Phosphine Oxide-Promoted Rh(I)-Catalyzed C–H Cyclization of Benzimidazoles with Alkenes
Abstract
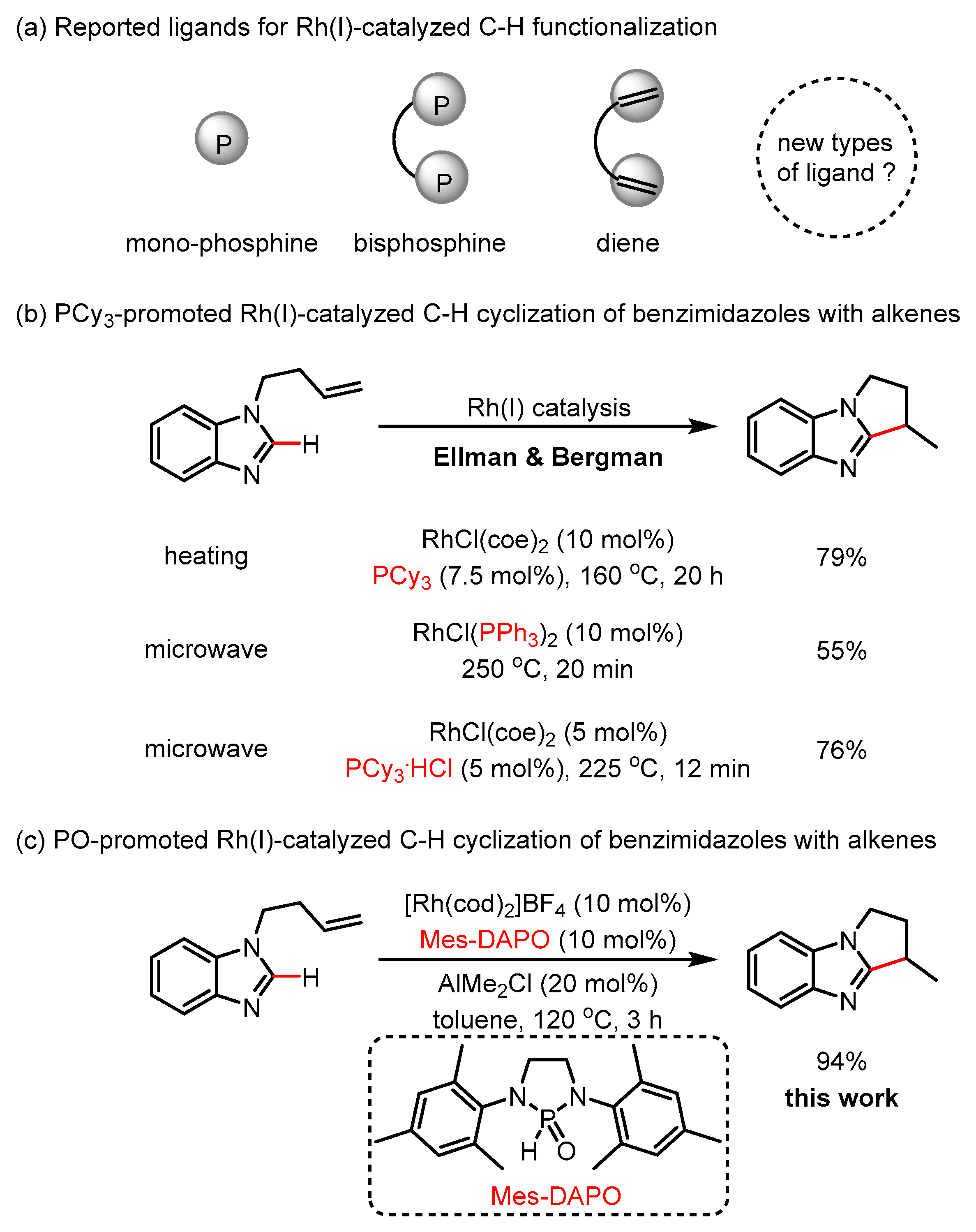
1. Introduction
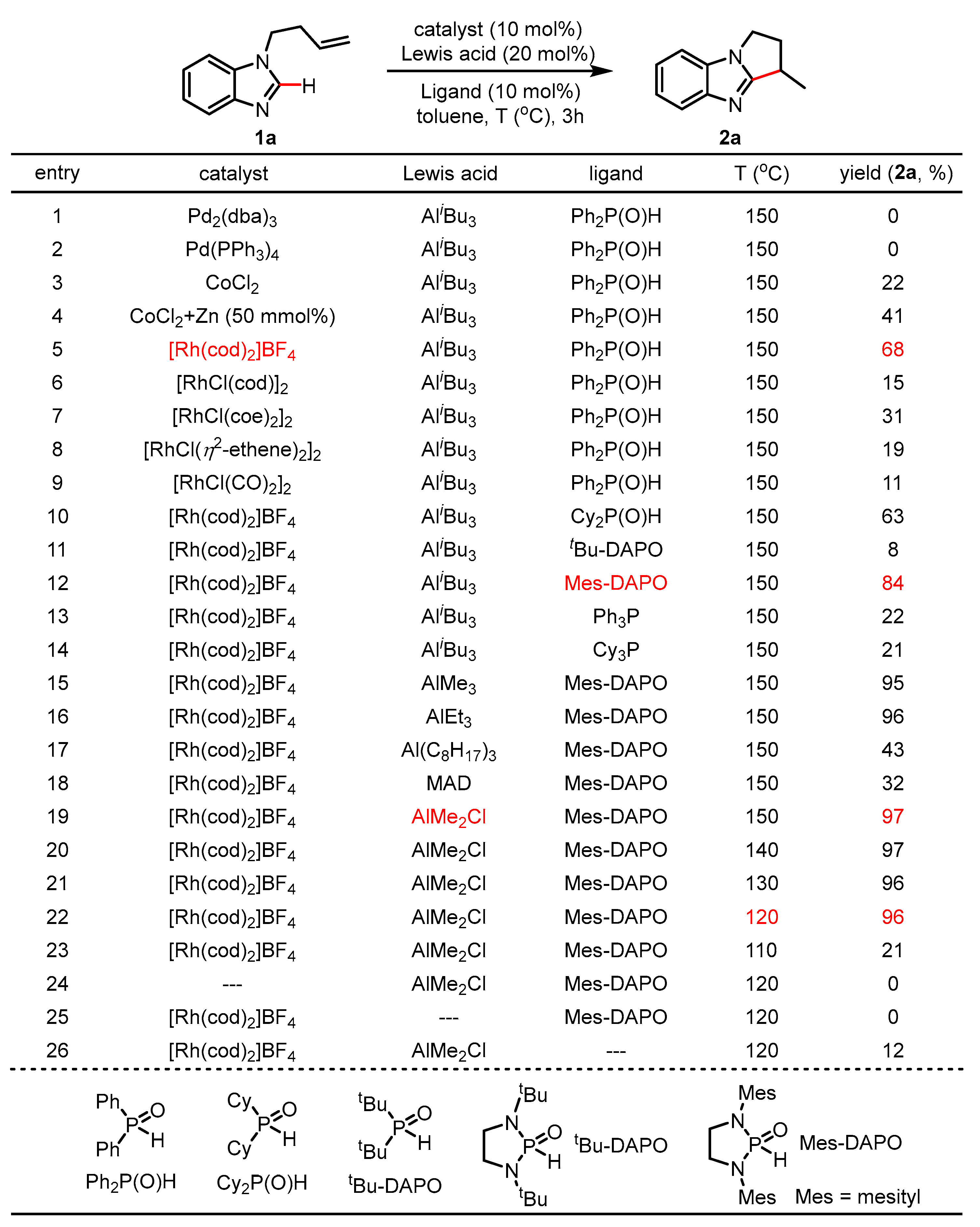
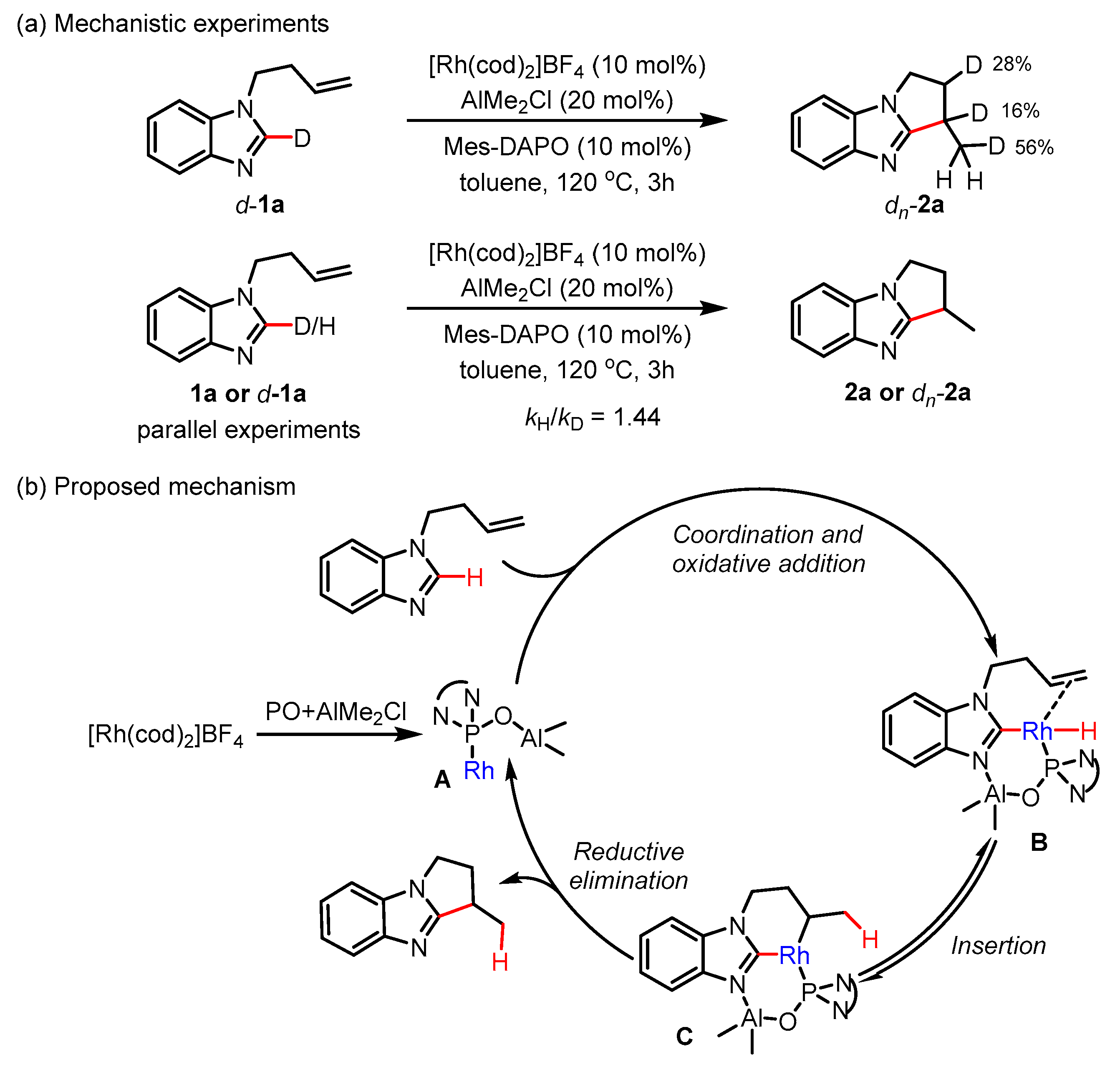
2. Results
3. Discussion
4. Materials and Methods
4.1. Typical Procedure for Rh-Catalyzed C–H Cyclization
4.1.1. 3-Methyl-2,3-dihydro-1H-benzo[d]pyrrolo[1,2-a]imidazole (2a) [39]
4.1.2. Methyl-3-methyl-2,3-dihydro-1H-benzo[d]pyrrolo[1,2-a]imidazole-6-carboxylate (2b) [39]
4.1.3. 3-Methyl-6-(trifluoromethyl)-2,3-dihydro-1H-benzo[d]pyrrolo[1,2-a]imidazole (2c) [39]
4.1.4. 6-Fluoro-3-methyl-2,3-dihydro-1H-benzo[d]pyrrolo[1,2-a]imidazole (2d) [39]
4.1.5. 6,7-Dichloro-3-methyl-2,3-dihydro-1H-benzo[d]pyrrolo[1,2-a]imidazole (2e) [39]
4.1.6. 7-Chloro-3-methyl-2,3-dihydro-1H-benzo[d]pyrrolo[1,2-a]imidazole (2f) [39]
4.1.7. 6-Chloro-3-methyl-2,3-dihydro-1H-benzo[d]pyrrolo[1,2-a]imidazole (2g) [39]
4.1.8. 6-Methyl-7,8-dihydro-6H-pyrrolo[2′,1′:2,3]imidazo[4,5-b]pyridine (2h) [39]
4.1.9. 3-Methyl-2,3-dihydro-1H-naphtho[2,3-d]pyrrolo[1,2-a]imidazole (2i) [39]
4.1.10. 3,7-Dimethyl-2,3-dihydro-1H-benzo[d]pyrrolo[1,2-a]imidazole (2j) [39]
4.1.11. 3,8-Dimethyl-2,3-dihydro-1H-benzo[d]pyrrolo[1,2-a]imidazole (2k) [39]
4.1.12. 3-Propyl-2,3-dihydro-1H-benzo[d]pyrrolo[1,2-a]imidazole (2l) [39]
4.2. Procedure for Enantioselective C–H Cyclization
4.2.1. Procedure for Enantioselective Rh-Catalyzed C–H Cyclization
4.2.2. Procedure for Enantioselective Co-Catalyzed C–H Cyclization
