Linum corymbulosum Protects Rats against CCl4-Induced Hepatic Injuries through Modulation of an Unfolded Protein Response Pathway and Pro-Inflammatory Intermediates
Abstract
1. Introduction
2. Results
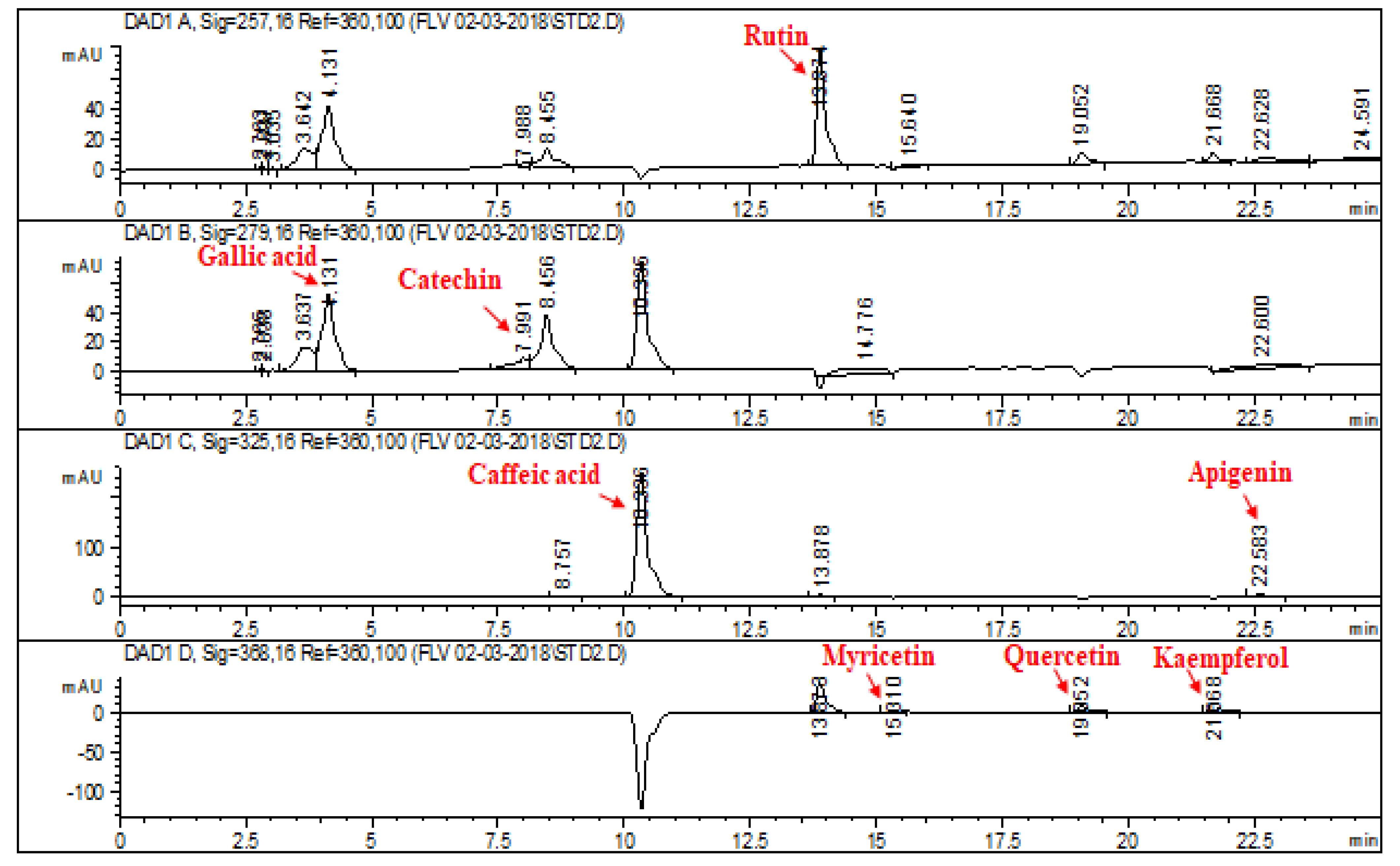
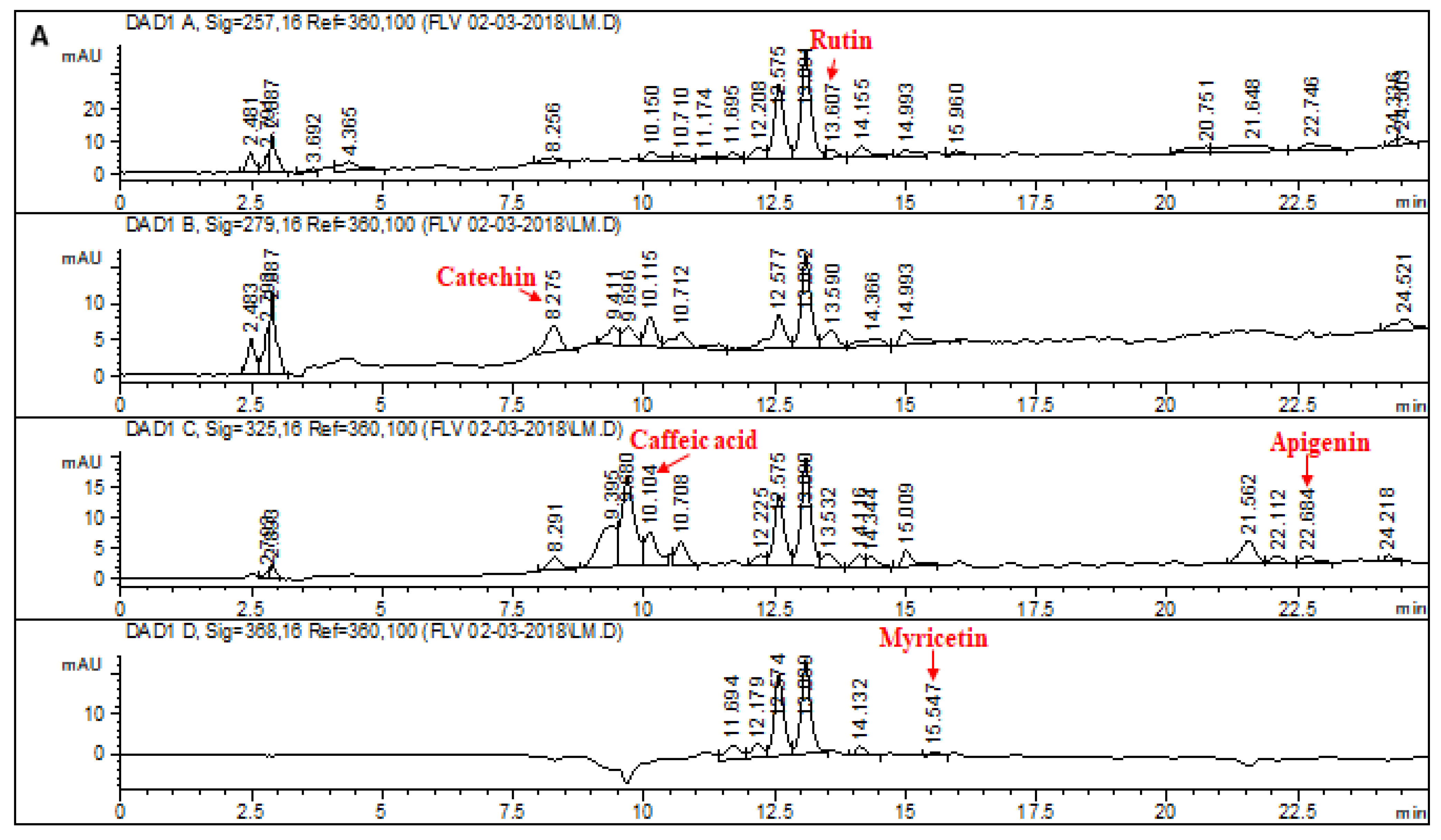
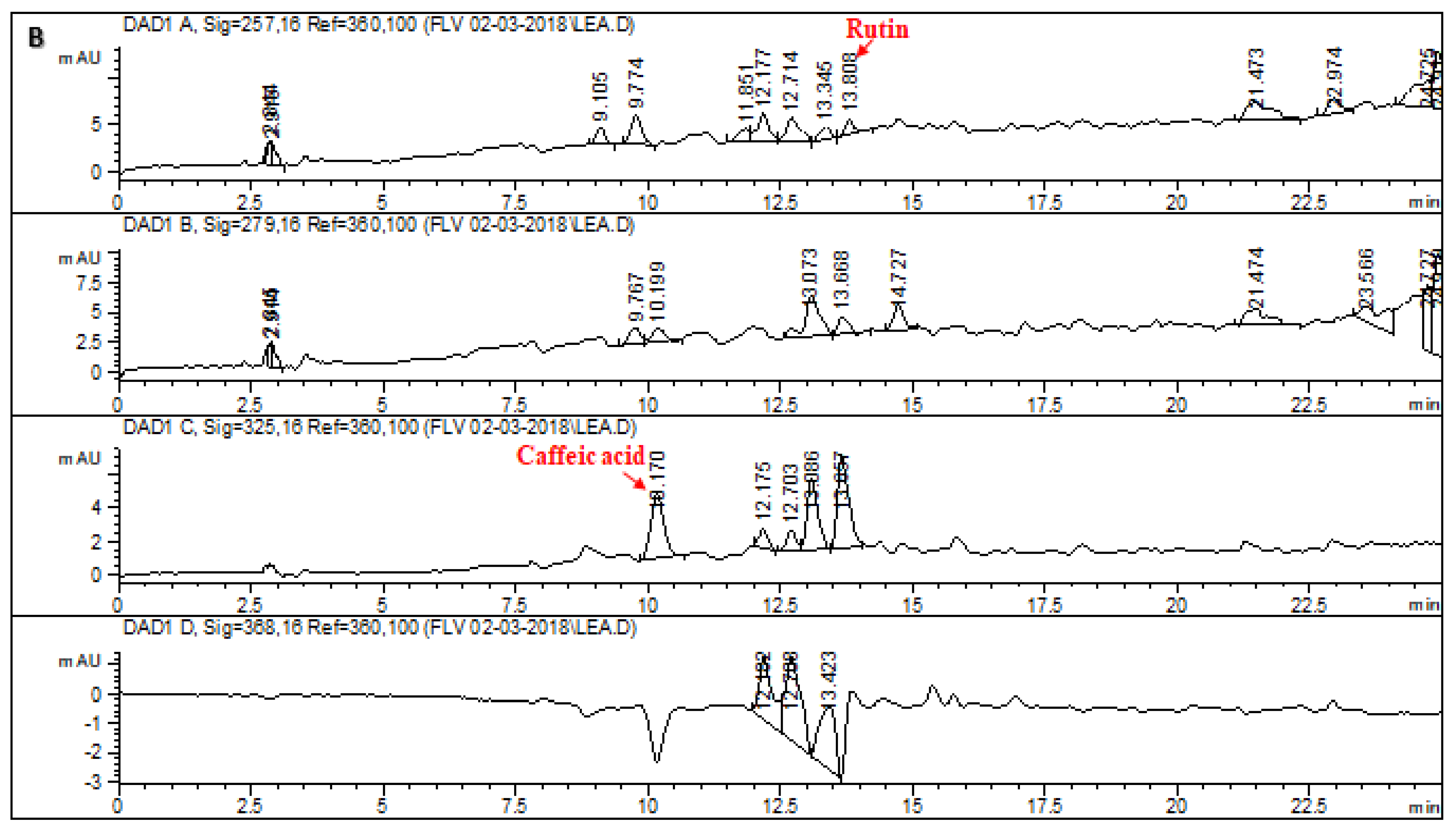
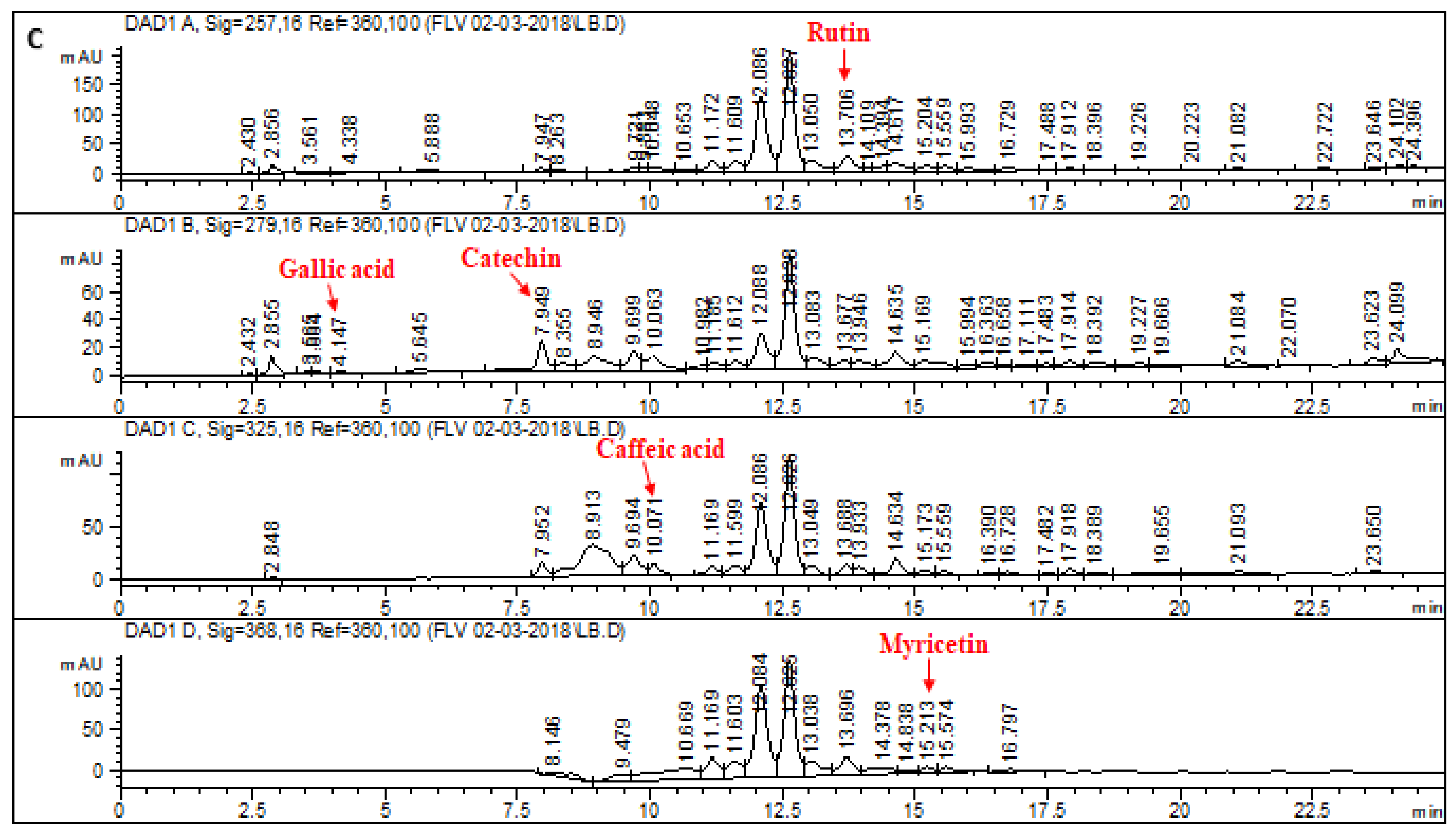
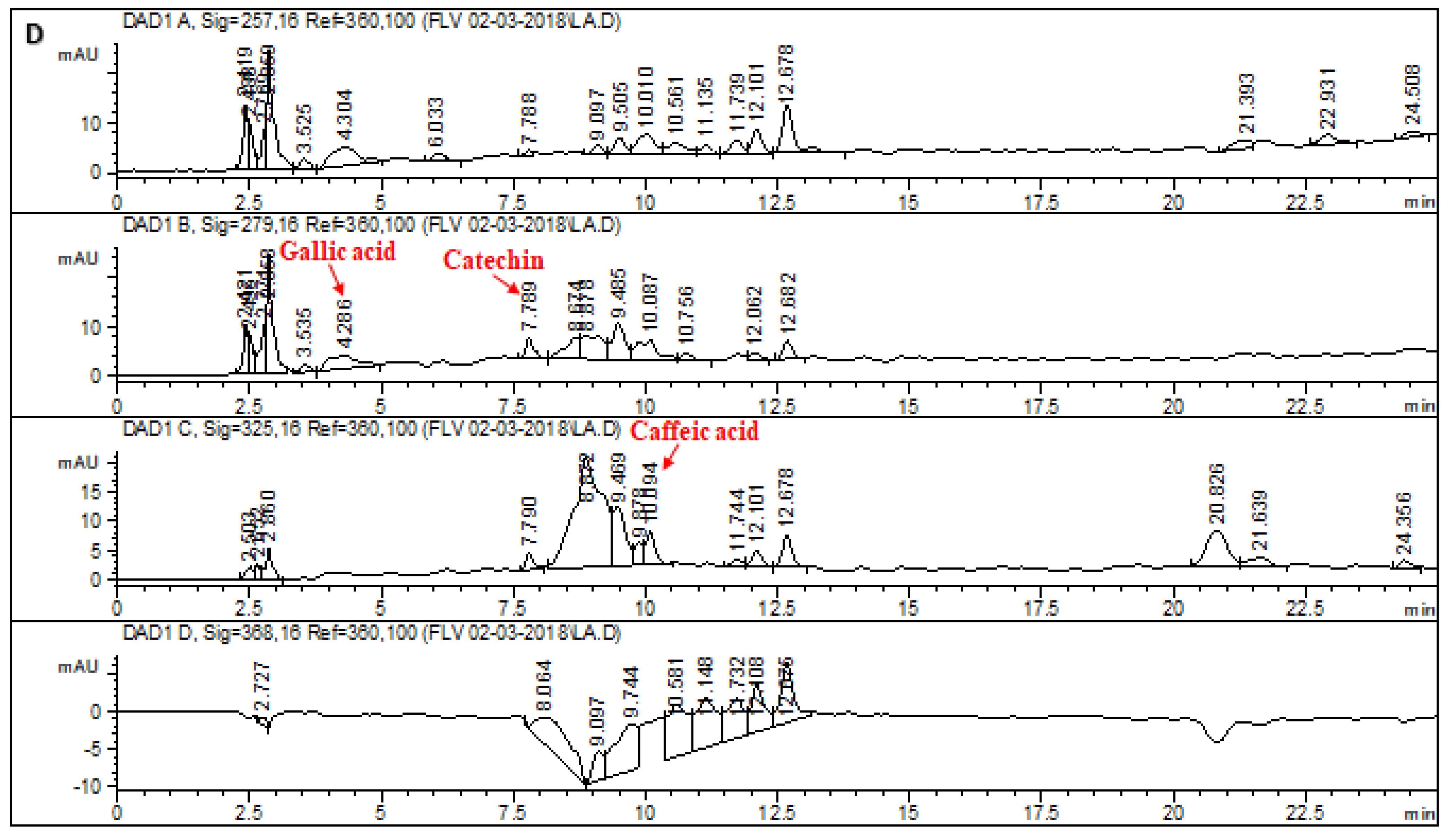
2.1. HPLC–DAD Analysis of L. corymbulosum Extracts
2.2. Acute Toxicity Studies of the effect of L. corymbulosum on Hematological Parameters
2.3. Effect of L. corymbulosum on Body Weight and Organ Weight
2.4. Effect of L. corymbulosum on Biochemical Serum Markers
2.5. Effect of L. corymbulosum on Hepatic Antioxidant Enzymes
2.6. Effect of L. corymbulosum on Hepatic Protein, TBARS, H2O2 and Nitrite Content
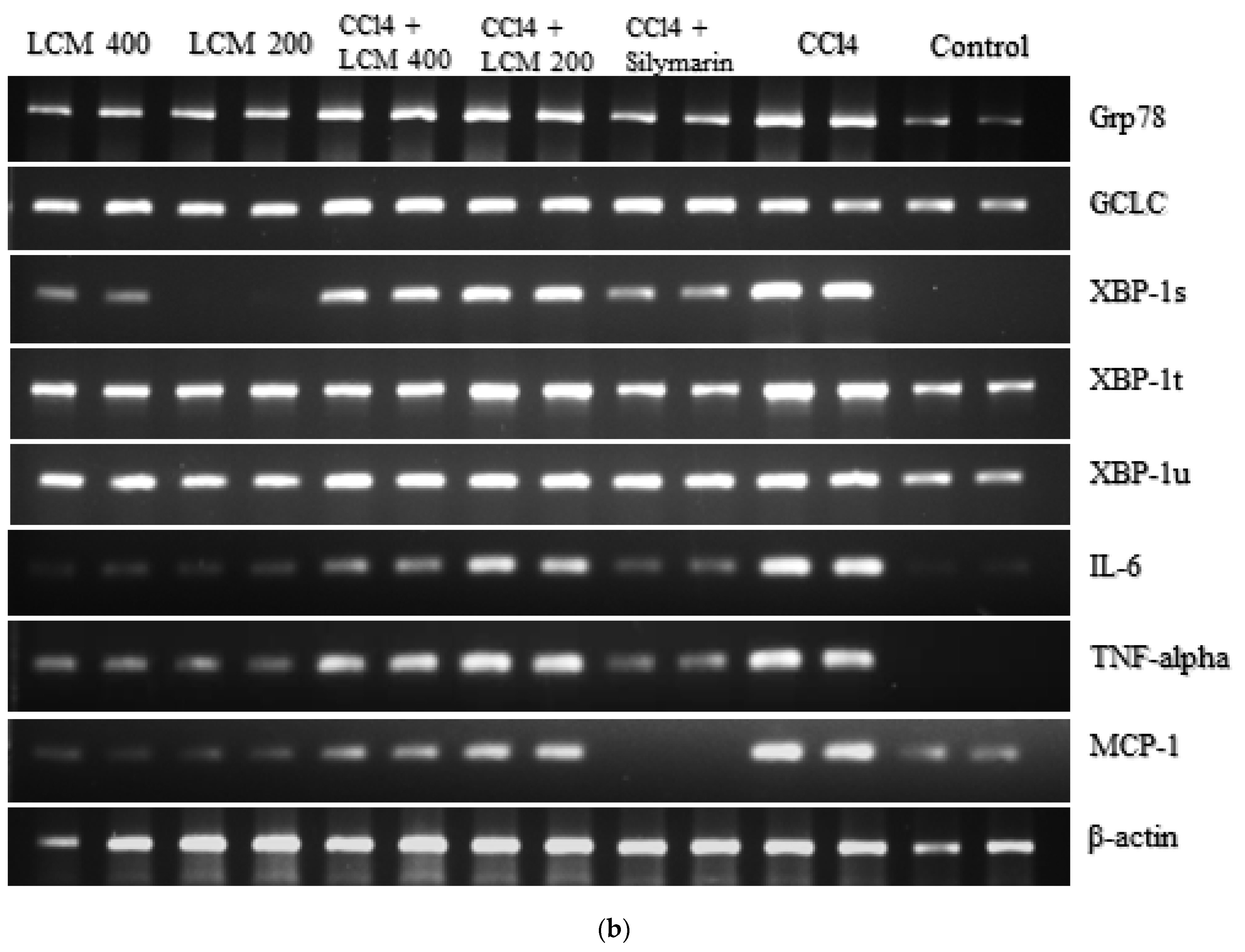
2.7. Effect of L. corymbulosum on ER-Stress Markers and Inflammatory Mediators
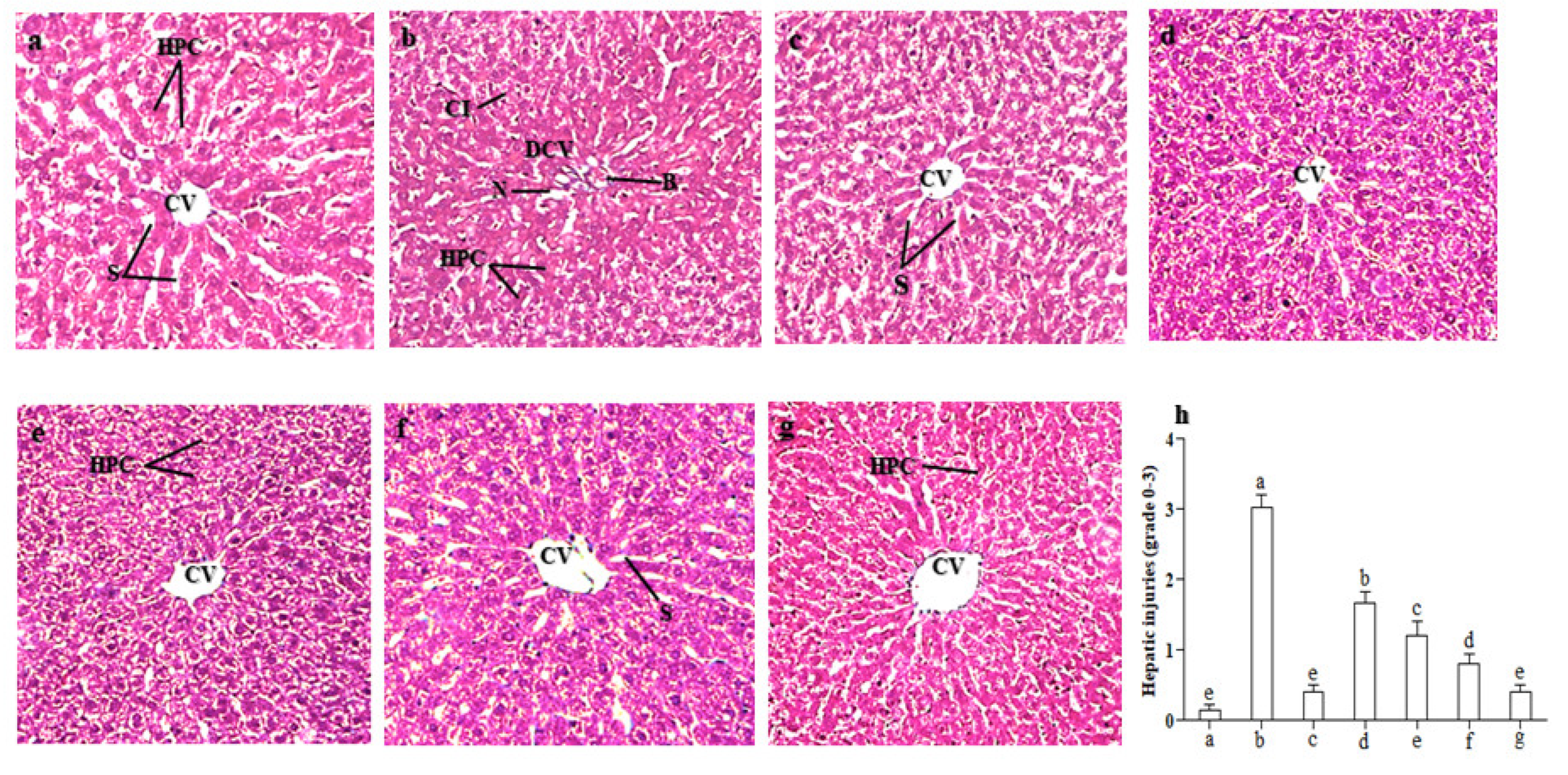
2.8. Effect of LCM on Histoarchitecture of the Liver
3. Discussion
4. Materials and Methods
4.1. Plant Collection
4.2. Preparation of Extracts
4.3. High Performance Liquid Chromatography (HPLC–DAD) Analysis
4.4. Animals
4.5. Acute Toxicity Assessment
4.6. Experimental Design
4.7. Dose Regimen
4.8. Body Weight and Organ Weight
4.9. Biochemical Studies of Serum
4.10. Biochemical Studies of Tissues
4.10.1. Catalase Assay (CAT)
4.10.2. Peroxidase Assay (POD)
4.10.3. Superoxide Dismutase Assay (SOD)
4.10.4. Reduced Glutathione Assay (GSH)
4.10.5. Estimation of Lipid Peroxidation Assay (TBARS)
4.10.6. Hydrogen Peroxide Assay (H2O2)
4.10.7. Nitrite Assay
4.10.8. Tissue Protein Assessment
4.11. Histopathological Studies
4.12. RNA Extraction
4.13. RT-PCR Analysis
4.14. Relative Quantification of Gene Expression
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Mahmoud, A.M.; Hozayen, W.G.; Hasan, I.H.; Shaban, E.; Bin-Jumah, M. Umbelliferone ameliorates ccl4-induced liver fibrosis in rats by upregulating pparγ and attenuating oxidative stress, inflammation, and tgf-β1/smad3 signaling. Inflammation 2019, 42, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Liu, Y.; Xiong, Y.; Wu, H.; Wang, G.; Sun, Z.; Chen, J.; Yan, X.; Pan, Z.; Xia, J. Curcumin protects against liver fibrosis by attenuating infiltration of gr1hi monocytes through inhibition of monocyte chemoattractant protein-1. Discov. Med. 2016, 21, 447–457. [Google Scholar] [PubMed]
- Wu, R.; Xiao, Z.; Zhang, X.; Liu, F.; Zhou, W.; Zhang, Y. The Cytochrome P450-Mediated Metabolism Alternation of Four Effective Lignans From Schisandra chinensis in Carbon Tetrachloride-Intoxicated Rats and Patients With Advanced Hepatocellular Carcinoma. Front. Pharmacol. 2018, 9, 229. [Google Scholar] [CrossRef]
