Investigation on the Removal Performances of Heavy Metal Copper (II) Ions from Aqueous Solutions Using Hydrate-Based Method
Abstract
1. Introduction
2. Results and Discussion
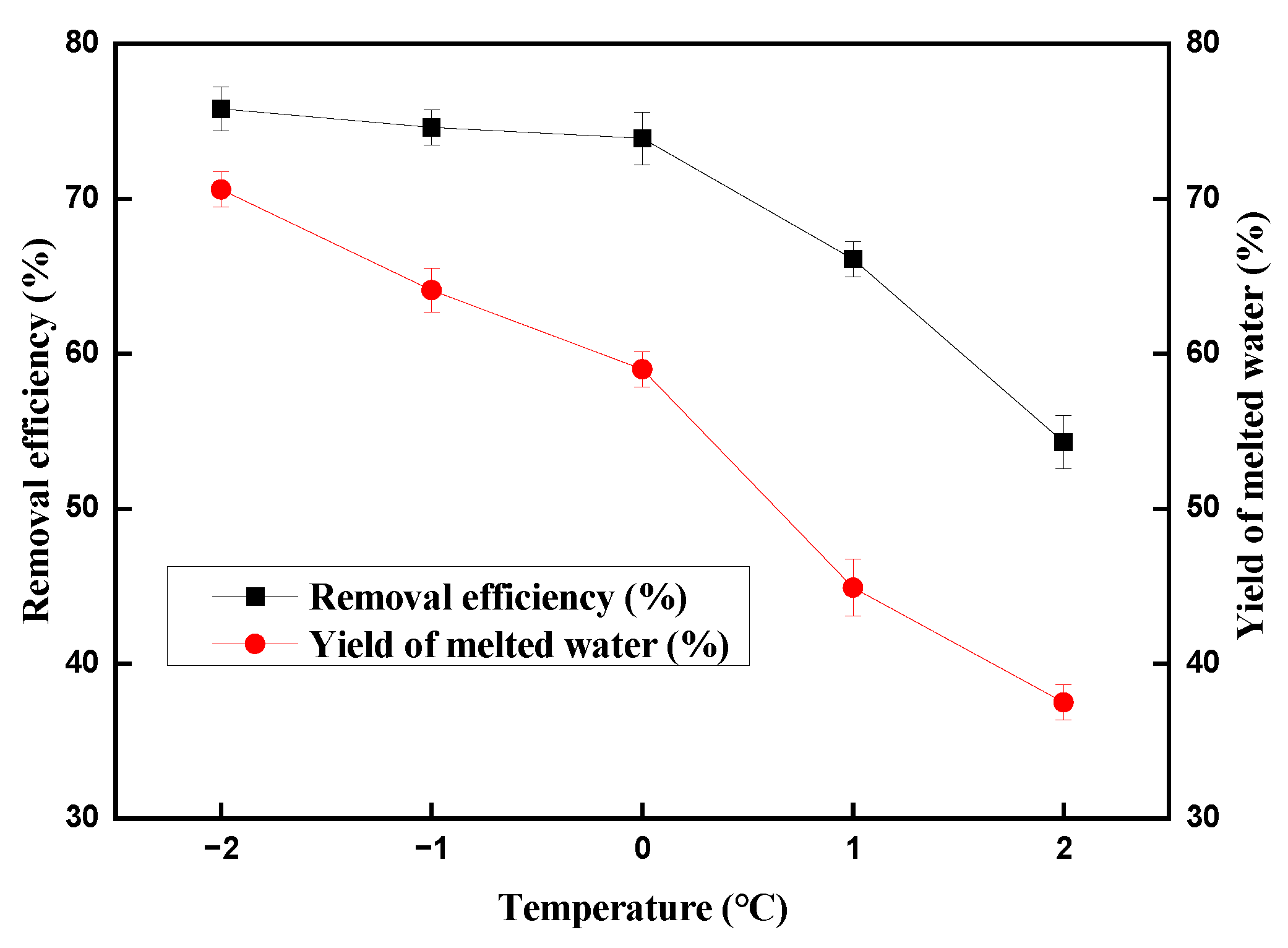
2.1. Effect of Temperature on the Copper (II) Ions Removal Efficiency
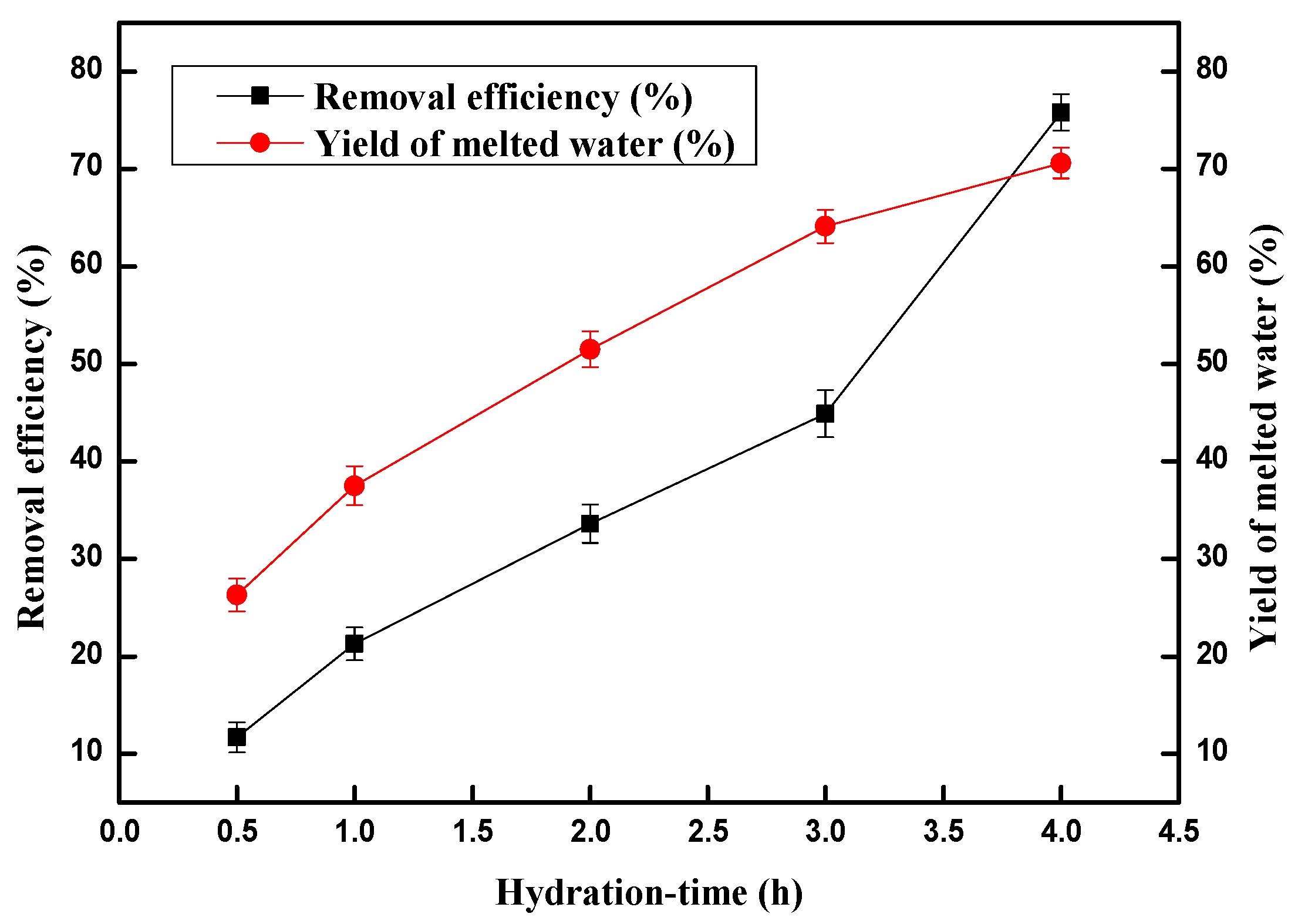
2.2. Effect of Hydration-Time on the Copper (II) Ion Removal Efficiency
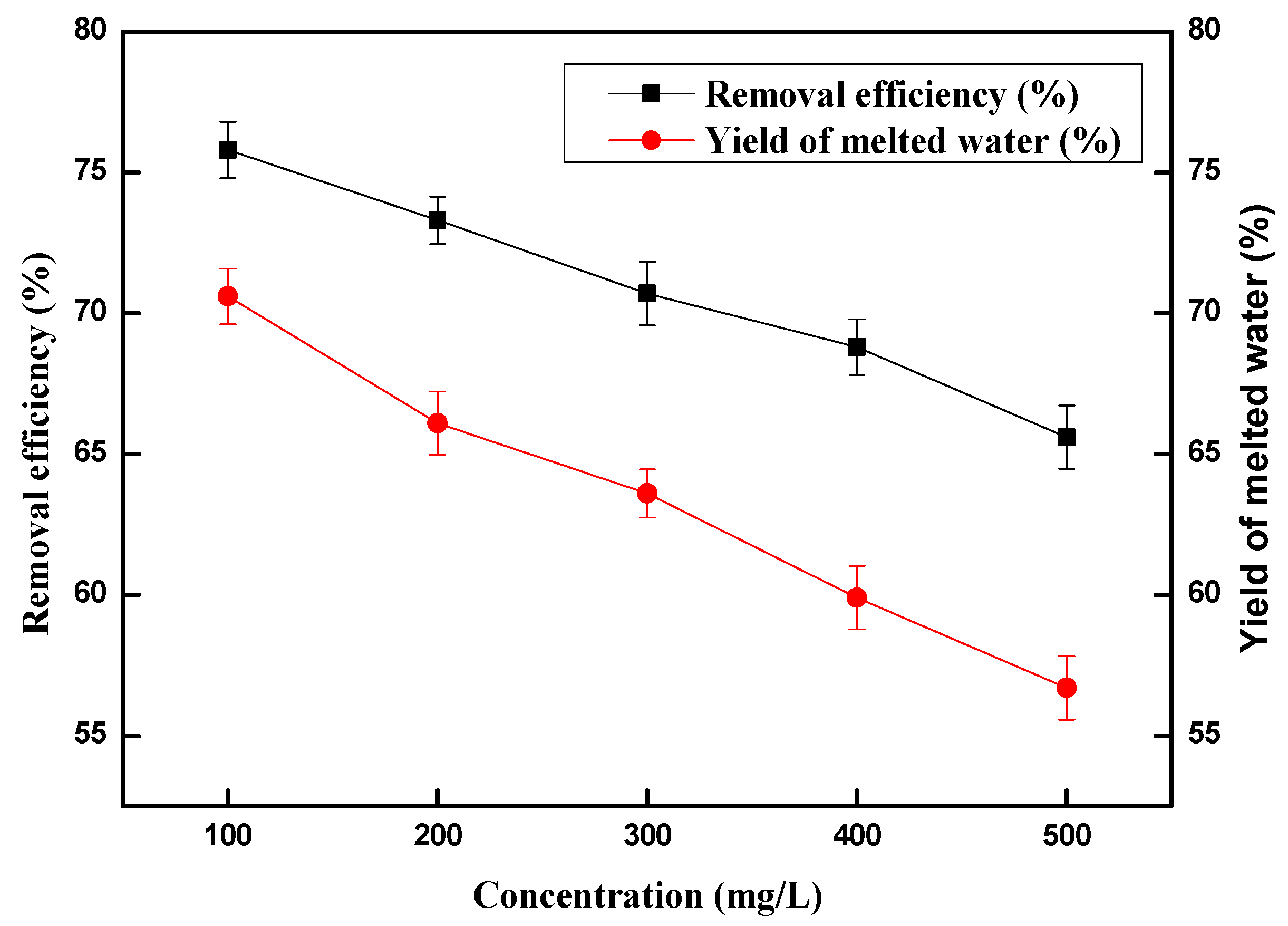
2.3. Effect of Copper (II) Ions Concentration on Removal Efficiency
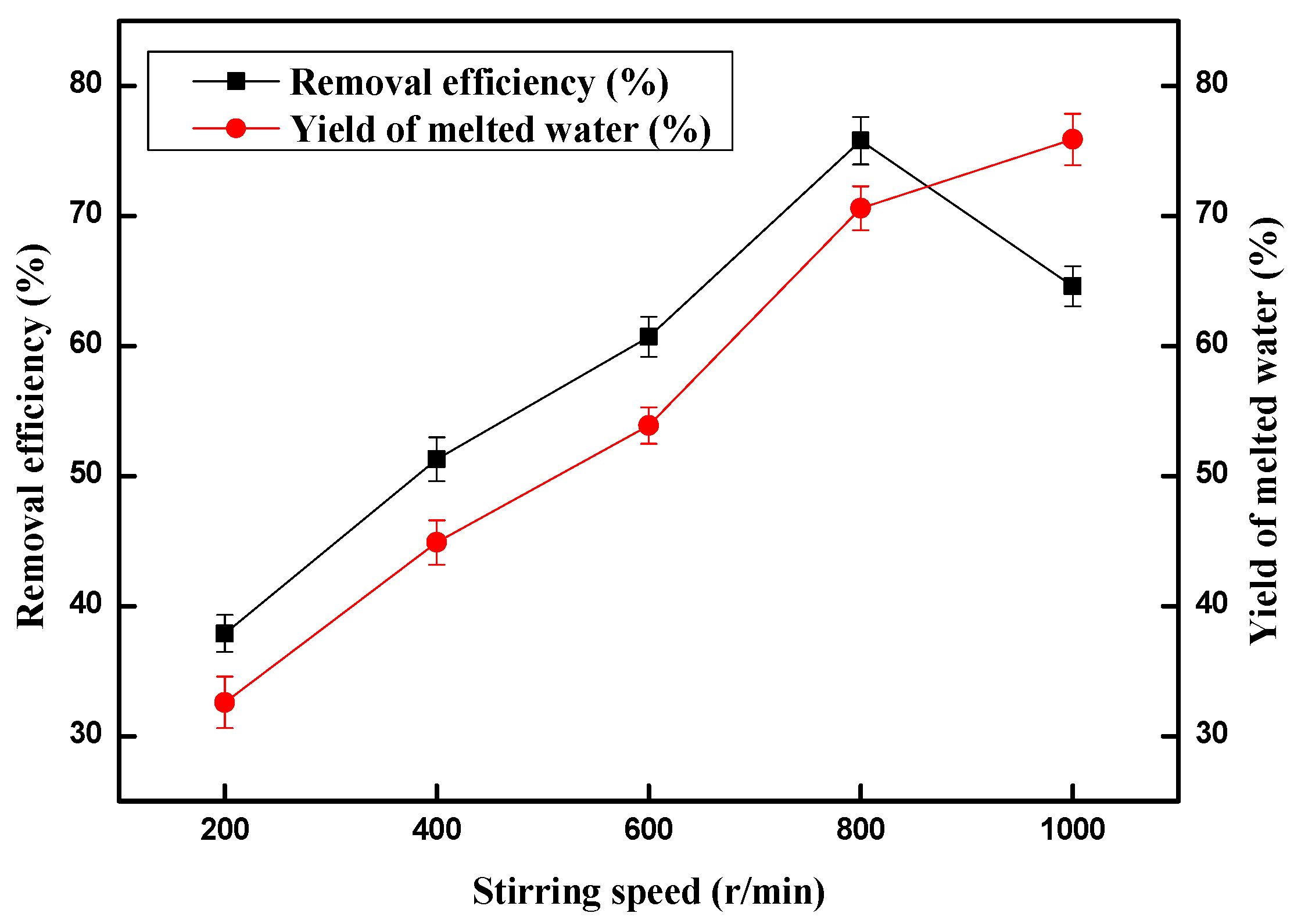
2.4. Effect of Stirring Speed on the Copper (II) Ions Removal Efficiency
3. Materials and Methods
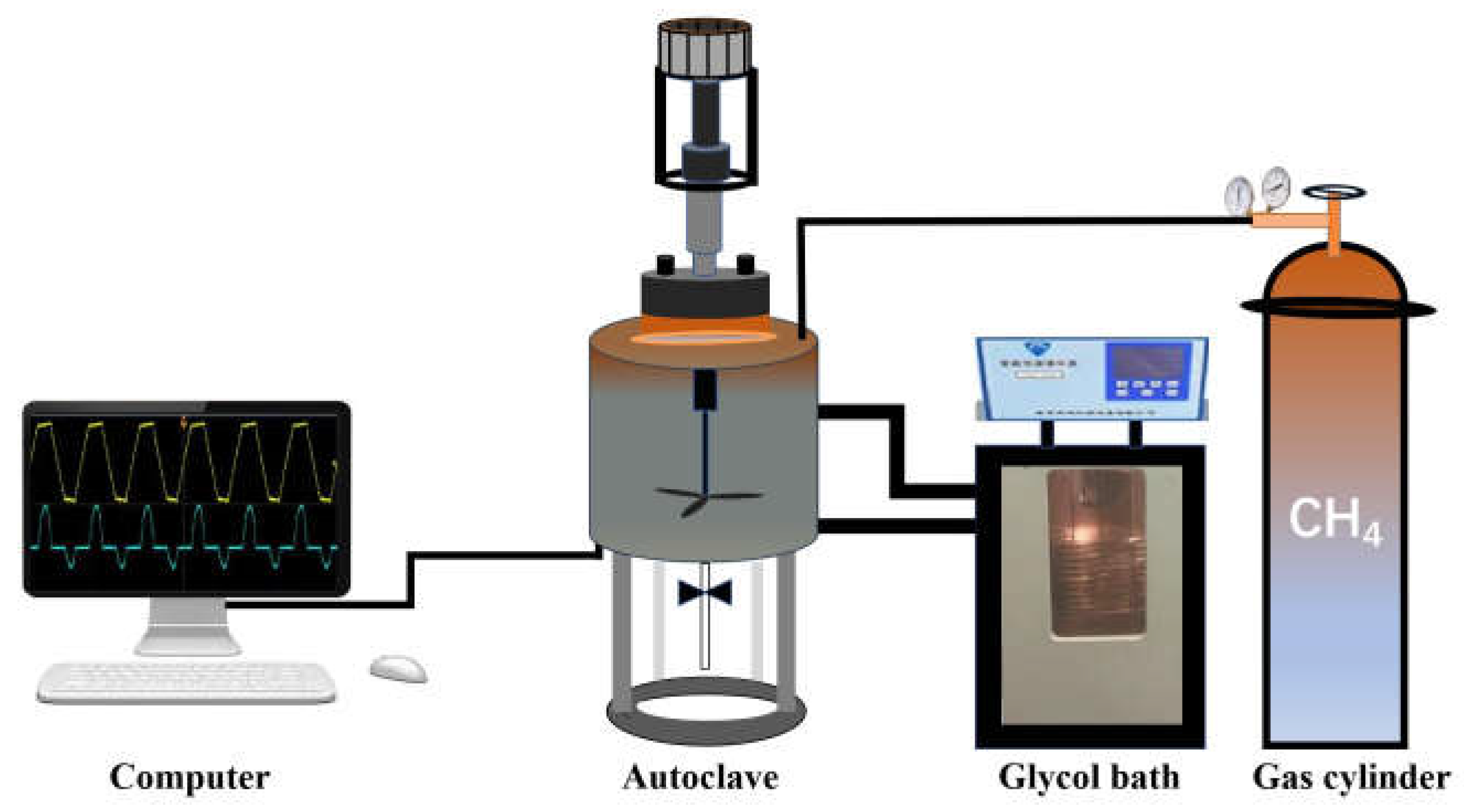
3.1. Materials and Apparatus
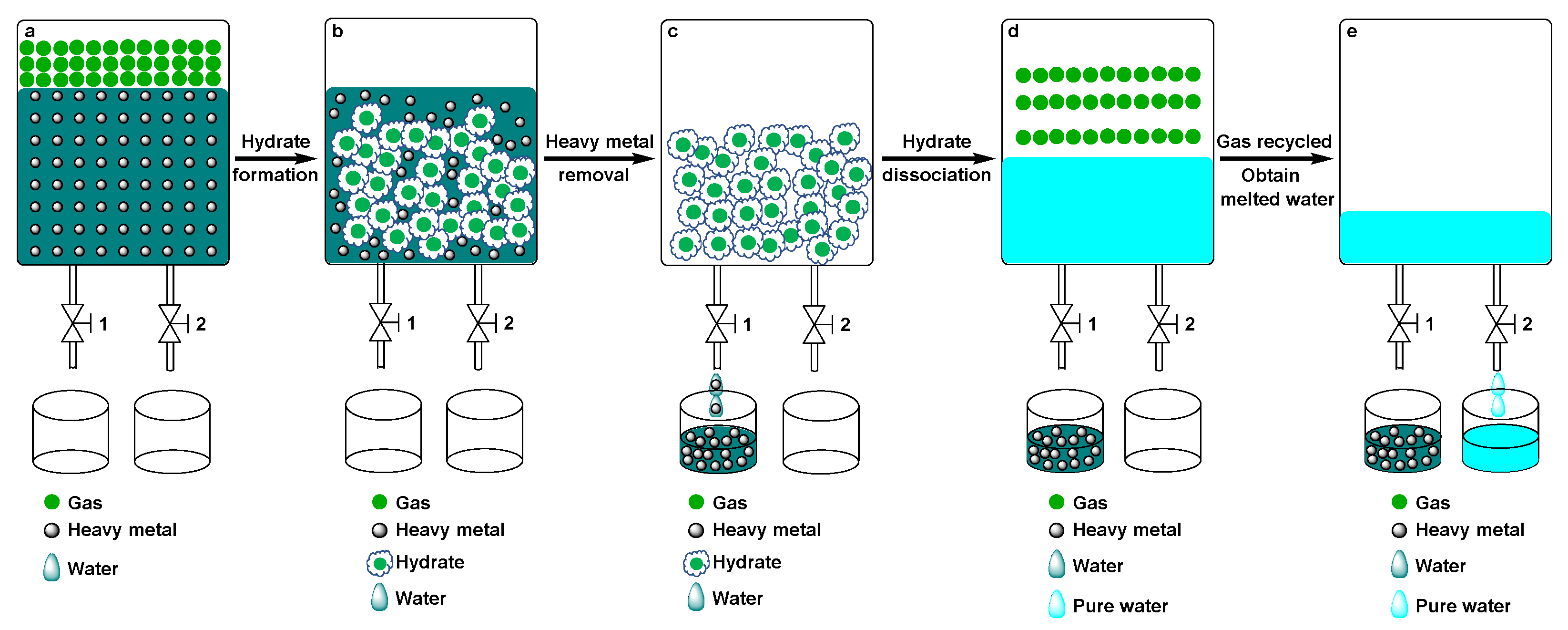
3.2. Experimental Procedure
3.3. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dharmapriya, T.N.; Li, D.; Chung, Y.-C.; Huang, P.-J. Green Synthesis of Reusable Adsorbents for the Removal of Heavy Metal Ions. ACS Omega 2021, 6, 30478–30487. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Chen, J.; Wang, X. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Parus, A. Copper(II) ions’ removal from aqueous solution using green horse-chestnut shell as a low-cost adsorbent. Chem. Ecol. 2018, 34, 56–69. [Google Scholar] [CrossRef]
