F2BMF (M = B and Al) Molecules: A Matrix Infrared Spectra and Theoretical Calculations Investigation
Abstract
1. Introduction
2. Results and Discussion
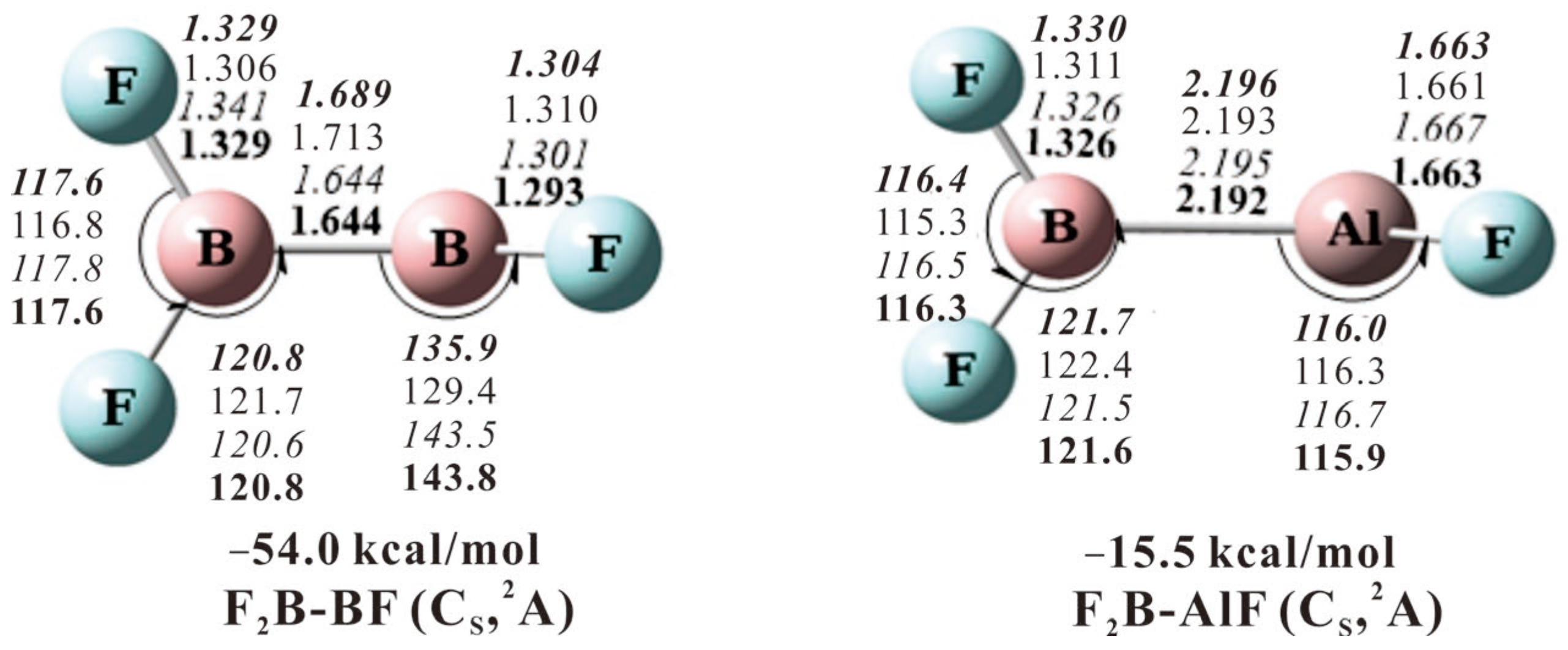
2.1. F2BBF
2.2. F2BAlF
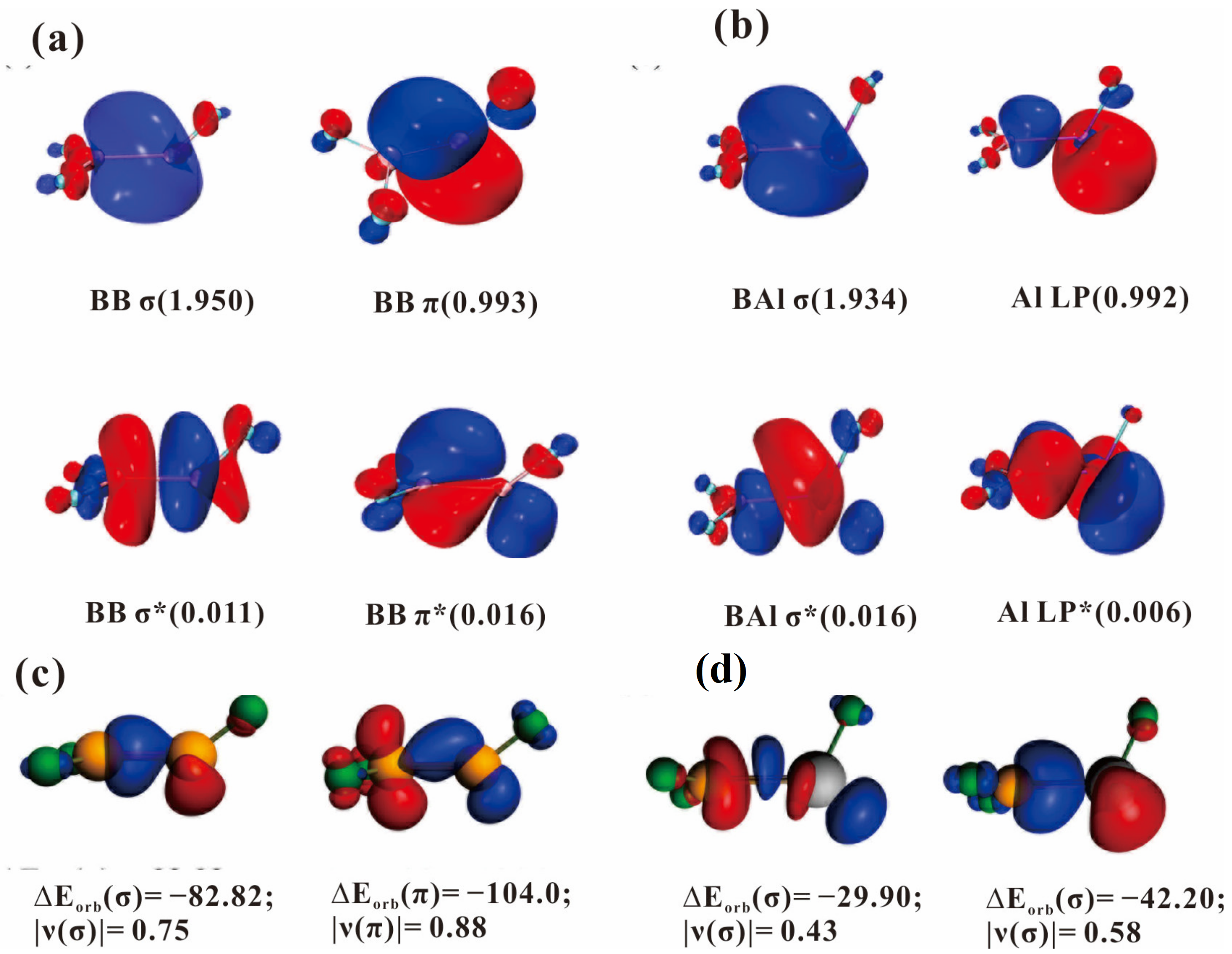
3. Reaction Product Comparison and Bonding Consideration
4. Experimental and Computational Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borthakur, R.; Saha, K.; Kar, S.; Ghosh, S. Recent advances in transition metal diborane(6), diborane(4) and diborene(2) chemistry. Coord. Chem. Rev. 2019, 399, 213021. [Google Scholar] [CrossRef]
- Trefonas, L.; Lipscomb, W.N. Crystal and Molecular Structure of Diboron Tetrafluoride, B2F4. J. Chem. Phys. 1958, 28, 54–55. [Google Scholar] [CrossRef]
- Nimon, L.A.; Seshadri, K.S.; Taylor, R.C.; White, D. Infrared Spectra and Geometry of Matrix-Isolated Diboron Tetrachloride and Tetrafluoride; Raman Spectra of the Liquids. J. Chem. Phys. 1970, 53, 2416–2427. [Google Scholar] [CrossRef]
- Finch, A.; Hyams, I.; Steele, D. The vibrational spectra of diboron compounds—I. Infrared spectra of diboron tetrafluoride. Spectrochim. Acta 1965, 21, 1423–1431. [Google Scholar] [CrossRef]
- Gayles, J.N.; Self, J. Infrared Spectrum of Diboron Tetrafluoride in the Gaseous and Solid States. J. Chem. Phys. 1964, 40, 3530–3539. [Google Scholar] [CrossRef]
