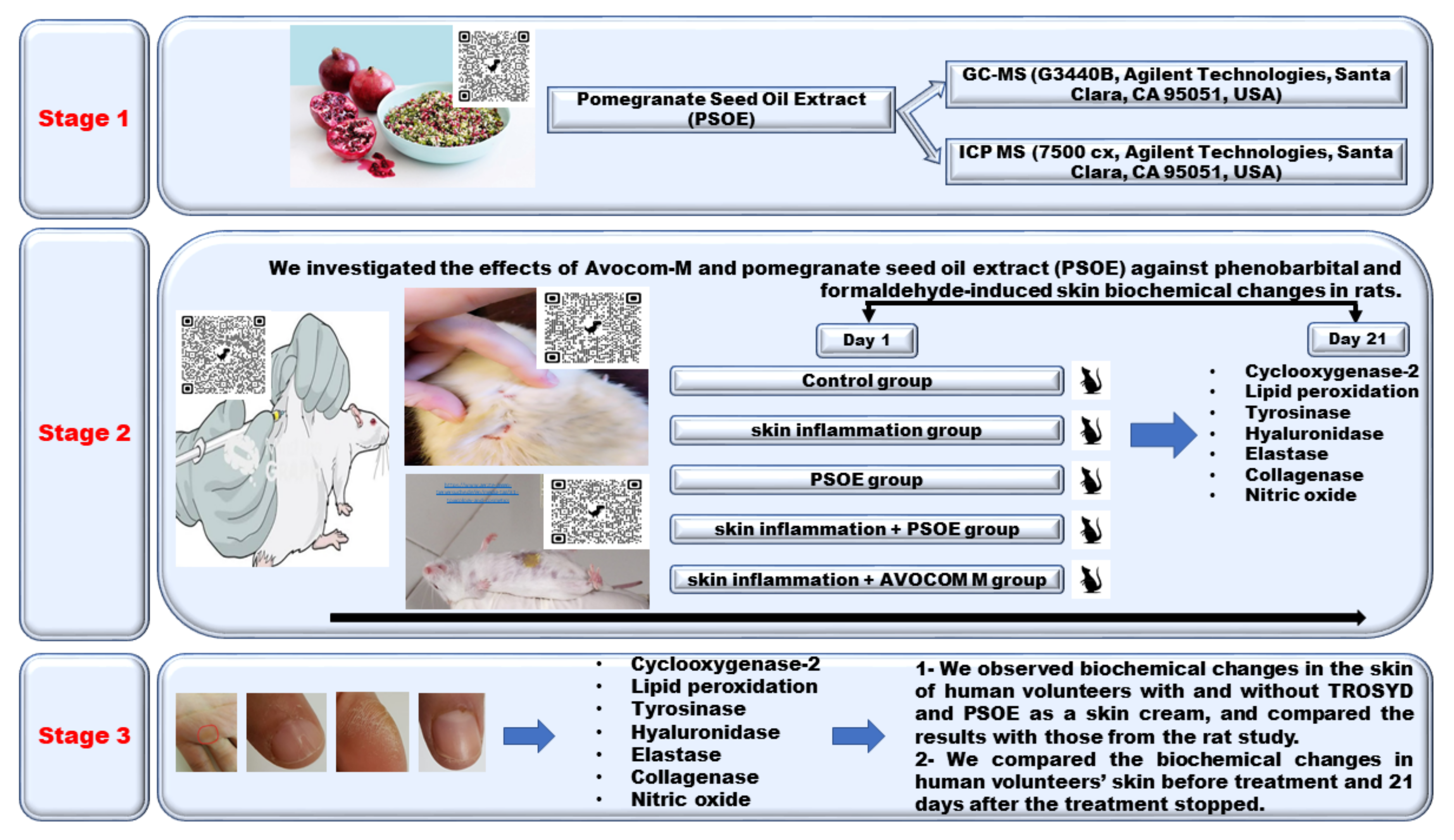
Biochemical Pilot Study on Effects of Pomegranate Seed Oil Extract and Cosmetic Cream on Neurologically Mediated Skin Inflammation in Animals and Humans: A Comparative Observational Study
Abstract
1. Introduction
2. Results
2.1. Metal Content Analysis of PSOE Using ICP–MS
2.2. GC-MS Analysis of PSOE
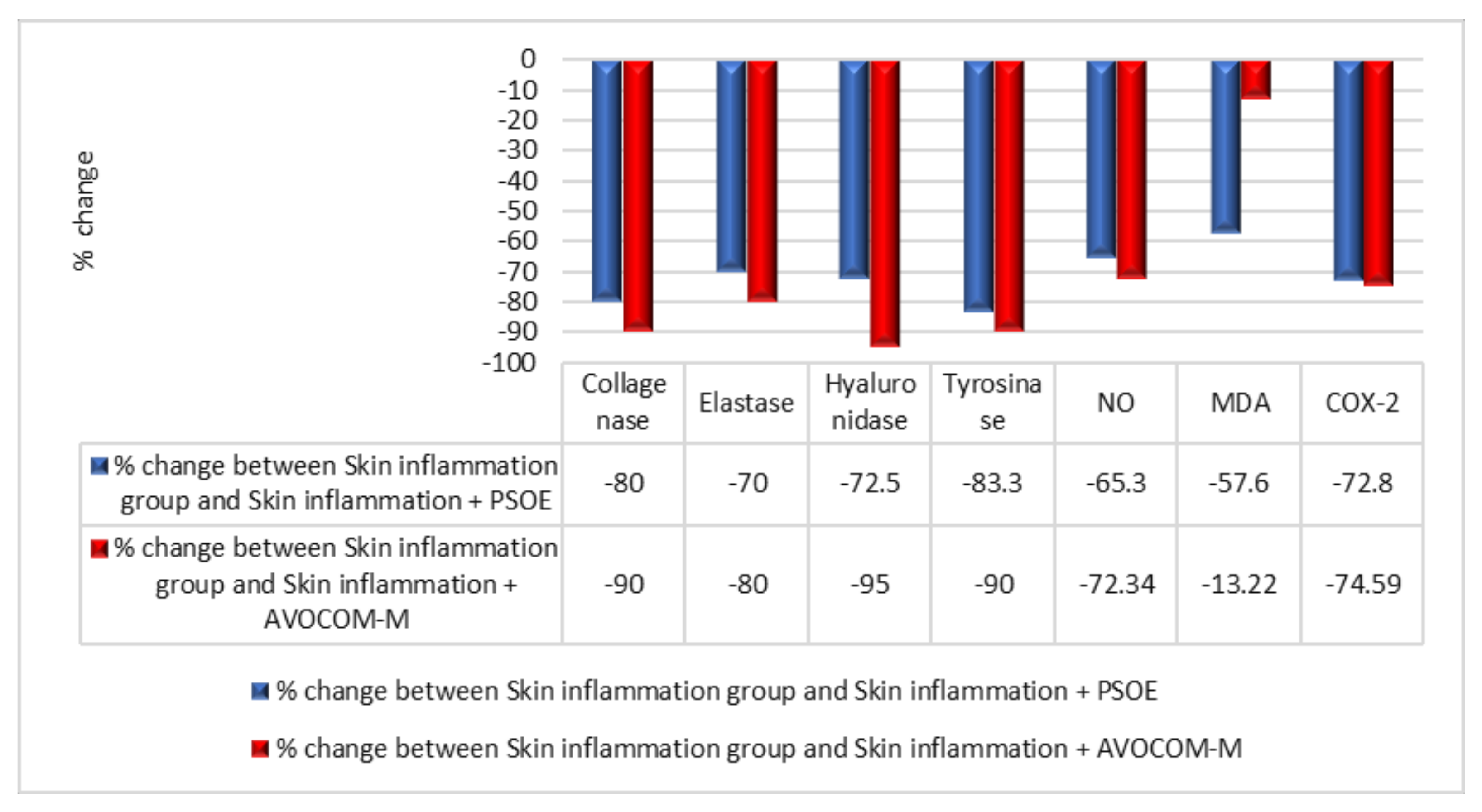
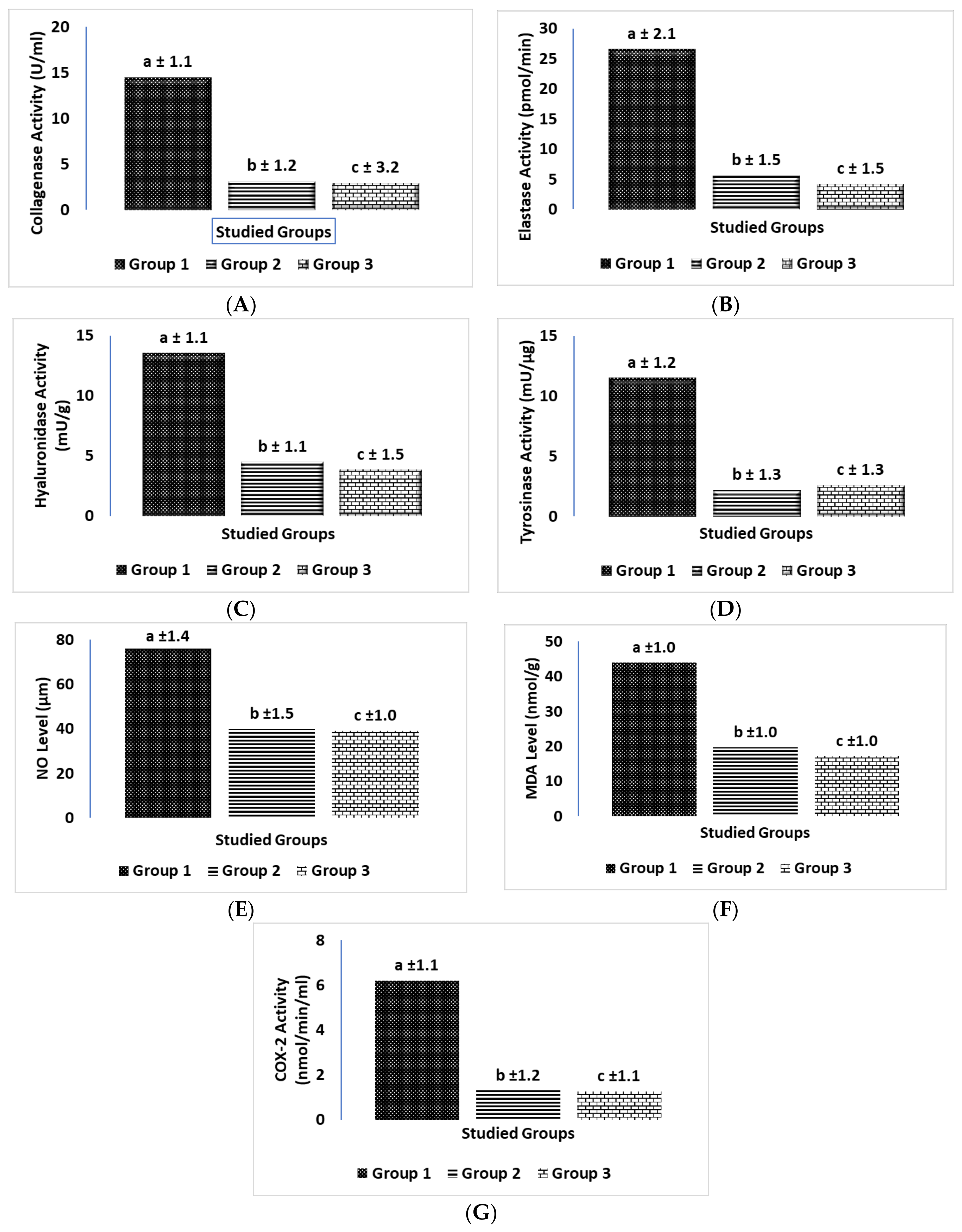
2.3. Biochemical Comparisons of Different Groups in Animal Skin Study
2.4. Patient Questionnaire Results
2.5. Biochemical Comparison of Three Groups in Human Investigations
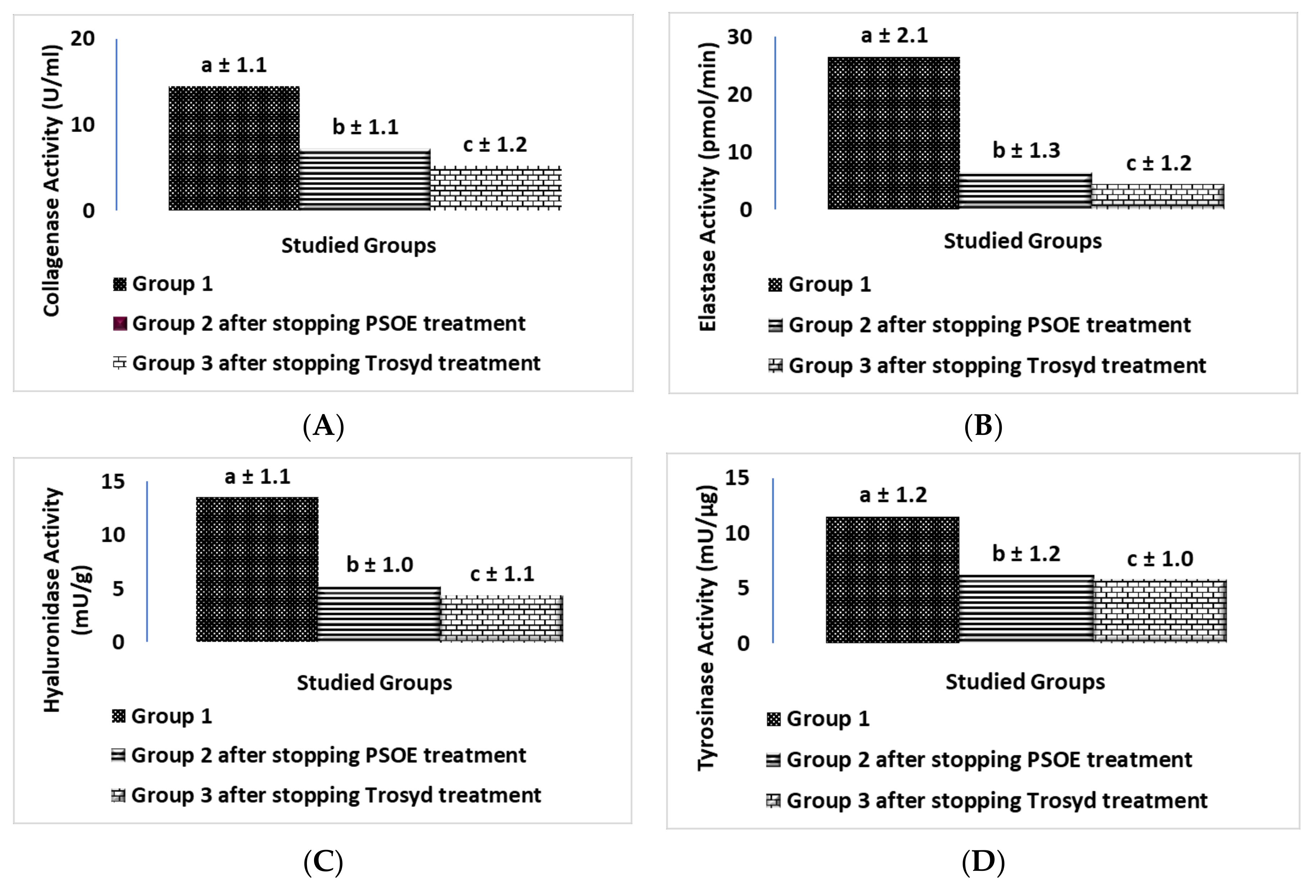
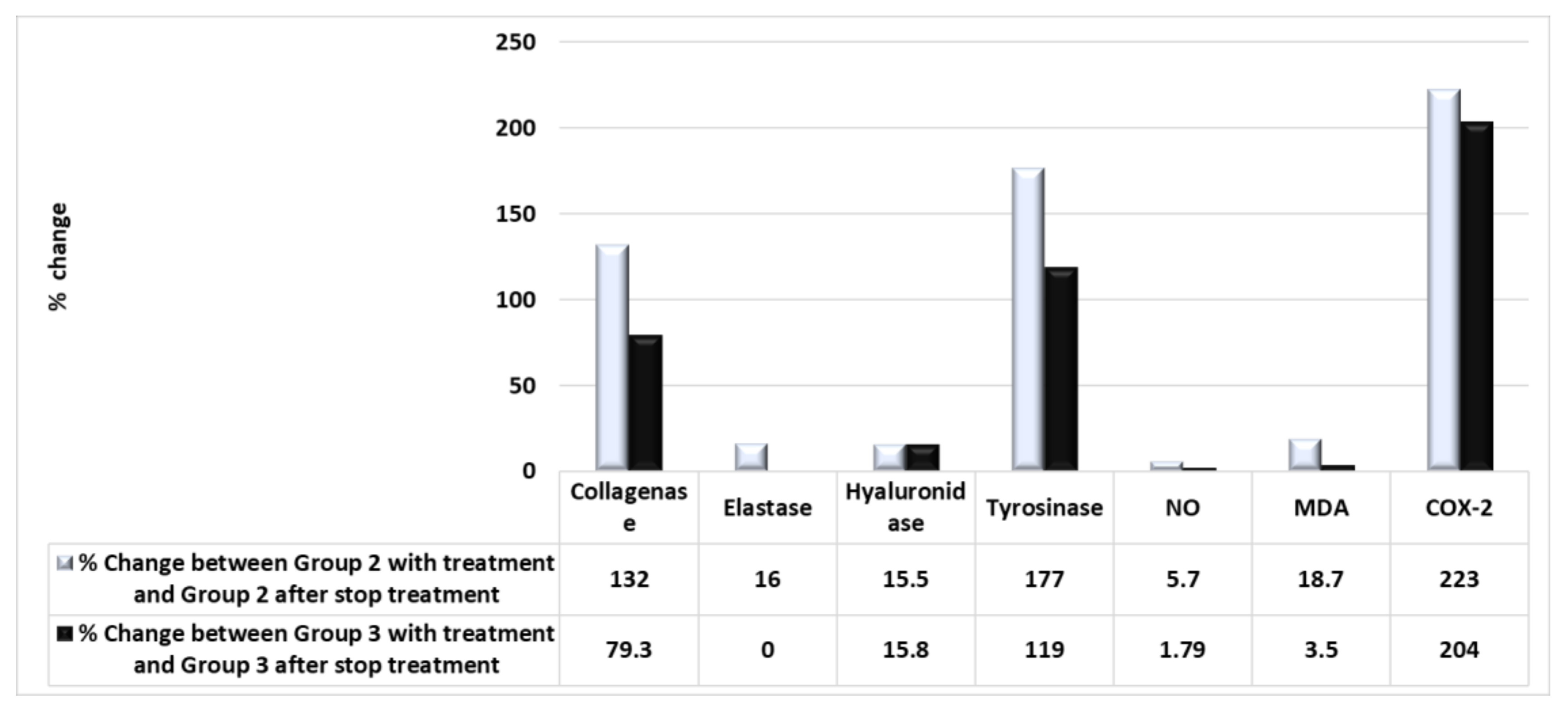
2.6. Biochemical Comparison of the Therapeutic Efficiency of PSOE between the Animal and Human Studies
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.1.1. Metal Analyses
4.1.2. Gas Chromatography–Mass Spectrometry Analysis
4.2. Chemicals
4.3. Animal Study
4.4. Human Study
Patient Population and Data Collection
4.5. Biochemical Assays
4.5.1. Collagenase
4.5.2. Elastase
4.5.3. Hyaluronidase
