Abstract
Quinolizidine and azaphenalene alkaloids are common in nature and exhibit a pharmaceutical activity, which stirs up increased interest in expanding the range of methods for the synthesis of the corresponding derivatives. In this work, we attempted to adapt our previously presented method for the synthesis of tetrahydropyridines to the preparation of potential precursors for these heterocycles as a separate development of a necessary intermediate stage. To this end, we studied the reactions of β-styrylmalonates with N-protected cross-conjugated azatrienes in the presence of Sn(OTf)2. Moreover, the regioselectivity of the process involving unsymmetrically substituted azatrienes was estimated. The diene character of vinyltetrahydropyridines was studied in detail with the participation of PTAD. Finally, for the Ts-protected highly functionalized vinyltetrahydropyridines synthesized, a detosylation method to give new desired azadiene structures as precursors of the quinolizidine core was suggested.
1. Introduction
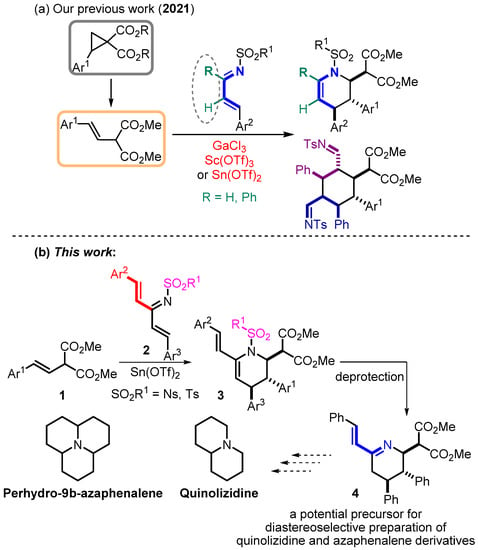
Quinolizidine motifs are widely distributed in natural products. These structures have long been of interest, primarily in the context of pharmaceuticals. Today, they continue to attract increasing attention from researchers [1,2,3,4,5,6], along with their related azaphenalene alkaloids [7,8,9]. Such acute attention prompts a search for new methods for the synthesis of compounds containing these structural cores. One of the possible strategies for synthesizing quinolizidine structures may involve the functionalization of an appropriate nitrogen-containing monocyclic precursor. We have recently presented a useful method for highly diastereoselective construction of 1,2,3,4-tetrahydropyridines from styrylmalonates [10,11,12,13,14] 1 and conformationally non-rigid 1-azadienes that undergo a formal (4+2)-cycloaddition in the presence of Lewis acids [15]. In order to expand the prospects for the synthetic application of the previously discovered reaction and to switch to quinolizidine precursors, modifying the substrate in such a way as to create the possibility of completing a second ring has been suggested.
One of the possible ways to develop this approach involves studying the reaction of cross-conjugated azatrienes 2 as initial substrates (Scheme 1). This method makes it possible to switch to the corresponding vinyltetrahydropyridines 3 containing a diene system, which opens access to further functionalization. Since we are interested in obtaining structures that contain a nodal nitrogen atom, it seems appropriate to “turn” the diene system in its direction by removing the protective group followed by migration of the double bond. It should be noted that such an unusual deprotection seems to be an interesting and promising challenge. It may result in a new class of azadienes 4 that retains the trans,trans-orientation of the substituents in the cyclic moiety. Compounds of this type can further undergo [4+2]-addition reactions, and thus the strategy makes it possible to use the features of the substrate and form stereocenters in the molecule due to cycloaddition processes that compensate for the loss of the leaving group in terms of atom economy.
Scheme 1.
Reactions of styrylmalonates 1 with cross-conjugated azatrienes 2 and further possible transformations of the products.
2. Results and Discussion
The implementation of the idea of synthesizing azadienes 4 is closely related to the choice of a removable protective group. In our previous work, substrates containing sulfonyl protective groups such as tosyl (Ts) and nosyl (Ns) were studied because of the availability of well-known and simple methods for the synthesis of the corresponding starting compounds, the acceptor effect that promotes the reaction, and the prominent stability of these groups. These circumstances prompted us to decide first to study the possibility of removing these protective sulfonyl groups in the corresponding substrates. Since the removal of the tosyl group usually requires rather drastic conditions, such as a strongly acidic medium (HBr/AcOH [16], TfOH [17]) or the presence of powerful reducing agents (Na/Hg [18,19], Na/NH3 [20], Na(Li)/naphthalene [21,22,23,24,25], SmI2 [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43], Mg/MeOH [15,27,44,45,46], etc.), the nosyl group was chosen as the most preferred one. For this purpose, protected azatriene 2a was obtained and then reacted with styrylmalonate in the presence of Sn(OTf)2 as a Lewis acid (Scheme 2). As a result, vinyltetrahydropyridine 3a was synthesized in a good yield, as those obtained earlier in the reaction with azadienes. Further, a number of attempts were made to remove the nosyl group [47,48]; however, due to side processes, the desired product was not identified in the reaction mixture, as well as any other products in preparative amounts. It is most probable that the thiolate anion is capable of reacting with the azadiene fragment of newly formed denosylated substrate 4 with the occurrence of further side processes. Thus, it was shown that nosyl protection was not an optimal choice for the chosen strategy, so we switched to the synthesis of tosyl derivatives.
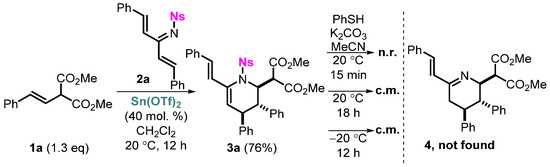
Scheme 2.
Preparation of vinyltetrahydropyridine 3a and attempts to remove the nosyl protection.
To study the scope of the reaction with the participation of sulfonyl-protected azatrienes 2a–c, we tested a number of starting styrylmalonates 1 (Scheme 3). All the corresponding vinyltetrahydropyridines 3 were obtained as single trans,trans-diastereomers. As in the case of azadienes studied in our previous work, the highest yields were achieved for para-methyl, -methoxy, and halo substituents in the aryl moiety of styrylmalonates. A noticeable decrease in yields was demonstrated for electron-withdrawing para-NO2C6H4- and 1-naphtyl-substituted styrylmalonates. Our attempt to involve an analogous bis(4-MeOC6H4)-substituted azatriene (see Supplementary Materials) in the reaction failed due to side reactions of the donor substrate in the presence of Sn(OTf)2, which was confirmed by mixing these substances in the absence of styrylmalonate; only traces of the desired product were detected in the 1H NMR spectrum. It is interesting that compound 3i showed a partial broadening of a set of signals in the NMR spectra; the same behavior was shown and studied in detail earlier in our previous work for a similar product of the reaction between the same ortho-ClC6H4-styrylmalonate and azadiene bearing a Ph group at the C=N double bond (see [15], Supplementary Materials). As in the case described in the previous work, the signals of some aliphatic (e.g., H(2), H(3), H(4) in the tetrahydropyridine ring) and aromatic protons that are spatially proximate to the ortho-ClC6H4 moiety are broadened, and apparently, those closest to the chlorine atom (especially H(2) and H(4)) undergo a downfield shift by up to 0.8 ppm that is quite noticeable in comparison with the chemical shifts of the corresponding signals of the other obtained tetrahydropyridines. Presumably, this effect can be explained by the proximity and direct influence of the chlorine atom.
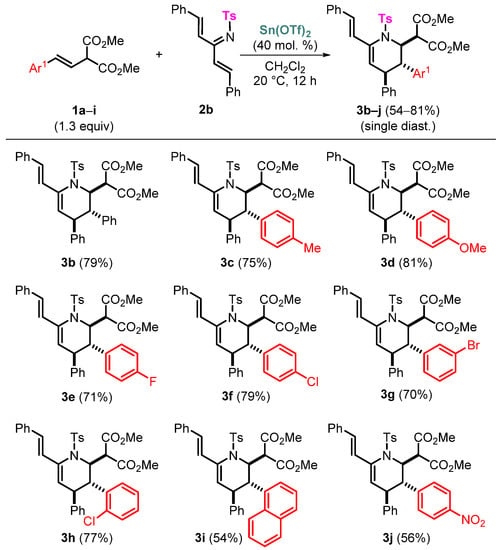
Scheme 3.
Scope of the reaction for vinyltetrahydropyridines 3.
To show the potential applications of our synthetic method, we carried out the reaction between styrylmalonate 1a and azatriene 2b on a gram scale under standard reaction conditions (Scheme 4). As shown by TLC, in this case, the reaction takes longer to achieve full conversion. Fortunately, the formation of the desired vinyltetrahydropyridine 3b occurred with only a slightly lower yield in comparison with the milligram-scale synthesis.
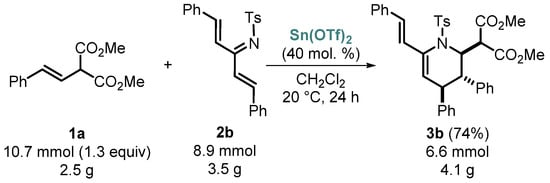
Scheme 4.
Gram-scale synthesis of vinyltetrahydropyridine 3b.
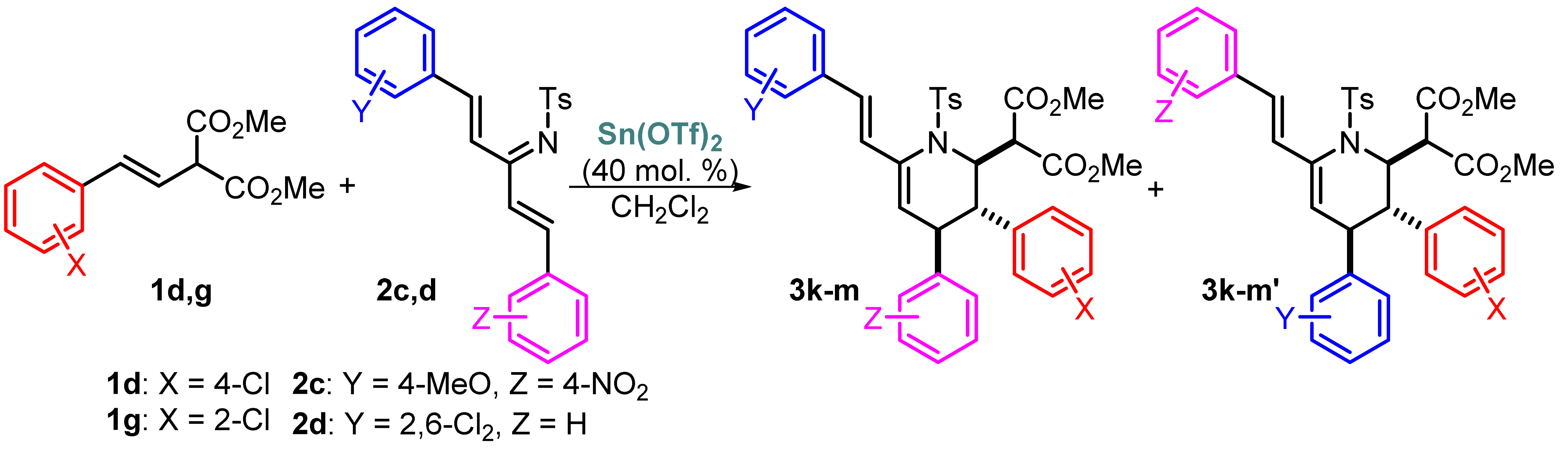
A study of the regioselectivity of the reaction in the presence of unsymmetrically substituted azatrienes is an obvious development of this research. To identify the potential effect of electronic and sterical factors, the corresponding azatrienes 2c,d were synthesized and used in the reaction with styrylmalonate 1d as the model substrate (Table 1). It was shown that the effect of strong electron-donating and electron-withdrawing groups is not sufficient to make the process regioselective (compounds 3k and 3k′), but the situation was slightly better in the case of the sterical impact of the 2,6-Cl2C6H3 moiety. This observation prompted us to increase steric hindrance by using a bulkier ortho-ClC6H4 substituent in the starting styrylmalonate 1g, which resulted in increasing the ratio of regioisomers 3m and 3m′ up to 4/1 at low temperature (entry 6).

Table 1.
The reaction of unsymmetrically substituted azatrienes 2c,d with styrylmalonates 1d,g.
