Reaction with ROO• and HOO• Radicals of Honokiol-Related Neolignan Antioxidants
Abstract
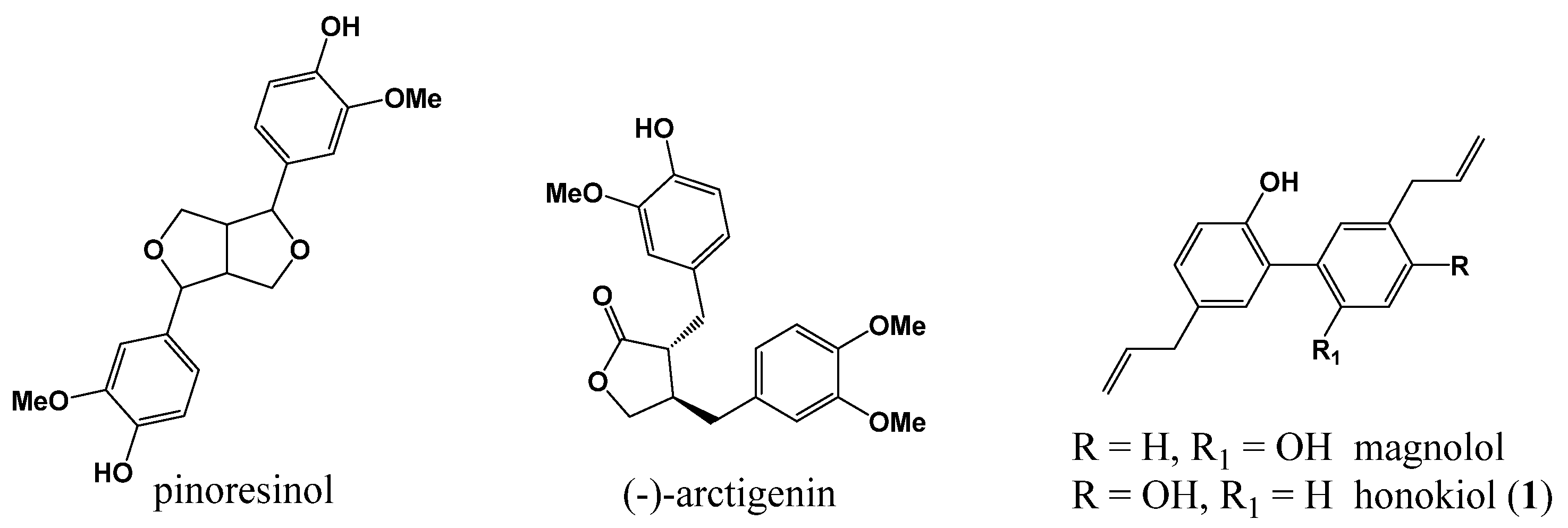
1. Introduction
2. Results and Discussion
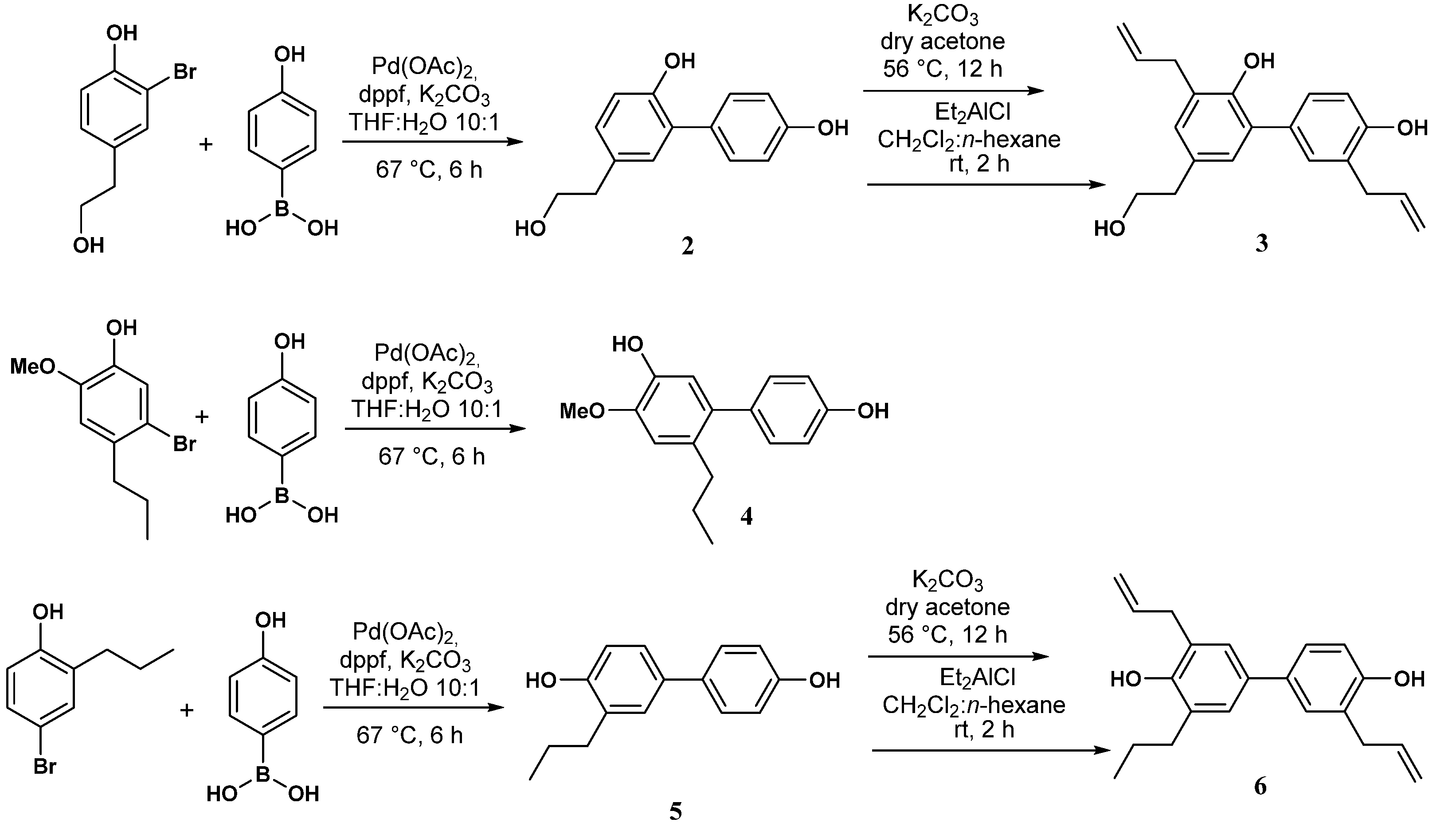
2.1. Synthesis of Bisphenol Neolignans 2–7
2.2. Kinetics and Stoichiometry of the Reaction with Peroxyl Radicals
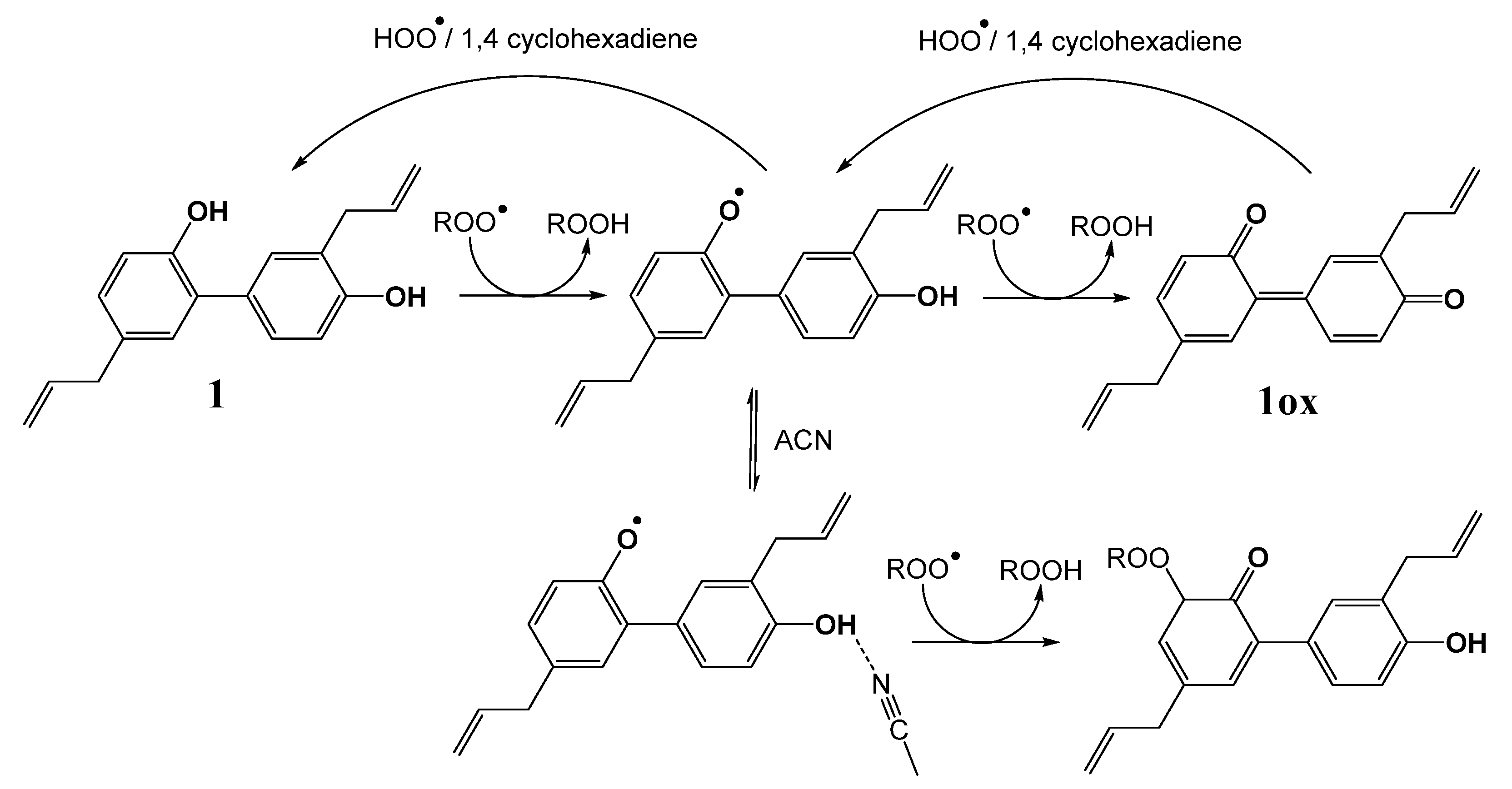
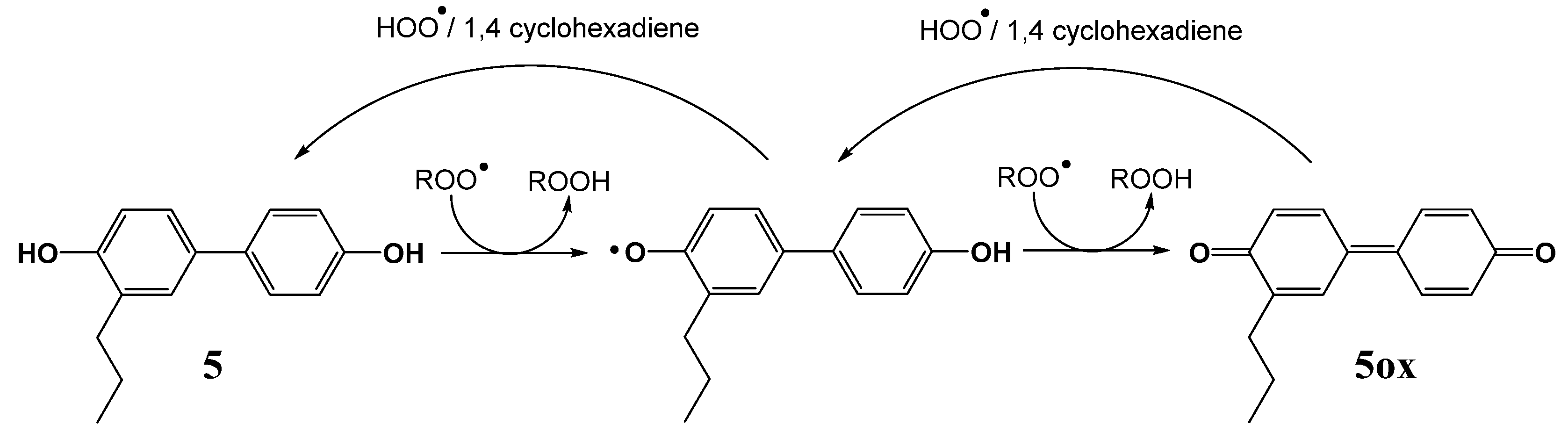
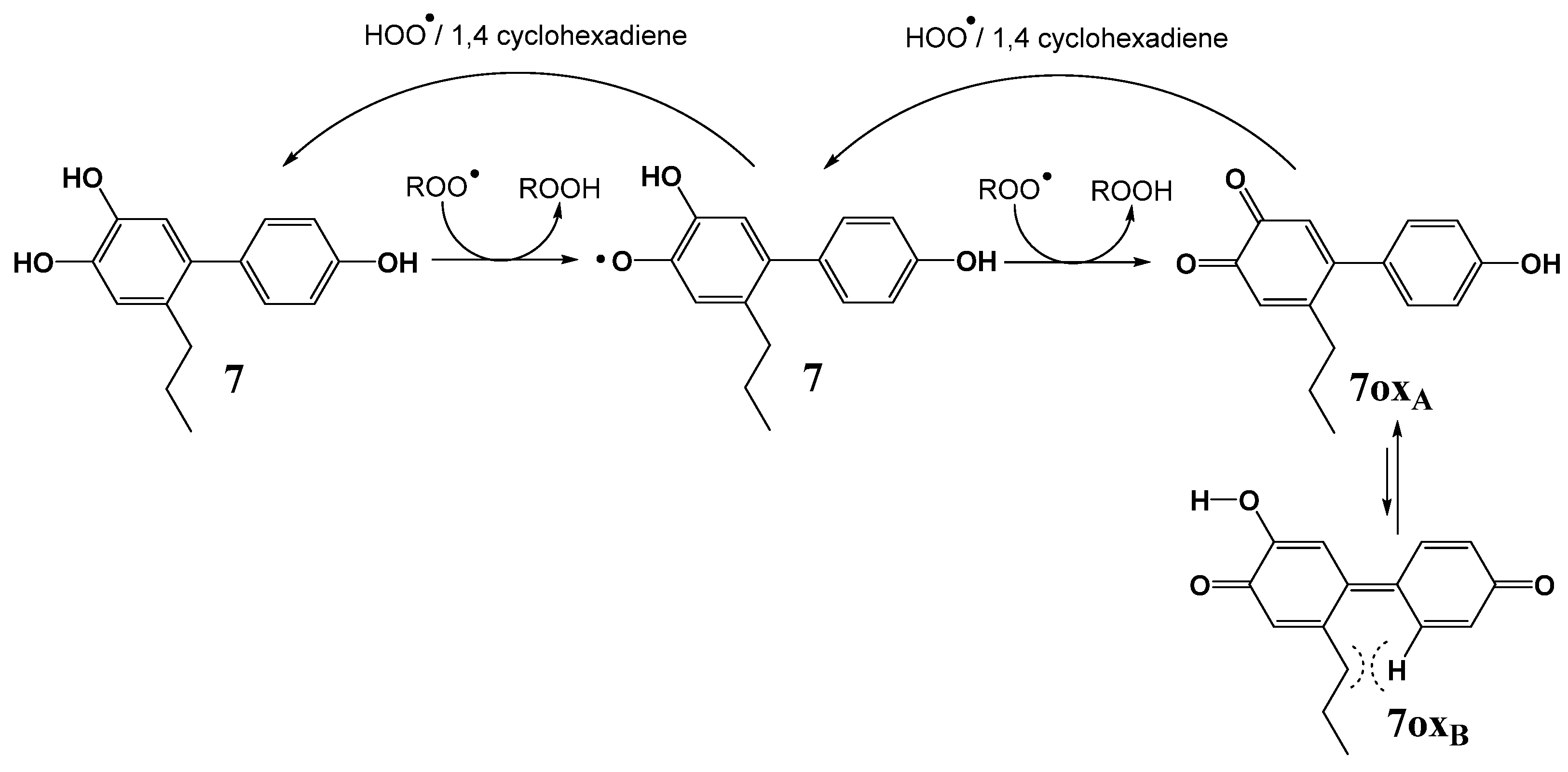
2.3. Hydrogen Atom Transfer from HOO• to Quinones
2.4. Theoretical Calculation of Bond Dissociation Enthaplies
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Bisphenol Neolignans
3.3. Inhibited Autoxidation Studies
3.4. Theoretical Calculations
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bernini, R.; Gualandi, G.; Crestini, C.; Barontini, M.; Belfiore, M.C.; Willfor, S.; Eklund, P.; Saladino, R. A novel and efficient synthesis of highly oxidized lignans by a methyltrioxorhenium/hydrogen peroxide catalytic system. Studies on their apoptogenic and antioxidant activity. Bioorg. Med. Chem. 2009, 17, 5676–5682. [Google Scholar] [CrossRef] [PubMed]
- Aldemir, H.; Richarz, R.; Gulder, T.A. The biocatalytic repertoire of natural biaryl formation. Angew. Chem. Int. Ed. 2014, 53, 8286–8293. [Google Scholar] [CrossRef] [PubMed]
- Cardullo, N.; Muccilli, V.; Tringali, C. Laccase-mediated synthesis of bioactive natural products and their analogues. RSC Chem. Biol. 2022, 3, 614–647. [Google Scholar] [CrossRef]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef]
- Zalesak, F.; Bon, D.J.D.; Pospisil, J. Lignans and Neolignans: Plant secondary metabolites as a reservoir of biologically active substances. Pharmacol. Res. 2019, 146, 104284. [Google Scholar] [CrossRef]
