Combination NIPS/TIPS Synthesis of α-Fe2O3 and α/γ-Fe2O3 Doped PVDF Composite for Efficient Piezocatalytic Degradation of Rhodamine B
Abstract
:1. Introduction
2. Experimental
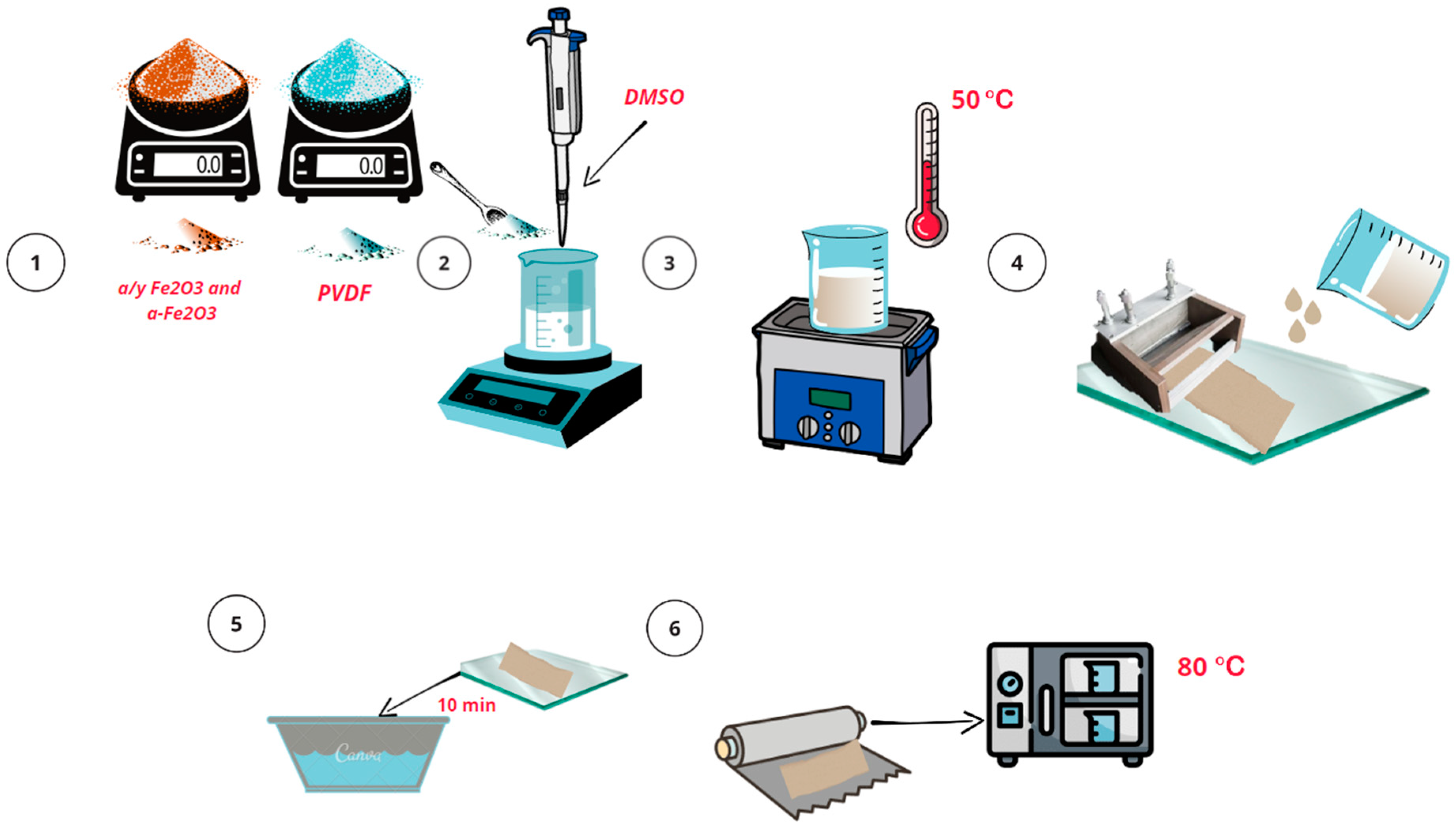
