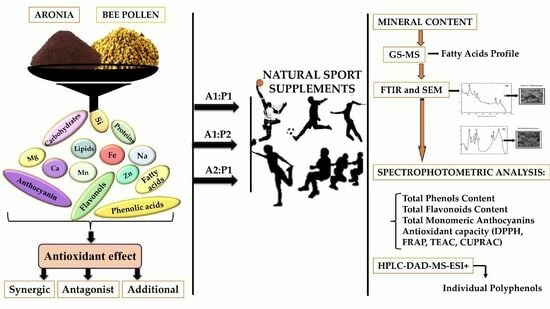
Designing New Sport Supplements Based on Aronia melanocarpa and Bee Pollen to Enhance Antioxidant Capacity and Nutritional Value
Abstract
:1. Introduction
2. Results
2.1. Characterization of Raw Biological Material
Proximate Composition: Total Carbohydrate, Lipid, Protein, Mineral Content and Fatty Acids Profile in Aronia and Bee Pollen Samples
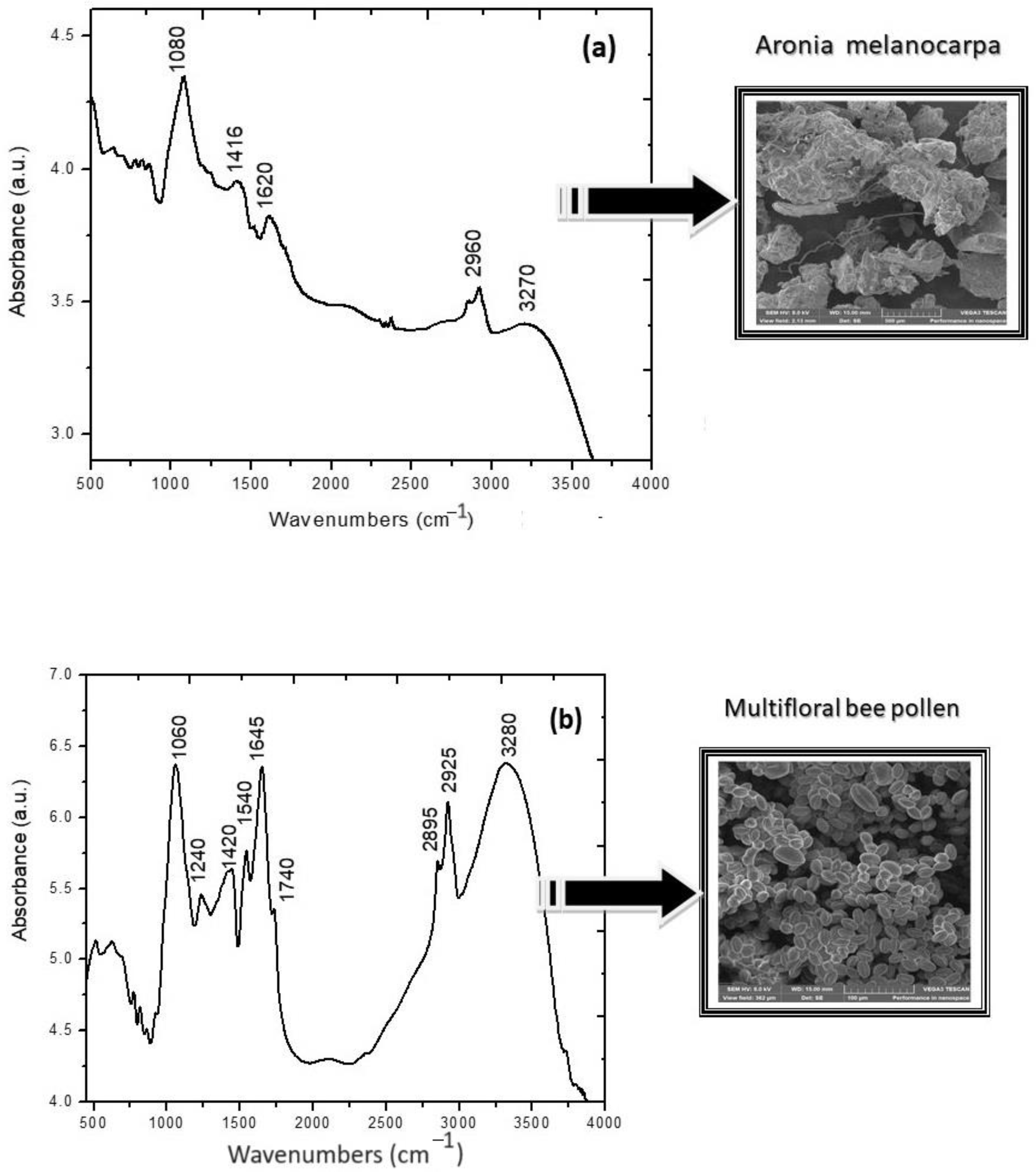
2.2. Structural Characterization via FTIR Spectroscopy
2.3. Determination of Bio-Active Compounds in Aronia and Bee Pollen Samples
2.4. Identification and Quantification of Individual Polyphenols via HPLC-DAD-MS-ESI+
2.5. Determination of Antioxidant Capacity of Biological Samples
3. Discussion
4. Materials and Methods
4.1. Biological Material and Experimental Design
4.2. Characterization of Raw Materials
4.2.1. Determination of Total Carbohydrate, Lipid and Protein Contents in Aronia and Bee Pollen Samples
4.2.2. Determination of Mineral Content
4.2.3. Determination of Fatty Acids Profile via GS-MS
4.2.4. Structural and Morphological Characterization via FTIR and SEM
4.3. Determination of Bio-Active Compounds in Aronia and Bee POLLEN Samples Using Spectrophotometric Methods
4.3.1. Extraction of Phenols from ARONIA and Bee Pollen
4.3.2. Total Phenols Content
4.3.3. Total Flavonoids Content
4.3.4. Total Monomeric Anthocyanins (Spectrophotometric Method)
4.4. Identification and Quantification of Individual Polyphenols via HPLC-DAD-MS-ESI+
4.5. Determination of Antioxidant Capacity of Biological Samples
4.5.1. DPPH (2,2-Diphenyl-1-picryl-hydrazyl-hydrate) Assay
4.5.2. FRAP (Ferric-Reducing Antioxidant Power) Assay
4.5.3. TEAC (Trolox Equivalent Antioxidant Capacity) Assay
4.5.4. CUPRAC (Cupric-Reducing Antioxidant Capacity) Assay
4.5.5. Preparation of Natural Sport Supplements and Determination of Nutritional Values
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC Consensus Statement: Dietary Supplements and the High-Performance Athlete. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 104–125. [Google Scholar] [CrossRef] [PubMed]
- Lindschinger, M.; Tatzber, F.; Schimetta, W.; Schmid, I.; Lindschinger, B.; Cvirn, G.; Fuchs, N.; Markolin, G.; Lamont, E.; Wonisch, W. Bioavailability of natural versus synthetic B vitamins and their effects on metabolic processes. MMW Fortschr. Med. 2020, 162, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, H.; Offord-Cavin, E.; Phothirath, P.; Horcajada, M.-N.; Romeis, P.; Mathis, G.A. 1,25-Dihydroxyvitamin D3-Glycoside of Herbal Origin Exhibits Delayed Release Pharmacokinetics When Compared to Its Synthetic Counterpart. J. Steroid Biochem. Mol. Biol. 2013, 136, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.G.; Lunkenbein, S.; Ströhle, A.; Hahn, A. Antioxidants in Food: Mere Myth or Magic Medicine? Crit. Rev. Food Sci. Nutr. 2012, 52, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and Therapeutic Properties of Bee Pollen: A Review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef] [PubMed]
- Dickson, J.H.; Oeggl, K.; Holden, T.G.; Handley, L.L.; O’Connell, T.C.; Preston, T. The Omnivorous Tyrolean Iceman: Colon Contents (Meat, Cereals, Pollen, Moss and Whipworm) and Stable Isotope Analyses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Martín-Muñoz, M.F.; Bartolome, B.; Caminoa, M.; Bobolea, I.; Garcia Ara, M.C.; Quirce, S. Bee Pollen: A Dangerous Food for Allergic Children. Identification of Responsible Allergens. Allergol. Immunopathol. 2010, 38, 263–265. [Google Scholar] [CrossRef]
- Algethami, J.S.; El-Wahed, A.A.A.; Elashal, M.H.; Ahmed, H.R.; Elshafiey, E.H.; Omar, E.M.; Naggar, Y.A.; Algethami, A.F.; Shou, Q.; Alsharif, S.M.; et al. Bee Pollen: Clinical Trials and Patent Applications. Nutrients 2022, 14, 2858. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Apitherapy for Age-Related Skeletal Muscle Dysfunction (Sarcopenia): A Review on the Effects of Royal Jelly, Propolis, and Bee Pollen. Foods 2020, 9, 1362. [Google Scholar] [CrossRef]