- López-Biedma, A.; Sánchez-Quesada, C.; Delgado-Rodríguez, M.; Gaforio, J.J. The biological activities of natural lignans from olives and virgin olive oils: A review. J. Funct. Foods 2016, 26, 36–47. [Google Scholar] [CrossRef]
- Schuster, C.; Wolpert, N.; Moustaid-Moussa, N.; Gollahon, L.S. Combinatorial Effects of the Natural Products Arctigenin, Chlorogenic Acid, and Cinnamaldehyde Commit Oxidation Assassination on Breast Cancer Cells. Antioxidants 2022, 11, 591. [Google Scholar] [CrossRef]
- Xu, Y.; Li, L.-Z.; Cong, Q.; Wang, W.; Qi, X.-L.; Peng, Y.; Song, S.-J. Bioactive lignans and flavones with in vitro antioxidant and neuroprotective properties from Rubus idaeus rhizome. J. Funct. Foods 2017, 32, 160–169. [Google Scholar] [CrossRef]
- Di Micco, S.; Spatafora, C.; Cardullo, N.; Riccio, R.; Fischer, K.; Pergola, C.; Koeberle, A.; Werz, O.; Chalal, M.; Vervandier-Fasseur, D.; et al. 2,3-Dihydrobenzofuran privileged structures as new bioinspired lead compounds for the design of mPGES-1 inhibitors. Bioorg. Med. Chem. 2016, 24, 820–826. [Google Scholar] [CrossRef]
- Hajduk, P.J.; Bures, M.; Praestgaard, J.; Fesik, S.W. Privileged molecules for protein binding identified from NMR-based screening. J. Med. Chem. 2000, 43, 3443–3447. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, Y.M.; Lee, C.K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. 2011, 130, 157–176. [Google Scholar] [CrossRef]
- Chen, J.H.; Kuo, H.C.; Lee, K.F.; Tsai, T.H. Magnolol protects neurons against ischemia injury via the downregulation of p38/MAPK, CHOP and nitrotyrosine. Toxicol. Appl. Pharmacol. 2014, 279, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Meng, A.P.; Guan, X.L.; Li, J.; Wu, Q.; Deng, S.P.; Su, X.J.; Yang, R.Y. Anti-hepatitis B virus lignans from the root of Streblus asper. Bioorg. Med. Chem. Lett. 2013, 23, 2238–2244. [Google Scholar] [CrossRef] [PubMed]
