Abstract
As an alternative to fossil volatile hydrocarbon solvents used nowadays in perfumery, investigation on essential oil of Commiphora wildii Merxm. oleo gum resin as a source of heptane is reported here. Heptane, representing up to 30 wt-% of this oleo gum resin, was successfully isolated from the C. wildii essential oil, using an innovative double distillation process. Isolated heptane was then used as a solvent in order to extract some noble plants of perfumery. It was found that extracts obtained with this solvent were more promising in terms of sensory analysis than those obtained from fossil-based heptane. In addition, in order to valorize the essential oil depleted from heptane, chemical composition of this oil was found to obtain, and potential biological activity properties were studied. A total of 172 different compounds were identified by GC-MS in the remaining oil. In vitro tests—including hyaluronidase, tyrosinase, antioxidant, elastase and lipoxygenase, as well as inhibitory tests against two yeasts and 21 bacterial strains commonly found on the skin—were carried out. Overall, bioassays results suggest this heptane-depleted essential oil is a promising active ingredient for cosmetic applications.
1. Introduction
Because of the large number of volatile compounds exhibiting pleasant odor, flavor and biological activities present in essential oils, the latter are valorized in various ways, such as aromas, perfumes and cosmetics, as well as pharmaceutical drugs [1,2]. When a compound inside an essential oil is relatively abundant, it can be physically isolated (e.g., fractional distillation). For instance, Chemat et al. reported the extraction of limonene from citrus peels essential oil and its valorization as a green solvent [3,4]. Similarly, menthol was extracted and further purified from the essential oil of Mentha arvensis by fractional distillation [5].
Commiphora wildii Merxm. is a Namibian desert resin tree. Its aromatic exudate is traditionally used as a perfume by women from the Ovahimba tribe, originating from Namibia. The resin is also used in combination with animal fat and ochre to protect skin against solar exposure [6]. Up to now, C. wildii essential oil has received little attention leading to limited knowledge about its phytochemical content, compared to studies reported for C. myrrha [7,8,9,10]. Very recently, two works described the terpenoid content of C. wildii [6,11]. The essential oil of this Namibian resin analyzed by Sheehama et al. [6] shows a surprisingly high level of heptane (24%) along with α-Pinene (50.0%) and β-Pinene (11.7%). Jemmali et al. [11] have characterized monoterpenoids, diterpenoids and triterpenoids in C. wildii resin after solvent extraction such as chloroform, and sample derivatization. Other species, such as Pinus oocarpa, P. jeffreyi and P. sabiniana contain large amounts of heptane in their essential oil obtained from exudates or from woods [12,13]. But to the best of our knowledge C. wildii is the only one in the Commiphora genus presenting such a high amount of natural heptane. C. wildii can thus be envisaged as a viable source of sustainable heptane to be applied as an extraction solvent in perfume industry.
There are three goals for this study: (i) to describe isolation of heptane from C. wildii essential oil; (ii) to evaluate sustainable heptane as an extraction solvent of raw materials for perfumery; and (iii) to study the chemical composition of the remaining essential oil, after heptane isolation, and its possible bioactivities. The latter goal could provide insights into the reasons for the traditional use of this plant and give possible ways of valorizing this extract. Such results have led to a patent recently filed by our research group [14].
2. Results
2.1. Chemical Composition of Essential Oil of C. wildii and Heptane Isolation
Isolation of heptane from C. wildii was achieved by using an innovative two-step process, so-called “double-distillation” process. The first step consists in a classical hydrodistillation from C. wildii resin, leading to the production of an essential oil (EO). In the second step, a fractional distillation of the essential oil leads to one enriched heptane fraction and one oily fraction corresponding to the remaining essential oil. In the essential oil from C. wildii resin obtained after the first step, 58 compounds were identified (98.5% of the total GC area; Table S1, Supplementary Information). Compounds were mainly monoterpene and sesquiterpene hydrocarbons, along with alkane. Oxygenated terpenes were also characterized. As shown in Table 1, most abundant compounds were α-pinene (43.4%), heptane (29.5%) and β-pinene (11%), in accordance with previous work [6].

Table 1.
Major compounds of C. wildii essential oil (GC/MS) before heptane isolation (compounds with relative percentage greater than 0.5%).
The proportion of heptane was determined by external calibration (protocol described in materials and methods section) and was found to be around 25 to 35% in mass, depending on the batch from the supplier. These results are in agreement with the one reported by Sheehama et al. (24 ± 7%) [6].
During the second step of the process, a fraction of around 35 wt% was separated from the rest of the essential oil by fractional vacuum-distillation. This fraction contained heptane (>99%), α-pinene (0.3%) and β-pinene (0.1%).
2.2. Heptane from C. wildii as An Extraction Solvent for Perfumery
Heptane obtained was evaluated as solvent for extracting three emblematic and historical flowers of French perfumery: rose (Rosa centiflolia L.), Madone lily (Lilium candidum L.) and jasmine (Jasminum grandiflorum L.). Both extractions were carried out under the same conditions. Exception made from two peaks was found on the chromatogram of concrete obtained using heptane from C. wildii, GC-MS analyses of each concrete led to similar chromatograms within experimental uncertainty. These two peaks were attributed to α-pinene and β-pinene, which are removed from the essential oil together with heptane during the double distillation process. Olfactory comparison (use of a panel trained in odor description) between concretes extracted with fossil heptane and those obtained from natural heptane was performed.
Results, collected in Table 2, reveal that different olfactory perceptions between concretes were obtained using heptane from C. wildii EO and those obtained using fossil heptane. Yields obtained using natural heptane appears to be higher than those obtained using fossil heptane, and to exhibit slightly different olfactive profiles, with a generally more floral note. Olfactive description was carried out with the help of an independent perfumist.

Table 2.
Comparison of olfactive evaluation obtained for the extraction of tree natural products, using fossil heptane and heptane from C. wildii.
Even if natural heptane extracted from this essential oil cannot be used in large quantities, it offers a natural alternative to petrochemical hydrocarbons, especially to extract noble fragrances from plants (i.e., concrete) [15] in limited productions.
2.3. C. wildii Essential Oil after Heptane Isolation
The remaining essential oil, after heptane isolation, represents nearly 70% of starting essential oil. This represents an important quantity that must be valorized.
The essential oil composition of C. wildii resin, after heptane isolation, was studied by GC-MS and GC/FID (Figure 1). To achieve this, the residual essential oil was analyzed directly and after fractionation by silica gel chromatography (Figure S1, Supplementary Materials). Compounds identified are presented in Table 3 along with their elution time. Chromatographic profiles of the essential oil before fractionation revealed 97 compounds. After fractionation by silica gel chromatography, 172 compounds were identified, representing 98.36% of total GC/FID area. This number is much larger than those previously identified by Sheehama et al. [6]. Qualitatively, mainly oxygenated monoterpenes (72), monoterpenes (24), sesquiterpenes (14), oxygenated sesquiterpenes (10), diterpenes (2), hemiterpene (1) but also acids (5), alkanes (6), alcohols (9), aldehydes (3), ketones (13), esters (6), phenols (3), aromatic hydrocarbon (1) and furans (3) were found.