- Choi, J.H.; Moon, C.M.; Shin, T.-S.; Kim, E.K.; McDowell, A.; Jo, M.-K.; Joo, Y.H.; Kim, S.-E.; Jung, H.-K.; Shim, K.-N. Lactobacillus paracasei-derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway. Exp. Mol. Med. 2020, 52, 423–437. [Google Scholar] [CrossRef]
- Dai, X.; Medzhitov, R. Inflammation: Memory beyond immunity. Nature 2017, 550, 460–461. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, J.; Khan, M.R. Evaluation of anti-asthmatic and antioxidant potential of Boerhavia procumbens in toluene diisocyanate (TDI) treated rats. J. Ethnopharmacol. 2015, 172, 377–385. [Google Scholar] [CrossRef]
- Foell, D.; Kessel, C. Means and Methods for Diagnosing and Treating Inflammatory Disorders. U.S. Patent Application No. 15/571,374, 2018. [Google Scholar]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Shah, S.A.; Yoon, G.H.; Kim, M.O. Protection of the Developing Brain with Anthocyanins Against Ethanol-Induced Oxidative Stress and Neurodegeneration. Mol. Neurobiol. 2014, 51, 1278–1291. [Google Scholar] [CrossRef]
- Bokhari, J.; Khan, M.R.; Shabbir, M.; Rashid, U.; Jan, S.; Zai, J.A. Evaluation of diverse antioxidant activities of galium aparine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 24–29. [Google Scholar] [CrossRef]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; De Freitas, V. Wine flavonoids in health and disease prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef]
- Talebi, S.M.; Sheidai, M.; Atri, M.; Sharifnia, F.; Noormohammadi, Z. Palynological study of the genus linum in iran (a taxonomic review). Phytol. Balc. 2012, 18, 293–303. [Google Scholar]
- Baker, J.G. Elementary Lessons in Botanical Geography; Lovell Reeve & Company: London, UK, 1875. [Google Scholar]
- Noori, M.; Talebi, S.M. A chemotaxonomic study of some iranian linum taxa. Phytol. Balc. Int. J. Balk. Flora Veg. 2017, 23, 23–29. [Google Scholar]
- Rehman, K.; Mashwani, Z.-U.; Khan, M.A.; Ullah, Z.; Chaudhary, H.J. An ethno botanical perspective of traditional medicinal plants from the Khattak tribe of Chonthra Karak, Pakistan. J. Ethnopharmacol. 2015, 165, 251–259. [Google Scholar] [CrossRef]
- Shin, S.; Cho, I.; Kim, S. Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3β inhibition downstream of poly(ADP-ribose) polymerase-LKB1 pathway. Mol. Pharmacol. 2009, 76, 884–895. [Google Scholar] [CrossRef]