4.3. Procedure for Mechanistic Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dalton, T.; Faber, T.; Glorius, F. C–H Activation: Toward Sustainability and Applications. ACS Central Sci. 2021, 7, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Achar, T.K.; Maiti, S.; Jana, S.; Maiti, D. Transition Metal Catalyzed Enantioselective C(sp2)–H Bond Functionalization. ACS Catal. 2020, 10, 13748–13793. [Google Scholar] [CrossRef]
- Gandeepan, P.; Muller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. 3d Transition Metals for C–H Activation. Chem. Rev. 2019, 119, 2192–2452. [Google Scholar] [PubMed]
- Wozniak, L.; Cramer, N. Enantioselective C-H Bond Functionalizations by 3d Transition-Metal Catalysts. Trends Chem. 2019, 1, 471–484. [Google Scholar] [CrossRef]
- Loup, J.; Dhawa, U.; Pesciaioli, F.; Wencel-Delord, J.; Ackermann, L. Enantioselective C−H Activation with Earth-Abundant 3d Transition Metals. Angew. Chem. Int. Ed. 2019, 58, 12803–12818. [Google Scholar] [CrossRef]
- Saint-Denis, T.G.; Zhu, R.-Y.; Chen, G.; Wu, Q.-F.; Yu, J.-Q. Enantioselective C(sp3)-H Bond Activation by Chiral Transition Metal Catalysts. Science 2018, 359, eaao4798. [Google Scholar] [CrossRef]
- Su, B.; Hartwig, J.F. Development of Chiral Ligands for the Transition-Metal-Catalyzed Enantioselective Silylation and Borylation of C–H Bonds. Angew. Chem. Int. Ed. 2022, 61, e202113343. [Google Scholar]
- Goswami, N.; Bhattacharya, T.; Maiti, D. Transient Directing Ligands for Selective Metal-Catalysed C-H Activation. Nat. Rev. Chem. 2021, 5, 646–659. [Google Scholar] [CrossRef]
