Matricaria chamomilla Essential Oils: Repellency and Toxicity against Imported Fire Ants (Hymenoptera: Formicidae)
Abstract
1. Introduction
2. Results
2.1. Chemical Composition
2.2. Digging Bioassay
2.3. Toxicity Bioassay
3. Discussion
4. Materials and Methods
4.1. Chemicals and GC-MS
4.2. Ants
4.3. Digging Bioassay
4.4. Toxicity Bioassay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, J.; Oi, D.H. Naturally occurring compounds/materials as alternatives to synthetic chemical insecticides for use in fire ant management. Insects 2020, 11, 758. [Google Scholar] [CrossRef]
- Lofgren, C.S.; Banks, W.A.; Glancey, B. Biology and control of imported fire ants. Annu. Rev. Entomol. 1975, 20, 1–30. [Google Scholar] [CrossRef]
- Morrison, J.E., Jr.; Williams, D.F.; Oi, D.H.; Potter, K.N. Damage to dry crop seed by red imported fire ant (Hymenoptera: Formicidae). J. Econ. Entomol. 1997, 90, 218–222. [Google Scholar] [CrossRef]
- Lard, C.; Willis, D.B.; Salin, V.; Robison, S. Economic assessments of red imported fire ant on Texas’ urban and agricultural sectors. Southwest. Entomol. 2002, 25, 123–137. [Google Scholar]
- Wu, D.; Zeng, L.; Lu, Y.; Xu, Y. Effects of Solenopsis invicta (Hymenoptera: Formicidae) and its interaction with aphids on the seed productions of mungbean and rapeseed plants. J. Econ. Entomol. 2014, 107, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Vinson, S.B. Impact of the invasion of the imported fire ant. Insect Sci. 2013, 20, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, L.; Simberloff, D. Interaction of hybrid imported fire ants (Solenopsis invicta × S. richteri) with native ants at baits in southeastern Tennessee. Southeast. Nat. 2005, 4, 303–320. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Bamisile, B.S.; Khan, M.M.; Islam, W.; Hafeez, M.; Bodlah, I.; Xu, Y. Impact of invasive ant species on native fauna across similar habitats under global environmental changes. Environ. Sci. Pollut. Res. 2021, 28, 54362–54382. [Google Scholar] [CrossRef]
- Taber, S.W. Fire Ants; Texas A&M University Press: College Station, TX, USA, 2000. [Google Scholar]
- Callcott, A. Range expansion of the imported fire ant—1918–2001. In Proceedings of the 2002 Annual Imported Fire Ant Research Conference, Athens, GA, USA, 24–26 March 2002. [Google Scholar]
- Oliver, J.B.; Vander Meer, R.K.; Ochieng, S.A.; Youssef, N.N.; Pantaleoni, E.; Mrema, F.A.; Vail, K.M.; Parkman, J.P.; Valles, S.M.; Haun, W.G. Statewide survey of imported fire ant (Hymenoptera: Formicidae) populations in Tennessee. J. Entomol. Sci. 2009, 44, 149–157. [Google Scholar] [CrossRef]