- Taha, E.-K.A.; Al-Kahtani, S.; Taha, R. Protein Content and Amino Acids Composition of Bee-Pollens from Major Floral Sources in Al-Ahsa, Eastern Saudi Arabia. Saudi J. Biol. Sci. 2019, 26, 232–237. [Google Scholar] [CrossRef]
- Jeannerod, L.; Carlier, A.; Schatz, B.; Daise, C.; Richel, A.; Agnan, Y.; Baude, M.; Jacquemart, A.-L. Some Bee-Pollinated Plants Provide Nutritionally Incomplete Pollen Amino Acid Resources to Their Pollinators. PLoS ONE 2022, 17, e0269992. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Dranca, F.; Ursachi, F. Characterization of Romanian Bee Pollen—An Important Nutritional Source. Foods 2022, 11, 2633. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Evans, S.P. Effects of Pollen Extract upon Adolescent Swimmers. Br. J. Sports Med. 1982, 16, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Steben, R.E.; Boudreaux, P. The Effects of Pollen and Protein Extracts on Selected Blood Factors and Performance of Athletes. J. Sports Med. Phys. Fit. 1978, 18, 221–226. [Google Scholar]
- Nazarian, A.; Azarbayjani, M.A.; Atashak, S.; Peeri, M. Effects of Resistance Training, Palm Pollen Grain Extracts, and Testosterone Injection on Luteinizing Hormone Receptors, Claudin-1, Cingulin, and Zonula Occludens in the Prostate Tissues of Adult Male Rats. Andrologia 2022, 54, e14394. [Google Scholar] [CrossRef] [PubMed]
- Ketkar, S.; Rathore, A.; Kandhare, A.; Lohidasan, S.; Bodhankar, S.; Paradkar, A.; Mahadik, K. Alleviating Exercise-Induced Muscular Stress Using Neat and Processed Bee Pollen: Oxidative Markers, Mitochondrial Enzymes, and Myostatin Expression in Rats. Integr. Med. Res. 2015, 4, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Salles, J.; Cardinault, N.; Patrac, V.; Berry, A.; Giraudet, C.; Collin, M.-L.; Chanet, A.; Tagliaferri, C.; Denis, P.; Pouyet, C.; et al. Bee Pollen Improves Muscle Protein and Energy Metabolism in Malnourished Old Rats through Interfering with the Mtor Signaling Pathway and Mitochondrial Activity. Nutrients 2014, 6, 5500–5516. [Google Scholar] [CrossRef]
- Elghouizi, A.; Al-Waili, N.; Elmenyiy, N.; Elfetri, S.; Aboulghazi, A.; Al-Waili, A.; Lyoussi, B. Protective Effect of Bee Pollen in Acute Kidney Injury, Proteinuria, and Crystalluria Induced by Ethylene Glycol Ingestion in Rats. Sci. Rep. 2022, 12, 8351. [Google Scholar] [CrossRef]
- Turner, K.K.; Nielsen, B.D.; O’Connor, C.I.; Burton, J.L. Bee Pollen Product Supplementation to Horses in Training Seems to Improve Feed Intake: A Pilot Study. J. Anim. Physiol. Anim. Nutr. 2006, 90, 414–420. [Google Scholar] [CrossRef]
- Jurendić, T.; Ščetar, M. Aronia Melanocarpa Products and By-Products for Health and Nutrition: A Review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef]
- Ren, Y.; Frank, T.; Meyer, G.; Lei, J.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health. Molecules 2022, 27, 7823. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-Induced Oxidative Stress: Friend or Foe? J. Sport. Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Zare, R.; Kimble, R.; Ali Redha, A.; Cerullo, G.; Clifford, T. How Can Chokeberry (Aronia) (Poly)Phenol-Rich Supplementation Help Athletes? A Systematic Review of Human Clinical Trials. Food Funct. 2023, 14, 5478–5491. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, B.; Cieślicka, M.; Mieszkowski, J.; Kochanowicz, A.; Niespodziński, B.; Szwarc, A.; Waldziński, T.; Reczkowicz, J.; Piskorska, E.; Petr, M.; et al. Effect of Supplementation with Black Chokeberry (Aronia melanocarpa) Extract on Inflammatory Status and Selected Markers of Iron Metabolism in Young Football Players: A Randomized Double-Blind Trial. Nutrients 2023, 15, 975. [Google Scholar] [CrossRef] [PubMed]
