Neuropharmacological Effects in Animal Models and HPLC-Phytochemical Profiling of Byrsonima crassifolia (L.) Kunth Bark Extracts
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Screening
2.2. Extractions Yields
2.3. Thin Layer Chromatography (TLC)
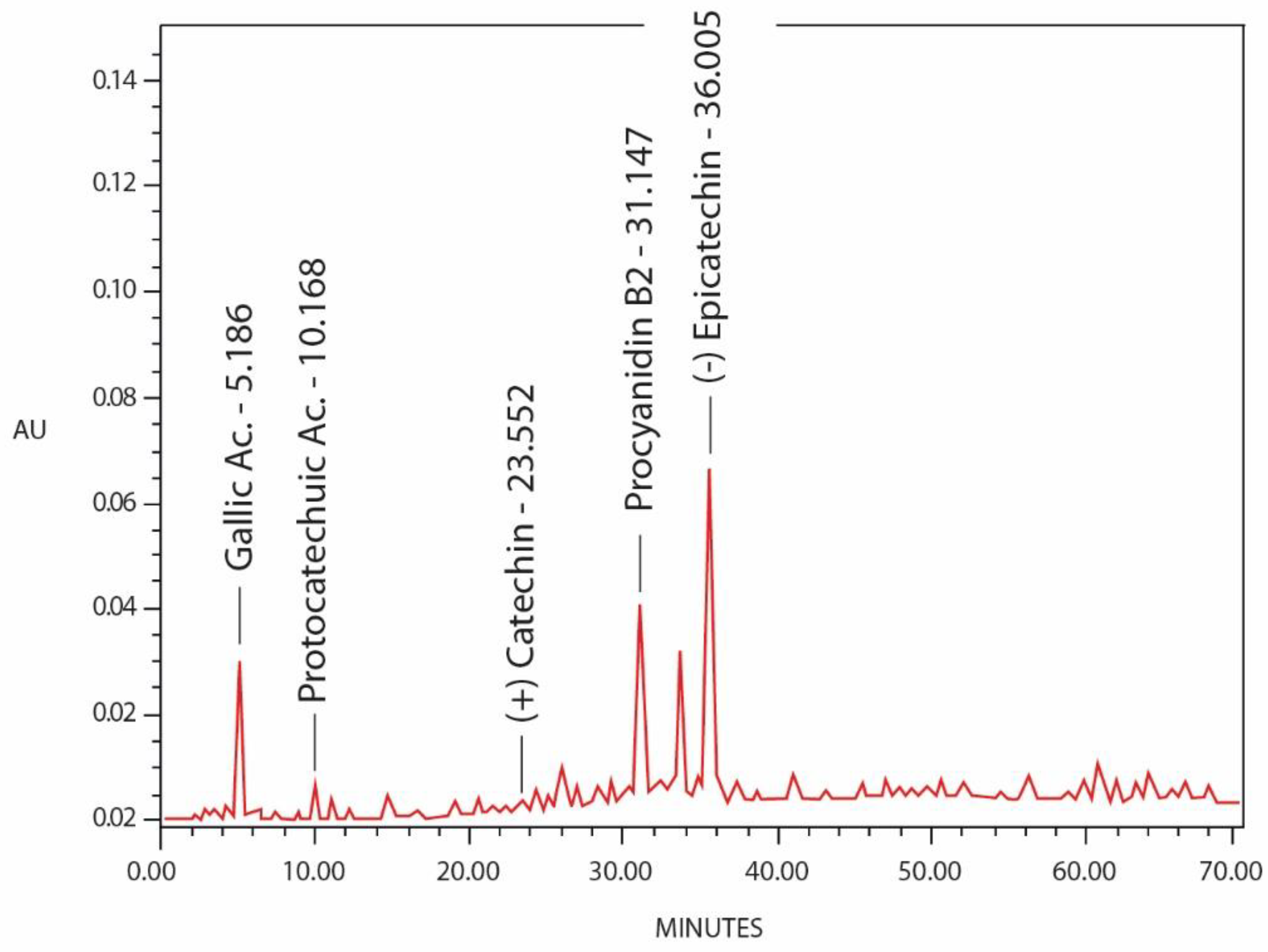
2.4. Identification of Phenolic Compounds by HPLC Analysis
Validation of the Chromatographic Method
2.5. Neuropharmacological Studies
2.5.1. Preliminary Neuropsychopharmacologic Screening
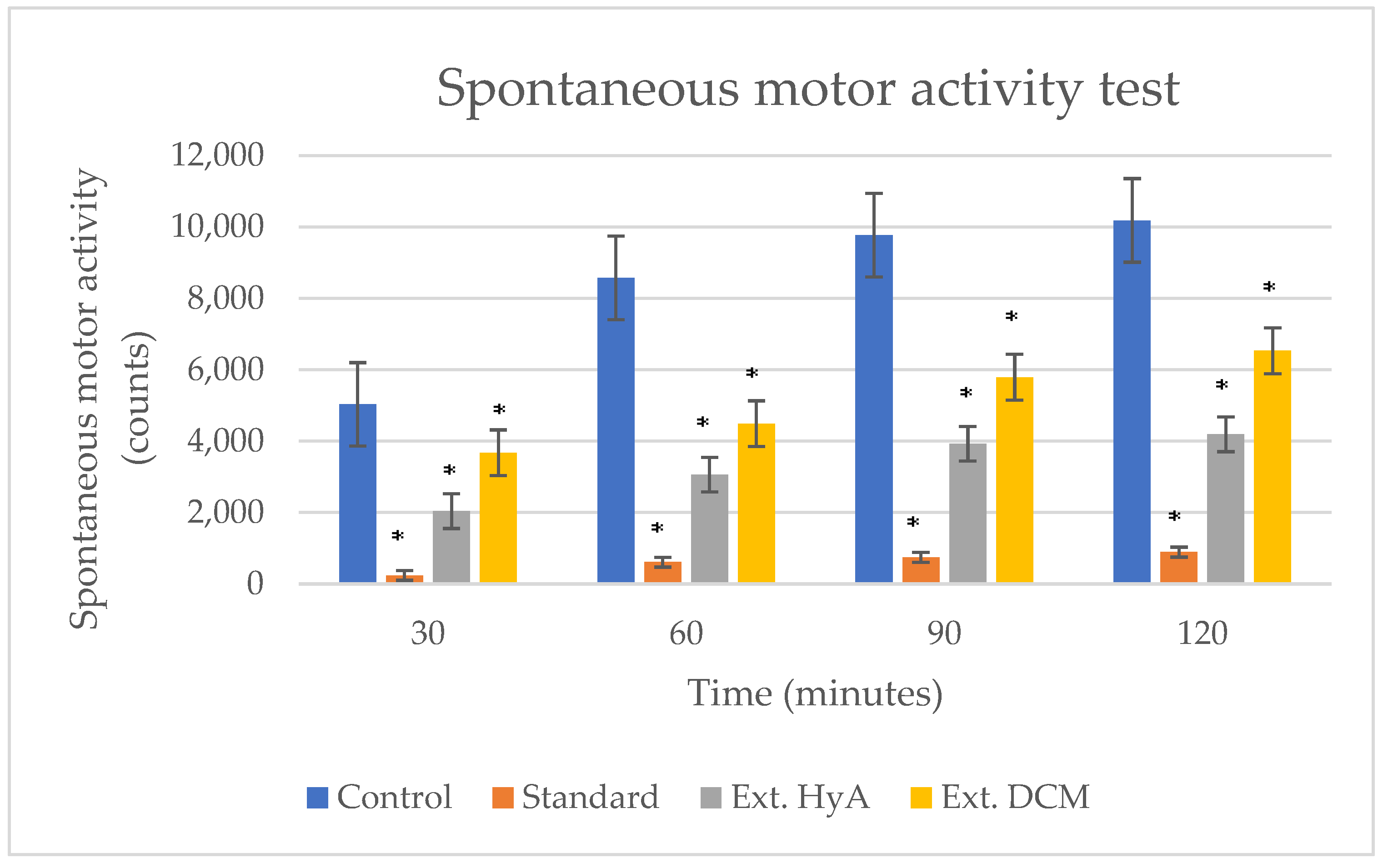
2.5.2. Locomotor Activity Measurement
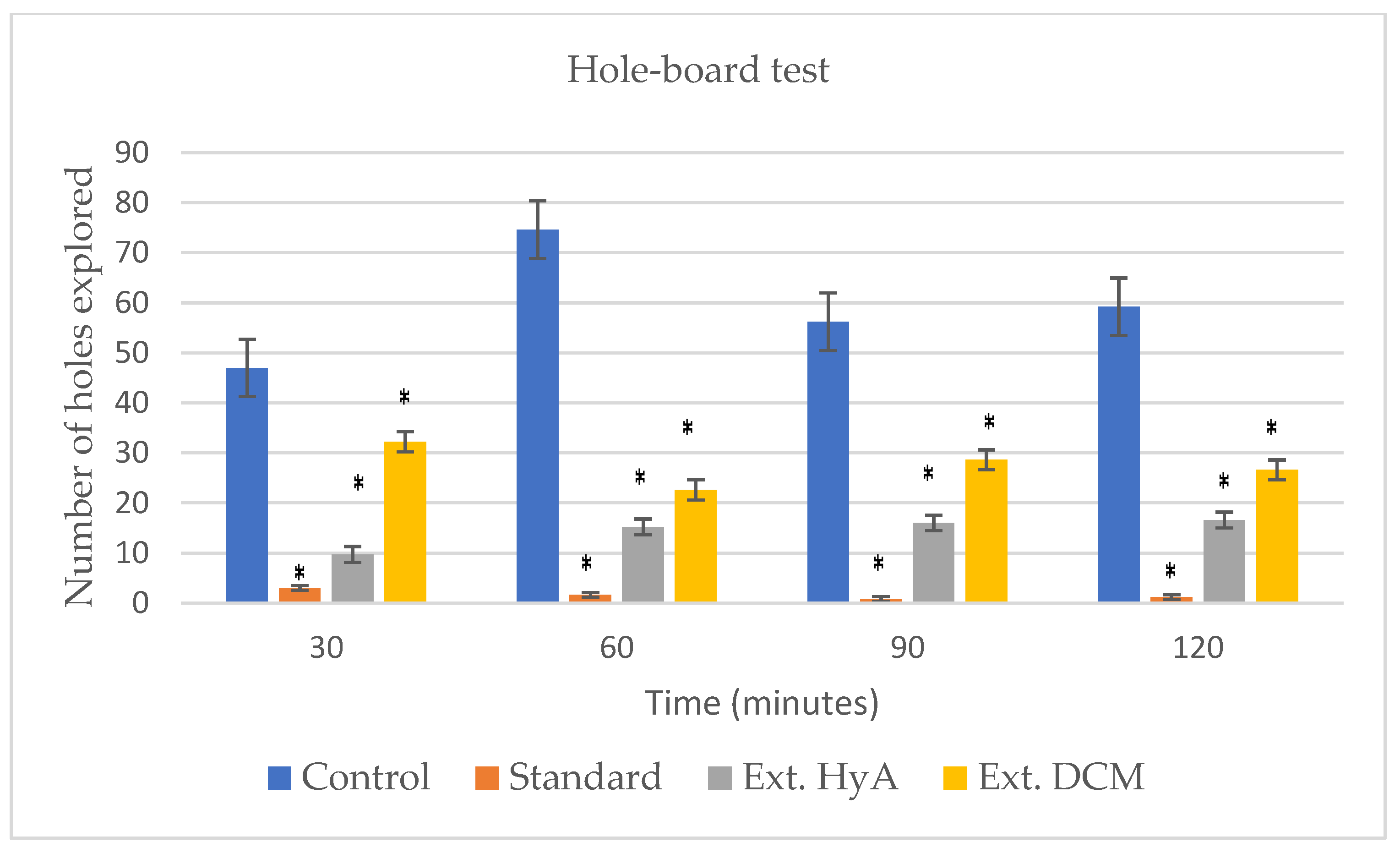
2.5.3. Hole-Board Test
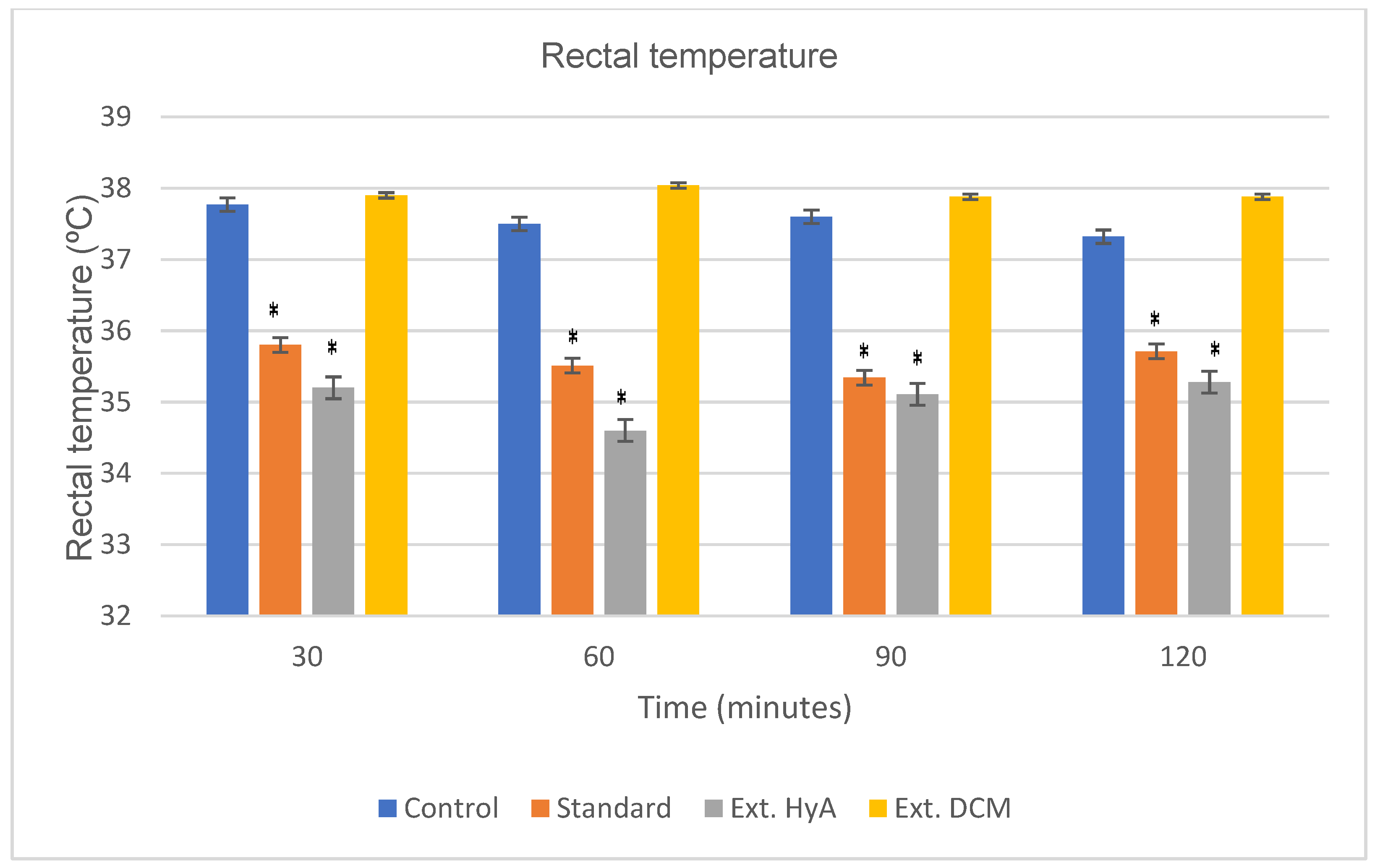
2.5.4. Rectal Temperature Test
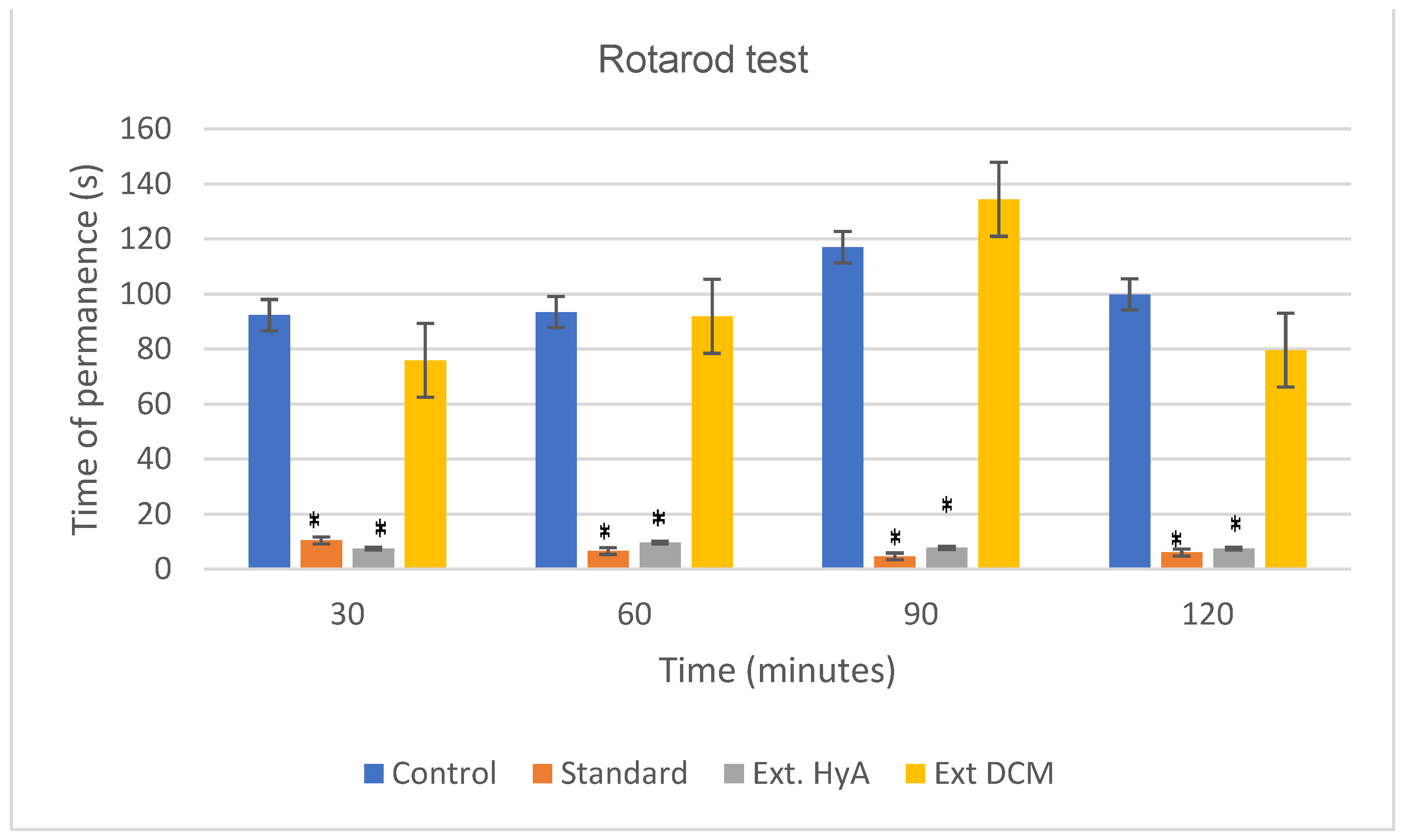
2.5.5. Motor Coordination (Rotarod Test)
2.5.6. Induced Sleeping Time Test
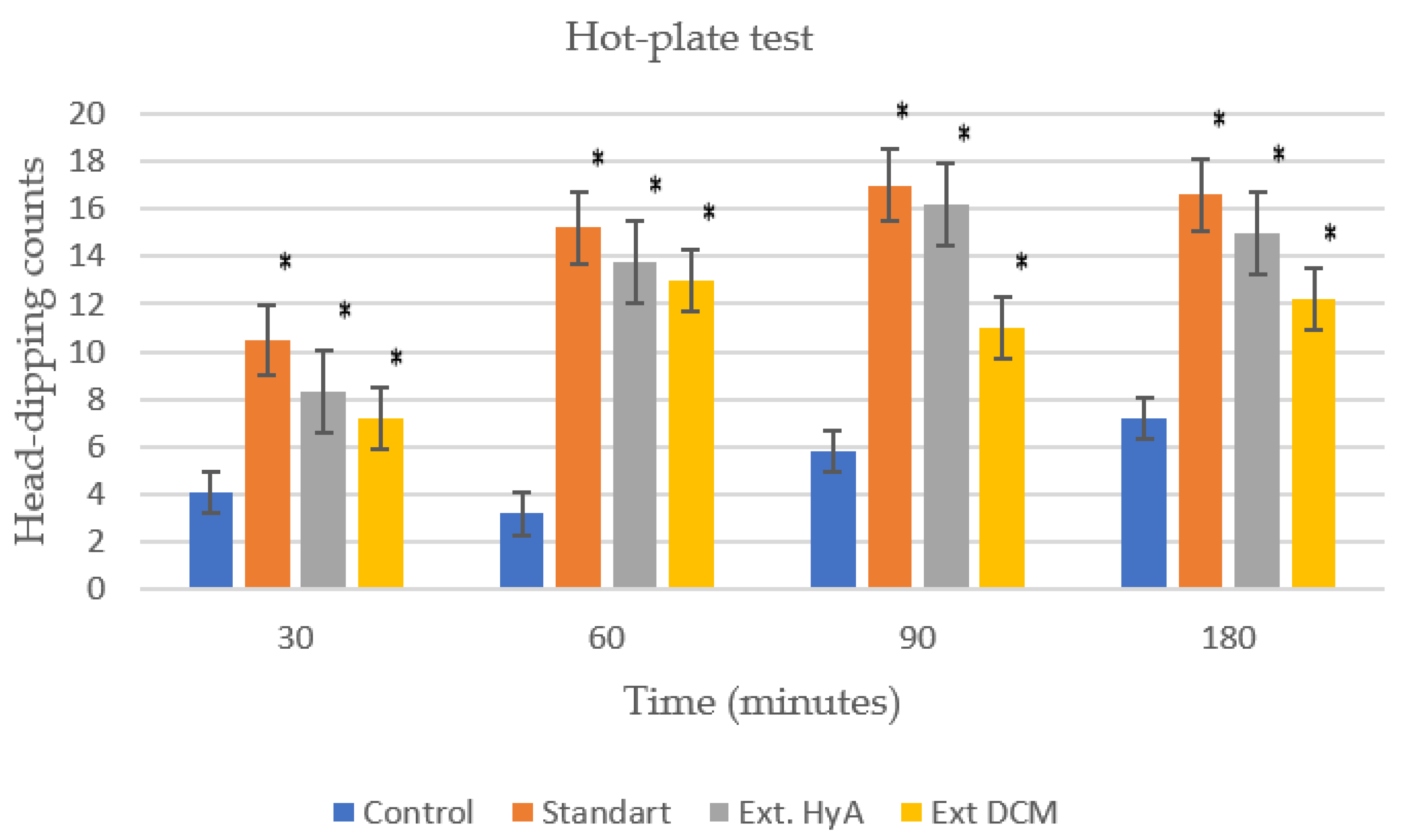
2.6. Analgesic Activity: Hot Plate Test
3. Materials and Methods
3.1. Plant Material
3.2. Extraction Procedure
3.3. Phytochemical Screening
3.4. Phytochemical Screening by Thin Layer Chromatography (TLC)
TLC Condition
3.5. HPLC Analysis
3.5.1. Regents and Standards
3.5.2. HPLC Analysis Conditions
3.5.3. Standard Solutions and Calibration Graphs
3.5.4. Evaluation of Peak Purity and Linearity
3.6. Test Animals
3.7. Drugs and Dosage
3.8. Initial Pharmacological Screening
3.9. Neuropharmacological Studies
3.9.1. Spontaneous Motor Activity Test
3.9.2. Hole-board Test
3.9.3. Rectal Temperature Test
3.9.4. Motor Coordination Test (Rotarod Test)
3.9.5. Pentobarbital-Induced Sleep Test
3.9.6. Analgesic Activity. Hot Plate Test
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bernal, H.Y.; Correa, J.E. Especies Vegetales Promisorias de Los Países del Convenio de Andrés Bello, 1st ed.; Ed. SECAB: Santa Fé de Bogotá, Colombia, 1998. [Google Scholar]
- Cáceres, A. Plantas de Uso Medicinal en Guatemala; Ed. Universitaria: Guatemala City, Guatemala, 1996. [Google Scholar]
