Effects of Hydrogen Bonds between Ethoxylated Alcohols and Sodium Oleate on Collecting Performance in Flotation of Quartz
Abstract
:1. Introduction
2. Results
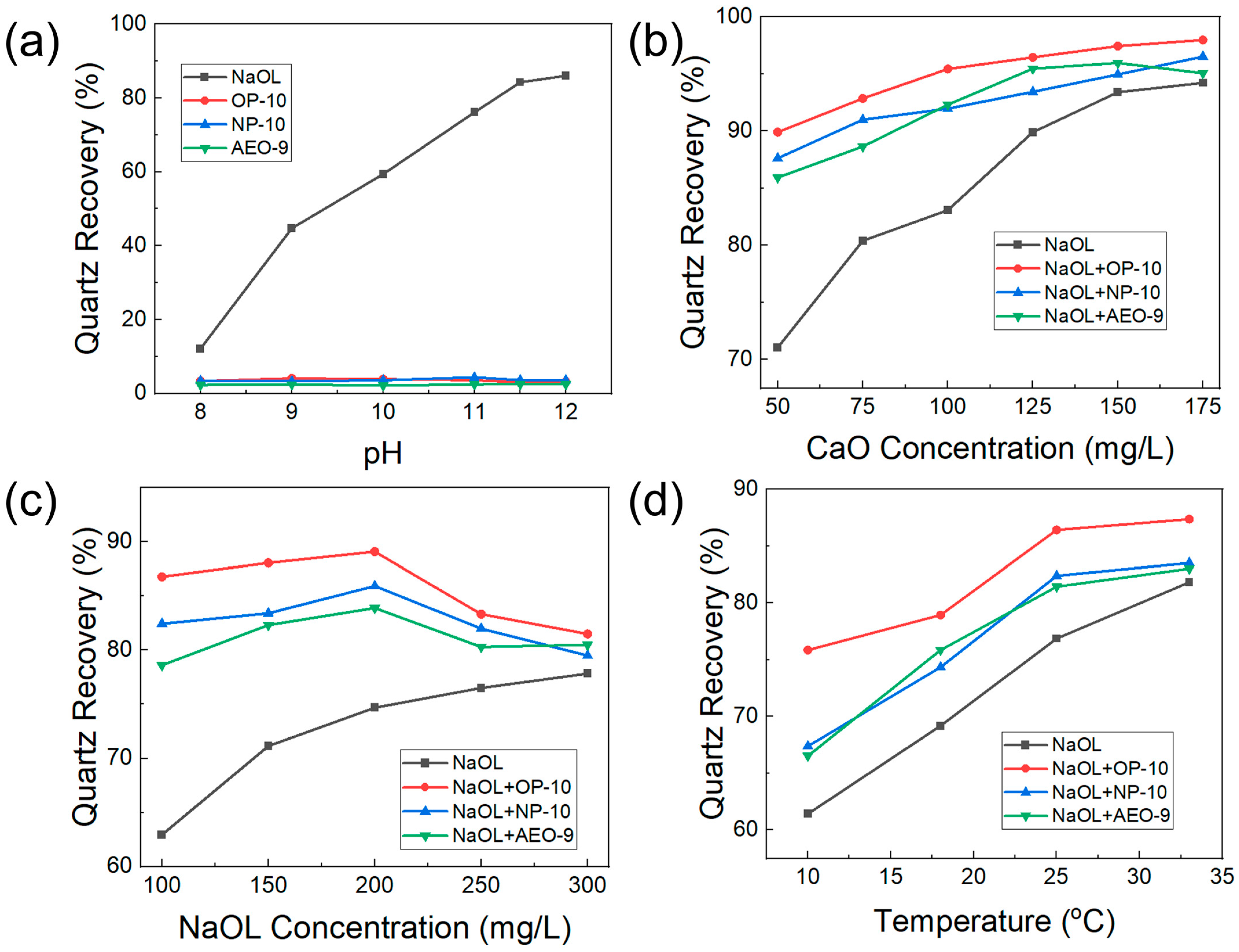
2.1. Flotation Behavior of Quartz with the Addition of Ethoxylated Alcohols
