Probing the Potential Energy Profile of the I + (H2O)3 → HI + (H2O)2OH Forward and Reverse Reactions: High Level CCSD(T) Studies with Spin-Orbit Coupling Included
Abstract
1. Introduction
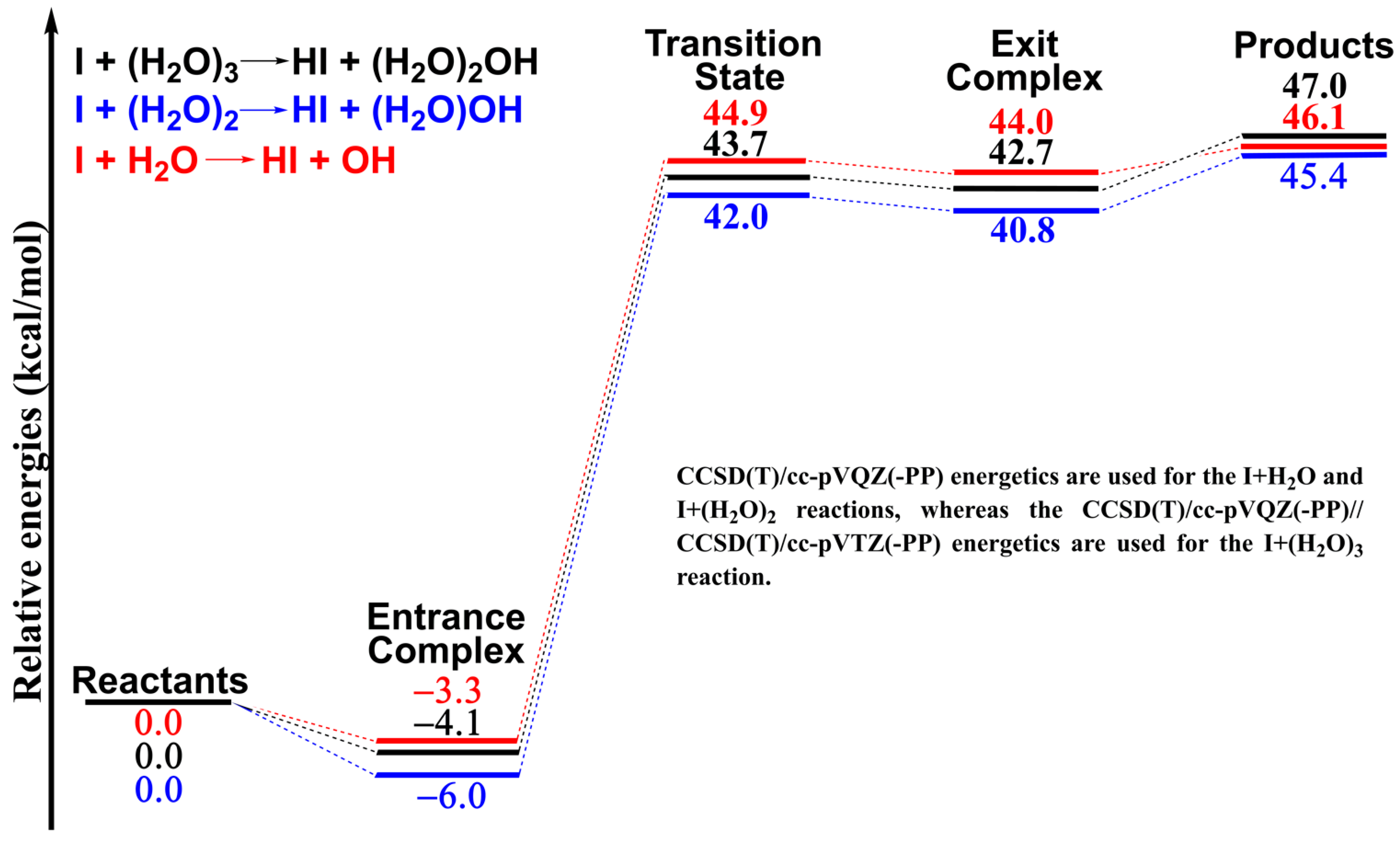
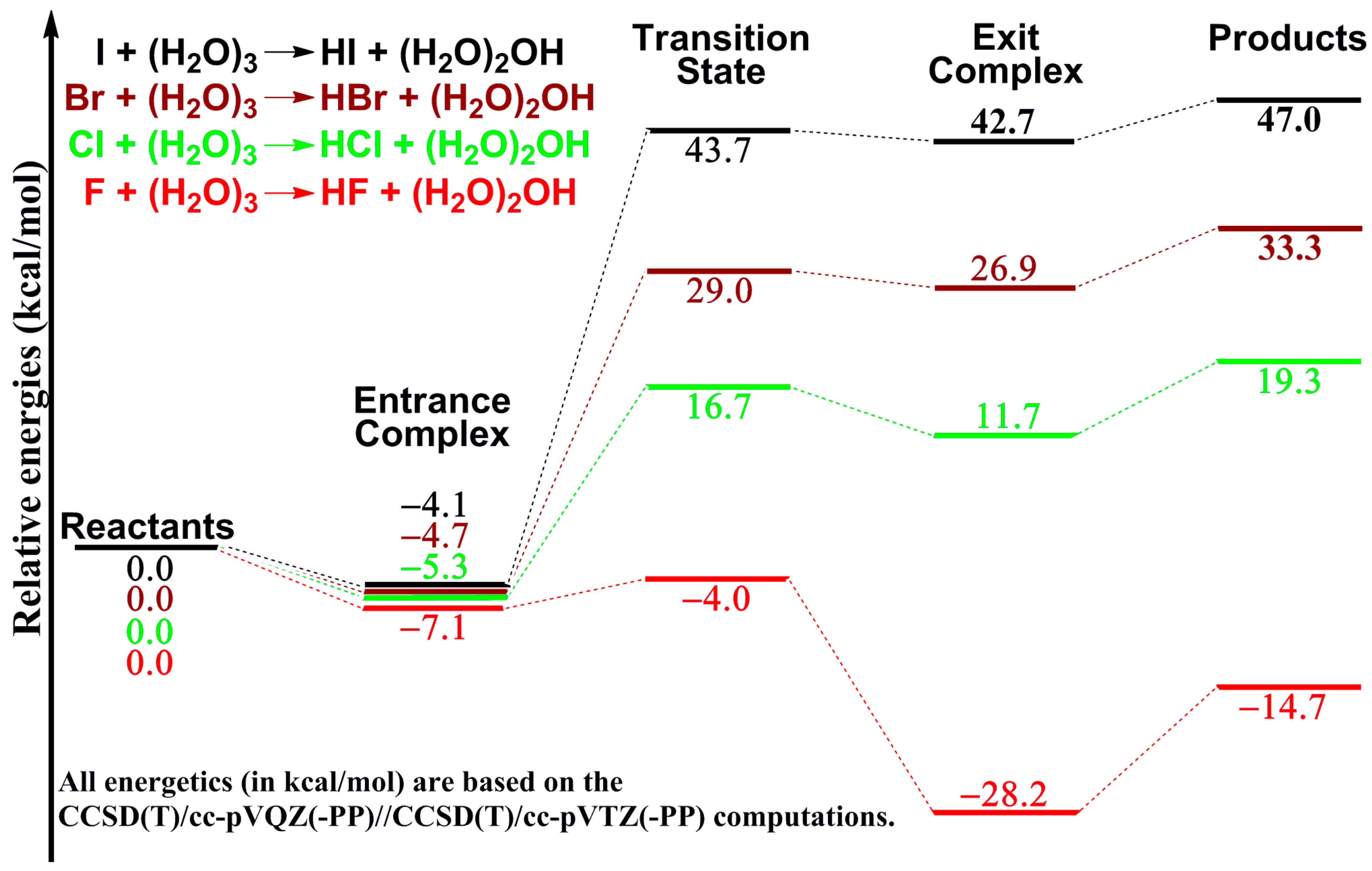
2. Results and Discussion
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lewis, T.R.; Gómez Martín, J.C.; Blitz, M.A.; Cuevas, C.A.; Plane, J.M.C.; Saiz-Lopez, A. Determination of the absorption cross sections of higher-order iodine oxides at 355 and 532 nm. Atmos. Chem. Phys. 2020, 20, 10865–10887. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, X.; Fung, J.C.H.; Sarwar, G.; Li, Z.; Li, Q.; Saiz-Lopez, A.; Lau, A.K.H. Effect of bromine and iodine chemistry on tropospheric ozone over Asia-Pacific using the CMAQ model. Chemosphere 2021, 262, 127595. [Google Scholar] [CrossRef] [PubMed]
- Dix, B.; Baidar, S.; Bresch, J.F.; Hall, S.R.; Schmidt, K.S.; Wang, S.; Volkamer, R. Detection of iodine monoxide in the tropical free troposphere. Proc. Natl. Acad. Sci. USA 2013, 110, 2035–2040. [Google Scholar] [CrossRef]
- Saiz-Lopez, A.; Mahajan, A.S.; Salmon, R.A.; Bauguitte, S.J.B.; Jones, A.E.; Roscoe, H.K.; Plane, J.M.C. Boundary layer halogens in coastal Antarctica. Science 2007, 317, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Chameides, W.L.; Davis, D.D. The free radical chemistry of cloud droplets and its impact upon the composition of rain. J. Geophys. Res. 1982, 87, 4863–4877. [Google Scholar] [CrossRef]
- Solomon, S.; Garcia, R.R.; Ravishankara, A.R. On the role of iodine in ozone depletion. J. Geophys. Res. 1994, 99, 20491. [Google Scholar] [CrossRef]