- Béjar, E.; Malone, M.H. Pharmacological and chemical screening of Byrsonima crassifolia, a medicinal tree from Mexico Part I. J. Ethnopharmacol. 1993, 39, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Martınez-Vázquez, M.; González-Esquinca, A.; Luna, L.C.; Gutiérrez, M.M.; Garcıa-Argáez, A. Antimicrobial activity of Byrsonima crassifolia (L.) H.B.K. J. Ethnopharmacol. 1999, 66, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Peraza-Sánchez, S.; Cen-Pacheco, F.; Noh-Chimal, A.; May-Pat, F.; Simá-Polanco, P.; Dumonteil, E.; García-Miss, M.; Mut-Martín, M. Leishmanicidal evaluation of extracts from native plants of the Yucatan peninsula. Fitoterapia 2007, 78, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Maldini, M.; Sosa, S.; Montoro, P.; Giangaspero, A.; Balick, M.; Pizza, C.; Della Loggia, R. Screening of the topical anti-inflammatory activity of the bark of Acacia cornigera Willdenow, Byrsonima crassifolia Kunth, Sweetia panamensis Yakovlev and the leaves of Sphagneticola trilobata Hitchcock. J. Ethnopharmacol. 2009, 122, 430–433. [Google Scholar] [CrossRef]
- Pires, F.C.S.; de Oliveira, J.C.; Menezes, E.G.O.; Silva, A.P.D.S.E.; Ferreira, M.C.R.; Siqueira, L.M.M.; Almada-Vilhena, A.O.; Pieczarka, J.C.; Nagamachi, C.Y.; Junior, R.N.D.C. Bioactive Compounds and Evaluation of Antioxidant, Cytotoxic and Cytoprotective Effects of Murici Pulp Extracts (Byrsonima crassifolia) Obtained by Supercritical Extraction in HepG2 Cells Treated with H2O2. Foods 2021, 10, 737. [Google Scholar] [CrossRef] [PubMed]
- Aniceto, A.; Montenegro, J.; Cadena, R.; Teodoro, A. Physicochemical Characterization, Antioxidant Capacity, and Sensory Properties of Murici (Byrsonima crassifolia (L.) Kunth) and Taperebá (Spondias mombin L.) Beverages. Molecules 2021, 26, 332. [Google Scholar] [CrossRef] [PubMed]