- Kuo, D.H.; Lai, Y.S.; Lo, C.Y.; Cheng, A.C.; Wu, H.; Pan, M.H. Inhibitory effect of magnolol on TPA-induced skin inflammation and tumor promotion in mice. J. Agric. Food. Chem. 2010, 58, 5777–5783. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.H.; Yao, C.J.; Lai, G.M.; Lee, L.M.; Whang-Peng, J.; Shih, P.H. Honokiol induces apoptotic cell death by oxidative burst and mitochondrial hyperpolarization of bladder cancer cells. Exp. Ther. Med. 2019, 17, 4213–4222. [Google Scholar] [CrossRef]
- Pulvirenti, L.; Muccilli, V.; Cardullo, N.; Spatafora, C.; Tringali, C. Chemoenzymatic Synthesis and alpha-Glucosidase Inhibitory Activity of Dimeric Neolignans Inspired by Magnolol. J. Nat. Prod. 2017, 80, 1648–1657. [Google Scholar] [CrossRef]
- Cassiano, C.; Esposito, R.; Tosco, A.; Casapullo, A.; Mozzicafreddo, M.; Tringali, C.; Riccio, R.; Monti, M.C. Chemical Proteomics-Guided Identification of a Novel Biological Target of the Bioactive Neolignan Magnolol. Front. Chem. 2019, 7, 53. [Google Scholar] [CrossRef]
- Maioli, M.; Basoli, V.; Carta, P.; Fabbri, D.; Dettori, M.A.; Cruciani, S.; Serra, P.A.; Delogu, G. Synthesis of magnolol and honokiol derivatives and their effect against hepatocarcinoma cells. PLoS ONE 2018, 13, e0192178. [Google Scholar] [CrossRef]
- Fuchs, A.; Baur, R.; Schoeder, C.; Sigel, E.; Muller, C.E. Structural analogues of the natural products magnolol and honokiol as potent allosteric potentiators of GABA(A) receptors. Bioorg. Med. Chem. 2014, 22, 6908–6917. [Google Scholar] [CrossRef]
- Chu, J.; Yang, R.; Cheng, W.; Cui, L.; Pan, H.; Liu, J.; Guo, Y. Semisynthesis, biological activities, and mechanism studies of Mannich base analogues of magnolol/honokiol as potential alpha-glucosidase inhibitors. Bioorg. Med. Chem. 2022, 75, 117070. [Google Scholar] [CrossRef]