2.1. Synthesis of α/γ-Fe2O3 and α-Fe2O3 Particles
2.2. Synthesis of PVDF Membrane
2.3. Methods
2.4. Piezocatalytic Experiment Methodology
2.5. Piezoelectric Nanogenerator Measurement
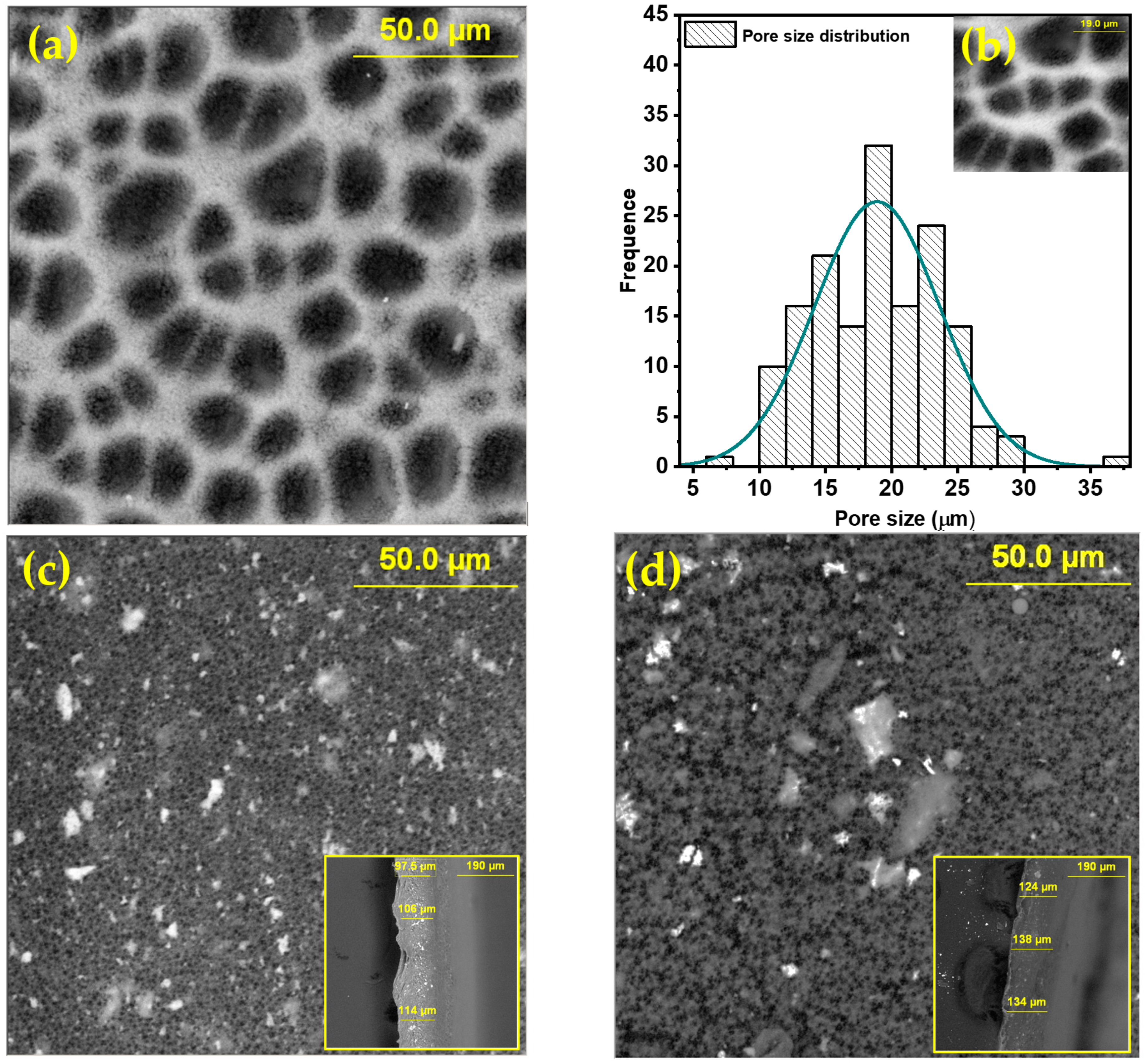
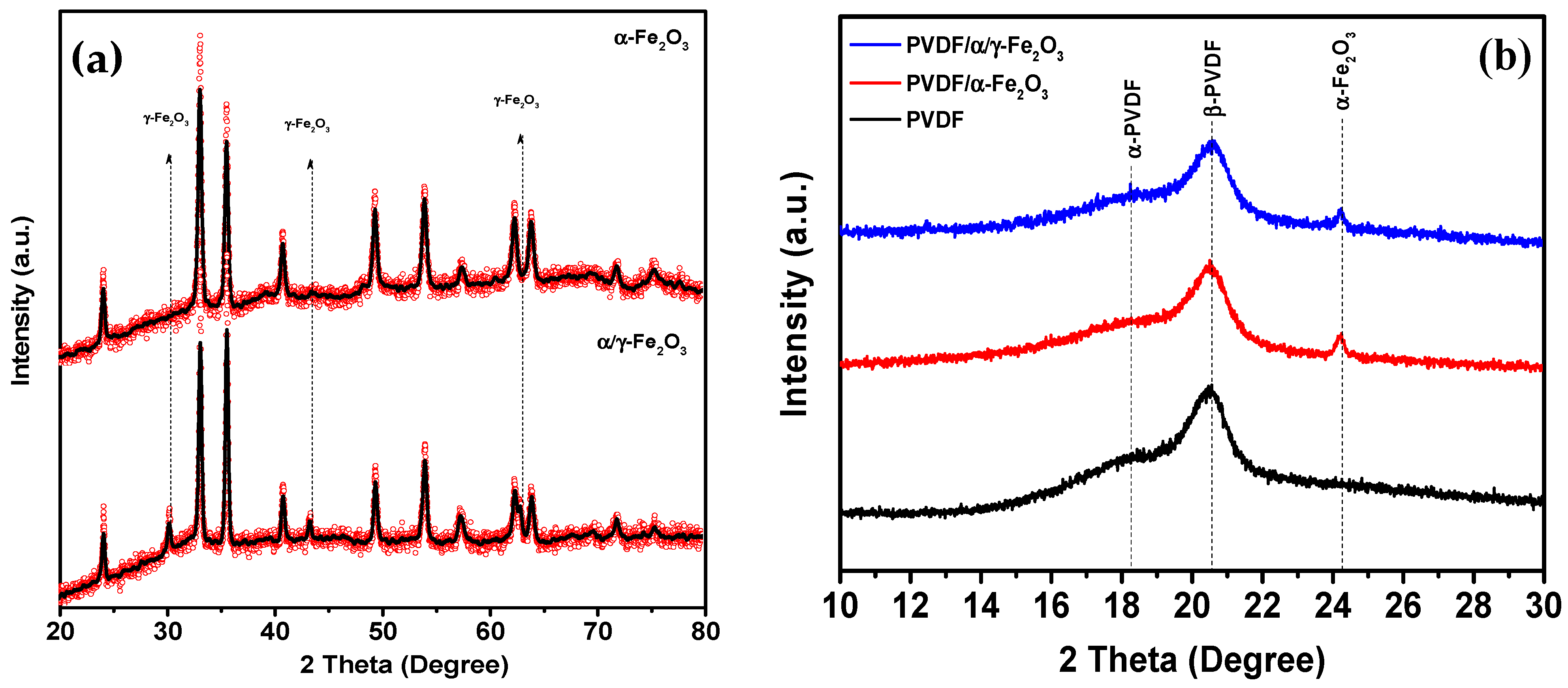
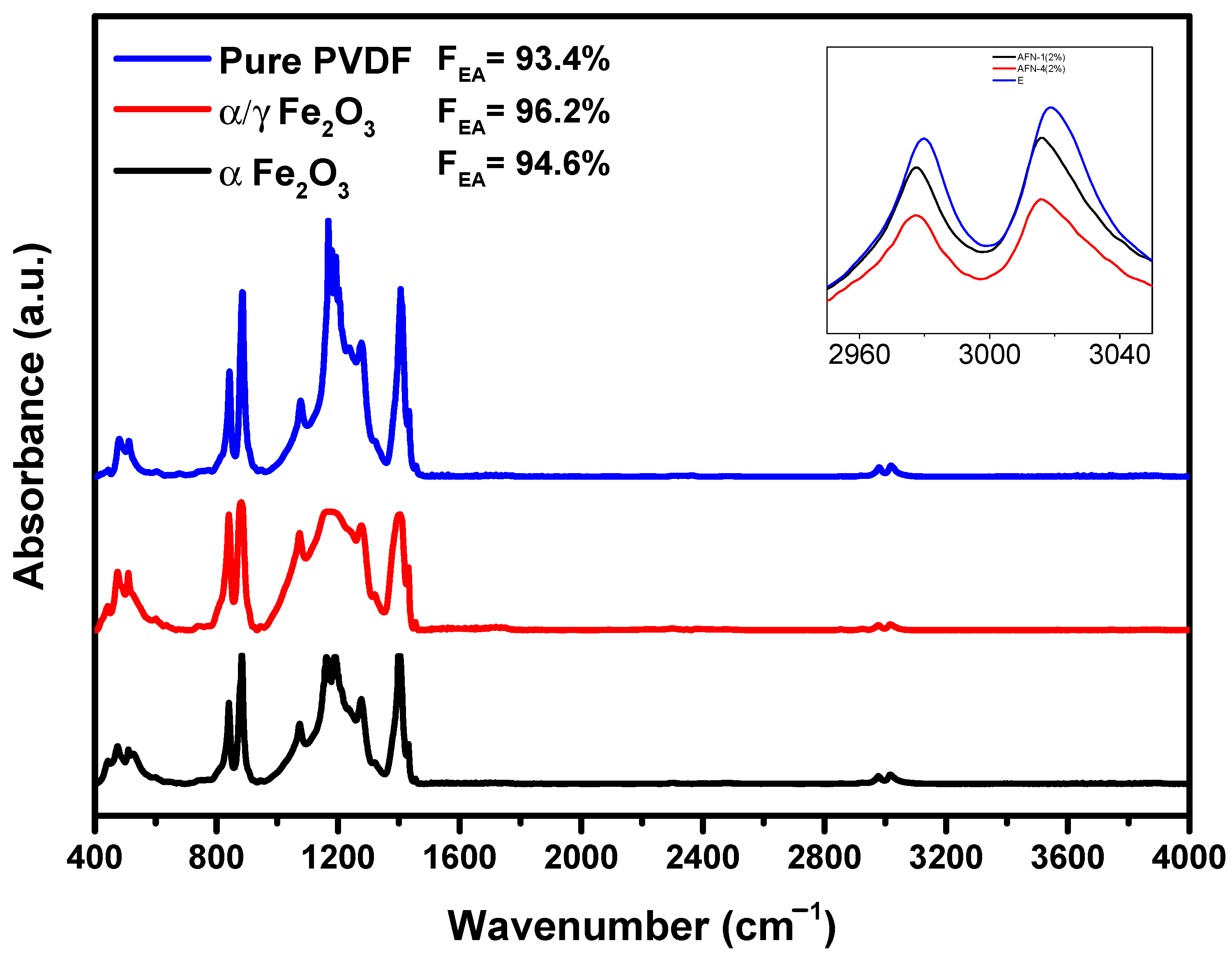
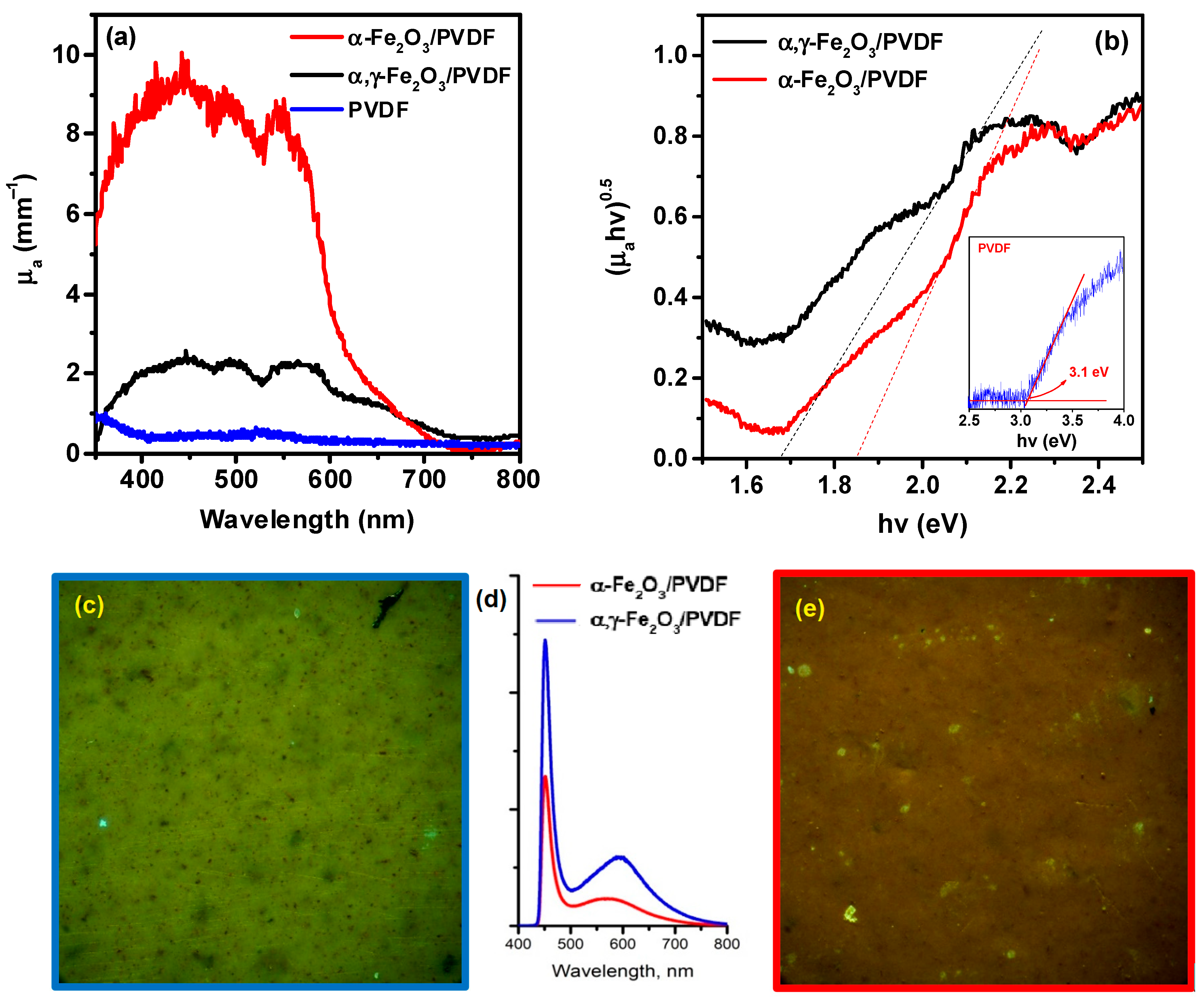
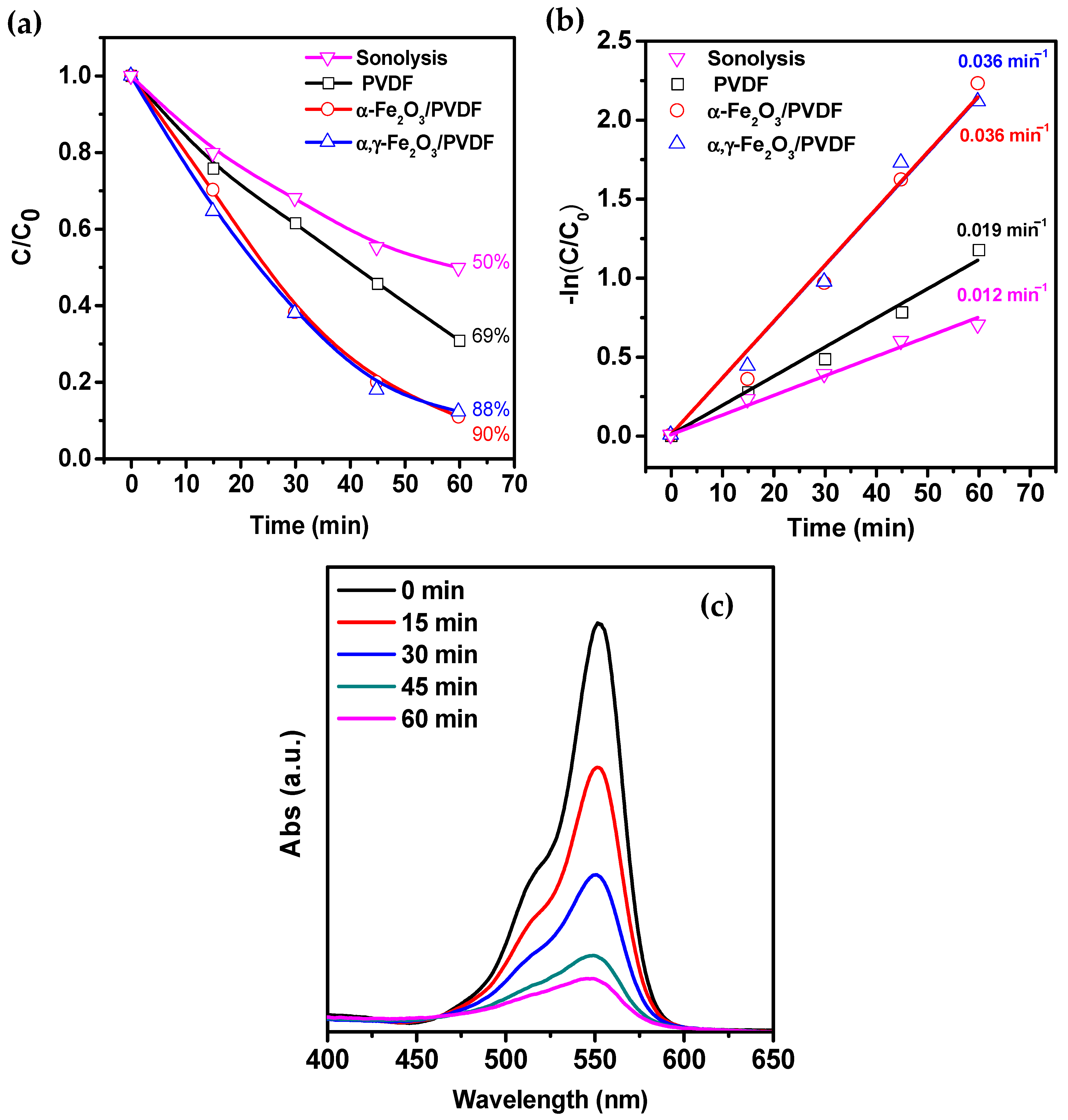