- Leod, H.M.; Balestra, C.; Jourdain, J.L.; Laverdet, G.; Bras, G.L. Kinetic study of the reaction OH + HI by laser photolysis-resonance fluorescence. Int. J. Chem. Kinet. 1990, 22, 1167–1176. [Google Scholar] [CrossRef]
- Takacs, G.A.; Glass, G.P. Reactions of hydroxyl radicals with some hydrogen halides. J. Phys. Chem. 1973, 77, 1948–1951. [Google Scholar] [CrossRef]
- Lancar, I.T.; Mellouki, A.; Poulet, G. Kinetics of the Reactions of hydrogen iodide with hydroxyl and nitrate radicals. Chem. Phys. Lett. 1991, 177, 554–558. [Google Scholar] [CrossRef]
- Campuzano-Jost, P.; Crowley, J.N. Kinetics of the reaction of OH with HI between 246 and 353 K. J. Phys. Chem. A 1999, 103, 2712–2719. [Google Scholar] [CrossRef]
- Moise, A.; Parker, D.H.; ter Meulen, J.J. State-to-state inelastic scattering of OH by HI: A comparison with OH-HCl and OH-HBr. J. Chem. Phys. 2007, 126, 124302. [Google Scholar] [CrossRef] [PubMed]
- Inada, Y.; Akagane, K. Non-empirical analysis of the chemical reactions of iodine with steam; I + H2O → HI + OH and I + H2O → IOH + H, in severe light water reactor accidents. J. Nucl. Sci.Technol. 1997, 34, 217–221. [Google Scholar] [CrossRef]
- Hao, Y.; Gu, J.; Guo, Y.; Zhang, M.; Xie, Y.; Schaefer, H.F. Spin-orbit corrected potential energy surface features for the I(2P3/2) + H2O → HI + OH forward and reverse reactions. Phys. Chem. Chem. Phys. 2014, 16, 2641–2646. [Google Scholar] [CrossRef] [PubMed]
- Lillington, J.N. Light Water Reactor Safety; Elsevier: New York, NY, USA, 1995. [Google Scholar]