- Béjar, E.; Reyes-Chilpa, R.; Jiménez-Estrada, M. Bioactive Compounds from Selected Plants used in the xvi Century Mexican Traditional Medicine. Stud. Nat. Prod. Chem. 2000, 24, 799–844. [Google Scholar] [CrossRef]
- Geiss, F.; Heinrich, M.; Hunkler, D.; Rimpler, H. Proanthocyanidins with (+)-epicatechin units from Byrsonima crassifolia bark. Phytochemistry 1995, 39, 635–643. [Google Scholar] [CrossRef]
- Silva, E.M.; Souza, J.N.S.; Rogez, H.; Rees, J.F.; Larondelle, Y. Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem. 2007, 101, 1012–1018. [Google Scholar] [CrossRef]
- Souza, J.N.S.; Silva, E.M.; Loir, A.; Rees, J.-F.; Rogez, H.; Larondelle, Y. Antioxidant capacity of four polyphenol-rich Amazonian plant extracts: A correlation study using chemical and biological in vitro assays. Food Chem. 2008, 106, 331–339. [Google Scholar] [CrossRef]
- Caceres, A.; Lopez, B.R.; Giron, M.A.; Logemann, H. Plants used in guatemala for the treatment of dermatophytic infections. 1. Screening for antimycotic activity of 44 plant extracts. J. Ethnopharmacol. 1991, 31, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.P. 270 Plantas Medicinales Iberoamericanas; Presencia: Cafayate, Argentina, 1995. [Google Scholar]
- Cáceres, A.; López, B.; Juárez, X.; del Aguila, J.; García, S. Plants used in Guatemala for the treatment of dermatophytic infections. 2. Evaluation of antifungal activity of seven American plants. J. Ethnopharmacol. 1993, 40, 207–213. [Google Scholar] [CrossRef]
- Perez, G.R.M.; Perez, L.J.A.; Garcia, D.L.M.; Sossa, M.H. Neuropharmacological activity of Solanum nigrum fruit. J. Ethnopharmacol. 1998, 62, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, C.; Gómez-Serranillos, M.; Iglesias, I.; del Fresno, A.V. Neuropharmacological profile of ethnomedicinal plants of Guatemala. J. Ethnopharmacol. 