Abstract
Cirsium japonicum DC. var. australe Kitam. has been used as an herbal remedy and often involves using the whole plant or roots. However, the bioactivities of different parts of the plant have been far less explored. This study aimed to evaluate the antioxidative ability of methanol extracts from the flowers, leaves, stems, and roots of the Cirsium plant and their possible active components against juglone-induced oxidative stress in the nematode Caenorhabditis elegans. The results showed that the highest dry weight (12.3 g per plant) was observed in leaves, which was followed by stems (8.0 g). The methanol extract yields from the flowers, leaves, and roots were all similar (13.0–13.8%), while the yield from stems was the lowest (8.6%). The analysis of the silymarin contents in the extracts indicated that the flowers, leaves, stems, and roots contained silychristin and taxifolin; however, silydianin was only found in the leaves, stems, and roots. The flower, leaf, and stem extracts, at a concentration of 10 mg/L, significantly reduced juglone-induced oxidative stress in C. elegans, which was potentially due to the presence of silychristin and taxifolin. Overall, C. japonicum DC. var. australe Kitam. contains a significant amount of silymarin and exhibits in vivo antioxidative activity, suggesting that the prospects for the plant in terms of health supplements or as a source of silymarin are promising.
1. Introduction
Cirsium is a genus that is categorized under the family Asteraceae and has been found in many regions, such as Asia, Europe, North America, and eastern and northern Africa [1]. In some Asian medical cultures, Cirsium can be used as an ingredient in traditional Chinese medicine and has been shown to cool blood, stop bleeding, and reduce liver injury [2]. Additionally, Cirsium extracts have been reported to have several beneficial effects; for instance, the extracts from Cirsium rivulare roots demonstrated in vitro antioxidative activity and exhibited antibacterial properties [3]. An in vitro study showed that the ethanol extract of Cirsium japonicum var. maackii could be utilized in the development of breast cancer treatments [4]. The methanolic extract from the aerial parts of Cirsium japonicum var. ussuriense could inhibit the production of inflammatory mediators and cytokines in cell-based experiments [5]. Although plenty of studies have reported on the pharmacological effects of Cirsium extracts [2], a recent study indicated that the chemical constituents in Cirsium japonicum extracts from different regions may vary due to the distinguishable molecular markers in the plants [6]. This suggests the importance of conducting research on native plant species to better understand their unique properties and potential benefits. Taiwanese Cirsium plants, with a total of eleven identified species [1,7,8], have been used as folk medicines. However, only a few studies support their medical properties; for instance, the extracts from Cirsium arisanense Kitam. roots and leaves reduced the hepatotoxicity induced by tacrine in Hep 3B cells and in mice [9]. Cirsium japonicum DC. var. australe Kitam. and Cirsium kawakamii Hayata also exerted hepatoprotective effects in mice, which may be associated with their antioxidant activities [10]. In addition, a previous study found that among seven Taiwanese Cirsium species, C. japonicum DC. var. australe Kitam. showed the best radical-scavenging activities in vitro [11], thereby suggesting that C. japonicum DC. var. australe Kitam. presents noticeable therapeutic potential; however, the antioxidant activities and phytochemical composition of the different parts of the C. japonicum DC. var. australe Kitam. plant remains unclear.
It has been noted that some phytochemicals identified in the Cirsium plants are the same as those found in silymarin, which is a well-known extract from Silybum marianum L. Gaertn. (milk thistle) that has been used to treat hepatic diseases [12,13]. The main phytochemicals in silymarin include silibinin, isosilibinin, silychristin, silydianin, and taxifolin [14]. Additionally, the key compounds silibinin, silychristin, and silydianin, found in silymarin, have been discovered in extracts from the C. japonicum DC. var. australe Kitam. flowers. Their antioxidant activities have also been examined in vitro and were found to be in the order of silychristin > silibinin > silydianin [11]. Another study indicated that silibinin diastereomers might contribute to the hepatoprotective effects that were observed in extracts from four Taiwanese Cirsium plants [10]. In addition, taxifolin is a precursor in the production of silymarin in C. japonicum var. spinossimum Kitam. [15], which implies that taxifolin may be an essential ingredient in Cirsium plants. Notably, taxifolin has been proven to exert anti-inflammatory [16], antioxidant [17], and hepatoprotective effects [18]. Therefore, to efficiently explore the health-promoting potential of C. japonicum DC. var. australe Kitam., an analysis of the key active constituents in different parts of the plant C. japonicum DC. var. australe Kitam. is required.
