Molecular Iodine Capture by Covalent Organic Frameworks
Abstract
1. Introduction
2. Mechanism of Iodine Capture by COFs
2.1. Methods for Studying Adsorption Mechanism
2.2. Physical Adsorption of Iodine by COFs
2.3. Synergistic Effect of Physical and Chemical Adsorption
3. Factors Affecting the Molecular Iodine Capture Performance of COFs
3.1. Structural Characteristics of COFs
3.1.1. Porosity
3.1.2. π-Conjugated Systems
3.1.3. Dimensions of COFs
3.1.4. Flexibility of the Skeleton
3.2. Electron-Rich Functional Groups
| COFs | BET (m2·g−1) | Pore Size (nm) | Pore Volume (cm3·g−1) | I2 Uptake (g·g−1) | Adsorption Equilibrium Time (h) | Adsorption Mechanism a | Ref. |
|---|---|---|---|---|---|---|---|
| PA-TT COF | 48.6 | 2.45 | 0.112 | 5.1 | 12 | P-C | [48] |
| PB-TT COF | 1305.3 | 3.67 | 0.986 | 5.97 | 72 | P-C | [48] |
| TTF-TAPT | 461 | / | 0.28 | 5.02 | 18 | P-C | [49] |
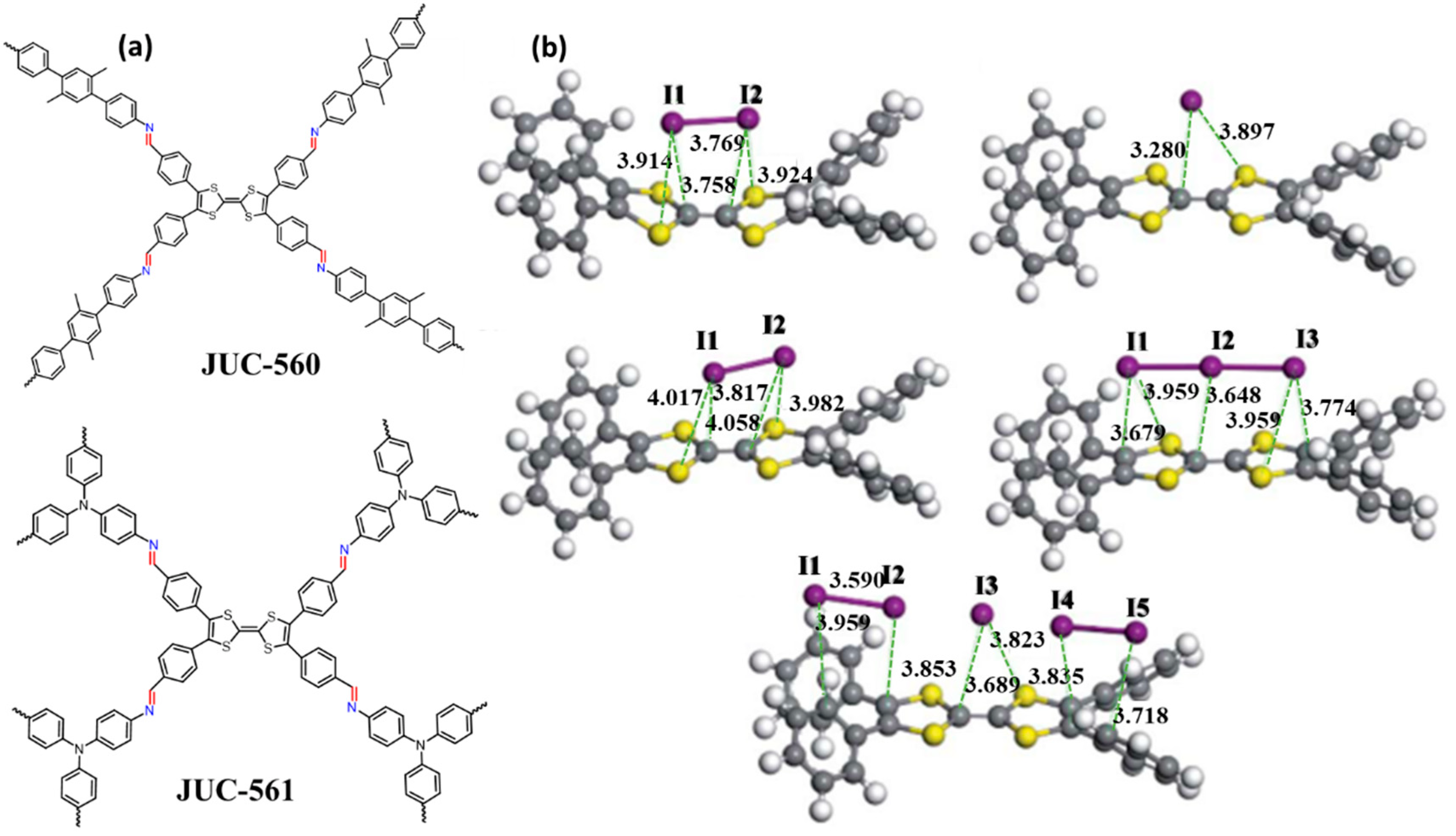
| JUC-560 | 1815 | 2.6 | 1.11 | 5.2 | 14 | P-C | [50] |
| JUC-561 | 2359 | 2.55 | 1.92 | 8.19 | 18 | P-C | [50] |
| TPB-DMTP | 1927 | 3.3 | 1.28 | 6.26 | 48 | P | [53] |
| TTA-TTB | 1733 | 2.2 | 1.01 | 4.95 | 96 | P-C | [53] |
| Micro-COF-1 | 816 | 1.6 | 0.59 | 2.9 | 75 | P-C | [54] |
| Micro-COF-2 | 1056 | 1.7 | 0.71 | 3.5 | 75 | P-C | [54] |
| Meso-COF-3 | 982 | 4.0 | 0.84 | 4 | 75 | P-C | [54] |
| Meso-COF-4 | 926 | 4.7 | 1.01 | 3.3 | 75 | P-C | [54] |
| COF-TpgDB | 209.6 | 6.8 | 0.36 | 2.75 | 65 | P-C | [58] |
| COF-TpgBD | 217.9 | 8.3 | 0.46 | 1.81 | 65 | P-C | [58] |
| QTD-COF-1 | / | 1.36/1.72 | / | 6.29 | 20 | P-C | [60] |
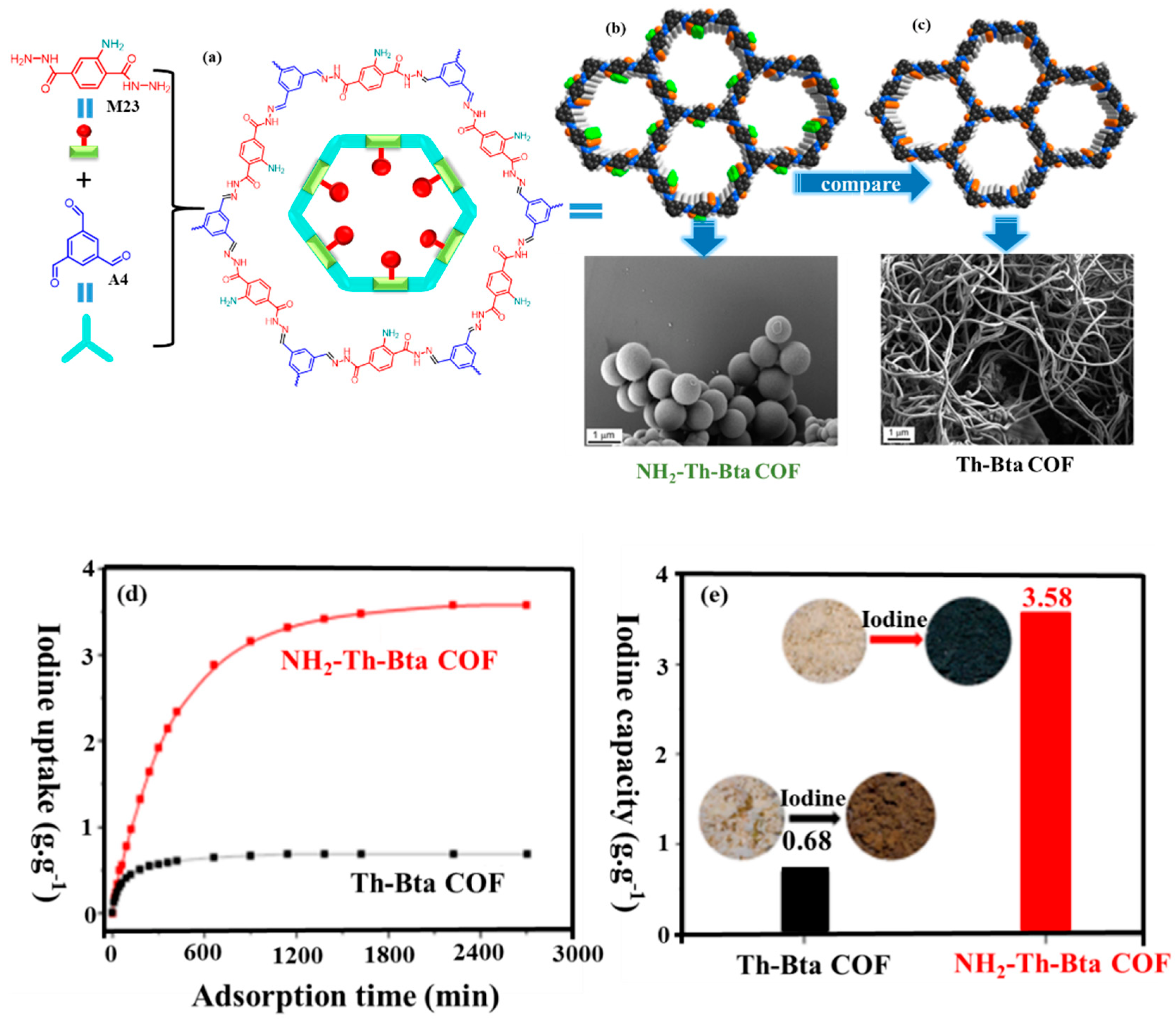
| NH2-Th-Bta | 10 | 2.4 | / | 3.58 | 37 | P-C | [75] |
| Th-Bta | 22 | 2.6 | / | 0.68 | 37 | P-C | [75] |
| TJNU-201 | 2510 | 1.4 | / | 5.625 | 96 | P-C | [77] |
| TJNU-202 | 714 | 1.7 | / | 4.82 | 96 | P-C | [77] |
| TJNU-203 | 1833 | 0.98 | / | 5.885 | 100 | P-C | [78] |
| TJNU-204 | 2048 | 0.89 | / | 5.335 | 100 | P-C | [78] |
| TPT-Azine | 1020 | 2.5 | 0.65 | 2.19 | / | P | [79] |
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ewing, R.C.; Hippel, F.N.V. Nuclear waste management in the United States—Starting over. Science 2009, 325, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Veliscek-Carolan, J. Separation of actinides from spent nuclear fuel: A review. J. Hazard. Mater. 2016, 318, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, K.S.; Sarma, D.; Malliakas, C.D.; Polychronopoulou, K.; Riley, B.J.; Pierce, D.A.; Chun, J.; Kanatzidis, M.G. Chalcogenide Aerogels as Sorbents for Radioactive Iodine. Chem. Mater. 2015, 27, 2619–2626. [Google Scholar] [CrossRef]
- Haefner, D.R.; Tranter, T.J. Methods of Gas Phase Capture of Iodine from Fuel Reprocessing Off-Gas: A Literature Survey; Idaho National Laboratory: Idaho Falls, ID, USA, 2007. [Google Scholar]