- Li, Z.H.; Fan, K.N. B2F4 molecule: A challenge for theoretical calculations. J. Phys. Chem. A 2002, 106, 6659–6664. [Google Scholar] [CrossRef]
- Danielson, D.D.; Patton, J.V.; Hedberg, K. The effect of Temperature on Structure of Gaseous Molecules. 3. Molecular-Structure and Barrier to Internal-Rotation for Diboron Tetrafluoride. J. Am. Chem. Soc. 1977, 99, 6484–6487. [Google Scholar] [CrossRef]
- Korkin, A.A.; Balkova, A.; Bartlett, R.J.; Boyd, R.J.; Schleyer, P.v.R. The 28-Electron Tetraatomic Molecules: N4, CN2O, BFN2, C2O2, B2F2, CBFO, C2FN, and BNO2. Challenges for Computational and Experimental Chemistry. J. Phys. Chem. 1996, 100, 5702–5714. [Google Scholar] [CrossRef]
- Xu, B.; Beckers, H.; Ye, H.Y.; Lu, Y.; Cheng, J.J.; Wang, X.F.; Riede, l.S. Cleavage of the N identical withN Triple Bond and Unpredicted Formation of the Cyclic 1,3-Diaza-2,4-Diborete (FB)2N2 from N2 and Fluoroborylene BF. Angew. Chem. Int. Ed. Engl. 2021, 60, 17205–17210. [Google Scholar] [CrossRef]
- Andrews, L.; Lanzisera, D.V.; Hassanzadeh, P. Reactions of Laser-Ablated Boron Atoms with Ethylene and Ethane. Infrared Spectra and DFT Calculations for Several Novel BC2Hx (x = 1, 2, 3, 4, 5) Molecules. J. Phys. Chem. A 1998, 102, 3259. [Google Scholar] [CrossRef]
- Lanzisera, D.V.; Andrews, L. Reactions of laser-ablated boron atoms with methylamines. Matrix infrared spectra and MP2 frequency calculations for isotopic product molecules. J. Phys. Chem. A 1997, 101, 824–830. [Google Scholar] [CrossRef]