Oxidative stress, which can be caused by an imbalance between the production of reactive species and the antioxidant defense system, has been linked to inflammation and several diseases, such as cancer, diabetes, and Alzheimer’s disease [19,20]. Although oxidative stress has been receiving great attention for about 35 years, scientists continue to explore promising therapies for alleviating oxidative damage [20]. To reduce oxidative damage and improve overall health status in humans, researchers have proposed that antioxidant therapies could be a good strategy for inhibiting oxidative damage [21]. Phytochemicals are abundant in plants, and they can confer protection against environmental threats and reverse oxidative stress [22]. For example, plant-derived non-enzymatic antioxidants such as curcumin can enhance the total antioxidant capacity in humans [23]. Synthesized curcumin analogues also exhibit in vitro antioxidant activity and can reduce scopolamine-caused oxidative stress in mice [24]. Although plant-derived antioxidants have been proven to exert a wide range of bioactivities [25], the assumption that phytochemicals can alleviate oxidative stress appears to be incorrect in some clinical situations, whereby endogenous antioxidant enzymes have the potential to react faster than exogenous antioxidants [19]. Given this, some researchers have attempted to utilize phytochemicals to regulate endogenous antioxidant enzymes. Consequently, to explore the beneficial effects of plant-derived compounds, there is a need to examine whether these phytochemicals can exert antioxidant activities in organisms.
The nematode Caenorhabditis elegans is widely used as an in vivo model organism. With a short life cycle, C. elegans takes only 3 days to grow from larvae into gravid adults. Moreover, C. elegans has a simple but well-investigated cell lineage [26]. The genome of C. elegans is highly homologous to humans, which makes it a versatile model for medical and biological studies [27]. Furthermore, C. elegans is a simplified and tractable system for studying the genetic and molecular aspects of stress responses; therefore, it has been used as a model organism to study stress response [28]. The highly conserved detoxification mechanisms involved in oxidative stress in C. elegans include the insulin signaling pathway, superoxide dismutase, and catalase [28,29]. Therefore, C. elegans also allows researchers to evaluate the protective effects of natural compounds through specific assays, such as oxidative stress resistance assay, and measurement of reactive oxygen species (ROS) levels [30]. In addition to aiding the exploration of the potential antioxidative activity of natural compounds, C. elegans can provide valuable insights into molecular and cellular information regarding the mechanisms of antioxidant activities exerted by natural compounds [30].
Herein, this study aimed to use C. elegans as a model to evaluate the antioxidative properties of methanol extracts from the flowers, leaves, stems, and roots of C. japonicum DC. var. australe Kitam., as well as the possible active components, silychristin and taxifolin, and silydianin during juglone-induced oxidative stress. Juglone (5-hydroxyl-1,4-naphthoquinone) is a natural toxin that induces cytotoxic effects, including ROS production, apoptosis, and DNA damage [31]; therefore, juglone has been used as a ROS-generating compound in C. elegans [32].
2. Results
2.1. C. japonicum DC. var. australe Kitam. Leaves Exhibit the Highest Dry Weight and Yield in Methanol Extracts
To screen the antioxidant potential of different parts of the C. japonicum DC. var. australe Kitam. plant, it was divided into four parts: flowers, leaves, stem, and roots. The results showed that the leaves exhibited the highest dry weight (12.3 g) per plant, followed by the stem (8.0 g), roots (6.4 g), and flowers (4.0 g) (Figure 1A). Similarly, the leaf ranked highest in terms of maximum dry weight percentage (39.9%), while the flowers had the lowest (13.1%) (Figure 1B). Methanol extraction was performed on these plant parts to obtain crude extracts. The results showed that similar yields of crude extracts were obtained from the flowers (13.3%), leaves (13.0%), and roots (13.8%), while the stem provided the lowest yield (8.6%) (Figure 1C). When considering the overall crude methanol extract yield, the highest crude methanol extract yield was found in the leaves, compared with the whole plant extract, followed by the stem, roots, and flowers (Figure 1D).
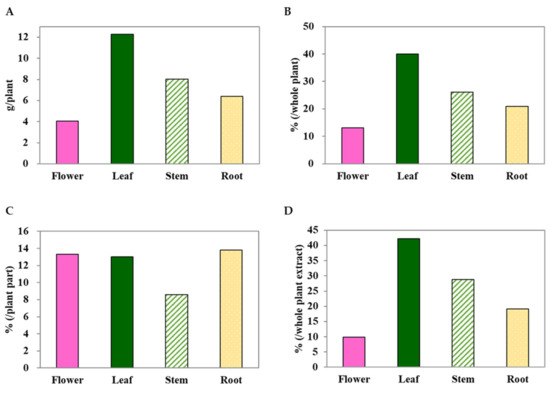
Figure 1.
The dry weights (A,B) and yields of the methanol extracts (C,D) from Cirsium japonicum DC. var. australe Kitam. flowers, leaves, stem, and roots.
2.2. Crude Methanol Extracts Enhance Oxidative Resistance in C. elegans
Next, the antioxidative activity of the crude methanol extract from the flowers, leaves, stem, and roots of C. japonicum DC. var. australe Kitam. was examined. In our study, a juglone-induced oxidative stress assay was applied to examine the oxidative resistance in C. elegans. Figure 2 shows that 10 mg/L of flower, leaf, and stem extracts increased the survival of the worms following exposure to 250 μM juglone for 3.5 h. Conversely, 10 mg/L of root extract did not show significant antioxidative activity (Figure 2). Moreover, 100 mg/L of the flower, leaf, stem, and root crude extracts also did not significantly increase the survival of the worms (Figure 2), thereby suggesting that a high concentration of the crude extracts could not provide beneficial effects and might exert toxic effects. Therefore, 10 mg/L of crude methanol extracts may alleviate the ROS production in C. elegans.