2001, 76, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Salisu, B.; Anua, S.M.; Ishak, W.R.W.; Mazlan, N. Development and validation of quantitative thin layer chromatographic technique for determination of total aflatoxins in poultry feed and food grains without sample clean-up. J. Adv. Veter-Anim. Res. 2021, 8, 656–670. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Teixeira, D.; Azevedo, J.; De Freitas, V.; Mateus, N.; Calhau, C. Flavonoid transport across RBE4 cells: A blood-brain barrier model. Cell. Mol. Biol. Lett. 2010, 15, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Irwin, S. Drug Screening and Evaluative Procedures. Science 1962, 136, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Moscardo, E.; Maurin, A.; Dorigatti, R.; Champeroux, P.; Richard, S. An optimised methodology for the neurobehavioural assessment in rodents. J. Pharmacol. Toxicol. Methods 2007, 56, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Amoateng, P.; Adjei, S.; Osei-Safo, D.; Kukuia, K.K.E.; Bekoe, E.O.; Karikari, T.K.; Kombian, S.B. Extract of Synedrella nodiflora (L.) Gaertn exhibits antipsychotic properties in murine models of psychosis. BMC Complement. Altern. Med. 2017, 17, 389. [Google Scholar] [CrossRef]
- Pothier, J.; Cheav, S.; Galand, N.; Dormeau, C.; Viel, C. A Comparative Study of the Effects of Sparteine, Lupanine and Lupin Extract on the Central Nervous System of the Mouse. J. Pharm. Pharmacol. 1998, 50, 949–954. [Google Scholar] [CrossRef]
- Jackson, H.C.; Nutt, D.J. Body temperature discriminates between full and partial benzodiazepine receptor agonists. Eur. J. Pharmacol. 1990, 185, 243–246. [Google Scholar] [CrossRef]
- Abramov, U.; Raud, S.; Innos, J.; Lasner, H.; Kurrikoff, K.; Türna, T.; Puussaar, T.; Õkva, K.; Matsui, T.; Vasar, E. Different housing conditions alter the behavioural phenotype of CCK2 receptor-deficient mice. Behav. Brain Res. 2008, 193, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Shiotsuki, H.; Yoshimi, K.; Shimo, Y.; Funayama, M.; Takamatsu, Y.; Ikeda, K.; Takahashi, R.; Kitazawa, S.; Hattori, N. A