- Lakhdhar, I.; Mangin, P.; Chabot, B. Copper (II) ions adsorption from aqueous solutions using electrospun chitosan/peonanofibres: Effects of process variables and process optimization. J. Water Process Eng. 2015, 7, 295–305. [Google Scholar] [CrossRef]
- Hojjati-Najafabadi, A.; Mansoorianfar, M.; Liang, T.; Shahin, K.; Karimi-Maleh, H. A review on magnetic sensors for monitoring of hazardous pollutants in water resources. Sci. Total Environ. 2022, 824, 153844. [Google Scholar] [CrossRef]
- Hojjati-Najafabadi, A.; Mansoorianfar, M.; Liang, T.; Shahin, K.; Wen, Y.; Bahrami, A.; Karaman, C.; Zare, N.; Karimi-Maleh, H.; Vasseghian, Y. Magnetic-MXene-based nanocomposites for water and wastewater treatment: A review. J. Water Process Eng. 2022, 47, 102696. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Shahin, K.; Hojjati–Najafabadi, A.; Pei, R. MXene–laden bacteriophage: A new antibacterial candidate to control bacterial contamination in water. Chemosphere 2022, 290, 133383. [Google Scholar] [CrossRef]
- Fu, Z.-J.; Jiang, S.-K.; Chao, X.-Y.; Zhang, C.-X.; Shi, Q.; Wang, Z.-Y.; Liu, M.-L.; Sun, S.-P. Removing miscellaneous heavy metals by all-in-one ion exchange-nanofiltration membrane. Water Res. 2022, 222, 118888. [Google Scholar] [CrossRef]