- Mushkacheva, G.; Rabinovich, E.; Privalov, V.; Povolotskaya, S.; Shorokhova, V.; Sokolova, S.; Turdakova, V.; Ryzhova, E.; Hall, P.; Schneider, A.B.; et al. Thyroid abnormalities associated with protracted childhood exposure to 131I from atmospheric emissions from the Mayak weapons facility in Russia. Radiat. Res. 2006, 166, 715–722. [Google Scholar] [CrossRef]
- Hoevea, J.E.T.; Jacobson, M.Z. Worldwide health effects of the Fukushima Daiichi nuclear accident. Energy Environ. Sci. 2012, 5, 8743–8757. [Google Scholar] [CrossRef]
- Taylor, D.M. The radiotoxicology of iodine. J. Radioanal. Chem. 1981, 65, 195–208. [Google Scholar] [CrossRef]
- Sisson, J.C.; Freitas, J.; McDougall, I.R.; Dauer, L.T.; Hurley, J.R.; Brierley, J.D.; Edinboro, C.H.; Rosenthal, D.; Thomas, M.J.; Wexler, J.A.; et al. Radiation Safety in the Treatment of Patients with Thyroid Diseases by Radioiodine 131I: Practice Recommendations of the American Thyroid Association. Thyroid 2011, 21, 335–346. [Google Scholar] [CrossRef]
- Shimamoto, Y.S.; Takahashi, Y.; Terada, Y. Formation of Organic Iodine Supplied as Iodide in a Soil–Water System in Chiba, Japan. Environ. Sci. Technol. 2011, 45, 2086–2092. [Google Scholar] [CrossRef]
- Hu, Q.; Zhao, P.; Moran, J.E.; Seaman, J.C. Sorption and transport of iodine species in sediments from the Savannah River and Hanford Sites. J. Contam. Hydrol. 2005, 78, 185–205. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Nakano, M.; Takamatsu, R.; Tanida, H. Inorganic iodine incorporation into soil organic matter: Evidence from iodine K-edge X-ray absorption near-edge structure. J. Environ. Radioact. 2010, 101, 451–457. [Google Scholar] [CrossRef]
- Liu, S.; Wang, N.; Zhang, Y.; Li, Y.; Han, Z.; Na, P. Efficient removal of radioactive iodide ions from water by three-dimensional Ag2O–Ag/TiO2 composites under visible light irradiation. J. Hazard. Mater. 2015, 284, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, K.S.; Malliakas, C.D.; Sarma, D.; Armatas, G.S.; Wu, J.; Kanatzidis, M.G. Ion-exchangeable molybdenum sulfide porous chalcogel: Gas adsorption and capture of iodine and mercury. J. Am. Chem. Soc. 2015, 137, 13943–13948. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.C.T.; Docao, S.; Hwang, I.C.; Song, M.K.; Choi, D.Y.; Moon, D.; Oleynikov, P.; Yoon, K.B. Capture of iodine and organic iodides using silica zeolites and the semiconductor behaviour of iodine in a silica zeolite. Energy Environ. Sci. 2016, 9, 1050–1062. [Google Scholar] [CrossRef]
- Chapman, K.W.; Chupas, P.J.; Nenoff, T.M. Radioactive iodine capture in silver-containing mordenites through nanoscale silver iodide formation. J. Am. Chem. Soc. 2010, 132, 8897–8899. [Google Scholar] [CrossRef] [PubMed]