4.5.4. Tyrosinase
4.5.5. NO Level
4.5.6. Lipid Peroxidation
4.5.7. COX-2
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamouda, A.F.; Hassan, A.; Ahmad, M.S. A Screening Pilot Study on the Relation between Body Mass Index, and Heavy Metal and Mineral Levels in College Students. Artic. Electron. J. Biol. 2019, 15, 79–89. [Google Scholar] [CrossRef]
- Jiratchayamaethasakul, C.; Ding, L.; Jiratchayamaethasakul, C.; Kim, A.; Kim, J.; Heo, J.; Lee, H. In vitro screening of elastase, collagenase, hyaluronidase, and tyrosinase inhibitory and antioxidant activities of 22 halophyte plant extracts for novel cosmeceuticals. Fish. Aquat. Sci. 2020, 23, 62–72. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the Skin. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef]
- Slominski, A.T.; Slominski, R.M.; Raman, C.; Chen, J.Y.; Athar, M.; Elmets, C. Neuroendocrine signaling in the skin with a special focus on the epidermal neuropeptides. Am. J. Physiol.-Cell Physiol. 2022, 323, C1757–C1776. [Google Scholar] [CrossRef] [PubMed]
- Kendall, A.C.; Nicolaou, A. Bioactive lipid mediators in skin inflammation and immunity. Prog. Lipid Res. 2013, 52, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef]
- Slominski, R.M.; Sarna, T.; Raman, C.; Płonka, P.M.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef]
- Kim, M.-J.; Hyun, J.M.; Seong, K.C.; Lim, C.K.; Kang, J.-S. In vitro Screening of Subtropical Plants Cultivated in Jeju Island for Cosmetic Ingredients. Orient. J. Chem. 2016, 32, 807–815. [Google Scholar] [CrossRef][Green Version]
- Horng, C.-T.; Wu, H.-C.; Chiang, N.-N.; Lee, C.-F.; Huang, Y.-S.; Wang, H.-Y.; Yang, J.-S.; Chen, F.-A. Inhibitory effect of burdock leaves on elastase and tyrosinase activity. Exp. Ther. Med. 2017, 14, 3247–3252. [Google Scholar] [CrossRef][Green Version]
- Ding, Y.; Jiratchayamaethasakul, C.; Kim, E.-A.; Kim, J.; Heo, S.-J.; Lee, S.-H. Hyaluronidase Inhibitory and Antioxidant Activities of Enzymatic Hydrolysate from Jeju Island Red Sea Cucumber (Stichopus japonicus) for Novel Anti-aging Cosmeceuticals. J. Mar. Biosci. Biotechnol. 2018, 10, 62–72. [Google Scholar] [CrossRef]
- Leach, J.B.; Bivens, K.A.; Patrick, C.W.; Schmidt, C.E. Photocrosslinked hyaluronic acid hydrogels: Natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 2003, 82, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Moragot, C.; Takeshi, Y.; Kenshi, Y.; Setsuya, A.; Anchalee, C. Anti melanogenic effect of Croton roxburghii and Croton sublyratus leaves in α-MSH stimulated B16F10 cells–DOAJ. J. Tradit. Complement. Med. 2019, 9, 66–72. [Google Scholar]
- Jevons, S.; Gymer, G.E.; Brammer, K.W.; Cox, D.A.; Leeming, M.R. Antifungal Activity of Tioconazole (UK-20,349), a New Imidazole Derivative. Antimicrob. Agents Chemother. 1979, 15, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Kashin, P.; Phyfferoen, M.C.; Gibbs, D.L. A comparative study of once versus twice daily treatment of superficial dermatophyte and yeast infections with tioconazole (1 %) cream. J. Int. Med. Res. 1985, 13, 88–95. [Google Scholar] [CrossRef] [PubMed]
- WebMD. Available online: https://www.webmd.com/drugs/2/drug-3841-787/miconazole-nitrate-topical/miconazole-topical/details (accessed on 12 October 2022).