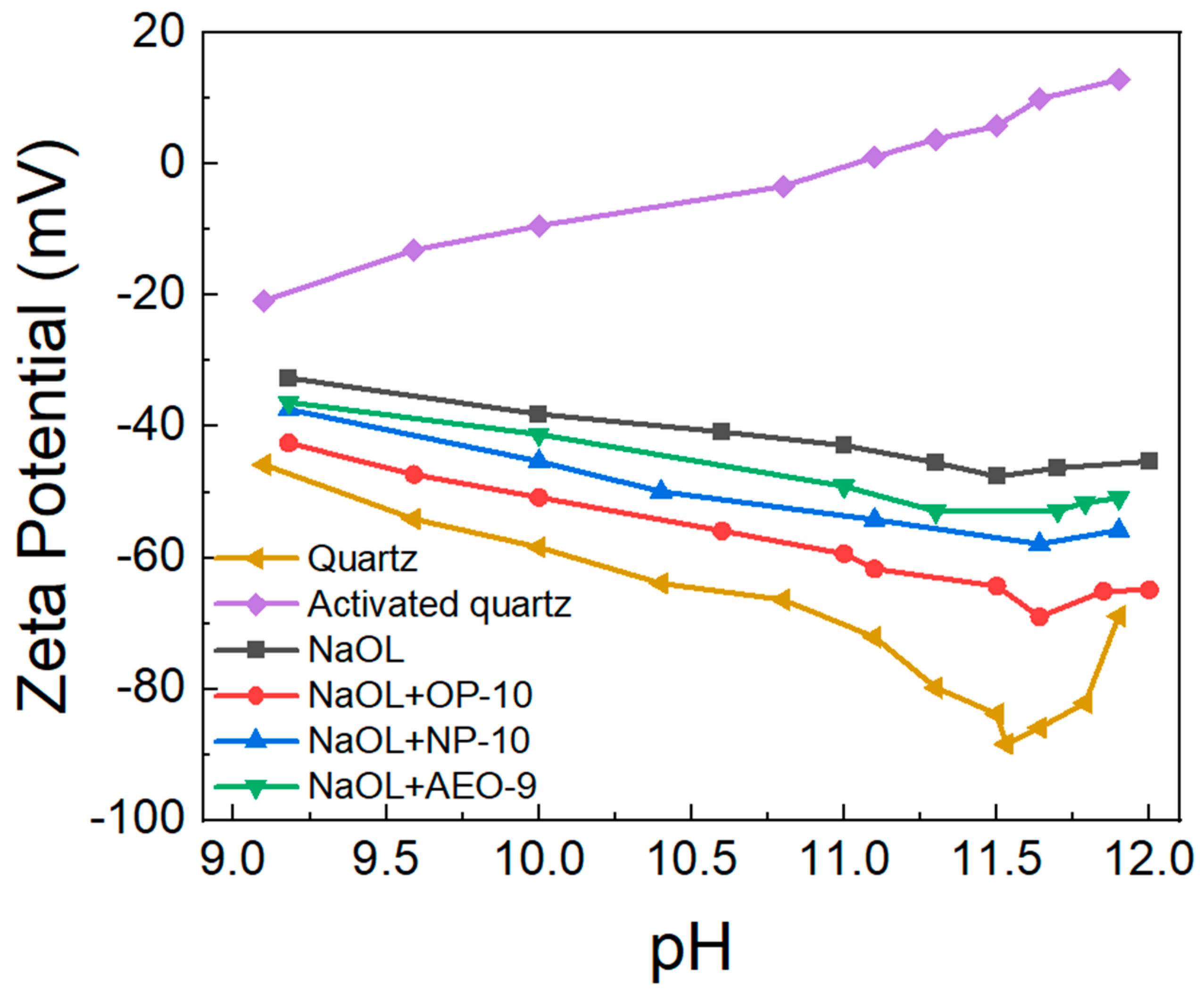
2.2. Adsorption Behavior of OL− on Quartz with the Participation of Ethoxylated Alcohols
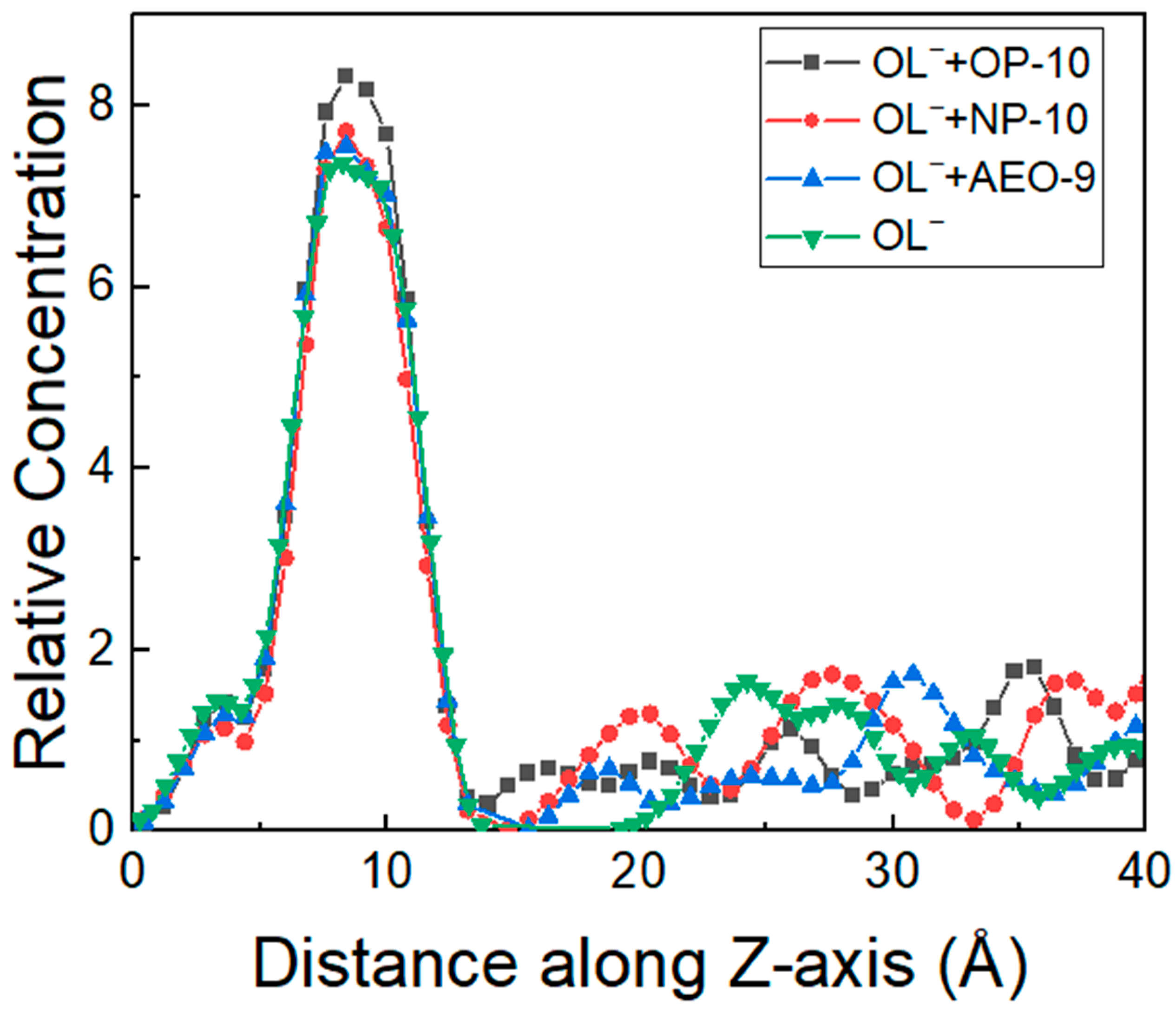
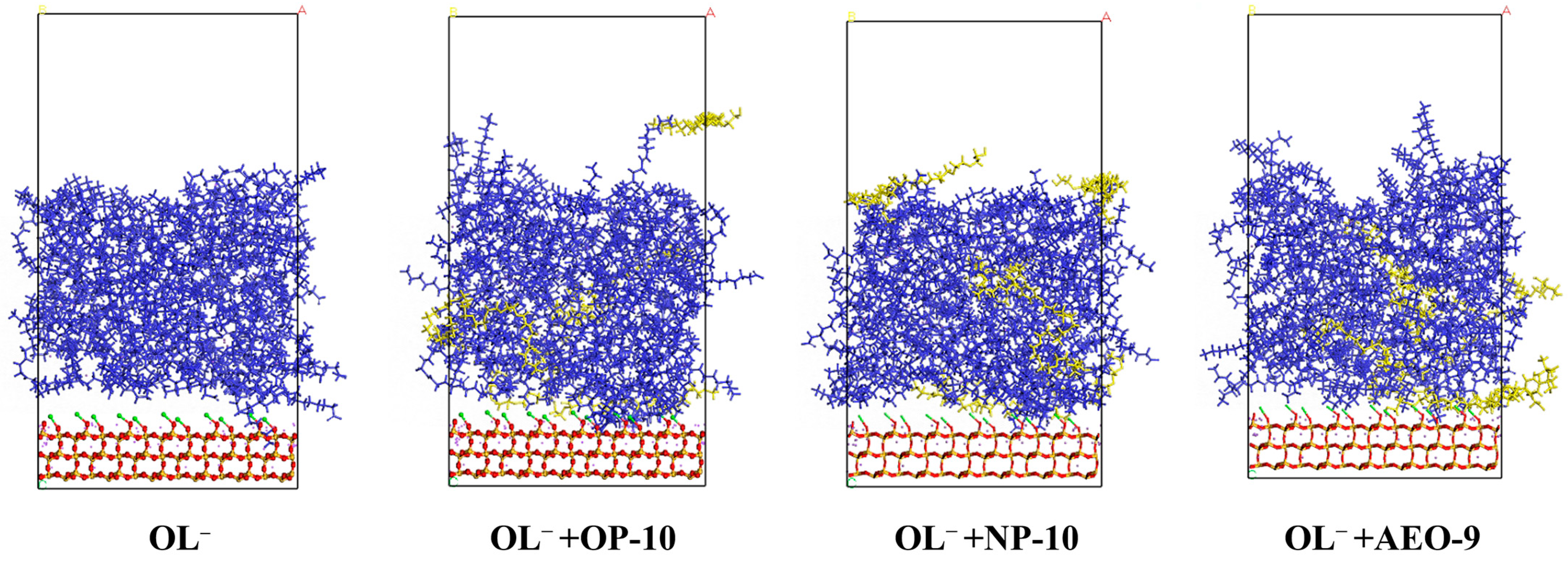
2.3. Hydrogen Bonds between Ethoxylated Alcohols and OL− and Their Effect on Collecting Behavior of OL−