- Deuber, H. Investigations on the Retention of Elemental Radioiodine by Activated Carbons at High Temperatures. Nucl. Technol. 2017, 72, 44–48. [Google Scholar] [CrossRef]
- Riley, B.J.; Vienna, J.D.; Strachan, D.M.; McCloy, J.S.; Jerden, J.L. Materials and processes for the effective capture and immobilization of radioiodine: A review. J. Nucl. Mater. 2016, 470, 307–326. [Google Scholar] [CrossRef]
- Xie, W.; Cui, D.; Zhang, S.-R.; Xu, Y.-H.; Jiang, D.-L. Iodine capture in porous organic polymers and metal-organic frameworks materials. Mater. Horiz. 2019, 6, 1571–1595. [Google Scholar] [CrossRef]
- Sava, D.F.; Rodriguez, M.A.; Chapman, K.W.; Chupas, P.J.; Greathouse, J.A.; Crozier, P.S.; Nenoff, T.M. Capture of Volatile Iodine, a Gaseous Fission Product, by Zeolitic Imidazolate Framework-8. J. Am. Chem. Soc. 2011, 133, 12398–12401. [Google Scholar] [CrossRef]
- Sava, D.F.; Chapman, K.W.; Rodriguez, M.A.; Greathouse, J.A.; Crozier, P.S.; Zhao, H.; Chupas, P.J.; Nenoff, T.M. Competitive I2 Sorption by Cu-BTC from Humid Gas Streams. Chem. Mater. 2013, 25, 2591–2596. [Google Scholar] [CrossRef]
- Zhang, X.; Silva, I.d.; Godfrey, H.G.W.; Callear, S.K.; Sapchenko, S.A.; Cheng, Y.; Vitórica-Yrezábal, I.; Frogley, M.D.; Cinque, G.; Tang, C.C.; et al. Confinement of Iodine Molecules into Triple-Helical Chains within Robust Metal–Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 16289–16296. [Google Scholar] [CrossRef]
- Brunet, G.; Safin, D.A.; Aghaji, M.Z.; Robeyns, K.; Korobkov, I.; Woo, T.K.; Murugesu, M. Stepwise crystallographic visualization of dynamic guest binding in a nanoporous framework. Chem. Sci. 2017, 8, 3171–3177. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Huang, H.; Li, J.; Xue, W.; Zhong, C. IL-induced formation of dynamic complex iodide anions in IL@MOF composites for efficient iodine capture. J. Mater. Chem. A 2019, 7, 18324–18329. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, L.; Lin, G.; Chen, G.; Liu, B.; Zhang, L.; Duan, T.; Lei, J. Controllable Synthesis of Porous Cu-BTC@polymer Composite Beads for Iodine Capture. ACS Appl. Mater. Interfaces 2019, 11, 4263–42645. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; He, X.; Pang, M.; Dong, X.; Zhao, S.; Zhang, W. Iodine Capture Using Zr-Based Metal—Organic Frameworks (Zr-MOFs): Adsorption Performance and Mechanism. ACS Appl. Mater. Interfaces 2020, 12, 20429–20439. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.B.; Budd, P.M. Polymers of intrinsic microporosity (PIMs): Organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem. Soc. Rev. 2006, 35, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Budd, P.M.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E. Polymers of intrinsic microporosity (PIMs): Robust, solution-processable, organic nanoporous materials. Chem. Commun. 2004, 230–231. [Google Scholar] [CrossRef]