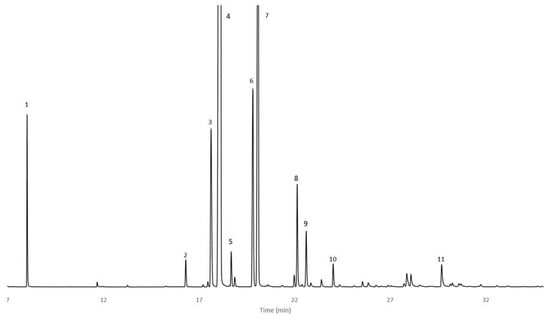
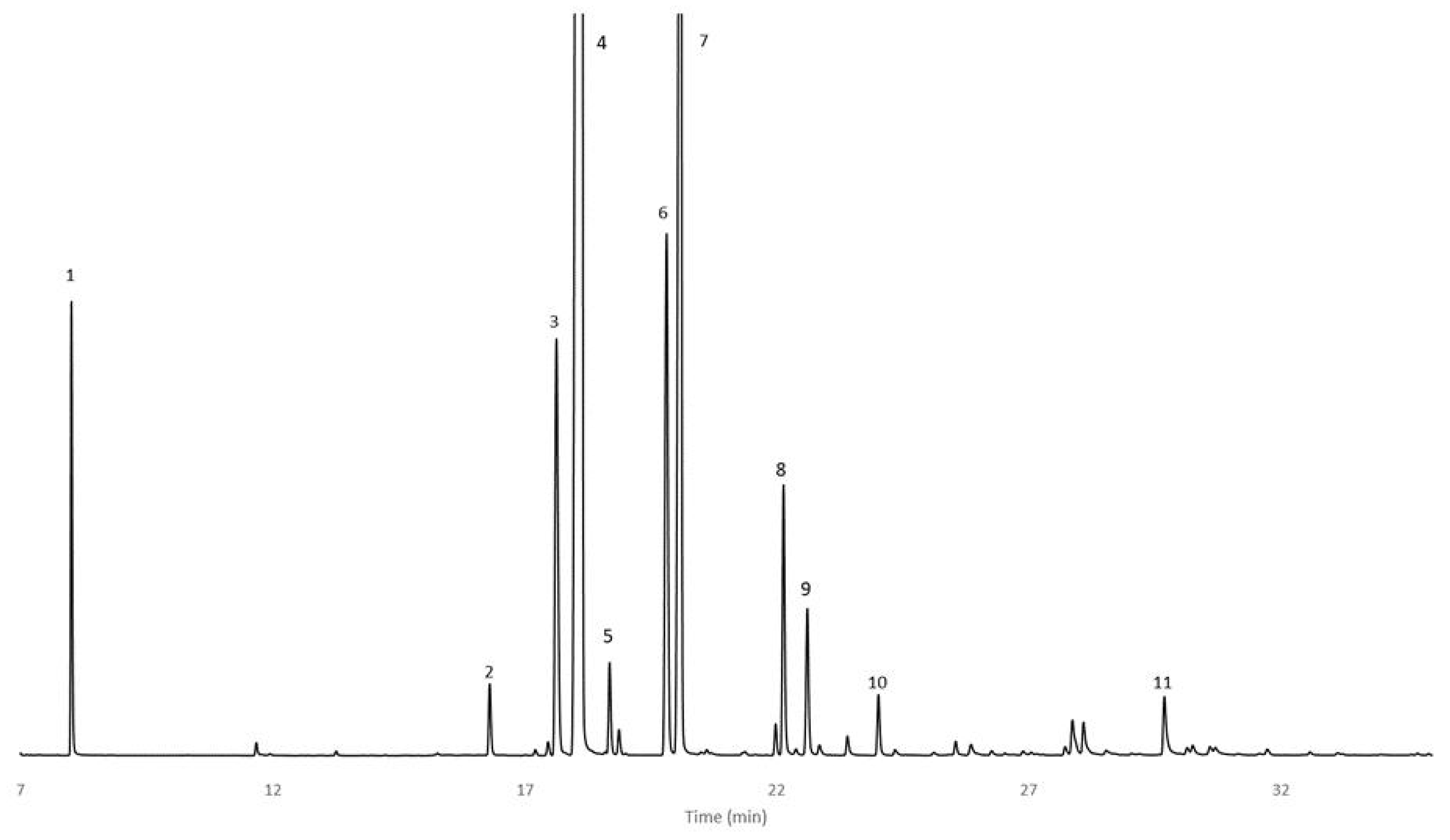
Figure 1.
GC/FID chromatogram of C. wildii doubly distillated essential oil from 7 to 35 min: 1 Heptane, 2 Nonane, 3 α-Thujene, 4 α-Pinene, 5 Camphene, 6 Sabinene, 7 β-Pinene, 8 p-Cymene, 9 Limonene, 10 γ-Terpinene, 11 Terpinene-4-ol (conditions in Section 4).

Table 3.
Constituents identified in the essential oil from C. wildii resin. Data reported according to their retention index values (RI) and relative abundance defined as a surface percentage of the FID chromatogram (% FID) and with the corresponding standard deviation (SD). Cal. and lit. stand for calculated and literature, respectively.
The most abundant chemical group is monoterpenes (94.04% of the FID total area), followed by alkanes (2.84%) and oxygenated monoterpenes (1.47%).
The ten major compounds of the residual essential oil are α-pinene (63.55%), β-pinene (15.95%), sabinene (5.01%), α-thujene (4.21%), heptane (2.31%), p-cymene (2.10%), limonene (1.22%), camphene (0.68%), terpinene-4-ol (0.64%) and nonane (0.53%).