The resulting vinyltetrahydropyridines 3 contain a diene moiety that can be involved in [4+2]-cycloaddition reactions and thereby provide access to the implementation of the diene-transmissive hetero Diels–Alder reaction strategy (DTHDA) [49]. To illustrate this approach, reactions of compounds 3a,b with a commonly used dienophile PTAD were carried out (Scheme 5). The process occurs very quickly with almost quantitative yields to give classical adducts 5a,b. Purification of the latter using column chromatography was found to be difficult due to the occurrence of side reactions on silica gel, the products of which were adsorbed and partially decomposed. However, the purity and yield of the compounds obtained make it possible to do without additional purification. Nevertheless, for one of the adducts, 5a, we attempted to isolate the most stable product of transformation by carrying out the reactions with SiO2 as an acidic reagent (Scheme 5, Table 2), and heterocycle 6 was isolated as the only product of double bond migration. Despite the low yield of this compound, it is of interest because a new asymmetric center is induced at a noticeable distance from the initial ones. In view of this, we tried to optimize the conditions for double bond migration, including the replacement of silica gel with neutral Al2O3, but we failed to achieve an improvement here (Table 2).
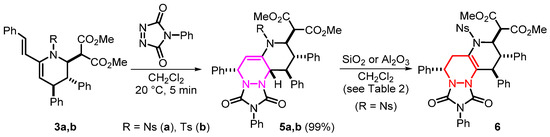
Scheme 5.
The reaction of vinyltetrahydropyridines 3a,b with PTAD to form adducts 5a,b and subsequent formation of isomer 6.

Table 2.
The optimization of conditions for obtaining the product 6.
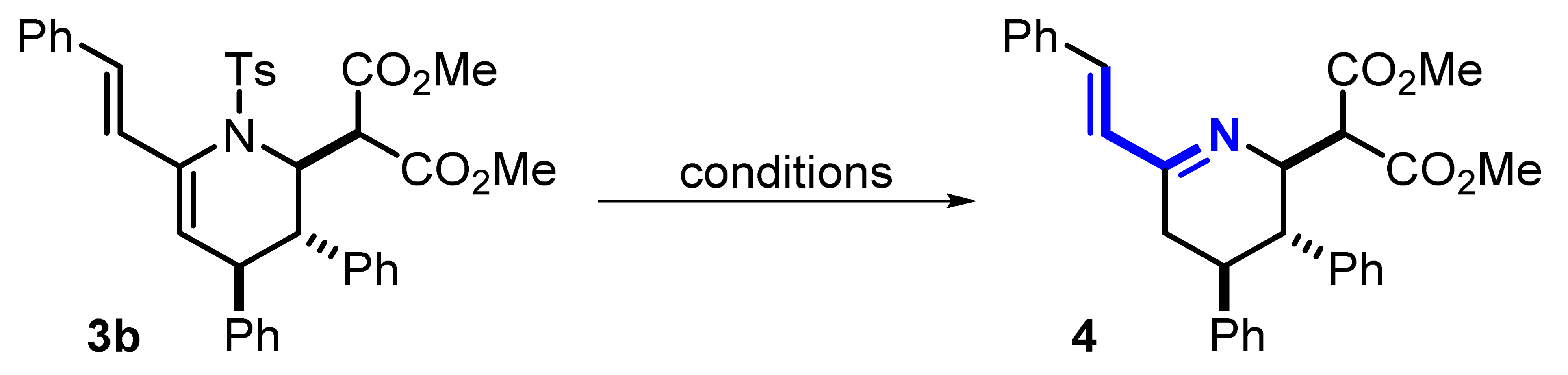
The next stage of the work was the selection of the method for the removal of tosyl protection. Vinyltetrahydropyridine 3b was chosen as a model substrate. It should be noted that the removal of tosyl protection is still an urgent problem of modern organic chemistry. Despite the existence of a set of deprotection methods discussed above, the type of substrate has a very strong influence, which requires careful selection and optimization of conditions in each specific case. We tested a variety of detosylation conditions described in the literature (Table 3). It was shown that the use of SmI2 in combination with miscellaneous additives was not effective (entries 1–4). In the case of using the Mg/MeOH system, it was not possible to stop the reduction at the stage of azadiene 4; the formation of traces of further reduction products were found (entries 5–7). An attempt at detosylation in the presence of TfOH or HBr/AcOH resulted in the formation of a complex mixture of products (entries 8–10). Finally, the formation of the desired azadiene 4 in an acceptable amount was achieved only if sodium naphthalenide was used (entries 11–13). Apparently, the rather low yields of compound 4 are due to the occurrence of side reactions under harsh reducing conditions, which indicates the need for further research to develop a milder approach to the preparation of such compounds. Nevertheless, this result shows that the formation of such complexly functionalized azadienes is possible. This fact opens up the possibility of further study of such substances in reactions with various ene compounds for the formation of the desired quinolizidines.

Table 3.
The optimization of reaction conditions for detosylation of vinyltetrahydropyridine 3b.
3. Materials and Methods
3.1. General Methods
All reagents and solvents used were purchased from Aldrich (St. Louis, MI, USA) or Acros Organics (Geel, Belgium) without additional purification. THF was purified by distillation over Na/benzophenone under an argon atmosphere just prior to use. All operations were performed under an argon atmosphere. Starting styrylmalonates 1 were synthesized from the corresponding D-A cyclopropanes [50,51]. TLC analysis was performed on Silufol chromatographic plates. For preparative chromatography, silica gel 60 (0.040−0.063 mm) was used. 1H, 13C, and 15N NMR spectra were recorded on a Bruker AM-300 (300.1, 75.5, and 30.4 MHz, respectively) spectrometers in CDCl3 or CD2Cl2 containing 0.05% Me4Si as the internal standard. Determinations of structures and stereochemistry of obtained compounds and assignments of 1H and 13C signals were made with the aid of 1D and 2D gradient/non-gradient DEPT-135, COSY, NOESY, HSQC, HMBC, and 1H–15N HMBC spectra. 19F NMR spectra were recorded on a 300 MHz spectrometer (282.4 MHz); standard—CFCl3. High-resolution mass spectra were obtained using simultaneous electrospray ionization (ESI) [52]. The melting points were determined using a Kofler hot-stage microscope.
3.2. Synthetic Procedures
3.2.1. General Synthetic Procedure and Spectroscopic Data for Azatrienes 2
A mixture of corresponding substituted 1,4-pentadien-3-one (10 mmol), Et3N (6.13 mL, 44 mmol), and p-nitrophenylsulfonamide or p-toluenesulfonamide (4.0 or 3.4 g, 20 mmol) in CH2Cl2 (50 mL) was cooled to 0 °C, and titanium tetrachloride (10 mL, 1.0 M solution in CH2Cl2, 10 mmol) was added dropwise. The reaction mixture was warmed to room temperature with stirring for 7 h, after which the reaction was quenched with saturated aqueous NaHCO3, and the mixture was extracted with CH2Cl2 (30 mL × 2). The combined extracts were washed with water and brine, dried over MgSO4, and concentrated in vacuo. Acetone (20 mL) was added to the residue, left for 10 min, and then the precipitate was filtered off, washed with cold acetone, and dried to afford the azatrienes 2.
N-((1E,4E)-1,5-diphenylpenta-1,4-dien-3-ylidene)-4-nitrobenzenesulfonamide (2a). Yellow solid (2.39 g, 57%). M.p. 153–155 °C. 1H NMR (300.1 MHz, CDCl3): δ 7.29–7.77 (m, 14H, Ph, CH=CH), 8.24 (d, J = 9.0 Hz, 2H), 8.38 (d, J = 9.0 Hz, 2H) ppm. 13C NMR (75.5 MHz, CDCl3): δ 122.9 (2=CH), 124.1 (2CH, m-Ns), 128.4 (2CH), 128.7 (4CH), 129.1 (6CH), 131.2 (2=CH), 134.5 (2Cq, Ph), 145.4 (Cq, Ns), 147.5 (Cq, Ns), 173.6 (C=N) ppm. HRMS (ESI) m/z: calcd for C23H19N2O4S+ [M+H]+ 419.1060; found: 419.1053.
N-((1E,4E)-1,5-diphenylpenta-1,4-dien-3-ylidene)-4-methylbenzenesulfonamide (2b). Yellow solid (2.36 g, 61%). M.p. 184–185 °C. 1H NMR (300.1 MHz, CDCl3): δ 2.42 (s, 3H, Ts), 7.24–7.61 (m, 16H, Ar, CH=CH), 7.94 (d, J = 8.2 Hz, Ts) ppm. Spectral data are consistent with literature data [49].
N-((1E,4E)-1-(4-methoxyphenyl)-5-(4-nitrophenyl)penta-1,4-dien-3-ylidene)-4-methylbenzenesulfonamide (2c). Orange solid (2.31 g, 50%). M.p. >250 °C. 1H NMR (300.1 MHz, CDCl3): δ 2.44 (s, 3H, Me), 3.86 (s, 3H, OMe), 6.93 (d, J = 8.7 Hz, 2H, 4-MeOC6H4), 7.20–8.00 (m, 4H, CH=CH), 7.34 (d, J = 8.2 Hz, 2H, Ts), 7.57 (d, J = 8.7 Hz, 2H, 4-MeOC6H4), 7.71 (d, J = 8.6 Hz, 2H, 4-NO2C6H4), 7.92 (d, J = 8.2 Hz, 2H, Ts), 8.24 (d, J = 8.6 Hz, 2H, 4-NO2C6H4) ppm. 13C NMR (75.5 MHz, CDCl3): δ 21.6 (Me), 55.5 (OMe), 114.6 (2CH, 4-MeOC6H4), 124.2 (2CH), 127.1 and 127.2 (2=CH), 127.1 (2CH), 128.8 (2CH), 129.5 (2CH), 130.7 (2CH), 138.8 (Cq), 139.4 (2=CH), 141.1 (Cq), 143.5 (Cq), 145.4 (2Cq), 148.4 (Cq), 162.4 (Cq–OMe), 171.4 (C=N) ppm. HRMS (ESI) m/z: calcd for C25H23N2O5S+ [M+H]+ 463.1322; found: 463.1323.
N-((1E,4E)-1-(2,6-dichlorophenyl)-5-phenylpenta-1,4-dien-3-ylidene)-4-methylbenzenesulfonamide (2d). Yellow solid (2.10 g, 46%). M.p. 167–169 °C. 1H NMR (300.1 MHz, CDCl3): δ 2.43 (s, 3H, Me), 7.00–8.20 (m,4H, CH=CH), 7.17–7.25 (t, J = 8.2 Hz, 1H, 2,6-Cl2C6H3), 7.33 (d, J = 8.2 Hz, Ts), 7.38 (d, J = 8.2 Hz, 2H, 2,6-Cl2C6H3), 7.38–7.46 (m, 3H, Ph), 7.57–7.66 (m, 2H, Ph), 7.94 (d, J = 8.2 Hz, Ts) ppm. 13C NMR (75.5 MHz, CDCl3): δ 21.6 (Me), 124.5 (2=CH), 127.2 (2CH), 128.7 (br.s, 2CH), 128.8 (2CH), 129.0 (2CH), 129.4 (2CH), 130.0 (CH), 131.0 (CH), 132.5 (=CH), 134.6 (Cq), 135.1 (Cq), 137.0 (Cq), 138.7 (=CH), 143.5 (Cq), 145.6 (Cq), 170.8 (C=N) ppm. HRMS (ESI) m/z: calcd for C24H20Cl2NO2S+ [M+H]+ 456.0586; found: 456.0574.
3.2.2. General Synthetic Procedure and Spectroscopic Data for Vinyltetrahydropyridines 3
A solution of styrlmalonate 1a–i (0.33 mmol, 1.3 eq.), azatriene 2a–e (0.25 mmol), and Sn(OTf)2 (40 mol. %, 42 mg) in dry CH2Cl2 (3 mL) was stirred 12 h at room temperature or 5 days at -20 °C for azatrienes 2c,d. Then the reaction mixture without any preliminary work-up was purified by column chromatography on silica gel (petroleum ether–EtOAc 20:1 to 1:1 or benzene–EtOAc, 100:1 to 10:1) to afford title compounds 3a–m,k’–m’.