- Liyanaarachchi, G.D.; Samarasekera, J.K.R.R.; Mahanama, K.R.R.; Hemalal, K.D.P. Tyrosinase, elastase, hyaluronidase, inhibitory and antioxidant activity of Sri Lankan medicinal plants for novel cosmeceuticals. Ind. Crops Prod. 2018, 111, 597–605. [Google Scholar] [CrossRef]
- Asmaa, H.; Shaban, N.Z. Short and Long Term Effects of Pomegranate (Punica granatum) Extracts on Apoptosis in Rat Kidney Induced by Diethylnitrosamine and Phenobarbital. J. Pharm. Pharmacol. 2016, 4, 52–63. [Google Scholar] [CrossRef][Green Version]
- Stefanou, V.; Timbis, D.; Kanellou, A.; Margari, D. Wound Healing Properties of Pomegranate Combined use of current molecular techniques and gold nanoparticle probes in the quality assurance of food View project Supramolecules View project. Arch Microbiol. Immunol. 2021, 5, 263–291. [Google Scholar] [CrossRef]
- Shaban, N.Z.; El-Kersh, M.A.R.; Bader-Eldin, M.M.; Kato, S.A.; Hamoda, A.F. Effect of Punica granatum (Pomegranate) Juice Extract on Healthy Liver and Hepatotoxicity Induced by Diethylnitrosamine and Phenobarbital in Male Rats. J. Med. Food 2014, 17, 339–349. [Google Scholar] [CrossRef]
- Hamouda, A.F.; Shaban, N.Z.; Talaat, I.M. Effects of Some Pyrimidine Derivatives and Pomegranate Juice on Male Rat kidney Injuries Induced by Diethylnitrosamine and Carbon Tetrachloride. Biol. Chem. Res. 2015, 2015, 215–229. Available online: http://www.ss-pub.org/wp-content/uploads/2015/07/BCR2015042201.pdf (accessed on 13 May 2022).
- Hamouda, A.F.; Farawilla, T.-L.M.; Attafi, I.M.; Khardali, I.A.; Attafi, M.A.; Oraiby, M.E.; Abualsail, F.M. Screening Pilot Study of Fruit Seed Compositions by GC-MS and Their Potential Scenario Anti ACE2 and 2rh1 Receptors as a Recycling Possibility in the Coronavirus Pandemic. J. Clin. Med. Res. 2021, 2, 1–65. [Google Scholar] [CrossRef]
- Hamouda, A.F. A Pilot Study of Antistress Effects of Vitamin B Complex and Agarwood Extract in an Animal Model with Parallel Observations on Depression in Human Subjects. J. Drug Alcohol Res. 2021, 11, 1–13. Available online: https://www.ashdin.com/articles/a-pilot-study-of-antistress-effects-of-vitamin-b-complex-and-agarwood-extract-in-an-animal-model-with-parallel-observations-on-dep-68282.html (accessed on 4 March 2021).
- Hamouda, A.F. Ethical to using rats in the scientific researches. Pharm. Pharmacol. Int. J. 2018, 6, 23–25. [Google Scholar] [CrossRef]
- Doctors Against Animal Experiments. Available online: https://www.aerzte-gegen-tierversuche.de/en/media-tag/31-toxicology-and-cosmetics (accessed on 8 October 2022).
- Abu-Serie, M.M.; Hamouda, A.F.; Habashy, N.H. Acacia senegal gum attenuates systemic toxicity in CCl4-intoxicated rats via regulation of the ROS/NF-κB signaling pathway. Sci. Rep. 2021, 11, 20316. [Google Scholar] [CrossRef]
- Grube, D.D.; Peraino, C.; Fry, R.J.M. The effect of dietary phenobarbital on the induction of skin tumors in hairless mice with 7,12 dimethylbenz [a] anthracene. J. Investig. Dermatol. 1975, 64, 258–262. [Google Scholar] [CrossRef][Green Version]
- Gedzelman, E.R.; Meador, K.J. Neurological and psychiatric sequelae of developmental exposure to antiepileptic drugs. Front. Neurol. 2012, 3, 182. [Google Scholar] [CrossRef]
- Manuyakorn, W.; Siripool, K.; Kamchaisatian, W.; Pakakasama, S.; Visudtibhan, A.; Vilaiyuk, S.; Rujirawat, T.; Benjaponpitak, S. Phenobarbital-induced severe cutaneous adverse drug reactions are associated with CYP2C19*2 in Thai children. Pediatr. Allergy Immunol. 2013, 24, 299–303. [Google Scholar] [CrossRef]
- Mungase, M.; Chaudhury, S.; Patil, A.; Jagtap, B.; Jain, V. Stress, anxiety, depression, and resilience in cancer patients on radiotherapy. Ind. Psychiatry J. 2021, 30, 346–352. [Google Scholar] [CrossRef]
- Bowden, G.; Slaga, T.; Shapas, B.; Boutwel, R. The Role of Aryl Hydrocarbon Hydroxylase in Skin Tumor Initiation by 7,12-Dimethylbenz(a)anthracene and 1,2,5.6-Dibenzanthracene Using DNA Binding and Thymidine-’H Incorporation into DNA as Criteria. Cancer Res. 1974, 34, 2634–2642. Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/referencespapers.aspx?referenceid=648420 (accessed on 1 December 2022).
