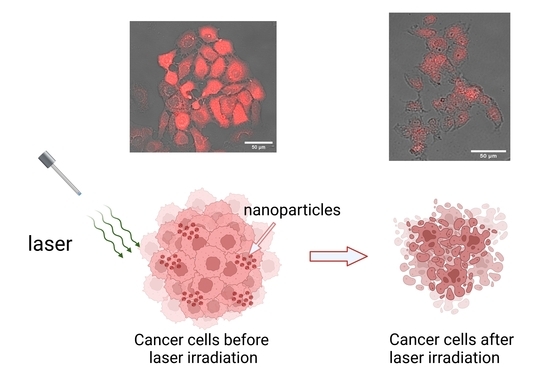
Photodynamic Treatment of Human Breast and Prostate Cancer Cells Using Rose Bengal-Encapsulated Nanoparticles
Abstract
:1. Introduction
2. Results
2.1. Scanning Electron Microscopy and Dynamic Light Scattering
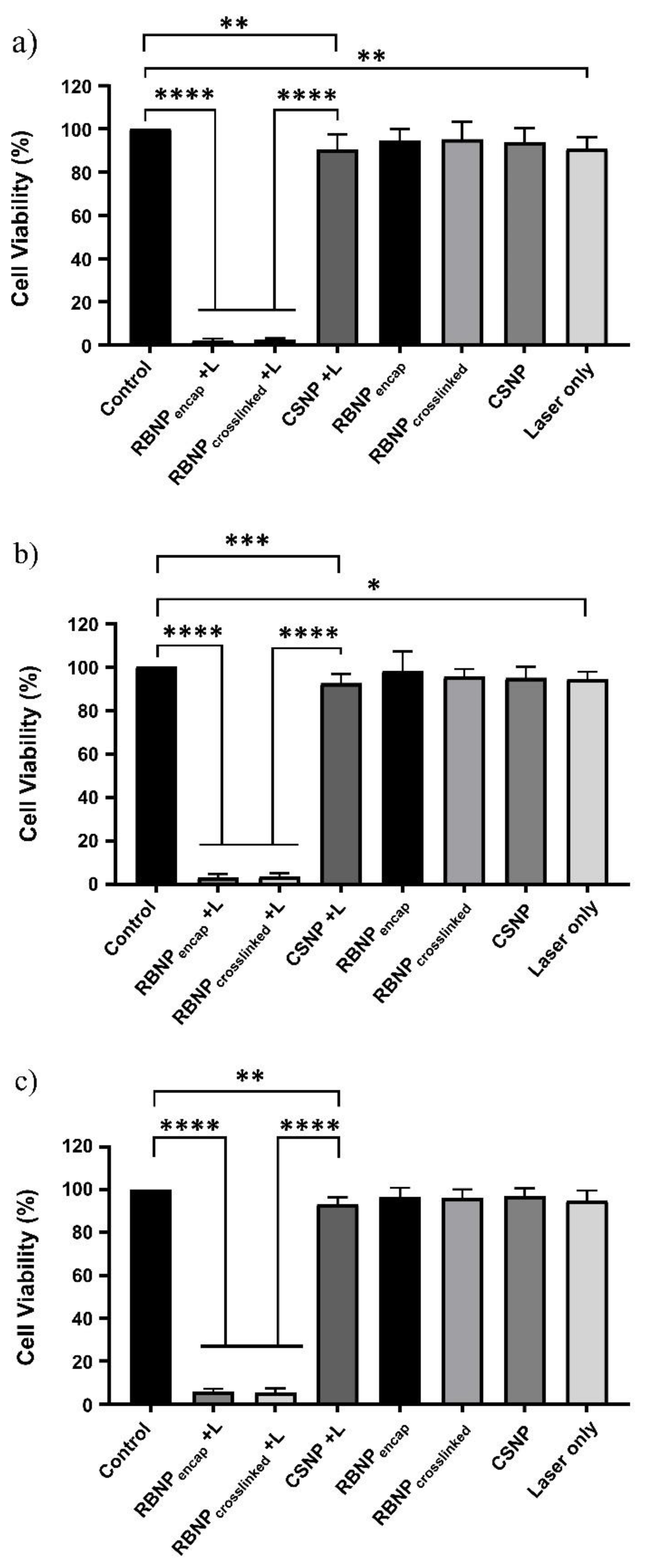
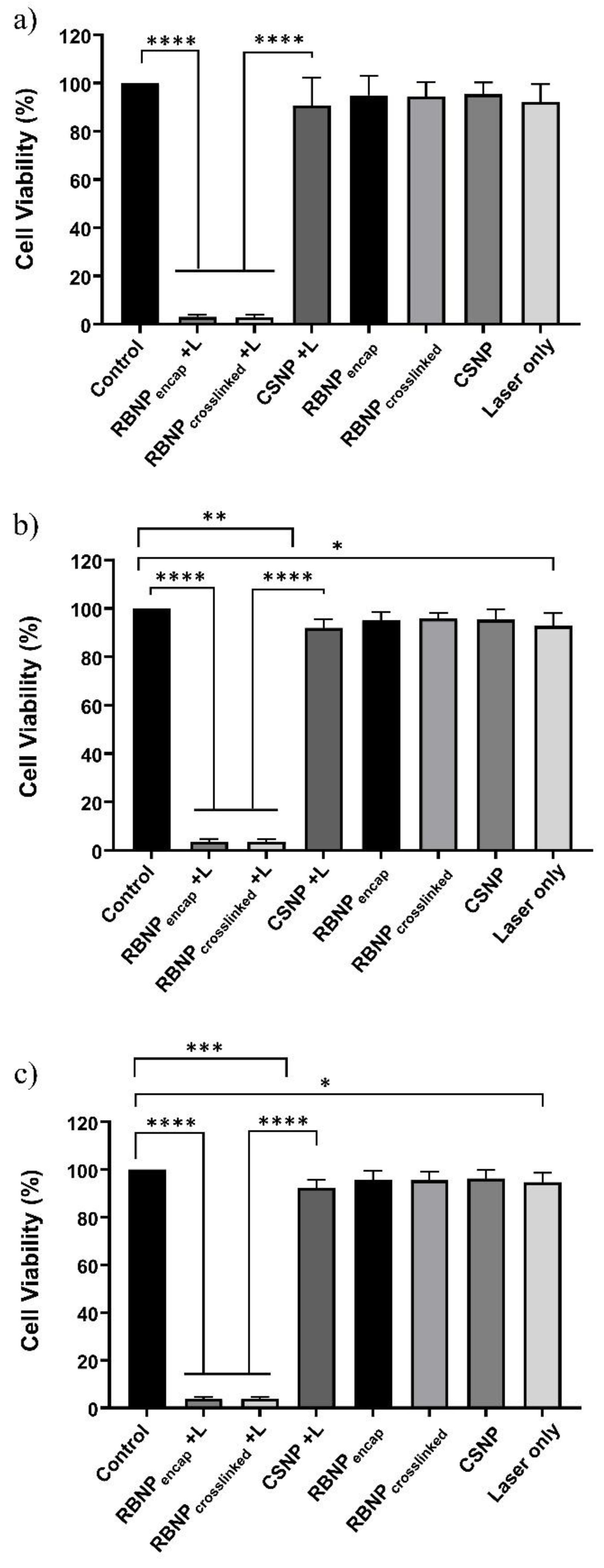
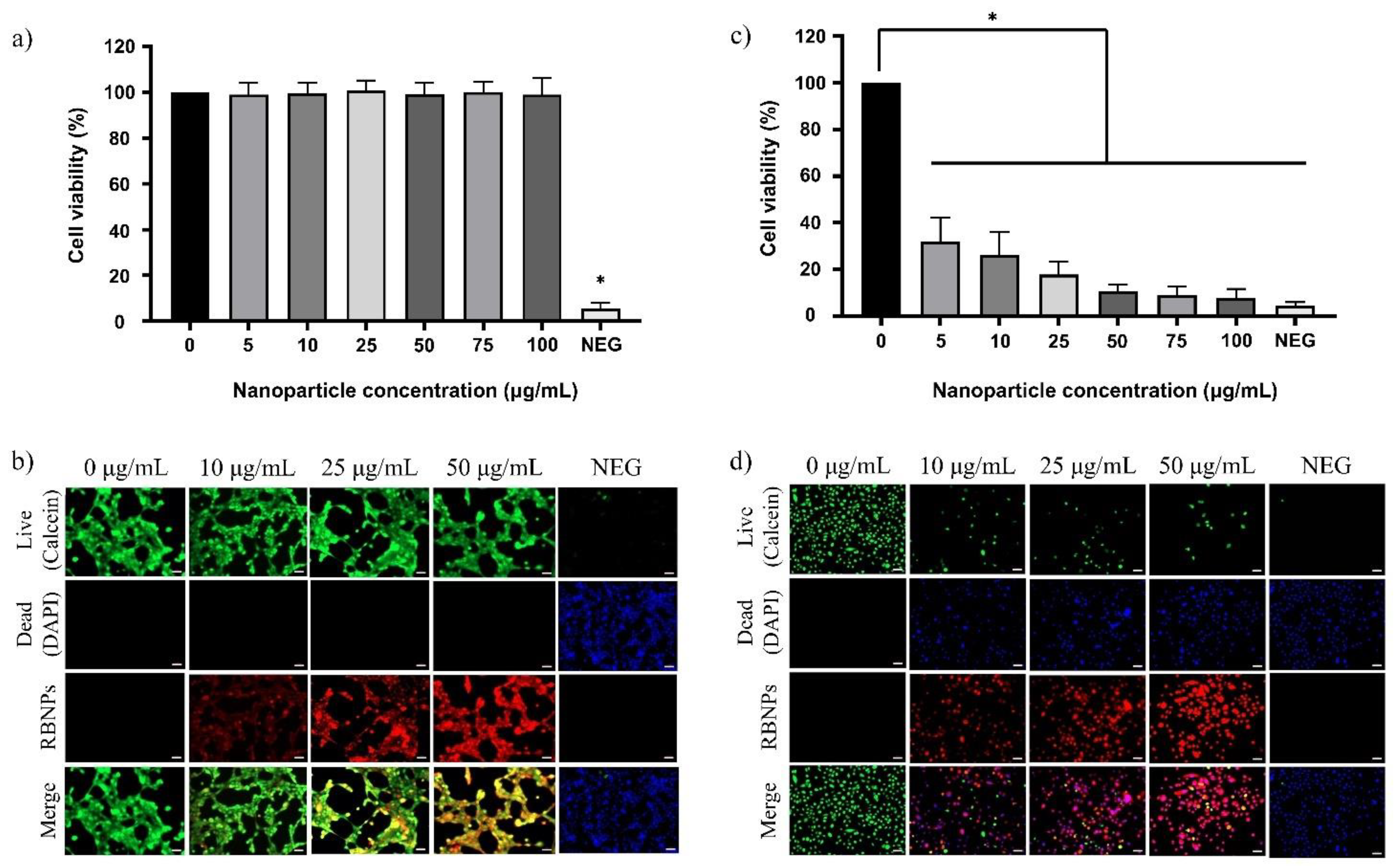
