A Double-Layer Hydrogel Dressing with High Mechanical Strength and Water Resistance Used for Drug Delivery
Abstract
1. Introduction
2. Results and Discussion
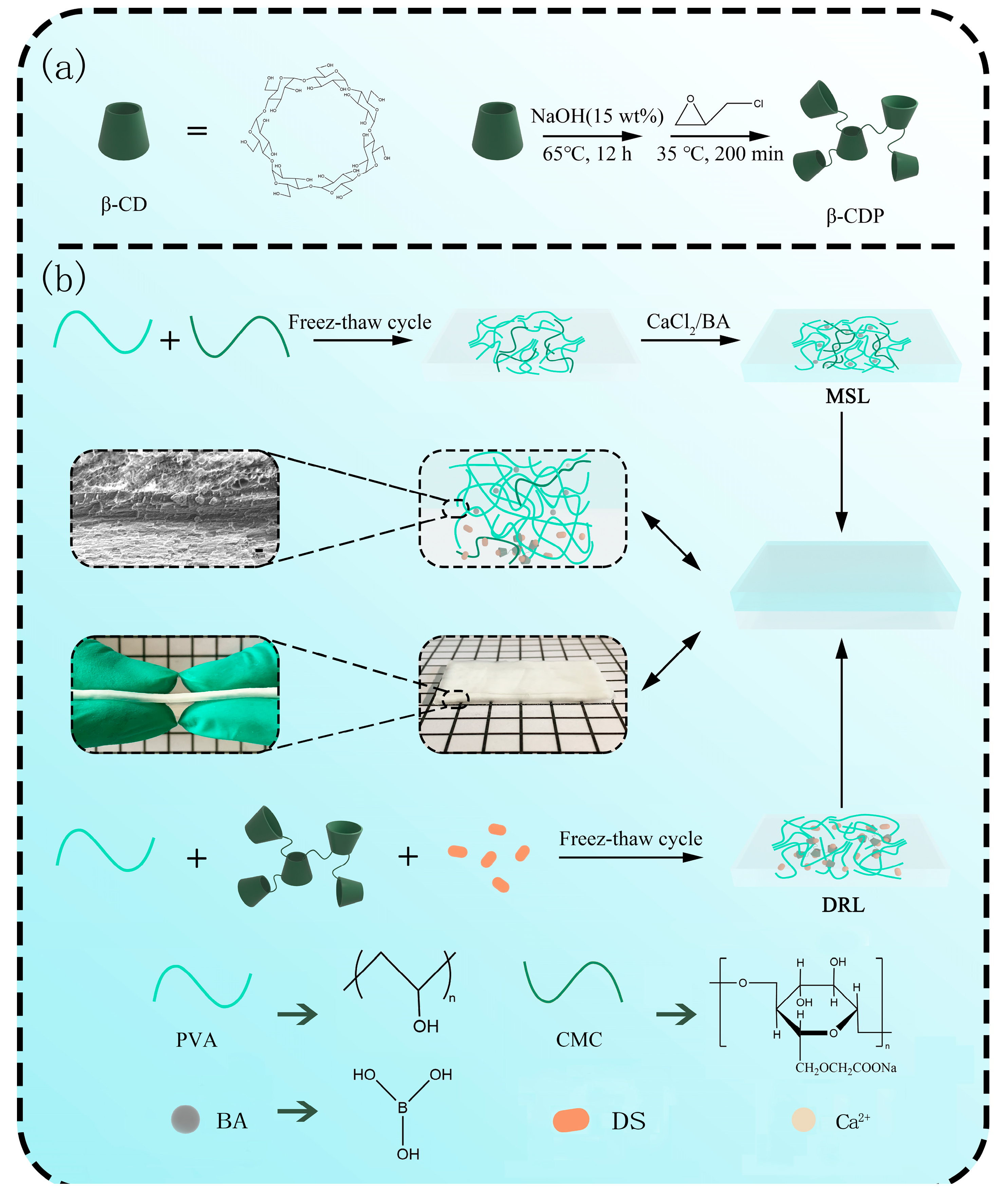
2.1. Synthesis and Characterization of β-CDP

2.2. Preparation and Mechanical Property Analysis of MSL
2.3. Preparation and Drug Release Behavior Analysis of DRL
2.4. Preparation and Characterization of DL Hydrogel
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Synthesis of β-CD Polymers (β-CDP) and DS/β-CDP
3.4. Preparation of Double-Layer (DL) Hydrogel
| Level | A (PVA/g) | B (β-CDP/g) | C (DS/g) | D (Freeze–Thaw Times) |
|---|---|---|---|---|
| 1 | 1.50 | 1.00 | 0.20 | 1 |
| 2 | 2.00 | 1.25 | 0.25 | 2 |
| 3 | 2.50 | 1.50 | 0.30 | 3 |
3.5. Determination the Solubility, Polymerization Degree, and Activity of β-CDP
3.6. Static Tensile Test of DL Hydrogel
3.7. Drug Release Analysis of DL Hydrogel
3.8. Rheological Measurements
3.9. Determination of Water Retention
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, Z.Y.; Zhu, M.M.; Chen, L.; Zhang, Y.Y.; Lu, T.; Deng, Y.K.; Ma, W.J.; Xu, J.H.; Huang, C.B.; Xiong, R.H. Design the molecule structures to achieve functional advantages of hydrogel wound dressings: Advances and strategies. Compos. Pt. B-Eng. 2022, 247, 20. [Google Scholar] [CrossRef]
- Jin, S.G. Production and Application of Biomaterials Based on Polyvinyl alcohol (PVA) as Wound Dressing. Chem.-Asian J. 2022, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Do, N.H.N.; Lac, H.D.; Nguyen, P.L.N.; Le, P.K. Synthesis, properties, and applications of chitosan hydrogels as anti-inflammatory drug delivery system. J. Porous Mater. 2022, 16, 1–16. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Li, L.; Xu, L.; Feng, N.; Wang, Y.; Fei, X.; Tian, J.; Li, Y. Novel Nonreleasing Antibacterial Hydrogel Dressing by a One-Pot Method. ACS Biomater. Sci. Eng. 2020, 6, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Noack, A.K.; Lucas, H.; Chytil, P.; Etrych, T.; Mader, K.; Mueller, T. Intratumoral Distribution and pH-Dependent Drug Release of High Molecular Weight HPMA Copolymer Drug Conjugates Strongly Depend on Specific Tumor Substructure and Microenvironment. Int. J. Mol. Sci. 2020, 21, 6029. [Google Scholar] [CrossRef]
- Lotfy, V.F.; Basta, A.H. Optimizing the chitosan-cellulose based drug delivery system for controlling the ciprofloxacin release versus organic/inorganic crosslinker, characterization and kinetic study. Int. J. Biol. Macromol. 2020, 165 Pt A, 1496–1506. [Google Scholar] [CrossRef]