- McFarland, D.C.; Walsh, L.E.; Miller, A.H. Depression, Inflammation, and Cancer. In Psycho-Oncology, 4th ed.; McFarland, D.C., Walsh, L.E., Breitbart, W., Butow, P., Jacobsen, P., Lam, W., Lazenby, M., Loscalzo, M., Eds.; Oxford Academic: Oxford, UK, 2021; Chapter 80; pp. 644–653. [Google Scholar] [CrossRef]
- Morgan, K.T. A brief review of formaldehyde carcinogenesis in relation to rat nasal pathology and human health risk assessment. Toxicol. Pathol. 1997, 25, 291–307. [Google Scholar] [CrossRef]
- Bogdanffy, M.S.; Morgan, P.H.; Starr, T.B.; Morgan, K.T. Binding of formaldehyde to human and rat nasal mucus and bovine serum albumin. Toxicol. Lett. 1987, 38, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lyga, J. Brain-Skin Connection: Stress, Inflammation and Skin Aging. Inflamm. Allergy Drug Targets 2014, 13, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, K.A.; Medeiros, S.C.; Neves, J.K.O.; da Silva, J.A.; Tomé, A.D.R.; Carvalho, A.L.M.; de Freitas, R.M. In vivo evaluation of anticonvulsant and antioxidant effects of phenobarbital microemulsion for transdermal administration in pilocarpine seizure rat model. Pharmacol. Biochem. Behav. 2015, 131, 6–12. [Google Scholar] [CrossRef][Green Version]
- Hamouda, A.F. A biochemical study of chronic stress and chronic inflammation fibromyalgia. Pharm. Pharmacol. Int. J. 2018, 6, 1. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.S.; Correia, A.; Esteves, A.C. Bacterial collagenases—A review. Crit. Rev. Microbiol. 2016, 42, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Watt, A.J.; Hentz, V.R. Collagenase clostridium histolyticum: A novel nonoperative treatment for Dupuytren’s disease. Int. J. Clin. Rheumtol. 2011, 6, 123–133. [Google Scholar] [CrossRef]
- Thring, T.S.A.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Wittenauer, J.; MäcKle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Ohshima, M.; Miyake, M.; Takeda, M.; Muto, T.; Ueda, N.; Ito, K.; Sakamoto, T. Development of mechanisms associated with neurogenic-mediated skin inflammation during the growth of rats. Pediatr. Res. 2010, 67, 363–368. [Google Scholar] [CrossRef]
- Odds, F.C. Laboratory evaluation of antifungal agents: A comparative study of five imidazole derivatives of clinical importance. J. Antimicrob. Chemother. 1980, 6, 749–761. [Google Scholar] [CrossRef]
- East, M.O.; Henderson, J.T.; Jevons, S. Tioconazole in the treatment of fungal infections of the skin. An international clinical research program. Dermatologica 1983, 166 (Suppl. 1), 20–33. [Google Scholar] [CrossRef]
- Beggs, W.H. Fungicidal activity of tioconazole in relation to growth phase of Candida albicans and Candida parapsilosis. Antimicrob. Agents Chemother. 1984, 26, 699–701. [Google Scholar] [CrossRef]
- Liyanaarachchi, G.; Perera, A.; Samarasekera, J.; Mahanama, R.; Hemalal, P.; Dlamini, S.; Perera, S.; Alhadidi, Q.; Shah, Z.; Tillekeratne, V. Bioactive constituents isolated from the Sri Lankan endemic plant Artocarpus nobilis and their potential to use in novel cosmeceuticals. Ind. Crops Prod. 2022, 184, 115076. [Google Scholar] [CrossRef]
- Atsü, A.N.; Tosuner, Z.; Bilgiç, T. Evaluation of the Effect of Pomegranate Seed Oil on Healing in a Rat Wound Model with Antioxidant, Vascular, and Histopathological Parameters. Int. J. Low. Extrem. Wounds 2021. [Google Scholar] [CrossRef]
- Trotta, V.; Scalia, S. Pulmonary delivery systems for polyphenols. Drug Dev. Ind. Pharm. 2017, 43, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Husari, A.; Hashem, Y.; Bitar, H.; Dbaibo, G.; Zaatari, G.; el Sabban, M. Antioxidant activity of pomegranate juice reduces emphysematous changes and injury secondary to cigarette smoke in an animal model and human alveolar cells. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 227–237. [Google Scholar] [CrossRef]
- Hamouda, A.F.; Felemban, S. A Pilot Study of the Amelioration of Avocado Seed Oil in Obese Female Rats Induced by Carbon Tetrachloride and Alloxan Monohydrate. J. Drug Alcohol Res. 2022, 11, 236177. [Google Scholar] [CrossRef]
- Hirano, T.; Higa, S.; Arimitsu, J.; Naka, T.; Shima, Y.; Ohshima, S.; Fujimoto, M.; Yamadori, T.; Kawase, I.; Tanaka, T. Flavonoids such as luteolin, fisetin and apigenin are inhibitors of interleukin-4 and interleukin-13 production by activated human basophils. Int. Arch. Allergy Immunol. 2004, 134, 135–140. [Google Scholar] [CrossRef]
- Felemban, S.; Hamouda, A.F. A Pilot Study of the Effects of Ajwa Date Seed Extract in a Diabetic Animal with Parallel Observations on Human Subjects. J. Pharm. Res. Int. 2022, 34, 23–33. [Google Scholar] [CrossRef]
- Asmaa, H. A biochemical study of agarwood on methanol injection in rat. J. Drug Alcohol Res. Ashdin Publ. Corp. 2019, 8, 236073. [Google Scholar]
- Morteza, S.A.; Sayid, M.M.; Masumeh, D.; Ehsan, N. Evaluation of Wound Healing Activities of Pomegranate (Punica granatum-Lythraceae) Peel and Pulp. J. Res. Med. Dent. Sci. 2018, 6, 230–236. Available online: https://doaj.org/article/2a61efbe63e143eba7e6eb1343664d9b (accessed on 15 May 2022).
- Ramírez-Bosca, A.; Carrion, M.; Lopez, V.N. Pomegranate Seed Oil Increases the Expression of Alpha 1 Type I Collagen, Elastin and Telomerase Reverse Transcriptase Genes in Oryzias Latipes Embryos. Adv. Cosmet. Dermatol. 2015, 1, 1–8. Available online: https://www.researchgate.net/publication/282908892_Pomegranate_seed_oil_increases_the_expression_of_alpha_1_type_I_collagen_elastin_and_telomerase_reverse_transcriptase_genes_in_Oryzias_latipes_embryos (accessed on 24 November 2022).