Dimethyl trans,trans-2-(1-((4-nitrophenyl)sulfonyl)-3,4-diphenyl-6-((E)-styryl)-1,2,3,4-tetrahydropyridin-2-yl)malonate (3a). Yellow solid (124 mg, 76%). Rf = 0.47 (petroleum ether–EtOAc 3:1). M.p. 127–129 °C. 1H NMR (300.1 MHz, CDCl3): δ 3.02 (dd, 3J3,4 = 9.5 Hz, 3J4,5 = 3.8 Hz, 1H, H(4)), 3.38 and 3.43 (both s, 2×3H, 2 CO2Me), 3.51 (dd, 3J3,4 = 9.5 Hz, 3J2,3 = 6.6 Hz, 1H, H(3)), 3.87 (d, 3J2,2′ = 8.1 Hz, 1H, H(2′)), 5.33 (dd, 3J2,2′ = 8.1 Hz, 3J2,3 = 6.6 Hz, 1H, H(2)), 6.31 (d, 3J4,5 = 3.8 Hz, 1H, H(5)), 6.54 (d, 3J1″,2″ = 16.0 Hz, 1H, H(2″)), 6.79–6.85 (m, 2H, 2×H(o″)), 6.84 (d, 3J1″,2″ = 16.0 Hz, 1H, H(1″)), 6.89–6.98 (m, 2H, 2×H(o’)), 7.04–7.15 (m, 6H, 2×H(m’), H(p’), 2×H(m″) and H(p″)), 7.24–7.37 (m, 5H, 2×H(o‴), 2×H(m‴) and H(p‴)), 8.04 (d, J = 8.9 Hz, 2H, 2×H(o)), 8.28 (d, J = 8.9 Hz, 2H, 2×H(m)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 46.3 (CH(4)), 52.38 and 52.41 (2 CO2Me), 55.2 (CH(3)), 56.1 (CH(2′)), 62.3 (CH(2)), 123.8 (2×CH(m)), 125.1 (CH(1″)), 126.6 (2×CH(o‴)), 126.7 (CH(p″)), 127.1 (CH(p’)), 127.9 (2×CH(o″)), 128.23, 128.27, 128.32, 128.36 and 128.36 (CH(5), 2×CH(o’), 2×CH(m’), 2×CH(m″) and CH(p‴)), 128.8 (2×CH(m‴)), 129.6 (2×CH(o)), 131.1 (CH(2″)), 136.0 (C(i‴)), 137.8 (C(6)), 140.2 (C(i’)), 141.1 (C(i″)), 144.7 (C(i)), 150.1 (C(p)), 166.7 and 166.8 (2 COO) ppm. HRMS (ESI) m/z: calcd for C36H33N2O8S+ [M+H]+ 653.1952; found: 653.1951.
Dimethyl trans,trans-2-(3,4-diphenyl-6-((E)-styryl)-1-tosyl-1,2,3,4-tetrahydropyridin-2-yl)malonate (3b). Yellow solid (122 mg, 79%). Rf = 0.46 (petroleum ether–EtOAc 3:1). M.p. 91–92 °C. 1H NMR (300.1 MHz, CDCl3): δ 2.43 (s, 3H, C(p)-Me), 2.53 (dd, 3J3,4 = 11.2 Hz, 3J4,5 = 3.9 Hz, 1H, H(4)), 3.11 and 3.49 (both s, 2×3H, 2 CO2Me), 3.65 (dd, 3J3,4 = 11.2 Hz, 3J2,3 = 8.3 Hz, 1H, H(3)), 4.06 (d, 3J2,2′ = 6.3 Hz, 1H, H(2′)), 5.17 (dd, 3J2,3 = 8.3 Hz, 3J2,2′ = 6.3 Hz, 1H, H(2)), 6.20 (d, 3J4,5 = 3.9 Hz, 1H, H(5)), 6.62–6.71 (m, 2H, 2×H(o″)), 6.75 and 6.78 (two doublets, 3J1″,2″ = 16.3 Hz, 2H, H(1″) and H(2″)), 6.77–6.85 (m, 2H, 2×H(o’)), 6.92–7.07 (m, 6H, 2×H(m’), H(p’), 2×H(m″) and H(p″)), 7.16–7.24 (m, 1H, H(p‴)), 7.25–7.33 (m, 2H, 2×H(m‴)), 7.32–7.42 (m, 2H, 2×H(o‴)), 7.35 (d, J = 8.2 Hz, 2H, 2×H(m)), 7.90 (d, J = 8.2 Hz, 2H, 2×H(o)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 21.3 (C(p)-Me), 46.7 (CH(4)), 51.7 and 52.1 (2 CO2Me), 55.4 (CH(3)), 56.1 (CH(2′)), 61.9 (CH(2)), 125.6 (CH(1″)), 126.2 (CH(p″)), 126.4 (2×CH(o‴)), 126.5 (CH(p’)), 127.6 (CH(p‴)), 127.7, 127.8 and 127.9 (2×CH(m’), 2×CH(o″) and 2×CH(m″)), 128.0 (2×CH(o)), 128.4 (2×CH(o’) and 2×CH(m‴)), 129.3 (2×CH(m)), 129.9 (CH(5)), 130.1 (CH(2″)), 135.3 (C(i)), 136.4 (C(i‴)), 138.1 (C(6)), 139.8 (C(i’)), 141.0 (C(i″)), 144.2 (C(p)), 166.4 and 167.1 (2 COO) ppm. HRMS (ESI) m/z: calcd for C37H36NO6S+ [M+H]+ 622.2258; found: 622.2259.
Dimethyl trans,trans-2-(3-(4-tolyl)-4-phenyl-6-((E)-styryl)-1-tosyl-1,2,3,4-tetrahydropyridin-2-yl)malonate (3c). Yellowish solid (119 mg, 75%). Rf = 0.36 (petroleum ether–EtOAc 3:1). M.p. 42–44 °C. 1H NMR (300.1 MHz, CDCl3): δ 2.17 (s, 3H, C(p’)-Me), 2.49 (s, 3H, C(p)-Me), 2.51 (dd, 3J3,4 = 11.1 Hz, 3J4,5 = 3.9 Hz, 1H, H(4)), 3.19 and 3.54 (both s, 2×3H, 2 CO2Me), 3.59 (dd, 3J3,4 = 11.1 Hz, 3J2,3 = 8.3 Hz, 1H, H(3)), 4.02 (d, 3J2,2′ = 6.2 Hz, 1H, H(2′)), 5.10 (dd, 3J2,3 = 8.3 Hz, 3J2,2′ = 6.3 Hz, 1H, H(2)), 6.17 (d, 3J4,5 = 3.9 Hz, 1H, H(5)), 6.63–6.73 (m, 4H, 2×H(o’) and 2×H(o″)), 6.76 (two doublets, 3J1″,2″ = 16.3 Hz, 2H, H(1″) and H(2″)), 6.81–6.89 (m, 2H, 2×H(m’)), 6.99–7.09 (m, 3H, 2×H(m″) and H(p″)), 7.19–7.25 (m, 1H, H(p‴)), 7.28–7.35 (m, 2H, 2×H(m‴)), 7.35–7.44 (m, 4H, 2×H(m) and 2×H(o‴)), 7.89 (d, J = 8.2 Hz, 2H, 2×H(o)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 20.9 (C(p’)-Me), 21.6 (C(p)-Me), 46.8 (CH(4)), 52.0 and 52.3 (2 CO2Me), 55.1 (CH(3)), 56.3 (CH(2′)), 62.3 (CH(2)), 125.9 (CH(1″)), 126.4 (CH(p″)), 126.7 (2×CH(o‴)), 127.8 (CH(p‴)), 128.1 and 128.5 (2×CH(o’) and 2×CH(o″)), 128.1 (2×CH(m″)), 128.3 (2×CH(o)), 128.6 (2×CH(m‴)), 128.7 (2×CH(m’)), 129.5 (2×CH(m)), 129.8 (CH(5)), 130.4 (CH(2″)), 135.7 (C(p)), 136.2 (C(p’)), 136.8 and 137.0 (C(i’) and C(i‴)), 138.3 (C(6)), 141.5 (C(i″)), 144.3 (C(i)), 166.8 and 167.5 (2 COO) ppm. HRMS (ESI) m/z: calcd for C38H37NNaO6S+ [M+Na]+ 658.2234; found: 658.2228.
Dimethyl trans,trans-2-(3-(4-methoxyphenyl)-4-phenyl-6-((E)-styryl)-1-tosyl-1,2,3,4-tetrahydropyridin-2-yl)malonate (3d). Beige solid (132 mg, 81%). Rf = 0.35 (petroleum ether–EtOAc 3:1). M.p. 65–67 °C. 1H NMR (300.1 MHz, CDCl3): δ 2.49 (s, 3H, C(p)-Me), 2.49 (dd, 3J3,4 = 11.2 Hz, 3J4,5 = 3.8 Hz, 1H, H(4)), 3.22 and 3.54 (both s, 2×3H, 2 CO2Me), 3.57 (dd, 3J3,4 = 11.2 Hz, 3J2,3 = 8.0 Hz, 1H, H(3)), 3.68 (s, 3H, OMe), 4.02 (d, 3J2,2′ = 6.3 Hz, 1H, H(2′)), 5.09 (dd, 3J2,3 = 8.0 Hz, 3J2,2′ = 6.3 Hz, 1H, H(2)), 6.18 (d, 3J4,5 = 3.8 Hz, 1H, H(5)), 6.58 (d, J = 8.6 Hz, 2H, 2×H(m’)), 6.64–6.75 (m, 4H, 2×H(o’) and 2×H(o″)), 6.72 and 6.77 (two doublets, 3J1″,2″ = 16.0 Hz, 2H, H(1″) and H(2″)), 6.98–7.12 (m, 3H, 2×H(m″) and H(p″)), 7.21–7.43 (m, 7H, 2×H(o‴), 2×H(m‴), H(p‴) and 2×H(m)), 7.90 (d, J = 8.3 Hz, 2H, 2×H(o)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 21.7 (C(p)-Me), 47.0 (CH(4)), 52.1 and 52.4 (2 CO2Me), 54.8 (CH(3)), 55.1 (OMe), 56.3 (CH(2′)), 62.3 (CH(2)), 113.4 (2×CH(m’)), 125.9 (CH(1″)), 126.4 (CH(p″)), 126.7 (2×CH(o‴)), 127.8 (CH(p‴)), 128.1 (2×CH(o″)), 128.2 (2×CH(m″)), 128.3 (2×CH(o)), 128.6 (2×CH(m‴)), 130.0 (CH(5)), 129.5 (2×CH(m)), 129.6 (2×CH(o’)), 130.4 (CH(2″)), 132.1 (C(i’), 135.7 (C(p)), 136.8 (C(i‴)), 138.3 (C(6)), 141.5 (C(i″)), 144.4 (C(i)), 158.2 (C(p’)), 166.8 and 167.5 (2 COO) ppm. HRMS (ESI) m/z: calcd for C38H38NO7S+ [M+H]+ 652.2363; found: 652.2350.
Dimethyl trans,trans-2-(3-(4-fluorophenyl)-4-phenyl-6-((E)-styryl)-1-tosyl-1,2,3,4-tetrahydropyridin-2-yl)malonate (3e). Beige solid (113 mg, 71%). Rf = 0.55 (petroleum ether–EtOAc 3:1). M.p. 58–60 °C. 1H NMR (300.1 MHz, CDCl3): δ 2.48 (s, 3H, C(p)-Me), 2.59 (dd, 3J3,4 = 11.1 Hz, 3J4,5 = 3.9 Hz, 1H, H(4)), 3.23 and 3.52 (both s, 2×3H, 2 CO2Me), 3.61 (dd, 3J3,4 = 11.1 Hz, 3J2,3 = 7.9 Hz, 1H, H(3)), 4.00 (d, 3J2,2′ = 6.6 Hz, 1H, H(2′)), 5.13 (dd, 3J2,3 = 7.9 Hz, 3J2,2′ = 6.6 Hz, 1H, H(2)), 6.21 (d, 3J4,5 = 3.9 Hz, 1H, H(5)), 6.65–6.73 (m, 2H, 2×H(o″)), 6.73 and 6.76 (two doublets, 3J1″,2″ = 16.0 Hz, 2H, H(1″) and H(2″)), 6.70–6.81 (m, 2H, 2×H(m’)), 6.74–6.85 (m, 2H, 2×H(o’)), 7.01–7.11 (m, 3H, 2×H(m″) and H(p″)), 7.21–7.28 (m, 1H, H(p‴)), 7.28–7.42 (m, 6H, 2×H(o‴), 2×H(m‴) and 2×H(m)), 7.89 (d, J = 8.3 Hz, 2H, 2×H(o)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 21.6 (C(p)-Me), 47.1 (CH(4)), 52.1 and 52.4 (2 CO2Me), 54.9 (CH(3)), 56.4 (CH(2′)), 62.1 (CH(2)), 114.9 (d, 2JC,F = 21.3 Hz, 2×CH(m’)), 125.7 (CH(1″)), 126.6 (CH(p″)), 126.7 (2×CH(o‴)), 127.9 (CH(p‴)), 128.0 (2×CH(o″)), 128.29 and 128.32 (2×CH(o) and 2×CH(m″)), 128.6 (2×CH(m‴)), 129.8 (CH(5)), 129.6 (2×CH(m)), 130.1 (d, 3JC,F = 7.9 Hz, 2×CH(o’)), 130.6 (CH(2″)), 135.8 (C(p)), 136.1 (d, 4JC,F = 3.3 Hz, C(i’)), 136.6 (C(i‴)), 138.5 (C(6)), 141.1 (C(i″)), 144.5 (C(i)), 161.5 (d, 1JC,F = 21.3 Hz, C(p’)), 166.7 and 167.4 (2 COO) ppm. 19F NMR (282.5 MHz, CDCl3): δ -115.4 (tt, 3JH,F = 8.4 Hz, 4JH,F = 5.2 Hz) ppm. HRMS (ESI) m/z: calcd for C37H34FNNaO6S+ [M+Na]+ 662.1983; found: 662.1973.