- Kim, S.; Jung, S. Biocompatible and self-recoverable succinoglycan dialdehyde-crosslinked alginate hydrogels for pH-controlled drug delivery. Carbohydr. Polym. 2020, 250, 116934. [Google Scholar] [CrossRef]
- Sharaf, S.; El-Naggar, M.E. Wound dressing properties of cationized cotton fabric treated with carrageenan/cyclodextrin hydrogel loaded with honey bee propolis extract. Int. J. Biol. Macromol. 2019, 133, 583–591. [Google Scholar] [CrossRef]
- Chen, H.; Peng, C.; Wang, L.; Li, X.; Yang, M.; Liu, H.; Qin, H.; Chen, W. Mechanically tough, healable hydrogels synergistically reinforced by UV-responsive crosslinker and metal coordination interaction for wound healing application. Chem. Eng. J. 2021, 403, 126341. [Google Scholar] [CrossRef]
- Yong, J.W.; Ge, L.; Ng, Y.F.; Tan, S.N. The chemical composition and biological properties of coconut (Cocos nucifera L.) water. Molecules 2009, 14, 5144–5164. [Google Scholar] [CrossRef]
- Graupner, N.; Labonte, D.; Humburg, H.; Buzkan, T.; Dorgens, A.; Kelterer, W.; Mussig, J. Functional gradients in the pericarp of the green coconut inspire asymmetric fibre-composites with improved impact strength, and preserved flexural and tensile properties. Bioinspir. Biomim. 2017, 12, 026009. [Google Scholar] [CrossRef]
- Li, C.; Xue, Y.; Han, M.; Palmer, L.C.; Rogers, J.A.; Huang, Y.; Stupp, S.I. Synergistic photoactuation of bilayered spiropyran hydrogels for predictable origami-like shape change. Matter 2021, 4, 1377–1390. [Google Scholar] [CrossRef]
- He, X.; Sun, Y.; Wu, J.; Wang, Y.; Chen, F.; Fan, P.; Zhong, M.; Xiao, S.; Zhang, D.; Yang, J.; et al. Dual-stimulus bilayer hydrogel actuators with rapid, reversible, bidirectional bending behaviors. J. Mater. Chem. C 2019, 7, 4970–4980. [Google Scholar] [CrossRef]
- Tavakoli, J.; Mirzaei, S.; Tang, Y. Cost-Effective Double-Layer Hydrogel Composites for Wound Dressing Applications. Polymers 2018, 10, 305. [Google Scholar] [CrossRef]
- Aycan, D.; Yayla, N.A.; Aydin, Y.A. Chitosan polyvinyl alcohol blend films for ibuprofen encapsulation: Fabrication, characterization and kinetics. Polym. Degrad. Stab. 2020, 181, 109346. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, D.; Li, Z.; Luo, D.; Hang, L.; Jing, J.; Shah, B.R. Controlled release of lysozyme based core/shells structured alginate beads with CaCO3 microparticles using Pickering emulsion template and in situ gelation. Colloids Surf. B Biointerfaces 2019, 183, 110410. [Google Scholar] [CrossRef]