- Pirbalouti, A.G.; Azizi, S.; Koohpayeh, A.; Hamedi, B. Wound Healing Activity of Malva Sylvestris and Punica granatum in Alloxan-Induced Diabetic Rats. Acta Pol. Pharm. 2010, 67, 511–516. Available online: https://pubmed.ncbi.nlm.nih.gov/20873419/ (accessed on 6 October 2022).
- Aslam, M.N.; Lansky, E.P.; Varani, J. Pomegranate as a cosmeceutical source: Pomegranate fractions promote proliferation and procollagen synthesis and inhibit matrix metalloproteinase-1 production in human skin cells. J. Ethnopharmacol. 2006, 103, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, A.F.; Hassan, A. A Short Communication Pilot Study on Stress and Its Chronic Consequences of College Students. CPQ Nutr. 2020, 4, 1–12. Available online: Chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cientperiodique.com/article/CPQNN-4-2-81.pdf (accessed on 1 January 2021).
- Hamouda, A.F. The Association between Lifestyle, Anthropometric Measurements, and Obesity in University Students. J. Pharm. Pharmacol. 2016, 4, 119–127. [Google Scholar] [CrossRef][Green Version]
- Daboor, S.M.; Budge, S.M.; Ghaly, A.E.; Brooks, S.L.; Dave, D. Extraction and Purification of Collagenase Enzymes: A Critical Review. Am. J. Biochem. Biotechnol. 2010, 6, 239–263. [Google Scholar] [CrossRef]
- Azmi, N.; Hashim, P.; Hashim, D.M.; Halimoon, N.; Majid, N.M.N. Anti-elastase, anti-tyrosinase and matrix metalloproteinase-1 inhibitory activity of earthworm extracts as potential new anti-aging agent. Asian Pac. J. Trop. Biomed. 2014, 4 (Suppl. 1), S348–S352. [Google Scholar] [CrossRef]
- Lansing, A.I.; Rosenthal, T.B.; Alex, M.; Dempsey, E.W. The structure and chemical characterization of elastic fibers as reveled by elastase and by electron microscopy. Anat. Rec. 1952, 114, 555–575. [Google Scholar] [CrossRef] [PubMed]
- Nadalian, M.; Kamaruzaman, N.; Yusop, M.S.M.; Babji, A.S.; Yusop, S.M. Isolation, Purification and Characterization of Antioxidative Bioactive Elastin Peptides from Poultry Skin. Food Sci. Anim. Resour. 2019, 39, 966–979. [Google Scholar] [CrossRef]
- Houghton, A.M.; Rzymkiewicz, D.M.; Ji, H.; Gregory, A.D.; Egea, E.E.; Metz, H.E.; Stolz, D.B.; Land, S.R.; Marconcini, L.A.; Kliment, C.R.; et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat. Med. 2010, 16, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Sirithunyalug, J.; Somwongin, S.; Punyoyai, C.; Laothaweerungsawat, N.; Marsup, P.; Neimkhum, W.; Yawootti, A. Enhancement of the Antioxidant, Anti-Tyrosinase, and Anti-Hyaluronidase Activity of Morus alba L. Leaf Extract by Pulsed Electric Field Extraction. Molecules 2020, 25, 2212. [Google Scholar] [CrossRef]
- Montgomery, H.A.C.; Dymock, J. The determination of nitrite in water. Analyst 1961, 86, 414–416. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Zhang, Y.; Talley, J.J. Pharmacological analysis of cyclooxygenase-1 in inflammation A High-Throughput Method for Screening Arabidopsis Mutants with Disordered Abiotic Stress-Induced Calcium Signal View project Amino acid isosteres View project. Proc. Natl. Acad. Sci. USA 1998, 95, 13313–13318. [Google Scholar] [CrossRef]




| Metal | Concentration (mg/kg) |
|---|---|
| Na | 2.92 |
| K | 220 |
| Ca2+ | 8.60 |
| Fe | 0.26 |
| Zn | 0.39 |
