Global Search for Stable C4H5NO Compounds—Guinness Molecules and Stability Islands
Abstract
1. Introduction
2. Results and Discussion
2.1. Computational Details
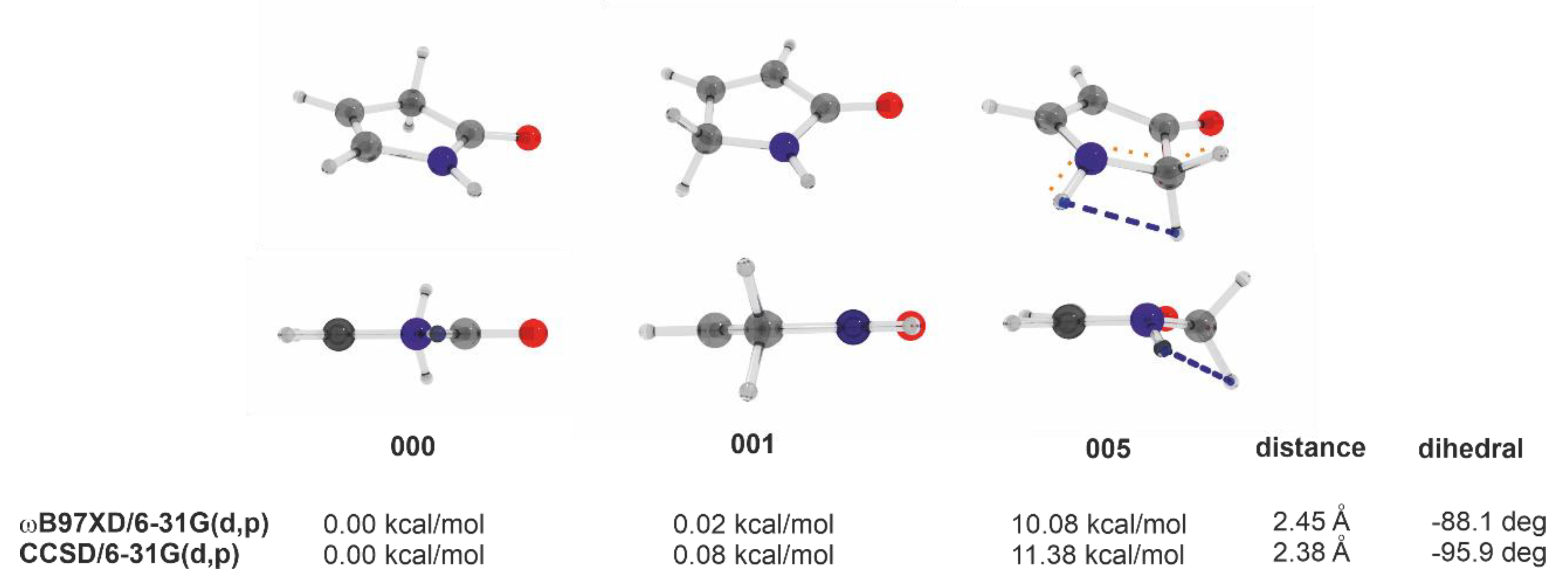
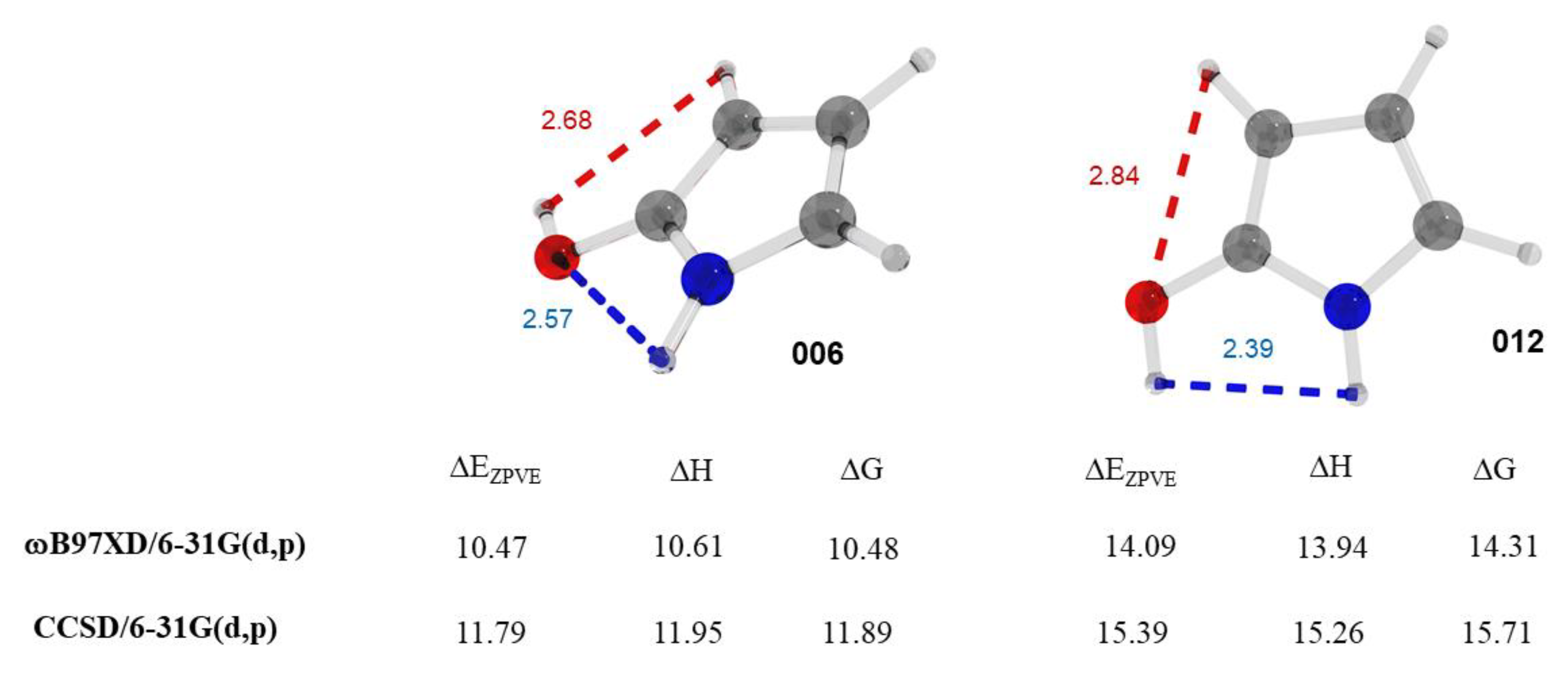
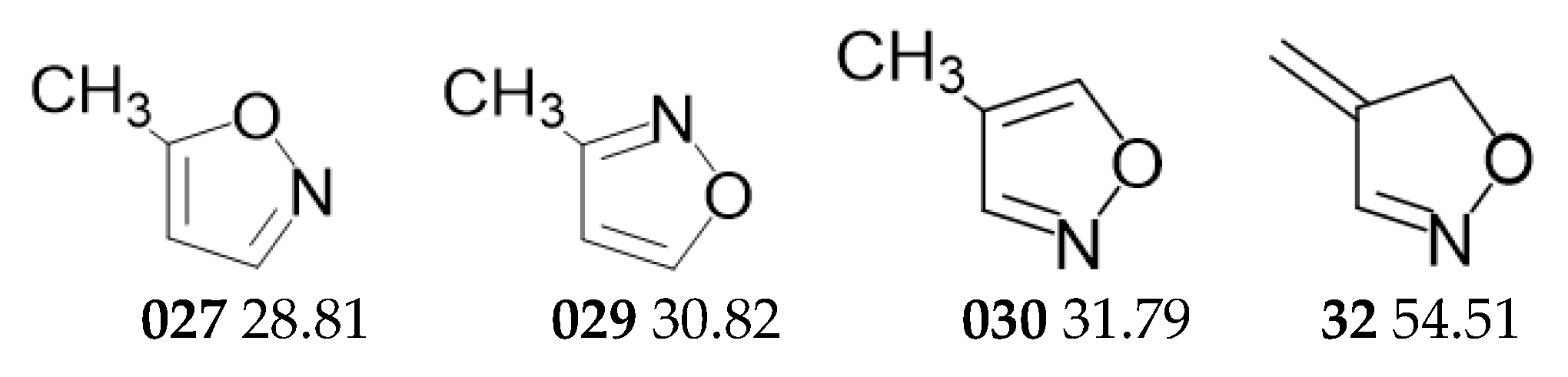
2.2. Guinness Molecules and 5-Membered Heterocycles of C4H5NO
2.3. 6-Membered Heterocycles of C4H5NO
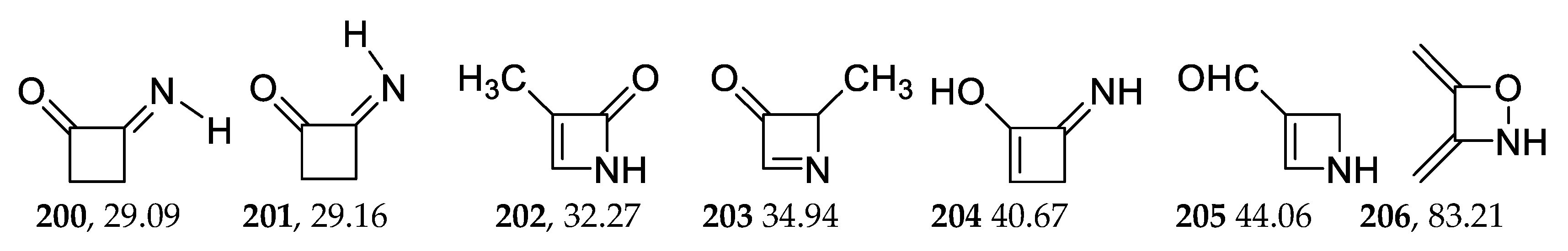
2.4. 4-Membered Heterocycles of C4H5NO2
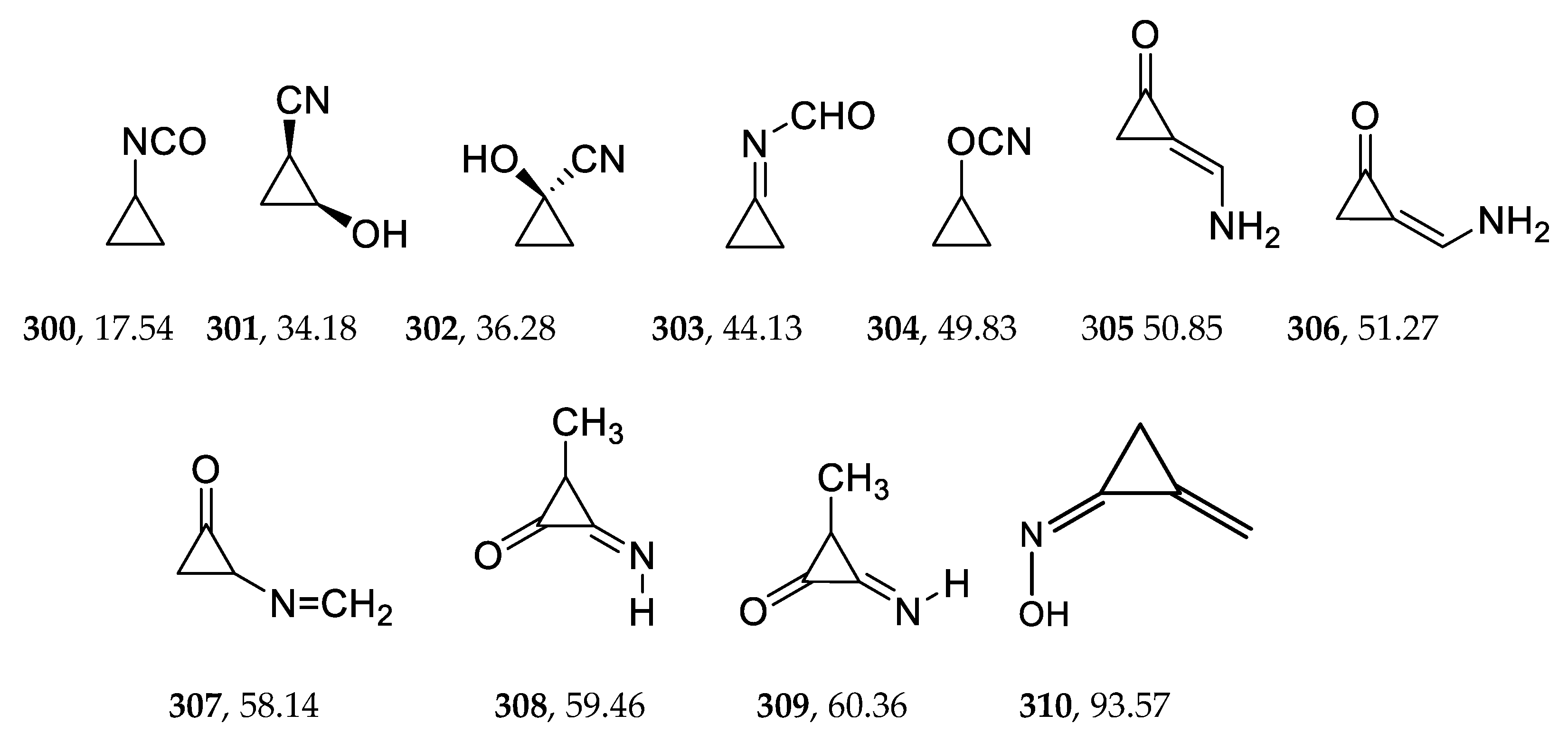
2.5. Saturated 3-Membered Heterocycles of C4H5NO
2.6. Unsaturated 3-Membered Heterocycles of C4H5NO
2.7. Bicyclic Compounds of C4H5NO
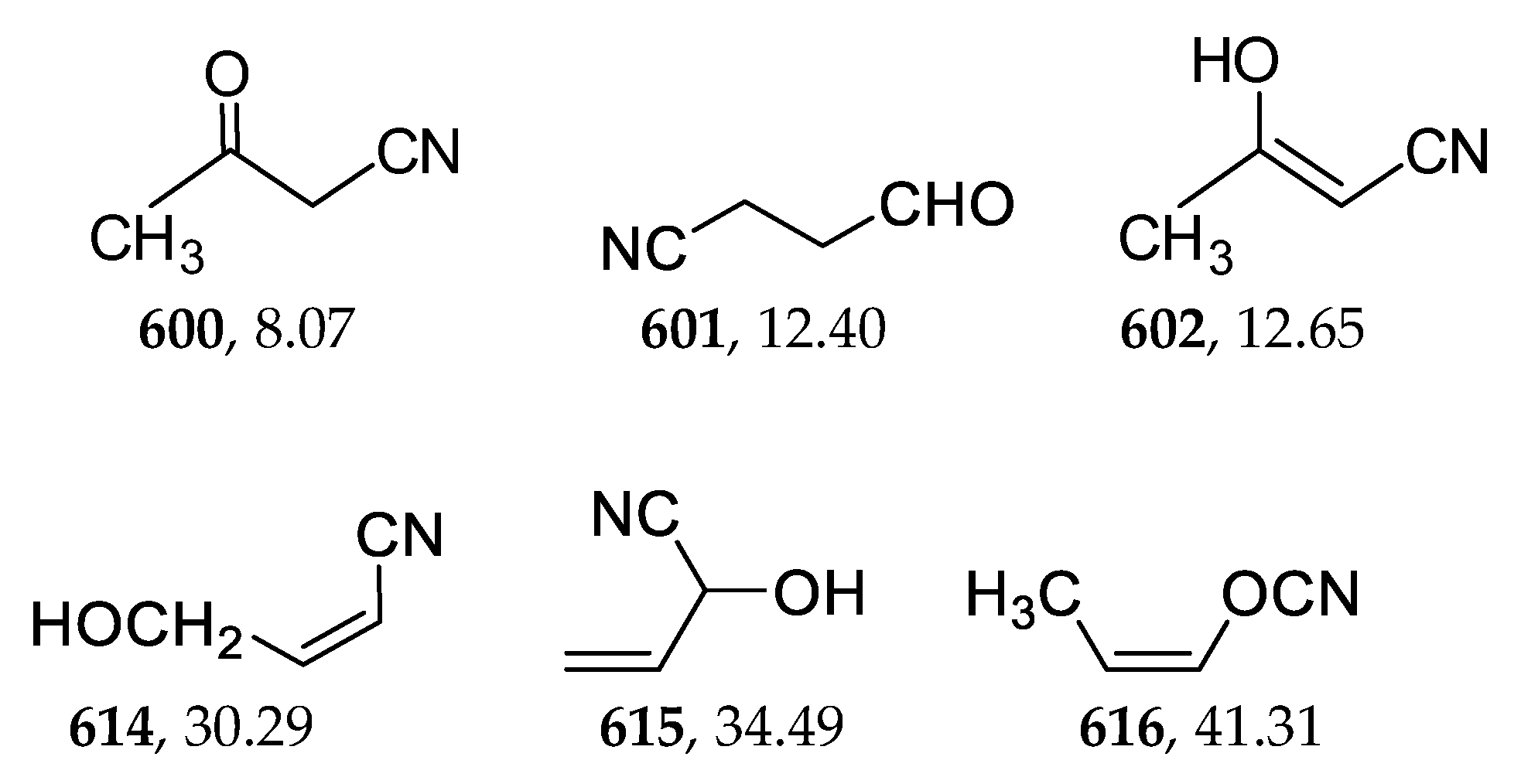
2.8. Acyclic Nitriles
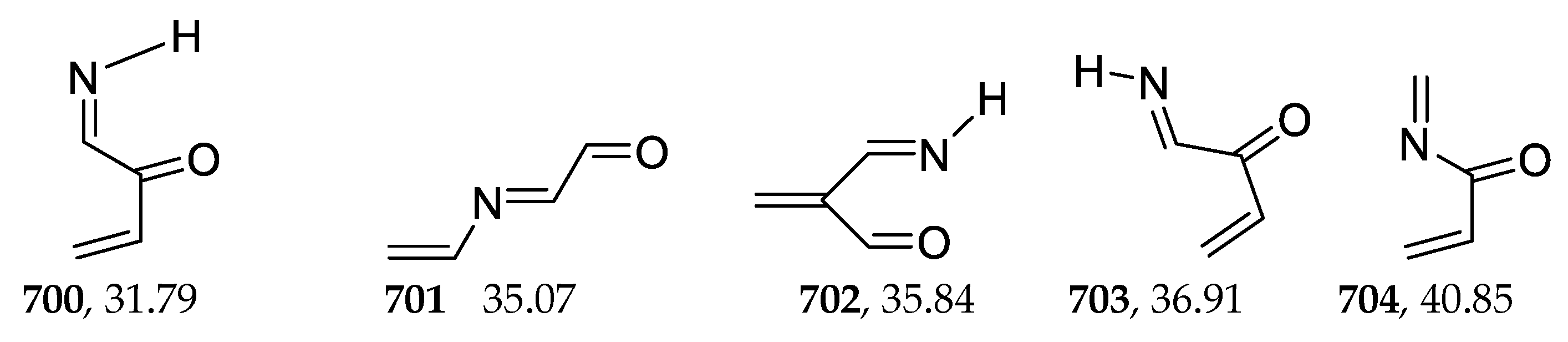
2.9. Trienes
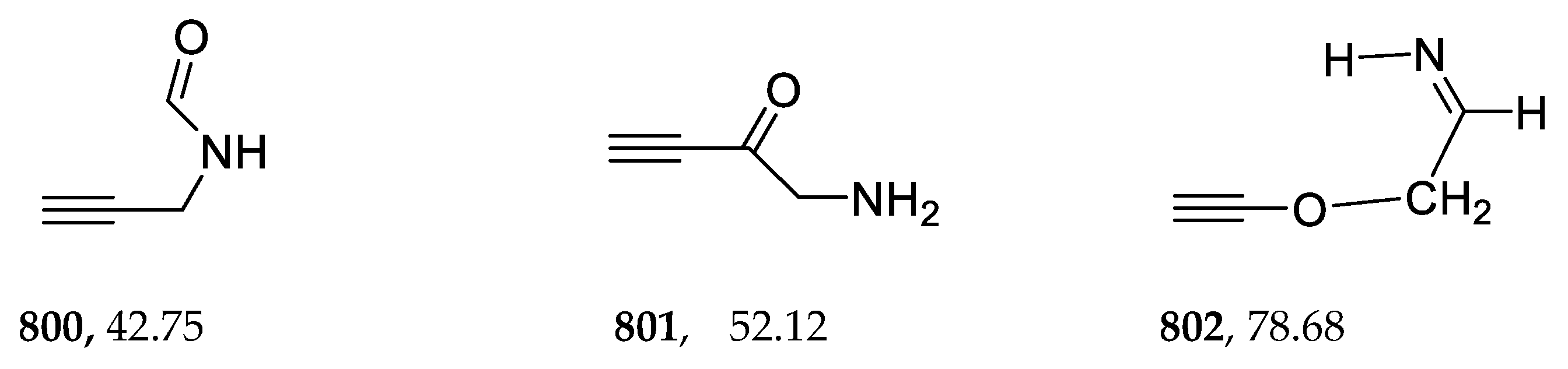
2.10. Acetylenes
2.11. Allenes and Ketenes
2.12. Isonitriles
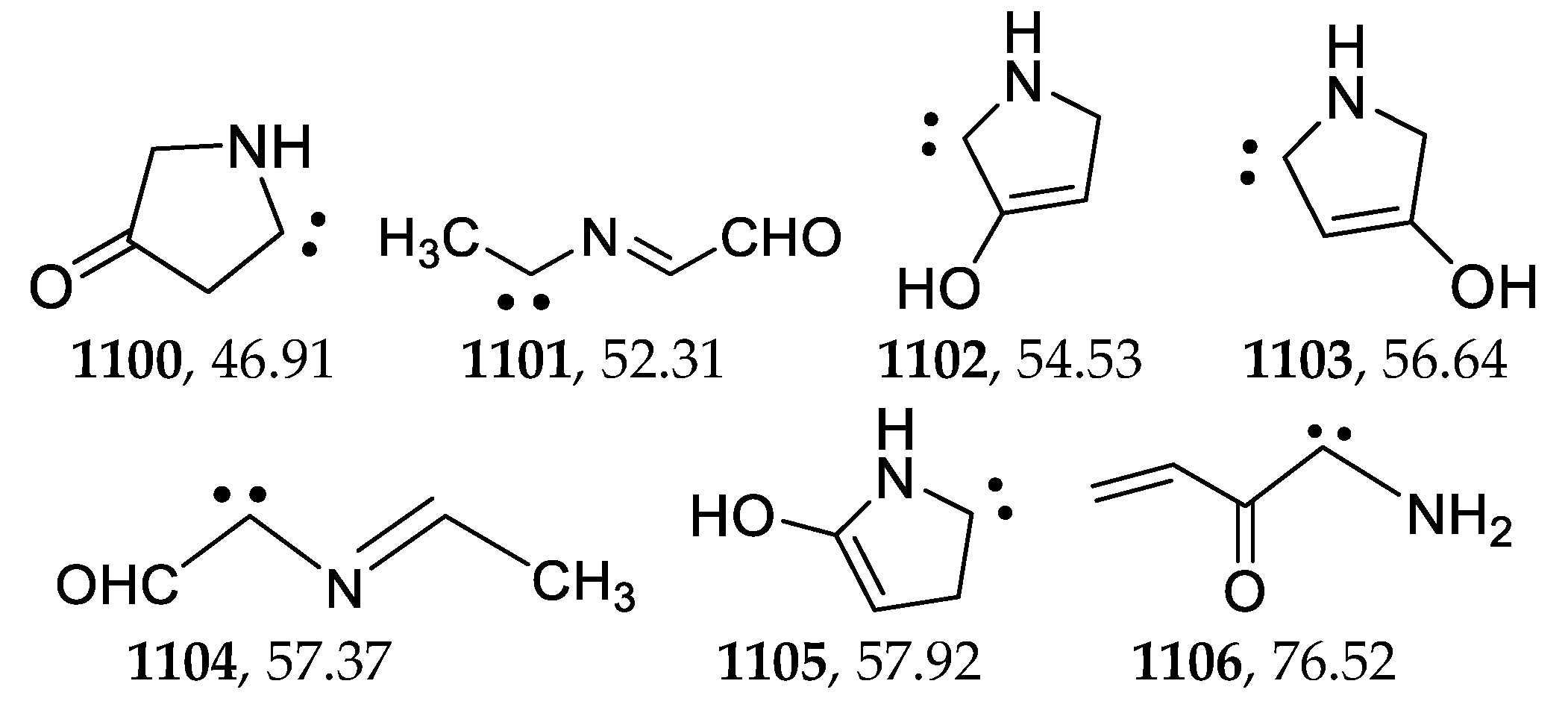
2.13. Carbenes
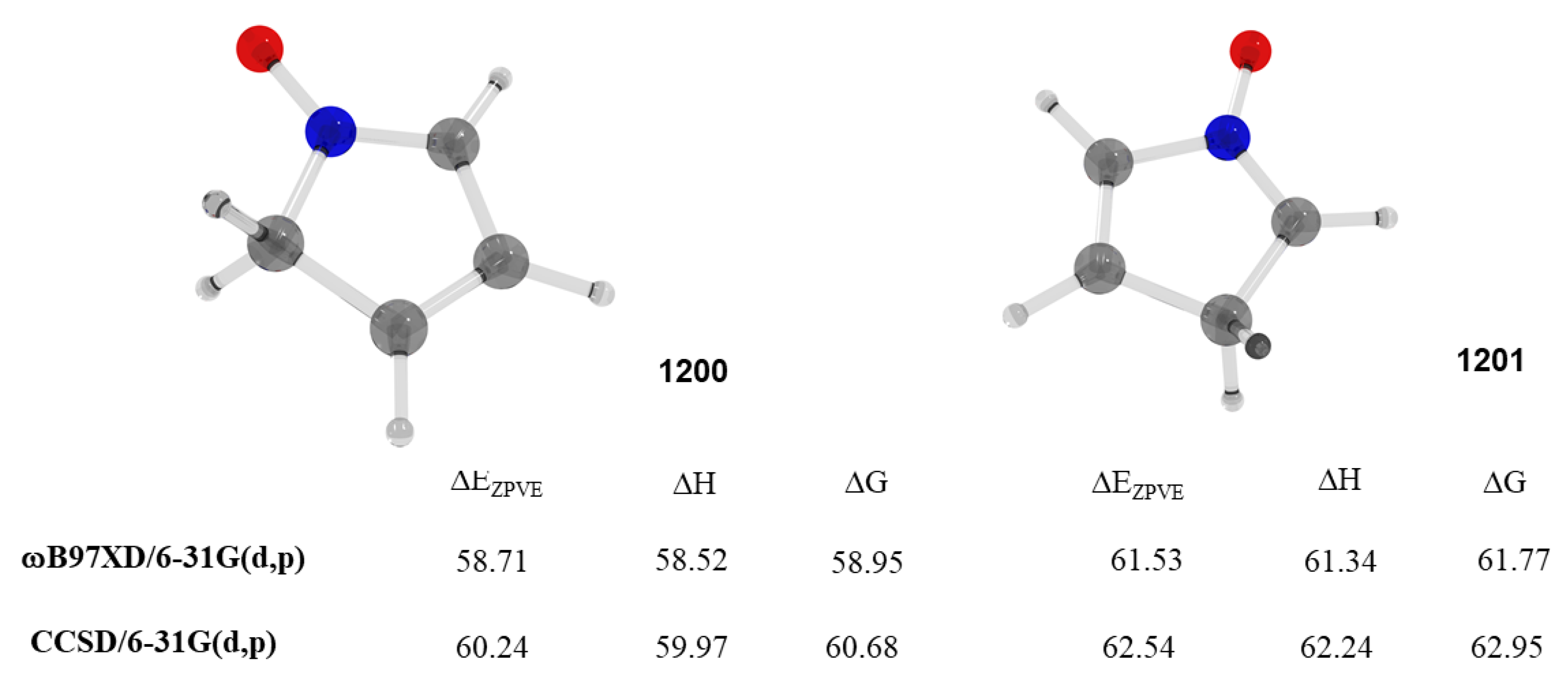
2.14. Bipolar Compounds
2.15. Molecular Associates
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suhm, M.A. Guinness molecules: Identifying lowest-energy structures. Angew. Chem. Int. Ed. Engl. 2014, 53, 1714–1715. [Google Scholar] [CrossRef] [PubMed]