- Xu, W.; Huang, L.; Jin, W.; Ge, P.; Shah, B.R.; Zhu, D.; Jing, J. Encapsulation and release behavior of curcumin based on nanoemulsions-filled alginate hydrogel beads. Int. J. Biol. Macromol. 2019, 134, 210–215. [Google Scholar] [CrossRef]
- Ijaz, M.; Matuszczak, B.; Rahmat, D.; Mahmood, A.; Bonengel, S.; Hussain, S.; Huck, C.W.; Bernkop-Schnurch, A. Synthesis and characterization of thiolated beta-cyclodextrin as a novel mucoadhesive excipient for intra-oral drug delivery. Carbohydr. Polym. 2015, 132, 187–195. [Google Scholar] [CrossRef]
- Mallard Favier, I.; Baudelet, D.; Fourmentin, S. VOC trapping by new crosslinked cyclodextrin polymers. J. Incl. Phenom. Macrocycl. Chem. 2010, 69, 433–437. [Google Scholar] [CrossRef]
- Sabadini, E.; Cosgrove, T.; Egidio, F.C. Solubility of cyclomaltooligosaccharides (cyclodextrins) in H2O and D2O: A comparative study. Carbohydr. Res. 2006, 341, 270–274. [Google Scholar] [CrossRef]
- Quintanilla de Stefano, J.C.; Abundis-Correa, V.; Herrera-Flores, S.D.; Alvarez, A.J. pH-Sensitive Starch-Based Hydrogels: Synthesis and Effect of Molecular Components on Drug Release Behavior. Polymers 2020, 12, 1974. [Google Scholar] [CrossRef] [PubMed]
- Colombo, P.; Bettini, R.; Santi, P.; Peppas, N.A. Swellable matrices for controlled drug delivery: Gel-layer behaviour, mechanisms and optimal performance. Pharm. Sci. Technol. Today 2000, 3, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Sasa, B.; Odon, P.; Stane, S.; Julijana, K. Analysis of surface properties of cellulose ethers and drug release from their matrix tablets. Eur. J. Pharm. Sci. 2006, 27, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, G.; Peng, L.; Guo, J.; Tao, L.; Yuan, J.; Chang, C.; Wei, Y.; Zhang, L. Highly Efficient Self-Healable and Dual Responsive Cellulose-Based Hydrogels for Controlled Release and 3D Cell Culture. Adv. Funct. Mater. 2017, 27, 1703174. [Google Scholar] [CrossRef]
- Guo, Z.; Ma, W.; Gu, H.; Feng, Y.; He, Z.; Chen, Q.; Mao, X.; Zhang, J.; Zheng, L. pH-Switchable and self-healable hydrogels based on ketone type acylhydrazone dynamic covalent bonds. Soft Matter 2017, 13, 7371–7380. [Google Scholar] [CrossRef]
- Huang, C.; Dong, L.; Zhao, B.; Lu, Y.; Huang, S.; Yuan, Z.; Luo, G.; Xu, Y.; Qian, W. Anti-inflammatory hydrogel dressings and skin wound healing. Clin. Transl. Med. 2022, 12, e1094. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr. Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef]
- Ye, H.; Owh, C.; Jiang, S.; Ng, C.Z.Q.; Wirawan, D.; Loh, X.J. A Thixotropic Polyglycerol Sebacate-Based Supramolecular Hydrogel as an Injectable Drug Delivery Matrix. Polymers 2016, 8, 130. [Google Scholar] [CrossRef]