- Yang, Y.; Du, R.; Shi, C.; Zhao, J.; Song, Y.; Zhang, L.; Ling, Z. Desalination and enrichment of phosphorus-containing wastewater via cyclopentane hydrate. J. Environ. Chem. Eng. 2021, 9, 105507. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Azam, M.; Wabaidur, S.M.; Khan, M.R.; Al-Resayes, S.I.; Islam, M.S. Removal of Chromium(III) and Cadmium(II) Heavy Metal Ions from Aqueous Solutions Using Treated Date Seeds: An Eco-Friendly Method. Molecules 2021, 26, 3718. [Google Scholar] [CrossRef] [PubMed]
- Nallakukkala, S.; Rehman, A.u.; Zaini, D.B.; Lal, B. Gas Hydrate-Based Heavy Metal Ion Removal from Industrial Wastewater: A Review. Water 2022, 14, 1171. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Alhoshan, M.; ArockiasamyDass, L.; Ali, F.A.A.; Muthumareeswaran, M.R.; Mishra, U.; Ansari, M.A. Removal of heavy metal ions using a carboxylated graphene oxide-incorporated polyphenylsulfone nanofiltration membrane. Environ. Sci. Water Res. Technol. 2018, 4, 438–448. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, H.; Zhang, Y.; Kang, C.; Chen, S.; Song, L.; Liu, D.; Zhong, C. A versatile MOF-based trap for heavy metal ion capture and dispersion. Nat. Commun. 2018, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, S.; Yang, Y.; Guo, L.; Lyu, B.; Yang, R.; Zhang, X.; Wang, Y.; Yang, F.; Chen, Q. Metal-algae interaction contributes to the water environment heterogeneity in an urbanized river. Ecol. Indic. 2022, 139, 108875. [Google Scholar] [CrossRef]