- McKeown, N.B.; Budd, P.M. Exploitation of Intrinsic Microporosity in Polymer-Based Materials. Macromolecules 2010, 43, 5163–5176. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, S.; Xu, H.; Nagai, A.; Jiang, D. Conjugated microporous polymers: Design, synthesis and application. Chem. Soc. Rev. 2013, 42, 8012–8031. [Google Scholar] [CrossRef]
- Zhuang, X.; Gehrig, D.; Forler, N.; Liang, H.; Wagner, M.; Hansen, M.R.; Laquai, F.; Zhang, F.; Feng, X. Conjugated Microporous Polymers with Dimensionality-Controlled Heterostructures for Green Energy Devices. Adv. Mater. 2015, 27, 3789–3796. [Google Scholar] [CrossRef]
- Gu, C.; Huang, N.; Gao, J.; Xu, F.; Xu, Y.; Jiang, D. Controlled Synthesis of Conjugated Microporous Polymer Films: Versatile Platforms for Highly Sensitive and Label-Free Chemo- and Biosensing. Angew. Chem. Int. Ed. 2014, 53, 4850–4855. [Google Scholar] [CrossRef]
- Geng, T.; Zhang, W.; Zhu, Z.; Kai, X. Triazine-based conjugated microporous polymers constructing triphenylamine and its derivatives with nitrogen as core for iodine adsorption and fluorescence sensing I2. Micropor. Mesopor. Mat. 2019, 273, 163–170. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Krishna, R.; Yao, K.; Hang, Y.; Wu, Z.; Ma, D.; Shi, Z.; Pham, T.; Space, B.; et al. Introduction of π-Complexation into Porous Aromatic Framework for Highly Selective Adsorption of Ethylene over Ethane. J. Am. Chem. Soc. 2014, 136, 8654–8660. [Google Scholar] [CrossRef]
- Wu, X.; Shaibani, M.; Smith, S.J.D.; Konstas, K.; Hill, M.R.; Wang, H.; Zhang, K.; Xie, Z. Microporous carbon from fullerene impregnated porous aromatic frameworks for improving the desalination performance of thin film composite forward osmosis membranes. J. Mater. Chem. A. 2018, 6, 11327–11336. [Google Scholar] [CrossRef]
- Ren, H.; Ben, T.; Sun, F.; Guo, M.; Jing, X.; Ma, H.; Cai, K.; Qiu, S.; Zhu, G. Synthesis of a porous aromatic framework for adsorbing organic pollutants application. J. Mater. Chem. 2011, 21, 10348–10353. [Google Scholar] [CrossRef]
- Feng, X.; Ding, X.; Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022. [Google Scholar] [CrossRef]