- Altnöder, J.; Krüger, K.; Borodin, D.; Reuter, L.; Rohleder, D.; Hecker, F.; Schulz, R.A.; Nguyen, X.T.; Preiß, H.; Eckhoff, M.; et al. The Guinness molecules for the carbohydrate formula. Chem. Rec. 2014, 14, 1116–1133. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Maeda, S. Global Reaction Route Mapping on Potential Energy Surfaces of Formaldehyde, Formic Acid, and Their Metal-Substituted Analogues. J. Phys. Chem. A 2006, 110, 8933–8941. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Ohno, K.; Morokuma, K. Systematic exploration of the mechanism of chemical reactions: The global reaction route mapping (GRRM) strategy using the ADDF and AFIR methods. Phys. Chem. Chem. Phys. 2013, 15, 3683. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K. Study of Potential Energy Surfaces towards Global Reaction Route Mapping. Chem. Rec. 2016, 16, 2198–2218. [Google Scholar] [CrossRef]
- Tokoyama, H.; Yamakado, H.; Maeda, S.; Ohno, K. Exploration of Isomers of Benzene by GRRM/SCC-DFTB. Chem. Lett. 2014, 43, 702–704. [Google Scholar] [CrossRef]
- Omori, K.; Nakayama, H.; Ishii, K. Diversity of the Dimer Structures of Toluene: Exploration by the GRRM Method. Chem. Lett. 2014, 43, 1803–1805. [Google Scholar] [CrossRef]
- Suzuki, S.; Maeda, S.; Morokuma, K. Exploration of Quenching Pathways of Multiluminescent Acenes Using the GRRM Method with the SF-TDDFT Method. J. Phys. Chem. A 2015, 119, 11479–11487. [Google Scholar] [CrossRef]
- Watanabe, K.; Kawashima, Y.; Mukai, C.; Takagi, T.; Suwa, Y.; Tian, Y.-S.; Kawashita, N. A Comparison between the Cycloadditions of Allenyl- and Vinyl-Cyclopentanes Using Density Functional Theory and GRRM Program. Chem. Pharm. Bull. 2020, 68, 737–741. [Google Scholar] [CrossRef]
- Ohno, K.; Kishimoto, N.; Iwamoto, T.; Satoh, H.; Watanabe, H. High Performance Global Exploration of Isomers and Isomerization Channels on Quantum Chemical Potential Energy Surface of H5C2NO2. J. Comput. Chem. 2021, 42, 192–204. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Chai, J.-D.