3. Materials and Methods
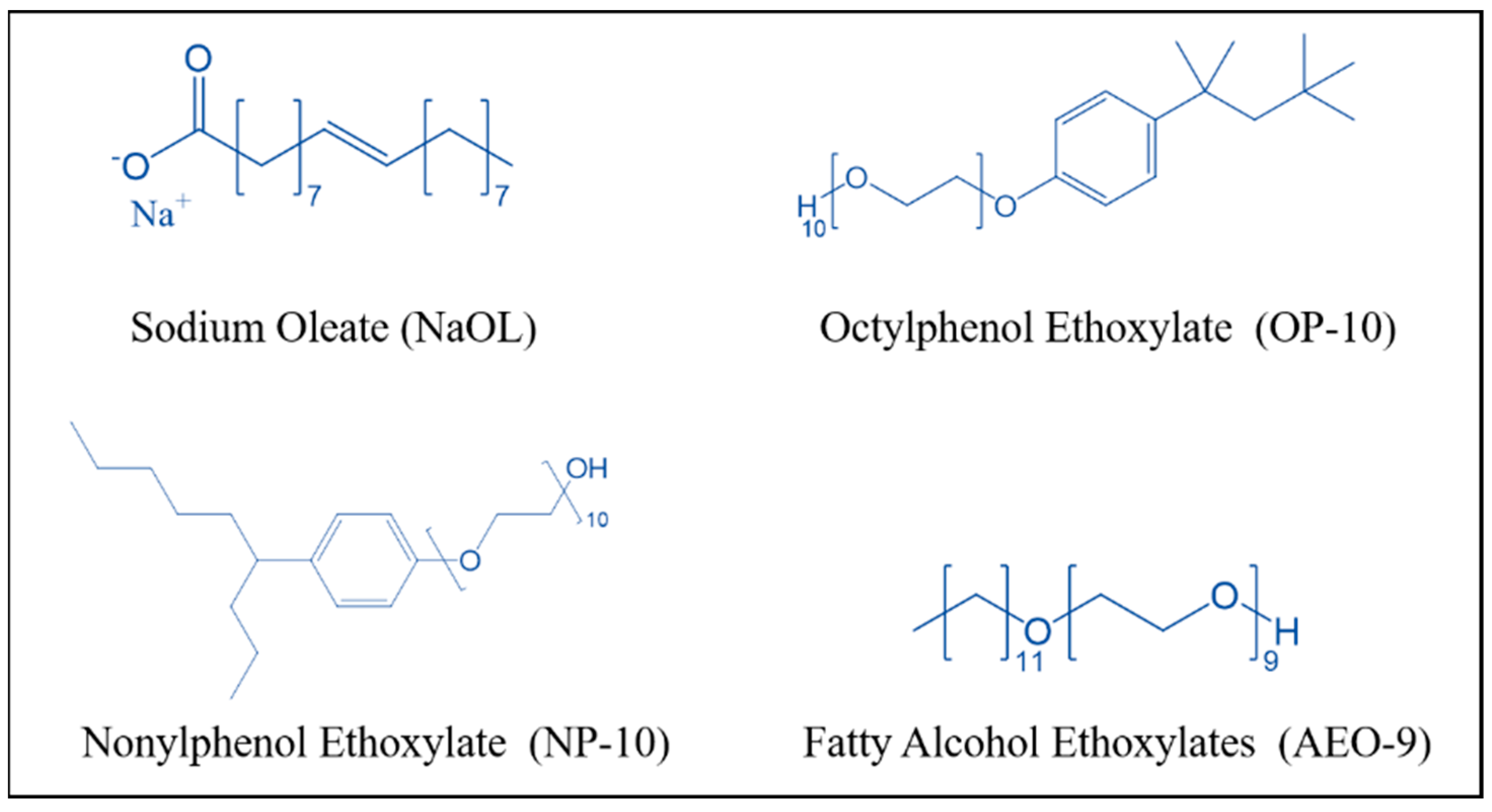
3.1. Materials
3.2. Flotation Test
3.3. Zeta Potential Test
3.4. Molecular Dynamic (MD) Simulation
4. Conclusions
- •
- The ethoxylated alcohols (OP-10, NP-10, AEO-9) had no collecting ability, but they could promote the flotation of quartz when sodium oleate was the collector. The dosage of the reagent including the activator CaO and the collector NaOL could be decreased with the addition of ethoxylated alcohols (OP-10, NP-10, AEO-9) to achieve the same flotation index. The promotion effect was in the order of OP-10 > NP-10 > AEO-9. The optimal dosage of reagent was 50 mg/L CaO, 150 mg/L NaOL and 7.5 mg/L ethoxylated alcohols (OP-10, NP-10, AEO-9), and the optimal temperature and pH were 25 °C and 11.5, respectively.