- Wang, H.; Li, G.; Li, Q.-S.; Xie, Y.; Schaefer, H.F. I + (H2O)2 → HI + (H2O)OH forward and reverse reactions. CCSD(T) studies including spin–orbit coupling. J. Phys. Chem. B 2016, 120, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Q.-S.; Xie, Y.; Schaefer, H.F. From gas-phase to liquid-water chemical reactions: The fluorine atom plus water trimer system. Angew. Chem. Int. Ed. 2015, 54, 11223–11226. [Google Scholar] [CrossRef]
- Li, G.; Yao, Y.; Lu, S.; Xie, Y.; Douberly, G.E.; Schaefer, H.F. Potential energy profile for the Cl + (H2O)3 → HCl + (H2O)2OH reaction. A CCSD(T) study. Phys. Chem. Chem. Phys. 2021, 23, 26837–26842. [Google Scholar] [CrossRef]
- Li, G.; Yao, Y.; Lin, Y.; Meng, Y.; Xie, Y.; Schaefer, H.F. The reaction between the bromine atom and the water trimer: High level theoretical studies. Phys. Chem. Chem. Phys. 2022, 24, 26164–26169. [Google Scholar] [CrossRef]
- Howard, J.C.; Tschumper, G.S. Wavefunction methods for the accurate characterization of water clusters. WIREs Comput. Mol. Sci. 2014, 4, 199–224. [Google Scholar] [CrossRef]
- Miliordos, E.; Aprà, E.; Xantheas, S.S. Optimal geometries and harmonic vibrational frequencies of the global minima of water clusters (H2O)n, n = 2–6, and several hexamer local minima at the CCSD(T) level of theory. J. Chem. Phys. 2013, 139, 114302. [Google Scholar] [CrossRef]
- Ch’ng, L.C.; Samanta, A.K.; Wang, Y.; Bowman, J.M.; Reisler, H. Experimental and theoretical investigations of the dissociation energy (D0) and dynamics of the water trimer, (H2O)3. J. Phys. Chem. A 2013, 117, 7207–7216. [Google Scholar] [CrossRef]
- Wang, Y.; Shepler, B.C.; Braams, B.J.; Bowman, J.M. Full-dimensional, ab initio potential energy and dipole moment surfaces for water. J. Chem. Phys. 2009, 131, 054511. [Google Scholar] [CrossRef] [PubMed]
- Keutsch, F.N.; Cruzan, J.D.; Saykally, R.J. The water trimer. Chem. Rev. 2003, 103, 2533–2577. [Google Scholar] [CrossRef]