Dimethyl trans,trans-2-(3-(4-chlorophenyl)-4-phenyl-6-((E)-styryl)-1-tosyl-1,2,3,4-tetrahydropyridin-2-yl)malonate (3f). Yellowish solid (129 mg, 79%). Rf = 0.57 (petroleum ether–EtOAc 3:1). M.p. 82–83 °C. 1H NMR (300.1 MHz, CDCl3): δ 2.48 (s, 3H, C(p)-Me), 2.62 (dd, 3J3,4 = 10.8 Hz, 3J4,5 = 3.8 Hz, 1H, H(4)), 3.25 and 3.51 (both s, 2×3H, 2 CO2Me), 3.62 (dd, 3J3,4 = 10.8 Hz, 3J2,3 = 7.8 Hz, 1H, H(3)), 3.99 (d, 3J2,2′ = 6.7 Hz, 1H, H(2′)), 5.12 (dd, 3J2,3 = 7.8 Hz, 3J2,2′ = 6.7 Hz, 1H, H(2)), 6.19 (d, 3J4,5 = 3.9 Hz, 1H, H(5)), 6.67–6.75 (m, 2H, 2×H(o″)), 6.70 and 6.77 (two doublets, 3J1″,2″ = 16.0 Hz, 2H, H(1″) and H(2″)), 6.74–6.82 (m, 2H, 2×H(o’)), 6.99–7.14 (m, 5H, 2×H(m’), 2×H(m″) and H(p″)), 7.21–7.28 (m, 1H, H(p‴)), 7.28–7.42 (m, 6H, 2×H(o‴), 2×H(m‴) and 2×H(m)), 7.86 (d, J = 8.3 Hz, 2H, 2×H(o)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 21.6 (C(p)-Me), 46.7 (CH(4)), 52.1 and 52.4 (2 CO2Me), 54.8 (CH(3)), 56.3 (CH(2′)), 61.9 (CH(2)), 125.6 (CH(1″)), 126.6 (CH(p″)), 126.7 (2×CH(o‴)), 127.9 (CH(p‴)), 127.9 (2×CH(o″)), 128.2 (2×CH(o)), 128.2 and 128.3 (2×CH(m’) and 2×CH(m″)), 128.6 (2×CH(m‴)), 129.2 (CH(5)), 129.5 (2×CH(m)), 129.9 (2×CH(o’)), 130.6 (CH(2″)), 132.5 (C(p’)), 135.8 (C(p)), 136.6 (C(i‴)), 138.5 (C(6)), 138.9 (C(i’), 141.0 (C(i″)), 144.5 (C(i)), 166.6 and 167.3 (2 COO) ppm. HRMS (ESI) m/z: calcd for C37H35ClNO6S+ [M+H]+ 656.1868; found: 656.1879.
Dimethyl trans,trans-2-(3-(3-bromophenyl)-4-phenyl-6-((E)-styryl)-1-tosyl-1,2,3,4-tetrahydropyridin-2-yl)malonate (3g). Yellowish solid (122 mg, 70%). Rf = 0.43 (petroleum ether–EtOAc 3:1). M.p. 45–46 °C. 1H NMR (300.1 MHz, CDCl3): δ 2.47 (dd, 3J3,4 = 11.2 Hz, 3J4,5 = 3.9 Hz, 1H, H(4)), 2.51 (s, 3H, C(p)-Me), 3.21 and 3.54 (both s, 2×3H, 2 CO2Me), 3.65 (dd, 3J3,4 = 11.1 Hz, 3J2,3 = 8.1 Hz, 1H, H(3)), 4.07 (d, 3J2,2′ = 6.2 Hz, 1H, H(2′)), 5.04 (dd, 3J2,3 = 8.1 Hz, 3J2,2′ = 6.3 Hz, 1H, H(2)), 6.18 (d, 3J4,5 = 3.9 Hz, 1H, H(5)), 6.64–6.69 (m, 2H, 2×H(o’)), 6.69 (ddd, 3J6′″,5′″ = 7.8, 4J6′″,2′″ = 1.8, 4J6′″,4′″ = 1.1 Hz, 1H, H(6′″)), 6.75 and 6.79 (two doublets, 3J1″,2″ = 16.0 Hz, 2H, H(1″) and H(2″)), 6.88 (t, 3J5′″,4′″ = 3J5′″,6′″ = 7.8 Hz, 1H, H(5′″)), 6.93 (t, 4J2′″,4′″ = 4J2′″,6′″ = 1.8, 1H, H(2′″)), 7.00–7.11 (m, 3H, 2×H(m’) and H(p’)), 7.14 (ddd, 3J4′″,5′″ = 7.8, 4J4′″,2′″ = 1.8, 4J4′″,6′″ = 1.1 Hz, 1H, H(4′″)), 7.20–7.28 (m, 1H, H(p″)), 7.28–7.36 (m, 2H, 2×H(m″)), 7.37–7.47 (m, 4H, 2×H(o″) and 2×H(m)), 7.91 (d, J = 8.3 Hz, 2H, 2×H(o)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 21.7 (C(p)-Me), 46.8 (CH(4)), 52.1 and 52.5 (2 CO2Me), 55.0 (CH(3)), 56.3 (CH(2′)), 61.9 (CH(2)), 122.1 (C(3′″)), 125.8 (CH(1″)), 126.7 (CH(p’)), 126.8 (2×CH(o’)), 127.8 and 127.9 (CH(6′″) and CH(p″)), 128.0 (2×CH(o″)), 128.2 (2×CH(o)), 128.4 (2×CH(m’)), 128.6 (2×CH(m″)), 129.2 (CH(5)), 129.7 (CH(5′″)), 129.9 (2×CH(m)), 129.9 (CH(4′″)), 130.7 (CH(2″)), 131.0 (CH(2′″)), 135.6 (C(p)), 136.7 (C(i″)), 138.6 (C(6)), 140.9 (C(i’)), 142.8 (C(1′″)), 144.7 (C(i)), 166.6 and 167.4 (2 COO) ppm. HRMS (ESI) m/z: calcd for C37H35BrNO6S+ [M+H]+ 700.1363; found: 700.1355.
Dimethyl trans,trans-2-(3-(2-chlorophenyl)-4-phenyl-6-((E)-styryl)-1-tosyl-1,2,3,4-tetrahydropyridin-2-yl)malonate (3h). Beige solid (126 mg, 77%). Rf = 0.40 (petroleum ether–EtOAc 3:1). M.p. 87–88 °C. 1H NMR (300.1 MHz, CDCl3): δ 2.46 (s, 3H, C(p)-Me), 2.67 (br.s, 1H, H(4)), 3.37 (br.s, 3H, C(3′)O2Me), 3.61 (s, 3H, C(1′)O2Me), 3.94 (br.s, 1H, H(2′)), 4.23 (dd, 3J3,4 = 10.9 Hz, 3J2,3 = 8.5 Hz, 1H, H(3)), 5.15 (br.s, 1H, H(2)), 6.27 (d, 3J4,5 = 3.9 Hz, 1H, H(5)), 6.70–6.79 (m, 4H, 2×H(o’), H(1″) and H(2″)), 6.91–7.10 (m, 5H, H(p’), H(3″), H(4″) and 2×H(m’)), 7.12–7.43 (m, 8H, H(5″), H(p″), 2×H(m″), 2×H(o″) and 2×H(m)), 7.90 (d, J = 8.3 Hz, 2H, 2×H(o)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 21.7 (C(p)-Me), 47.4 (br.s, CH(4)), 51.0 (br.s, CH(3)), 52.2 (C(3′)O2Me), 52.6 (C(1′)O2Me), 56.5 (CH(2′)), 62.5 (CH(2)), 125.7 (CH(1″)), 126.7 (CH(p’)), 126.8 (2×CH(o″)), 127.0 (br.s, CH(5′″)), 127.9 (CH(p″)), 128.0 (2×CH(m’), CH(4′″) and CH(6′″)), 128.3 (2×CH(o’)), 128.5 (2×CH(o)), 128.6 (2×CH(m″)), 129.3 (br.s, CH(3′″)), 129.6 (2×CH(m)), 129.9 (CH(5)), 130.7 (CH(2″)), 134.5 (br.s, C(2′″)), 135.9 (C(p)), 136.7 (C(i″)), 138.4 (br.s, C(1′″)), 138.8 (br.s, C(6)), 140.1 (br.s, C(i’)), 144.5 (C(i)), 166.7 and 167.2 (2 COO) ppm. HRMS (ESI) m/z: calcd for C37H35ClNO6S+ [M+H]+ 656.1868; found: 656.1853.
Dimethyl trans,trans-2-(3-(naphthalen-1-yl)-4-phenyl-6-((E)-styryl)-1-tosyl-1,2,3,4-tetrahydropyridin-2-yl)malonate (3i). Yellowish solid (90 mg, 54%). Rf = 0.52 (petroleum ether–EtOAc 3:1). M.p. 51–53 °C. 1H NMR (300.1 MHz, CDCl3): δ 2.48 (s, 3H, C(p)-Me), 2.88 and 3.42 (both s, 2×3H, 2 CO2Me), 2.91 (dd, 3J3,4 = 9.7 Hz, 3J4,5 = 3.9 Hz, 1H, H(4)), 4.06 (d, 3J2,2′ = 6.5 Hz, 1H, H(2′)), 4.78 (dd, 3J3,4 = 9.7 Hz, 3J2,3 = 7.5 Hz, 1H, H(3)), 5.31 (dd, 3J2,3 = 7.5 Hz, 3J2,2′ = 6.5 Hz, 1H, H(2)), 6.29 (d, 3J4,5 = 3.9 Hz, 1H, H(5)), 6.74–6.80 (m, 2H, 2×H(o’)), 6.84 and 6.88 (two doublets, 3J1″,2″ = 16.0 Hz, 2H, H(1″) and H(2″)), 6.83–6.94 (m, 3H, H(p’) and 2×H(m’)), 7.20–7.39 (m, 9H, 2×H(m), 2×H(m″), H(p″), H(2′″), H(3′″), H(6′″), H(7′″)), 7.40–7.50 (m, 2H, 2×H(o″)), 7.57 (d, 3J4′″,3′″ = 7.6 Hz, 1H, H(4′″)), 7.60–7.66 (m, 1H, H(5′″)), 7.80 (d, J = 8.3 Hz, 2H, 2×H(o)), 7.89–7.97 (m, 1H, H(8′″)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 21.7 (C(p)-Me), 47.3 (CH(3) and CH(4)), 51.9 and 52.4 (2 CO2Me), 56.0 (CH(2′)), 63.3 (CH(2)), 123.3 (CH(8′″)), 125.1 and 125.2 (CH(2′″) and CH(3′″)), 125.3 (CH(6′″)), 125.8 (CH(7′″)), 126.0 (CH(1″)), 126.3 (CH(p’)), 126.8 (2×CH(o″)), 127.4 (CH(4′″)), 127.5 (CH(5)), 127.9 (CH(p″)), 127.9 (2×CH(m’)), 128.0 (2×CH(o’)), 128.2 (CH(5′″)), 128.3 (2×CH(o)), 128.7 (2×CH(m″)), 129.6 (2×CH(m)), 130.6 (CH(2″)), 132.0 (C(8′″a)), 133.3 (C(4′″a)), 136.1 (C(p)), 136.8 (C(i″)), 137.8 (C(1′″)), 138.5 (C(6)), 141.3 (C(i’)), 144.3 (C(i)), 166.6 and 167.9 (2 COO) ppm. HRMS (ESI) m/z: calcd for C41H38NO6S+ [M+H]+ 672.2414; found: 672.2399.