- Apostolou, A.; Stagosa, D.; Galitsioua, E.; Spyroua, A.; Haroutounian, S.; Portesisa, N.; Trizogloua, I.; Hayescd, A.W.; Tsatsakis, A.M.; Kouretas, D. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food Chem. Toxicol. 2013, 61, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Al-Olayan, E.M.; El-Khadragy, M.F.; Aref, A.M.; Othman, M.S.; Kassab, R.B.; Abdel Moneim, A.E. The potential protective effect of physalis peruviana l. Against carbon tetrachloride-induced hepatotoxicity in rats is mediated by suppression of oxidative stress and downregulation of mmp-9 expression. Oxidative Med. Cell. Longev. 2014, 2014, 381413. [Google Scholar] [CrossRef]
- Boll, M.; Weber, L.W.; Becker, E.; Stampfl, A. Hepatocyte damage induced by carbon tetrachloride: Inhibited lipoprotein secretion and changed lipoprotein composition. Z. Naturforsch. C 2001, 56, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhan, Q.; Li, Y.; Sun, S.; Zhao, L.; Zhang, H.; Zhang, G. Schisandra lignan extract protects against carbon tetrachloride-induced liver injury in mice by inhibiting oxidative stress and regulating the nf-κb and jnk signaling pathways. Evid.-Based Complement. Altern. Med. 2017, 2017, 5140297. [Google Scholar] [CrossRef]
- Liu, J.; Fu, Y.; Zhang, H.; Wang, J.; Zhu, J.; Wang, Y.; Guo, Y.; Wang, G.; Xu, T.; Chu, M. The hepatoprotective effect of the probiotic Clostridium butyricum against carbon tetrachloride-induced acute liver damage in mice. Food Funct. 2017, 8, 4042–4052. [Google Scholar] [CrossRef]
- Zu, Y.; Fu, Y.; Li, S.; Sun, R.; Li, Q.; Schwarz, G. Rapid separation of four main taxoids in Taxus species by a combined LLP-SPE-HPLC (PAD) procedure. J. Sep. Sci. 2006, 29, 1237–1244. [Google Scholar] [CrossRef]
- Shahid, A.; Ali, R.; Ali, N.; Hasan, S.K.; Bernwal, P.; Afzal, S.M.; Vafa, A.; Sultana, S. Modulatory effects of catechin hydrate against genotoxicity, oxidative stress, inflammation and apoptosis induced by benzo (a) pyrene in mice. Food Chem. Toxicol. 2016, 92, 64–74. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; Tavares-Da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Khan, M.R.; Shah, N.A.; Shah, S.A.; Ismail, H.; Younis, T.; Zahra, Z. Phytochemical, antioxidant and hepatoprotective effects of Alnus nitida bark in carbon tetrachloride challenged Sprague Dawley rats. BMC Complement. Altern. Med. 2016, 16, 268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, G.; Liao, Y.; Gong, D. Myricetin inhibits the generation of superoxide anion by reduced form of xanthine oxidase. Food Chem. 2017, 221, 1569–1577. [Google Scholar] [CrossRef]
- Jeong, G.S.; Lee, S.H.; Jeong, S.N.; Kim, Y.C.; Kim, E.C. Anti-inflammatory effects of apigenin on nicotine- and lipopolysaccharide-stimulated human periodontal ligament cells via heme oxygenase-1. Int. Immunopharmacol. 2009, 9, 1374–1380. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Apigenin: A Promising Molecule for Cancer Prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef]