2.4. Bioactivities of the Remaining Essential Oil
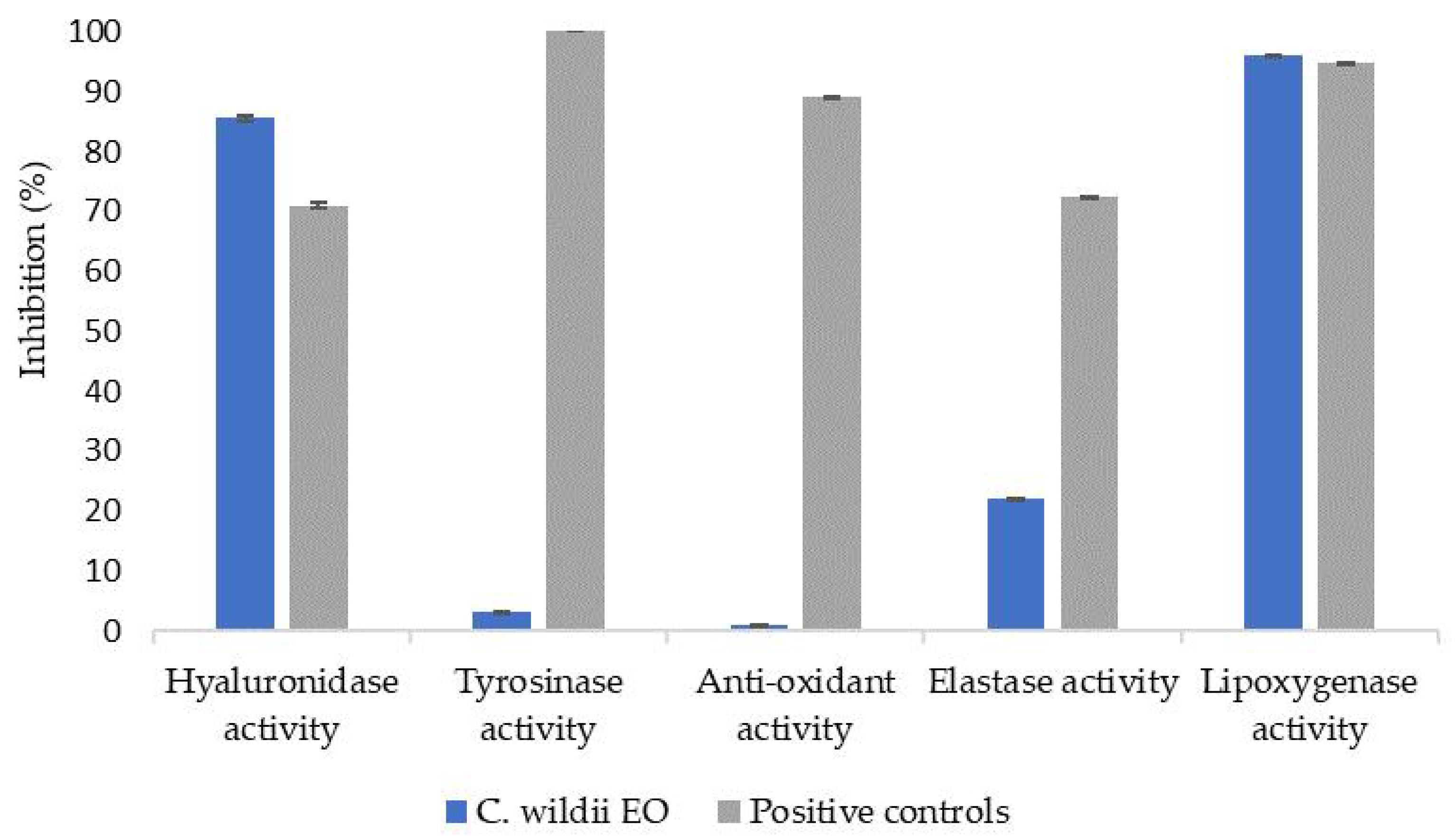
Because of the amount of compounds found in residual C. wildii essential oil after heptane isolation and in order to find potential applications of such an essential oil, cosmetic potential and antimicrobial potential was studied. To that end, several bioactivity assays, such as hyaluronidase, tyrosinase, antioxidant, elastase and lipoxygenase assays [16] were carried out. Results are presented in Figure 2. Finally, activity of such an essential oil on several fungi and bacterial strains was measured. Results are presented in Table 4 and Table 5.
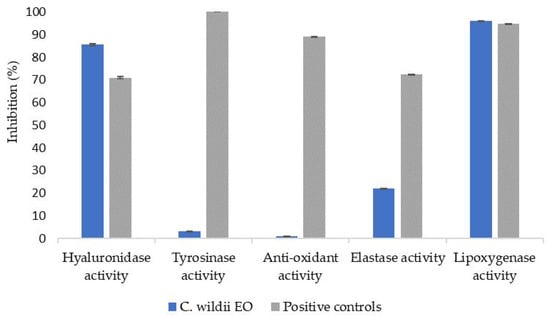
Figure 2.
Bioactivity tested for essential oil of C. wildii resin after heptane isolation, compared with bioactivity of commercial cosmetic ingredients (positive controls: hyaluronidase: commercial hydraberry extract, tyrosinase: SymWhite ingredient, Anti-oxidant: commercial rosemary extract, elastase: Berryflux vita (VITALAB), lipoxygenase: resveratrol).

Table 4.
Antifungal activities of C. wildii expressed as diameter of inhibition (mm) and as MIC80 (µg/mL) on two Candida species.

Table 5.
Antimicrobial activity expressed as MIC (mg/mL) of C. wildii essential oil on 21 Gram positive and negative bacteria strains.
2.4.1. Bioassays Results
Bioassay results obtained for doubly distilled C. wildii essential oil are presented in Figure 2. These results show that this essential oil exhibits significant hyaluronidase and lipoxygenase activities but no tyrosinase nor antioxidant activities. Finally, elastase activity is low compared to positive response since its inhibition reaches 22% compared to 72% obtained for the positive response.
2.4.2. Antimicrobial Tests
Antifungal activity of the doubly distillated essential oil was tested on two current yeasts and according to two different methods, namely agar diffusion and broth dilution method. Inhibition results are presented in Table 4.
Essential oil of C. wildii exhibited an inhibitory effect against both species of Candida albicans and glatrata, the inhibition diameter for the latter being slightly larger than that obtained on C. albicans. Nevertheless, the same MICs values were obtained. It can be noticed that essential oils from other species of the genus Commiphora seem to have a similar behavior against C. albicans [17,18,19].
On the other hand, the antibacterial activity of C. wildii essential oil has also been evaluated against various bacterial strains capable of colonizing mainly the oral cavity or skin (Table 5).
An interesting activity from 30 mg/mL on all strains of Methicillin-resistant S. aureus and S. pyogenes was observed. An inhibition is also detected on C. tuberculostearicum and P. asaccharolytica strains from a value of 100 mg/mL. However, no activity was found at the concentration tested on all other bacteria.
3. Discussion
Because of the amount of heptane found in the essential oil of C. wildii, typically 30%, it is conceivable to use this renewable source of heptane as an alternative source of natural solvent for the extraction of very valuable raw materials for perfumery. Extraction of three noble plants, as shown in Table 2, reveals that concrete yields are generally higher when heptane from C. wildii is used compared to fossil heptane. This is most probably due to the presence of odorous compounds, such as 0.3% α-pinene and 0.1% β-pinene, that are present in this heptane and that, because of a high volatility of these compounds, remain with heptane throughout the double distillation process. Once extraction from a plant such as Rosa centifolia or Jasminium grandifolium is carried out using this heptane, α-pinene and β-pinene, and possibly other molecules present in smaller amounts in heptane, remain within concrete after heptane removal, thus leading to a yield higher than expected.
Moreover, slight olfactory note variations are observed as well. These variations, once again, are most probably due to the presence of α-pinene and β-pinene remaining in concrete after removal of heptane from C. wildii., thus modifying slightly the olfactive profile of the concrete, compared to that obtained using fossil heptane.
To the best of our knowledge, except for antioxidant activity, biological activities of C. wildii essential oil, were not reported previously [6]. Hyaluronidase is an enzyme responsible for depolymerization of hyaluronic acid [20,21]. This acid, with its distinctive feature of retaining 6 L of water in 1 g of hyaluronic acid [22,23], ensures the hydration and softness of the skin, promotes wound healing and the reduction of wrinkles [21,22,23]. Degradation of hyaluronic acid by hyaluronidase causes the viscosity of body fluids to decrease and the permeability of connective tissues to increase [21,24]. Thus, hyaluronidase inhibitors, by their effective regulatory factors, preserve moisture and skin smoothness, by regulating and balancing both catabolism and anabolism processes [23]. Our experiments showed that the essential oil demonstrated an inhibitory activity of 86% in the hyaluronidase activity, a value greater than that of hydraberry commercial extract, that was used as a control (71% inhibition) for this test. This significantly high inhibitory effect might explain traditional use of C. wildii as a cosmetic in order to restore the skin.