Dimethyl trans,trans-2-(3-(4-nitrophenyl)-4-phenyl-6-((E)-styryl)-1-tosyl-1,2,3,4-tetrahydropyridin-2-yl)malonate (3j). Orange solid (93 mg, 56%). Rf = 0.36 (petroleum ether–EtOAc 3:1). M.p. 39–40 °C. 1H NMR (300.1 MHz, CDCl3): δ 2.48 (s, 3H, C(p)-Me), 2.84 (dd, 3J3,4 = 10.7 Hz, 3J4,5 = 3.8 Hz, 1H, H(4)), 3.28 and 3.50 (both s, 2×3H, 2 CO2Me), 3.72 (dd, 3J3,4 = 10.7 Hz, 3J2,3 = 7.3 Hz, 1H, H(3)), 3.96 (d, 3J2,2′ = 7.1 Hz, 1H, H(2′)), 5.25 (dd, 3J2,2′ = 7.1 Hz, 3J2,3 = 7.3 Hz, 1H, H(2)), 6.25 (d, 3J4,5 = 3.9 Hz, 1H, H(5)), 6.64 (d, 3J1″,2″ = 16.0 Hz, 1H, H(2″)), 6.70–6.79 (m, 2H, 2×H(o″)), 6.76 (d, 3J1″,2″ = 16.0 Hz, 1H, H(1″)), 7.05–7.14 (m, 3H, 2×H(m″) and H(p″)), 7.07 (d, J = 8.7 Hz, 2H, 2×H(o’)), 7.23–7.29 (m, 1H, H(p‴)), 7.30–7.36 (m, 4H, 2×H(o‴), 2×H(m‴)), 7.37 (d, J = 8.2 Hz, 2H, 2×H(m)), 7.87 (d, J = 8.2 Hz, 2H, 2×H(o)), 7.94 (d, J = 8.7 Hz, 2H, 2×H(m’)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 21.6 (C(p)-Me), 46.9 (CH(4)), 52.2 and 52.5 (2 CO2Me), 55.7 (CH(3)), 56.5 (CH(2′)), 61.5 (CH(2)), 123.2 (2×CH(m’)), 125.2 (CH(1″)), 126.7 (2×CH(o‴)), 127.0 (CH(p″)), 127.8 (2×CH(o″)), 128.1 (CH(p‴)), 128.3 (2×CH(o)), 128.5 and 128.6 (2×CH(m″) and 2×CH(m‴)), 128.9 (CH(5)), 129.4 (2×CH(o’)), 129.6 (2×CH(m)), 131.0 (CH(2″)), 136.0 (C(p)), 136.3 (C(i‴)), 138.8 (C(6)), 140.5 (C(i″)), 144.7 (C(i)), 146.6 (C(p’)), 148.4 (C(i’)), 166.5 and 167.1 (2 COO) ppm. HRMS (ESI) m/z: calcd for C37H34N2NaO8S+ [M+Na]+ 689.1928; found: 689.1918.
Dimethyl trans,trans-2-(3-(4-chlorophenyl)-4-(4-nitrophenyl)-6-((E)-4-methoxystyryl)-1-tosyl-1,2,3,4-tetrahydropyridin-2-yl)malonate (3k) and dimethyl trans,trans-2-(3-(4-chlorophenyl)-4-(4-methoxyphenyl)-6-((E)-4-nitrostyryl)-1-tosyl-1,2,3,4-tetrahydropyridin-2-yl)malonate (3k’). Orange thick oil (131 mg, 72%, rr 1/1 at rt, 12 h; 111 mg, 61%, rr 1/1 at -20 °C, 5 d). Rf = 0.34 (petroleum ether–EtOAc 3:1). HRMS (ESI) m/z: calcd for C38H36ClN2O9S+ [M+H]+ 731.1825; found: 731.1819. 3k: 1H NMR (300.1 MHz, CDCl3): δ 2.50 (s, 3H, C(p)-Me), 2.87 (dd, 3J3,4 = 11.2 Hz, 3J4,5 = 3.9 Hz, 1H, H(4)), 3.23 and 3.51 (both s, 2×3H, 2 CO2Me), 3.67 (dd, 3J3,4 = 11.2 Hz, 3J2,3 = 7.7 Hz, 1H, H(3)), 3.83 (s, 3H, OMe), 3.94 (d, 3J2,2′ = 6.6 Hz, 1H, H(2′)), 5.17 (dd, 3J2,3 = 7.7 Hz, 3J2,2′ = 6.6 Hz, 1H, H(2)), 6.07 (d, 3J4,5 = 3.9 Hz, 1H, H(5)), 6.59 (two doublets, 3J1″,2″ = 16.0 Hz, 2H, H(1″) and H(2″)), 6.80 (d, J = 8.6 Hz, 2H, 2×H(o’)), 6.86 (d, J = 8.9 Hz, 2H, 2×H(m‴)), 6.91 (d, J = 8.8 Hz, 2H, 2×H(o″)), 7.07 (d, J = 8.6 Hz, 2H, 2×H(m’)), 7.28 (d, J = 8.9 Hz, 2H, 2×H(o‴)), 7.40 (d, J = 8.3 Hz, 2H, 2×H(m)), 7.89 (d, J = 8.3 Hz, 2H, 2×H(o)), 7.96 (d, J = 8.8 Hz, 2H, 2×H(m″)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 21.68 (C(p)-Me), 47.1 (CH(4)), 52.19 and 52.53 (2 CO2Me), 55.0 (CH(3)), 55.3 (OMe), 56.3 (CH(2′)), 61.9 (CH(2)), 114.2 (2×CH(m‴)), 122.9 (CH(1″)), 123.6 (2×CH(m″)), 126.5 (CH(5)), 128.08 (2×CH(o‴)), 128.4 and 128.6 (2×CH(o) and 2×CH(m’)), 129.0 (2×CH(o″)), 129.6 and 129.7 (2×CH(o’) and 2×CH(m)), 131.0 (CH(2″)), 132.7 (C(i‴)), 133.1 (C(p’)), 135.9 (C(p)), 138.2 (C(i’)), 139.6 (C(6)), 144.7 (C(i)), 146.8 (C(p″)), 148.9 (C(i″)), 159.8 (C(p‴)), 166.52 and 167.3 (2 COO) ppm. 3k’: 1H NMR (300.1 MHz, CDCl3): δ 2.40 (dd, 3J3,4 = 11.4 Hz, 3J4,5 = 3.9 Hz, 1H, H(4)), 2.52 (s, 3H, C(p)-Me), 3.22 and 3.56 (both s, 2×3H, 2 CO2Me), 3.61 (dd, 3J3,4 = 11.4 Hz, 3J2,3 = 8.4 Hz, 1H, H(3)), 3.69 (s, 3H, OMe), 4.03 (d, 3J2,2′ = 5.9 Hz, 1H, H(2′)), 4.98 (dd, 3J2,3 = 8.4 Hz, 3J2,2′ = 5.9 Hz, 1H, H(2)), 6.27 (d, 3J4,5 = 3.9 Hz, 1H, H(5)), 6.54 (d, J = 8.9 Hz, 2H, 2×H(o″)), 6.61 (d, J = 8.9 Hz, 2H, 2×H(m″)), 6.69 (d, J = 8.3 Hz, 2H, 2×H(o’)), 6.84 and 6.93 (two doublets, 3J1″,2″ = 16.0 Hz, 2H, H(1″) and H(2″)), 7.04 (d, J = 8.3 Hz, 2H, 2×H(m’)), 7.43 (d, J = 8.3 Hz, 2H, 2×H(m)), 7.54 (d, J = 8.8 Hz, 2H, 2×H(o‴)), 7.88 (d, J = 8.3 Hz, 2H, 2×H(o)), 8.20 (d, J = 8.8 Hz, 2H, (2×H(m‴))) ppm. 13C NMR (75.5 MHz, CDCl3): δ 21.70 (C(p)-Me), 46.1 (CH(4)), 52.16 and 52.46 (2 CO2Me), 54.7 (CH(3)), 55.2 (OMe), 56.0 (CH(2′)), 62.0 (CH(2)), 113.8 (2×CH(m″)), 124.1 (2×CH(m‴)), 127.1 (2×CH(o‴)), 128.1 (CH(1″)), 128.2 (2×CH(o)), 128.3 (2×CH(m’)), 128.8 (2×CH(o″)), 129.8 (2×CH(m)), 129.9 (2×CH(o’)), 130.36 (CH(2″)), 132.5 (C(i″)), 132.5 (C(p’), 132.8 (CH(5)), 135.3 (C(p)), 138.0 (C(6)), 138.6 (C(i’), 143.3 (C(i‴)), 144.9 (C(i)), 147.0 (C(p‴)), 158.3 (C(p″)), 166.53 and 167.5 (2 COO) ppm.
Dimethyl trans,trans-2-(3-(4-chlorophenyl)-4-phenyl-6-((E)-2,6-dichlorostyryl)-1-tosyl-1,2,3,4-tetrahydropyridin-2-yl)malonate (3l) and dimethyl trans,trans-2-(3-(4-chlorophenyl)-4-(2,6-dichlorophenyl)-6-((E)-styryl)-1-tosyl-1,2,3,4-tetrahydropyridin-2-yl)malonate (3l’). Yellowish thick oil (94 mg, 52%, rr 1/1 at rt, 12 h; 101 mg, 56%, rr 1.2/1 at -20 °C, 5 d). Rf = 0.53 (petroleum ether–EtOAc 3:1). HRMS (ESI) m/z: calcd for C37H33Cl3NO6S+ [M+H]+ 724.1089; found: 724.1086. 3l: 1H NMR (300.1 MHz, CDCl3): δ 2.48 (s, 3H, C(p)-Me), 2.58 (dd, 3J3,4 = 10.3 Hz, 3J4,5 = 3.8 Hz, 1H, H(4)), 3.30 and 3.58 (both s, 2×3H, 2 CO2Me), 3.64 (dd, 3J3,4 = 10.3 Hz, 3J2,3 = 7.9 Hz, 1H, H(3)), 3.98 (d, 3J2,2′ = 6.6 Hz, 1H, H(2′)), 5.07 (dd, 3J2,3 = 7.9 Hz, 3J2,2′ = 6.6 Hz, 1H, H(2)), 6.24 (d, 3J4,5 = 3.8 Hz, 1H, H(5)), 6.68–6.75 (m, 2H, 2×H(o″)), 6.77 (d, J = 8.5 Hz, 2H, 2×H(o’)), 6.92 and 7.00 (two doublets, 3J1″,2″ = 16.0 Hz, 2H, H(1″) and H(2″)), 7.02 (d, J = 8.5 Hz, 2H, 2×H(m’)), 7.05–7.12 (m, 4H, 2×H(m″), H(p″) and H(p‴)), 7.34 (d, J = 8.1 Hz, 2H, 2×H(m‴)), 7.37 (d, J = 8.3 Hz, 2H, 2×H(m)), 7.88 (d, J = 8.3 Hz, 2H, 2×H(o)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 21.7 (C(p)-Me), 46.4 (CH(4)), 52.2 and 52.4 (2 CO2Me), 54.6 (CH(3)), 56.1 (CH(2′)), 61.8 (CH(2)), 124.1 (CH(1″)), 126.7 (CH(p″)), 127.9 (2×CH(o″)), 128.1 (2×CH(o)), 128.2 (CH(p‴)), 128.3 and 128.4 (2×CH(m’) and 2×CH(m″)), 128.7 (2×CH(m‴)), 129.1 (CH(5)), 129.6 (2×CH(m)), 129.8 (2×CH(o’)), 132.7 (C(p’)), 133.7 (C(i‴)), 134.4 (CH(2″)), 134.7 (2×CCl(o‴)), 135.5 (C(p)), 138.3 (C(6)), 138.9 (C(i’), 141.0 (C(i″)), 144.4 (C(i)), 166.7 and 167.4 (2 COO) ppm. 3l’: 1H NMR (300.1 MHz, CDCl3): δ 2.44 (s, 3H, C(p)-Me), 3.21 and 3.60 (both s, 2×3H, 2 CO2Me), 3.73 (dd, 3J3,4 = 11.6 Hz, 3J4,5 = 4.1 Hz, 1H, H(4)), 4.17 (d, 3J2,2′ = 6.3 Hz, 1H, H(2′)), 4.56 (dd, 3J3,4 = 11.6 Hz, 3J2,3 = 8.7 Hz, 1H, H(3)), 5.15 (dd, 3J2,3 = 8.7 Hz, 3J2,2′ = 6.3 Hz, 1H, H(2)), 6.24 (d, 3J4,5 = 4.1 Hz, 1H, H(5)), 6.73 (two doublets, 3J1″,2″ = 16.0 Hz, 2H, H(1″) and H(2″)), 6.90–6.95 (m, 2H, 2×H(m″)), 6.93–7.00 (m, 2H, 2×H(o’)), 7.02–7.08 (m, 2H, 2×H(m’)), 7.14–7.20 (m, 1H, H(p″)), 7.24–7.43 (m, 5H, 2×H(o‴), 2×H(m‴) and H(p‴)), 7.40 (d, J = 8.3 Hz, 2H, 2×H(m)), 7.93 (d, J = 8.3 Hz, 2H, 2×H(o)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 21.6 (C(p)-Me), 42.9 (CH(4)), 49.8 (CH(3)), 52.0 and 52.5 (2 CO2Me), 56.7 (CH(2′)), 62.5 (CH(2)), 125.8 (CH(1″)), 126.8 (2×CH(o‴)), 127.3 (CH(5)), 127.8 (CH(p‴)), 128.05 (2×CH(o)), 128.05 (2×CH(m’)), 128.14 and 128.7 (2×CH(m″)), 128.6 (2×CH(m‴)), 129.90 (2×CH(m)), 129.92 (CH(p″)), 129.95 (2×CH(o’)), 130.1 (CH(2″)), 133.0 (C(p’)), 134.55 and 134.68 (2×CCl(o″)), 135.6 (C(p)), 136.0 (C(i″)), 136.7 (C(i‴)), 137.7 (C(6)), 137.8 (C(i’), 144.5 (C(i)), 166.7 and 167.2 (2 COO) ppm.