- Rashid, U.; Khan, M.R. Fagonia olivieri prevented hepatorenal injuries induced with gentamicin in rat. Biomed. Pharmacother. 2017, 88, 469–479. [Google Scholar] [CrossRef]
- Weber, L.W.D.; Boll, M.; Stampfl, A. Hepatotoxicity and Mechanism of Action of Haloalkanes: Carbon Tetrachloride as a Toxicological Model. Crit. Rev. Toxicol. 2003, 33, 105–136. [Google Scholar] [CrossRef] [PubMed]
- Seyer, J.M.; Hutcheson, E.T.; Kang, A.H. Collagen polymorphism innormal and cirrhotic human liver. J. Clin. Investig. 1977, 59, 241–248. [Google Scholar] [CrossRef]
- Lieber, C.S.; Jones, D.P.; Decarli, L.M. Effects of prolonged ethanolintake: Production of fatty liver despite adequate diets. J. Clin. Investig. 1965, 44, 1009–1021. [Google Scholar] [CrossRef]
- Lee, C.-P.; Shih, P.-H.; Hsu, C.-L.; Yen, G.-C. Hepatoprotection of tea seed oil (Camellia oleifera Abel.) against CCl4-induced oxidative damage in rats. Food Chem. Toxicol. 2007, 45, 888–895. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.; Kim, M.; Lee, Y.; Seo, J.; Ha, N.; Kim, K. Hepatoprotective effect of Bifidobacterium adolescentis SPM0212 on carbon tetrachloride induced hepatotoxicity. Korean J. Microbiol. 2015, 51, 280–287. [Google Scholar] [CrossRef]
- Ali, S.; Khan, M.R.; Sajid, M. Protective potential of parrotiopsis jacquemontiana (decne) rehder on carbon tetrachloride induced hepatotoxicity in experimental rats. Biomed. Pharmacother. 2017, 95, 1853–1867. [Google Scholar] [CrossRef]
- El-Sayed, Y.S.; Lebda, M.A.; Hassinin, M.; Neoman, S.A. Chicory (Cichorium intybus L.) root extract regulates the oxidative status and antioxidant gene transcripts in ccl4-induced hepatotoxicity. PLoS ONE 2015, 10, e0121549. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.M.; Al-Kahtani, M.A.; El-Kersh, M.A.; Al-Omair, M.A. Free radical-scavenging, anti-inflammatory/anti-fibrotic and hepatoprotective actions of taurine and silymarin against ccl4 induced rat liver damage. PLoS ONE 2015, 10, e0144509. [Google Scholar] [CrossRef] [PubMed]
- Gite, S.; Yadav, S.; Nilegaonkar, S.; Agte, V. Evaluation of hepatoprotective potential of functional food formulations using in vitro and in vivo models of ccl4 radical induced toxicity. IJIMS 2014, 1, 6–13. [Google Scholar]
- Suzek, H.; Celik, I.; Dogan, A.; Yildirim, S. Protective effect and antioxidant role of sweetgum (Liquidambar orientalis) oil against carbon tetrachloride-induced hepatotoxicity and oxidative stress in rats. Pharm. Biol. 2016, 54, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Hosokawa, N.; Wada, I.; Nagata, K. Edem as an acceptor of terminally misfolded glycoproteins released from calnexin. Science 2003, 299, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