- Streett, D.; Freeland, T.B., Jr.; Vander Meer, R.K. Survey of imported fire ant (Hymenoptera: Formicidae) populations in Mississippi. Fla. Entomol. 2006, 89, 91–92. [Google Scholar] [CrossRef]
- Pandey, M.; Addesso, K.; Archer, R.; Valles, S.; Baysal-Gurel, F.; Ganter, P.; Youssef, N.; Oliver, J. Worker size, geographical distribution, and introgressive hybridization of invasive Solenopsis invicta and Solenopsis richteri (Hymenoptera: Formicidae) in Tennessee. Environ. Entomol. 2019, 48, 727–732. [Google Scholar] [CrossRef]
- Tschinkel, W. The Fire Ants; The Belknap Press of Harvard University Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Drees, B.M.; Calixto, A.A.; Nester, P.R. Integrated pest management concepts for red imported fire ants Solenopsis invicta (Hymenoptera: Formicidae). Insect Sci. 2013, 20, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Banks, W. Chemical Control of the Imported Fire Ants; CRC Press: Boca Raton, FL, USA, 2019; pp. 596–603. [Google Scholar]
- Siddiqui, J.A.; Zhang, Y.; Luo, Y.; Bamisile, B.S.; Rehman, N.U.; Islam, W.; Qasim, M.; Jiang, Q.; Xu, Y. Comprehensive detoxification mechanism assessment of red imported fire ant (Solenopsis invicta) against indoxacarb. Molecules 2022, 27, 870. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Shen, L.; Chen, J.; Zhang, J.; Feng, Y.; Wang, Z.; Chen, X.; Cai, J.; Wang, L.; He, Y. Red imported fire ants cover the insecticide-treated surfaces with particles to reduce contact toxicity. J. Pest Sci. 2022, 95, 1135–1150. [Google Scholar] [CrossRef]
- Pan, F.; Lu, Y.; Wang, L. Toxicity and sublethal effects of sulfoxaflor on the red imported fire ant, Solenopsis invicta. Ecotoxicol. Environ. Saf. 2017, 139, 377–383. [Google Scholar] [CrossRef]
- Zhang, B.-Z.; Kong, F.-C.; Wang, H.-T.; Gao, X.-W.; Zeng, X.-N.; Shi, X.-Y. Insecticide induction of O-demethylase activity and expression of cytochrome P450 genes in the red imported fire ant (Solenopsis invicta Buren). J. Integr. Agric. 2016, 15, 135–144. [Google Scholar] [CrossRef]
- Rehman, J.U.; Ali, A.; Khan, I.A. Plant based products: Use and development as repellents against mosquitoes: A review. Fitoterapia 2014, 95, 65–74. [Google Scholar] [CrossRef] [PubMed]
- George, D.R.; Finn, R.D.; Graham, K.M.; Sparagano, O.A. Present and future potential of plant-derived products to control arthropods of veterinary and medical significance. Parasites Vectors 2014, 7, 28. [Google Scholar] [CrossRef]
- Shah, F.M.; Razaq, M.; Ali, A.; Han, P.; Chen, J. Comparative role of neem seed extract, moringa leaf extract and imidacloprid in the management of wheat aphids in relation to yield losses in Pakistan. PLoS ONE 2017, 12, e0184639. [Google Scholar] [CrossRef]