Melanogenesis is the process of production of the melanin by cells called melanocytes [25]. Melanin is a main determinant of skin color, and it provides protection to the skin by absorbing 50% to 75% of ultraviolet rays. Also, these pigments have the ability to scavenge reactive oxygen species (ROS) [26,27]. In melanocytes, melanin synthesis is catalyzed by Tyrosinase. This enzyme is responsible for the hydroxylation of L-tyrosine into 3,4-dihydroxyphenylalanine (L-DOPA) and the subsequence oxidation of L-DOPA to dopaquinone. The latter is a highly reactive compound able to polymerize spontaneously to build melanin. In any case, studies [23,28] have concluded that in the skin, an excessive production of melanin can lead to melanoma and hyperpigmentation and can be genotoxic. However, the European commission restricts the use of a large list of synthetic molecules despite their strong bleaching ability [29,30]. Subsequently, in the field of cosmetics and pharmaceuticals, tyrosinase inhibitors have become molecules of great importance as lightening agents [23]. Regarding lightening activity, the essential oil tested here shows a low inhibitory activity (3%), much lower than that of SymWhite control (100%). Our results are in agreement with those commonly obtained for plant extracts [31,32,33,34].
C. wildii essential oil exhibits an anti-elastase activity of 22%, a value significantly lower than that of Berryflux vita control, which exhibits a value of 72,42%. Previous work reported an inhibition activity of α-pinene and limonene on elastase [35]. Because α-pinene is the major compound of C. wildii essential oil after heptane isolation, and because limonene is also among the 10 most abundant compounds found in this essential oil, they are probably responsible for this non negligible anti-elastase activity.
The lipoxygenase activity for this essential oil is high, reaching a typical value of 96%. This value is slightly higher than that of Resveratrol control. Recent studies [36,37,38,39] report that α-pinene has an anti-inflammatory effect. The presence of this compound in large proportion in the essential oil obtained in this study most probably explains the high lipoxygenase activity observed for C. wildii essential oil.
Starting for the results obtained here, it appears that C. wildii essential oil obtained after the double distillation process has a cosmetic potential, such as an active ingredient in the development of anti-aging or skin repairing cosmetic active.
Cutibacterium acnes belongs to largely commensal species and is an integral part of the cutaneous flora present on the skin of most healthy adult humans [40]. Species of the genus Prevotella sp. are part of the oral, vaginal and intestinal microbiota and are often found after an anaerobic infection of the respiratory tract. Gemella morbillorum is rarely a cause of disease in humans, though it may be found benignly in the oropharyngeal area. It has been reported to be among the most common bacteria present in teeth with cysts that do not resolve after repeated treatments [41]. Porphyromonas sp. is a pathogenic bacterium that causes periodontal disease. This genus lives in the oral cavity of man and is part of the salivary microbiome. Finally, Streptococcus pyogenes and Staphylococcus aureus are commensal bacteria responsible for potentially serious infections in humans. It appears that the essential oil of C. wildii shows preferentially an activity on pathogenic and not commensal strains of the skin or oral cavity.
Sheehama et al. (2018) [6] measured the antibacterial activity of the essential oil of C. wildii in vitro against three bacteria (E. coli, K. pseudomoniae and S. aureus) and one fungi (C. albicans). The MICs measured were 10 mg/mL for all strains except for S. aureus was the best antibacterial activity measured with an MIC 8 mg/mL. This result for C. albicans is greater than that obtained in our experiment. It is also important to note that MIC (Minimum inhibitory concentration) obtained in our study for antibacterial activity is at least three times higher than that obtained by Sheehama et al.
A recent report [42] showed that (+)-α-pinene and (+)-β-pinene exhibited a high toxicity against C. albicans, 100% of the inoculum killed in 60 min. The presence of these compounds in large proportion in the residual essential oil reported in this study could explain the results observed with C. albicans. [42].
In conclusion, the essential oil of C. wildii obtained after the double distillation process has shown biological activities such as hyaluronidase and lipoxygenase inhibition, antifungal activities against C. albicans and C. glabrata and antibacterial activity against some strains of methicillin-resistant S. aureus and S. pyogenes. It can be noticed that the two fungi studied here are responsible of dermal infections. Therefore, considering the biological and antimicrobial activities, this product could be used as a raw material in the development of cures or drugs against skin infections without unbalancing the natural microbiota, and for its antiaging and skin repairing properties.
4. Materials and Methods
4.1. Plant Material, Chemicals and Reagents
Essential oil of Commiphora wildii was obtained from BeHave (Nantes, France). As the bark of this tree is very thin, and the resin-secreting channels are located just underneath, it is possible to recover this resin by incision on the low part of the trunk. The exudate used in this study was collected after some weeks so that the resin was dry [14].
Analytical grade solvents (i.e., pentane, diethyl ether, methanol) as well as dimethyl sulfoxide (DMSO), amphotericin B, and (YPDA) agar containing 0.5 g/L of chloramphenicol were purchased from Sigma–Aldrich (Saint Quentin Fallavier, France).
4.2. Isolation of Natural Heptane
Bio-sourced heptane was obtained from the commercial essential oil by vacuum distillation. Essential oil (4 kg) was introduced in a 6 L round-bottom flask overcome by a Sulzer® packed column 1 m long with a diameter of 10 cm. The distillation temperature was set at 30 °C; eight fractions were collected then analyzed by GC/MS.
4.3. Fractionation of Residual Essential Oil (Silica Gel Chromatography)
Essential oil obtained after heptane isolation (5 g) was fractionated on a silica gel column (50 g). Five fractions were obtained: F1 (300 mL pentane), F2 (200 mL pentane/diethyl ether 90/10 v/v), F3 (200 mL pentane/diethyl ether 50/50 v/v), F4 (200 mL diethyl ether) and F5 (100 mL methanol) (Figure S2).
4.4. Extraction and Evaluation of Concretes
Isolated heptane was used as a solvent for extracting natural fragrant raw materials. The extractions were carried out under the same conditions for three plants, obtained from the Jardin du Musée International de la Parfumerie (Mouans-Sartoux), (Rosa centifolia L., Lilium candidum L., Jasminum grandiflorum L.). Frozen plant material (5 g) was extracted in 50 mL of heptane, natural or fossil. After 4 h of stirring, a filtration of the macerate on a Büchner then an evaporation of the heptane from the filtrate in a rotary evaporator were carried out in order to obtain a concrete.
Concrete was then washed with 2 mL of ethanol and then placed in the freezer for 2 h. The solidification of waxes was provided by the glassing step; then filtration separated them from the rest of the extract. Absolute was obtained after final evaporation of the filtrate.
An olfactory comparison was made between the extracts obtained and those derived from fossil heptane, from a petrochemical source. These olfactory evaluations were performed by trained perfumers.