- Malhi, H.; Kaufman, R.J. Endoplasmic reticulum stress in liver disease. J. Hepatol. 2011, 54, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, D.T.; Wu, J.; Back, S.-H.; Callaghan, M.U.; Ferris, S.P.; Iqbal, J.; Clark, R.; Miao, H.; Hassler, J.R.; Fornek, J. Upr pathways combine to prevent hepatic steatosis caused by er stress-mediated suppression of transcriptional master regulators. Dev. Cell 2008, 15, 829–840. [Google Scholar] [CrossRef]
- Lee, G.H.; Bhandary, B.; Lee, E.M.; Park, J.K.; Jeong, K.S.; Kim, I.K.; Kim, H.R.; Chae, H.J. The roles of ER stress and P450 2E1 in CCl4-induced steatosis. Int. J. Biochem. Cell Biol. 2011, 43, 1469–1482. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zheng, S.; Lin, J.; Ryerse, J.; Chen, A. Curcumin protects the rat liver from ccl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol. Pharmacol. 2007, 73, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.F.; Safhi, M.M.; Anwer, T.; Siddiqui, R.; Khan, G.; Moni, S.S. Therapeutic potential of vanillylacetone against ccl4 induced hepatotoxicity by suppressing the serum marker, oxidative stress, inflammatory cytokines and apoptosis in swiss albino mice. Exp. Mol. Pathol. 2018, 105, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, I.; Zhu, W.; Li, K.-K.; Li, C.-M. Polyphenols of mulberry fruits as multifaceted compounds: Compositions, metabolism, health benefits, and stability—A structural review. J. Funct. Foods 2018, 40, 28–43. [Google Scholar] [CrossRef]
- Atsumi, T.; Iwakura, I.; Kashiwagi, Y.; Fujisawa, S.; Ueha, T. Free radical scavenging activity in the nonenzymatic fraction of human saliva: A simple dpph assay showing the effect of physical exercise. Antioxid. Redox Signal. 1999, 1, 537–546. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Y.; Wen, J.; Fan, G.; Wu, Y.; Zhang, C. A rapid and simple HPLC method for the determination of curcumin in rat plasma: Assay development, validation and application to a pharmacokinetic study of curcumin liposome. Biomed. Chromatogr. 2009, 23, 1201–1207. [Google Scholar] [CrossRef]
- Handa, S.; Sharma, A. Hepatoprotective activity of andrographolide from Andrographis paniculata against carbontetrachloride. Indian J. Med. Res. 1990, 92, 276–283. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Mahboob, S.A.; Apte, K.; Bhagwat, G.B. Hepatoprotective Potential of Acaro calamus against Carbon tetrachloride induced Liver damage in Rats. Int. J. PharmTech Res. 2014, 6, 1315–1321. [Google Scholar]
- Vargas-Mendoza, N.; Madrigal-Santillán, E.; Morales-González, A.; Esquivel-Soto, J.; Esquivel-Chirino, C.; González-Rubio, M.G.; Gayosso-de-Lucio, J.A.; Morales-González, J.A. Hepatoprotective effect of silymarin. J. Hepatol. 2014, 6, 144. [Google Scholar] [CrossRef]