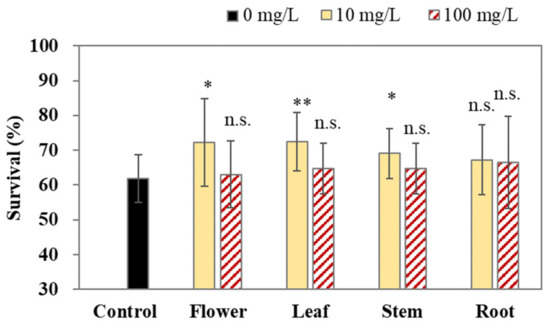
Figure 2.
Antioxidative effects of methanol extracts from Cirsium japonicum DC. var. australe Kitam. flowers, leaves, stem, and roots in C. elegans. L1 larvae were treated with crude methanol extracts (0, 10, and 100 mg/L) from C. japonicum DC. var. australe Kitam. flowers, leaves, stem, and roots for 72 h, followed by the 250 μM juglone challenge for 3.5 h; the survival of the worms was scored thereafter. The assay was performed for at least 3 biological trials and at least 60 worms were scored in each trial. Data are presented as the mean ± SD, and the results from the statistical analysis are labeled with an asterisk (p < 0.05), two asterisks (p < 0.01), or n.s. (p > 0.05).
2.3. Development of a Chromatographic Method for the Analysis of Five Target Phytochemicals in the Methanol Extracts
It has been reported that flavonoglignan (including silibinin, silychristin, and silydianin) and flavonoid (such as taxifolin) are major phytochemical compounds in Cirsium plants [11,15]. To further investigate these compounds, the chromatographic separation of a mixture of compounds (silibinin A and B, silychristin, silydianin, and taxifolin) was conducted using the HPLC-PDA system. Based on the HPLC chromatogram peak retention times, the standard compounds could be well separated, and these peaks were identified as follows: taxifolin (peak 1), silychristin (peak 2), silydianin (peak 3), silibinin A, and silibinin B (peak 4 and peak 5) (Figure 3), revealing that the chromatographic method can be used for rapidly screening target phytochemicals in Cirsium plants. Since the established method was suitable for separating all the target compounds, the qualitative and quantitative analyses of the target compounds in the methanolic extracts were analyzed under the same conditions.
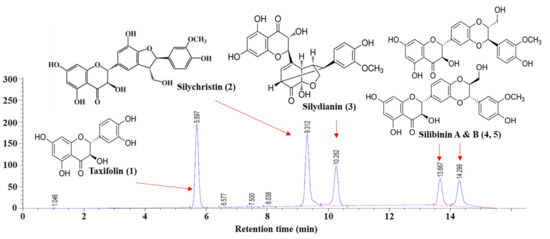
Figure 3.
HPLC chromatogram and chemical structures of major phytochemical compounds. The concentration of each compound was 400 mg/L.
2.4. Flower, Leaf, Stem, and Root Methanol Extracts from C. japonicum DC. var. australe Kitam. Contain Rich Contents of Silymarin
Silymarin is an extract from milk thistle (S. marianum L. Gaertn.) and contains flavonolignans (silybins A and B, isosilybins A and B, silychristin, isosilychristin, and silydianin) and a flavonoid (taxifolin) [33]. In this study, taxifolin and silychristin were found in the flowers, leaves, stem, and roots of C. japonicum DC. var. australe Kitam.; however, silydianin was only detected in the leaf, stem, and root extracts (Figure 4). Silibinin A and B were completely absent in C. japonicum DC. var. australe Kitam. (Figure 4). Meanwhile, the root contained the highest amount of taxifolin, silychristin, and silydianin, while the flower was second for silychristin abundance (Figure 4). Moreover, the levels of taxifolin, silychristin, and silydianin in the leaves were almost identical to those in the stem (Figure 4B,C). Thus, the results suggest that the flowers, leaves, stem, and roots of C. japonicum DC. var. australe Kitam. hold potential as rich sources of silymarin, while the root contained the highest amount of silymarin.
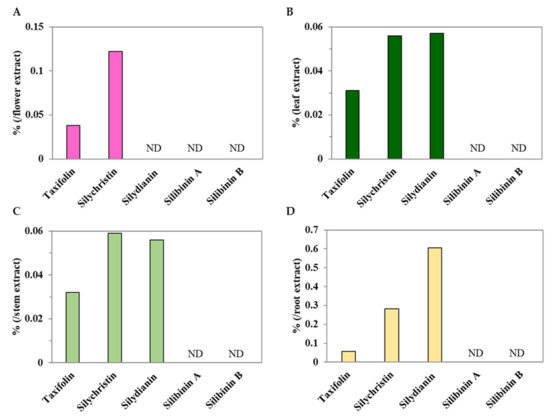
Figure 4.