rotarod test for evaluation of motor skill learning. J. Neurosci. Methods 2010, 189, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.G.; Vogel, W.H. Psychotropic and neurotropic activity. In Drug Discovery and Evaluation; Springer: Berlin/Heidelberg, Germany, 1997; pp. 204–316. [Google Scholar]
- Alonso-Castro, A.J.; Gasca-Martínez, D.; Cortez-Mendoza, L.V.; Alba-Betancourt, C.; Padilla, A.J.R.; Zapata-Morales, J.R. Evaluation of the neuropharmacological effects of Gardenin A in mice. Drug Dev. Res. 2020, 81, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Domer, F. Characterization of the analgesic activity of ketorolac in mice. Eur. J. Pharmacol. 1990, 177, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, E.; Abad, A.; Montenegro, G.; del Olmo, E.; López-Pérez, J.L.; Feliciano, A.S. Analgesic and anti-inflammatory activity of podophyllotoxin derivatives. Pharm. Biol. 2013, 51, 566–572. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chem. 2006, 99, 835–841. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Khushi, S.; Mahadhi Hasan, M.; Lokman Hossain, M.; Kumar Sadhu, S. Medicinal Activity of Avicennia officinalis: Evaluation of Phytochemical and Pharmacological Properties. Saudi J. Med. Pharm. Sci. 2016, 2, 250–255. [Google Scholar] [CrossRef]
- Wagner, H.; Bladt, S. Plant Drugs Analisis, a Thin Layer Chromatography, 2nd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2001. [Google Scholar]
- Mathiasen, J.R.; Moser, V.C. The Irwin Test and Functional Observational Battery (FOB) for Assessing the Effects of Compounds on Behavior, Physiology, and Safety Pharmacology in Rodents. Curr. Protoc. Pharmacol. 2018, 83, e43. [Google Scholar] [CrossRef]
- Boissier, P.S.J.R. Le Test de La Curiosité Pour l’etude Du Psicolépticos. Therapie 1967, 22, 467–471. [Google Scholar] [PubMed]