- Cikiriz, N.; Milosavljevic, I.; Jakovljevic, B.; Bolevich, S.; Jeremic, J.; Nikolic Turnic, T.; Mitrovic, M.; Srejovic, I.; Bolevich, S.; Jakovljevic, V. The Influences of Chokeberry Extract Supplementation on Redox Status and Body Composition in Handball Players during Competition Phase. Can. J. Physiol. Pharmacol. 2021, 99, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Pilaczynska-Szczesniak, L.; Skarpanska-Steinborn, A.; Deskur, E.; Basta, P.; Horoszkiewicz-Hassan, M. The Influence of Chokeberry Juice Supplementation on the Reduction of Oxidative Stress Resulting from an Incremental Rowing Ergometer Exercise. Int. J. Sport. Nutr. Exerc. Metab. 2005, 15, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Tirla, A.; Islam, F.; Islam, M.R.; Ioana Vicas, S.; Cavalu, S. New Insight and Future Perspectives on Nutraceuticals for Improving Sports Performance of Combat Players: Focus on Natural Supplements, Importance and Advantages over Synthetic Ones. Appl. Sci. 2022, 12, 8611. [Google Scholar] [CrossRef]
- Timmons, J.S.; Weiss, W.P.; Palmquist, D.L.; Harper, W.J. Relationships Among Dietary Roasted Soybeans, Milk Components, and Spontaneous Oxidized Flavor of Milk1. J. Dairy. Sci. 2001, 84, 2440–2449. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Ćujić, N.; Trifković, K.; Bugarski, B.; Ibrić, S.; Pljevljakušić, D.; Šavikin, K. Chokeberry (Aronia melanocarpa L.) Extract Loaded in Alginate and Alginate/Inulin System. Ind. Crops Prod. 2016, 86, 120–131. [Google Scholar] [CrossRef]
- Prđun, S.; Svečnjak, L.; Valentić, M.; Marijanović, Z.; Jerković, I. Characterization of Bee Pollen: Physico-Chemical Properties, Headspace Composition and FTIR Spectral Profiles. Foods 2021, 10, 2103. [Google Scholar] [CrossRef] [PubMed]
- Lahlali, R.; Jiang, Y.; Kumar, S.; Karunakaran, C.; Liu, X.; Borondics, F.; Hallin, E.; Bueckert, R. ATR–FTIR Spectroscopy Reveals Involvement of Lipids and Proteins of Intact Pea Pollen Grains to Heat Stress Tolerance. Front. Plant Sci. 2014, 5, 747. [Google Scholar] [CrossRef] [PubMed]
- Memete, A.R.; Miere (Groza), F.; Laslo, V.; Purcarea, C.; Vicas, L.; Ganea, M.; Antonescu, A.; Vicas, S.I. An In Vitro Study of the Healing Potential of Black Mulberry (Morus nigra L.) Extract in a Liposomal Formulation. Appl. Sci. 2023, 13, 1041. [Google Scholar] [CrossRef]
- Aylanc, V.; Tomás, A.; Russo-Almeida, P.; Falcão, S.I.; Vilas-Boas, M. Assessment of Bioactive Compounds under Simulated Gastrointestinal Digestion of Bee Pollen and Bee Bread: Bioaccessibility and Antioxidant Activity. Antioxidants 2021, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Edreva, A.M.; Velikova, V.B.; Tsonev, T.D. Phenylamides in Plants. Russ. J. Plant Physiol. 2007, 54, 287–301. [Google Scholar] [CrossRef]
- Stokes, T.; Hector, A.J.; Morton, R.W.; McGlory, C.; Phillips, S.M. Recent Perspectives Regarding the Role of Dietary Protein for the Promotion of Muscle Hypertrophy with Resistance Exercise Training. Nutrients 2018, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Poulios, A.; Georgakouli, K.; Draganidis, D.; Deli, C.K.; Tsimeas, P.D.; Chatzinikolaou, A.; Papanikolaou, K.; Batrakoulis, A.; Mohr, M.; Jamurtas, A.Z.; et al. Protein-Based Supplementation to Enhance Recovery in Team Sports: What Is the Evidence? J. Sports Sci. Med. 2019, 18, 523–536. [Google Scholar]