- Yu, B.; Li, X.; He, M.; Li, Y.; Ding, J.; Zhong, Y.; Zhang, H. Selective production of singlet oxygen for harmful cyanobacteria inactivation and cyanotoxins degradation: Efficiency and mechanisms. J. Hazard. Mater. 2023, 441, 129940. [Google Scholar] [CrossRef]
- Chu, S.; Feng, X.; Liu, C.; Wu, H.; Liu, X. Advances in Chelating Resins for Adsorption of Heavy Metal Ions. Ind. Eng. Chem. Res. 2022, 61, 11309–11328. [Google Scholar] [CrossRef]
- Gupta, K.; Joshi, P.; Gusain, R.; Khatri, O.P. Recent advances in adsorptive removal of heavy metal and metalloid ions by metal oxide-based nanomaterials. Coord. Chem. Rev. 2021, 445, 214100. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, Y.; Jia, D.; Chen, W.; Cui, Z.; Wang, X. Layered silicate RUB-15 for efficient removal of UO22+ and heavy metal ions by ion-exchange. Environ. Sci. Nano 2017, 4, 1851–1858. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Chung, T.-S. Nanometric Graphene Oxide Framework Membranes with Enhanced Heavy Metal Removal via Nanofiltration. Environ. Sci. Technol. 2015, 49, 10235–10242. [Google Scholar] [CrossRef]
- Mao, M.; Yan, T.; Shen, J.; Zhang, J.; Zhang, D. Capacitive Removal of Heavy Metal Ions from Wastewater via an Electro-Adsorption and Electro-Reaction Coupling Process. Environ. Sci. Technol. 2021, 55, 3333–3340. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, X.; Guo, L.; Lan, J.; Zhang, L.; Cao, D. Heavy metal ion removal of wastewater by zeolite-imidazolate frameworks. Sep. Purif. Technol. 2018, 194, 462–469. [Google Scholar] [CrossRef]
- Lu, S.; Shen, L.; Li, X.; Yu, B.; Ding, J.; Gao, P.; Zhang, H. Advances in the photocatalytic reduction functions of graphitic carbon nitride-based photocatalysts in environmental applications: A review. J. Clean. Prod. 2022, 378, 134589. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Xu, J.; Yuan, Q.; Deng, B.; Chen, C.; Li, Z. Experiments and insights of desalination by a freezing/thawing method at low subcooling. Chin. J. Chem. Eng. 2020, 28, 3011–3017. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, C.; Liu, Q.; Chen, J. Separation of C3H8 and C3H6 from Butyl Alcohol–Octyl Alcohol Vent Gas Mixture via Hydrate Formation in the Presence of SDS and THF in Tap-Water System. J. Chem. Eng. Data 2019, 64, 1244–1249. [Google Scholar] [CrossRef]
- Horii, S.; Ohmura, R. Continuous separation of CO2 from a H2 + CO2 gas mixture using clathrate hydrate. Appl. Energy 2018, 225, 78–84. [Google Scholar] [CrossRef]
- Fan, S.; Long, X.; Lang, X.; Wang, Y.; Chen, J. CO2 Capture from CH4/CO2 Mixture Gas with Tetra-n-butylammonium Bromide Semi-clathrate Hydrate through a Pressure Recovery Method. Energy Fuels 2016, 30, 8529–8534. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, Y.-S.; Yu, X.-Y.; Yuan, Q.; Wang, T.; Deng, B.; Yan, K.-L.; Jiang, J.-H.; Tao, L.-M.; Chen, C.-Z. A covering liquid method to intensify self-preservation effect for safety of methane hydrate storage and transportation. Pet. Sci. 2022, 19, 1411–1419. [Google Scholar] [CrossRef]
- Warrier, P.; Khan, M.N.; Srivastava, V.; Maupin, C.M.; Koh, C.A. Overview: Nucleation of clathrate hydrates. J. Chem. Phys. 2016, 145, 211705. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Zeng, Y.; Liang, Z.; Chen, G.; Liu, B.; Li, Z.; Deng, B. Self-preservation effect exceeding 273.2 K by introducing deuteriumoxide to form methane hydrate. Chem. Eng. J. 2022, 433, 134591. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, Y.; Liu, C.; Kang, M.; Chen, G.; Deng, B.; Zeng, F. Methane hydrate dissociation from anti-agglomerants containing oil dominated dispersed systems. Fuel 2021, 294, 120561. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, J.; Yu, X.; Wang, T.; Deng, B.; Zeng, F.; Li, J. Suppression of methane hydrate dissociation from SDS-dry solution hydrate formation system by a covering liquid method. Fuel 2020, 277, 118222. [Google Scholar] [CrossRef]
- Montazeri, S.M.; Kolliopoulos, G. Hydrate based desalination for sustainable water treatment: A review. Desalination 2022, 537, 115855. [Google Scholar] [CrossRef]