- Shah, F.M.; Razaq, M.; Ali, Q.; Ali, A.; Shad, S.A.; Aslam, M.; Hardy, I.C.W. Action threshold development in cabbage pest management using synthetic and botanical insecticides. Entomol. Gen. 2020, 40, 157–172. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides in the twenty-first century—Fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Razaq, M.; Shah, F.M. Biopesticides for management of arthropod pests and weeds. In Biopesticides; Rakshit, A., Meena, V.S., Abhilash, P.C., Sarma, B.K., Singh, H.B., Fraceto, L., Parihar, M., Singh, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 7–18. [Google Scholar]
- Shah, F.M.; Razaq, M.; Ali, Q.; Shad, S.A.; Aslam, M.; Hardy, I.C.W. Field evaluation of synthetic and neem-derived alternative insecticides in developing action thresholds against cauliflower pests. Sci. Rep. 2019, 9, 7684. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Sparks, J.M.; Duke, S.O. Natural product-based crop protection compounds─ origins and future prospects. J. Agric. Food Chem. 2023, 71, 2259–2269. [Google Scholar] [CrossRef]
- Kemp, S.F.; DeShazo, R.D.; Moffitt, J.E.; Williams, D.F.; Buhner II, W.A. Expanding habitat of the imported fire ant (Solenopsis invicta): A public health concern. J. Allergy Clin. Immunol. 2000, 105, 683–691. [Google Scholar] [CrossRef]
- Singh, B.; Singh, P.R.; Mohanty, M.K. Toxicity of a plant based mosquito repellent/killer. Interdiscip. Toxicol. 2012, 5, 184–191. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Xu, Y. Safe chemical repellents to prevent the spread of invasive ants. Pest Manag. Sci. 2019, 75, 821–827. [Google Scholar] [CrossRef]
- Chen, J. Assessment of repellency of nine phthalates against red imported fire ant (Hymenoptera: Formicidae) workers using ant digging behavior. J. Entomol. Sci. 2005, 40, 368–377. [Google Scholar] [CrossRef]
- Vogt, J.T.; Shelton, T.G.; Merchant, M.E.; Russell, S.A.; Tanley, M.J.; Appel, A.G. Efficacy of three citrus oil formulations against Solenopsis invicta buren (Hymenoptera: Formicidae), the red imported fire ant. J. Agric. Urban Entomol. 2002, 19, 159–171. [Google Scholar]
- Appel, A.G.; Gehret, M.J.; Tanley, M.J. Repellency and toxicity of mint oil granules to red imported fire ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2004, 97, 575–580. [Google Scholar] [CrossRef]
- Ali, A.; Chen, J.; Khan, I.A. Toxicity and repellency of Magnolia grandiflora seed essential oil and selected pure compounds against the workers of hybrid imported fire ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2022, 115, 412–416. [Google Scholar] [CrossRef]