- Jin, E.; Asada, M.; Xu, Q.; Dalapati, S.; Addicoat, M.A.; Brady, M.A.; Xu, H.; Nakamura, T.; Heine, T.; Chen, Q.; et al. Two-dimensional sp2 carbon–conjugated covalent organic frameworks. Science 2017, 357, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, L.A.; Crowe, J.W.; Pyles, D.A.; McGrier, P.L. Metalation of a Mesoporous Three-Dimensional Covalent Organic Framework. J. Am. Chem. Soc. 2016, 138, 15134–15137. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yang, H.; Whiteley, J.M.; Wan, S.; Jin, Y.; Lee, S.H.; Zhang, W. Ionic Covalent Organic Frameworks with Spiroborate Linkage. Angew. Chem. Int. Ed. 2016, 55, 1737–1741. [Google Scholar] [CrossRef] [PubMed]
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Zhao, X.; Pachfule, P.; Thomas, A. Covalent organic frameworks (COFs) for electrochemical applications. Chem. Soc. Rev. 2021, 50, 6871–6913. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Chen, Y.; Zhang, Z.; Ma, S. Covalent organic frameworks for separation applications. Chem. Soc. Rev. 2020, 49, 708–735. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, R.; Mondal, S.; Pal, S.C.; Mukherjee, D.; Das, M.C. Covalent-Organic Frameworks (COFs) as Proton Conductors. Adv. Energy Mater. 2021, 11, 2102300. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhuang, S.T. Covalent organic frameworks (COFs) for environmental applications. Coord. Chem. Rev. 2019, 400, 213046. [Google Scholar] [CrossRef]
- Cai, Y.; Ling, Q.; Yi, Y.; Chen, Z.; Yang, H.; Hu, B.; Liang, L.; Wang, X. Application of covalent organic frameworks in environmental pollution management. Appl. Catal. A Gen. 2022, 643, 118733. [Google Scholar] [CrossRef]
- Li, Y.; Song, X.; Zhang, G.; Wang, L.; Liu, Y.; Chen, W.; Chen, L. 2D Covalent Organic Frameworks toward Efficient Photocatalytic Hydrogen Evolution. ChemSusChem 2022, 15, e202200901. [Google Scholar] [CrossRef]
- Yin, Z.-J.; Xu, S.-Q.; Zhan, T.-G.; Qi, Q.-Y.; Wu, Z.-Q.; Zhao, X. Ultrahigh volatile iodine uptake by hollow microspheres formed from a heteropore covalent organic framework. Chem. Commun. 2017, 53, 7266–7269. [Google Scholar] [CrossRef]
- Yan, X.; Yang, Y.X.; Li, G.R.; Zhang, J.H.; He, Y.; Wang, R.; Lin, Z.; Cai, Z.W. Thiophene-based covalent organic frameworks for highly efficient iodine capture. Chinese Chem. Lett. 2023, 34, 107201. [Google Scholar] [CrossRef]
- Wang, G.; Xie, K.; Zhu, F.; Kan, J.; Li, S.; Geng, Y.; Dong, Y. Construction of Tetrathiafulvalene-based Covalent Organic Frameworks for Superior Iodine Capture. Chem. Res. Chinese Univ. 2022, 38, 409–414. [Google Scholar] [CrossRef]
- Chang, J.H.; Li, H.; Zhao, J.; Guan, X.Y.; Li, C.M.; Yu, G.T.; Valtchev, V.; Yan, Y.S.; Qiu, S.L.; Fang, Q.R. Tetrathiafulvalene-based covalent organic frameworks for ultrahigh iodine capture. Chem. Sci. 2021, 12, 8452–8457. [Google Scholar] [CrossRef]
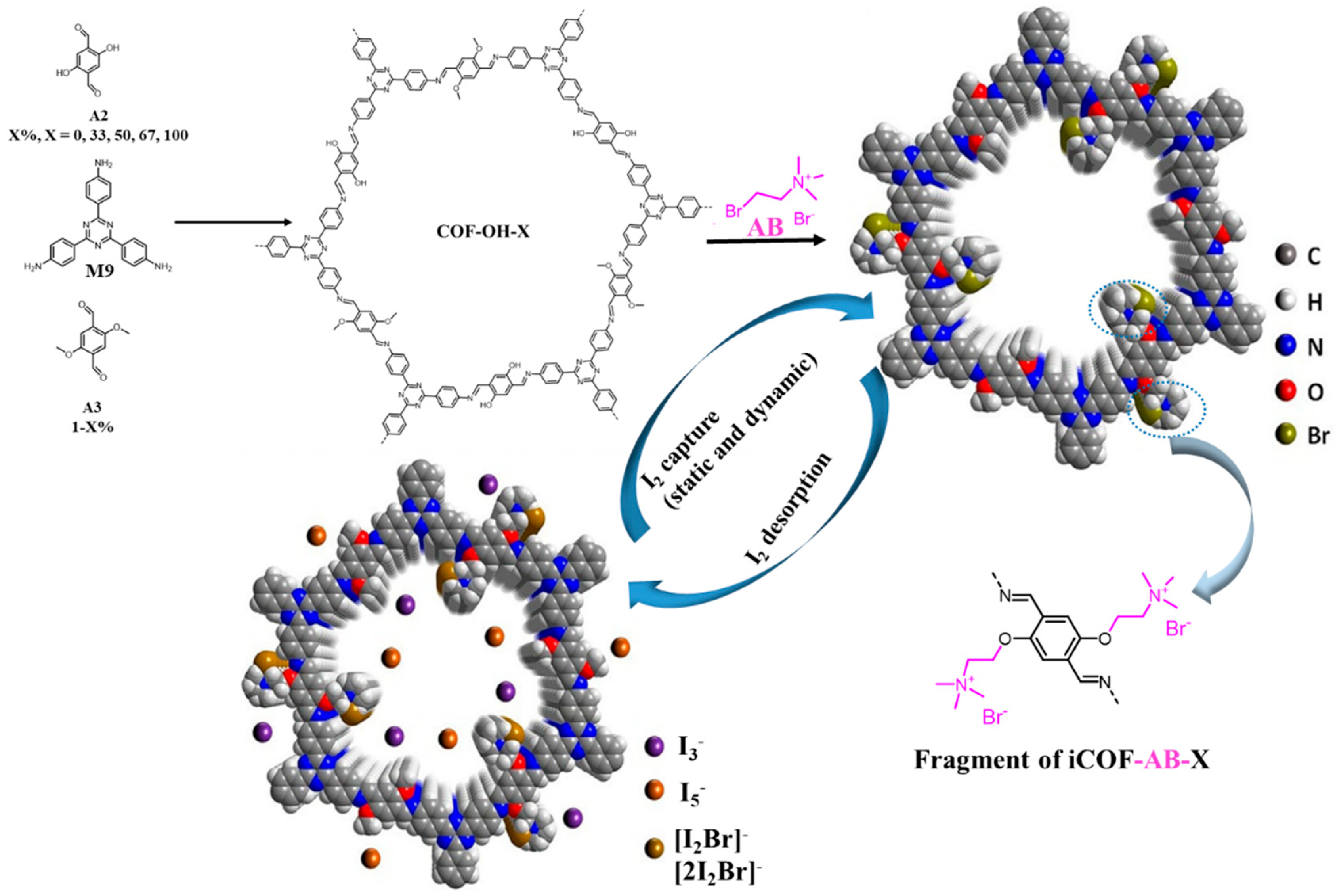