- Takeda, H.; Tsuji, M.; Matsumiya, T. Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur. J. Pharmacol. 1998, 350, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Dunham, N.; Miya, T. A Note on a Simple Apparatus for Detecting Neurological Deficit in Rats and Mice**College of Pharmacy, University of Nebraska, Lincoln 8. J. Am. Pharm. Assoc. 1957, 46, 208–209. [Google Scholar] [CrossRef]
- Corrêa, C.R.; Kyle, D.J.; Chakraverty, S.; Calixto, J.B. Antinociceptive profile of the pseudopeptide B2 bradykinin receptor antagonist NPC 18688 in mice. Br. J. Pharmacol. 1996, 117, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, F.; Ghazavi, H.; Vahedi, M.M.; Tarah, K.; Yavari, Z.; Hosseini, A.; Aghaee, A.; Rakhshandeh, H. Tanacetum parthenium enhances pentobarbital-induced sleeping behaviors. Avicenna J. Phytomed. 2019, 10, 70–77. [Google Scholar] [CrossRef]
- Anzoise, M.L.; Marrassini, C.; Ferraro, G.; Gorzalczany, S. Hydroalcoholic extract of Urtica circularis: A neuropharmacological profile. Pharm. Biol. 2013, 51, 1236–1242. [Google Scholar] [CrossRef]
- Hunskaar, S.; Berge, O.-G.; Hole, K. A modified hot-plate test sensitivie to mild analgesics. Behav. Brain Res. 1986, 21, 101–108. [Google Scholar] [CrossRef]
- Connor, J.; Makonnen, E.; Rostom, A. Comparison of Analgesic Effects of Khat (Catha edulis Forsk) Extract, D-Amphetamine and Ibuprofen in Mice. J. Pharm. Pharmacol. 2000, 52, 107–110. [Google Scholar] [CrossRef]
| Extract | Yield (%) |
|---|---|
| Dichloromethane (DCM) | 2.48 |
| Hhydroalcoholic (HyA) | 30.5 |
| Phenols | Retention Time (tR ± SD) (min) | Concentration (µg/100 mg) |
|---|---|---|
| Gallic Ac. | 5.186 ± 0.07 | 0.205 ± 0.08 |
| Protocathechuic Ac. | 10.168 ± 0.10 | 0.092 ± 0.11 |
| (+) Catechin | 23.552 ± 0.14 | 0.101 ± 0.16 |
| Procyanidin B2 | 31.147 ± 0.18 | 1.200 ± 0.21 |
| (−) Epicatechin | 36.006 ± 0.09 | 0.405 ± 0.07 |
| Time (min) | Control | Standard | Ext. HyA | Ext. DCM |
|---|---|---|---|---|
