholar] [CrossRef]
- Liu, N.; Li, Z.; Meng, B.; Bian, W.; Li, X.; Wang, S.; Cao, X.; Song, Y.; Yang, M.; Wang, Y.; et al. Accelerated Wound Healing Induced by a Novel Amphibian Peptide (OA-FF10). Protein Pept. Lett. 2019, 26, 261–270. [Google Scholar] [CrossRef]
- Cao, X.; Wang, Y.; Wu, C.; Li, X.; Fu, Z.; Yang, M.; Bian, W.; Wang, S.; Song, Y.; Tang, J.; et al. Author Correction: Cathelicidin-OA1, a novel antioxidant peptide identified from an amphibian, accelerates skin wound healing. Sci. Rep. 2018, 8, 15906. [Google Scholar] [CrossRef]
- Sun, Y.; Dong, W.B.; Sun, L.; Ma, L.J.; Shang, D.J. Insights into the membrane interaction mechanism and antibacterial properties of chensinin-1b. Biomaterials 2015, 37, 299–311. [Google Scholar] [CrossRef]
- Bian, W.; Meng, B.; Li, X.; Wang, S.; Cao, X.; Liu, N.; Yang, M.; Tang, J.; Wang, Y.; Yang, X. OA-GL21, a novel bioactive peptide from Odorrana andersonii, accelerated the healing of skin wounds. Biosci. Rep. 2018, 38, BSR20180215. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, C.; Wang, S.; Wang, Y.; Fu, Z.; Zhang, Y.; Sun, H.; Xie, C.; Fu, Y.; Tao, J.; et al. A novel amphibian-derived peptide alleviated ultraviolet B-induced photodamage in mice. Biomed. Pharmacother. 2021, 136, 111258. [Google Scholar] [CrossRef]
- Woodhams, D.C.; Rollins-Smith, L.A.; Reinert, L.K.; Lam, B.A.; Harris, R.N.; Briggs, C.J.; Vredenburg, V.T.; Patel, B.T.; Caprioli, R.M.; Chaurand, P.; et al. Probiotics Modulate a Novel Amphibian Skin Defense Peptide That Is Antifungal and Facilitates Growth of Antifungal Bacteria. Microb. Ecol. 2020, 79, 192–202. [Google Scholar] [CrossRef]
- Qin, D.; Lee, W.H.; Gao, Z.; Zhang, W.; Peng, M.; Sun, T.; Gao, Y. Protective effects of antioxidin-RL from Odorrana livida against ultraviolet B-irradiated skin photoaging. Peptides 2018, 101, 124–134. [Google Scholar] [CrossRef]
- Qin, P.; Meng, Y.; Yang, Y.; Gou, X.Y.; Liu, N.X.; Yin, S.G.; Hu, Y.; Sun, H.L.; Fu, Z.; Wang, Y.L.; et al. Mesoporous polydopamine nanoparticles carrying peptide RL-QN15 show potential for skin wound therapy. J. Nanobiotechnol. 2021, 19, 309. [Google Scholar] [CrossRef]
- Crack, L.R.; Jones, L.; Malavige, G.N.; Patel, V.; Ogg, G.S. Human antimicrobial peptides LL-37 and human beta-defensin-2 reduce viral replication in keratinocytes infected with varicella zoster virus. Clin. Exp. Dermatol. 2012, 37, 534–543. [Google Scholar] [CrossRef]
- Pemmari, T.; Ivanova, L.; May, U.; Lingasamy, P.; Tobi, A.; Pasternack, A.; Prince, S.; Ritvos, O.; Makkapati, S.; Teesalu, T.; et al. Exposed CendR Domain in Homing Peptide Yields Skin-Targeted Therapeutic in Epidermolysis Bullosa. Mol. Ther. 2020, 28, 1833–1845. [Google Scholar] [CrossRef]
- Wollenberg, A.; Folster-Holst, R.; Saint Aroman, M.; Sampogna, F.; Vestergaard, C. Effects of a protein-free oat plantlet extract on microinflammation and skin barrier function in atopic dermatitis patients. J. Eur. Acad. Dermatol. Venereol. 2018, 32 (Suppl. 1), 1–15. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Trujillo-Paez, J.V.; Umehara, Y.; Yue, H.N.; Peng, G.; Kiatsurayanon, C.; Chieosilapatham, P.; Song, P.; Okumura, K.; Ogawa, H.; et al. Role of Antimicrobial Peptides in Skin Barrier Repair in Individuals with Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 7607. [Google Scholar] [CrossRef]
- Kiatsurayanon, C.; Peng, G.; Niyonsaba, F. Opposing Roles of Antimicrobial Peptides in Skin Cancers. Curr. Pharm. Design. 2022, 28, 248–258. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, T.X.; Cheng, N.N.; Wu, S.T.; Huang, J.C.; Chen, X.L.; Chen, T.B.; Zhou, M.; Wang, L.; Shaw, C. Evaluation of antimicrobial and anticancer activities of three peptides identified from the skin secretion of Hylarana latouchii. Acta Biochim. Biophys. Sin. 2021, 53, 1469–1483. [Google Scholar] [CrossRef] [PubMed]