- Xie, Y.Q.; Pan, T.T.; Lei, Q.; Chen, C.L.; Dong, X.L.; Yuan, Y.Y.; Shen, J.; Cai, Y.C.; Zhou, C.H.; Pinnau, I.; et al. Ionic Functionalization of Multivariate Covalent Organic Frameworks to Achieve an Exceptionally High Iodine-Capture Capacity. Angew. Chem. Int. Ed. 2021, 60, 22432–22440. [Google Scholar] [CrossRef]
- An, S.; Zhu, X.; He, Y.; Yang, L.; Wang, H.; Jin, S.; Hu, J.; Liu, H. Porosity Modulation in Two-Dimensional Covalent Organic Frameworks Leads to Enhanced Iodine Adsorption Performance. Ind. Eng. Chem. Res. 2019, 58, 10495–10502. [Google Scholar] [CrossRef]
- Wang, P.; Xu, Q.; Li, Z.; Jiang, W.; Jiang, Q.; Jiang, D. Exceptional Iodine Capture in 2D Covalent Organic Frameworks. Adv. Mater. 2018, 30, 1801991. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, X.; Li, Y.; Xia, M.; Xia, T.; Sun, H.; Sui, Z.; Hu, X.-M.; Chen, Q. Ultra-stable fluorescent 2D covalent organic framework for rapid adsorption and selective detection of radioiodine. Micropor. Mesopor. Mat. 2021, 319, 111046. [Google Scholar] [CrossRef]
- Zhai, L.; Sun, S.; Chen, P.; Zhang, Y.; Sun, Q.; Xu, Q.; Wu, Y.; Nie, R.; Li, Z.; Mi, L. Constructing cationic covalent organic frameworks by a post-function process for an exceptional iodine capture via electrostatic interactions. Mater. Chem. Front. 2021, 5, 5463–5470. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Z.; Munyentwali, A.; Li, C.; Shui, H.; Li, H. Highly Conjugated Two-dimensional Covalent Organic Frameworks for Efficient Iodine Uptake. Chem. Asian J. 2022, e202200358. [Google Scholar] [CrossRef]
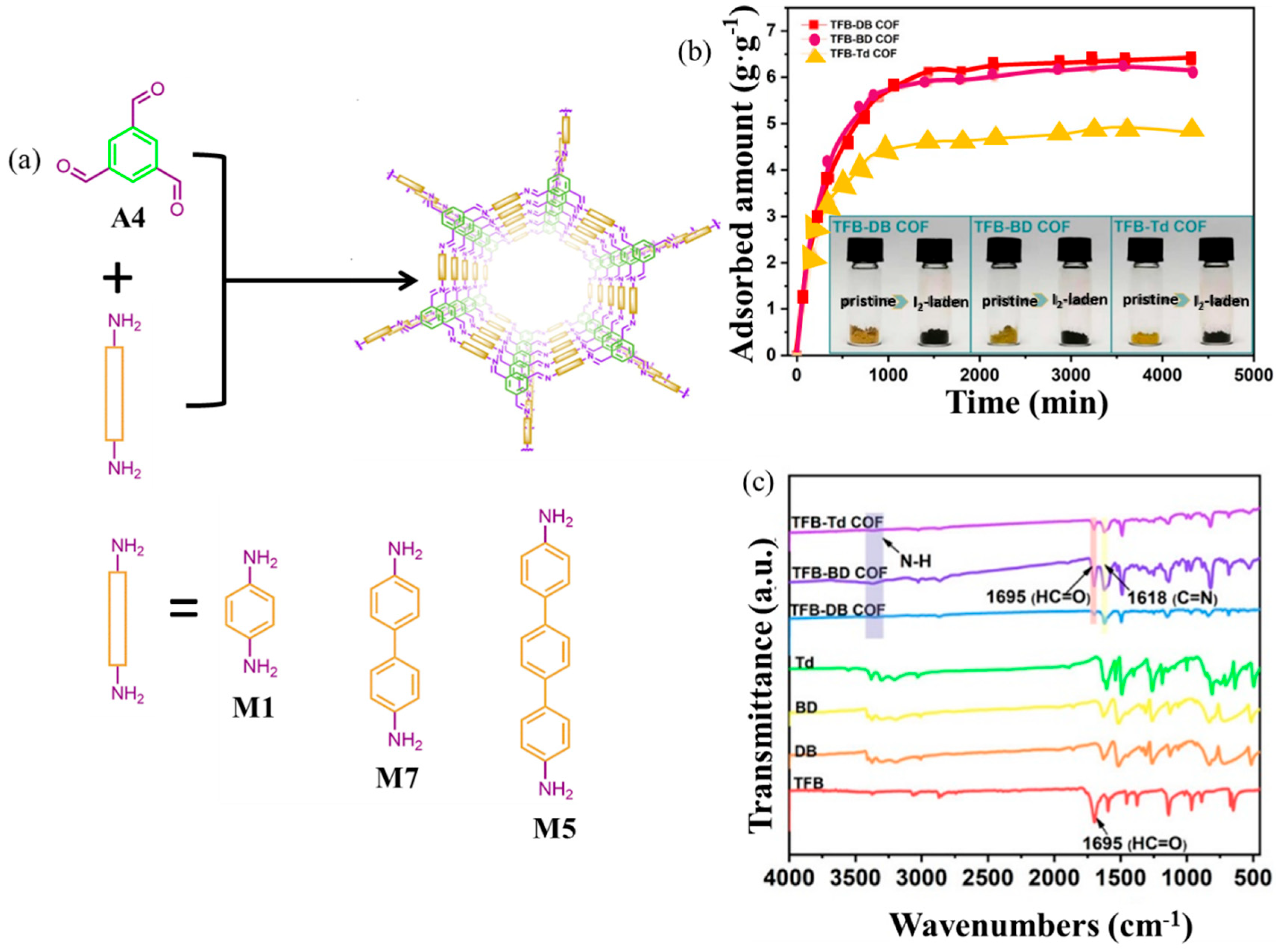
- Yang, Y.; Xiong, X.; Fan, Y.; Lai, Z.; Xu, Z.; Luo, F. Insight into volatile iodine uptake properties of covalent organic frameworks with different conjugated structures. J Solid State Chem. 2019, 279, 120979. [Google Scholar] [CrossRef]
- Sun, Y.; Song, S.; Xiao, D.; Gan, L.; Wang, Y. Easily Constructed Imine-Bonded COFs for Iodine Capture at Ambient Temperature. ACS Omega 2020, 5, 24262–24271. [Google Scholar] [CrossRef]
- Song, S.; Shi, Y.; Liu, N.; Liu, F. Theoretical Screening and Experimental Synthesis of Ultrahigh-Iodine Capture Covalent Organic Frameworks. ACS Appl. Mater. Interfaces 2021, 13, 10513–10523. [Google Scholar] [CrossRef]
- Guo, X.; Li, Y.; Zhang, M.; Cao, K.; Tian, Y.; Qi, Y.; Li, S.; Li, K.; Yu, X.; Ma, L. Colyliform Crystalline 2D Covalent Organic Frameworks (COFs) with Quasi-3D Topologies for Rapid I2 Adsorption. Angew. Chem. Int. Ed. 2020, 59, 22697–22705. [Google Scholar] [CrossRef]
- Lan, Y.; Tong, M.; Yang, Q.; Zhong, C. Computational screening of covalent organic frameworks for the capture of radioactive iodine and methyl iodide. CrystEngComm 2017, 19, 4920–4926. [Google Scholar] [CrossRef]
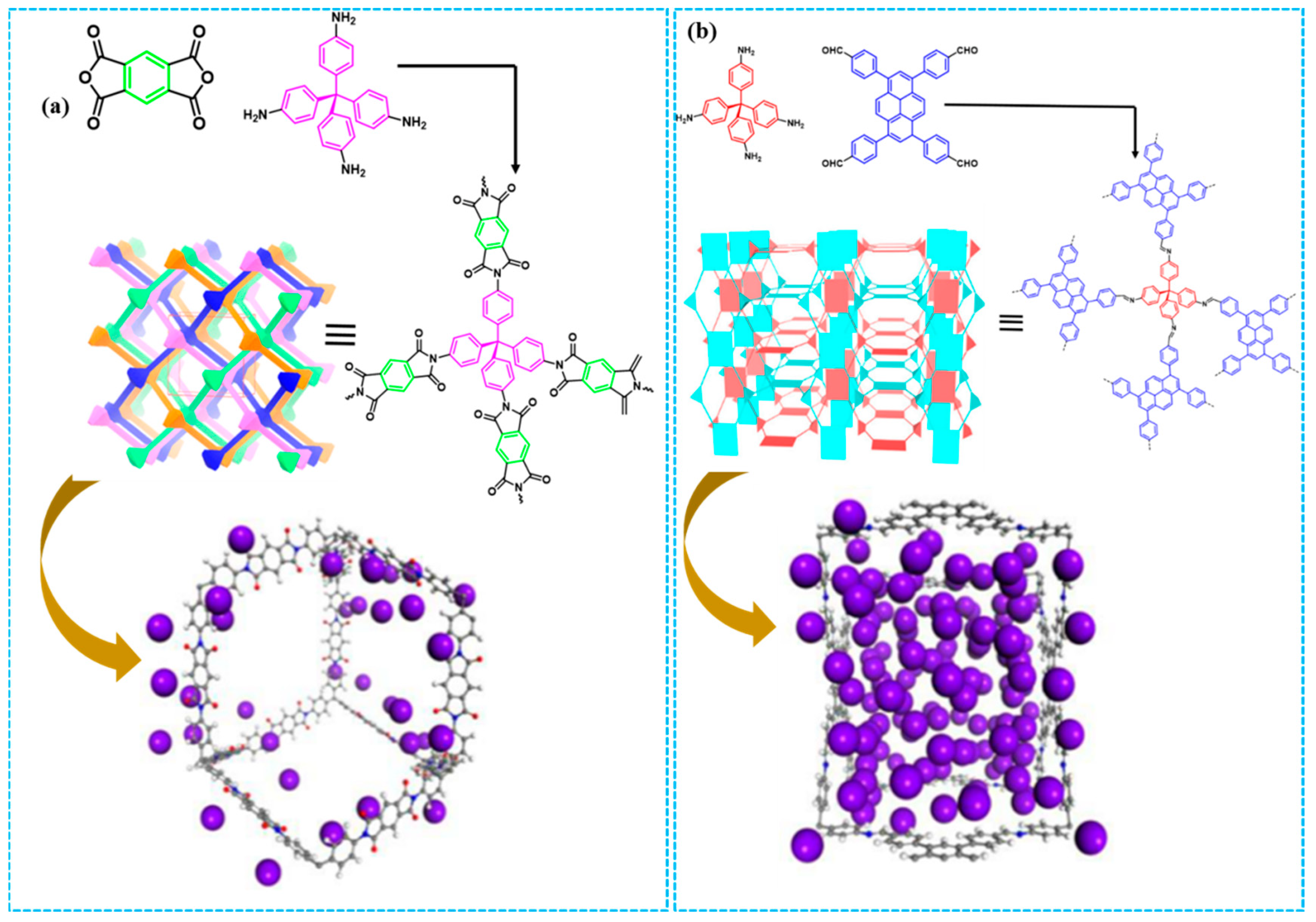
