Discovery of a Novel Trifluoromethyl Diazirine Inhibitor of SARS-CoV-2 Mpro
Abstract
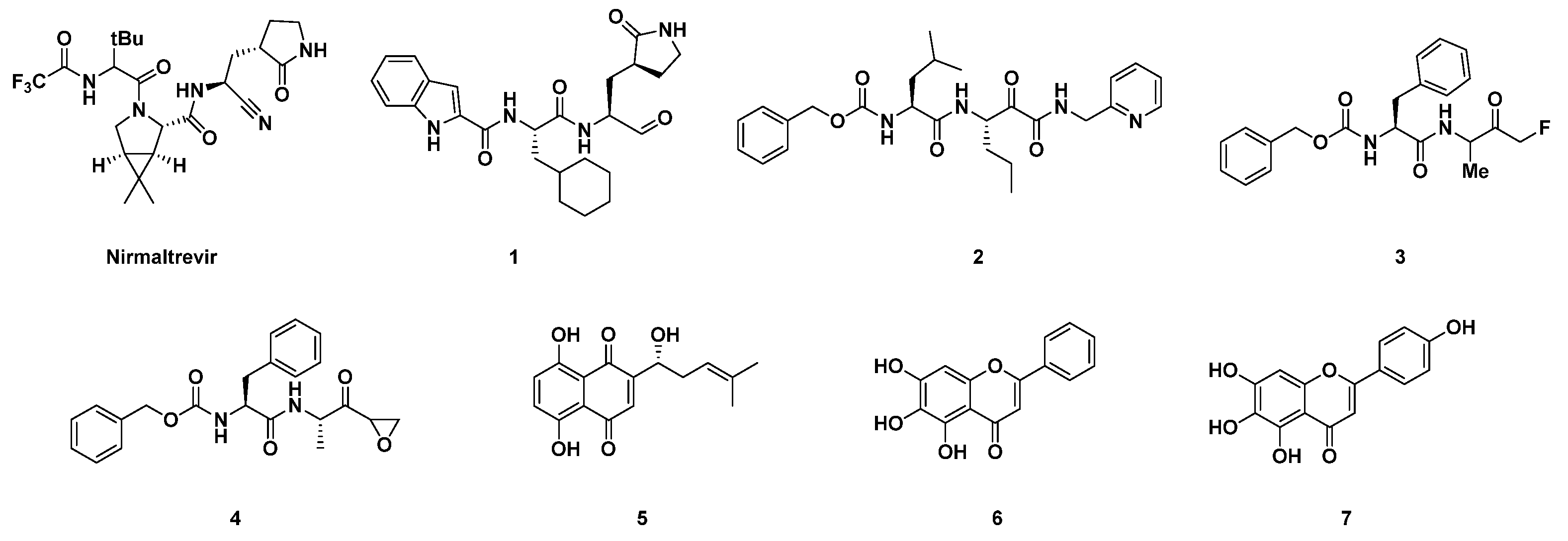
1. Introduction
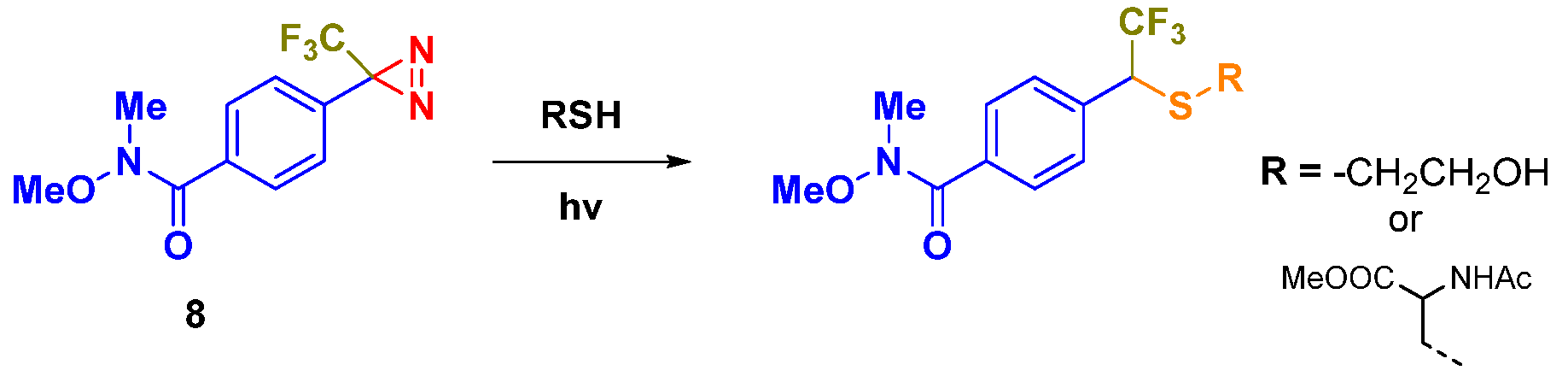
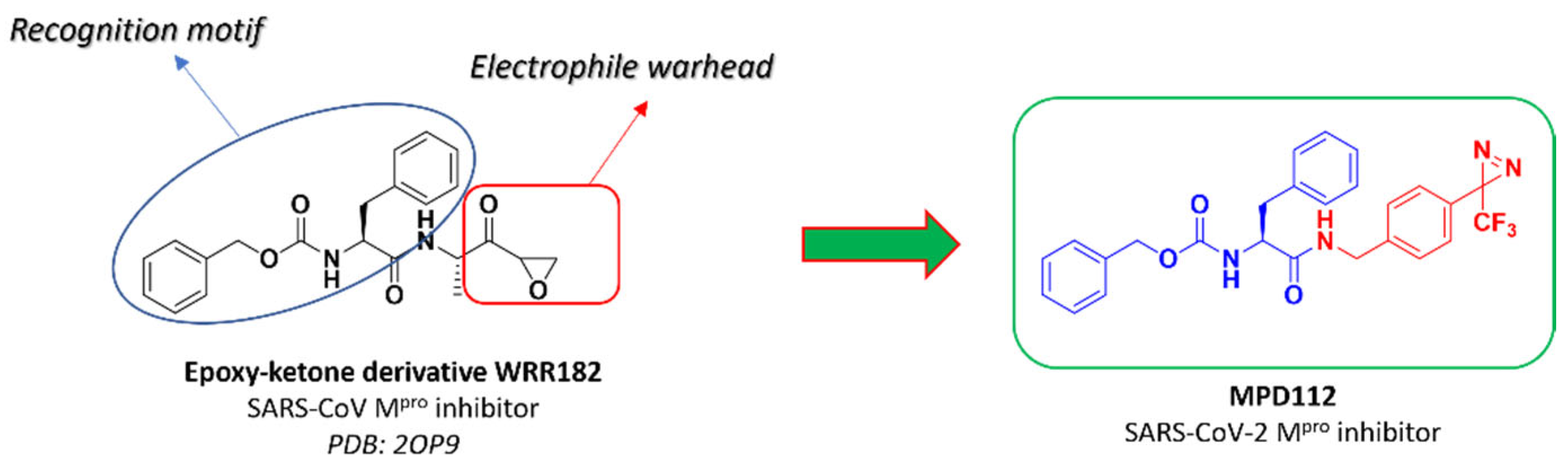
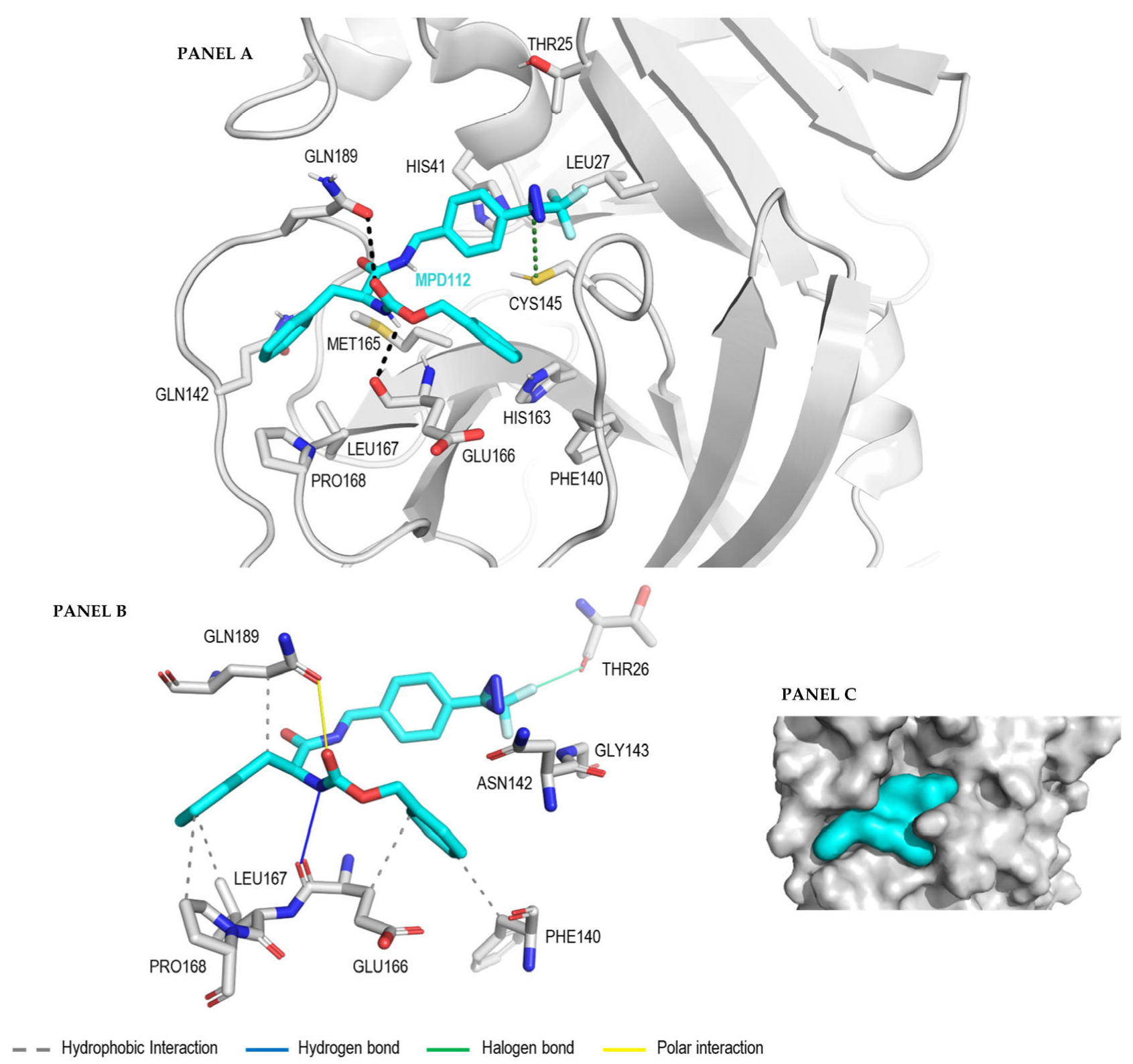
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Information
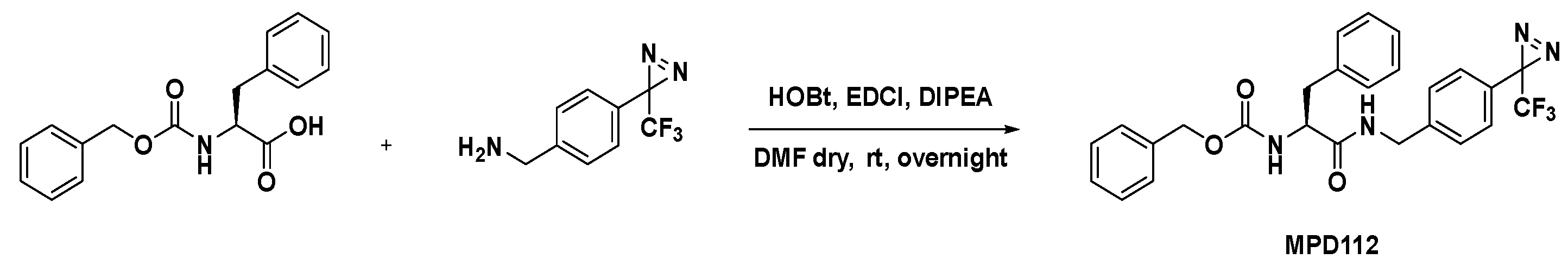
3.2. Synthesis of MPD112
3.3. Enzymatic Assay
3.4. Docking
3.5. Cells and Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Banerjee, R.; Perera, L.; Tillekeratne, L.V. Potential SARS-CoV-2 main protease inhibitors. Drug Discov. Today 2021, 26, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Citarella, A.; Scala, A.; Piperno, A.; Micale, N. SARS-CoV-2 M(pro): A Potential Target for Peptidomimetics and Small-Molecule Inhibitors. Biomolecules 2021, 11, 607. [Google Scholar] [CrossRef]
- Yan, S.; Wu, G. Spatial and temporal roles of SARS-CoV PLpro—A snapshot. FASEB J. 2021, 35, e21197. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Zhong, N.; Zhang, S.; Zou, P.; Chen, J.; Kang, X.; Li, Z.; Liang, C.; Jin, C.; Xia, B. Without its N-finger, the main protease of severe acute respiratory syndrome coronavirus can form a novel dimer through its C-terminal domain. J. Virol. 