- Zeng, N.; Dumortier, G.; Maury, M.; Mignet, N.; Boudy, V. Influence of additives on a thermosensitive hydrogel for buccal delivery of salbutamol: Relation between micellization, gelation, mechanic and release properties. Int. J. Pharm. 2014, 467, 70–83. [Google Scholar] [CrossRef]
- Shen, Z.; Cai, N.; Xue, Y.; Chan, V.; Yu, B.; Wang, J.; Song, H.; Deng, H.; Yu, F. Engineering Sustainable Antimicrobial Release in Silica-Cellulose Membrane with CaCO3-Aided Processing for Wound Dressing Application. Polymers 2019, 11, 808. [Google Scholar] [CrossRef]
- Wang, Y.; Delgado-Fukushima, E.; Fu, R.X.; Doerk, G.S.; Monclare, J.K. Controlling Drug Absorption, Release, and Erosion of Photopatterned Protein Engineered Hydrogels. Biomacromolecules 2020, 21, 3608–3619. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Wang, Y.; Garcia, C.R.; Ding, Z.; Gabrilska, R.; Rumbaugh, K.P.; Wu, J.; Liu, Q.; Li, W. Adhesive, Self-Healing, and Antibacterial Chitosan Hydrogels with Tunable Two-Layer Structures. ACS Sustain. Chem. Eng. 2020, 8, 18006–18014. [Google Scholar] [CrossRef]
- Abubakr, N.; Jayemanne, A.; Audrey, N.; Lin, S.X.; Chen, X.D. Effects of encapsulation process parameters of calcium alginate beads on Vitamin B12drug release kinetics. Asia-Pac. J. Chem. Eng. 2009, 5, 804–810. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, X.; Li, H.; Wei, C.X.; Wu, C.W. Onion-structure bionic hydrogel capsules based on chitosan for regulating doxorubicin release. Carbohydr. Polym. 2019, 209, 152–160. [Google Scholar] [CrossRef]
- Liu, B.; Huang, W.; Yang, G.; An, Y.; Yin, Y.; Wang, N.; Jiang, B. Preparation of gelatin/poly (gamma-glutamic acid) hydrogels with stimulated response by hot-pressing preassembly and radiation crosslinking. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111259. [Google Scholar] [CrossRef]
- Sharma, S.; Jain, P.; Tiwari, S. Dynamic imine bond based chitosan smart hydrogel with magnified mechanical strength for controlled drug delivery. Int. J. Biol. Macromol. 2020, 160, 489–495. [Google Scholar] [CrossRef]
- Koopmans, C.; Ritter, H. Formation of Physical Hydrogels via Host−Guest Interactions of β-Cyclodextrin Polymers and Copolymers Bearing Adamantyl Groups. Macromolecules 2008, 41, 7418–7422. [Google Scholar] [CrossRef]
- Wang, T.; Li, B.; Si, H.; Lin, L. Investigation on surface activity of cyclodextrins grafting cellulose beads through phenolphthalein probe molecule. Surf. Interface Anal. 2011, 43, 1532–1538. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef] [PubMed]