- Lanzisera, D.V.; Andrews, L. Reactions of laser-ablated boron atoms with methanol. Infrared spectra and ab initio calculations of CH3BO, CH2BOH, and CH2BO in solid argon. J. Phys. Chem. A 1997, 101, 1482–1487. [Google Scholar] [CrossRef]
- Lanzisera, D.V.; Andrews, L. Reactions of Laser-Ablated Boron Atoms with Methyl Halides in Excess Argon. Infrared Spectra and Density Functional Theory Calculations on CH3BX, CH2BX, and CHBX (X = F, Cl, Br). J. Phys. Chem. A 2000, 104, 9295–9301. [Google Scholar] [CrossRef]
- Xu, B.; Li, L.; Yu, W.J.; Huang, T.F.; Wang, X.F. Matrix Infrared Spectra and Theoretical Calculations of Fluoroboryl Complexes F2B-MF (M = C, Si, Ge, Sn and Pb). J. Phys. Chem. A 2018, 122, 7301–7311. [Google Scholar] [CrossRef]
- Apeloig, Y.; Pauncz, R.; Karni, M.; West, R.; Steiner, W.; Chapman, D. Why Is Methylene a Ground State Triplet while Silylene Is a Ground State Singlet? Organometallics 2003, 22, 3250–3256. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Andrews, L. Reaction of halogens with laser-ablated boron: Infrared spectra of BXn (X = F, Cl, Br, I; n = 1, 2, 3) in solid argon. J. Phys. Chem. 1993, 97, 4910–4915. [Google Scholar] [CrossRef]
- Wang, Y.; Quillian, B.; Wei, P.; Wannere, C.S.; Xie, Y.; King, R.B.; Schaefer, H.F., 3rd; Schleyer, P.V.; Robinson, G.H. A stable, neutral diborene containing a B=B double bond. J. Am. Chem. Soc. 2007, 129, 12412–12413. [Google Scholar] [CrossRef]