2008, 82, 4227–4234. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. COVID-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ 2021, 375, n2713. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yuan, S.; Wei, X.; Sun, H. Metal-based strategies for the fight against COVID-19. Chem. Commun. 2022, 58, 7466–7482. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chan, J.F.; Wang, S.; Li, H.; Zhao, J.; Ip, T.K.; Zuo, Z.; Yuen, K.Y.; Yuan, S.; Sun, H. Orally administered bismuth drug together with N-acetyl cysteine as a broad-spectrum anti-coronavirus cocktail therapy. Chem. Sci. 2022, 13, 2238–2248. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, J. A review of the latest research on M(pro) targeting SARS-COV inhibitors. RSC Med. Chem. 2021, 12, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, X.; Zhang, Y.; Zhong, F.; Lin, C.; McCormick, P.J.; Jiang, F.; Luo, J.; Zhou, H.; Wang, Q.; et al. Crystal structure of SARS-CoV-2 main protease in complex with the natural product inhibitor shikonin illuminates a unique binding mode. Sci. Bull. 2021, 66, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky, L.; Krom, B.P.; Meijler, M.M. Diazirine based photoaffinity labeling. Bioorg. Med. Chem. 2012, 20, 554–570. [Google Scholar] [CrossRef] [PubMed]
- Ollevier, T.; Carreras, V. Emerging Applications of Aryl Trifluoromethyl Diazoalkanes and Diazirines in Synthetic Transformations. ACS Org. Inorg. Au 2022, 2, 83–98. [Google Scholar] [CrossRef]
- Tuley, A.; Fast, W. The Taxonomy of Covalent Inhibitors. Biochemistry 2018, 57, 3326–3337. [Google Scholar] [CrossRef] [PubMed]
- West, A.V.; Muncipinto, G.; Wu, H.Y.; Huang, A.C.; Labenski, M.T.; Jones, L.H.; Woo, C.M. Labeling Preferences of Diazirines with Protein Biomolecules. J. Am. Chem. Soc. 2021, 143, 6691–6700. [Google Scholar] [CrossRef] [PubMed]
- Goetz, D.H.; Choe, Y.; Hansell, E.; Chen, Y.T.; McDowell, M.; Jonsson, C.B.; Roush, W.R.; McKerrow, J.; Craik, C.S. Substrate Specificity Profiling and Identification of a New Class of Inhibitor for the Major Protease of the SARS Coronavirus. Biochemistry 2007, 46, 8744–8752. [Google Scholar] [CrossRef] [PubMed]
- Citarella, A.; Gentile, D.; Rescifina, A.; Piperno, A.; Mognetti, B.; Gribaudo, G.; Sciortino, M.T.; Holzer, W.; Pace, V.; Micale, N. Pseudo-Dipeptide Bearing α,α-Difluoromethyl Ketone Moiety as Electrophilic Warhead with Activity against Coronaviruses. Int. J. Mol. Sci. 2021, 22, 1398. [Google Scholar] [CrossRef] [PubMed]