- Sierra-Galicia, M.I.; Rodríguez-de Lara, R.; Orzuna-Orzuna, J.F.; Lara-Bueno, A.; Ramírez-Valverde, R.; Fallas-López, M. Effects of Supplementation with Bee Pollen and Propolis on Growth Performance and Serum Metabolites of Rabbits: A Meta-Analysis. Animals 2023, 13, 439. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Wang, Q.; Xin, B.; Wang, H. Trophic Effect of Bee Pollen on Small Intestine in Broiler Chickens. J. Med. Food 2007, 10, 276–280. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Alagawany, M.; Farag, M.R.; Elnesr, S.S. Beneficial Impacts of Bee Pollen in Animal Production, Reproduction and Health. J. Anim. Physiol. Anim. Nutr. 2019, 103, 477–484. [Google Scholar] [CrossRef]
- HaščíK, P.; Pavelková, A.; Bobko, M.; Trembecká, L.; Elimam, I.O.E.; Capcarová, M. The Effect of Bee Pollen in Chicken Diet. World’s Poult. Sci. J. 2017, 73, 643–650. [Google Scholar] [CrossRef]
- Williams, C.; Rollo, I. Carbohydrate Nutrition and Team Sport Performance. Sports Med. 2015, 45, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.T.; Wallis, G.A. Carb-Conscious: The Role of Carbohydrate Intake in Recovery from Exercise. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 364–371. [Google Scholar] [CrossRef]
- Newell, M.; Wallis, G.; Hunter, A.; Tipton, K.; Galloway, S. Metabolic Responses to Carbohydrate Ingestion during Exercise: Associations between Carbohydrate Dose and Endurance Performance. Nutrients 2018, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, D.S.; Houltham, S.; Musa-Veloso, K.; Brown, F.; Paulionis, L.; Bailey, D. Fructose-Glucose Composite Carbohydrates and Endurance Performance: Critical Review and Future Perspectives. Sports Med. 2015, 45, 1561–1576. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.J.; Gonzalez, J.T.; Loon, L.J.C. Fructose Co-ingestion to Increase Carbohydrate Availability in Athletes. J. Physiol. 2019, 597, 3549–3560. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, M.; Hanus, P.; Lamparski, G.; Sawicki, T. Characterizing the Volatile and Sensory Profiles, and Sugar Content of Beeswax, Beebread, Bee Pollen, and Honey. Molecules 2021, 26, 3410. [Google Scholar] [CrossRef]
- Thielecke, F.; Blannin, A. Omega-3 Fatty Acids for Sport Performance—Are They Equally Beneficial for Athletes and Amateurs? A Narrative Review. Nutrients 2020, 12, 3712. [Google Scholar] [CrossRef]
- Zevenbergen, H.; de Bree, A.; Zeelenberg, M.; Laitinen, K.; van Duijn, G.; Flöter, E. Foods with a High Fat Quality Are Essential for Healthy Diets. Ann. Nutr. Metab. 2009, 54, 15–24. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef]
- Burke, L.M.; Ross, M.L.; Garvican-Lewis, L.A.; Welvaert, M.; Heikura, I.A.; Forbes, S.G.; Mirtschin, J.G.; Cato, L.E.; Strobel, N.; Sharma, A.P.; et al. Low Carbohydrate, High Fat Diet Impairs Exercise Economy and Negates the Performance Benefit from Intensified Training in Elite Race Walkers. J. Physiol. 2017, 595, 2785–2807. [Google Scholar] [CrossRef] [PubMed]
- Goldenshluger, A.; Constantini, K.; Goldstein, N.; Shelef, I.; Schwarzfuchs, D.; Zelicha, H.; Yaskolka Meir, A.; Tsaban, G.; Chassidim, Y.; Gepner, Y. Effect of Dietary Strategies on Respiratory Quotient and Its Association with Clinical Parameters and Organ Fat Loss: A Randomized Controlled Trial. Nutrients 2021, 13, 2230. [Google Scholar] [CrossRef]
- Philpott, J.D.; Witard, O.C.; Galloway, S.D.R. Applications of Omega-3 Polyunsaturated Fatty Acid Supplementation for Sport Performance. Res. Sports Med. 2019, 27, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Moussavi Javardi, M.S.; Madani, Z.; Movahedi, A.; Karandish, M.; Abbasi, B. The Correlation between Dietary Fat Quality Indices and Lipid Profile with Atherogenic Index of Plasma in Obese and Non-Obese Volunteers: A Cross-Sectional Descriptive-Analytic Case-Control Study. Lipids Health Dis. 2020, 19, 213. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Fell, J.; Williams, A. Exercise Causing Thrombosis. Physician Sportsmed. 2009, 37, 124–130. [Google Scholar] [CrossRef]