- Clevenger, A.L.; Stolley, R.M.; Aderibigbe, J.; Louie, J. Trends in the Usage of Bidentate Phosphines as Ligands in Nickel Catalysis. Chem. Rev. 2020, 120, 6124–6196. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, G.; Szostak, M.; Nolan, S.P. N-Heterocyclic Carbene (NHC) Complexes in C-H Activation Reactions. Chem. Rev. 2020, 120, 1981–2048. [Google Scholar] [CrossRef]
- Davison, R.T.; Kuker, E.L.; Dong, V.M. Teaching Aldehydes New Tricks Using Rhodium-and Cobalt- Hydride Catalysis. Acc. Chem. Res. 2021, 54, 1236–1250. [Google Scholar] [CrossRef] [PubMed]
- Dongbang, S.; Confair, D.N.; Ellman, J.A. Rhodium-Catalyzed C−H Alkenylation/Electrocyclization Cascade Provides Dihydropyridines That Serve as Versatile Intermediates to Diverse Nitrogen Heterocycles. Acc. Chem. Res. 2021, 54, 1766–1778. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, C.-X.; Gu, Q.; You, S.-L. Chiral CpxRh complexes for C–H functionalization reactions. Sci. Bull. 2021, 66, 210–213. [Google Scholar] [CrossRef]
- Zhang, Z.; Durand, N.; Soulé, J.-F. How Rhodium(I)-Catalyzed Phosphorus(III)-Directed C–H Bond Functionalizations Can Improve the Catalytic Activities of Phosphines. Synlett 2022, 33, 705–712. [Google Scholar]
- Coulter, M.M.; Kou, K.G.M.; Galligan, B.; Dong, V.M. Regioand Enantioselective Intermolecular Hydroacylation: Substrate-Directed Addition of Salicylaldehydes to Homoallylic Sulfides. J. Am. Chem. Soc. 2010, 132, 16330–16333. [Google Scholar] [CrossRef]
- Tran, D.N.; Cramer, N. syn-Selective rhodium(I)-catalyzed allylations of ketimines proceeding through a directed C-H activation/allene addition sequence. Angew. Chem. Int. Ed. 2010, 49, 8181–8184. [Google Scholar] [CrossRef]
- Duttwyler, S.; Lu, C.; Rheingold, A.L.; Bergman, R.G.; Ellman, J.A. Highly Diastereoselective Synthesis of Tetrahydropyridines by a C−H Activation−Cyclization−Reduction Cascade. J. Am. Chem. Soc. 2012, 134, 4064–4067. [Google Scholar] [CrossRef]