Major phytochemical compounds in methanol extracts from Cirsium japonicum DC. var. australe Kitam. flowers, leaves, stem, and roots. Major phytochemical compounds in methanol extracts from (A) flowers, (B) leaves, (C) stem, and (D) roots. ND: not detectable.
2.5. Silychristin and Taxifolin May Contribute to the Antioxidative Activity of Crude Methanol Extracts in C. elegans
We further investigated the potential key phytochemical compounds to which the antioxidative activity observed in C. elegans might be attributed by analyzing the crude methanol extracts (Figure 2). Based on the results observed in Figure 4, showing that silibinin A and silibinin B were absent in the crude extracts of C. japonicum DC. var. australe Kitam., we tested the antioxidative activity of taxifolin, silychristin, and silydianin under juglone-induced oxidative stress in C. elegans. Figure 5A showed that 1 mg/L and 10 mg/L of taxifolin increased the survival of C. elegans. Moreover, silychristin exhibited the lowest observed effect level (LOEL) of 0.1 mg/L, which enhanced the survival of C. elegans (Figure 5B). In contrast, silydianin did not show oxidative resistance in C. elegans at any of the examined concentrations (Figure 5C). This suggests that silychristin and taxifolin may contribute to the antioxidative activity, derived from the C. japonicum DC. var. australe Kitam. crude methanol extracts, in C. elegans.
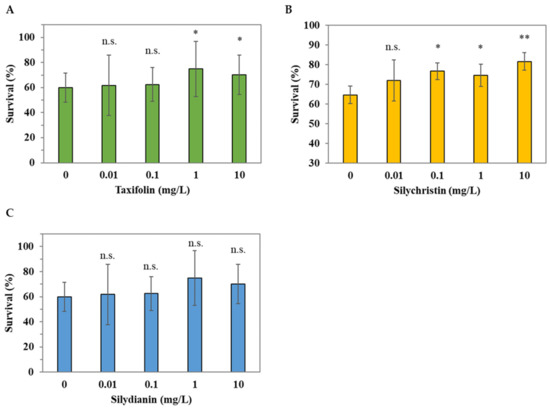
Figure 5.
Antioxidative effects of taxifolin, silychristin, and silydianin in C. elegans. L1 larvae were treated with different concentrations (0, 0.01, 0.1, 1, and 10 mg/L) of (A) taxifolin, (B) silychristin, and (C) silydianin for 72 h followed by the 250 μM juglone challenge for 3.5 h; the survival of worms was scored thereafter. The assay was performed for at least 3 biological trials and at least 60 worms were scored in each trial. Data are presented as the mean ± SD, and the results from the statistical analysis are labeled with an asterisk (p < 0.05), two asterisks (p < 0.01), or n.s. (p > 0.05).
3. Discussion
The extraction yield of a crude extract is fundamental for screening bioactive compounds and for drug discovery. This study showed that the crude extracts from the C. japonicum DC. var. australe Kitam. flowers, leaves, stem, and roots all yielded about 13% (Figure 1), which is a similar value to the one presented in a previous study, where a yield of 10% was extracted from the flowers of C. japonicum DC. var. australe Kitam. [10]. To compare yields among different Cirsium species, we also calculated the crude extract yield for the aerial part of C. japonicum DC. var. australe Kitam., which amounted to 11.0%. It has been reported that the methanolic extract yields from the aerial parts of C. arisanense and C. kawakamii were 23.9% and 4.5%, respectively [10]. Therefore, in comparison to C. arisanense and C. kawakamii, the yield from C. japonicum DC. var. australe Kitam. in this study was deemed moderate. This information provides valuable insights into the extraction efficiency of C. japonicum DC. var. australe Kitam. in relation to other Cirsium species for further bioactive compound exploration.
Next, we investigated the antioxidative potential of the crude methanol extracts obtained from various parts of C. japonicum DC. var. australe Kitam. The extracts from the flowers, leaves, and stem led to a notable increase in the survival of C. elegans upon juglone exposure for 3.5 h (Figure 2). The result aligns with a previous study [11] that reported C. japonicum DC. var. australe Kitam. as having the highest contents of total phenolic compounds and the best in vitro radical-scavenging capacity among seven Taiwanese Cirsium species. In comparison to the aerial parts of C. japonicum DC. var. australe Kitam., both our study and the aforementioned research [11] indicate that the roots exhibit the lowest antioxidant potential. These observations shed light on the inherent variations in antioxidative properties within different plant components of C. japonicum DC. var. australe Kitam. and suggest the most suitable plant parts for future antioxidant-related investigations.