- Wang, Z.X.; Chen, Z.F.; Jiao, H.J.; Schleyer, P.V. Isolobal boron carbonyl carbocation analogs. J. Theor. Comput. Chem. 2005, 4, 669–688. [Google Scholar] [CrossRef]
- Zhao, L.; von Hopffgarten, M.; Andrada, D.M.; Frenking, G. Energy decomposition analysis. WIREs Comput. Mol. Sci. 2017, 8, e1345. [Google Scholar] [CrossRef]
- Xu, B.; Li, W.J.; Yu, W.J.; Pu, Z.; Tan, Z.; Cheng, J.J.; Wang, X.F.; Andrews, L. Boron-Transition-Metal Triple-Bond FB identical with MF2 Complexes. Inorg. Chem. 2019, 58, 13418–13425. [Google Scholar] [CrossRef]
- Mitoraj, M.P.; Michalak, A.; Ziegler, T. A Combined Charge; Energy Decomposition Scheme for Bond Analysis. J. Chem. Theory Comput. 2009, 5, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Mitoraj, M.; Michalak, A. Donor–Acceptor Properties of Ligands from the Natural Orbitals for Chemical Valence. Organometallics 2007, 26, 6576–6580. [Google Scholar] [CrossRef]
- Michalak, A.; Mitoraj, M.; Ziegler, T. Bond Orbitals from Chemical Valence Theory. J. Phys. Chem. A 2008, 112, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron-Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09; Carnegie Mellon University: Pittsburgh, PA, USA, 2009. [Google Scholar]