- Korshunova, G.A.; Sumbatyan, N.V.; Topin, A.N.; Mtchedlidze, M.T. Photoactivatable Reagents Based on Aryl(trifluoromethyl)diazirines: Synthesis and Application for Studying Nucleic Acid–Protein Interactions. Mol. Biol. 2000, 34, 823–839. [Google Scholar] [CrossRef]
- Jackson, P.A.; Widen, J.C.; Harki, D.A.; Brummond, K.M. Covalent Modifiers: A Chemical Perspective on the Reactivity of α,β-Unsaturated Carbonyls with Thiols via Hetero-Michael Addition Reactions. J. Med. Chem. 2017, 60, 839–885. [Google Scholar] [CrossRef] [PubMed]
- Amendola, G.; Ettari, R.; Previti, S.; Di Chio, C.; Messere, A.; Di Maro, S.; Hammerschmidt, S.J.; Zimmer, C.; Zimmermann, R.A.; Schirmeister, T.; et al. Lead Discovery of SARS-CoV-2 Main Protease Inhibitors through Covalent Docking-Based Virtual Screening. J. Chem. Inf. Model. 2021, 61, 2062–2073. [Google Scholar] [CrossRef] [PubMed]
- Calistri, A.; Luganini, A.; Mognetti, B.; Elder, E.; Sibille, G.; Conciatori, V.; Del Vecchio, C.; Sainas, S.; Boschi, D.; Montserrat, N.; et al. The New Generation hDHODH Inhibitor MEDS433 Hinders the In Vitro Replication of SARS-CoV-2 and Other Human Coronaviruses. Microorganisms 2021, 9, 1731. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Citarella, A.; Moi, D.; Pedrini, M.; Pérez-Peña, H.; Pieraccini, S.; Stagno, C.; Micale, N.; Schirmeister, T.; Sibille, G.; Gribaudo, G.; et al. Discovery of a Novel Trifluoromethyl Diazirine Inhibitor of SARS-CoV-2 Mpro. Molecules 2023, 28, 514. https://doi.org/10.3390/molecules28020514
Citarella A, Moi D, Pedrini M, Pérez-Peña H, Pieraccini S, Stagno C, Micale N, Schirmeister T, Sibille G, Gribaudo G, et al. Discovery of a Novel Trifluoromethyl Diazirine Inhibitor of SARS-CoV-2 Mpro. Molecules. 2023; 28(2):514. https://doi.org/10.3390/molecules28020514
Chicago/Turabian StyleCitarella, Andrea, Davide Moi, Martina Pedrini, Helena Pérez-Peña, Stefano Pieraccini, Claudio Stagno, Nicola Micale, Tanja Schirmeister, Giulia Sibille, Giorgio Gribaudo, and et al. 2023. "Discovery of a Novel Trifluoromethyl Diazirine Inhibitor of SARS-CoV-2 Mpro" Molecules 28, no. 2: 514. https://doi.org/10.3390/molecules28020514
APA StyleCitarella, A., Moi, D., Pedrini, M., Pérez-Peña, H., Pieraccini, S., Stagno, C., Micale, N., Schirmeister, T., Sibille, G., Gribaudo, G., Silvani, A., Passarella, D., & Giannini, C. (2023). Discovery of a Novel Trifluoromethyl Diazirine Inhibitor of SARS-CoV-2 Mpro. Molecules, 28(2), 514. https://doi.org/10.3390/molecules28020514