- He, Y.; Zhang, J.; Shen, L.; Wang, L.; Qian, C.; Lyu, H.; Yi, C.; Cai, J.; Chen, X.; Wen, X. Eugenol derivatives: Strong and long-lasting repellents against both undisturbed and disturbed red imported fire ants. J. Pest Sci. 2022, 96, 327–344. [Google Scholar] [CrossRef]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Höferl, M.; Wanner, J.; Tabanca, N.; Ali, A.; Gochev, V.; Schmidt, E.; Kaul, V.K.; Singh, V.; Jirovetz, L. Biological activity of Matricaria chamomilla essential oils of various chemotypes. Planta Medica Int. Open 2020, 7, e114–e121. [Google Scholar] [CrossRef]
- Tabanca, N.; Demirci, B.; Avonto, C.; Wang, M.; Ali, A.; Bernier, U.R.; Raman, V.; Khan, I. Mosquito repellent activity of essential oils and extracts from Chamomile flowers. Planta Med. 2014, 80, PD53. [Google Scholar] [CrossRef]
- El Mihyaoui, A.; Esteves da Silva, J.C.; Charfi, S.; Candela Castillo, M.E.; Lamarti, A.; Arnao, M.B. Chamomile (Matricaria chamomilla L.): A review of ethnomedicinal use, phytochemistry and pharmacological uses. Life 2022, 12, 479. [Google Scholar] [CrossRef]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J. Chemical composition, antioxidant and antimicrobial activity of chamomile flowers essential oil (Matricaria chamomilla L.). J. Essent. Oil Bear. Plants 2016, 19, 2017–2028. [Google Scholar] [CrossRef]
- Pekić, B.; Zeković, Z.; Petrović, L.; Tolić, A. Behavior of (–)-α-Bisabolol and (–)-α-Bisabololoxides A and B in camomile flower extraction with supercritical carbon dioxide. Sep. Sci. Technol. 1995, 30, 3567–3576. [Google Scholar] [CrossRef]
- Kim, S.; Jung, E.; Kim, J.-H.; Park, Y.-H.; Lee, J.; Park, D. Inhibitory effects of (−)-α-bisabolol on LPS-induced inflammatory response in RAW264. 7 macrophages. Food Chem. Toxicol. 2011, 49, 2580–2585. [Google Scholar] [CrossRef]
- Firat, Z.; Demirci, F.; Demirci, B. Antioxidant activity of chamomile essential oil and main components. Nat. Volatiles Essent. Oils 2018, 5, 11–16. [Google Scholar]
- Ali, A.; Tabanca, N.; Raman, V.; Avonto, C.; Yang, X.; Demirci, B.; Chittiboyina, A.G.; Khan, I.A. Chemical compositions of essential oils from German, Roman, and Chinese chamomile flowers and their biological activities against three economically important insect pests. Rec. Nat. Prod. 2023, in press. [CrossRef]
- Padin, S.B.; Fuse, C.B.; Urrutia, M.I.; Dal Bello, G. Toxicity and repellency of nine medicinal plants against Tribolium castaneum in stored wheat. Bull. Insectology 2013, 66, 45–49. [Google Scholar]
- Al-Jabr, A.M. Toxicity and repellency of seven plant essential oils to Oryzaephilus surinamensis (Coleoptera: Silvanidae) and Tribolium castaneum (Coleoptera: Tenebrioidae). Sci. J. King Faisal Univ. 2006, 7, 49–60. [Google Scholar]