This study also examined characteristic phytochemical compounds akin to silymarin in each of these methanolic extracts. We found that the order of the highest silymarin contents was found to be root > flower = leaf = stem (Figure 4). This result suggests that the root is the best source from which to obtain silymarin compounds. Interestingly, silibinin A and silibinin B, which are the major compounds in silymarin from milk thistle, were not found in any of the crude methanol extracts in this study (Figure 4), which is different to previous studies [10,11]. This suggests that silibinin may not be the key component in the crude methanol extracts from C. japonicum DC. var. australe Kitam. In addition, in comparison with the C. japonicum DC. var. australe Kitam. flower extracts [10,11], our study provided further information on the contents of taxifolin in crude methanol extracts from C. japonicum DC. var. australe Kitam., as our results revealed that taxifolin was abundant in the roots and the aerial parts of the Cirsium plant (Figure 3). This information is valuable for future extraction processes aimed at harnessing the phytochemical potential of these plant components.
We investigated the antioxidative effect of silymarin in vivo and found that silychristin and taxifolin were potentially the main antioxidants in the crude methanol extracts (Figure 5). Previous studies have reported that taxifolin has several pharmacological activities, including antioxidant, anti-inflammatory, hepatoprotective, and antihyperglycemic properties [34,35]. Taxifolin may inhibit ROS generation and the induction of cell apoptosis by H2O2, which is associated with Nrf2 translocation [17]. Another study found that taxifolin may scavenge ROS and repress the genes involved in the apoptotic pathway [36]. The antioxidant activity of taxifolin may result from its structure, which consists of two phenolic groups [37]. As for silychristin, previous research has shown that silychristin may inhibit α-glucosidase activity and protect pancreatic β cells from apoptosis [38]. Moreover, silychristin may suppress apoptosis via the Nrf2 pathway in GLUTag cells [39]. The antioxidant capacity of silychristin may further increase multidrug resistance by inhibiting ABC transporters [40]. Overall, our study revealed that C. japonicum DC. var. australe Kitam. contains characteristic silymarin compounds in the root, flower, leaf, and stem, while the root is the best source from which to obtain silymarin compounds. Furthermore, our study identified that silychristin and taxifolin played a crucial role in the plant’s antioxidative ability in vivo. This study suggests that C. japonicum DC. var. australe Kitam. could be a rich source of silymarin and has the potential to be applied within the medical field or health supplements.
In this study, we explored the potential application of C. japonicum DC. var. australe Kitam. and found that the plant extracts and some ingredients exhibited health-promoting potential. It is important to acknowledge, however, that our study has certain limitations. For instance, we did not include an optimization of the processes and preparations for these plant extracts. Although the solvent was chosen according to previous research [10,11], the residual methanol may pose a health concern in the medical application of the extracts. In future research, there is an opportunity to enhance our processes by drawing on insights from successful studies related to milk thistle or silymarin [41,42], which have demonstrated effective optimization strategies. Additionally, we should note that our study did not examine the biological activity or toxicity of other ingredients; therefore, future studies should evaluate the antioxidant potential of these additional components.
4. Materials and Methods
4.1. Chemicals
All chemical standards, including (2R,3R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydrochromen-4-one (taxifolin), (2R,3R)-3,5,7-trihydroxy-2-[(2R,3S)-7-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3-(hydroxymethyl)-2,3-dihydro-1-benzofuran-5-yl]-2,3-dihydrochromen-4-one (silychristin), (1R,3R,6R,7R,10R)-3-hydroxy-10-(4-hydroxy-3-methoxyphenyl)-8-[(2R,3R)-3,5,7-trihydroxy-4-oxo-2,3-dihydrochromen-2-yl]-4-oxatricyclo[4.3.1.03,7]dec-8-en-2-one (silydianin), (2R,3R)-3,5,7-trihydroxy-2-[(2R,3R)-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl]-2,3-dihydrochromen-4-one (silibinin A), and (2R,3R)-3,5,7-trihydroxy-2-[(2S,3S)-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl]-2,3-dihydrochromen-4-one (silibinin B), were purchased from Sigma-Aldrich (St. Louis, MO, USA), and the solvents used were of high-performance liquid chromatography (HPLC) grade.
4.2. Plants and Extraction
The plants were identified as C. japonicum DC. var. australe Kitam. by Yen-Hsueh Tseng, collected by Chih-Yi Chang from Chiayi, and cultivated by Jeng-Chuann Yang in nurseries at Taiwan Forestry Research Institute. Plants were divided into flowers, leaves, stems, and roots, which were rinsed, and dried at 50 °C until their weight was stabilized, with no variations exceeding 0.01 g. Subsequently, the dried samples were powdered and extracted using methanol (MeOH) according to previous studies [10,11], and the crude extracts were concentrated under vacuum until the residue did not differ by more than 0.01 g. The residue of crude extract was then suspended in dimethyl sulfoxide (DMSO).
4.3. Identification and Quantification of Active Components in Methanol Extracts
For the calibration curves, all chemical standards were dissolved in DMSO and analyzed using binary gradient elution with mobile phase A (95% water, 5% acetonitrile, and 0.1% formic acid) and B (20% water, 80% methanol, and 0.1% formic acid). The gradient started with 30% of B at 0 min, followed by 60% B at 12 min, 60% B at 13 min, and 30% B at 14 min until 16.5 min, using an HPLC system (Shimadzu, Japan) with a monolithic Chromolith RP-C18 column (100 × 3 mm). The signals were acquired using a photodiode array (PDA) and extracted at 285 nm. The injection volume was 2 µL.