- Patrick-Iwuanyanwu, K.C.; Onyeike, E.N.; Wegwu, M.O. Hepatoprotective effects of methanolic extract and fractions of african mistletoe Tapinanthus bangwensis (engl. & k. Krause) from nigeria. EXCLI J. 2010, 9, 187. [Google Scholar] [PubMed]
- Chance, B.; Maehly, A. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Wright, D.J. Host resistance to insecticides can confer protection to endo-larval parasitoids. Bull. Entomol. Res. 1996, 86, 721–723. [Google Scholar] [CrossRef]
- Pick, E.; Keisari, Y. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages—Induction by multiple nonphagocytic stimuli. Cell. Immunol. 1981, 59, 301–318. [Google Scholar] [CrossRef]
- Majid, M.; Khan, M.R.; Shah, N.A.; Haq, I.U.; Farooq, M.A.; Ullah, S.; Sharif, A.; Zahra, Z.; Younis, T.; Sajid, M. Studies on phytochemical, antioxidant, anti-inflammatory and analgesic activities of Euphorbia dracunculoides. BMC Complement. Altern. Med. 2015, 15, 349. [Google Scholar] [CrossRef]
- Bensadoun, A.; Weinstein, D. Assay of proteins in the presence of interfering materials. Anal. Biochem. 1976, 70, 241–250. [Google Scholar] [CrossRef]
- Stevens, S.S. On the Theory of Scales of Measurement. Science 1946, 103, 677–680. [Google Scholar] [CrossRef]
- Chomczynski, P. A reagent for the single-step simultaneous isolation of rna, DNA and proteins from cell and tissue samples. Biotechniques 1993, 15, 532–534. [Google Scholar]
- Schmittgen, T.D.; Zakrajsek, B.A. Effect of experimental treatment on housekeeping gene expression: Validation by real-time, quantitative RT-PCR. J. Biochem. Biophys. Methods 2000, 46, 69–81. [Google Scholar] [CrossRef] [PubMed]
 Control,
Control,  CCl4,
CCl4,  CCl4 + silymarin (200 mg/kg),
CCl4 + silymarin (200 mg/kg),  CCl4 + LCM (200 mg/kg),
CCl4 + LCM (200 mg/kg),  CCl4 + LCM (400 mg/kg),
CCl4 + LCM (400 mg/kg),  LCM (200 mg/kg),
LCM (200 mg/kg),  LCM (400 mg/kg). LCM; Linum corymbulosum methanol extract. The representation of letters (a-e) specify significance at p < 0.05 (b): Gel electrophoresis of cDNA after RT-PCR analysis.
LCM (400 mg/kg). LCM; Linum corymbulosum methanol extract. The representation of letters (a-e) specify significance at p < 0.05 (b): Gel electrophoresis of cDNA after RT-PCR analysis.
 Control,
Control,  CCl4,
CCl4,  CCl4 + silymarin (200 mg/kg),
CCl4 + silymarin (200 mg/kg),  CCl4 + LCM (200 mg/kg),
CCl4 + LCM (200 mg/kg),  CCl4 + LCM (400 mg/kg),
CCl4 + LCM (400 mg/kg),  LCM (200 mg/kg),
LCM (200 mg/kg),  LCM (400 mg/kg). LCM; Linum corymbulosum methanol extract. The representation of letters (a-e) specify significance at p < 0.05 (b): Gel electrophoresis of cDNA after RT-PCR analysis.