- Tran, D.N.; Cramer, N. Enantioselective Rhodium(I)-Catalyzed [3 + 2] Annulations of Aromatic Ketimines Induced by Directed C-H Activations. Angew. Chem. Int. Ed. 2011, 50, 11098–11102. [Google Scholar] [CrossRef]
- Kuninobu, Y.; Yamauchi, K.; Tamura, N.; Seiki, T.; Takai, K. Rhodium-Catalyzed Asymmetric Synthesis of Spirosilabifluorene Derivatives. Angew. Chem. Int. Ed. 2013, 52, 1520–1522. [Google Scholar] [CrossRef]
- Zhang, Q.W.; An, K.; Liu, L.C.; Yue, Y.; He, W. Rhodiumcatalyzed Enantioselective Intramolecular C-H Silylation for the Syntheses of Planar-Chiral Metallocene Siloles. Angew. Chem. Int. Ed. 2015, 54, 6918–6921. [Google Scholar] [CrossRef]
- Lee, T.; Wilson, T.W.; Berg, R.; Ryberg, P.; Hartwig, J.F. Rhodium-Catalyzed Enantioselective Silylation of Arene C-H Bonds: Desymmetrization of Diarylmethanols. J. Am. Chem. Soc. 2015, 137, 6742–6745. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Chen, Z.; Dong, V.M. Rhodium-Catalyzed Enantioselective Cycloisomerization to Cyclohexenes Bearing Quaternary Carbon Centers. J. Am. Chem. Soc. 2016, 138, 3310–3313. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Shizuno, T.; Sasaki, T. Enantioselective Synthesis of Planar-Chiral Benzosiloloferrocenes by Rh-Catalyzed Intramolecular C–H Silylation. Chem. Commun. 2015, 51, 7802–7804. [Google Scholar] [CrossRef]