- Roos, B.O. Advances in Chemical Physics. In Ab Initio Methods in Quantum Chemistry-II; Lawley, K.P., Ed.; John Wiley & Sons Ltd.: New York, NY, USA, 1987; Chapter 69; pp. 399–445. [Google Scholar]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Purvis, G.D.; Bartlett, R.J. A Full Coupled-Cluster Singles and Doubles Model-the Inclusion of Disconnected Triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Raghavachari, K.; Trucks, G.W.; Pople, J.A.; Head-Gordon, M. A Fifth-Order Perturbation Comparison of Electron Correlation Theories. Chem. Phys. Lett. 1989, 157, 479. [Google Scholar] [CrossRef]
- Bartlett, R.J.; Musial, M. Coupled-Cluster Theory in Quantum Chemistry. Rev. Mod. Phys. 2007, 79, 291–352. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev.-Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev.-Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural-Population Analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Malrieu, J.P. N-electron valence state perturbation theory: A spinless formulation and an efficient implementation of the strongly contracted and of the partially contracted variants. J. Chem. Phys. 2002, 117, 9138–9153. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Malrieu, J.P. N-electron valence state perturbation theory: A fast implementation of the strongly contracted variant. Chem. Phys. Lett. 2001, 350, 297–305. [Google Scholar] [CrossRef]
- Pastore, M.; Angeli, C.; Cimiraglia, R. A multireference perturbation theory study on the vertical electronic spectrum of thiophene. Theor. Chem. Acc. 2007, 118, 35–46. [Google Scholar] [CrossRef]