- Tonk, M.; Vilcinskas, A.; Rahnamaeian, M. Insect antimicrobial peptides: Potential tools for the prevention of skin cancer. Appl. Microbiol. Biotechnol. 2016, 100, 7397–7405. [Google Scholar] [CrossRef]
- Lee, R.H.; Oh, J.D.; Hwang, J.S.; Lee, H.K.; Shin, D. Antitumorigenic effect of insect-derived peptide poecilocorisin-1 in human skin cancer cells through regulation of Sp1 transcription factor. Sci. Rep. 2021, 11, 18445. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.; Sharma, R.; Preet, S. Augmented therapeutic efficacy of 5-fluorouracil in conjunction with lantibiotic nisin against skin cancer. Biochem. Biophys. Res. Commun. 2019, 520, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Sunderland, K.; Mao, C. Virus-Derived Peptides for Clinical Applications. Chem. Rev. 2017, 117, 10377–10402. [Google Scholar] [CrossRef]
- Dostert, M.; Belanger, C.R.; Hancock, R.E.W. Design and Assessment of Anti-Biofilm Peptides: Steps toward Clinical Application. J. Innate Immun. 2019, 11, 193–204. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, J.; Li, Z.; Fu, C.; Xu, M.; Yang, J.; Jiang, X.; Zhou, B.; Ye, X.; Xu, C. Exploring and exploiting plant cyclic peptides for drug discovery and development. Med. Res. Rev. 2021, 41, 3096–3117. [Google Scholar] [CrossRef]
- Ding, Y.; Ting, J.P.; Liu, J.; Al-Azzam, S.; Pandya, P.; Afshar, S. Impact of non-proteinogenic amino acids in the discovery and development of peptide therapeutics. Amino Acids 2020, 52, 1207–1226. [Google Scholar] [CrossRef]
- Kang, H.J.; Chen, N.Z.; Dash, B.C.; Hsia, H.C.; Berthiaume, F. Self-Assembled Nanomaterials for Chronic Skin Wound Healing. Adv. Wound Care 2021, 10, 221–233. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, C.; Lan, M.; Guo, Q.; Du, X.; Zhou, S.; Cui, P.; Hong, T.; Jiang, P.; Wang, J.; et al. Antibacterial Photodynamic Gold Nanoparticles for Skin Infection. ACS Appl. Bio Mater. 2021, 4, 3124–3132. [Google Scholar] [CrossRef]
- Aditya, A.; Chattopadhyay, S.; Gupta, N.; Alam, S.; Veedu, A.P.; Pal, M.; Singh, A.; Santhiya, D.; Ansari, K.M.; Ganguli, M. ZnO Nanoparticles Modified with an Amphipathic Peptide Show Improved Photoprotection in Skin. ACS Appl. Mater. Interfaces 2019, 11, 56–72. [Google Scholar] [CrossRef]
- Ouyang, Q.Q.; Hu, Z.; Lin, Z.P.; Quan, W.Y.; Deng, Y.F.; Li, S.D.; Li, P.W.; Chen, Y. Chitosan hydrogel in combination with marine peptides from tilapia for burns healing. Int. J. Biol. Macromol. 2018, 112, 1191–1198. [Google Scholar] [CrossRef]
- Sawicka, J.; Ilowska, E.; Deptula, M.; Sosnowski, P.; Sass, P.; Czerwiec, K.; Chmielewska, K.; Szymanska, A.; Pietralik-Molinska, Z.; Kozak, M.; et al. Functionalized Peptide Fibrils as a Scaffold for Active Substances in Wound Healing. Int. J. Mol. Sci. 2021, 22, 3818. [Google Scholar] [CrossRef]
- Stern, D.; Cui, H.G. Crafting Polymeric and Peptidic Hydrogels for Improved Wound Healing. Adv. Healthc. Mater. 2019, 8, 1900104. [Google Scholar] [CrossRef]
- Sannasimuthu, A.; Ramani, M.; Paray, B.A.; Pasupuleti, M.; Al-Sadoon, M.K.; Alagumuthu, T.S.; Al-Mfarij, A.R.; Arshad, A.; Mala, K.; Arockiaraj, J. Arthrospira platensis transglutaminase derived antioxidant peptide-packed electrospun chitosan/poly (vinyl alcohol) nanofibrous mat accelerates wound healing, in vitro, via inducing mouse embryonic fibroblast proliferation. Colloid Surf. B 2020, 193, 111124. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).