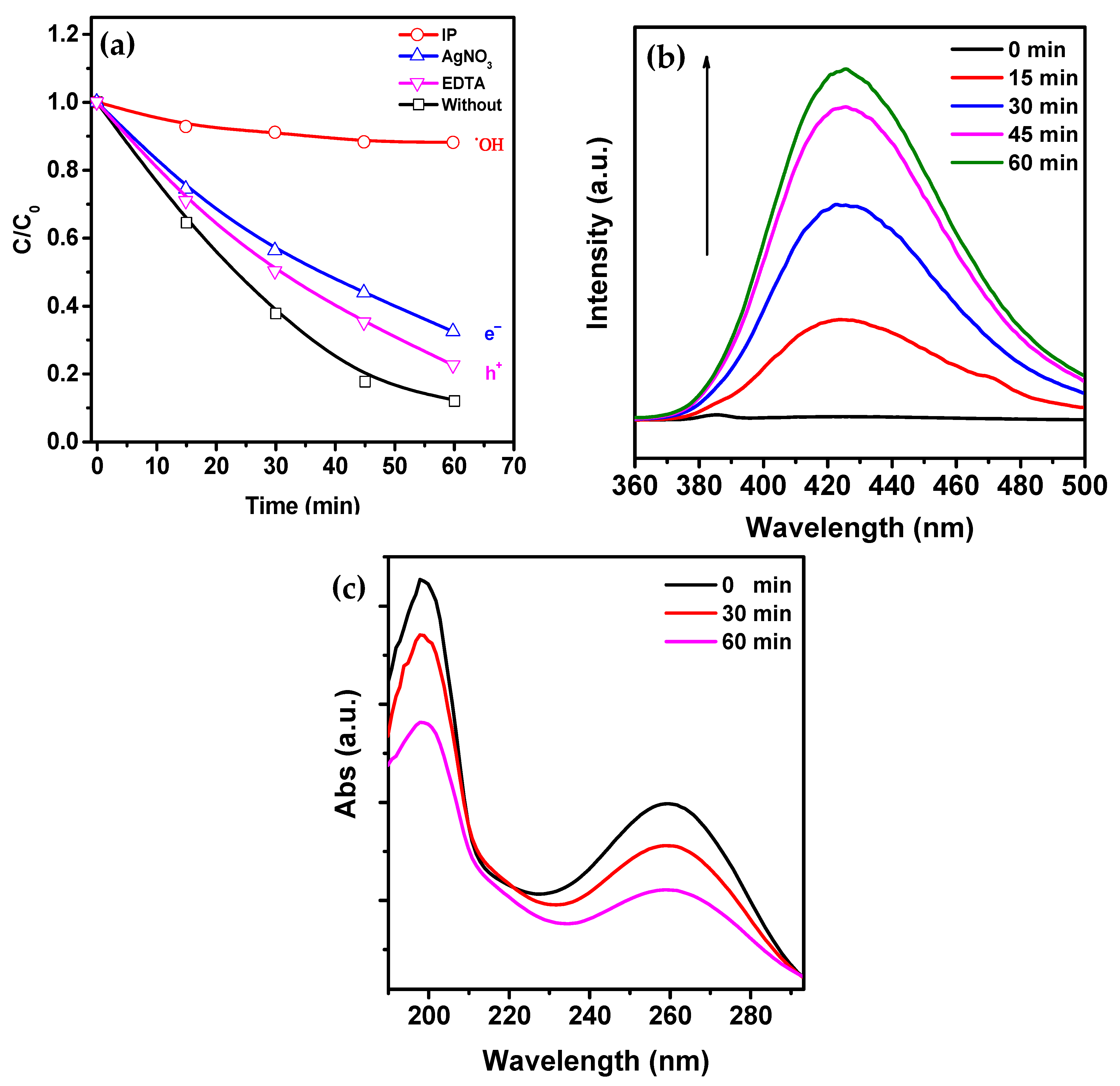
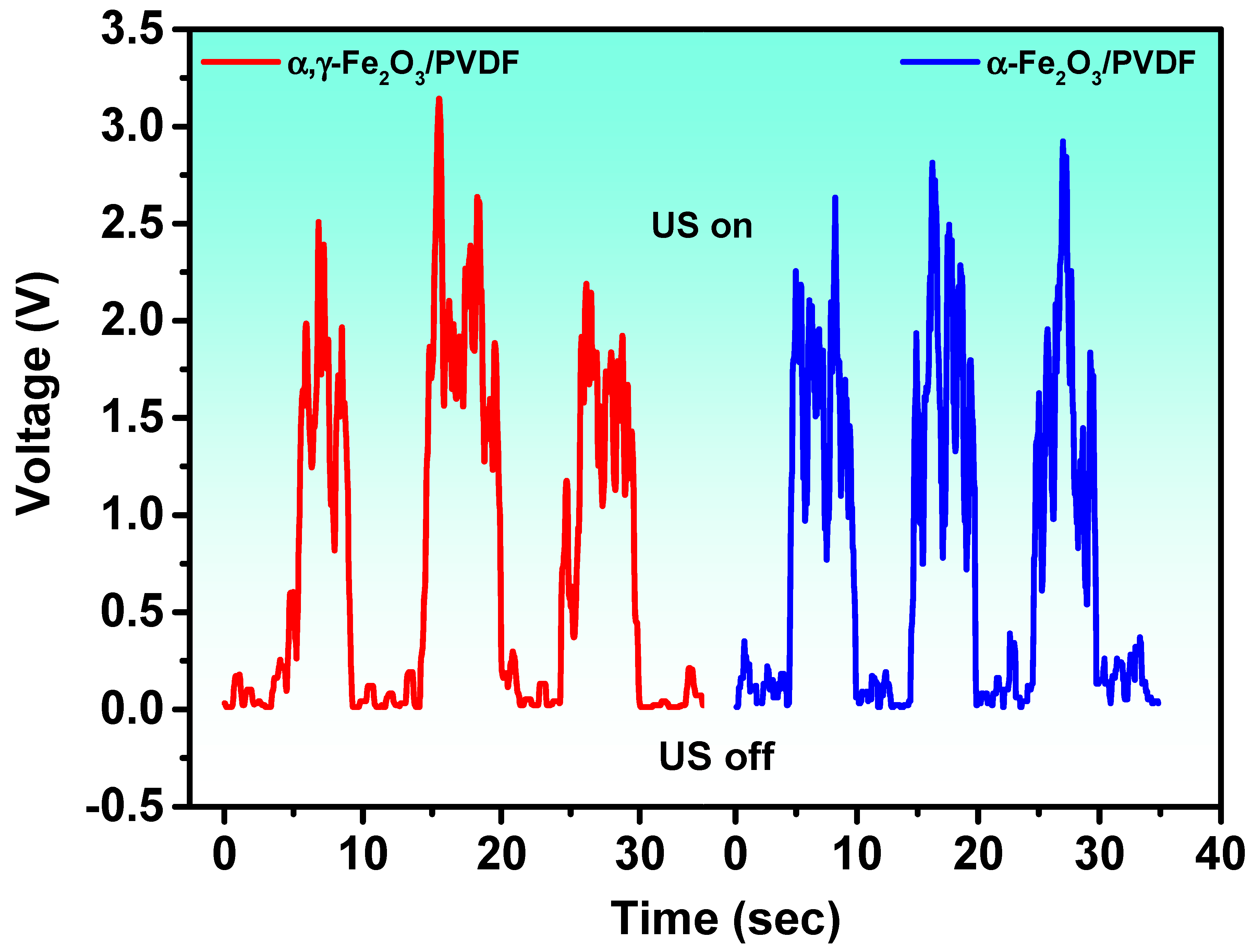
3. Results and Discussion
- Calculation of initial approximations for the coefficients and based on the and data, using the relationships:where d is the thickness of the samples.
- Calculation of the parameters and based on the obtained values of the coefficients and , using the Monte Carlo method.