- Fang, Q.R.; Wang, J.H.; Gu, S.; Kaspar, R.B.; Zhuang, Z.B.; Zheng, J.; Guo, H.; Qiu, S.L.; Yan, Y. 3D Porous Crystalline Polyimide Covalent Organic Frameworks for Drug Delivery. J. Am. Chem. Soc. 2015, 137, 8352–8355. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Ding, H.; Yuan, D.; Wang, B.; Wang, C. A Pyrene-Based Fluorescent Three-Dimensional Covalent Organic Framework. J. Am. Chem. Soc. 2016, 138, 3302–3305. [Google Scholar] [CrossRef] [PubMed]
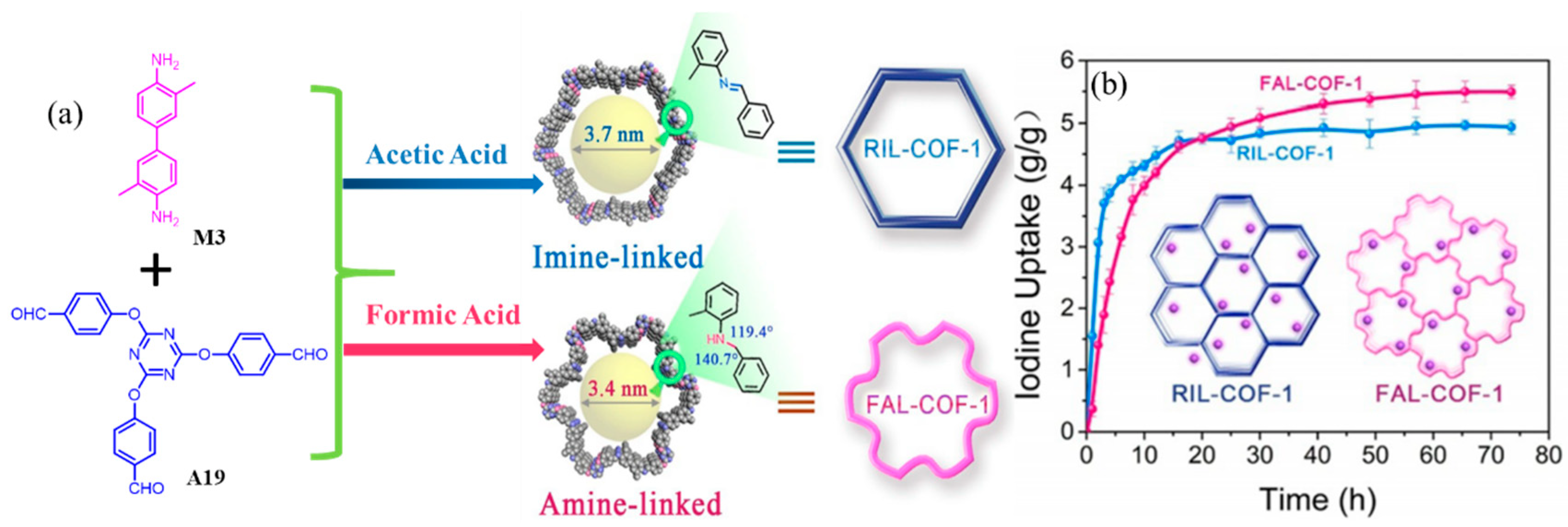
- Zhang, M.; Li, Y.; Yuan, W.; Guo, X.; Bai, C.; Zou, Y.; Long, H.; Qi, Y.; Li, S.; Tao, G.; et al. Construction of Flexible Amine-linked Covalent Organic Frameworks by Catalysis and Reduction of Formic Acid via the Eschweiler-Clarke Reaction. Angew. Chem. Int. Ed. 2021, 60, 12396–12405. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, N.; Dinari, M. Developing novel amine-linked covalent organic frameworks towards reversible iodine capture. Sep. Purif. Technol. 2022, 301, 121948. [Google Scholar] [CrossRef]
- Guo, X.; Tian, Y.; Zhang, M.; Li, Y.; Wen, R.; Li, X.; Li, X.; Xue, Y.; Ma, L.; Xia, C.; et al. Mechanistic Insight into Hydrogen-Bond-Controlled Crystallinity and Adsorption Property of Covalent Organic Frameworks from Flexible Building Blocks. Chem. Mater. 2018, 30, 2299–2308. [Google Scholar] [CrossRef]
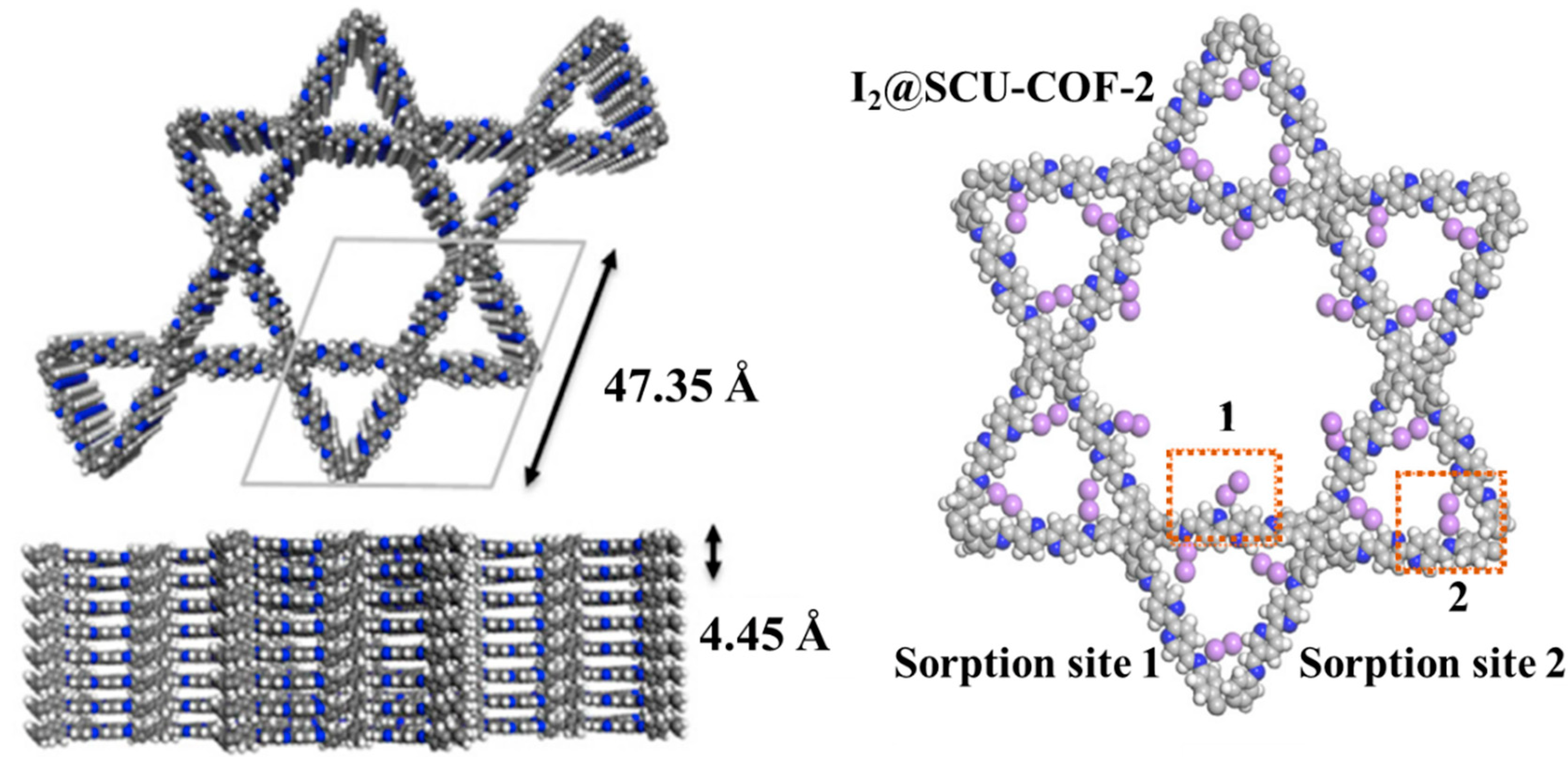
- Wang, C.; Wang, Y.; Ge, R.; Song, X.; Xing, X.; Jiang, Q.; Lu, H.; Hao, C.; Guo, X.; Gao, Y.; et al. A 3D Covalent Organic Framework with Exceptionally High Iodine Capture Capability. Chem. Eur. J. 2018, 24, 585–589. [Google Scholar] [CrossRef]
- Chen, R.; Hu, T.; Zhang, W.; He, C.; Li, Y. Synthesis of nitrogen-containing covalent organic framework with reversible iodine capture capability. Micropo. Mesopor. Mat. 2021, 312, 110739. [Google Scholar] [CrossRef]
- He, L.; Chen, L.; Dong, X.; Zhang, S.; Zhang, M.; Dai, X.; Liu, X.; Lin, P.; Li, K.; Chen, C.; et al. A nitrogen-rich covalent organic framework for simultaneous dynamic capture of iodine and methyl iodide. Chem 2021, 7, 699–714. [Google Scholar] [CrossRef]
- Jiang, B.; Qi, Y.; Li, X.; Guo, X.; Jia, Z.; Zhang, J.; Li, Y.; Ma, L. Efficient gaseous iodine capture enhanced by charge-induced effect of covalent organic frameworks with dense tertiary-amine nodes. Chin. Chem. Lett. 2022, 33, 3556–3560. [Google Scholar] [CrossRef]
- Pan, X.; Qin, X.; Zhang, Q.; Ge, Y.; Ke, H.; Cheng, G. N- and S-rich covalent organic framework for highly efficient removal of indigo carmine and reversible iodine capture. Micropor. Mesopor. Mat. 2020, 296, 109990. [Google Scholar] [CrossRef]