- Sun, H.; Sun, L.; Zhao, Y.; Yang, S.; Zhang, L.; Dong, H.; Yuan, H.; Ling, Z.; Zhao, J.; Song, Y. A combined hydrate-based method for removing heavy metals from simulated wastewater with high concentrations. J. Environ. Chem. Eng. 2021, 9, 106633. [Google Scholar] [CrossRef]
- Gaikwad, N.; Nakka, R.; Khavala, V.; Bhadani, A.; Mamane, H.; Kumar, R. Gas Hydrate-Based Process for Desalination of Heavy Metal Ions from an Aqueous Solution: Kinetics and Rate of Recovery. ACS EST Water 2021, 1, 134–144. [Google Scholar]
- Dong, H.; Zhang, L.; Ling, Z.; Zhao, J.; Song, Y. The Controlling Factors and Ion Exclusion Mechanism of Hydrate-Based Pollutant Removal. ACS Sustain. Chem. Eng. 2019, 7, 7932–7940. [Google Scholar] [CrossRef]
- Song, Y.; Dong, H.; Yang, L.; Yang, M.; Li, Y.; Ling, Z.; Zhao, J. Hydrate-based heavy metal separation from aqueous solution. Sci. Rep. 2016, 6, 21389. [Google Scholar] [CrossRef]
- Wu, J.-J.; Kang, M.-Q.; Hu, F.-L.; Yan, Y.-H.; Liu, C.-Z.; Chen, J.; Liang, Z.-K.; Zeng, Y.-S.; Jiang, J.-H.; Deng, B. Comparing hydrate-based method with freezing/thawing method for chromium hydroxide sulfate removal close to the melting point of ice. Sep. Purif. Technol. 2021, 266, 118523. [Google Scholar] [CrossRef]