- Elnabawy, E.-S.M.; Hassan, S.; Taha, E.-K.A. Repellent and toxicant effects of eight essential oils against the red flour beetle, Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Biology 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Tang, L.; Hu, W.; Wang, K.; Zhou, Y.; Li, H.; Huang, C.; Chun, J.; Zhang, Z. Insecticidal, fumigant, and repellent activities of sweet wormwood oil and its individual components against red imported fire ant workers (Hymenoptera: Formicidae). J. Insect Sci. 2014, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Tang, L.; Li, W.; Wang, K.; Cheng, D.; Zhang, Z. Fumigant toxicity and repellence activity of camphor essential oil from Cinnamonum camphora Siebold against Solenopsis invicta workers (Hymenoptera: Formicidae). J. Insect Sci. 2015, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Yoshimura, M.; Huang, R.-N. Wasabi versus red imported fire ants: Preliminary test of repellency of microencapsulated allyl isothiocyanate against Solenopsis invicta (Hymenoptera: Formicidae) using bait traps in Taiwan. Appl. Entomol. Zool. 2019, 54, 193–196. [Google Scholar] [CrossRef]
- Chen, J.; Cantrell, C.; Duke, S.; Allen, M. Repellency of callicarpenal and intermedeol against workers of imported fire ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2008, 101, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Aimad, A.; Bourhia, M.; Hana, H.; Sanae, R.; Salamatullah, A.M.; Soufan, W.; Rihan, H.Z.; Ouahmane, L.; Youness, E.A.; Noureddine, E. Essential oils from Artemisia herba alba Asso., Maticaria recutita L., and Dittrichia viscosa L.(Asteraceae): A promising source of eco-friendly agents to control Callosobruchus maculatus Fab. warehouse pest. J. Chem. 2022, 2022, 2373460. [Google Scholar] [CrossRef]
- Ross, K.G.; Meer, R.K.V.; Fletcher, D.J.; Vargo, E.L. Biochemical phenotypic and genetic studies of two introduced fire ants and their hybrid (Hymenoptera: Formicidae). Evolution 1987, 41, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Deletre, E.; Schatz, B.; Bourguet, D.; Chandre, F.; Williams, L.; Ratnadass, A.; Martin, T. Prospects for repellent in pest control: Current developments and future challenges. Chemoecology 2016, 26, 127–142. [Google Scholar] [CrossRef]
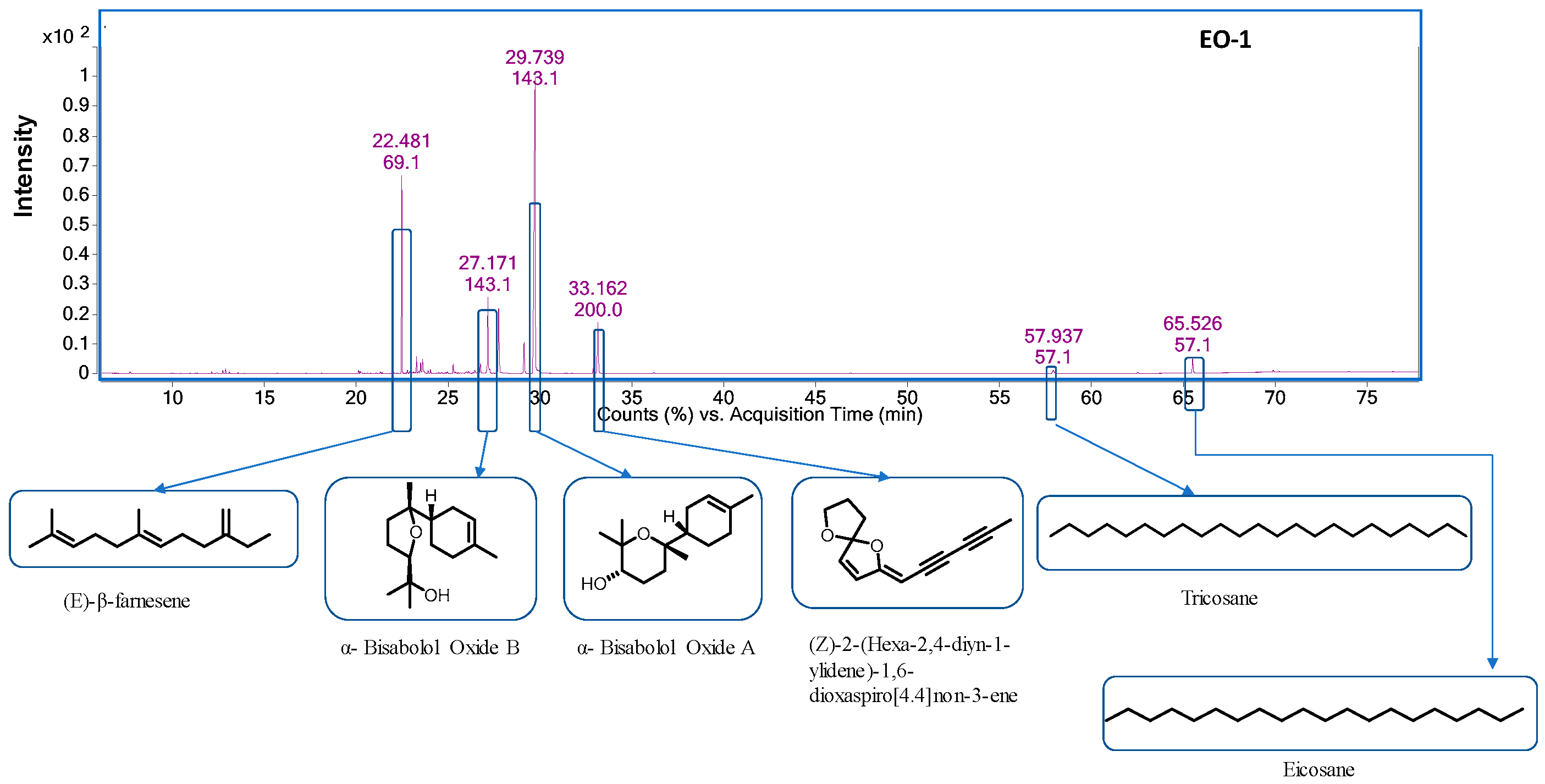
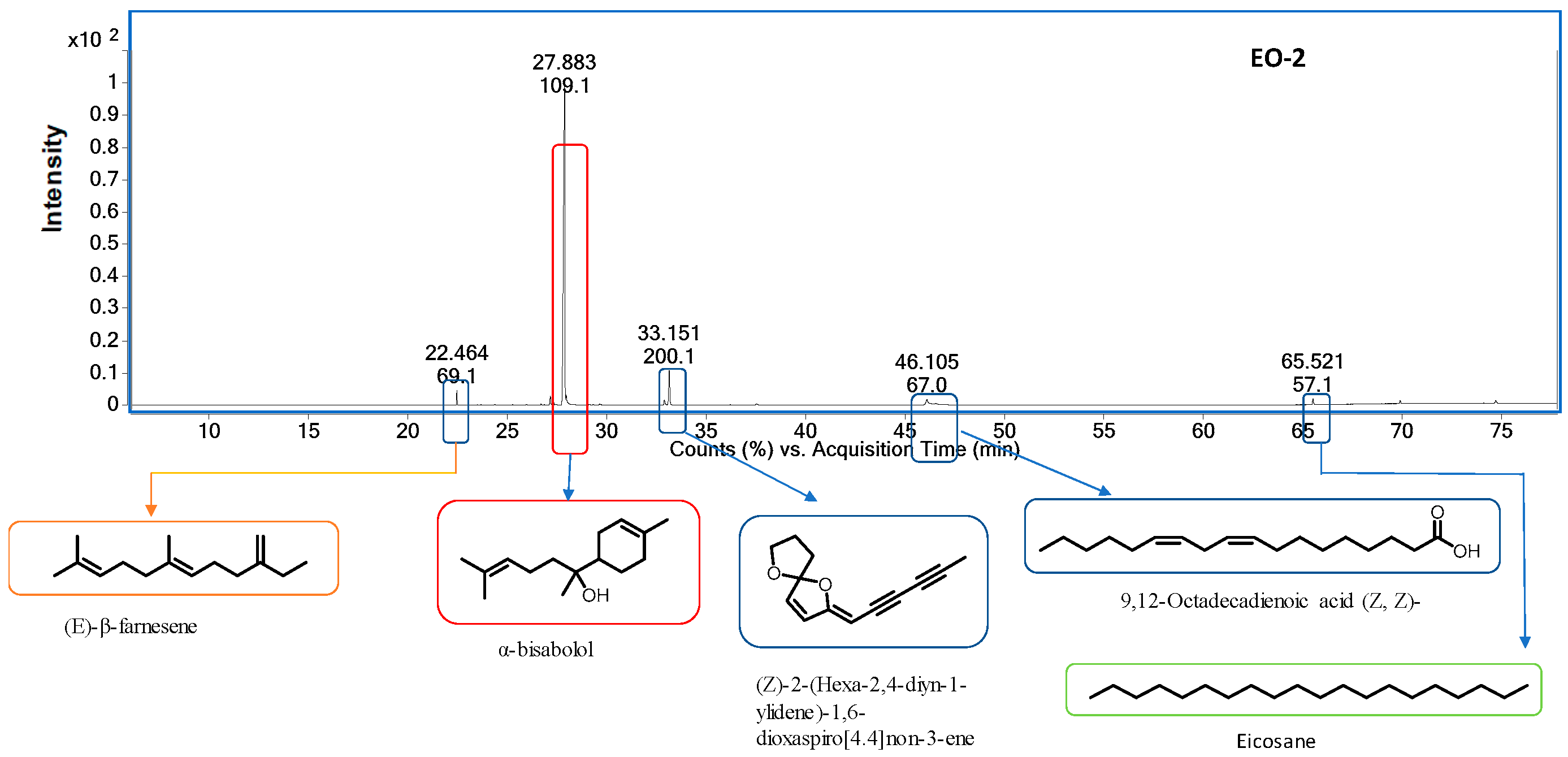
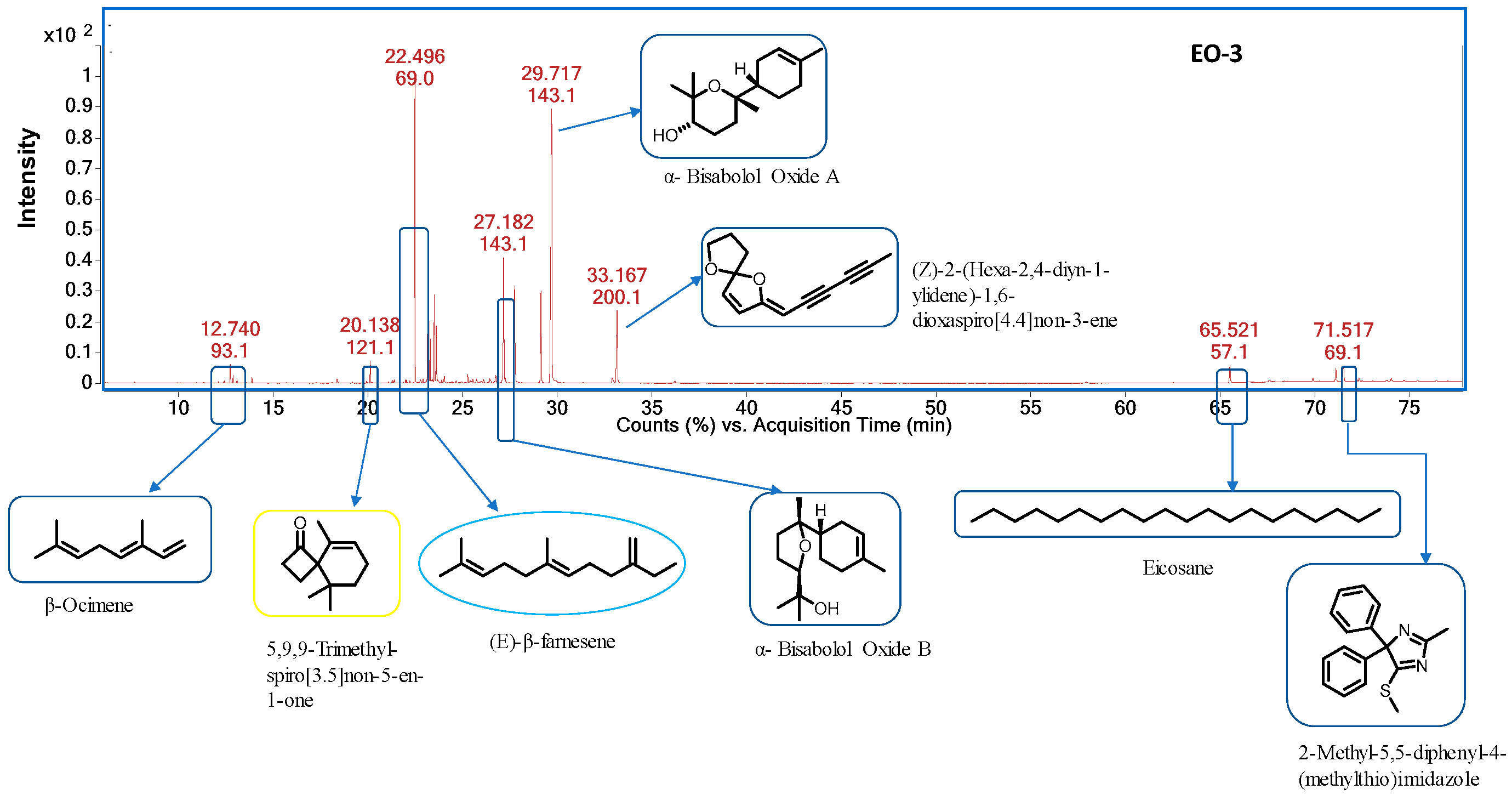
| S. No | RT a | RI b (Exp.) | RI c (Lit.) | Compound Name | Content% | ID d | ||
|---|---|---|---|---|---|---|---|---|
| EO-1 | EO-2 | EO-3 | ||||||
| 1 | 12.74 | 1039 | 1044 | β-Ocimene | − | − | 0.80 | RI, MS |