- ADF; SCM. Theoretical Chemistry; Vrije Universiteit: Amsterdam, The Netherlands, 2017. [Google Scholar]
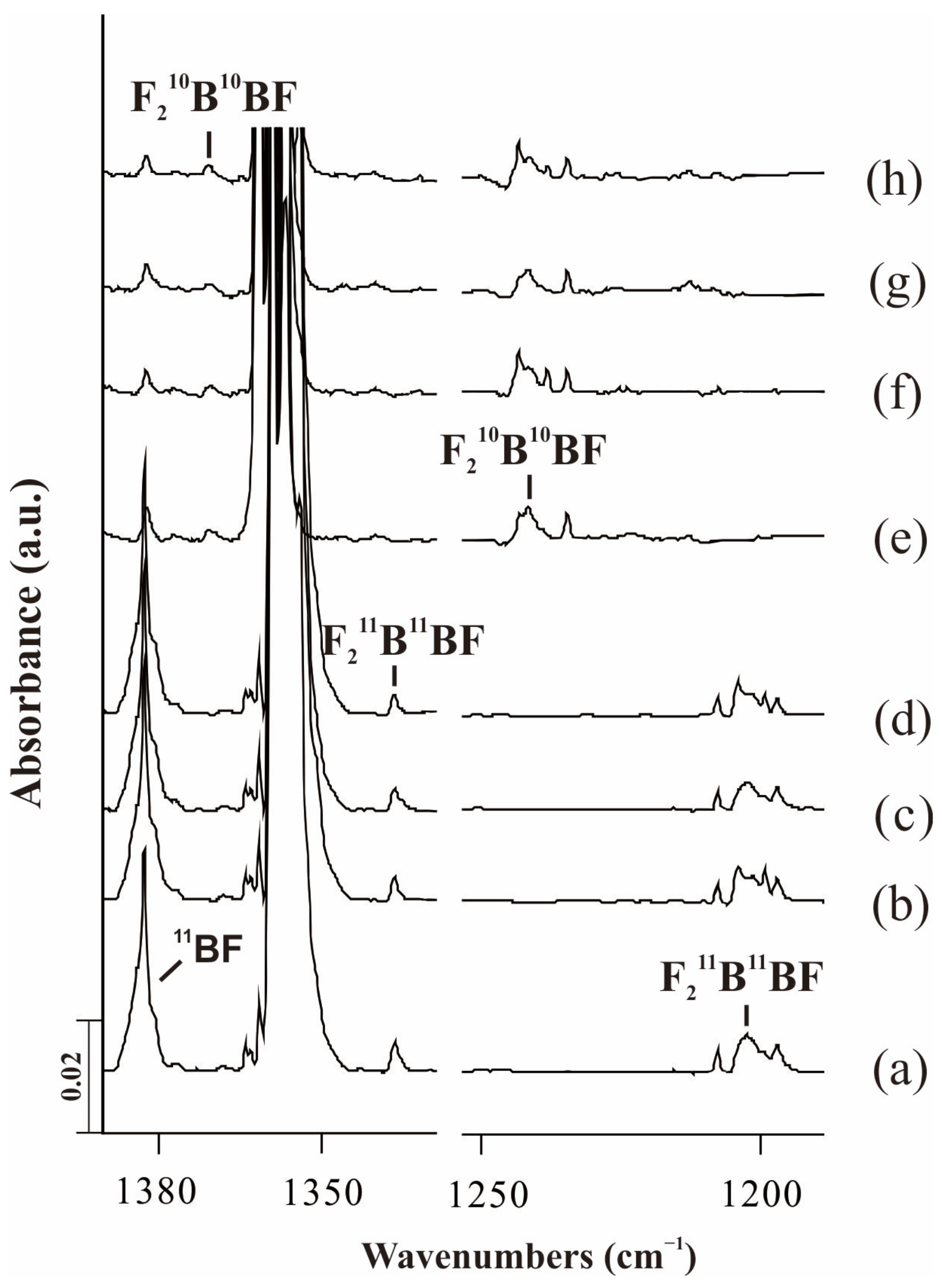
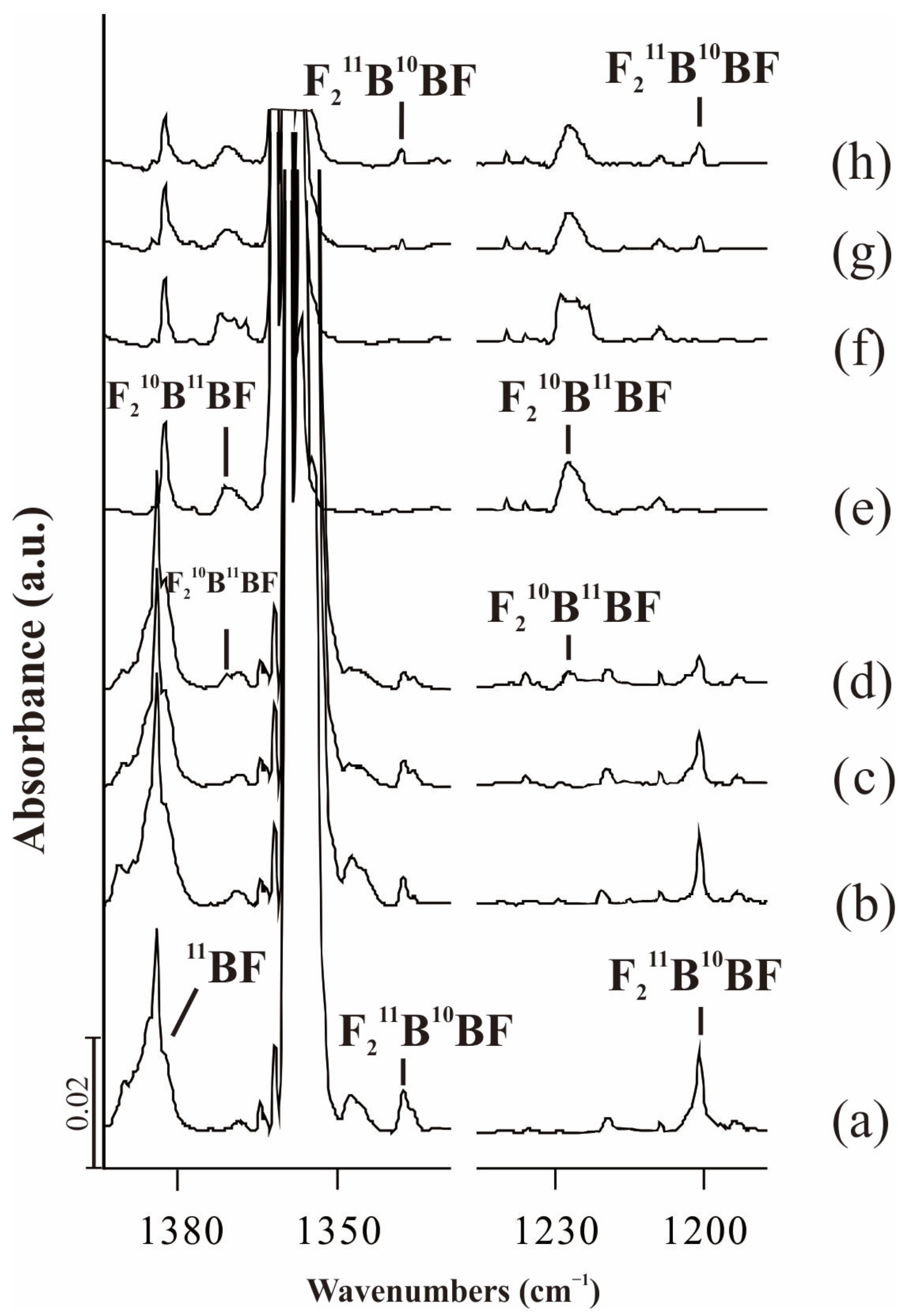


| Approximate Description | Obs(Ne) | Cal(int) a | Cal(int) b | Cal(int) c | Obs(Ne) | Cal(int) a | Cal(int) b | Cal(int) c |
|---|---|---|---|---|---|---|---|---|
| BBFantisymstr | / | 1491.2(52) | 1461.7(50) | 1466.2 | / | 1501.4(41) | 1471.7(37) | 1477.8 |
| BF2antisymstr | 1336.9 | 1332.3(294) | 1286.7(265) | 1376.3 | 1369.4 | 1378.8(317) | 1331.6(284) | 1425.1 |
| BF2symstr | 1202.4 | 1188.9(465) | 1150.9(427) | 1213.6 | 1223.8 | 1221.3(505) | 1182.6(465) | 1245.4 |
| F211B10BF | F210B10BF | |||||||
| BBFantisymstr | / | 1538.6(70) | 1508.0(63) | 1511.4 | / | 1546.82(56) | 1516.6(49) | 1520.8 |
| BF2antisymstr | 1338.7 | 1332.3(294) | 1286.7(265) | 1376.3 | 1370.6 | 1378.90(324) | 1331.6(284) | 1425.1 |
| BF2symstr | 1200.8 | 1192.3(462) | 1154.2(424) | 1217.9 | 1241.6 | 1226.32(505) | 1187.3(463) | 1251.9 |
| F211BAlF | F210BAlF | |||||||
| BF2antisymmstr | 1281.5 | 1312.0(277) | 1270.9(257) | 1342.1 | 1324.6 | 1357.4(299) | 1314.9(275) | 1389.1 |
| BF2symstr | 1180.8 | 1172.2(320) | 1133.9(309) | 1190.3 | 1217.1 | 1208.2(378) | 1168.8(336) | 1227.4 |
| AlFstr | 819.6 | 806.7(106) | 780.1(98) | 814.5 | 819.6 | 806.8(106) | 780.2(98) | 814.6 |