- Tirla, A.; Vesa, C.M.; Cavalu, S. Severe Cardiac and Metabolic Pathology Induced by Steroid Abuse in a Young Individual. Diagnostics 2021, 11, 1313. [Google Scholar] [CrossRef]
- Grozenski, A.; Kiel, J. Basic Nutrition for Sports Participation, Part 2: Vitamins and Minerals. Curr. Sports Med. Rep. 2020, 19, 508–510. [Google Scholar] [CrossRef]
- Heffernan, S.; Horner, K.; De Vito, G.; Conway, G. The Role of Mineral and Trace Element Supplementation in Exercise and Athletic Performance: A Systematic Review. Nutrients 2019, 11, 696. [Google Scholar] [CrossRef]
- Houston, M.C.; Harper, K.J. Potassium, Magnesium, and Calcium: Their Role in Both the Cause and Treatment of Hypertension. J. Clin. Hypertens. 2008, 10, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Belenguer-Varea, Á.; Tarazona-Santabalbina, F.J.; Avellana-Zaragoza, J.A.; Martínez-Reig, M.; Mas-Bargues, C.; Inglés, M. Oxidative Stress and Exceptional Human Longevity: Systematic Review. Free Radic. Biol. Med. 2020, 149, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liang, M.; Ugbolue, U.C.; Fekete, G.; Gu, Y. Effect of Physical Exercise Under Different Intensity and Antioxidative Supplementation for Plasma Superoxide Dismutase in Healthy Adults: Systematic Review and Network Meta-Analysis. Front. Physiol. 2022, 13, 707176. [Google Scholar] [CrossRef]
- Cobley, J.N.; Close, G.L.; Bailey, D.M.; Davison, G.W. Exercise Redox Biochemistry: Conceptual, Methodological and Technical Recommendations. Redox Biol. 2017, 12, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chen, C.; Teo, E.-C.; Zhang, Y.; Huang, J.; Xu, Y.; Gu, Y. Intracellular Oxidative Stress Induced by Physical Exercise in Adults: Systematic Review and Meta-Analysis. Antioxidants 2022, 11, 1751. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Redox Basis of Exercise Physiology. Redox Biol. 2020, 35, 101499. [Google Scholar] [CrossRef] [PubMed]
- Canals-Garzón, C.; Guisado-Barrilao, R.; Martínez-García, D.; Chirosa-Ríos, I.J.; Jerez-Mayorga, D.; Guisado-Requena, I.M. Effect of Antioxidant Supplementation on Markers of Oxidative Stress and Muscle Damage after Strength Exercise: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 1803. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef]
- Bakour, M.; Laaroussi, H.; Ferreira-Santos, P.; Genisheva, Z.; Ousaaid, D.; Teixeira, J.A.; Lyoussi, B. Exploring the Palynological, Chemical, and Bioactive Properties of Non-Studied Bee Pollen and Honey from Morocco. Molecules 2022, 27, 5777. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, W.; Latimer, G. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2006; p. 18. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Memete, A.R.; Sărac, I.; Teusdea, A.C.; Budău, R.; Bei, M.; Vicas, S.I. Bioactive Compounds and Antioxidant Capacity of Several Blackberry (Rubus Spp.) Fruits Cultivars Grown in Romania. Horticulturae 2023, 9, 556. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1–F2. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Vicaş, S.I.; Bandici, L.; Teuşdea, A.C.; Turcin, V.; Popa, D.; Bandici, G.E. The Bioactive Compounds, Antioxidant Capacity, and Color Intensity in Must and Wines Derived from Grapes Processed by Pulsed Electric Field. CyTA J. Food 2017, 15, 553–562. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Ranjbar Nedamani, E.; Sadeghi Mahoonak, A.; Ghorbani, M.; Kashaninejad, M. Evaluation of Antioxidant Interactions in Combined Extracts of Green Tea (Camellia sinensis), Rosemary (Rosmarinus officinalis) and Oak Fruit (Quercus branti). J. Food Sci. Technol. 2015, 52, 4565–4571. [Google Scholar] [CrossRef]
- Merrill, A.L.; Watt, B.K. Energy Value of Foods: Basis and Derivation; Human Nutrition Research Branch, Agricultural Research Service, US Department of Agriculture: Beltsville, MD, USA, 1955.