| 2 | 12.89 | 1045 | 1,5-Heptadien-4-one, 3,3,6-trimethyl- | − | − | 0.33 | RI, MS | |
| 3 | 12.89 | 1045 | 1056 | Artemisia ketone | 0.30 | − | − | RI, MS |
| 4 | 13.88 | 1083 | 1086 | α-Terpinolene | − | − | 0.26 | RI, MS |
| 5 | 20.13 | 1342 | 5,9,9-Trimethyl-spiro [3,5] non-5-en-1-one | − | − | 0.99 | RI, MS | |
| 6 | 20.13 | 1342 | 1335 | Elemene isomer | 0.22 | − | − | RI, MS |
| 7 | 22.48 | 1450 | 1454 | (E)-β-Farnesene | 13.98 | 1.39 | 16.56 | RI, MS |
| 8 | 23.16 | 1481 | 3H-Pyrazol-3-one, 2,4-dihydro-2-methyl-5-phenyl- | − | − | 2.98 | RI, MS | |
| 9 | 23.28 | 1487 | 1430 | (-)-β-Copaene | 1.11 | − | − | RI, MS |
| 10 | 23.29 | 1488 | 1480 | Germacrene D | − | 2.93 | RI, MS | |
| 11 | 23.51 | 1498 | 1505 | α-Farnesene | 0.67 | − | 4.06 | RI, MS |
| 12 | 23.61 | 1503 | 3,6-Dihydrochamazulene | 1.15 | − | − | RI, MS | |
| 13 | 23.62 | 1503 | 1500 | Bicyclogermacrene | − | 2.84 | RI, MS | |
| 14 | 23.91 | 1516 | 1478 | γ-Muurolene | 0.25 | − | − | RI, MS |
| 15 | 24.05 | 1522 | 1522 | Cadinene | − | 0.53 | RI, MS | |
| 16 | 25.27 | 1575 | 1577 | Spathulenol | 0.69 | − | 0.53 | RI, MS |
| 17 | 26.75 | 1633 | 1-epi-Bicyclosesquiphellandrene | 0.86 | − | − | RI, MS | |
| 18 | 26.76 | 1633 | Bicyclo [4.4.0] dec-1-ene, 2-isopropyl-5-methyl-9-methylene | − | − | 0.57 | RI, MS | |
| 19 | 27.17 | 1648 | 1656 | α- Bisabolol Oxide B | 7.55 | 1.46 | 8.80 | RI, MS |
| 20 | 27.30 | 1653 | Cyclofenchene | − | 0.96 | RI, MS | ||
| 21 | 27.75 | 1670 | 1684 | Bisabolone oxide A | 7.38 | 7.13 | RI, MS | |
| 22 | 27.88 | 1674 | 1685 | α-Bisabolol | − | 81.85 | − | RI, MS |
| 23 | 27.96 | 1677 | 7-Methoxycoumarin | − | 2.60 | − | RI, MS | |
| 24 | 29.13 | 1717 | 1730 | Chamazulene | 3.30 | − | 6.89 | RI, MS |
| 25 | 29.73 | 1735 | 1748 | α-Bisabolol oxide A | 49.19 | − | 27.83 | RI, MS |
| 26 | 32.92 | 1827 | (Z)-2-(Hexa-2,4-diyn-1-ylidene)-1,6-dioxaspiro [4,4] non-3-ene | 6.84 | 6.98 | 6.73 | RI, MS | |
| 27 | 46.11 | 2106 | 2132 | 9,12-Octadecadienoic acid (Z,Z)- | − | 1.39 | RI, MS | |
| 28 | 65.52 | 2500 | 2000 | Eicosane | 0.71 | 1.02 | 1.35 | RI, MS |
| 29 | 71.12 | 2753 | Methacrylic acid, 2,2,2-trichloroethyl ester | − | − | 1.03 | RI, MS | |
| 30 | 71.51 | 2771 | 2-Methyl-5,5-diphenyl-4-(methylthio) imidazole | − | − | 1.26 | RI, MS | |
| Conc. (µg/g) | Mean ± SE † | F-Value | p-Value | Mean ± SE † | F-Value | p-Value | Mean ± SE † | F-Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| RIFA | BIFA | HIFA | |||||||
| EO-1 | |||||||||
| Control | 1.66 ± 0.17 a | 11.74 | 0.003 | 1.54 ± 0.37 a | 8.36 | 0.008 | - | ||
| 15.6 | 0.00 ± 0.00 bc | 0.00 ± 0.00 c | - | ||||||
| 7.8 | 0.39 ± 0.16 b | 0.50 ± 0.27 bc | - | ||||||