- Fowler, J.E.; Schaefer, H.F. Detailed study of the water trimer potential energy surface. J. Am. Chem. Soc. 1995, 177, 446–452. [Google Scholar] [CrossRef]
- Pugliano, N.; Saykally, R.J. Measurement of quantum tunneling between chiral isomers of the cyclic water trimer. Science 1992, 257, 1937–1940. [Google Scholar] [CrossRef]
- Burnham, C.J.; Xantheas, S.S.; Miller, M.A.; Applegate, B.E.; Miller, R.E. The formation of cyclic water complexes by sequential ring insertion: Experiment and theory. J. Chem. Phys. 2002, 117, 1109–1122. [Google Scholar] [CrossRef]
- Tsuji, K.; Shibuya, K. Infrared spectroscopy and quantum chemical calculations of OH-(H2O)n complexes. J. Phys. Chem. A 2009, 113, 9945. [Google Scholar] [CrossRef]
- Moudens, A.; Georges, R.; Goubet, M.; Makarewicz, J.; Lokshtanov, S.E.; Vigasin, A.A. Direct absorption spectroscopy of water clusters formed in a continuous slit nozzle expansion. J. Chem. Phys. 2009, 131, 204312. [Google Scholar] [CrossRef]
- Huber, K.P.; Herzberg, G. Molecular Spectra and Molecular Structure: IV. Constants of Diatomic Molecules; van Nostrand Reinhold: New York, NY, USA, 1979. [Google Scholar]
- Berning, A.; Schweizer, M.; Werner, H.-J.; Knowles, P.J.; Palmieri, P. Spin-orbit matrix elements for internally contracted multireference configuration interaction wavefunctions. Mol. Phys. 2000, 98, 1823–1833. [Google Scholar] [CrossRef]
- Werner, H.-J.; Knowles, P.J.; Knizia, G.; Manby, F.R.; Schütz, M.; Celani, P.; Korona, T.; Lindh, R.; Mitrushenkov, A.; Rauhut, G.; et al. MOLPRO, Version 2015.1, a Package of Ab Initio Programs. 2015. Available online: http://www.molpro.net (accessed on 22 March 2018).