4.5. GC-MS and GC/FID Analyses of Complete and Residual Essential Oil
Analysis of the residual essential oil was performed by GC-MS and GC/FID using an Agilent 6890N gas chromatograph (Palo Alto, CA) equipped with an Agilent MSD5973N mass selective detector, a flame ionization detector (FID), an electronic pressure control (EPC) injector and a multifunction automatic sampler (Combi-Pal, CTC Analytics, Zwingen, Swiss). Separations were achieved either on an apolar HP-1 capillary column (100% polydimethylpolysiloxane; 50 m × 200 µm, 0.33 µm film thickness, Agilent Technologies) or a polar column HP-Innowax 50 m × 200 µm, 0.4 µm film thickness, Agilent Technologies. 1μL of sample (80 mg/mL) was injected in split mode (1/25), and helium (carrier gas) was used at a flowrate of 0.8 mL/min. The injector temperature was set to 250 °C, and the oven temperature was programmed from 40 °C to 270 °C at 3 °C/min for the apolar column; the oven temperature was programmed from 40 °C for 5 min, then 40 °C to 220 °C at 2 °C/min for the polar column. For GC-MS, a solvent delay of 5 min was selected. Mass spectra were recorded in electronic ionization (EI) mode at 70 eV scanning the m/z 35–500 range (3.15 scan/s). For GC-FID, samples were injected in triplicate for quantification. The average of these three values and the standard deviation were determined for each identified compound.
C. wildii essential oil and the concretes extracted with natural heptane were identified by GC-MS using apolar column (Supelco SLB—5MS; 30 m × 250 µm, 0.33 µm film thickness). 1μL of sample (80 mg/mL) was injected in split mode (1/100), and helium was used at a flowrate of 1 mL/min. The injector temperature was set to 250 °C, and the oven temperature was first programmed from 40 °C to 220 °C at 2 °C/min and then from 220 °C to 270 °C at 20 °C/min.
4.6. Identification of Complete and Residual Essential Oil Compounds
Data treatment was performed using MSD ChemStation (E02.02) software, Agilent Technologies. The identification of compounds involved a comparison of mass spectra with those recorded by internal or commercial mass-spectral libraries (Flora, NIST and Wiley), as well as comparison of linear retention indices (LRI) with those available in the literature (NIST, ESO) and articles for data missing from databases mentioned above [2,43,44,45,46,47,48,49,50]. Retention indices (RI) were calculated using a formula according to van Den Dool and Kratz [51] and according to the retention times of standard n-alkanes C6-C27 homemade mixture. Alkanes mixture diluted to 10% in diethyl ether was analyzed by GC-MS and GC/FID, according to the above-described methods.
4.7. Heptane Assay in Complete Essential Oil
A heptane assay method has been developed using GC-FID in order to quickly check heptane content in samples. A calibration curve with fossil heptane in hexane at five chosen concentrations (0%; 20%; 40%; 60%; 80% m/m) was performed with linear regression (Figure S2, Supplementary Information).
Heptane assays by GC/FID were performed on an Agilent Intuvo 9000 chromatograph with a G4513A autosampler equipped with a flame ionization detector (temperature set at 250 °C, air flow at 300 mL/min, H2 flow at 30 mL/min). The column used is an apolar column HP-5 MS 30 m × 0.25 mm × 0.25 μm, with H2 as carrier gas set at 1 mL/min. A 0.1 µL volume injection volume was used, with a split ratio of 1/100. Temperature program mode was used for the oven: 1 °C/min from 40 °C to 50 °C and then 20 °C/min up to 270 °C, followed by 20 min at 270 °C.
4.8. Activity Tests
4.8.1. Bioassays
Bioassays were carried out as presented in previous studies [52,53]. Samples, such as extracts, standards and controls, were prepared at a concentration of 3.433 mg/mL in dimethyl sulfoxide (DMSO). Positive controls used for all bioassays are collected in Table 6. In each plate, a negative control corresponding to neat DMSO (OD control, with OD statin for optical density), exhibited no activity for all bioassays.

Table 6.
Positive controls included in bioassays.
Instrumentation
Bioassays were conducted in untreated 96-well UV-transparent plates obtained from Costar, Sigma-Aldrich (Saint-Quentin Fallavier, Auvergne-Rhone-Alpes, France) or purchased from Thermo Nunc (Villebon-sur-Yvette, Ile-de-France, France). In order to seal all 96-well plates during incubation, adhesive sealing films (Greiner Bio-One, Courtaboeuf, Ȋle de France, France) were used. Bioassays were conducted using an automated pipetting system epMotion 5075 from Eppendorf. Absorbance of 96-well plates was recorded using a microplate reader Spectramax Plus 384 from Molecular Devices, Wokingham, Berkshire, UK. Data acquisition was provided by SoftMaxPro software (Molecular devices, Wokingham, Berkshire, UK) and the inhibition percentages calculated using Prism software (GraphPad Software, La Jolla, CAS, USA). Unless specified, results are reported as inhibition percentages (I%) calculated as follows for the DPPH radical scavenging assay, and tyrosinase, elastase and lipoxygenase assays:
I% = ((OD control − OD sample)/OD control) × 100 (with OD starting for optical density).
Or as follows (for the hyaluronidase assay):
I% = (OD sample/OD blank-OD control) × 100
Likewise, after correction of each OD (except those for hyaluronidase) using the blank measurement related to the sample absorbance value, the substrate was added.
Hyaluronidase Assay
Assay was carried out as follows: in each well, 150 µL of a solution of hyaluronidase (13.3 U/mL in hyaluronidase buffer) was deposited along with 7.5 µL of extract. Incubation for the sealed plate was performed at 37 °C for 20 min, and a first OD reading was carried out at 405 nm. Next, in each well, 100 µL of a solution of hyaluronic acid (150 µg/mL in pH 5.35 buffer) was added. 50 µL of cetyltrimethylammonium bromide (40 mM in a 2% NaOH solution), after 30 min incubation at 37 °C, was distributed in each well, and a final OD reading was carried out.
Tyrosinase Assays
Assay was carried out as follows: in each well, 150 µL of a solution of mushroom tyrosinase (171.66 U/mL in phosphate buffer) was added along with 7.5 µL of extract. Incubation of the sealed plate was performed at RT for 20 min. Next, in each well, 100 µL of a solution of substrate (either L-tyrosine or L-DOPA, 1 mM in phosphate buffer) was deposited. After 20 min of incubation, the final OD reading was carried out at 480 nm
DPPH Radical Scavenging Assay
Evaluation of the antioxidant activity of an extracts was carried out by studying the scavenging activity of 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) (4,5,6). In each well, 150 µL of a solution of ethanol/acetate buffer 0.1 M (50/50) was added, with 7.5 µL of a sample.
At 517 nm, a first OD reading was made (OD blank). Then 100 µL of a DPPH solution (386.25 µM in ethanol) were deposited in each well. After incubation of the sealed plate at RT for 30 min in the dark, a final OD reading was performed.
Elastase Assay
In each well, 150 µL of a solution of porcine pancreatic elastase (0.171 U/mL in Tris buffer) was deposited, with 7.5 µL of the extracts. Incubation of the sealed plate was performed at RT for 20 min. After recording a first OD reading at 410 nm, 100 µL of a solution of N-succinyl-Ala-Ala-Ala-p-nitroanilide (2.06 mM in Tris buffer) was added. Then, a 40-min incubation was carried out before final OD reading.