LCM (400 mg/kg). LCM; Linum corymbulosum methanol extract. The representation of letters (a-e) specify significance at p < 0.05 (b): Gel electrophoresis of cDNA after RT-PCR analysis.
| Reference Flavonoids/Phenolics | Signal Wavelength | Retention Time (min) | Regression Analysis | R2 |
|---|---|---|---|---|
| Rutin | 257 | 13.874 | y = 8.336x + 22.21 | 0.9899 |
| Gallic acid | 279 | 4.131 | y = 24.85x − 47.147 | 0.9928 |
| Catechin | 279 | 7.991 | y = 7.987x − 16.913 | 0.9967 |
| Caffeic acid | 325 | 10.336 | y = 10.24x + 26.27 | 0.9875 |
| Apigenin | 325 | 22.583 | y = 12.53x + 77.54 | 0.9854 |
| Myricetin | 368 | 15.310 | y = 2.04x − 10.16 | 0.9897 |
| Quercetin | 368 | 19.052 | y = 12.21x − 20.34 | 0.9993 |
| Kaempferol | 368 | 21.668 | y = 8.78x + 120.24 | 0.9994 |
| Extracts | Polyphenolics (μg/mg of Extract) | |||||||
|---|---|---|---|---|---|---|---|---|
| Rutin | Catechin | Gallic Acid | Caffeic Acid | Myricetin | Apigenin | Quercetin | Kaempferol | |
| LCM | 1.92 | 9.94 | - | 7.38 | 3.37 | 2.64 | - | - |
| LCE | 4.71 | - | - | 6.21 | - | - | - | - |
| LCB | 51.48 | 43.7 | 3.11 | 1.43 | 21.82 | - | - | - |
| LCA | - | 7.97 | 6.11 | 6.4 | - | - | - | - |
| Treatment Groups | WBC (103/mm3) | RBC (106/mm3) | HGB (g/dL) | MCV (fL) | PLT (103/mL) | LYM (103/mm3) | MCHC (g/dL) |
|---|---|---|---|---|---|---|---|
| Control | 6.82 ± 0.1 b | 7.33 ± 0.08 ab | 13.78 ± 0.07 a | 42.36 ± 0.30 c | 170.4 ± 1.0 b | 5.24 ± 0.12 c | 34.5 ± 0.92 b |
| LCM (2000 mg/kg) | 12.67 ± 0.26 a | 7.10 ± 0.12 b | 13.3± 0.15 a | 47.44 ± 0.83 b | 159.1 ± 1.31 c | 7.50 ± 0.37 b | 37.7 ± 0.55 a |
| LCM (4000 mg/kg) | 13.13 ± 0.35 a | 7.57 ± 0.23 a | 14.3 ± 0.69 a | 51.6 ± 1.05 a | 186.23 ± 1.20 a | 9.75 ± 0.22 a | 39.2 ± 0.52 a |
| Treatment Groups | Initial Body Weight (g) | Final Body Weight (g) | % Increase | Absolute Liver Weight (g) | Relative Liver Weight (mg/g) |
|---|---|---|---|---|---|
| Control | 153.9 ± 1.5 | 249.3 ± 2.0 | 61.95 ± 2.6 a | 7.65 ± 0.22 d | 30.69 ± 0.8 e |
| CCl4 (1 mL/kg) | 160.4 ± 1.8 | 213.3 ± 0.62 | 32.98 ± 1.3 e | 9.42 ± 0.04 a | 44.16 ± 0.2 a |
| CCl4 + silymarin (200 mg/kg) | 158.2 ± 1.3 | 247.3 ± 0.9 | 56.32 ± 1.8 b | 7.96 ± 0.13 d | 32.18 ± 0.6 e |
| CCl4 + LCM (200 mg/kg) | 159.6 ± 1.4 | 220.6 ± 0.6 | 38.23 ± 1.17 d | 8.98 ± 0.12 b | 40.74 ± 0.58 b |
| CCl4 + LCM (400 mg/kg) | 156.0 ± 1.4 | 221.7 ± 1.6 | 42.16 ± 2.0 cd | 8.5 ± 0.23 c | 38.33 ± 1.29 c |
| LCM(200 mg/kg) | 161.3 ± 1.2 | 237.6 ± 1.2 | 47.31 ± 1.8 c | 8.41 ± 0.11 c | 35.4 ± 0.54 d |
| LCM(400 mg/kg) | 161.4 ± 0.7 | 251.3 ± 1.4 | 55.72 ± 1.5 b | 8.07 ± 0.12 cd | 32.13 ± 0.28 e |
| Treatment Groups | ALT (U/L) | AST (U/L) | ALP (U/L) | Albumin (mg/dL) | Total Bilirubin (mg/dL) |
|---|---|---|---|---|---|
| Control | 44.43 ± 0.25 g | 57.13 ± 0.44 g | 66.76 ± 0.78 g | 4.76 ± 0.22 a | 0.45 ± 0.02 f |
| CCl4 (1 mL/kg) | 156.8 ± 0.37 a | 171.8 ± 1.11 a | 147.8 ± 1.07 a | 1.98 ± 0.08 f | 1.80 ± 0.02 a |
| CCl4 + silymarin (200 mg/kg) | 55.65 ± 0.43 e | 73.40 ± 0.74 f | 78.39 ± 0.46 f | 4.22 ± 0.10 b | 0.66 ± 0.04 e |
| CCl4 + LCM (200 mg/kg) | 111.8 ± 0.84 b | 131.2 ± 1.36 b | 120.4 ± 1.06 b | 2.57 ± 0.10 e | 1.19 ± 0.05 b |
| CCl4 + LCM (400 mg/kg) | 93.97 ± 0.41 c | 108.8 ± 0.65 c | 112.8 ± 0.76 c | 2.99 ± 0.12 d | 0.93 ± 0.02 c |
| LCM (200 mg/kg) | 53.38 ± 0.53 d | 98.11 ± 0.73 d | 98.02 ± 0.56 d | 3.78 ± 0.13 c | 0.82 ± 0.02 d |
| LCM (400 mg/kg) | 46.17 ± 0.72 f | 78.54 ± 0.96 e | 87.79 ± 0.66 e | 3.90 ± 0.06 c | 0.71 ± 0.04 e |
| Treatment Groups | CAT (U/min) | POD (U/min) | SOD (U/mg Protein) | GSH (µmol/mg) |
|---|---|---|---|---|
| Control | 9.82 ± 0.70 a | 12.11 ± 1.37 a | 4.78 ± 0.63 a | 17.05 ± 0.94 a |
| CCl4 (1 mL/kg) | 1.58 ± 0.37 f | 3.88 ± 0.35 f | 0.84 ± 0.18 e | 4.90 ± 0.45 f |
| CCl4 + silymarin (200 mg/kg) | 8.20 ± 0.68 b | 11.52 ± 1.5 ab | 3.57 ± 0.74 b | 14.04 ± 1.6 b |
| CCl4 + LCM (200 mg/kg) | 2.94 ± 0.32 e | 5.54 ± 0.62 e | 1.58 ± 0.18 de | 7.12 ± 0.62 e |
| CCl4 + LCM (400 mg/kg) | 4.46 ± 0.31 d | 7.22 ± 0.55 d | 1.94 ± 0.61 cd | 8.68 ± 0.45 d |
| LCM (200 mg/kg) | 6.9 ± 0.80 c | 8.84 ± 0.65 c | 2.74 ± 0.55 bc | 11.38 ± 0.90 c |
| LCM (400 mg/kg) | 7.54 ± 0.57 bc | 10.24 ± 0.42 bc | 3.47 ± 0.65 b | 13.47 ± 0.92 b |
| Treatment Groups | Protein (μg/mg Tissue) | TBARS (nM/min/mg Protein) | H2O2 (nM/min/mg Tissue) | Nitrite Content (μM/mL) |