- •
- Based on the change in the Zeta potential of the quartz surface after modification, ethoxylated alcohols (OP-10, NP-10, AEO-9) promoted the adsorption of oleate on the quartz surface in the order of OP-10 > NP-10 > AEO-9.
- •
- Based on the MD simulation results, it was shown that there were hydrogen bonds between ethoxylated alcohols and oleate molecules, which decreased the repulsion between oleate in micelles, increased the activity of oleate and increased the attraction force between oleate and the activated quartz surface. As a result, the adsorption density of oleate increased with the synergy of ethoxylated alcohols in the order of OP-10 > NP-10 > AEO-9, which explained the results of the Zeta potential and flotation well.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crawford, C.B.; Quinn, B. 9—Microplastic Separation Techniques. In Microplastic Pollutants; Crawford, C.B., Quinn, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 203–218. [Google Scholar]
- Cui, W.; Chen, J. Insight into mineral flotation fundamentals through the DFT method. Int. J. Min. Sci. Technol. 2021, 31, 983–994. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, W.; Wen, S.; Wang, H.; Zhao, W.; Han, G. Flotation of copper oxide minerals: A review. Int. J. Min. Sci. Technol. 2022, 32, 1351–1364. [Google Scholar] [CrossRef]
- Aarab, I.; Derqaoui, M.; Abidi, A.; Yaacoubi, A.; El Amari, K.; Etahiri, A.; Baçaoui, A. Direct flotation of low-grade Moroccan phosphate ores: A preliminary micro-flotation study to develop new beneficiation routes. Arab. J. Geosci. 2020, 13, 1252. [Google Scholar] [CrossRef]
- Ren, Z.; Shen, Y.; Gao, H.; Chen, H.; Liu, C.; Chen, Z. Comparison of Sodium Oleate and Sodium Petroleum Sulfonate for Low-Temperature Flotation of Fluorite and the Collecting Mechanisms. Min. Metall. Explor. 2021, 38, 2527–2536. [Google Scholar] [CrossRef]
- Qiu, H.; Wu, B.; Deng, J.; Sun, X.; Hu, M.; Cai, J.; Zheng, C. The effect of collectors on froth stability of frother: Atomic-scale study by experiments and molecular dynamics simulations. J. Mol. Liq. 2022, 364, 120035. [Google Scholar] [CrossRef]
- Xiong, D.; Lu, L.; Holmes, R.J. 9—Developments in the physical separation of iron ore: Magnetic separation. In Iron Ore; Lu, L., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 283–307. [Google Scholar]
- Ozkan, A.; Ucbeyiay, H.; Duzyol, S. Comparison of stages in oil agglomeration process of quartz with sodium oleate in the presence of Ca(II) and Mg(II) ions. J. Colloid Interface Sci. 2009, 329, 81–88. [Google Scholar] [CrossRef]
- Hao, H.; Li, L.; Yuan, Z.; Liu, J. Molecular arrangement of starch, Ca2+ and oleate ions in the siderite-hematite-quartz flotation system. J. Mol. Liq. 2018, 254, 349–356. [Google Scholar] [CrossRef]
- Wang, Y.; Ahmed Khoso, S.; Luo, X.; Tian, M. Understanding the depression mechanism of citric acid in sodium oleate flotation of Ca2+-activated quartz: Experimental and DFT study. Miner. Eng. 2019, 140, 105878. [Google Scholar] [CrossRef]