4.4. C. elegans and Oxidative Stress Assay
This study used wild-type N2 strain C. elegans acquired from the Caenorhabditis Genetics Center. We followed the standard protocol, maintaining the C. elegans at 20 °C to obtain synchronized L1 larvae. The oxidative stress assay was performed as described by [43]. Briefly, L1 larvae were treated with crude extracts, compounds, or 0.1% DMSO (solvent control), which could be safely used for drug delivery in C. elegans [44], for 72 h, and then washed three times with M9 buffer. Next, adult worms were randomly selected and exposed to 250 μM juglone for 3.5 h before their survival was scored. The assay was performed in at least 3 biological trials with at least 60 worms in each trial.
4.5. Data Analysis
Data were presented as the mean ± SD from at least three independent biological replicates, and one-way analysis of variance with Tukey’s post hoc test using SPSS 22.0 (IBM, Inc., New York, NY, USA) to compare any differences between groups. An asterisk (p < 0.05), two asterisks (p < 0.01), and n.s. (p > 0.05) are used to indicate statistical analysis results.
5. Conclusions
In summary, in this study we conducted a comprehensive investigation into the antioxidant potential of different parts of the Cirsium japonicum DC. var. australe Kitam. plant, including the flowers, leaves, stem, and roots. We observed variations in the dry weight across the different plant parts. While the yields of crude extracts from flowers, leaves, and roots exhibited similarities, the stem yielded the smallest amount. Additionally, leaves demonstrated the highest crude methanol extract yield, followed by the stem, roots, and flowers. Our results demonstrated that 10 mg/L of flower, leaf, and stem extracts significantly increased the survival of C. elegans under juglone-induced oxidative stress. We further conducted chromatographic analysis and successfully separated key compounds, including taxifolin, silychristin, silydianin, silibinin A, and silibinin B. We found that taxifolin and silychristin existed across all plant parts of C. japonicum DC. var. australe Kitam., whereas silydianin was exclusive to the leaf, stem, and root extracts. Silibinin A and B were absent in the crude methanol extracts of the Cirsium plant. Taxifolin, at concentrations of 1 mg/L and 10 mg/L, significantly increased the survival of C. elegans while silychristin exhibited antioxidative effects at the lowest observed effect level of 0.1 mg/L. Silydianin did not demonstrate significant antioxidative activity at the tested concentrations. Taken together, these findings indicate that taxifolin and silychristin are pivotal compounds responsible for the antioxidative activity of the crude methanol extracts from C. japonicum DC. var. australe Kitam. in C. elegans. These results offer insights into the potential applications of the Cirsium plant in the fields of health and medicine.
Author Contributions
Conceptualization, F.-L.H. and V.H.-C.L.; Methodology, P.-L.Y., T.-A.L., W.L.C. and F.-L.H.; Investigation, P.-L.Y., T.-A.L., W.L.C., C.-Y.H. and F.-L.H.; Resources, C.-Y.C., Y.-H.T., J.-C.Y. and V.H.-C.L.; Writing—original draft, P.-L.Y., T.-A.L. and W.L.C.; Writing—review and editing, F.-L.H. and V.H.-C.L.; Visualization, P.-L.Y.; Supervision, V.H.-C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Agriculture, Executive Yuan, Taiwan Forestry Research Institute (111AS-7.4.3-FI-G1) and Forestry and Nature Conservation Agency (TFBK-1120508).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Peng, C.I.; Chung, K.F.; Li, H.L. Cirsium. In Flora of Taiwan, 2nd ed.; Huang, T.C., Ed.; Editorial Committee of the Flora of Taiwan: Taipei, Taiwan, 1998; Volume 4, pp. 903–913. [Google Scholar]
- Luo, W.; Wu, B.; Tang, L.; Li, G.; Chen, H.; Yin, X. Recent research progress of Cirsium medicinal plants in China. J. Ethnopharmacol. 2021, 280, 114475. [Google Scholar]
- Strawa, J.; Wajs-Bonikowska, A.; Leszczyńska, K.; Ściepuk, M.; Nazaruk, J. Chemical composition and antioxidant, antibacterial activity of Cirsium rivulare (Jacq) All. roots. Nat. Prod. Res. 2016, 30, 2730–2733. [Google Scholar] [PubMed]
- Park, J.Y.; Kim, H.Y.; Shibamoto, T.; Jang, T.S.; Lee, S.C.; Shim, J.S.; Hahm, D.H.; Lee, H.J.; Lee, S.; Kang, K.S. Beneficial effects of a medicinal herb, Cirsium japonicum var. maackii, extract and its major component, cirsimaritin on breast cancer metastasis in MDA-MB-231 breast cancer cells. Bioorg. Med. Chem. Lett. 2017, 27, 3968–3973. [Google Scholar]