- Zhu, D.-X.; Xia, H.; Liu, J.-G.; Chung, L.W.; Xu, M.-H. Regiospecific and Enantioselective Arylvinylcarbene Insertion of a C-H Bond of Aniline Derivatives Enabled by a Rh(I)-Diene Catalyst. J. Am. Chem. Soc. 2021, 143, 2608–2619. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.L.; Bergman, R.G.; Ellman, J.A. Annulation of Alkenyl-Substituted Heterocycles via Rhodium-Catalyzed Intramolecular C–H Activated Coupling Reactions. J. Am. Chem. Soc. 2001, 123, 2685–2686. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.L.; Vasudevan, A.; Bergman, R.G.; Ellman, J.A.; Souers, A.J. Microwave-Assisted C−H Bond Activation: A Rapid Entry into Functionalized Heterocycles. Org. Lett. 2003, 5, 2131–2134. [Google Scholar] [CrossRef]
- Tan, K.L.; Bergman, R.G.; Ellman, J.A. Intermediacy of an N-Heterocyclic Carbene Complex in the Catalytic C−H Activation of a Substituted Benzimidazole. J. Am. Chem. Soc. 2002, 124, 3202–3203. [Google Scholar] [CrossRef]
- Wiedemann, S.H.; Lewis, J.C.; Ellman, J.A.; Bergman, R.G. Experimental and Computational Studies on the Mechanism of N-Heterocycle C−H Activation by Rh(I). J. Am. Chem. Soc. 2006, 128, 2452–2462. [Google Scholar] [CrossRef]
- Lewis, J.C.; Bergman, R.G.; Ellman, J.A. Direct Functionalization of Nitrogen Heterocycles via Rh-Catalyzed C−H Bond Activation. Acc. Chem. Res. 2008, 41, 1013–1025. [Google Scholar] [CrossRef]
- Tsai, A.S.; Wilson, R.M.; Harada, H.; Bergman, R.G.; Ellman, J.A. Rhodium Catalyzed Enantioselective Cyclization of Substituted Imidazoles via C–H Bond Activation. Chem. Commun. 2009, 26, 3910–3912. [Google Scholar] [CrossRef]
- Ackermann, L.; Born, R.; Spatz, J.H.; Althammer, A.; Gschrei, C.J. Air-Stable Phosphine Oxides as Preligands for Catalytic Activation Reactions of C–Cl, C–F, and C–H Bonds. Pure Appl. Chem. 2006, 78, 209–214. [Google Scholar] [CrossRef]