- Construction of the objective function and its minimization:
- The minimization procedure was performed based on the Nelder-Mead simplex method until the condition is met:
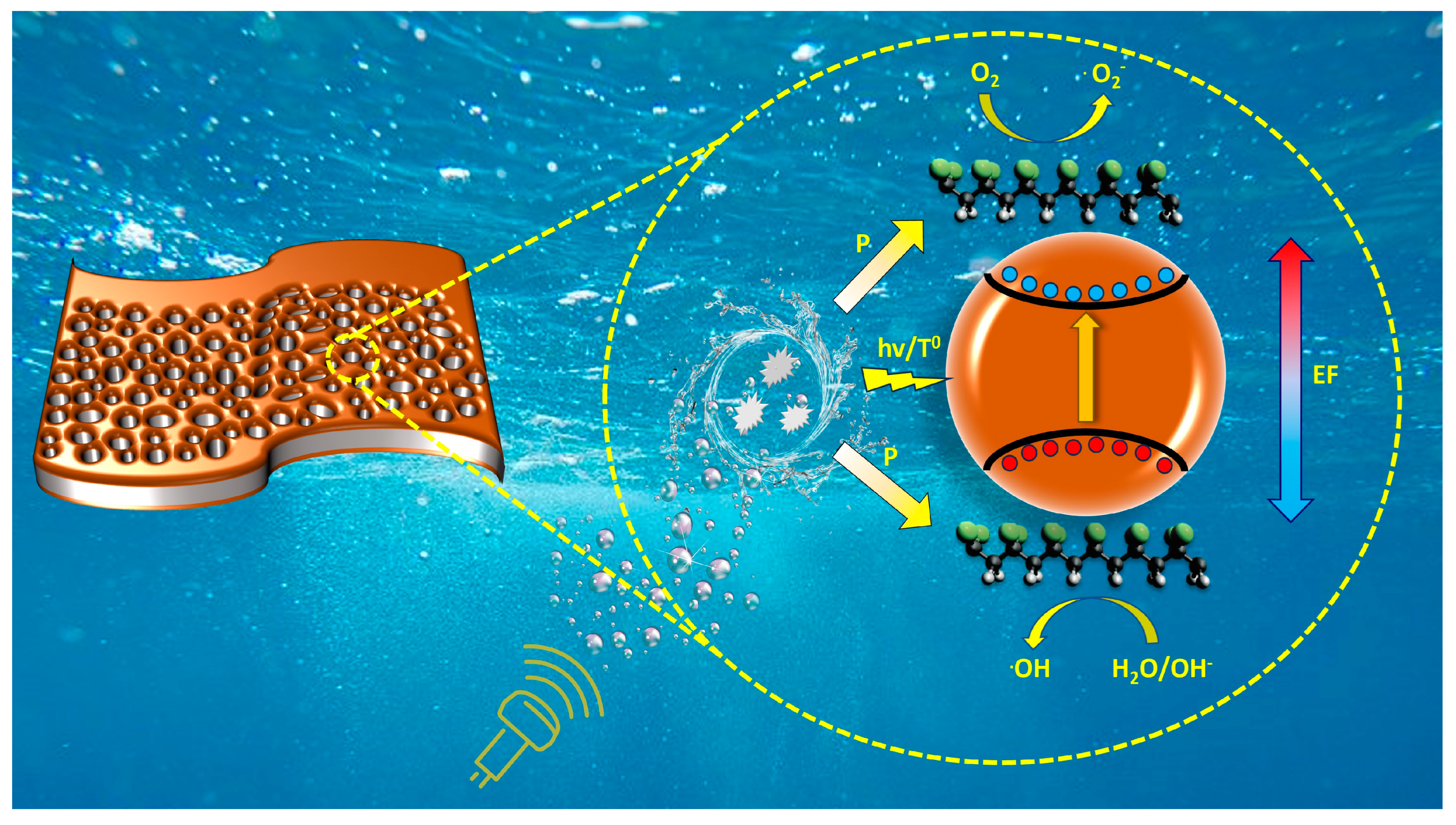
- Photocatalytic mechanism (PCM), in which sonoluminescence is generated during cavitation collapse, can contribute to the formation of electron-hole pairs in iron oxide.
- Thermocatalytic mechanism (TCM), in which, according to the hot spot theory, the local high temperature during cavitation collapse can promote the formation of electron-hole pairs in iron oxide through thermo-induced thermal excitation of the semiconductor.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sui, S.; Quan, H.; Hu, Y.; Hou, M.; Guo, S. A Strategy of Heterogeneous Polyurethane-Based Sponge for Water Purification: Combination of Superhydrophobicity and Photocatalysis to Conduct Oil/Water Separation and Dyes Degradation. J. Colloid Interface Sci. 2021, 589, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Youssef, Z.; Colombeau, L.; Yesmurzayeva, N.; Baros, F.; Vanderesse, R.; Hamieh, T.; Toufaily, J.; Frochot, C.; Roques-Carmes, T. Dye-Sensitized Nanoparticles for Heterogeneous Photocatalysis: Cases Studies with TiO2, ZnO, Fullerene and Graphene for Water Purification. Dye. Pigment. 2018, 159, 49–71. [Google Scholar] [CrossRef]
- Magomedova, A.; Isaev, A.; Orudzhev, F.; Alikhanov, N.; Emirov, R.; Rabadanov, M.; Mingshan, Z. Electrochemical Synthesis of Superparamagnetic Fe3O4 Nanoparticles for the Photo-Fenton Oxidation of Rhodamine B. ChemistrySelect 2023, 8, e202301694. [Google Scholar] [CrossRef]
- Mondal, D.; Roy, S.; Bardhan, S.; Roy, J.; Kanungo, I.; Basu, R.; Das, S. Recent Advances in Piezocatalytic Polymer Nanocomposites for Wastewater Remediation. Dalton Trans. 2022, 51, 451–462. [Google Scholar] [CrossRef]
- Wu, J.; Qin, N.; Bao, D. Effective Enhancement of Piezocatalytic Activity of BaTiO3 Nanowires under Ultrasonic Vibration. Nano Energy 2018, 45, 44–51. [Google Scholar] [CrossRef]
- Kumar, P.; Vaish, R.; Sung, H.; Hwang, W.; Kwang, H.; Park, B.; Kumar, A.; Kebaili, I.; Boukhris, I.; Kumar, P.; et al. Effect of Poling on Photocatalysis, Piezocatalysis, and Photo–Piezo Catalysis Performance of BaBi4Ti4O15 Ceramics. Glob. Chall. 2023, 7, 2200142. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, Y.; Zhang, S.; Fu, J.; Li, Y.; Zhang, Y.; Ye, W. (1 − x)Bi0.5Na0.5TiO3–XBiFeO3 Solid Solutions with Enhanced Piezocatalytic Dye Degradation. Sep. Purif. Technol. 2022, 290, 120831. [Google Scholar] [CrossRef]
- Hu, C.; Huang, H.; Chen, F.; Zhang, Y.; Yu, H.; Ma, T. Coupling Piezocatalysis and Photocatalysis in Bi4NbO8X (X = Cl, Br) Polar Single Crystals. Adv. Funct. Mater. 2020, 30, 1908168. [Google Scholar] [CrossRef]
- Wu, L.; Jin, Z.; Liu, Y.; Ning, H.; Liu, X.; Alamusi; Hu, N. Recent Advances in the Preparation of PVDF-Based Piezoelectric Materials. Nanotechnol. Rev. 2022, 11, 1386–1407. [Google Scholar] [CrossRef]
- Lu, L.; Ding, W.; Liu, J.; Yang, B. Flexible PVDF Based Piezoelectric Nanogenerators. Nano Energy 2020, 78, 105251. [Google Scholar] [CrossRef]
- Zhu, G.D.; Zeng, Z.G.; Zhang, L.; Yan, X.J. Piezoelectricity in β-Phase PVDF Crystals: A Molecular Simulation Study. Comput. Mater. Sci. 2008, 44, 224–229. [Google Scholar] [CrossRef]
- Salimi, A.; Yousefi, A.A. Analysis Method: FTIR Studies of β-Phase Crystal Formation in Stretched PVDF Films. Polym. Test. 2003, 22, 699–704. [Google Scholar] [CrossRef]
- Orudzhev, F.; Sobola, D.; Ramazanov, S.; Částková, K.; Papež, N.; Selimov, D.A.; Abdurakhmanov, M.; Shuaibov, A.; Rabadanova, A.; Gulakhmedov, R.; et al. Piezo-Enhanced Photocatalytic Activity of the Electrospun Fibrous Magnetic PVDF/BiFeO3 Membrane. Polymers 2023, 15, 246. [Google Scholar] [CrossRef] [PubMed]
- Orudzhev, F.F.; Sobola, D.S.; Ramazanov, S.M.; Častková, K.; Selimov, D.A.; Rabadanova, A.A.; Shuaibov, A.O.; Gulakhmedov, R.R.; Abdurakhmanov, M.G.; Giraev, K.M. Hydrogen Bond-Induced Activation of Photocatalytic and Piezophotocatalytic Properties in Calcium Nitrate Doped Electrospun PVDF Fibers. Polymers 2023, 15, 3252. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Na, B.; Lv, R.; Li, C.; Zhu, J.; Yu, Z. Polar Phase Formation in Poly(Vinylidene Fluoride) Induced by Melt Annealing. J. Polym. Sci. B Polym. Phys. 2012, 50, 1433–1437. [Google Scholar] [CrossRef]