Dimethyl trans,trans-2-(3-(2-chlorophenyl)-4-phenyl-6-((E)-2,6-dichlorostyryl)-1-tosyl-1,2,3,4-tetrahydropyridin-2-yl)malonate (3m) and dimethyl trans,trans-2-(3-(2-chlorophenyl)-4-(2,6-dichlorophenyl)-6-((E)-styryl)-1-tosyl-1,2,3,4-tetrahydropyridin-2-yl)malonate (3m’). Yellowish thick oil (134 mg, 74%, rr 2/1 at rt; 98 mg, rr 4/1 54% at -20 °C). Rf = 0.33 (petroleum ether–EtOAc 3:1). HRMS (ESI) m/z: calcd for C37H33Cl3NO6S+ [M+H]+ 724.1089; found: 724.1085. Major isomer (3m): 1H NMR (300.1 MHz, CDCl3): δ 2.48 (s, 3H, C(p)-Me), 2.50 (br.s, 1H, H(4)), 3.44 (br.s, 3H, C(3′)O2Me), 3.69 (s, 3H, C(1′)O2Me), 3.89 (br.s, 1H, H(2′)), 4.25 (br.s, 1H, H(3)), 5.02 (br.s, 1H, H(2)), 6.28 (d, 3J4,5 = 3.8 Hz, 1H, H(5)), 6.68–6.77 (m, 2H, 2×H(o’)), 6.99 (two doublets, 3J1″,2″ = 16.0 Hz, 2H, H(1″) and H(2″)), 6.92–7.36 (m, 10H, 2×H(m’), H(p’), 2×H(m″), H(p″), H(3′″), H(4′″), H(5′″), H(6′″)), 7.40 (d, J = 8.3 Hz, 2H, 2×H(m)), 7.95 (d, J = 8.3 Hz, 2H, 2×H(o)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 21.7 (C(p)-Me), 47.1 (br.s, CH(4)), 52.3 (C(3′)O2Me), 52.6 (C(1′)O2Me), 56.1 (CH(3)), 56.1 (CH(2′)), 62.6 (CH(2)), 124.4 (CH(1″)), 126.7 (CH(p’)), 124.36 (CH(1″)), 126.7 (C(p’)), 127.0 and 127.1 (CH(4′″) and CH(5′″)), 128.0 (2×CH(m’)), 128.1 (CH(p″)), 128.20 (2×CH(o’)), 128.37 (2×CH(o)), 128.71 (2×CH(m″)), 128.82 (CH(6′″)), 129.42 (CH(3′″)), 129.55 (2×CH(m)), 130.5 (CH(5)), 133.9 (C(i″)), 134.2 (CH(2″)), 134.6 (C(2′″)–Cl) , 134.7 (C(o″)–Cl), 135.3 (C(p)), 138.7 (C(6) and C(1′″)), 140.1 (C(i’)), 144.5 (C(i)), 166.7 (C(3′)O2Me) and 167.3 (br.s, C(1′)O2Me) ppm. Minor isomer (3m’): 1H NMR (300.1 MHz, CDCl3): δ 2.39 (s, 3H, C(p)-Me), 3.57 and 3.61 (both s, 2×3H, 2 CO2Me), 3.96 (d, 3J2,2′ = 8.2 Hz, 1H, H(2′)), 4.04 (dd, 3J3,4 = 11.4 Hz, 3J4,5 = 3.8 Hz, 1H, H(4)), 4.63 (dd, 3J3,4 = 11.4 Hz, 3J2,3 = 6.2 Hz, 1H, H(3)), 5.38 (dd, 3J2,3 = 8.2 Hz, 3J2,2′ = 6.2 Hz, 1H, H(2)), 6.37 (d, 3J4,5 = 3.8 Hz, 1H, H(5)), 6.59 and 6.67 (two doublets, 3J1″,2″ = 16.0 Hz, 2H, H(1″) and H(2″)), 6.92–7.36 (m, 14H, 2×H(m’), H(p’), 2×H(o″), 2×H(m″), H(p″), H(3′″), H(4′″), H(5′″), H(6′″) and 2×H(m)), 7.92 (m, 2H, 2×H(o)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 21.6 (C(p)-Me), 43.3 (CH(4)), 48.6 (CH(3)), 52.5 and 52.7 (2 CO2Me), 58.1 (CH(2′)), 63.3 (CH(2)), 125.5 (CH(1″)), 126.7 (2×CH(o″)), 127.8 (CH(5′″)), 127.9 (CH(p″)), 128.1 (CH(5)), 128.16 (CH(p’)), 128.17 (CH(4′″)), 128.44 (2×CH(o)), 128.56 (2×CH(m’)), 128.78 (2×CH(m″)), 129.42 (CH(3′″)), 129.6 (2×CH(m)), 130.3 (CH(2″)), 130.5 (CH(6′″)), 134.1 (C(2′″)–Cl), 134.2 (C(i’)), 136.3 (C(p)), 136.7 (2×CCl(o’) and C(i″)), 137.5 (C(6)), 138.5 (C(1′″)), 144.4 (C(i)), 166.6 and 166.8 (2 COO) ppm.
3.2.3. General Synthetic Procedure and Spectroscopic Data for Heterocycles 5
To a solution of vinyltetrahydropyridine 3 (0.046 mmol) in CH2Cl2 (1 mL) PTAD (8 mg, 0.046 mmol, 1.0 equiv) was added. After 15 min, the final solution was removed under vacuum to afford corresponding heterocycles 5a,b.
Dimethyl 2-((5SR,8RS,9SR,10SR,10aSR)-7-((4-nitrophenyl)sulfonyl)-1,3-dioxo-2,5,9,10-tetraphenyl-2,3,5,7,8,9,10,10a-octahydro-1H-pyrido[3,2-c][1,2,4]triazolo[1,2-a]pyridazin-8-yl)malonate (5a). Yellow solid (38 mg, 99%). Rf = 0.34 (petroleum ether–EtOAc 3:1). M.p. >250 °C. 1H NMR (300.1 MHz, CDCl3): δ 3.66 and 3.67 (both s, 2×3H, 2 CO2Me), 3.68 (dd, 3J10,10a = 11.3 Hz, 3J9,10 = 9.1 Hz, 1H, H(10)), 3.91 (dd, 3J9,10 = 9.1 Hz, 3J8,9 = 5.7 Hz, H(9)), 4.42 (d, 3J8,2′ = 9.1 Hz, 1H, H(2′)), 5.12 (dd, 3J8,2′ = 9.1 Hz, 3J8,9 = 5.7 Hz, H(8)), 5.31 (t, 3J5,6 = 5J5,10a = 2.1 Hz, H(5)), 5.38 (ddd, 3J10,10a = 11.3 Hz, 5J5,10a = 2.1 Hz, 4J6,10a = 1.2 Hz, 1H, H(10a)), 5.80 (dd, 3J5,6 = 2.1 Hz, 4J6,10a = 1.2 Hz, 1H, H(6)), 7.02–7.13 (m, 7H, 2×H(oA), 2×H(oD), 2×H(mD) and H(pD)), 7.14–7.22 (m, 5H, 2×H(oE), 2×H(mE) and H(pE)), 7.22–7.35 (m, 3H, 2×H(mA) and H(pA)), 7.41–7.49 (m, 5H, 2×H(oB), 2×H(mB) and H(pB)), 7.72 (d, J = 9.0 Hz, 2H, 2×H(oC)), 8.04 (d, J = 9.0 Hz, 2H, 2×H(mC)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 51.2 (CH(9)), 53.14 and 53.17 (2 CO2Me), 53.9 (CH(10)), 55.0 (CH(10a)), 57.0 (CH(2′)), 61.9 (CH(5)), 66.2 (CH(8)), 116.6 (CH(6)), 124.1 (2×CH(mC)), 125.3 (2×CH(oA)), 127.1 (2×CH(oB)), 127.6 (CH(pD)), 128.1 (CH(pE)), 128.2 (CH(pA)), 128.60 and 128.62 (2×CH(oE) and 2×CH(mE)), 128.7 and 128.80 (2×CH(oD) and 2×CH(mD)), 128.84 (2×CH(mA)), 128.9 (CH(pB)), 129.1 (2×CH(mB)), 129.2 (2×CH(oC)), 130.8 (C(iA)), 134.1 (C(6a)), 137.3 (C(iE)), 138.1 (C(iB)), 138.9 (C(iD)), 143.9 (C(iC)), 148.5 and 155.2 (C(1) and C(3)), 150.3 (C(pC)), 166.6 and 167.5 (2 COO) ppm. HRMS (ESI) m/z: calcd for C44H38N5O10S+ [M+H]+ 828.2334; found: 828.2321.
Dimethyl 2-((5SR,8RS,9SR,10SR,10aSR)-1,3-dioxo-2,5,9,10-tetraphenyl-7-tosyl-2,3,5,7,8,9,10,10a-octahydro-1H-pyrido[3,2-c][1,2,4]triazolo[1,2-a]pyridazin-8-yl)malonate (5b). Yellowish solid (37 mg, 99%). Rf = 0.35 (petroleum ether–EtOAc 3:1). M.p. 181–183 °C. 1H NMR (300.1 MHz, CDCl3): δ 2.45 (s, 3H, C(p’)-Me), 3.58 and 3.59 (both s, 2×3H, 2 CO2Me), 3.63 (dd, 3J10,10a = 11.2, 3J9,10 = 9.8 Hz, 1H, H(10)), `4.08 (dd, 3J9,10 = 9.8 Hz, 3J8,9 = 7.2 Hz, 1H, H(9)), 4.51 (d, 3J8,2′ = 8.9 Hz, 1H, H(2′)), 5.03 (dd, 3J8,2′ = 8.9 Hz, 3J8,9 = 7.2 Hz, 1H, H(8)), 5.33 (m, 1H, H(5)), 5.40 (d, 3J10,10a = 11.2 Hz, 1H, H(10a)), 5.89 (m, 1H, H(6)), 7.01–7.15 (m, 7H, 2×H(oA), 2×H(oD), 2×H(mD) and H(pD)), 7.15–7.25 (m, 7H, 2×H(mC), 2×H(oE), 2×H(mE) and H(pE)), 7.25–7.37 (m, 3H, 2×H(mA) and H(pA)), 7.45–7.55 (m, 5H, 2×H(oB), 2×H(mB) and H(pB)), 7.61 (d, J = 8.2 Hz, 2H, 2×H(oC)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 21.8 (C(p’)-Me), 50.9 (CH(9)), 53.13 and 53.15 (2 CO2Me), 55.3 (CH(10a)), 55.6 (CH(10)), 56.5 (CH(2′)), 61.9 (CH(5)), 66.2 (CH(8)), 117.8 (CH(6)), 125.4 (2×CH(oA)), 127.3 (CH(pD)), 127.4 (2×CH(oB)), 128.0 (CH(pE)), 128.07 (2×CH(oC)), 128.11 (CH(pA)), 128.4, 128.6, 128.83, 128.85 and 128.9 (2×CH(oD), 2×CH(mD), 2×CH(oE), 2×CH(mE) and CH(pB)), 128.9 (2×CH(mA)), 129.2 (2×CH(mB)), 129.8 (2×CH(mC)), 130.7 (C(iA)), 135.1 (C(6a)), 135.6 (C(pC)), 137.4 (C(iE)), 138.1 (C(iB)), 138.7 (C(iD)), 144.8 (C(iC)), 148.5 and 155.3 (C(1) and C(3)), 167.1 and 167.7 (2 COO) ppm. 15N NMR (30.4 MHz, CDCl3; reconstructed from 1H–15N HMBC): δ 120.3 (s, N(7)), 126.5 (s, N(4)), 128.9 (s, N(11)), 147.9 (s, N(2)) ppm. HRMS (ESI) m/z: calcd for C45H41N4O8S+ [M+H]+ 797.2640; found: 797.2640.
3.2.4. General Synthetic Procedure and Spectroscopic Data for Heterocycle 6
To a solution of heterocycle 5a (0.046 mmol, 38 mg) in CH2Cl2 (1 mL) at −20 °C SiO2 (50 mg) was added. After 12 h, the final solution was evaporated and purified using column chromatography on silica gel (petroleum ether–EtOAc 10:1 to 1:1) to afford title compound 6.