| Orbitals | F2BBF (2A) | Orbitals | F2BAlF (2A) |
|---|---|---|---|
| ΔEint | −148.3 | ΔEint | −28.84 |
| ΔEPauli | 97.5 | ΔEPauli | 111.5 |
| ΔEelstat | −53.0 (21.5%) | ΔEelstat | −60.4 (42.8%) |
| ΔEorb | −193.9 (78.5%) | ΔEorb | −80.7 (57.2%) |
| ΔEσ | −82.82 (42.7%) | ΔEσ | −29.9 (37.1%) |
| ΔEπ | −104.0 (53.6%) | ΔEσ | −42.2 (52.3%) |
| ΔEorb(rest) | −7.1 (3.7%) | ΔEorb(rest) | −8.6 (15.6%) |
| ΔEdist | 1.1 | ΔEdist | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Cai, L.; Pu, Z.; Xu, B.; Wang, X. F2BMF (M = B and Al) Molecules: A Matrix Infrared Spectra and Theoretical Calculations Investigation. Molecules 2023, 28, 554. https://doi.org/10.3390/molecules28020554
Cheng J, Cai L, Pu Z, Xu B, Wang X. F2BMF (M = B and Al) Molecules: A Matrix Infrared Spectra and Theoretical Calculations Investigation. Molecules. 2023; 28(2):554. https://doi.org/10.3390/molecules28020554
Chicago/Turabian StyleCheng, Juanjuan, Liyan Cai, Zhen Pu, Bing Xu, and Xuefeng Wang. 2023. "F2BMF (M = B and Al) Molecules: A Matrix Infrared Spectra and Theoretical Calculations Investigation" Molecules 28, no. 2: 554. https://doi.org/10.3390/molecules28020554
APA StyleCheng, J., Cai, L., Pu, Z., Xu, B., & Wang, X. (2023). F2BMF (M = B and Al) Molecules: A Matrix Infrared Spectra and Theoretical Calculations Investigation. Molecules, 28(2), 554. https://doi.org/10.3390/molecules28020554