- Fried, L.E.; Arbiser, J.L. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid. Redox Signal. 2009, 11, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, Z.Q. Comparison of antioxidant abilities of magnolol and honokiol to scavenge radicals and to protect DNA. Biochimie 2011, 93, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Zotova, J.; Baschieri, A.; Valgimigli, L. Antioxidant Activity of Magnolol and Honokiol: Kinetic and Mechanistic Investigations of Their Reaction with Peroxyl Radicals. J. Org. Chem. 2015, 80, 10651–10659. [Google Scholar] [CrossRef] [PubMed]
- Helberg, J.; Pratt, D.A. Autoxidation vs. antioxidants-the fight for forever. Chem. Soc. Rev. 2021, 50, 7343–7358. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Farmer, L.A.; Zilka, O.; Van Kessel, A.T.M.; Pratt, D.A. Beyond DPPH: Use of Fluorescence-Enabled Inhibited Autoxidation to Predict Oxidative Cell Death Rescue. Cell Chem. Biol. 2019, 26, 1594–1607.e7. [Google Scholar] [CrossRef] [PubMed]
- Baschieri, A.; Pulvirenti, L.; Muccilli, V.; Amorati, R.; Tringali, C. Chain-breaking antioxidant activity of hydroxylated and methoxylated magnolol derivatives: The role of H-bonds. Org. Biomol. Chem. 2017, 15, 6177–6184. [Google Scholar] [CrossRef]
- Baschieri, A.; Amorati, R. Methods to Determine Chain-Breaking Antioxidant Activity of Nanomaterials beyond DPPH(*). A Review. Antioxidants 2021, 10, 1551. [Google Scholar] [CrossRef]
- Cardullo, N.; Barresi, V.; Muccilli, V.; Spampinato, G.; D’Amico, M.; Condorelli, D.F.; Tringali, C. Synthesis of Bisphenol Neolignans Inspired by Honokiol as Antiproliferative Agents. Molecules 2020, 25, 733. [Google Scholar] [CrossRef]