- Capuano, E.; Oliviero, T.; Fogliano, V.; Pellegrini, N. Role of the Food Matrix and Digestion on Calculation of the Actual Energy Content of Food. Nutr. Rev. 2018, 76, 274–289. [Google Scholar] [CrossRef]
| Component | Aronia | Bee Pollen | A1:P1 | A1:P2 | A2:P1 |
|---|---|---|---|---|---|
| Carbohydrates/100 g | 9.80 ± 0.09 e | 31.69 ± 0.3 a | 20.75 ± 0.2 c | 24.39 ± 0.2 b | 17.10 ± 0.2 d |
| Lipids/100 g | 13.95 ± 0.9 b | 20.44 ± 0.2 a,c | 17.20 ± 0.2 d,e | 18.28 ± 0.2 c,e,f | 16.11 ± 0.2 b,d,f |
| Proteins/100 g | 5.16 ± 0.05 e | 23.6 ± 0.2 a | 14.38 ± 0.1 c | 17.45 ± 0.2 b | 11.31 ± 0.1 d |
| Energy (kcal/100 g) | 202.246 ± 2.13 d | 402.520 ± 3.89 a | 293.448 ± 1.87 c,d,b | 329.890 ± 2.31 b | 257.005 ± 3.56 d |
| Minerals (mg/Kg/ppm) | Aronia | Bee Pollen | A1:P1 | A1:P2 | A2:P1 |
|---|---|---|---|---|---|
| Ca | 1270.28 | 557.32 | 913.80 | 794.97 | 1032.63 |
| K | 4054.04 | 1934.64 | 2994.34 | 2641.11 | 3347.57 |
| Mg | 463.88 | 312.32 | 388.10 | 362.84 | 413.36 |
| Na | 200.52 | 164.28 | 182.40 | 176.36 | 188.44 |
| Fe | 28.56 | 25.68 | 27.12 | 26.64 | 27.60 |
| Zn | 2.616 | 6.824 | 4.72 | 5.42 | 4.02 |
| Mn | 17.52 | 9.44 | 13.48 | 12.13 | 14.83 |
| Si | 33.12 | 26.92 | 30.02 | 28.99 | 31.05 |
| NaCl% | 6.76 | 3.72 | 5.24 | 4.73 | 5.75 |
| Fatty Acids | Aronia | Pollen | A1:P1 | A1:P2 | A2:P1 |
|---|---|---|---|---|---|
| Caprylic acid (C8:0) | 0.91 | 0.18 | 1.09 | 1.27 | 2.00 |
| Capric acid (C10:0) | 0.58 | nd | nd | nd | nd |
| Lauric acid (C12:0) | 0.42 | 0.76 | 1.19 | 1.95 | 1.61 |
| Myristic acid (C14:0) | 0.83 | 0.39 | 1.22 | 1.61 | 2.05 |
| Pentadecenoic acid (C15:1) | 0.83 | nd | nd | nd | nd |
| Palmitic acid (C16:0) | 8.10 | 22.12 | 30.22 | 52.34 | 38.32 |
| Palmitoleic acid (C16:1) | 15.08 | 0.15 | 15.23 | 15.38 | 30.31 |
| Heptadecanoic Acid (C17:0) | nd | 0.12 | nd | nd | nd |
| Heptadecenoic acid (C17:1) | nd | 0.18 | nd | nd | nd |
| Stearic acid (C18:0) | 1.37 | 1.60 | 2.97 | 4.57 | 4.35 |
| Oleic acid (C18:1C+T) | 15.61 | 5.48 | 21.09 | 26.56 | 36.70 |
| Linoleic acid (C18:2C+T) Ɯ-6 | 43.88 | 22.76 | 66.64 | 89.40 | 110.52 |
| Linolenic acid (C18:3n3) Ɯ-3 | 4.28 | 34.93 | 39.20 | 74.13 | 43.48 |
| Arachidic acid (C20:0) | nd | 0.32 | nd | nd | nd |
| 9-cis eicosenoic acid (C20:1n9) | nd | 0.31 | nd | nd | nd |
| Eicosadienoic acid (C20:2) | nd | 0.53 | nd | nd | nd |
| Behenic acid (C22:0) | 8.11 | 0.31 | 8.42 | 8.72 | 16.53 |
| Erucic acid (C22:1n9) | nd | 0.43 | nd | nd | nd |
| Docosadienoic acid (C22:2) | nd | 10.21 | nd | nd | nd |
| Σ SFA | 20.32 | 25.03 | 45.36 | 70.39 | 65.68 |
| Σ MUFA | 30.99 | 6.55 | 37.54 | 44.09 | 68.53 |
| Σ PUFA | 48.16 | 67.89 | 116.05 | 183.94 | 164.20 |
| Σ SMCFA | 1.91 | 0.18 | 2.09 | 2.27 | 4.00 |
| Σ LCFA | 118.41 | 99.82 | 218.23 | 318.06 | 336.64 |
| PUFAs/SFAs | 2.37 | 2.71 | 2.56 | 2.61 | 2.50 |
| Ɯ-6/n-Ɯ 3 FA | 10.26 | 0.65 | 1.70 | 1.21 | 2.54 |