- Minnhagen, L. The energy levels of neutral atomic iodine. Ark. Fys. 1962, 21, 415–478. [Google Scholar]
- Lynch, B.J.; Fast, P.L.; Harris, M.; Truhlar, D.G. Adiabatic connection kinetics. J. Phys. Chem. A 2000, 104, 4811–4815. [Google Scholar] [CrossRef]
- Li, G.; Zhou, L.; Li, Q.-S.; Xie, Y.; Schaefer, H.F. The entrance complex, transition state, and exit complex for the F + H2O → HF + OH reaction. Definitive predictions. Comparison with popular density functional methods. Phys. Chem. Chem. Phys. 2012, 14, 10891–10895. [Google Scholar] [CrossRef] [PubMed]
- Purvis, G.D.; Bartlett, R.J.A. Full coupled-cluster singles and doubles model: The inclusion of disconnected triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Scuseria, G.E.; Janssen, C.L.; Schaefer, H.F. An efficient reformulation of the closed-shell coupled cluster single and double excitation (CCSD) equations. J. Chem. Phys. 1988, 89, 7382–7387. [Google Scholar] [CrossRef]
- Raghavachari, K.; Trucks, G.W.; Pople, J.A.; Head-Gordon, M. A fifth-order perturbation comparison of electron correlation theories. Chem. Phys. Lett. 1989, 157, 479–483. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations I: The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Peterson, K.A.; Shepler, B.C.; Figgen, D.; Stoll, H. On the spectroscopic and thermochemical properties of ClO, BrO, IO, and their anions. J. Phys. Chem. A 2006, 110, 13877–13883. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Hratchian, H.P.; Schlegel, H.B. Accurate reaction paths using a hessian based predictor-corrector integrator. J. Chem. Phys. 2004, 120, 9918–9924. [Google Scholar] [CrossRef]
- Hratchian, H.P.; Schlegel, H.B. Theory and Applications of Computational Chemistry: The First 40 Years; Dykstra, C.E., Frenking, G., Kim, K.S., Scuseria, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Hratchian, H.P.; Schlegel, H.B. Using hessian updating to increase the efficiency of a hessian based predictor-corrector reaction path following method. J. Chem. Theory Comput. 2005, 1, 61–69. [Google Scholar] [CrossRef]
- Stanton, J.F.; Gauss, J.; Harding, M.E.; Szalay, P.G.; Auer, A.A.; Bartlett, R.J.; Benedikt, U.; Berger, C.; Bernholdt, D.E.; Bomble, Y.J.; et al. CFOUR. A Quantum Chemical Program Package; Watts and the Integral Packages MOLECULE (Almlöf, J.; Taylor, P.R.), PROPS (Taylor, P.R.), ABACUS (Helgaker, T.; Jensen, H.J. Aa.; Jørgensen, P.; Olsen, J.), and ECP Routines by Mitin, A.V.; van Wüllen., C.; Gaussian Inc.: Wallingford, CT, USA, 2010; Available online: https://cfour.uni-mainz.de/cfour/ (accessed on 13 December 2022).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Chen, X.; Lin, Y.; Meng, Y.; Li, G.; Xie, Y.; Schaefer, H.F., III. Probing the Potential Energy Profile of the I + (H2O)3 → HI + (H2O)2OH Forward and Reverse Reactions: High Level CCSD(T) Studies with Spin-Orbit Coupling Included. Molecules 2023, 28, 904. https://doi.org/10.3390/molecules28020904
Zhang X, Chen X, Lin Y, Meng Y, Li G, Xie Y, Schaefer HF III. Probing the Potential Energy Profile of the I + (H2O)3 → HI + (H2O)2OH Forward and Reverse Reactions: High Level CCSD(T) Studies with Spin-Orbit Coupling Included. Molecules. 2023; 28(2):904. https://doi.org/10.3390/molecules28020904
Chicago/Turabian StyleZhang, Xinyuan, Xiaoting Chen, Yan Lin, Yan Meng, Guoliang Li, Yaoming Xie, and Henry F. Schaefer, III. 2023. "Probing the Potential Energy Profile of the I + (H2O)3 → HI + (H2O)2OH Forward and Reverse Reactions: High Level CCSD(T) Studies with Spin-Orbit Coupling Included" Molecules 28, no. 2: 904. https://doi.org/10.3390/molecules28020904
APA StyleZhang, X., Chen, X., Lin, Y., Meng, Y., Li, G., Xie, Y., & Schaefer, H. F., III. (2023). Probing the Potential Energy Profile of the I + (H2O)3 → HI + (H2O)2OH Forward and Reverse Reactions: High Level CCSD(T) Studies with Spin-Orbit Coupling Included. Molecules, 28(2), 904. https://doi.org/10.3390/molecules28020904