- Bizzarri, B.M.; Botta, L.; Capecchi, E.; Celestino, I.; Checconi, P.; Palamara, A.T.; Nencioni, L.; Saladino, R. Regioselective IBX-Mediated Synthesis of Coumarin Derivatives with Antioxidant and Anti-influenza Activities. J. Nat. Prod. 2017, 80, 3247–3254. [Google Scholar] [CrossRef]
- Frigerio, M.; Santagostino, M.; Sputore, S. A User-Friendly Entry to 2-Iodoxybenzoic Acid (IBX). J. Org. Chem. 1999, 64, 4537–4538. [Google Scholar] [CrossRef]
- Burton, G.W.; Doba, T.; Gabe, E.; Hughes, L.; Lee, F.L.; Prasad, L.; Ingold, K.U. Autoxidation of biological molecules. 4. Maximizing the antioxidant activity of phenols. J. Am. Chem. Soc. 1985, 107, 7053–7065. [Google Scholar] [CrossRef]
- Amorati, R.; Baschieri, A.; Valgimigli, L. Measuring Antioxidant Activity in Bioorganic Samples by the Differential Oxygen Uptake Apparatus: Recent Advances. J. Chem. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Amorati, R.; Pedulli, G.F.; Valgimigli, L.; Attanasi, O.A.; Filippone, P.; Fiorucci, C.; Saladino, R. Absolute rate constants for the reaction of peroxyl radicals with cardanol derivatives. J. Chem. Soc., Perkin Trans. II 2001, 2, 2142–2146. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Timokhin, V.I.; Zaborovskiy, A.B.; Lutsyk, D.S.; Prystansky, R.E. Rate constants for the reaction of cumylperoxyl radicals with group 14 hydrides. J. Chem. Soc., Perkin Trans. II 2000, 577–582. [Google Scholar] [CrossRef]
- Denisov, E.T.; Khudyakov, I.V. Mechanisms of action and reactivities of the free radicals of inhibitors. Chem. Rev. 2002, 87, 1313–1357. [Google Scholar] [CrossRef]
- Denisov, E.T. Liquid-Phase Reaction Rate Constants; Springer: New York, NY, USA, 1995. [Google Scholar]
- Lucarini, M.; Pedulli, G.F.; Valgimigli, L.; Amorati, R.; Minisci, F. Thermochemical and Kinetic Studies of a Bisphenol Antioxidant. J. Org. Chem. 2001, 66, 5456–5462. [Google Scholar] [CrossRef]