Dimethyl 2-((5SR,8RS,9SR,10SR)-7-((4-nitrophenyl)sulfonyl)-1,3-dioxo-2,5,9,10-tetraphenyl-2,3,5,6,7,8,9,10-octahydro-1H-pyrido[3,2-c][1,2,4]triazolo[1,2-a]pyridazin-8-yl)malonate (6). Yellow solid (10 mg, 26%). Rf = 0.69 (petroleum ether–EtOAc 1:1). M.p. >250 °C. 1H NMR (300.1 MHz, CDCl3): δ 3.38 and 3.78 (both s, 2×3H, 2 CO2Me), 3.46 (ddd, 2J6′,6″ = 17.9 Hz, 3J5,6′ = 2.0 Hz, J = 1.8 Hz, 1H, H(6′)), 3.70 (ddd, 2J6′,6″ = 17.9 Hz, 3J5,6″ = 7.4 Hz, J = 2.0 1H, H(6″)), 4.08 (br.s, 1H, H(9)), 4.15 (d, 3J8,2′ = 11.4 Hz, 1H, H(2′)), 5.38 (br.s, 1H, H(10)), 5.55 (dd, 3J8,2′ = 11.4 Hz, 3J8,9 = 1.7 Hz, 1H, H(8)), 5.65 (dd, 3J5,6″ = 7.4 Hz, 3J5,6′ = 2.0 Hz, 1H, H(5)), 6.88–6.96 (m, 2H, 2×CH(oA)), 7.00 (d, J = 8.8 Hz, 2H, 2×H(oC)), 7.23–7.51 (m, 18H, Ar), 7.85 (d, J = 8.8 Hz, 2H, 2×H(mC)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 32.6 (CH2(6)), 38.2 (CH(10)), 45.9 (CH(9)), 52.6 and 53.5 (2 CO2Me), 53.3 (CH(2′)), 56.2 (CH(5)), 61.2 (CH(8)), 119.9 (C(10a)), 120.1 (C(6a)), 124.2 (2×CH(mC)), 125.9 (2×CH(oA)), 127.1 (2×CH(oB) and CH(pD)), 128.0 (2×CH(oD) and CH(pA)), 128.7 (2×CH(oC)), 129.0 (2×CH(mA) and 2×CH(oE)), 129.2 (2×CH(mB), 2×CH(mD) and 2×CH(mE)), 129.20 (CH(pB)), 129.6 (CH(pE)), 130.0 (C(iA)), 136.5 (C(iB)), 141.8 (C(iD)), 142.3 (C(iE)), 145.1 (C(iC)), 146.5 and 152.3 (C(1) and C(3)), 149.3 (C(pC)), 167.1 and 167.3 (2 COO) ppm. 15N NMR (30.4 MHz, CDCl3; reconstructed from 1H–15N HMBC): δ 116.9 (s, N(7)), 132 and 132.5 (two singlets, N(4) and N(11)), 150.2 (s, N(2)), 365.5 (s, NO2) ppm. HRMS (ESI) m/z: calcd for C44H38N5O10S+ [M+H]+ 828.2334; found: 828.2317.
3.2.5. General Synthetic Procedure and Spectroscopic Data for Azadiene 4
To a solution of 3b (80 mg, 12.9 µmol) in THF (5 mL) at −78 °C was added dropwise (during 15–20 min) a freshly prepared ~0.35 M solution of sodium naphthalenide in THF (2.5 mL, 6 equiv) and the reaction mixture stirred 15 min. The reaction was then quenched by the addition of saturated ammonium chloride (2 mL) and warmed to room temperature. The whole was poured onto saturated ammonium chloride (20 mL) and extracted with ethyl acetate (3×20 mL). The combined organic extracts were dried over Na2SO4, filtered, and concentrated in vacuo. The residual naphthalene was then removed by filtration through a short pad of deactivated silica gel using petroleum ether—EtOAc (1:1) as the eluent. The resulting solution was evaporated, and the residue was purified using column chromatography on deactivated silica gel (benzene–EtOAc 50:1 to 10:1) to afford the desired azadiene 4 as a beige solid. A stock solution of sodium naphthalenide in THF was prepared by dissolving naphthalene (980 mg, 7.65 mmol) in freshly dried THF (20 mL) under an argon atmosphere, to which sodium metal (160 mg, 6.96 mmol) was added in small pieces with vigorous stirring for 2 h until the mixture had formed a homogeneous dark green solution.
Dimethyl trans,trans-2-(3,4-diphenyl-6-((E)-styryl)-2,3,4,5-tetrahydropyridin-2-yl)malonate (4). Beige solid (24 mg, 40%). Rf = 0.31 (petroleum ether–EtOAc 3:1). M.p. 62–63 °C. 1H NMR (300.1 MHz, CDCl3): δ 2.70 (m, 1H, H(5′)), 3.07 (m, 1H, H(5″)), 3.23 (m, 2H, H(3) and H(4)), 3.60 (m, 1H, H(2′)), 3.63 (two s, 2×3H, 2 CO2Me), 4.71 (m, 1H, H(2)), 6.90 (d, 3J1″,2″ = 16.3 Hz, 1H, H(1″)), 6.97 (d, 3J1″,2″ = 16.3 Hz, 1H, H(2″)), 6.96–7.21 (m, 10H, 2×H(o), 2×H(m), H(p), 2×H(o’), 2×H(m’) and H(p’)), 7.21–7.40 (m, 3H, 2×H(m″) and H(p″)), 7.42–7.50 (m, 2H, 2×H(o″)) ppm. 13C NMR (75.5 MHz, CDCl3): δ 35.2 (CH2(5)), 44.5 (CH(4)), 48.7 (CH(3)), 52.1 and 52.3 (2 CO2Me), 55.2 (CH(2′)), 66.2 (CH(2)), 126.4 and 126.7 (CH(p) and CH(p’)), 127.2 (2×CH(o″)), 127.6 and 128.3 (2×CH(o), 2×CH(o’), 2×CH(m) and 2×CH(m’)), 128.4 (CH(p″)), 128.7 (CH(m″)), 131.0 (CH(1″)), 135.0 (CH(2″)), 136.1 (C(i″)), 140.1 and 142.8 (C(i) and C(i’)), 166.3 (C(6)), 168.4 and 169.1 (2 COO) ppm. 1H NMR (300.1 MHz, CD2Cl2): δ 2.70 (ddd, 2J5′,5″ = 17.9 Hz, 3J4,5′ = 11.6 Hz, 5J2,5′ = 3.4 Hz, 1H, H(5′)), 3.11 (ddd, 2J5′,5″ = 17.9 Hz, 3J4,5″ = 5.5 Hz, 5J2,5″ = 1.6 Hz, 1H, H(5″)), 3.20 (dd, 3J3,4 = 11.6 Hz, 3J2,3 = 10.3 Hz, 1H, H(3)), 3.35 (td, 3J3,4 = 3J4,5′ = 11.6 Hz, 3J4,5″ = 5.5 Hz, 1H, H(4)), 3.54 (d, 3J2,2′ = 4.7 Hz, H(2′)), 3.63 and 3.72 (two s, 2×3H, 2 CO2Me), 4.61–4.73 (m, 1H, H(2)), 6.91 (d, 3J1″,2″ = 16.8 Hz, 1H, H(1″)), 7.08 (d, 3J1″,2″ = 16.8 Hz, 1H, H(2″)), 7.08–7.26 (m, 10H, 2×H(o), 2×H(m), H(p), 2×H(o’), 2×H(m’) and H(p’)), 7.32–7.45 (m, 3H, 2×H(m″) and H(p″)), 7.51–7.59 (m, 2H, 2×H(o″)) ppm. 13C NMR (75.5 MHz, CD2Cl2): δ 35.7 (CH2(5)), 44.3 (CH(4)), 48.7 (CH(3)), 51.9 and 52.2 (2 CO2Me), 55.3 (CH(2′)), 66.5 (CH(2)), 126.4 and 126.7 (CH(p) and CH(p’)), 127.2 (2×CH(o″)), 127.8 and 128.3 (2×CH(o), 2×CH(o’), 2×CH(m) and 2×CH(m’)), 128.5 (CH(p″)), 128.7 (CH(m″)), 130.6 (CH(1″)), 135.1 (CH(2″)), 136.1 (C(i″)), 140.4 and 143.2 (C(i) and C(i’)), 165.9 (C(6)), 168.3 and 169.0 (2 COO) ppm. HRMS (ESI) m/z: calcd for C30H30NO4+ [M+H]+ 468.2169; found: 468.2164.
3.2.6. Gram-Scale Synthetic Procedure for Vinyltetrahydropyridine 3b
A solution of styrlmalonate 1a (10.7 mmol, 1.3 eq., 2.5 g), azatriene 2b (8.9 mmol, 3.5 g), and Sn(OTf)2 (40 mol. %, 1.5 g) in dry CH2Cl2 (100 mL) was stirred 24 h at room temperature. Then the reaction mixture was filtrated through a short pad of silica gel, evaporated, and purified by column chromatography on silica gel (petroleum ether–EtOAc 15:1 to 3:1) to afford title compound 3b; yield 4.1 g (74%).
4. Conclusions
In conclusion, the Lewis acid-catalyzed reactions of formal (4+2)-cycloaddition between styrylmalonates and N-sulfonyl-protected cross-conjugated azatrienes have been studied. The reaction occurs in good yields and makes it possible to obtain the corresponding vinyltetrahydropyridines as single trans,trans-diastereomers. The regioselectivity of the reaction was studied in the case of unsymmetrically substituted azatrienes, where the maximum ratio of regioisomers reached 4/1 at best. The resulting vinyltetrahydropyridines were tested as dienes in the Diels–Alder reaction with PTAD, which is an implementation of the DTHDA concept. Finally, the optimal detosylation conditions were found to obtain a new type of highly functionalized azadienes from the vinyltetrahydropyridines discussed above. In the future, we plan to study related new precursors in order to develop an approach to the diastereoselective synthesis of polycyclic saturated nitrogen-containing heterocycles, in particular, quinolizidines and azaphenalenes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28010088/s1, copies of NMR spectra.
Author Contributions
Conceptualization, P.G.S.; methodology, P.G.S.; investigation, P.G.S.; writing—original draft preparation, P.G.S.; writing—review and editing, R.A.N. and Y.V.T.; supervision, R.A.N. and Y.V.T.; project administration, R.A.N. and Y.V.T.; funding acquisition, R.A.N. and Y.V.T. All authors have read and agreed to the published version of the manuscript.
Funding
The main part of the research (after April 2022), including asymmetric synthesis and biological studies, was funded by a grant from the Russian Science Foundation (RSF grant no. 22-13-00418). The initial part of the research (before May 2022) was funded by the completed grant of the Russian Science Foundation (RSF grant no. 19-73-10210).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data obtained are contained in this article.