Lipoxygenase Assay
In each well, 150 µL of a solution of soybean lipoxygenase (686.66 U/mL in phosphate buffer) was added, along with 7.5 µL of an extract. Incubation of sealed plate was performed for 10 min in the dark. An incubation was carried out in the dark for 2 min, before a first OD reading at 235 nm; after an additional incubation of 50 min, the final OD reading was carried out.
4.8.2. Antimicrobial Activity
Antifungal Activity
Antifungal activity of extracts was tested against human pathogenic fungi, two yeasts (Candida albicans and C. glabrata). These yeasts were obtained from Parasitology and Mycology laboratory of CHU d’Angers (France). They were incubated at 37 °C on yeast extract-peptone-dextrone (YPDA) agar containing 0.5 g/L of chloramphenicol for a period of 48 h. Classically, the extracts or the compounds tested were dissolved in DMSO to obtain an initial concentration of 10 mg/mL. In our case, the essential oil was tested as pure without being diluted in DMSO. The fungal strains tested are listed in Table 4.
Antifungal Evaluation
- Disk diffusion testing
This method is performed according to a method traditionally used for yeasts [54]. Candida suspensions are obtained by incorporating a 2 mm colony in sterile distilled water [corresponding to 3 × 106 CFU per mL (Colony Forming Unit) for C. albicans, and 5 × 106 CFU per for C. glabrata]. A volume of 25 µL of essential oil was deposited on 12 mm diameter paper disks (Prat Dumas, France). After drying a Petri dish (diameter 90 mm) containing Casitone agar, the disks are placed at the center previously inoculated with 10 mL spore suspensions. Amphotericin B was used as a positive control and DMSO without compounds served as a negative control. Evaluation was done after 48 h of incubation by measurement of size of growth inhibition zones (mm) around the disk papers.
Broth Dilution Testing
Tests were carried out according to the recommendations of the CLSI in accordance with the reference method M27-A3 [55] for yeasts. Briefly, after the required culture time, Petri dishes are scraped with 2 × 10 mL of sterile distillated water and then centrifuged. The pellet is then washed, and suspensions are spectrophotometrically adjusted at 630 nm to a final concentration of approximately 0.5 × 103 to 2.5 × 103 CFU per mL for the yeasts. The suspensions are prepared in RPMI-1640 medium supplemented with 2 L-glutamine and buffered to 0.165 M with MPSO (3-(N-morpholino) propanesulfonic acid). Tests are carried out in sterile 96-well plates. From the essential oil tested, serial dilutions to half are made in DMSO. The solutions prepared are distributed in triplicate in the wells at a rate of 5 µL in final volume of 200 µL. Amphoterecin B is used as positive control. The growth control is realized in triplicate, using the spore suspension supplemented with 5 µL of DMSO without compounds.
After 48 h at 37 °C for C. albicans and C. glabrata, the minimum inhibitory concentration (MIC) is determined via solution turbidity as being the minimum concentration causing an inhibition equal to or greater than 80% of the inhibition caused by growth control.
For DMSO, the final concentration is less than 2.5%, which does not significantly affect fungal growth.
Antibacterial Activity
Antibacterial activity was evaluated on 21 clinical isolates collected by the Laboratory of Bacteriology at the University Hospital of Angers, France. The bacterial strains tested were: five Cutibacterium acnes, three Prevotella buccae, two methicillin-resistant Staphylococcus aureus, three methicillin-susceptible, two Corynebacterium tuberculostearicum, one Gemella morbillorum, one Gemella haemolysans, one Porphyromonas asaccharolytica and three Streptococcus pyogenes.
Tests were performed using a modified methodology described by Alomar et al. and adapted to anaerobic bacterial strains [56].
Briefly, a stock solution of C. wildii essential oil was prepared at 4 g/mL in DMSO under sterile conditions. The concentrations tested were 3, 30, 100 mg/mL, and tests were performed in Petri plates in a final volume of 20 mL Mueller-Hinton agar (Merck, Darmstadt, Germany) and 1 mL of horse serum.
For each bacterial isolate, a concentration corresponding to a 0.5 MF was prepared in sterile physiological serum, using a densitometer. This suspension corresponds approximately to 108 bacteria/mL. Then, 4 µL of each suspension were inoculated using an automatic inoculator (multipoint inoculator AQS), on the Petri dishes containing the MH agar with the three concentrations of C. wildii extract to be tested. Control Petri dishes without C. wildii essential oil were also used as growth control. After incubation for 24 h at 37 °C under anaerobic conditions, the minimum inhibitory concentration (MICs mg/mL) of C. wildii against each bacterial strain was determined. The MIC corresponds to the lowest concentration leading to bacterial growth inhibition. The experiment was carried out in duplicate.
5. Conclusions
For the first time, C. wildii was used as a source of heptane naturally present in its essential oil. The latter was found to contain up to 30% heptane. A simple double distillation process was proposed, yielding heptane of high purity, containing 0.3% α-pinene and 0.1% β-pinene. Heptane was used as an extraction solvent in order to obtain concrete from three emblematic and historical flowers of french perfumery. Because of the olfactive impurities present in heptane isolated from C. wildii essential oil and remaining after evaporation of heptane, yields for concretes obtained from such natural heptane were found to be higher than those obtained using fossil heptane. Furthermore, olfactive notes for concretes obtained using the so-called natural heptane were slightly different, in particular somehow more floral, compared to those obtained using fossil heptane, offering a new potential range of raw material for perfumer.
In addition, investigation on the composition of the remaining essential oil after heptane isolation revealed ten compounds were found in majority in the essential oil, α-pinene being the most abundant compound.
Finally, resulting essential oil after removal of heptane exhibited important hyaluronidase and lipoxygenase activities but low elastase activity. These activities suggest possible interesting antiaging and skin repairing cosmetics properties. These properties seem to conform to the traditional usage of the resin of C. wildii.
Tests against several strains of bacteria and two Candida species revealed inhibition preferentially on pathogenic and not commensal human bacterial strains of the skin or oral cavity, a medium effect against C. albicans and a potent inhibitor of C. glabrata. This study has shown different biological properties for the doubly distillated essential oil of C. wildii, which could be valorized as a raw material for cosmetic usage, after the removal of the natural heptane for perfumery usage.
The overall small amount of heptane extractable from C. wildii implies necessarily its application to luxury perfumery or other small scale production units.
6. Patents
Bouville, A.-S.; Dieffoldo, C.; Fernandez, X.; Piquart, S. Heptane from a Plant Source, for the Extraction of Natural Products; 9 December 2020, EP3746038.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28020891/s1, Figure S1: fractionation protocol; Figure S2: heptane assay calibration curve; Table S1: chemical composition of C. wildii essential oil.
Author Contributions
Investigation—writing and original draft preparation, D.M.; investigation, writing—review and editing, A.L. and M.K.; review and editing, C.D.S.J. and T.M.; investigation, B.R.; resources, S.A.; review and editing, N.P.; resources, conceptualization, supervision, writing—review and editing, X.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is stored in our laboratory.