- Amorati, R.; Lucarini, M.; Mugnaini, V.; Pedulli, G.F. Antioxidant activity of o-bisphenols: The role of intramolecular hydrogen bonding. J. Org. Chem. 2003, 68, 5198–5204. [Google Scholar] [CrossRef]
- Valgimigli, L.; Pratt, D.A. Antioxidants in Chemistry and Biology. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Amorati, R.; Baschieri, A.; Morroni, G.; Gambino, R.; Valgimigli, L. Peroxyl Radical Reactions in Water Solution: A Gym for Proton-Coupled Electron-Transfer Theories. Chem. Eur. J. 2016, 22, 7924–7934. [Google Scholar] [CrossRef]
- Foti, M.C.; Barclay, L.R.; Ingold, K.U. The role of hydrogen bonding on the h-atom-donating abilities of catechols and naphthalene diols and on a previously overlooked aspect of their infrared spectra. J. Am. Chem. Soc. 2002, 124, 12881–12888. [Google Scholar] [CrossRef]
- Howard, J.A.; Ingold, K.U. Absolute rate constants for hydrocarbon autoxidation. V. The hydroperoxy radical in chain propagation and termination. Can. J. Chem. 1967, 45, 785–792. [Google Scholar] [CrossRef]
- Sawyer, D.T.; McDowell, M.S.; Yamaguchi, K.S. Reactivity of perhydroxyl (HOO•) with 1,4-cyclohexadiene (model for allylic groups in biomembranes). Chem. Res. Toxicol. 1988, 1, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Baschieri, A.; Amorati, R.; Valgimigli, L.; Sambri, L. 1-Methyl-1,4-cyclohexadiene as a Traceless Reducing Agent for the Synthesis of Catechols and Hydroquinones. J. Org. Chem. 2019, 84, 13655–13664. [Google Scholar] [CrossRef] [PubMed]