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom–Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, R.J.; Purvis, G.D., III. Many-body perturbation-theory, coupled-pair many-electron theory, and importance of quadruple excitations for correlation problem. Int. J. Quantum Chem. 1978, 14, 561–581. [Google Scholar] [CrossRef]
- Purvis, G.D., III; Bartlett, R.J. A full coupled-cluster singles and doubles model—The inclusion of disconnected triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Hobza, P. Calculations on Noncovalent Interactions and Databases of Benchmark Interaction Energies. Acc. Chem. Res. 2012, 45, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Narasimhan, B. A comprehensive review on biological activities of oxazole derivatives. BMC Chem. 2019, 13, 16. [Google Scholar] [CrossRef]
- Cao, J.; Xie, Z.-Z.; Yu, X. Excited-state dynamics of oxazole: A combined electronic structure calculations and dynamic simulations study. Chem. Phys. 2016, 474, 25–35. [Google Scholar] [CrossRef]
- Wheeler, D.; Tannir, S.; Smith, E.; Tomlinson, A.; Jeffries-EL, M. A computational and experimental investigation of deep-blue light-emitting tetraaryl-benzobis[1,2-d:4,5-d′]oxazoles. Mater. Adv. 2022, 3, 3842–3852. [Google Scholar] [CrossRef]