|---|---|---|---|---|
| Control | 11.47 ± 0.74 a | 41.40 ± 1.36 g | 5.70 ± 0.29 d | 57.80 ± 1.52 f |
| CCl4 (1 mL/kg) | 5.91 ± 0.31 f | 99.44 ± 2.04 a | 11.02 ± 0.55 a | 95.48 ± 3.06 a |
| CCl4 + silymarin (200 mg/kg) | 10.72 ± 0.67 a | 46.28 ± 1.84 f | 6.33 ± 0.38 d | 65.74 ± 2.09 e |
| CCl4 + LCM (200 mg/kg) | 6.86 ± 0.37 e | 86.86 ± 2.11 b | 9.39 ± 0.64 b | 87.88 ± 2.72 b |
| CCl4 + LCM (400 mg/kg) | 7.99 ± 0.31 d | 79.14 ± 2.77 c | 8.0 ± 0.31 c | 81.88 ± 1.12 c |
| LCM (200 mg/kg) | 8.86 ± 0.42 c | 63.42 ± 2.56 d | 7.89 ± 0.36 c | 77.67 ± 1.98 d |
| LCM (400 mg/kg) | 9.74 ± 0.36 b | 51.14 ± 2.12 e | 6.34 ± 0.35 d | 68.65 ± 2.0 e |
| Treatment Groups | GRP78 | XBPt | XBPs | XBPu | GCLC | MCP-1 | IL-6 | TNF-ɑ |
|---|---|---|---|---|---|---|---|---|
| Control | 1.0 ± 0.0 f | 1.0 ± 0.0 e | 1.0 ± 0.0 f | 1.0 ± 0.0 e | 1.0 ± 0.0 e | 1.0 ± 0.0 e | 1.0 ± 0.0 e | 1.0 ± 0.0 f |
| CCl4 | 6.89 ± 0.17 a | 2.83 ± 0.10 a | 7.10 ± 0.13 a | 2.46 ± 0.10 a | 2.24 ± 0.10 d | 4.14 ± 0.09 a | 3.63 ± 0.12 a | 8.96 ± 0.18 a |
| CCl4 + silymarin (200 mg/kg) | 1.15 ± 0.13 ef | 0.96 ± 0.10 e | 1.18 ± 0.12 f | 1.27 ± 0.11 de | 3.66 ± 0.12 a | 1.23 ± 0.10 e | 0.98 ± 0.10 e | 1.39 ± 0.13 f |
| CCl4 + LCM (200 mg/kg) | 4.46 ± 0.18 b | 2.46 ± 0.09 b | 6.02 ± 0.18 b | 2.26 ± 0.13 a | 3.02 ± 0.11 b | 3.27 ± 0.11 b | 2.97 ± 0.08 b | 5.88 ± 0.15 b |
| CCl4 + LCM (400 mg/kg) | 3.38 ± 0.13 c | 1.93 ± 0.10 c | 4.76 ± 0.14 c | 1.73 ± 0.11 bc | 3.82 ± 0.12 c | 2.57 ± 0.10 c | 2.24 ± 0.10 c | 4.32 ± 0.17 c |
| LCM (200 mg/kg) | 2.14 ± 0.15 d | 1.55 ± 0.11 d | 2.99 ± 0.13 d | 1.82 ± 0.12 b | 2.34 ± 0.16 d | 1.70 ± 0.12 d | 1.67 ± 0.11 d | 2.53 ± 0.18 d |
| LCM (400 mg/kg) | 1.47 ± 0.07 e | 1.20 ± 0.09 e | 1.86 ± 0.13 e | 1.48 ± 0.08 cd | 2.67± 0.09 c | 1.58 ± 0.13 d | 1.24 ± 0.07 e | 1.91 ± 0.15 e |
| Gene | Primer Sequences (5′-3′) | Product Length (bp) | |
|---|---|---|---|
| GRP78 | Forward | GAAATTTCTGCTATGGTTCTCACT | 292 |
| Reverse | GAAGTAAGCTGGTACAGTCACA | ||
| GCLC | Forward | GTGGACACCCGATGCAGTAT | 192 |
| Reverse | TCATCCACCTGGCAACAGTC | ||
| XBP-1s | Forward | TGAGTCCGCAGCAGGTGCA | 155 |
| Reverse | ACAGGGTCCAACTTGTCCAGAA | ||
| XBP-1t | Forward | CCCTGGTTACTGAAGAGGTC | 238 |
| Reverse | GTCCAACTTGTCCAGAATGC | ||
| XBP-1u | Forward | AAAGCGCTGCGGAGGAAA | 170 |
| Reverse | AGCTGGAGTTTCTGGTTCTCT | ||
| TNF-ɑ | Forward | GCTCCCTCTCATCAGTTCCA | 265 |
| Reverse | GGTTGTCTTTGAGATCCATGC | ||
| IL-6 | Forward | GTCAACTCCATCTGCCCTTC | 126 |
| Reverse | ACTGGTCTGTTGTGGGTGGT | ||
| MCP | Forward | CAAGATGTGCGCTGAGGACA | 113 |
| Reverse | TTCTCTATTGGTGGCAGACC | ||
| β-actin | Forward | CCTCTATGCCAACACAGTGC | 178 |
| Reverse | CATCGTACTCCTGCTTGCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batool, R.; Khan, M.R.; Ijaz, M.U.; Naz, I.; Batool, A.; Ali, S.; Zahra, Z.; Gul, S.; Uddin, M.N.; Kazi, M.; et al. Linum corymbulosum Protects Rats against CCl4-Induced Hepatic Injuries through Modulation of an Unfolded Protein Response Pathway and Pro-Inflammatory Intermediates. Molecules 2023, 28, 2257. https://doi.org/10.3390/molecules28052257
Batool R, Khan MR, Ijaz MU, Naz I, Batool A, Ali S, Zahra Z, Gul S, Uddin MN, Kazi M, et al. Linum corymbulosum Protects Rats against CCl4-Induced Hepatic Injuries through Modulation of an Unfolded Protein Response Pathway and Pro-Inflammatory Intermediates. Molecules. 2023; 28(5):2257. https://doi.org/10.3390/molecules28052257
Chicago/Turabian StyleBatool, Riffat, Muhammad Rashid Khan, Muhammad Umar Ijaz, Irum Naz, Afsheen Batool, Saima Ali, Zartash Zahra, Safia Gul, Mohammad N. Uddin, Mohsin Kazi, and et al. 2023. "Linum corymbulosum Protects Rats against CCl4-Induced Hepatic Injuries through Modulation of an Unfolded Protein Response Pathway and Pro-Inflammatory Intermediates" Molecules 28, no. 5: 2257. https://doi.org/10.3390/molecules28052257
APA StyleBatool, R., Khan, M. R., Ijaz, M. U., Naz, I., Batool, A., Ali, S., Zahra, Z., Gul, S., Uddin, M. N., Kazi, M., & Khan, R. (2023). Linum corymbulosum Protects Rats against CCl4-Induced Hepatic Injuries through Modulation of an Unfolded Protein Response Pathway and Pro-Inflammatory Intermediates. Molecules, 28(5), 2257. https://doi.org/10.3390/molecules28052257