- Orudzhev, F.; Alikhanov, N.; Amirov, A.; Rabadanova, A.; Selimov, D.; Shuaibov, A.; Gulakhmedov, R.; Abdurakhmanov, M.; Magomedova, A.; Ramazanov, S.; et al. Porous Hybrid PVDF/BiFeO3 Smart Composite with Magnetic, Piezophotocatalytic, and Light-Emission Properties. Catalysts 2023, 13, 874. [Google Scholar] [CrossRef]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the Non-Solvent Induced Phase Separation (NIPS) Effect during the Fabrication of Microporous PVDF Membranes via Thermally Induced Phase Separation (TIPS). J. Memb. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Yeow, M.L.; Liu, Y.T.; Li, K. Morphological Study of Poly(Vinylidene Fluoride) Asymmetric Membranes: Effects of the Solvent, Additive, and Dope Temperature. J. Appl. Polym. Sci. 2004, 92, 1782–1789. [Google Scholar] [CrossRef]
- Yu, H.; Shangguan, S.; Yang, H.; Rong, H.; Qu, F. Chemical Cleaning and Membrane Aging of Poly(Vinylidene Fluoride) (PVDF) Membranes Fabricated via Non-Solvent Induced Phase Separation (NIPS) and Thermally Induced Phase Separation (TIPS). Sep. Purif. Technol. 2023, 313, 123488. [Google Scholar] [CrossRef]
- Liu, C.; Xu, H.; Huo, B.; Wang, J.; Wang, Z.; Chen, X.; Meng, F.; Sun, C.; Wang, Y. Research Progress of PVDF Based Piezoelectric Polymer Composites in Water Pollution Remediation. J. Water Process Eng. 2023, 55, 104181. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, W.; Song, W.; Cao, T.; Liu, Y.; Wang, L.; Wang, Z.; Zhang, Y. Piezocatalytic Performance Enhancement Using the Sandwich Structure of a PVDF-HFP/Graphene Film. J. Mater. Chem. A Mater. 2023, 11, 4280–4291. [Google Scholar] [CrossRef]
- Wan, L.; Tian, W.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. Hydrophilic Porous PVDF Membrane Embedded with BaTiO3 Featuring Controlled Oxygen Vacancies for Piezocatalytic Water Cleaning. Nano Energy 2022, 94, 106930. [Google Scholar] [CrossRef]
- Bagchi, B.; Hoque, N.A.; Janowicz, N.; Das, S.; Tiwari, M.K. Re-Usable Self-Poled Piezoelectric/Piezocatalytic Films with Exceptional Energy Harvesting and Water Remediation Capability. Nano Energy 2020, 78, 105339. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Chen, X.; Tang, Y.; Zhu, M.; Xie, H.; Xin, Y.; Lin, Y.; Fan, X. Enhanced Piezocatalytic Reactive Oxygen Species Production Activity and Recyclability of the Dual Piezoelectric Cu3B2O6/PVDF Composite Membrane. ACS Appl. Mater. Interfaces 2022, 15, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.H.; Zhang, X.; Wang, Y.X.; Sun, J.Y.; Zhang, H.; Liu, W.L.; Li, M.P.; Ma, X.H.; Xu, Z.L. Fe3O4/PVDF Catalytic Membrane Treatment Organic Wastewater with Simultaneously Improved Permeability, Catalytic Property and Anti-Fouling. Environ. Res. 2020, 187, 109617. [Google Scholar] [CrossRef]
- Pang, Y.L.; Lim, S.; Ong, H.C.; Chong, W.T. Synthesis, Characteristics and Sonocatalytic Activities of Calcined γ-Fe2O3 and TiO2 Nanotubes/γ-Fe2O3 Magnetic Catalysts in the Degradation of Orange G. Ultrason. Sonochem. 2016, 29, 317–327. [Google Scholar] [CrossRef]
- Alikhanov, N.M.R.; Rabadanov, M.K.; Orudzhev, F.F.; Gadzhimagomedov, S.K.; Emirov, R.M.; Sadykov, S.A.; Kallaev, S.N.; Ramazanov, S.M.; Abdulvakhidov, K.G.; Sobola, D. Size-Dependent Structural Parameters, Optical, and Magnetic Properties of Facile Synthesized Pure-Phase BiFeO3. J. Mater.Sci. Mater. Electron. 2021, 32, 13323–13335. [Google Scholar] [CrossRef]
- Orudzhev, F.F.; Alikhanov, N.M.R.; Ramazanov, S.M.; Sobola, D.S.; Murtazali, R.K.; Ismailov, E.H.; Gasimov, R.D.; Aliev, A.S.; Ţălu, Ş. Morphotropic Phase Boundary Enhanced Photocatalysis in Sm Doped BiFeO3. Molecules 2022, 27, 7029. [Google Scholar] [CrossRef]
- Magomedova, A.; Isaev, A.; Orudzhev, F.; Sobola, D.; Murtazali, R.; Rabadanova, A.; Shabanov, N.S.; Zhu, M.; Emirov, R.; Gadzhimagomedov, S.; et al. Magnetically Separable Mixed-Phase α/γ-Fe2O3 Catalyst for Photo-Fenton-like Oxidation of Rhodamine B. Catalysts 2023, 13, 872. [Google Scholar] [CrossRef]
- Bui, V.T.; Nguyen, V.T.; Nguyen, N.A.; Umapathi, R.; Larina, L.L.; Kim, J.H.; Kim, H.S.; Choi, H.S. Multilayered Pvdf-Hfp Porous Separator via Phase Separation and Selective Solvent Etching for High Voltage Lithium-Ion Batteries. Membranes 2021, 11, 41. [Google Scholar] [CrossRef]
- Ma, W.; Yao, B.; Zhang, W.; He, Y.; Yu, Y.; Niu, J. Fabrication of PVDF-based piezocatalytic active membrane with enhanced oxytetracycline degradation efficiency through embedding few-layer E-MoS2 nanosheets. Chem. Eng. J. 2021, 415, 129000. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, M.; Vaish, R. Flexible Ag@ LiNbO3/PVDF composite film for piezocatalytic dye/pharmaceutical degradation and bacterial disinfection. ACS Appl. Mater. Interfaces 2021, 13, 22914–22925. [Google Scholar] [CrossRef]