- Park, J.C.; Yoo, H.; Kim, C.E.; Shim, S.Y.; Lee, M. Hispidulin-7-O-neohesperidoside from Cirsium japonicum var. ussuriense attenuates the production of inflammatory mediators in LPS-induced Raw 264.7 cells and HT-29 Cells. Pharmacogn. Mag. 2017, 13, 707–711. [Google Scholar] [PubMed]
- Tian, L.; Jiang, M.; Chen, H.; Li, J.; Huang, L.; Liu, C. Comparative analysis of the complete chloroplast genomes of Cirsium japonicum from China and Korea. Mitochondrial DNA B Resour. 2021, 6, 1468–1470. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Tzeng, H.Y.; Tseng, Y.H. Cirsium taiwanense (Compositae, Cirsium sect. Onotrophe, subsect. Australicirsium), a new species from Taiwan. Phytokeys 2021, 183, 37–53. [Google Scholar]
- Chang, C.Y.; Tzeng, H.Y.; Tseng, Y.H. Cirsium tatakaense (Compositae), a new species from Taiwan. Phytokeys 2019, 117, 119–132. [Google Scholar] [CrossRef]
- Ku, K.L.; Tsai, C.T.; Chang, W.M.; Shen, M.L.; Wu, C.T.; Liao, H.F. Hepatoprotective effect of Cirsium arisanense Kitamura in tacrine-treated hepatoma Hep 3B cells and C57BL mice. Am. J. Chin. Med. 2008, 36, 355–368. [Google Scholar] [CrossRef]
- Zhao, Z.-W.; Chang, J.-C.; Lin, L.-W.; Tsai, F.-H.; Chang, H.-C.; Wu, C.-R. Comparison of the hepatoprotective effects of four endemic Cirsium species extracts from Taiwan on CCl4-induced acute liver damage in C57BL/6 mice. Int. J. Mol. Sci. 2018, 19, 1329. [Google Scholar]
- Zhao, Z.-W.; Chang, H.-C.; Ching, H.; Lien, J.-C.; Huang, H.-C.; Wu, C.-R. Antioxidant effects and phytochemical properties of seven Taiwanese Cirsium species extracts. Molecules 2021, 26, 3935. [Google Scholar]
- Gillessen, A.; Schmidt, H.H. Silymarin as supportive treatment in liver diseases: A narrative review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, G.; Kaur, G.; Bhardwaj, G.; Mutreja, V.; Sohal, H.S.; Nayik, G.A.; Bhardwaj, A.; Sharma, A. Traditional uses, phytochemical composition, pharmacological properties, and the biodiscovery potential of the genus Cirsium. Chemistry 2022, 4, 1161–1192. [Google Scholar] [CrossRef]
- Křenek, K.; Marhol, P.; Peikerová, Ž.; Křen, V.; Biedermann, D. Preparatory separation of the silymarin flavonolignans by Sephadex LH-20 gel. Food Res. Int. 2014, 65, 115–120. [Google Scholar] [CrossRef]
- Roy, N.S.; Kim, J.A.; Choi, A.Y.; Ban, Y.W.; Park, N.I.; Park, K.C.; Yang, H.S.; Choi, I.Y.; Kim, S. RNA-Seq de novo assembly and differential transcriptome analysis of Korean medicinal herb Cirsium japonicum var. spinossimum. Genom. Inform. 2018, 16, e34. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Wang, Y.J.; Yang, G.T.; Gao, Q.L.; Tang, M.X. Taxifolin inhibits receptor activator of NF-κB ligand-induced osteoclastogenesis of human bone marrow-derived macrophages in vitro and prevents lipopolysaccharide-induced bone loss in vivo. Pharmacology 2019, 103, 101–109. [Google Scholar] [CrossRef]
- Xie, X.; Feng, J.; Kang, Z.; Zhang, S.; Zhang, L.; Zhang, Y.; Li, X.; Tang, Y. Taxifolin protects RPE cells against oxidative stress-induced apoptosis. Mol. Vis. 2017, 23, 520–528. [Google Scholar]
- Chen, J.; Sun, X.; Xia, T.; Mao, Q.; Zhong, L. Pretreatment with dihydroquercetin, a dietary flavonoid, protected against concanavalin A-induced immunological hepatic injury in mice and TNF-α/ActD-induced apoptosis in HepG2 cells. Food Funct. 2018, 9, 2341–2352. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.Q. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Xin, X.; Xiang, X.; Xin, Y.; Li, Q.; Ma, H.; Liu, X.; Hou, Y.; Yu, W. Global trends in research on oxidative stress associated with periodontitis from 1987 to 2022: A bibliometric analysis. Front. Immunol. 2022, 13, 979675. [Google Scholar] [CrossRef]
- Guan, R.; Van Le, Q.; Yang, H.; Zhang, D.; Gu, H.; Yang, Y.; Sonne, C.; Lam, S.S.; Zhong, J.; Jianguang, Z.; et al. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere 2021, 271, 129499. [Google Scholar] [CrossRef]
- Dumanovic, J.; Nepovimova, E.; Natic, M.; Kuca, K.; Jacevic, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [PubMed]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant potential of curcumin-A meta-analysis of randomized clinical trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Ahmad, S.; Shah, S.W.A.; Ullah, A.; Rahman, S.U.; Ahmad, M.; Almehmadi, M.; Abdulaziz, O.; Allahyani, M.; Alsaiari, A.A.; et al. Synthetic mono-carbonyl curcumin analogues attenuate oxidative stress in mouse models. Biomedicines 2022, 10, 2597. [Google Scholar]