| Samples | k | n | |
|---|---|---|---|
| Without β-CDP | 0.88 | 0.036 | 0.9972 |
| With β-CDP | 0.64 | 0.14 | 0.9717 |
| Model | k | n | |||||
|---|---|---|---|---|---|---|---|
| Ritger–Peppa model | 0.2868 | 0.2072 | - | - | - | - | 0.5963 |
| Diffusion–relaxation model | - | - | 0.3166 | 0.04741 | - | - | 0.7944 |
| Diffusion–relaxation–corrosion model | - | - | 0.03668 | 0.1558 | 0.01459 | 0.00036 | 0.9902 |
| Diffusion Exponent (m) | Mechanism | ||
|---|---|---|---|
| Film | Cylinder | Sphere | |
| 0.50 | 0.45 | 0.43 | Fickian diffusion |
| 0.50 < m < 1.00 | 0.45 < m < 0.89 | 0.43 < m < 0.85 | Anomalous transport |
| 1.00 | 0.89 | 0.85 | Case Ⅱ transport |
| Matrix | Structure and Morphology | G′ | Water Retention | Drug Releasing Kinetic |
|---|---|---|---|---|
| AA, HEMA, AAm | Single-layer film | 104 Pa | - | Ritger-Peppas model [21] |
| CHI, ACHI, PA | Double-layer film | 102 Pa | - | -[33] |
| Ca-Alg | Single-layer sphere | - | - | Diffusion-relaxation model [34] |
| CS, TPP, HA | Multiple-layer sphere | - | - | Ritger-Peppas model [35] |
| Gel, γ-PGA | Single-layer film | - | 48 h, 37% | -[36] |
| CS, cuminaldehyde | Single-layer film | 103 Pa | - | -[37] |
| This work | Double-layer film | 2.5 × 104 Pa | 48 h, 67% | Diffusion-relaxation-erosion model |
| Level | A (PVA/g) | B (CMC/g) | C (Soaking Time/h) |
|---|---|---|---|
| 1 | 2.50 | 0.10 | 0.50 |
| 2 | 3.00 | 0.20 | 1.00 |
| 3 | 3.50 | 0.30 | 1.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Wang, Z.; Guo, H.; Li, H.; Chen, Y.; Guan, S. A Double-Layer Hydrogel Dressing with High Mechanical Strength and Water Resistance Used for Drug Delivery. Molecules 2023, 28, 499. https://doi.org/10.3390/molecules28020499
Liu F, Wang Z, Guo H, Li H, Chen Y, Guan S. A Double-Layer Hydrogel Dressing with High Mechanical Strength and Water Resistance Used for Drug Delivery. Molecules. 2023; 28(2):499. https://doi.org/10.3390/molecules28020499
Chicago/Turabian StyleLiu, Fangzhe, Zihan Wang, Hui Guo, Haichao Li, Yulan Chen, and Shuang Guan. 2023. "A Double-Layer Hydrogel Dressing with High Mechanical Strength and Water Resistance Used for Drug Delivery" Molecules 28, no. 2: 499. https://doi.org/10.3390/molecules28020499
APA StyleLiu, F., Wang, Z., Guo, H., Li, H., Chen, Y., & Guan, S. (2023). A Double-Layer Hydrogel Dressing with High Mechanical Strength and Water Resistance Used for Drug Delivery. Molecules, 28(2), 499. https://doi.org/10.3390/molecules28020499