- Liu, C.; Jin, Y.; Yu, Z.H.; Gong, L.; Wang, H.L.; Yu, B.Q.; Zhang, W.; Jiang, J.Z. Transformation of Porous Organic Cages and Covalent Organic Frameworks with Efficient Iodine Vapor Capture Performance. J. Am. Chem. Soc. 2022, 144, 12390–12399. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Li, J.; Liu, W.; Cheng, G.; Ke, H. Phosphine-based covalent organic framework for highly efficient iodine capture. Micropor. Mesopor. Mat. 2021, 325, 111351. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Dong, X.L.; Yin, J.; Li, Z.-G.; Li, X.; Zhang, D.L.; Pan, T.T.; Lei, Q.; Liu, X.L.; Xie, Y.Q.; et al. Chemically Stable Guanidinium Covalent Organic Framework for the Efficient Capture of Low-Concentration Iodine at High Temperatures. J. Am. Chem. Soc. 2022, 144, 6821–6829. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Tang, X.-H.; Yan, Y.-L.; Li, S.-Q.; Zheng, S.; Fan, J.; Li, X.; Zhang, W.-G.; Cai, S. Facile and Site-Selective Synthesis of an Amine-Functionalized Covalent Organic Framework. ACS Macro Lett. 2021, 10, 1590–1596. [Google Scholar] [CrossRef]
- Sen, A.; Sharma, S.; Dutta, S.; Shirolkar, M.M.; Dam, G.K.; Let, S.; Ghosh, S.K. Functionalized Ionic Porous Organic Polymers Exhibiting High Iodine Uptake from Both the Vapor and Aqueous Medium. ACS Appl. Mater. Interfaces 2021, 13, 34188–34196. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Zhang, L.; Wang, K.; Wang, Z.; Liu, G.; Zhao, Y.; Zeng, Y. Two-dimensional covalent-organic frameworks for ultrahigh iodine capture. J. Mater. Chem. A 2020, 8, 9523–9527. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Zhang, H.; Liu, Y.; Cui, Y.; Jin, F.; Wang, K.; Liu, G.; Zhao, Y.; Zeng, Y. High iodine uptake in two-dimensional covalent organic frameworks. Chem. Commun. 2021, 57, 5558–5561. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Hao, W.; Li, Y.; Chen, L. Covalent Organic Frameworks Constructed from Flexible Building Blocks with High Adsorption Capacity for Pollutants. ACS Appl. Nano Mater. 2018, 1, 4756–4761. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Tu, C.; Yin, H.; Liu, J.; Cheng, F.; Luo, F. Molecular Iodine Capture by Covalent Organic Frameworks. Molecules 2022, 27, 9045. https://doi.org/10.3390/molecules27249045
Yang Y, Tu C, Yin H, Liu J, Cheng F, Luo F. Molecular Iodine Capture by Covalent Organic Frameworks. Molecules. 2022; 27(24):9045. https://doi.org/10.3390/molecules27249045
Chicago/Turabian StyleYang, Yuting, Changzheng Tu, Hongju Yin, Jianjun Liu, Feixiang Cheng, and Feng Luo. 2022. "Molecular Iodine Capture by Covalent Organic Frameworks" Molecules 27, no. 24: 9045. https://doi.org/10.3390/molecules27249045
APA StyleYang, Y., Tu, C., Yin, H., Liu, J., Cheng, F., & Luo, F. (2022). Molecular Iodine Capture by Covalent Organic Frameworks. Molecules, 27(24), 9045. https://doi.org/10.3390/molecules27249045