- Baschieri, A.; Valgimigli, L.; Gabbanini, S.; DiLabio, G.A.; Romero-Montalvo, E.; Amorati, R. Extremely Fast Hydrogen Atom Transfer between Nitroxides and HOO• Radicals and Implication for Catalytic Coantioxidant Systems. J. Am. Chem. Soc. 2018, 140, 10354–10362. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Baschieri, A.; Mollica, F.; Valgimigli, L.; Cedrowski, J.; Litwinienko, G.; Amorati, R. Hydrogen Atom Transfer from HOO• to ortho-Quinones Explains the Antioxidant Activity of Polydopamine. Angew. Chem. Int. Ed. 2021, 60, 15220–15224. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter. 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Valgimigli, L.; Amorati, R.; Fumo, M.G.; DiLabio, G.A.; Pedulli, G.F.; Ingold, K.U.; Pratt, D.A. The unusual reaction of semiquinone radicals with molecular oxygen. J. Org. Chem. 2008, 73, 1830–1841. [Google Scholar] [CrossRef]
- Howard, J.A.; Ingold, K.U. Absolute rate constants for hydrocarbon autoxidation. VI. Alkyl aromatic and olefinic hydrocarbons. Can. J. Chem. 1967, 45, 793–802. [Google Scholar] [CrossRef]
- Enes, R.F.; Tome, A.C.; Cavaleiro, J.A.; Amorati, R.; Fumo, M.G.; Pedulli, G.F.; Valgimigli, L. Synthesis and antioxidant activity of [60]fullerene-BHT conjugates. Chem. Eur. J. 2006, 12, 4646–4653. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. The shikimate pathway: Aromatic amino acids and phenylpropanoids. In Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2009; pp. 137–186. [Google Scholar] [CrossRef]







| Sample | Chlorobenzene | Acetonitrile | KSE 3 | ||
|---|---|---|---|---|---|
| kinh /M−1s−1 | n | kinh /M−1s−1 | n | ||
| 1 | (1.2 ± 0.2) × 104 | 2.3 ± 0.2 | (8.2 ± 0.3) × 103 4 | 3.5 ± 0.5 | 1.5 |
| 2 | (6.7 ± 0.3) × 103 | 1.9 ± 0.1 | (3.2 ± 0.2) × 103 | 2.4 ± 0.4 | 2.1 |
| 3 | (6.1 ± 0.4) × 103 | 1.9 ± 0.1 | (4.0 ± 0.4) × 103 | 2.1 ± 0.2 | 1.5 |
| 4 | (1.1 ± 0.2) × 104 first OH (2.3 ± 0.4) × 103 s OH | 3.8 ± 0.3 | (5.6 ± 0.3) × 103 | 2.6 ± 0.4 | 2.0 |
| 5 | (2.5 ± 0.2) × 104 | 2.0 ± 0.1 | (7.6 ± 0.3) × 103 | 2.1 ± 0.1 | 3.3 |