- Ivashkin, P.E.; Sukhorukov, A.Y.; Eliseev, O.L.; Lesiv, A.V.; Khomutov, Y.R.; Ioffe, S.L. A Convenient Procedure for the Synthesis of N-Acetyl-5,6-dihydro-2H-1,2-oxazines. Synthesis 2007, 3461–3468. [Google Scholar] [CrossRef]
- Martin, W.R.; Ball, D.W. Small organic fulminates as high energy materials. Fulminates of acetylene, ethylene, and allene. J. Energ. Mater. 2019, 37, 70–79. [Google Scholar] [CrossRef]
- Radchenko, S.I.; Petrov, A.A. Acetylenic ethers and their analogues. Russ. Chem. Rev. 1989, 58, 948–980. [Google Scholar] [CrossRef]
- Nenajdenko, V.G. Isocyanide Chemistry; Wiley-VCH Verlag & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Vignolle, J.; Cattoën, X.; Bourissou, D. Stable Noncyclic Singlet Carbenes. Chem. Rev. 2009, 109, 3333–3384. [Google Scholar] [CrossRef] [PubMed]






| Structure | E (Zpve), a.u. | H, a.u. | G (298), a.u. | ΔG (kcal/mol) | |
|---|---|---|---|---|---|
| 1. 5-membered heterocycles | |||||
 | 000 | −285.229335 | −285.22337 | −285.257581 | 0.00 |
 | 032 | −285.142275 | −285.136263 | −285.170717 | 54.51 |
| 2. 6-membered heterocycles | |||||