- Cao, S.; Yin, W.; Yang, B.; Zhu, Z.; Sun, H.; Sheng, Q.; Chen, K. Insights into the influence of temperature on the adsorption behavior of sodium oleate and its response to flotation of quartz. Int. J. Min. Sci. Technol. 2022, 32, 399–409. [Google Scholar] [CrossRef]
- Liu, A.; Fan, P.-P.; Qiao, X.-X.; Li, Z.-H.; Wang, H.-F.; Fan, M.-Q. Synergistic effect of mixed DDA/surfactants collectors on flotation of quartz. Miner. Eng. 2020, 159, 106605. [Google Scholar] [CrossRef]
- Cao, Q.; Cheng, J.; Wen, S.; Li, C.; Bai, S.; Liu, D. A mixed collector system for phosphate flotation. Miner. Eng. 2015, 78, 114–121. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Kou, J.; Sun, C. Synergy Effect between Sodium Oleate and Ethoxylated alcohols on the Reverse Flotation of Quartz. Minerals 2023, 13, 93. [Google Scholar] [CrossRef]
- Espeso, M.B.; Corada-Fernández, C.; García-Delgado, M.; Candela, L.; González-Mazo, E.; Lara-Martín, P.A.; Jiménez-Martínez, J. Structural control of the non-ionic surfactant ethoxylated alcohols (AEOs) on transport in natural soils. Environ. Pollut. 2021, 269, 116021. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, J.; Zhang, Y.; Liang, H.; Sun, J.; Liu, Y.; D’Errico, G.; Sun, Y.; Di Serio, M. Synthesis and Properties of Primary Ethoxylated alcohols Using Different Catalytic Systems. ACS Omega 2021, 6, 29774–29780. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Cheng, X.; Zhao, O.; Liu, S.; Liu, C.; Wang, J.; Huang, J. The evolution of self-assemblies in the mixed system of oleic acid–diethylenetriamine based on the transformation of electrostatic interactions and hydrogen bonds. Soft Matter 2014, 10, 8023–8030. [Google Scholar] [CrossRef]
- Ghosh, S.; Ray, A.; Pramanik, N. Self-assembly of surfactants: An overview on general aspects of amphiphiles. Biophys. Chem. 2020, 265, 106429. [Google Scholar] [CrossRef]
- An, M.; Liao, Y.; Cao, Y.; Hao, X.; Ma, L. Improving Low Rank Coal Flotation Using a Mixture of Oleic Acid and Dodecane as Collector: A New Perspective on Synergetic Effect. Processes 2021, 9, 404. [Google Scholar] [CrossRef]
- Li, H.; Chai, W.; Cao, Y.; Wu, Y.; Yang, S. Synergistic collection mechanism of D-phenylalanine and sodium oleate in flotation of diaspore from kaolinite. Appl. Surf. Sci. 2021, 538, 147937. [Google Scholar] [CrossRef]
- Ayoub, A.T.; Tuszynski, J.; Klobukowski, M. Estimating hydrogen bond energies: Comparison of methods. Theor. Chem. Acc. 2014, 133, 1520. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Y.; Wen, S.; Ma, M.; Sun, C.; Yin, W.; Ma, Y. Effect of carbonate minerals on quartz flotation behavior under conditions of reverse anionic flotation of iron ores. Int. J. Miner. Process. 2016, 152, 1–6. [Google Scholar] [CrossRef]
- Kou, J.; Xu, S.; Sun, T.; Sun, C.; Guo, Y.; Wang, C. A study of sodium oleate adsorption on Ca2+ activated quartz surface using quartz crystal microbalance with dissipation. Int. J. Miner. Process. 2016, 154, 24–34. [Google Scholar] [CrossRef]
- Hou, Y.; Sobhy, A. New Insights on Sodium Oleate Adsorption on Quartz for Iron Direct Flotation under Weak-Acidic Condition. Tenside Surfactants Deterg. 2021, 58, 237–242. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, Y.; Cao, Y. Reverse Flotation of Quartz From Magnetite Ore with Modified Sodium Oleate. Miner. Process. Extr. Metall. Rev. 2013, 34, 320–330. [Google Scholar] [CrossRef]