- Shaikh, T.M.; Weng, C.-M.; Hong, F.-E. Secondary Phosphine Oxides: Versatile Ligands in Transition Metal-Catalyzed Cross-Coupling Reactions. Coord. Chem. Rev. 2016, 256, 771–803. [Google Scholar] [CrossRef]
- Van Leeuwen, P.W.N.M.; Cano, I.; Freixa, Z. Secondary Phosphine Oxides: Bifunctional Ligands in Catalysis. ChemCatChem 2020, 12, 3982–3994. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Ye, M. Recent Advances in Ni−Al Bimetallic Catalysis for Unreactive Bond Transformation. Sci. China Chem. 2018, 61, 1004–1013. [Google Scholar] [CrossRef]
- Li, J.-F.; Luan, Y.-X.; Ye, M. Bimetallic anchoring catalysis for C–H and C–Cactivation. Sci. China Chem. 2021, 64, 1923–1937. [Google Scholar] [CrossRef]
- Luan, Y.-X.; Ye, M. Ligand-ligated Ni–Al bimetallic catalysis for C–H and C–C bond activation. Chem. Commun. 2022, 58, 12260–12273. [Google Scholar] [CrossRef] [PubMed]
- Donets, P.A.; Cramer, N. Diaminophosphine Oxide Ligand Enabled Asymmetric Nickel-Catalyzed Hydrocarbamoylations of Alkenes. J. Am. Chem. Soc. 2013, 135, 11772–11775. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Wang, D.-Y.; Yang, Z.-J.; Luan, Y.-X.; Yang, J.-F.; Li, J.-F.; Pu, Y.-G.; Ye, M. Ni−Al Bimetallic Catalyzed Enantioselective Cycloaddition of Cyclopropyl Carboxamide with Alkyne. J. Am. Chem. Soc. 2017, 139, 18150–18153. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Qi, S.-L.; Luan, Y.-X.; Han, X.-W.; Wang, S.; Chen, H.; Ye, M. Enantioselective Ni−Al Bimetallic Catalyzed exo-Selective C−H Cyclization of Imidazoles with Alkenes. J. Am. Chem. Soc. 2018, 140, 5360–5364. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.-X.; Luan, Y.-X.; Ye, M. Enantioselective Twofold C−H Annulation of Formamides and Alkynes without Built-in Chelating Groups. Angew. Chem. Int. Ed. 2020, 59, 9428–9432. [Google Scholar] [CrossRef]