 | 100 | −285.186593 | −285.180805 | −285.215020 | 26.71 |
 | 107 | −285.115525 | −285.109668 | −285.143535 | 71.57 |
| 3. 4-membered heterocycles | |||||
 | 200 | −285.182089 | −285.175665 | −285.211225 | 29.09 |
 | 206 | −285.090918 | −285.089974 | −285.124975 | 83.21 |
| 4. Saturated 3-membered cycles | |||||
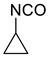 | 300 | −285.199137 | −285.192356 | −285.229628 | 17.54 |
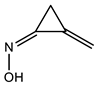 | 310 | −285.079089 | −285.072104 | −285.108475 | 93.57 |
| 5. Unsaturated 3-membered cycles | |||||
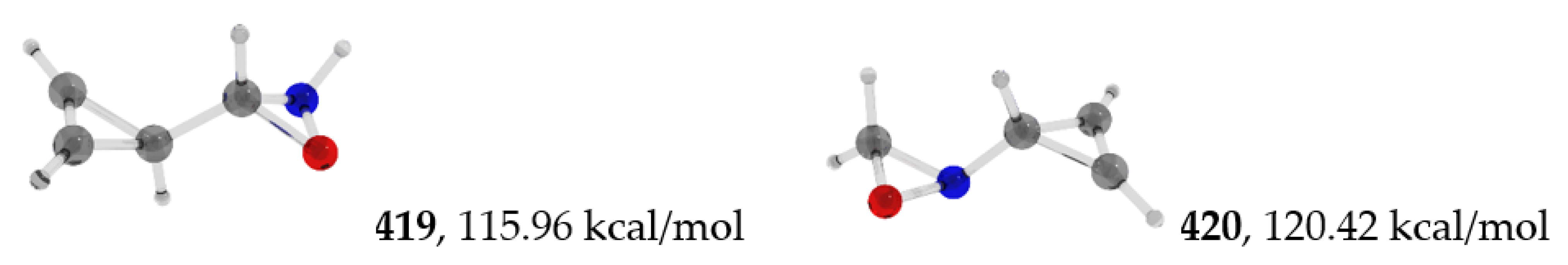
 | 400 | −285.167990 | −285.159793 | −285.202046 | 34.83 |
 | 420 | −285.036752 | −285.030302 | −285.065684 | 120.42 |
| 6. Bicyclic compounds | |||||
 | 500 | −285.149514 | −285.142922 | −285.178297 | 49.73 |
 | 506 | −285.053587 | −285.047092 | −285.082369 | 109.95 |
| 7. Acyclic nitriles | |||||
 | 600 | −285.215399 | −285.208703 | −285.244724 | 8.05 |
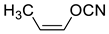 | 617 | −285.161402 | −285.154494 | −285.191744 | 41.29 |
| 8. Conjugated trienes | |||||
 | 700 | −285.177734 | −285.171310 | −285.206924 | 31.77 |