- Ben-Tal, N.; Sitkoff, D.; Topol, I.A.; Yang, A.-S.; Burt, S.K.; Honig, B. Free Energy of Amide Hydrogen Bond Formation in Vacuum, in Water, and in Liquid Alkane Solution. J. Phys. Chem. B 1997, 101, 450–457. [Google Scholar] [CrossRef]
- Sweetman, A.M.; Jarvis, S.P.; Sang, H.; Lekkas, I.; Rahe, P.; Wang, Y.; Wang, J.; Champness, N.R.; Kantorovich, L.; Moriarty, P. Mapping the force field of a hydrogen-bonded assembly. Nat. Commun. 2014, 5, 3931. [Google Scholar] [CrossRef]
- Zhou, Q.; Rosen, M.J. Molecular Interactions of Surfactants in Mixed Monolayers at the Air/Aqueous Solution Interface and in Mixed Micelles in Aqueous Media: The Regular Solution Approach. Langmuir 2003, 19, 4555–4562. [Google Scholar] [CrossRef]
- Holland, P.M.; Rubingh, D.N. Mixed surfactant systems: An overview. In Mixed Surfactant Systems; ACS Publications: Washington, DC, USA, 1992. [Google Scholar]
- Sun, H. Compass: An ab initio force-field optimized for condensed-phase applications—Overview with details on alkane and benzene compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Liu, W.; Shen, Y.; Duan, H.; Qiu, J.; Gu, X. Understanding adsorption of amine surfactants on the solvated quartz (101) surface by a jointed Dreiding-ClayFF force field. Appl. Surf. Sci. 2021, 566, 150737. [Google Scholar] [CrossRef]
- Luo, X.; Qi, L.; Wen, S.; Wang, Y.; Lai, H.; Lin, Q.; Zhou, Y.; Wu, X.; Song, Z. Adsorption configuration of dodecylamine at gas–liquid interface and its relationship with foam stability: MD simulation and ToF-SIMS investigation. Miner. Eng. 2021, 164, 106830. [Google Scholar] [CrossRef]


| Alcohol Ethoxylate | Hydrogen Bond Energy in Vacuum (kcal/mol) | Hydrogen Bond Energy in Water (kcal/mol) |
|---|---|---|
| OP-10 | 21.94 | 4.57 |
| NP-10 | 10.23 | 2.30 |
| AEO-9 | 7.58 | 1.46 |
| Alcohol Ethoxylate (AE) | Energy Change after Addition of AE (kcal/mol) |
|---|---|
| OP-10 | −2264.9 |
| NP-10 | −1386.9 |
| AEO-9 | −1234.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Li, J.; Kou, J.; Sun, C. Effects of Hydrogen Bonds between Ethoxylated Alcohols and Sodium Oleate on Collecting Performance in Flotation of Quartz. Molecules 2023, 28, 6945. https://doi.org/10.3390/molecules28196945
Zhang N, Li J, Kou J, Sun C. Effects of Hydrogen Bonds between Ethoxylated Alcohols and Sodium Oleate on Collecting Performance in Flotation of Quartz. Molecules. 2023; 28(19):6945. https://doi.org/10.3390/molecules28196945
Chicago/Turabian StyleZhang, Na, Jiajia Li, Jue Kou, and Chunbao Sun. 2023. "Effects of Hydrogen Bonds between Ethoxylated Alcohols and Sodium Oleate on Collecting Performance in Flotation of Quartz" Molecules 28, no. 19: 6945. https://doi.org/10.3390/molecules28196945
APA StyleZhang, N., Li, J., Kou, J., & Sun, C. (2023). Effects of Hydrogen Bonds between Ethoxylated Alcohols and Sodium Oleate on Collecting Performance in Flotation of Quartz. Molecules, 28(19), 6945. https://doi.org/10.3390/molecules28196945