- Veeralingam, S.; Badhulika, S. Rapid Degradation of Organic Dyes via Ultrasound Triggered Piezo-Catalysis Using PVDF/ZnSnO3/MoS2 Nanocomposite. ACS Appl. Nano Mater. 2023. [Google Scholar] [CrossRef]
- Qian, W.; Zhao, K.; Zhang, D.; Bowen, C.R.; Wang, Y.; Yang, Y. Piezoelectric material-polymer composite porous foam for efficient dye degradation via the piezo-catalytic effect. ACS Appl. Mater. Interfaces 2019, 11, 27862–27869. [Google Scholar] [CrossRef]
- Porwal, C.; Verma, S.; Chauhan, V.S.; Vaish, R. Bismuth zinc borate-Polyacrylonitrile nanofibers for photo-piezocatalysis. J. Ind. Eng. Chem. 2023, 124, 358–367. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive Phases of Poly(Vinylidene Fluoride): Determination, Processing and Applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Kanik, M.; Aktas, O.; Sen, H.S.; Durgun, E.; Bayindir, M. Spontaneous High Piezoelectricity in Poly(Vinylidene Fluoride) Nanoribbons Produced by Iterative Thermal Size Reduction Technique. ACS Nano 2014, 8, 9311–9323. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A Critical Analysis of the α, β and γ Phases in Poly(Vinylidene Fluoride) Using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef]
- Chipara, D.; Kuncser, V.; Lozano, K.; Alcoutlabi, M.; Ibrahim, E.; Chipara, M. Spectroscopic Investigations on PVDF-Fe2O3 Nanocomposites. J. Appl. Polym. Sci. 2020, 137, 48907. [Google Scholar] [CrossRef]
- Tewatia, K.; Sharma, A.; Kumar, A.; Kumar, K.; Sowjanya Pali, L.; Lal, S. Enhanced Optical Properties of Recycled Fe2O3 Reinforced in PVDF Nanocomposite Thin Films for Energy Harvesting. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Tamang, A.; Ghosh, S.K.; Garain, S.; Alam, M.M.; Haeberle, J.; Henkel, K.; Schmeisser, D.; Mandal, D. DNA-Assisted β-Phase Nucleation and Alignment of Molecular Dipoles in PVDF Film: A Realization of Self-Poled Bioinspired Flexible Polymer Nanogenerator for Portable Electronic Devices. ACS Appl. Mater. Interfaces 2015, 7, 16143–16147. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Lu, Q.; Wang, Y.; Wang, Y.; Yang, B. Degradation of Organic Wastewater by Hydrodynamic Cavitation Combined with Acoustic Cavitation. Ultrason. Sonochem. 2018, 43, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.L.; Abdullah, A.Z.; Bhatia, S. Review on Sonochemical Methods in the Presence of Catalysts and Chemical Additives for Treatment of Organic Pollutants in Wastewater. Desalination 2011, 277, 1–14. [Google Scholar] [CrossRef]
- Abdurahman, M.H.; Abdullah, A.Z. Mechanism and Reaction Kinetic of Hybrid Ozonation-Ultrasonication Treatment for Intensified Degradation of Emerging Organic Contaminants in Water: A Critical Review. Chem. Eng. Process.-Process Intensif. 2020, 154, 108047. [Google Scholar] [CrossRef]
- Kumar, Y.; Kumar, R.; Raizada, P.; Khan, A.A.P.; Singh, A.; Van Le, Q.; Nguyen, V.H.; Selvasembian, R.; Thakur, S.; Singh, P. Current Status of Hematite (α-Fe2O3) Based Z-Scheme Photocatalytic Systems for Environmental and Energy Applications. J. Environ. Chem. Eng. 2022, 10, 107427. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magomedova, A.G.; Rabadanova, A.A.; Shuaibov, A.O.; Selimov, D.A.; Sobola, D.S.; Rabadanov, K.S.; Giraev, K.M.; Orudzhev, F.F. Combination NIPS/TIPS Synthesis of α-Fe2O3 and α/γ-Fe2O3 Doped PVDF Composite for Efficient Piezocatalytic Degradation of Rhodamine B. Molecules 2023, 28, 6932. https://doi.org/10.3390/molecules28196932
Magomedova AG, Rabadanova AA, Shuaibov AO, Selimov DA, Sobola DS, Rabadanov KS, Giraev KM, Orudzhev FF. Combination NIPS/TIPS Synthesis of α-Fe2O3 and α/γ-Fe2O3 Doped PVDF Composite for Efficient Piezocatalytic Degradation of Rhodamine B. Molecules. 2023; 28(19):6932. https://doi.org/10.3390/molecules28196932
Chicago/Turabian StyleMagomedova, Asiyat G., Alina A. Rabadanova, Abdulatip O. Shuaibov, Daud A. Selimov, Dinara S. Sobola, Kamil Sh. Rabadanov, Kamal M. Giraev, and Farid F. Orudzhev. 2023. "Combination NIPS/TIPS Synthesis of α-Fe2O3 and α/γ-Fe2O3 Doped PVDF Composite for Efficient Piezocatalytic Degradation of Rhodamine B" Molecules 28, no. 19: 6932. https://doi.org/10.3390/molecules28196932
APA StyleMagomedova, A. G., Rabadanova, A. A., Shuaibov, A. O., Selimov, D. A., Sobola, D. S., Rabadanov, K. S., Giraev, K. M., & Orudzhev, F. F. (2023). Combination NIPS/TIPS Synthesis of α-Fe2O3 and α/γ-Fe2O3 Doped PVDF Composite for Efficient Piezocatalytic Degradation of Rhodamine B. Molecules, 28(19), 6932. https://doi.org/10.3390/molecules28196932