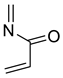 | 704 | −285.162554 | −285.155721 | −285.192477 | 40.83 |
| 9. Acetylenes | |||||
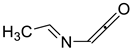 | 800 | −285.170278 | −285.162663 | −285.201201 | 35.36 |
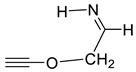 | 802 | −285.101478 | −285.093917 | −285.132200 | 78.66 |
| 10. Allenes | |||||
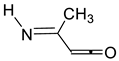 | 900 | −285.191324 | −285.183876 | −285.222050 | 22.28 |
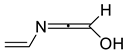 | 906 | −285.128969 | −285.121207 | −285.159660 | 61.43 |
| 11. Acyclic isonitriles | |||||
 | 1000 | −285.177682 | −285.170656 | −285.207548 | 31.38 |
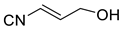 | 1009 | −285.143396 | −285.136367 | −285.173026 | 53.04 |
| 12. Carbenes | |||||
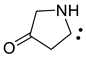 | 1100 | −285.153903 | −285.147722 | −285.182830 | 46.89 |
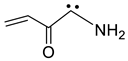 | 1106 | −285.105616 | −285.098696 | −285.135632 | 76.50 |
| 13. Bipolar compounds | |||||
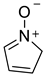 | 1200 | −285.135776 | −285.130117 | −285.163639 | 58.52 |