| Cd | Not detected |
| Pb | Not detected |
| Al | Not detected |
| Sr. No. | Name | Base Peak | RT * | Chromatogram (Device Print Out) |
|---|---|---|---|---|
| 1. | 5-Hydroxymethylfurfural | 97 | 5.702–6.087 |  |
| 2. | Pentanoic acid, 5-hydroxy-, 2,4-di-t-butylphenyl esters | 191.2 | 8.227–8.291 | 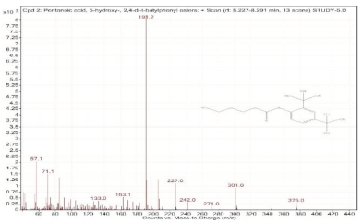 |
| 3. | Dodecyl acrylate | 55 | 9.554–9.591 | 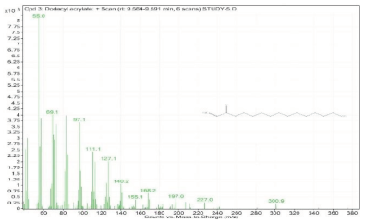 |
| 4. | n-Hexadecanoic acid | 73 | 11.527–11.628 | 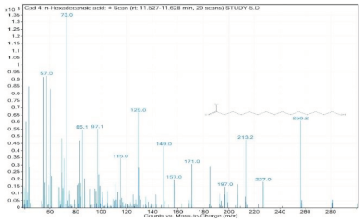 |
| 5. | Oleanitrile | 55 | 12.302–12.351 | 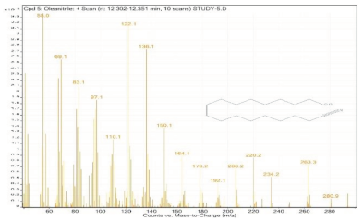 |
| 6. | 9-Octadecenoic acid, methyl ester, (E)- | 55.1 | 12.351–12.383 | 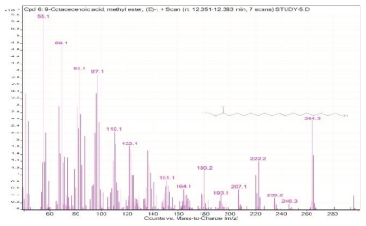 |
| 7. | 9,12-Octadecadienoic acid (Z,Z)- | 55 | 12.575–12.778 | 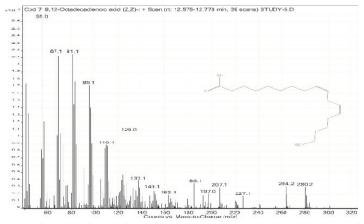 |
| 8. | Octadecanoic acid | 55 | 12.778–12.880 | 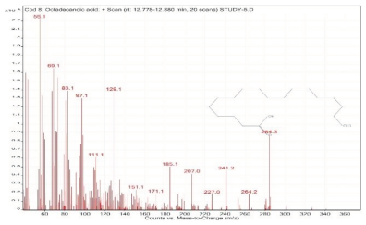 |
| 9. | Hexadecanamide | 59 | 12.934–12.960 | 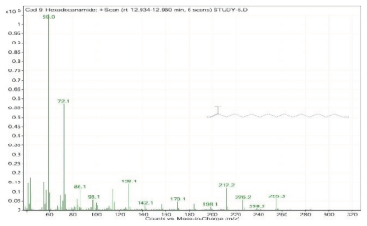 |
| 10. | 7-Hexadecenal, (Z)- | 55 | 13.522–13.624 | 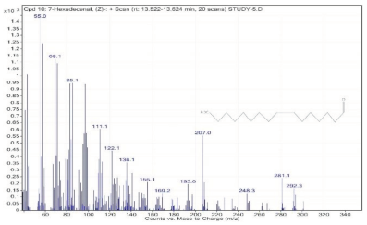 |
| 11. | 9-Octadecenamide, (Z)- | 59 | 14.003–14.073 | 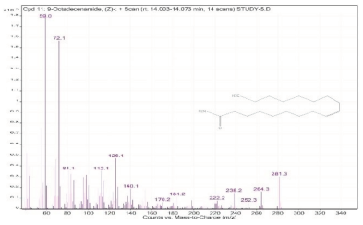 |
| 12. | 9-Octadecenamide, (Z)- | 59 | 14.073–14.126 | 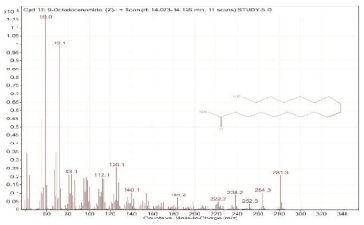 |
| 13. | Octadecanamide | 59 | 14.126–14.180 |  |
| 14. | Oleamide | 59 | 14.239–14.340 |  |
| 15. | Erucic acid | 55.1 | 14.645–14.677 |  |
| 16. | cis-11-Eicosenamide | 59 | 15.127–15.164 |  |
| 17. | cis-11-Eicosenamide | 59 | 15.164–15.223 |  |
| 18. | 9-Octadecenamide, (Z)- | 59 | 15.233–15.292 |  |
| 19. | 13-Docosenamide, (Z)- | 59 | 16.458–16.506 |  |
| 20. | Squalene | 69.1 | 16.517–16.565 |  |
| 21. | Stigmastan-3,5-diene | 145.1 | 18.213–18.277 | 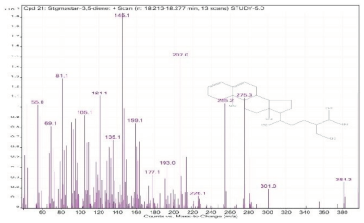 |
| 22. | gamma-Tocopherol | 416.4 | 19.015–19.101 | 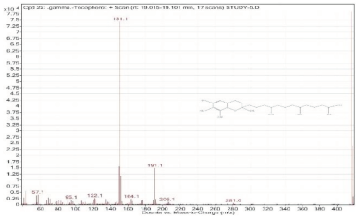 |
| 23. | Stigmastan-3,5-diene | 396.4 | 19.785–19.865 | 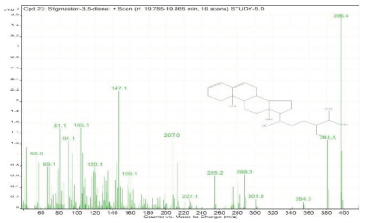 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamouda, A.F.; Felemban, S. Biochemical Pilot Study on Effects of Pomegranate Seed Oil Extract and Cosmetic Cream on Neurologically Mediated Skin Inflammation in Animals and Humans: A Comparative Observational Study. Molecules 2023, 28, 903. https://doi.org/10.3390/molecules28020903
Hamouda AF, Felemban S. Biochemical Pilot Study on Effects of Pomegranate Seed Oil Extract and Cosmetic Cream on Neurologically Mediated Skin Inflammation in Animals and Humans: A Comparative Observational Study. Molecules. 2023; 28(2):903. https://doi.org/10.3390/molecules28020903
Chicago/Turabian StyleHamouda, Asmaa Fathi, and Shifa Felemban. 2023. "Biochemical Pilot Study on Effects of Pomegranate Seed Oil Extract and Cosmetic Cream on Neurologically Mediated Skin Inflammation in Animals and Humans: A Comparative Observational Study" Molecules 28, no. 2: 903. https://doi.org/10.3390/molecules28020903
APA StyleHamouda, A. F., & Felemban, S. (2023). Biochemical Pilot Study on Effects of Pomegranate Seed Oil Extract and Cosmetic Cream on Neurologically Mediated Skin Inflammation in Animals and Humans: A Comparative Observational Study. Molecules, 28(2), 903. https://doi.org/10.3390/molecules28020903