| 3.9 | 0.94 ± 0.35 ab | 0.92 ± 0.19 ab | - | ||||||
| Control | 1.05 ± 0.04 a | 86.18 | <0.001 | 2.02 ± 0.14 a | 180.09 | <0.001 | 2.33 ± 0.21 a | 4.15 | 0.023 |
| 125 | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 1.01 ± 0.20 b | ||||||
| 62.5 | 0.00 ± 0.00 b | 0.01 ± 0.01 b | 0.93 ± 0.45 ab | ||||||
| 31.25 | 0.10 ± 0.10 b | 0.06 ± 0.06 b | 1.16 ± 0.35 ab | ||||||
| EO-2 | |||||||||
| Control | 1.70 ± 0.11 a | 7.33 | 0.011 | 1.68 ± 0.26 a | 2.99 | 0.096 | 2.60 ± 0.17 a | 2.79 | 0.109 |
| 15.6 | 0.60 ± 0.15 b | 0.68 ± 0.33 a | 1.65 ± 0.29 a | ||||||
| 7.8 | 1.05 ± 0.25 ab | 0.83 ± 0.27 a | 2.00 ± 0.30 a | ||||||
| 3.9 | 1.31 ± 0.13 a | 1.25 ± 0.13 a | 1.94 ± 0.13 a | ||||||
| Control | 1.71 ± 0.18 a | 29.95 | <0.001 | 1.85 ± 0.40 a | 21.36 | <0.001 | 2.19 ± 0.12 a | 38.97 | <0.001 |
| 125 | 0.01 ± 0.01 b | 0.02 ± 0.02 b | 0.37± 0.15 c | ||||||
| 62.5 | 0.32 ± 0.22 b | 0.02 ± 0.02 b | 0.28 ± 0.14 c | ||||||
| 31.25 | 0.27 ± 0.02 b | 0.13 ± 0.08 b | 0.93± 0.15 b | ||||||
| EO-3 | |||||||||
| Control | - | 1.12 ± 0.21 a | 1.265 | 0.35 | - | ||||
| 1.95 | - | 0.28 ± 0.28 a | - | ||||||
| 0.97 | - | 0.53 ± 0.33 a | - | ||||||
| 0.48 | - | 0.55 ± 0.42 a | - | ||||||
| Control | 1.40 ± 0.41 a | 6.166 | 0.018 | 1.52 ± 0.22 a | 10.06 | 0.004 | - | ||
| 15.6 | 0.08 ± 0.08 b | 0.03 ± 0.03 b | - | ||||||
| 7.8 | 0.43 ± 0.16 b | 0.56 ± 0.28 b | - | ||||||
| 3.9 | 0.62 ± 0.03 ab | 0.21 ± 0.21 b | - | ||||||
| Control | 1.14 ± 0.21 a | 29.45 | <0.001 | 1.78 ± 0.19 a | 73.35 | <0.001 | 2.45 ± 0.13 a | 14.3 | 0.0014 |
| 125 | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 1.05 ± 0.18 b | ||||||
| 62.5 | 0.00 ± 0.00 b | 0.02 ± 0.02 b | 0.88 ± 0.21 b | ||||||
| 31.25 | 0.02 ± 0.02 b | 0.06 ± 0.06 b | 1.89 ± 0.24 ab | ||||||
| α-bisabolol | |||||||||
| Control | 1.57 ± 0.11 a | 2.235 | 0.162 | 1.55 ± 0.22 a | 2.07 ± 0.08 a | 21.91 | <0.001 | ||
| 15.6 | 0.66 ± 0.29 a | 0.27 ± 0.27 b | 0.80 ± 0.06 c | ||||||
| 7.8 | 1.01 ± 0.20 a | 0.39 ± 0.19 b | 1.36 ± 0.02 b | ||||||
| 3.9 | 0.85 ± 0.37 a | 0.90 ± 0.15 ab | 1.70 ± 0.05 ab | ||||||
| Control | 1.97 ± 0.22 a | 36.49 | <0.001 | 1.63 ± 0.29 a | 29.65 | <0.001 | 2.36 ± 0.07 a | 70.63 | <0.0001 |
| 125 | 0.05 ± 0.03 b | 0.03 ± 0.03 b | 0.37 ± 0.15 c | ||||||
| 62.5 | 0.01 ± 0.00 b | 0.00 ± 0.00 b | 0.45 ± 0.06 c | ||||||
| 31.25 | 0.52 ± 0.21 b | 0.06 ± 0.05 b | 0.86 ± 0.13 b | ||||||
| DEET | |||||||||
| Control | - | - | 1.26 ± 0.19 a | 0.24 | 0.87 | ||||
| 15.6 | - | - | 0.98 ± 0.49 a | ||||||
| 7.8 | - | - | 1.37 ± 0.28 a | ||||||
| 3.9 | - | - | 1.16 ± 0.29 a | ||||||
| Control | 1.43 ± 0.19 a | 16.24 | 0.001 | 1.38 ± 0.25 a | 8.9 | 0.006 | 1.58 ± 0.11 a | 9.71 | 0.005 |