- Hu, F.; Yang, Y.; Kang, M.; Chen, J.; Liu, Q.; Liu, J.; Li, J.; Wu, J.; Deng, B. Hydrate-Based Method to Remove Cr(III) and Ni(II) in Chromium Hydroxide Sulfate and Nickel Sulfate Hexahydrate Solutions. J. Chem. Eng. Data 2021, 66, 4248–4253. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, X.; Chen, J.; Xie, Y.; Hu, F.; Chen, C.; Li, D.; Jiang, J.; Deng, B. Investigation on the Removal Performances of Heavy Metal Copper (II) Ions from Aqueous Solutions Using Hydrate-Based Method. Molecules 2023, 28, 469. https://doi.org/10.3390/molecules28020469
Lan X, Chen J, Xie Y, Hu F, Chen C, Li D, Jiang J, Deng B. Investigation on the Removal Performances of Heavy Metal Copper (II) Ions from Aqueous Solutions Using Hydrate-Based Method. Molecules. 2023; 28(2):469. https://doi.org/10.3390/molecules28020469
Chicago/Turabian StyleLan, Xiaobing, Jun Chen, Yang Xie, Fenglong Hu, Changzhong Chen, Dongdong Li, Jianhong Jiang, and Bin Deng. 2023. "Investigation on the Removal Performances of Heavy Metal Copper (II) Ions from Aqueous Solutions Using Hydrate-Based Method" Molecules 28, no. 2: 469. https://doi.org/10.3390/molecules28020469
APA StyleLan, X., Chen, J., Xie, Y., Hu, F., Chen, C., Li, D., Jiang, J., & Deng, B. (2023). Investigation on the Removal Performances of Heavy Metal Copper (II) Ions from Aqueous Solutions Using Hydrate-Based Method. Molecules, 28(2), 469. https://doi.org/10.3390/molecules28020469