| 30 | 47 ± 2.43 | 3 ± 1.58 (*) | 9.70 ± 6.26 (*) | 32.20 ± 6.26 (*) |
| 60 | 74.60 ± 1.70 | 1.60 ± 1.80 (*) | 15.20 ± 1.270 (*) | 22.60 ± 5.60 (*) |
| 90 | 56.20 ± 1.90 | 0.80 ± 1.70 (*) | 16 ± 8.40 (*) | 23.60 ± 1.00 (*) |
| 120 | 59.20 ± 1.99 | 1.20 ± 1.30 (*) | 16.5 ± 8.40 (*) | 26.6 ± 12.02 (*) |
| Time (min) | Control | Standard | Ext HyA | Ext DCM |
|---|---|---|---|---|
| 30 | 37.77 ± 0.22 | 35.88 ± 1.02 (*) | 34.27 ± 0.78 (*) | 37.92 ± 0.40 |
| 60 | 37.58 ± 0.34 | 35.51 ± 1 (*) | 35.6 ± 0.72 (*) | 38.04 ± 0.13 |
| 90 | 37.65 ± 0.39 | 35.34 ± 0.41 (*) | 35.11 ± 0.55 (*) | 37.88 ± 0.19 |
| 120 | 37.32 ± 0.44 | 35.71 ± 0.28 (*) | 35.28 ± 0.90 (*) | 37.88 ± 0.21 |
| Time (min) | Control | Standard | Ext HyA | Ext DCM |
|---|---|---|---|---|
| 30 | 92.28 ± 66 | 10.42 ± 5.70 (*) | 7.42 ± 2.37 (*) | 75.85 ± 65.70 |
| 60 | 93.42 ± 75.10 | 6.57 ± 1.81 (*) | 9.57 ± 5.30 (*) | 91.85 ± 67.10 |
| 90 | 117 ± 74.20 | 4.57 ± 0.70 (*) | 7.71 ± 2.70 (*) | 134.4 ± 73.60 |
| 120 | 99.85 ± 79.59 | 6 ± 2.23 (*) | 7.42 ± 3.86 (*) | 79.57 ± 50.40 |
| Pentobarbital (60 mg/kg i.p.) | ||
|---|---|---|
| Treatment | Latency Time (min) | Sleeping Time (min) |
| Saline solution | 4 ± 0.50 | 60.09 ± 1.40 |
| Hydroalcoholic extract | 2.10 ± 0.70 * | 115.35 ± 1.70 * |
| Dichloromethane extract | 6 ± 1.20 | 75.60 ± 3.20 |
| Time (min) | Control | Standard | Ext HyA | Ext DCM |
|---|---|---|---|---|
| 30 | 4.10 ± 1.20 | 10.50 ± 1 (*) | 8.30± 2.58 (*) | 7.20± 1.10 (*) |
| 60 | 3.20 ± 2 | 15.20± 0.59 (*) | 13.80± 1.75 (*) | 13± 2.43 (*) |
| 90 | 5.80 ± 2.10 | 17 ± 2.20 (*) | 16.20 ± 2.35 (*) | 11 ± 2.62 (*) |
| 180 | 7.20 ± 1.50 | 16.60 ± 1.63 (*) | 15 ± 1.48 (*) | 12.20 ± 3.20 (*) |
| Chemical Group | Mobile Phase (v/v) | Detection Reagents |
|---|---|---|
| Alkaloids | Toluene/Acetone (80:20) | Mayer Dragendorff |
| Flavonoids | Ethyl Acetate/Methanol/Water (100:13.5:10) | NEU |
| Saponins | Methanol/Water (80/20) | Sulfuric vanillin |