| 125 | 0.08 ± 0.04 c | 0.00 ± 0.00 b | 0.42 ± 0.25 b | ||||||
| 62.5 | 0.74 ± 0.18 b | 1.22 ± 0.04 a | 0.87 ± 0.13 b | ||||||
| 31.25 | 1.14 ± 0.10 ab | 0.79 ± 0.33 ab | 0.84 ± 0.04 b | ||||||
| E. oil/Compound | n † | Slope ± SE | LC50 (95% CI) ‡ | LC90 (95% CI) ‡ | χ2 | df |
|---|---|---|---|---|---|---|
| RIFA | ||||||
| EO-1 | 30 | 1.42 ± 0.19 | 93.60 (76.50–113.29) | 230.75 (180.47–336.06) | 7.44 | 13 |
| EO-2 | 30 | 1.64 ± 0.22 | 98.11 (81.98–117.46) | 213.63 (169.94–304.02) | 12.25 | 13 |
| EO-3 | 30 | 1.75 ± 0.24 | 142.92 (119.80–169.81) | 296.88 (239.67–411.08) | 11.46 | 13 |
| α-bisabolol | 30 | 1.27 ± 0.17 | 159.23 (129.74–196.81) | 434.90 (326.84–679.06) | 16.84 | 13 |
| Bifenthrin | 40 | 1.21 ± 0.18 | 0.03 (0.023 ± 0.04) | 0.09 (0.06 ± 0.16) | 42 | 19 |
| BIFA | ||||||
| EO-1 | 30 | 1.29 ± 0.17 | 188.11 (153.61–234.68) | 504.74 (375.77–800.96) | 13.02 | 13 |
| EO-2 | 30 | 1.59 ± 0.21 | 138.40 (115.23–166.04) | 309.21 (245.52–438.35) | 4.74 | 13 |
| EO-3 | 30 | 2.78 ± 0.47 | 202.49 (176.09–233.29) | 320.83 (271.53–427.61) | 4.28 | 13 |
| α-bisabolol § | 30 | - | 30% | - | - | - |
| Bifenthrin | 40 | 1.36 ± 0.23 | 0.032 (0.023 ± 0.044) | 0.08 (0.06 ± 0.15) | 34 | 19 |
| HIFA *** | ||||||
| EO-1 § | - | - | 73% | - | - | - |
| EO-2 § | - | - | 20% | - | - | - |
| EO-3 § | - | - | 40% | - | - | - |
| α-bisabolol § | - | - | 80% | - | - | - |
| Bifenthrin | 40 | 0.86 ± 0.13 | 0.018 (0.013 ± 0.024) | 0.07 (0.05 ± 0.17) | 42.4 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, F.M.; Guddeti, D.K.; Paudel, P.; Chen, J.; Li, X.-C.; Khan, I.A.; Ali, A. Matricaria chamomilla Essential Oils: Repellency and Toxicity against Imported Fire Ants (Hymenoptera: Formicidae). Molecules 2023, 28, 5584. https://doi.org/10.3390/molecules28145584
Shah FM, Guddeti DK, Paudel P, Chen J, Li X-C, Khan IA, Ali A. Matricaria chamomilla Essential Oils: Repellency and Toxicity against Imported Fire Ants (Hymenoptera: Formicidae). Molecules. 2023; 28(14):5584. https://doi.org/10.3390/molecules28145584
Chicago/Turabian StyleShah, Farhan Mahmood, Dileep Kumar Guddeti, Pradeep Paudel, Jian Chen, Xing-Cong Li, Ikhlas A. Khan, and Abbas Ali. 2023. "Matricaria chamomilla Essential Oils: Repellency and Toxicity against Imported Fire Ants (Hymenoptera: Formicidae)" Molecules 28, no. 14: 5584. https://doi.org/10.3390/molecules28145584
APA StyleShah, F. M., Guddeti, D. K., Paudel, P., Chen, J., Li, X.-C., Khan, I. A., & Ali, A. (2023). Matricaria chamomilla Essential Oils: Repellency and Toxicity against Imported Fire Ants (Hymenoptera: Formicidae). Molecules, 28(14), 5584. https://doi.org/10.3390/molecules28145584