Acknowledgments
The authors thank Stéphane Piquart for providing C. wildii essential oil and for our constructive discussions.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
C. wildii essential oil doubly distilled is available in our laboratory.
References
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Kerdudo, A.; Gonnot, V.; Ellong, E.N.; Boyer, L.; Chandre, F.; Adenet, S.; Rochefort, K.; Michel, T.; Fernandez, X. Composition and Bioactivity of Pluchea Carolinensis (Jack.) G. Essential Oil from Martinique. Ind. Crops Prod. 2016, 89, 295–302. [Google Scholar] [CrossRef]
- Chemat, S.; Tomao, V.; Chemat, F. Limonene as Green Solvent for Extraction of Natural Products. In Green Solvents I: Properties and Applications in Chemistry; Mohammad, A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 175–186. ISBN 978-94-007-1712-1. [Google Scholar]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.-S.F. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, E3007. [Google Scholar] [CrossRef]
- Batool, I.; Nisar, S.; Hamrouni, L.; Jilani, M. Extraction, Production and Analysis Techniques for Menthol: A Review. Int. J. Chem. Biochem. Sci. 2018, 14, 71–76. [Google Scholar]
- Sheehama, J.T.; Mukakalisa, C.; Amakali, T.; Uusiku, L.N.; Hans, R.H.; Nott, K.; Nott, A.; Louw, S. Chemical Characterization and in Vitro Antioxidant and Antimicrobial Activities of Essential Oil from Commiphora wildii Merxm. (Omumbiri) Resin. Flavour Fragrance J. 2019, 34, 241–251. [Google Scholar] [CrossRef]
- Arin, E.; Önem, E.; Tabur, M.A. Characterization of Myrrh Essential Oil wıth GC-MS and Investigation Antibacterıal Effects on Salmonella spp. Süleyman Demirel Üniversitesi Fen Edeb. Fakültesi Fen Dergisi 2021, 16, 319–327. [Google Scholar] [CrossRef]
- Hosseinkhani, A.; Ghavidel, F.; Mohagheghzadeh, A.; Zarshenas, M.M. Analysis of Six Populations of Commiphora myrrha (Nees) Engl. Oleo-Gum Resin. Trends Pharm. Sci. 2017, 3, 7–12. [Google Scholar]
- Mohamed, A.A.; Ali, S.I.; EL-Baz, F.K.; Hegazy, A.K.; Kord, M.A. Chemical Composition of Essential Oil and in Vitro Antioxidant and Antimicrobial Activities of Crude Extracts of Commiphora myrrha Resin. Ind. Crops Prod. 2014, 57, 10–16. [Google Scholar] [CrossRef]
- Marongiu, B.; Piras, A.; Porcedda, S.; Scorciapino, A. Chemical Composition of the Essential Oil and Supercritical CO2 Extract of Commiphora myrrha (Nees) Engl. and of Acorus calamus L. J. Agric. Food Chem. 2005, 53, 7939–7943. [Google Scholar] [CrossRef]
- Jemmali, Z.; Chartier, A.; Elfakir, C. Development of a Gas Chromatography–Mass Spectrometry Method to Monitor in a Single Run, Mono- to Triterpenoid Compounds Distribution in Resinous Plant Materials. J. Chromatogr. A 2016, 1443, 241–253. [Google Scholar] [CrossRef]
- Adams, R.P.; Wright, J.W. Alkanes and Terpenes in Wood and Leaves of Pinus jeffreyi and P. sabiniana. J. Essent. Oil Res. 2012, 24, 435–440. [Google Scholar] [CrossRef]
- Velásquez, J.; Toro, M.E.; Encinas, O.; Rojas, L.; Usubillaga, A. Chemical Composition of the Essential Oils of Exudates from Pinus oocarpa Schiede. Flavour Fragr. J. 2000, 15, 432–433. [Google Scholar] [CrossRef]
- Bouville, A.-S.; Dieffoldo, C.; Fernandez, X.; Piquart, S. Heptane from a Plant Source, for the Extraction of Natural Products. EP Patent EP3746038, 2020. [Google Scholar]
- Burger, P.; Plainfossé, H.; Brochet, X.; Chemat, F.; Fernandez, X. Extraction of Natural Fragrance Ingredients: History Overview and Future Trends. Chem. Biodivers. 2019, 16, e1900424. [Google Scholar] [CrossRef]
- Burger, P.; Landreau, A.; Watson, M.; Janci, L.; Cassisa, V.; Kempf, M.; Azoulay, S.; Fernandez, X. Vetiver Essential Oil in Cosmetics: What Is New? Medicines 2017, 4, 41. [Google Scholar] [CrossRef]
- Carvalhinho, S.; Costa, A.M.; Coelho, A.C.; Martins, E.; Sampaio, A. Susceptibilities of Candida Albicans Mouth Isolates to Antifungal Agents, Essentials Oils and Mouth Rinses. Mycopathologia 2012, 174, 69–76. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial Activity of Essential Oils and Other Plant Extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef]
- Gebrehiwot, M.; Asres, K.; Bisrat, D.; Mazumder, A.; Lindemann, P.; Bucar, F. Effects of Resin and Essential Oil from Commiphora myrrha Engl. on Wound Healing. Ethiop. Pharm. J. 2016, 32, 85–100. [Google Scholar] [CrossRef]
- Rini, P.; Ohtani, Y.; Ichiura, H. Antioxidant, Anti-Hyaluronidase and Antifungal Activities of Melaleuca leucadendron Linn. Leaf Oils. J. Wood Sci. 2012, 58, 429–436. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic Acid (Hyaluronan): A Review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Jegasothy, S.M.; Zabolotniaia, V.; Bielfeldt, S. Efficacy of a New Topical Nano-Hyaluronic Acid in Humans. J. Clin. Aesthet. Dermatol. 2014, 7, 27–29. [Google Scholar] [PubMed] [PubMed Central]
- Liyanaarachchi, G.D.; Samarasekera, J.K.R.R.; Mahanama, K.R.R.; Hemalal, K.D.P. Tyrosinase, Elastase, Hyaluronidase, Inhibitory and Antioxidant Activity of Sri Lankan Medicinal Plants for Novel Cosmeceuticals. Ind. Crops Prod. 2018, 111, 597–605. [Google Scholar] [CrossRef]
- Stern, R.; Jedrzejas, M.J. Hyaluronidases: Their Genomics, Structures, and Mechanisms of Action. Chem. Rev. 2006, 106, 818–839. [Google Scholar] [CrossRef]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Parvez, S.; Kang, M.; Chung, H.-S.; Bae, H. Naturally Occurring Tyrosinase Inhibitors: Mechanism and Applications in Skin Health, Cosmetics and Agriculture Industries. Phytother. Res. 2007, 21, 805–816. [Google Scholar] [CrossRef]
- Tada, M.; Kohno, M.; Niwano, Y. Scavenging or Quenching Effect of Melanin on Superoxide Anion and Singlet Oxygen. J Clin. Biochem. Nutr. 2010, 46, 224–228. [Google Scholar] [CrossRef]
- Anna, B.; Blazej, Z.; Jacqueline, G.; Andrew, C.J.; Jeffrey, R.; Andrzej, S. Mechanism of UV-Related Carcinogenesis and Its Contribution to Nevi/Melanoma. Expert Rev. Dermatol. 2007, 2, 451–469. [Google Scholar]