- Cui, X.J.; Lin, Q.L.; Liang, Y. Plant-derived antioxidants protect the nervous system from aging by inhibiting oxidative stress. Front. Aging Neurosci. 2020, 12, 209. [Google Scholar]
- Potts, M.B.; Cameron, S. Cell lineage and cell death: Caenorhabditis elegans and cancer research. Nat. Rev. Cancer 2011, 11, 50–58. [Google Scholar] [CrossRef]
- Lai, C.H.; Chou, C.Y.; Ch’ang, L.Y.; Liu, C.S.; Lin, W. Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res. 2000, 10, 703–713. [Google Scholar] [CrossRef]
- Rodriguez, M.; Snoek, L.B.; De Bono, M.; Kammenga, J.E. Worms under stress: C. elegans stress response and its relevance to complex human disease and aging. Trends Genet. 2013, 29, 367–374. [Google Scholar]
- Hu, Q.; D’Amora, D.R.; MacNeil, L.T.; Walhout, A.J.M.; Kubiseski, T.J. The Caenorhabditis elegans oxidative stress response requires the NHR-49 transcription factor. Genes Genom. Genet. 2018, 8, 3857–3863. [Google Scholar]
- Liao, V.H.C. Use of Caenorhabditis elegans to study the potential bioactivity of natural compounds. J. Agric. Food Chem. 2018, 66, 1737–1742. [Google Scholar] [CrossRef]
- Ahmad, T.; Suzuki, Y.J. Juglone in oxidative stress and cell signaling. Antioxidants 2019, 8, 91. [Google Scholar] [CrossRef]
- Senchuk, M.M.; Dues, D.J.; Van Raamsdonk, J.M. Measuring oxidative stress in Caenorhabditis elegans: Paraquat and juglone sensitivity assays. Bio. Protoc. 2017, 7, e2086. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.C.; Graf, T.N.; Sparacino, C.M.; Wani, M.C.; Wall, M.E. Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum). Org. Biomol. Chem. 2003, 1, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- Sunil, C.; Xu, B. An insight into the health-promoting effects of taxifolin (dihydroquercetin). Phytochemistry 2019, 166, 112066. [Google Scholar] [PubMed]
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological basis and new insights of taxifolin: A comprehensive review. Biomed. Pharmacother. 2021, 142, 112004. [Google Scholar]
- Sun, X.; Chen, R.C.; Yang, Z.H.; Sun, G.B.; Wang, M.; Ma, X.J.; Yang, L.J.; Sun, X.B. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem. Toxicol. 2014, 63, 221–232. [Google Scholar] [PubMed]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gulcin, I.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity-structure relationship. J. Enzyme Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Hu, X.; Li, S.; Wang, J.; Li, Z.; Li, D.; Xu, F.; Gao, M.; Hua, H. Hypoglycemic effect of silychristin A from Silybum marianum fruit via protecting pancreatic islet β cells from oxidative damage and inhibiting α-glucosidase activity in vitro and in rats with type 1 diabetes. J. Funct. Foods 2017, 38, 168–179. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Zhang, L.; Yan, T.; Wu, B.; Xu, F.; Jia, Y. Silychristin A activates Nrf2-HO-1/SOD2 pathway to reduce apoptosis and improve GLP-1 production through upregulation of estrogen receptor α in GLUTag cells. Eur. J. Pharmacol. 2020, 881, 173236. [Google Scholar]
- Viktorova, J.; Dobiasova, S.; Rehorova, K.; Biedermann, D.; Kanova, K.; Seborova, K.; Vaclavikova, R.; Valentova, K.; Ruml, T.; Kren, V.; et al. Antioxidant, anti-Inflammatory, and multidrug resistance modulation activity of silychristin derivatives. Antioxidants 2019, 8, 303. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H. Milk thistle oil extracted by enzyme-mediated assisted solvent extraction compared with n-hexane and cold-pressed extraction. Molecules 2023, 28, 2591. [Google Scholar] [CrossRef]
- Wianowska, D.; Wiśniewski, M. Simplified procedure of silymarin extraction from Silybum marianum L. Gaertner. J. Chromatogr. Sci. 2015, 53, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.; Wu, Y.C.; Liao, V.H. Early developmental nanoplastics exposure disturbs circadian rhythms associated with stress resistance decline and modulated by DAF-16 and PRDX-2 in C. elegans. J. Hazard Mater. 2022, 423 Pt A, 127091. [Google Scholar] [CrossRef]
- AlOkda, A.; Van Raamsdonk, J.M. Effect of DMSO on lifespan and physiology in C. elegans: Implications for use of DMSO as a solvent for compound delivery. MicroPubl. Biol. 2022, 2022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).