| 6 | (1.5 ± 0.2) × 104 | 1.8 ± 0.2 | (1.0 ± 0.3) × 104 | 2.1 ± 0.1 | 1.5 |
| 75 | (1.2 ± 0.1) × 106 | 2.0 ± 0.1 | (2.5 ± 0.2) × 104 | 2.0 ± 0.1 | 48 |
| Sample | Slope A 1 without Inhibitors + 26 mM CHD d[O2]/dt (μMs−1) | Slope B Oxidized Form + 26 mM CHD d[O2]/dt (μMs−1) | Slope Reduction A/B |
|---|---|---|---|
| 7 | 2.0 ± 0.2 | 0.123 | 16.3 |
| 5 | 0.227 | 8.8 | |
| 1 | 0.271 | 7.4 | |
| 4 | 0.657 | 3.0 |
| Compound | BDE(OH)/kcal/mol | ||
|---|---|---|---|
| 1-OH | 2-OH | ||
| 1 | 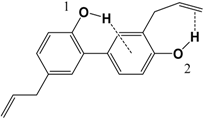 | 83.6 | 85.1 |
| 2 |  | 83.8 | 85.3 |
| 3 | 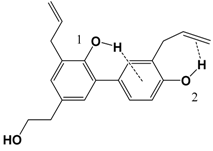 | 83.2 | 85.8 |
| 4 | 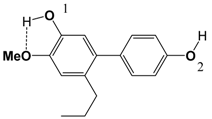 | 82.8 | 84.2 |
| 5 |  | 81.5 | 82.5 |
| 6 | 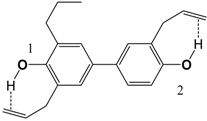 | 81.9 | 83.0 |
| 7 |  | 77.0 | 84.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardullo, N.; Monti, F.; Muccilli, V.; Amorati, R.; Baschieri, A. Reaction with ROO• and HOO• Radicals of Honokiol-Related Neolignan Antioxidants. Molecules 2023, 28, 735. https://doi.org/10.3390/molecules28020735
Cardullo N, Monti F, Muccilli V, Amorati R, Baschieri A. Reaction with ROO• and HOO• Radicals of Honokiol-Related Neolignan Antioxidants. Molecules. 2023; 28(2):735. https://doi.org/10.3390/molecules28020735
Chicago/Turabian StyleCardullo, Nunzio, Filippo Monti, Vera Muccilli, Riccardo Amorati, and Andrea Baschieri. 2023. "Reaction with ROO• and HOO• Radicals of Honokiol-Related Neolignan Antioxidants" Molecules 28, no. 2: 735. https://doi.org/10.3390/molecules28020735
APA StyleCardullo, N., Monti, F., Muccilli, V., Amorati, R., & Baschieri, A. (2023). Reaction with ROO• and HOO• Radicals of Honokiol-Related Neolignan Antioxidants. Molecules, 28(2), 735. https://doi.org/10.3390/molecules28020735