| UI | 52.43 | 92.61 | 145.05 | 237.66 | 197.48 |
| AI | 0.15 | 0.38 | 0.25 | 0.29 | 0.22 |
| TI | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| h/H | 6.82 | 3.15 | 4.20 | 3.77 | 4.79 |
| Sample | Total Phenols Content (mg GAE/g dw) | Total Flavonoids Content (mg QE/g dw) | Total Monomeric Anthocyanin Pigment (MAP) Content (mg/L) |
|---|---|---|---|
| Aronia | 17.56 ± 3.49 a | 87.68 ± 9.86 a | 192.371 ± 22.67 |
| Pollen | 22.62 ± 0.88 a | 85.75 ± 15.30 a | - |
| A1:P1 (v/v) | 24.80 ± 4.99 a | 87.17 ± 15.55 a | - |
| A1:P2 (v/v) | 24.08 ± 6.52 a | 92.99 ± 14.26 a | - |
| A2:P1 (v/v) | 22.27 ± 5.76 a | 104.17 ± 19.81 a | - |
| Peak No. | Rt (min) | UV λmax (nm) | [M+H]+ (m/z) | Compound | Subclass | Sample: Aronia * (mg/g) |
|---|---|---|---|---|---|---|
| 1 | 2.99 | 280 | 139 | 2-Hydroxybenzoic acid | Hydroxybenzoic acid | 1.531 ± 0.09 |
| 2 | 4.03 | 280 | 155 | Dihydroxybenzoic acid | Hydroxybenzoic acid | 0.675 ± 0.03 |
| 3 | 10.21 | 280 | 155 | Protocatechuic acid | Hydroxybenzoic acid | 1.094 ± 0.07 |
| 4 | 10.99 | 520,280 | 449 | Cyanidin-glucoside | Anthocyanin | 0.257 ± 0.03 |
| 5 | 11.70 | 519,279 | 419,287 | Cyanidin-arabinoside | Anthocyanin | 0.239 ± 0.01 |
| 6 | 12.43 | 519,279 | 419,287 | Cyanidin-xyloside | Anthocyanin | 0.199 ± 0.01 |
| 7 | 13.04 | 332 | 355 | 5-Caffeoylquinic acid (Chlorogenic acid) | Hydroxycinnamic acid | 3.421 ± 0.2 |
| 8 | 13.58 | 330 | 181,163 | Caffeic acid | Hydroxycinnamic acid | 4.971 ± 0.2 |
| 9 | 14.89 | 333 | 369 | 3-Feruloylquinic acid | Hydroxycinnamic acid | 3.130 ± 0.1 |
| 10 | 15.55 | 333 | 369 | 5-Feruloylquinic acid | Hydroxycinnamic acid | 9.049 ± 0.08 |
| 11 | 15.83 | 355,250 | 611,303 | Quercetin-rutinoside (Rutin) | Flavonol | 0.906 ± 0.07 |
| 12 | 16.39 | 354,250 | 465,303 | Quercetin-glucoside | Flavonol | 2.115 ± 0.01 |
| 13 | 21.81 | 356,251 | 303 | Quercetin | Flavonol | 0.431 ± 0.05 |
| Total phenols | 28.017 |
| Peak No. | Rt (min) | UV λmax (nm) | [M+H]+ (m/z) | Compound | Subclass | Sample: Pollen * (mg/g) |
|---|---|---|---|---|---|---|
| 1 | 3.00 | 280 | 139 | 2-Hydroxybenzoic acid | Hydroxybenzoic acid | 2.753 ± 0.19 |
| 2 | 4.05 | 280 | 155 | Dihydroxybenzoic acid | Hydroxybenzoic acid | 0.843 ± 0.01 |
| 3 | 12.96 | 332 | 355 | 5-Caffeoylquinic acid (Chlorogenic acid) | Hydroxycinnamic acid | 0.304 ± 0.02 |
| 4 | 14.58 | 354, 250 | 627,303 | Quercetin-diglucoside | Flavonol | 4.416 ± 0.65 |
| 5 | 15.07 | 350, 255 | 641,317 | Isorhamnetin-diglucoside | Flavonol | 1.509 ± 0.9 |
| 6 | 15.41 | 350, 250 | 757,611, 449,287 | Kaempferol-glucoside-glucoside-rhamnoside | Flavonol | 4.272 ± 0.11 |
| 7 | 15.81 | 355, 250 | 611,303 | Quercetin-rutinoside (Rutin) | Flavonol | 4.654 ± 0.13 |
| 8 | 16.09 | 350, 250 | 595,287 | Kaempferol-rutinoside | Flavonol | 3.548 ± 0.14 |
| 9 | 16.54 | 330 | 339 | p-Coumaroylquinic acid | Hydroxycinnamic acid | 0.629 ± 0.02 |