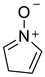 | 1201 | −285.131281 | −285.125617 | −285.159143 | 61.75 |
| 14. Intermolecular associates | |||||
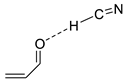 | 1300 | −285.167303 | −285.158443 | −285.200968 | 35.53 |
 | 1301 | −285.164966 | −285.155864 | −285.200403 | 46.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhaylov, O.A.; Gridnev, I.D. Global Search for Stable C4H5NO Compounds—Guinness Molecules and Stability Islands. Molecules 2023, 28, 728. https://doi.org/10.3390/molecules28020728
Mikhaylov OA, Gridnev ID. Global Search for Stable C4H5NO Compounds—Guinness Molecules and Stability Islands. Molecules. 2023; 28(2):728. https://doi.org/10.3390/molecules28020728
Chicago/Turabian StyleMikhaylov, Oleg A., and Ilya D. Gridnev. 2023. "Global Search for Stable C4H5NO Compounds—Guinness Molecules and Stability Islands" Molecules 28, no. 2: 728. https://doi.org/10.3390/molecules28020728
APA StyleMikhaylov, O. A., & Gridnev, I. D. (2023). Global Search for Stable C4H5NO Compounds—Guinness Molecules and Stability Islands. Molecules, 28(2), 728. https://doi.org/10.3390/molecules28020728