Acknowledgments
High-resolution mass spectra were recorded in the Department of Structural Studies of N. D. Zelinsky Institute of Organic Chemistry RAS, Moscow.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Karunakaran, T.; Ngew, K.Z.; Zailan, A.A.D.; Mian, J.V.Y.; Abu Bakar, M.H. The chemical and pharmacological properties of mitragynine and its diastereomers: An insight review. Front. Pharmacol. 2022, 13, 805986–805997. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-H.; Yu, Z.-P.; Capon, R.J.; Zhang, H. Natural enantiomers: Occurrence, biogenesis and biological properties. Molecules 2022, 27, 1279–1352. [Google Scholar] [CrossRef] [PubMed]
- Althagbi, H.I.; Alarif, W.M.; Al-Footy, K.O.; Abdel-Lateff, A. Marine-derived macrocyclic alkaloids (MDMAs): Chemical and biological diversity. Mar. Drugs 2020, 18, 368–402. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.P. The Alkaloids: Chemistry and Biology, 1st ed.; Knölker, H.-J., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 75, pp. 1–498. [Google Scholar]
- Li, Y.; Wang, G.; Liu, J.; Ouyang, L. Quinolizidine alkaloids derivatives from Sophora alopecuroides Linn: Bioactivities, structure-activity relationships and preliminary molecular mechanisms. Eur. J. Med. Chem. 2020, 188, 111972–111997. [Google Scholar] [CrossRef] [PubMed]
- Toya, H.; Satoh, T.; Okano, K.; Takasu, K.; Ihara, M.; Takahashi, A.; Tanaka, H.; Tokuyama, H. Stereocontrolled total synthesis and biological evaluation of (−)- and (+)-petrosin and its derivatives. Tetrahedron 2014, 70, 8129–8141. [Google Scholar] [CrossRef]
- Guerola, M.; Sánchez-Roselló, M.; Mulet, C.; del Pozo, C.; Fustero, S. Asymmetric intramolecular aza-Michael reaction in desymmetrization processes. Total synthesis of hippodamine and epi-hippodamine. Org. Lett. 2015, 17, 960–963. [Google Scholar] [CrossRef] [PubMed]
- Alujas-Burgos, S.; Oliveras-González, C.; Álvarez-Larena, Á.; Bayón, P.; Figueredo, M. Iterative synthetic strategy for azaphenalene alkaloids. Total synthesis of (−)-9a epi-hippocasine. J. Org. Chem. 2018, 83, 5052–5057. [Google Scholar] [CrossRef]
- Alujas-Burgos, S.; Bayón, P.; Figueredo, M. Recent advances in the synthesis of azaphenalene alkaloids: First enantioselective approaches. Org. Biomol. Chem. 2018, 16, 8218–8229. [Google Scholar] [CrossRef]
- Borisov, D.D.; Novikov, R.A.; Tomilov, Y.V. GaCl3-Mediated reactions of donor–acceptor cyclopropanes with aromatic aldehydes. Angew. Chem. Int. Ed. 2016, 55, 12233–12237. [Google Scholar] [CrossRef]
- Borisov, D.D.; Novikov, R.A.; Eltysheva, A.S.; Tkachev, Y.V.; Tomilov, Y.V. Styrylmalonates as an alternative to donor–acceptor cyclopropanes in the reactions with aldehydes: A route to 5,6-dihydropyran-2-ones. Org. Lett. 2017, 19, 3731–3734. [Google Scholar] [CrossRef]
- Novikov, R.A.; Levina, A.A.; Borisov, D.D.; Volodin, A.D.; Korlyukov, A.A.; Tkachev, Y.V.; Platonova, Y.B.; Tomilova, L.G.; Tomilov, Y.V. Synthesis of the cationic gallium phthalocyanines and their catalytic application in gallium (III)-activated processes for donor–acceptor substrates. Organometallics 2020, 39, 2580–2593. [Google Scholar] [CrossRef]
- Borisov, D.D.; Novikov, R.A.; Tomilov, Y.V. Reactions of styrylmalonates with aromatic aldehydes: Detailed synthetic and mechanistic studies. J. Org. Chem. 2021, 86, 4457–4471. [Google Scholar] [CrossRef] [PubMed]
- Borisov, D.D.; Chermashentsev, G.R.; Novikov, R.A.; Tomilov, Y.V. Coupling of styrylmalonates with furan and benzofuran carbaldehydes: Synthesis and chemistry of substituted (4-oxocyclopent-2-enyl) malonates. J. Org. Chem. 2021, 86, 8489–8499. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, P.G.; Novikov, R.A.; Tomilov, Y.V. Lewis acid-catalyzed formal (4 + 2)- and (2 + 2 + 2)-cycloaddition between 1-azadienes and styrylmalonates as analogues of donor-acceptor cyclopropanes. Adv. Synth. Catal. 2021, 363, 5292–5299. [Google Scholar] [CrossRef]
- Snyder, H.R.; Heckert, R.E. A method for the rapid cleavage of sulfonamides. J. Am. Chem. Soc. 1952, 74, 2006–2009. [Google Scholar] [CrossRef]
- Javorskis, T.; Orentas, E. Chemoselective deprotection of sulfonamides under acidic conditions: Scope, sulfonyl group migration, and synthetic applications. J. Org. Chem. 2017, 82, 13423–13439. [Google Scholar] [CrossRef]
- Birkinshaw, T.N.; Holmes, A.B. Synthesis of (±)-isoprosopinines A and B. Tetrahedron Lett. 1987, 28, 813–816. [Google Scholar] [CrossRef]
- Tanner, D.; Ming, H.H.; Bergdahl, M. Stereocontrolled synthesis of the spirocyclic alkaloid (±)-nitramine. Tetrahedron Lett. 1988, 29, 6493–6495. [Google Scholar] [CrossRef]
- Roemmele, R.C.; Rapoport, H. Removal of N-arylsulfonyl groups from hydroxy α-amino acids. J. Org. Chem. 1988, 53, 2367–2371. [Google Scholar] [CrossRef]
- Ji, S.; Gortler, L.B.; Waring, A.; Battisti, A.J.; Bank, S.; Closson, W.D.; Wriede, P.A. Cleavage of sulfonamides with sodium naphthalene. J. Am. Chem. Soc. 1967, 89, 5311–5312. [Google Scholar] [CrossRef]
- Alonso, E.; Ramon, D.J.; Yus, M. Reductive deprotection of allyl, benzyl and sulfonyl substituted alcohols, amines and amides using a naphthalene-catalysed lithiation. Tetrahedron 1997, 53, 14355–14368. [Google Scholar] [CrossRef]
- Teng, M.; Zi, W.; Ma, D. Total synthesis of the monoterpenoid indole alkaloid (±)-aspidophylline A. Angew. Chem. Int. Ed. 2014, 53, 1814–1817. [Google Scholar] [CrossRef] [PubMed]
- Blair, L.M.; Sperry, J. Total syntheses of (±)-spiroindimicins B and C enabled by a late-stage Schöllkopf–Magnus–Barton–Zard (SMBZ) reaction. Chem. Commun. 2016, 52, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Wua, P.; Zhoub, Q.; Liua, X.-Y.; Xuea, F.; Qin, Y. Synthetic studies towards (–)-deserpidine: Total synthesis of the stereoisomer and derivative of (–)-deserpidine. Chin. Chem. Lett. 2021, 32, 401–404. [Google Scholar] [CrossRef]
- Vedejs, E.; Lin, S. Deprotection of arenesulfonamides with samarium iodide. J. Org. Chem. 1994, 59, 1602–1603. [Google Scholar] [CrossRef]
- Alonso, D.A.; Andersson, P.G. Deprotection of sulfonyl aziridines. J. Org. Chem. 1998, 63, 9455–9461. [Google Scholar] [CrossRef]
- Hayashi, T.; Kawai, M.; Tokunaga, N. Asymmetric synthesis of diarylmethyl amines by rhodium-catalyzed asymmetric addition of aryl titanium reagents to imines. Angew. Chem. Int. Ed. 2004, 43, 6125–6128. [Google Scholar] [CrossRef]
- Kuriyama, M.; Soeta, T.; Hao, X.; Chen, Q.; Tomioka, K. N-Boc-L-valine-connected amidomonophosphane rhodium (I) catalyst for asymmetric arylation of N-tosylarylimines with arylboroxines. J. Am. Chem. Soc. 2004, 126, 8128–8129. [Google Scholar] [CrossRef]
- Grach, G.; Santos, J.S.-d.O.; Lohier, J.; Mojovic, L.; Plé, N.; Turck, A.; Reboul, V.; Metzner, P. Diastereoselective addition of enantiopure lithium tert-butylsulfinylferrocene to imines. J. Org. Chem. 2006, 71, 9572–9579. [Google Scholar] [CrossRef]
- Duan, H.; Jia, Y.; Wang, L.; Zhou, Q. Enantioselective Rh-catalyzed arylation of N-tosylarylimines with arylboronic acids. Org. Lett. 2006, 8, 2567–2569. [Google Scholar] [CrossRef]
- Ankner, T.; Hilmersson, G. Instantaneous deprotection of tosylamides and esters with SmI2/amine/water. Org. Lett. 2009, 11, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Berhal, F.; Wu, Z.; Zhang, Z.; Ayad, T.; Ratovelomanana-Vidal, V. Enantioselective synthesis of 1-aryl-tetrahydroisoquinolines through iridium catalyzed asymmetric hydrogenation. Org. Lett. 2012, 14, 3308–3311. [Google Scholar] [CrossRef] [PubMed]
- Ritzen, B.; Hoekman, S.; Verdasco, E.D.; van Delft, F.L.; Rutjes, F.P.J.T. Enantioselective chemoenzymatic synthesis of cis- and trans-2,5-disubstituted morpholines. J. Org. Chem. 2010, 75, 3461–3464. [Google Scholar] [CrossRef] [PubMed]
- Goulaouic-Dubois, C.; Guggisberg, A.; Hesse, M. Protection of amines by the pyridine-2-sulfonyl group and its cleavage under mild conditions (SmI2 or electrolysis). J. Org. Chem. 1995, 60, 5969–5972. [Google Scholar] [CrossRef]
- Aaseng, J.E.; Gautun, O.R. Synthesis of substituted (S)-2-aminotetralins via ring-opening of aziridines prepared from l-aspartic acid β-tert-butyl ester. Tetrahedron 2010, 66, 8982–8991. [Google Scholar] [CrossRef][Green Version]
- Nakamura, Y.; Tanaka, S.; Serizawa, R.; Morohashi, N.; Hattori, T. Synthesis of mono-and 1,3-diaminocalix[4]arenes via Ullmann-type amination and amidation of 1,3-bistriflate esters of calix [4] arenes J. Org. Chem. 2011, 76, 2168–2179. [Google Scholar] [CrossRef]
- Kumar, V.; Ramesh, N.G. Iodine catalyzed one-pot diamination of glycals with chloramine-T: A new approach to 2-amino-β-glycosylamines for applications in N-glycopeptide synthesis. Chem. Commun. 2006, 4952–4954. [Google Scholar] [CrossRef]
- Kumar, V.; Ramesh, N.G. A versatile strategy for the synthesis of N-linked glycoamino acids from glycals. Org. Biomol. Chem. 2007, 5, 3847–3858. [Google Scholar] [CrossRef]
- Jensen, K.L.; Franke, P.T.; Nielsen, L.T.; Daasbjerg, K.; Jørgensen, K.A. Anodic oxidation and organocatalysis: Direct regio- and stereoselective access to meta-substituted anilines by α-arylation of aldehydes. Angew. Chem. Int. Ed. 2010, 49, 129–133. [Google Scholar] [CrossRef]
- Yamagishi, T.; Ichikawa, H.; Haruki, T.; Yokomatsu, T. Diastereoselective synthesis of α,β’-disubstituted aminomethyl (2-carboxyethyl) phosphinates as phosphinyl dipeptide isosteres. Org. Lett. 2008, 10, 4347–4350. [Google Scholar] [CrossRef]
- Blay, G.; Cardona, L.; Climent, E.; Pedro, J.R. Highly enantioselective zinc/binol-catalyzed alkynylation of N-sulfonyl aldimines. Angew. Chem. Int. Ed. 2008, 47, 5593–5596. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.C.; Flugge, L.A.; Petillo, P.A. SmI2-promoted deprotection of N-(arylsulfonyl) glucosamines. J. Org. Chem. 1997, 62, 4864–4866. [Google Scholar] [CrossRef]
- Gaston, J.J.; Tague, A.J.; Smyth, J.E.; Butler, N.M.; Willis, A.C.; van Eikema Hommes, N.; Yu, H.; Clark, T.; Keller, P.A. The detosylation of chiral 1,2-bis (tosylamides). J. Org. Chem. 2021, 86, 9163–9180. [Google Scholar] [CrossRef]
- Okabe, K.; Natsume, M. The second generation synthesis of a tumor promoter pendolmycin. Tetrahedron 1991, 47, 7615–7624. [Google Scholar] [CrossRef]
- Pak, C.S.; Lim, D.S. Deprotection of 2-pyridyl sulfonyl group from pyridine-2-sulfonamides by magnesium in methanol. Synth. Commun. 2001, 31, 2209–2214. [Google Scholar] [CrossRef]
- Kan, T.; Fukuyama, T. Ns strategies: A highly versatile synthetic method for amines. Chem. Commun. 2004, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, T.; Jow, C.-K.; Cheung, M. 2- and 4-Nitrobenzenesulfonamides: Exceptionally versatile means for preparation of secondary amines and protection of amines. Tetrahedron Lett. 1995, 36, 6373–6374. [Google Scholar] [CrossRef]
- Kobayashi, S.; Furuya, T.; Otani, T.; Saito, T. A novel and facile stereocontrolled synthetic method for polyhydro-quinolines and pyridopyridazines via a diene-transmissive Diels–Alder reaction involving inverse electron-demand hetero Diels–Alder cycloaddition of cross-conjugated azatrienes. Tetrahedron 2008, 64, 9705–9716. [Google Scholar] [CrossRef]
- Borisov, D.D.; Chermashentsev, G.R.; Novikov, R.A.; Tomilov, Y.V. Synthesis of substituted β-styrylmalonates by sequential isomerization of 2-arylcyclopropane-1,1-dicarboxylates and (2-arylethylidene) malonates. Synthesis 2021, 53, 2253–2259. [Google Scholar] [CrossRef]
- Chagarovskiy, A.O.; Ivanova, O.A.; Rakhmankulov, E.R.; Budynina, E.M.; Trushkov, I.V.; Melnikov, M.Y. Lewis acid-catalyzed isomerization of 2-arylcyclopropane-1,1-dicarboxylates: A new efficient route to 2-styrylmalonates. Adv. Synth. Catal. 2010, 352, 3179–3184. [Google Scholar] [CrossRef]
- Tsedilin, A.M.; Fakhrutdinov, A.N.; Eremin, D.B.; Zalesskiy, S.S.; Chizhov, A.O.; Kolotyrkina, N.G.; Ananikov, V.P. How sensitive and accurate are routine NMR and MS measurements? Mendeleev Commun. 2015, 25, 454–456. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).