| 10 | 17.25 | 350, 255 | 479,317 | Isorhamnetin-glucoside | Flavonol | 4.125 ± 0.21 |
| 11 | 17.54 | 350, 250 | 449,287 | Kaempferol-glucoside | Flavonol | 1.051 ± 0.099 |
| 12 | 18.54 | 350, 250 | 565,287 | Kaempferol-rhamnoside-arabinoside | Flavonol | 3.333 ± 0.13 |
| 13 | 19.23 | 322 | 674 | Triferuloyl spermidine | Hydroxycinnamic acid amide derivative | 4.422 ± 0.28 |
| 14 | 20.28 | 322 | 644 | Diferuloyl-coumaroyl spermidine | Hydroxycinnamic acid amide derivative | 11.926 ± 0.99 |
| 15 | 21.15 | 320 | 584 | Tricoumaroyl spermidine | Hydroxycinnamic acid amide derivative | 3.409 ± 0.26 |
| 16 | 22.31 | 321 | 614 | Feruloyl-dicoumaroyl spermidine | Hydroxycinnamic acid amide derivative | 5.002 ± 0.13 |
| Total phenols | 56.197 |
| Aronia | Pollen | A1:P1 (v/v) | A1:P2 (v/v) | A2:P1 (v/v) | |
|---|---|---|---|---|---|
| DPPH (mmolTE/g) | 350.95 ± 0.49 d | 413.85 ± 0.51 a | 380.61 ± 8.32 b,c | 391.27 ± 0.52 b,c | 373.62 ± 2.60 b |
| EAC | - | - | 0.995 | 1.023 | 0.977 |
| Additional | Synergic | Additional | |||
| FRAP (µmolTE/g) | 66.32 ± 8.65 a | 36.56 ± 11.26 b | 71.57 ± 0.26 a,b,c | 61.27 ± 0.78 a,b | 76.72 ± 0.78 a,c |
| EAC | - | - | 1.39 | 1.19 | 1.49 |
| Synergic | Synergic | Synergic | |||
| TEAC (µmol TE/g) | 63.24 ± 16.06 d | 86.06 ± 1.53 c | 85.56 ± 7.37 a,b,c,d | 89.40 ± 1.16 a | 65.00 ± 8.53 b |
| EAC | - | - | 1.146 | 1.198 | 0.871 |
| Synergic | Synergic | Antagonist | |||
| CUPRAC (µmol TE/g) | 189.66 ± 0.87 c | 155.18 ± 23.63 c | 209.36 ± 26.07 a | 176.71 ± 13.26 c | 160.35 ± 9.24 b,c |
| EAC | - | - | 1.214 | 1.025 | 0.930 |
| Synergic | Synergic | Antagonist |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirla, A.; Timar, A.V.; Becze, A.; Memete, A.R.; Vicas, S.I.; Popoviciu, M.S.; Cavalu, S. Designing New Sport Supplements Based on Aronia melanocarpa and Bee Pollen to Enhance Antioxidant Capacity and Nutritional Value. Molecules 2023, 28, 6944. https://doi.org/10.3390/molecules28196944
Tirla A, Timar AV, Becze A, Memete AR, Vicas SI, Popoviciu MS, Cavalu S. Designing New Sport Supplements Based on Aronia melanocarpa and Bee Pollen to Enhance Antioxidant Capacity and Nutritional Value. Molecules. 2023; 28(19):6944. https://doi.org/10.3390/molecules28196944
Chicago/Turabian StyleTirla, Adrian, Adrian Vasile Timar, Anca Becze, Adriana Ramona Memete, Simona Ioana Vicas, Mihaela Simona Popoviciu, and Simona Cavalu. 2023. "Designing New Sport Supplements Based on Aronia melanocarpa and Bee Pollen to Enhance Antioxidant Capacity and Nutritional Value" Molecules 28, no. 19: 6944. https://doi.org/10.3390/molecules28196944
APA StyleTirla, A., Timar, A. V., Becze, A., Memete, A. R., Vicas, S. I., Popoviciu, M. S., & Cavalu, S. (2023). Designing New Sport Supplements Based on Aronia melanocarpa and Bee Pollen to Enhance Antioxidant Capacity and Nutritional Value. Molecules, 28(19), 6944. https://doi.org/10.3390/molecules28196944