- Qi, S.-L.; Liu, Y.-P.; Luan, Y.-X.; Ye, M. Ni-Catalyzed Hydroarylation of Alkynes with Unactivated β-C(sp2)−H Bonds. Nat. Commun. 2022, 13, 2938–2946. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Zhang, F.-P.; Chen, H.; Li, Y.; Li, J.-F.; Ye, M. Enantioselective Nickel-Catalyzed C(sp3)−H Activation of Formamides. Angew. Chem. Int. Ed. 2022, 61, e202209625. [Google Scholar]
- Li, J.-F.; Pan, D.; Wang, H.-R.; Zhang, T.; Li, Y.; Huang, G.; Ye, M. Enantioselective C2−H Alkylation of Pyridines with 1,3-Dienes via Ni−Al Bimetallic Catalysis. J. Am. Chem. Soc. 2022, 144, 18810–18816. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.; Xu, W.; Ye, M. Phosphine Oxide-Promoted Rh(I)-Catalyzed C–H Cyclization of Benzimidazoles with Alkenes. Molecules 2023, 28, 736. https://doi.org/10.3390/molecules28020736
Lu M, Xu W, Ye M. Phosphine Oxide-Promoted Rh(I)-Catalyzed C–H Cyclization of Benzimidazoles with Alkenes. Molecules. 2023; 28(2):736. https://doi.org/10.3390/molecules28020736
Chicago/Turabian StyleLu, Mingzhen, Weiwei Xu, and Mengchun Ye. 2023. "Phosphine Oxide-Promoted Rh(I)-Catalyzed C–H Cyclization of Benzimidazoles with Alkenes" Molecules 28, no. 2: 736. https://doi.org/10.3390/molecules28020736
APA StyleLu, M., Xu, W., & Ye, M. (2023). Phosphine Oxide-Promoted Rh(I)-Catalyzed C–H Cyclization of Benzimidazoles with Alkenes. Molecules, 28(2), 736. https://doi.org/10.3390/molecules28020736