- Buzek, J.; Ask, B. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union L 2009, 342, 59–209. [Google Scholar]
- Plainfosse, H. Recherche et Développement D’ingrédients Cosmétiques Innovants Favorisant la Réparation Cutanée à Partir de Matières Premieres Naturelles D’origine Méditerranéenne. Ph.D. Thesis, Université Cote d’Azur, Nice, France, 2019. [Google Scholar]
- Baurin, N.; Arnoult, E.; Scior, T.; Do, Q.T.; Bernard, P. Preliminary Screening of Some Tropical Plants for Anti-Tyrosinase Activity. J. Ethnopharmacol. 2002, 82, 155–158. [Google Scholar] [CrossRef]
- Kamkaen, N.; Mulsri, N.; Treesak, C. Screening of Some Tropical Vegetables for Anti-Tyrosinase Activity. Thail. Pharm. Health Sci. J. 2007, 2, 15–19. [Google Scholar]
- Chiari, M.E.; Joray, M.B.; Ruiz, G.; Palacios, S.M.; Carpinella, M.C. Tyrosinase Inhibitory Activity of Native Plants from Central Argentina: Isolation of an Active Principle from Lithrea molleoides. Food Chem. 2010, 120, 10–14. [Google Scholar] [CrossRef]
- Muddathir, A.M.; Yamauchi, K.; Batubara, I.; Mohieldin, E.A.M.; Mitsunaga, T. Anti-Tyrosinase, Total Phenolic Content and Antioxidant Activity of Selected Sudanese Medicinal Plants. S. Afr. J. Bot. 2017, 109, 9–15. [Google Scholar] [CrossRef]
- Fraternale, D.; Flamini, G.; Ascrizzi, R. In Vitro Anticollagenase and Antielastase Activities of Essential Oil of Helichrysum italicum subsp. Italicum (Roth) G. Don. J. Med. Food 2019, 22, 1041–1046. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-Inflammatory and Chondroprotective Activity of (+)-α-Pinene: Structural and Enantiomeric Selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lee, H.-J.; Jeon, Y.-D.; Han, Y.-H.; Kee, J.-Y.; Kim, H.-J.; Shin, H.-J.; Kang, J.; Lee, B.S.; Kim, S.-H.; et al. Alpha-Pinene Exhibits Anti-Inflammatory Activity Through the Suppression of MAPKs and the NF-ΚB Pathway in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef]
- Tümen, İ.; Akkol, E.K.; Taştan, H.; Süntar, I.; Kurtca, M. Research on the Antioxidant, Wound Healing, and Anti-Inflammatory Activities and the Phytochemical Composition of Maritime Pine (Pinus Pinaster Ait). J. Ethnopharmacol. 2018, 211, 235–246. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A Never-Ending Story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Brüggemann, H.; Henne, A.; Hoster, F.; Liesegang, H.; Wiezer, A.; Strittmatter, A.; Hujer, S.; Dürre, P.; Gottschalk, G. The Complete Genome Sequence of Propionibacterium Acnes, a Commensal of Human Skin. Science 2004, 305, 671–673. [Google Scholar] [CrossRef]
- Signoretti, F.G.C.; Gomes, B.P.F.A.; Montagner, F.; Jacinto, R.C. Investigation of Cultivable Bacteria Isolated from Longstanding Retreatment-Resistant Lesions of Teeth with Apical Periodontitis. J. Endodontics 2013, 39, 1240–1244. [Google Scholar] [CrossRef]
- da Silva, A.C.R.; Lopes, P.M.; de Azevedo, M.M.B.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological Activities of α-Pinene and β-Pinene Enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Tümen, G.; Tabanca, N.; Demirci, F. Composition and Antibacterial Activity of the Essential Oils from Satureja wiedemanniana (Lallem.) Velen. Z. Naturforsch. C 2001, 56, 731–738. [Google Scholar] [CrossRef]
- Barbeni, M.; Guarda, P.A.; Villa, M.; Cabella, P.; Pivetti, F.; Ciaccio, F. Identification and Sensory Analysis of Volatile Constituents of Babaco Fruit (Carica Pentagona Heilborn). Flavour Fragr. J. 1990, 5, 27–32. [Google Scholar] [CrossRef]
- Judzentiene, A.; Butkiene, R.; Budiene, J.; Tomi, F.; Casanova, J. Composition of Seed Essential Oils of Rhododendron tomentosum. Nat. Prod. Commun. 2012, 7, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Lutz, D.; Alviano, D.S.; Alviano, C.S.; Kolodziejczyk, P.P. Screening of Chemical Composition, Antimicrobial and Antioxidant Activities of Artemisia Essential Oils. Phytochemistry 2008, 69, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Radoias, G.; Bosilcov, A. Composition of the Essential Oil from the Flowers of Solandra maxima (Sessé & Moc.) P.S. Green: Essential Oil from the Flowers of Solandra maxima. Flavour Fragrance J. 2013, 28, 389–392. [Google Scholar] [CrossRef]
- Bouville, A.; Erlich, G.; Azoulay, S.; Fernandez, X. Forgotten Perfumery Plants—Part I: Balm of Judea. Chem. Biodivers. 2019, 16, e190056. [Google Scholar] [CrossRef]
- Kerdudo, A.; Ellong, E.N.; Burger, P.; Gonnot, V.; Boyer, L.; Chandre, F.; Adenet, S.; Rochefort, K.; Michel, T.; Fernandez, X. Chemical Composition, Antimicrobial and Insecticidal Activities of Flowers Essential Oils of Alpinia zerumbet (Pers.) B.L. BURTT & R.M. SM. from Martinique Island. Chem. Biodivers. 2017, 14, e1600344. [Google Scholar] [CrossRef]
- van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatog. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Plainfossé, H.; Burger, P.; Verger-Dubois, G.; Azoulay, S.; Fernandez, X. Design Methodology for the Development of a New Cosmetic Active Based on Prunus domestica L. Leaves Extract. Cosmetics 2019, 6, 8. [Google Scholar] [CrossRef]
- Plainfossé, H.; Burger, P.; Azoulay, S.; Landreau, A.; Verger-Dubois, G.; Fernandez, X. Development of a Natural Anti-Age Ingredient Based on Quercus pubescens Willd. Leaves Extract—A Case Study. Cosmetics 2018, 5, 15. [Google Scholar] [CrossRef]
- Barry, A.L.; Brown, S.D. Fluconazole Disk Diffusion Procedure for Determining Susceptibility of Candida Species. J. Clin. Microbiol. 1996, 34, 2154–2157. [Google Scholar] [CrossRef] [PubMed]
- Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; ISBN 978-1-56238-666-5.
- Alomar, K.; Landreau, A.; Kempf, M.; Khan, M.A.; Allain, M.; Bouet, G. Synthesis, Crystal Structure, Characterization of Zinc(II), Cadmium(II) Complexes with 3-Thiophene Aldehyde Thiosemicarbazone (3TTSCH). Biological Activities of 3TTSCH and Its Complexes. J. Inorg. Biochem. 2010, 104, 397–404. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).