| Tannins | Toluene/Chloroform/Acetone (40:25:35) | Ferric salts Cl3Fe. |
| Cumarinas | Ethyl Acetate/Ethanoll/Water/Ammonia (65:25:9:1) | Bornträger |
| Triterpenes | Ethyl Acetate/Methanol/Water (77:15:8) | Ehrlich |
| Step | Time (min) | Mobile Phase (%) | ||
|---|---|---|---|---|
| A | B | C | ||
| 1 | 0.00 | 100.0 | 0.0 | 0.0 |
| 2 | 55.00 | 20.0 | 80.0 | 0.0 |
| 3 | 57.00 | 10.0 | 90.0 | 0.0 |
| 4 | 70.00 | 10.0 | 90.0 | 0.0 |
| 5 | 80.00 | 5.0 | 95.0 | 0.0 |
| 6 | 90.00 | 0.0 | 100.0 | 0.0 |
| 7 | 100.00 | 0.0 | 20.0 | 80.0 |
| 8 | 105.00 | 0.0 | 0.0 | 100.0 |
| 9 | 110.00 | 100.0 | 0.0 | 0.0 |
| 10 | 120.00 | 100.0 | 0.0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Cabeza Fernández, M.; Sánchez, M.; Caceres, A.; Iglesias, I.; Gómez-Serranillos, M.P. Neuropharmacological Effects in Animal Models and HPLC-Phytochemical Profiling of Byrsonima crassifolia (L.) Kunth Bark Extracts. Molecules 2023, 28, 764. https://doi.org/10.3390/molecules28020764
de la Cabeza Fernández M, Sánchez M, Caceres A, Iglesias I, Gómez-Serranillos MP. Neuropharmacological Effects in Animal Models and HPLC-Phytochemical Profiling of Byrsonima crassifolia (L.) Kunth Bark Extracts. Molecules. 2023; 28(2):764. https://doi.org/10.3390/molecules28020764
Chicago/Turabian Stylede la Cabeza Fernández, María, Marta Sánchez, Armando Caceres, Irene Iglesias, and Maria Pilar Gómez-Serranillos. 2023. "Neuropharmacological Effects in Animal Models and HPLC-Phytochemical Profiling of Byrsonima crassifolia (L.) Kunth Bark Extracts" Molecules 28, no. 2: 764. https://doi.org/10.3390/molecules28020764
APA Stylede la Cabeza Fernández, M., Sánchez, M., Caceres, A., Iglesias, I., & Gómez-Serranillos, M. P. (2023). Neuropharmacological Effects in Animal Models and HPLC-Phytochemical Profiling of Byrsonima crassifolia (L.) Kunth Bark Extracts. Molecules, 28(2), 764. https://doi.org/10.3390/molecules28020764