2.2. Photodynamic Treatment of Breast and Prostate Cancer Cells
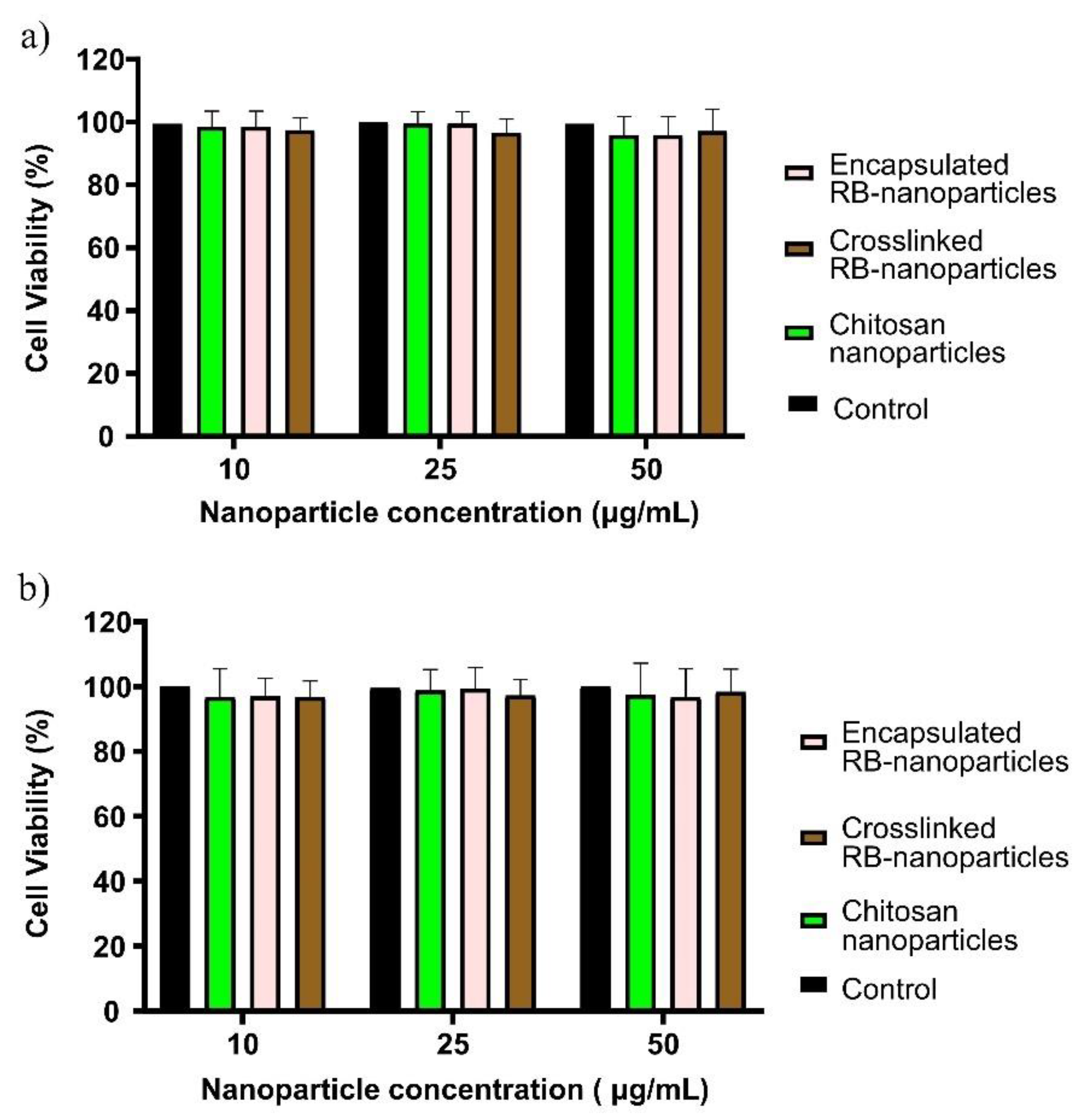
2.3. Dark Toxicity in Breast and Prostate Cancer Cells
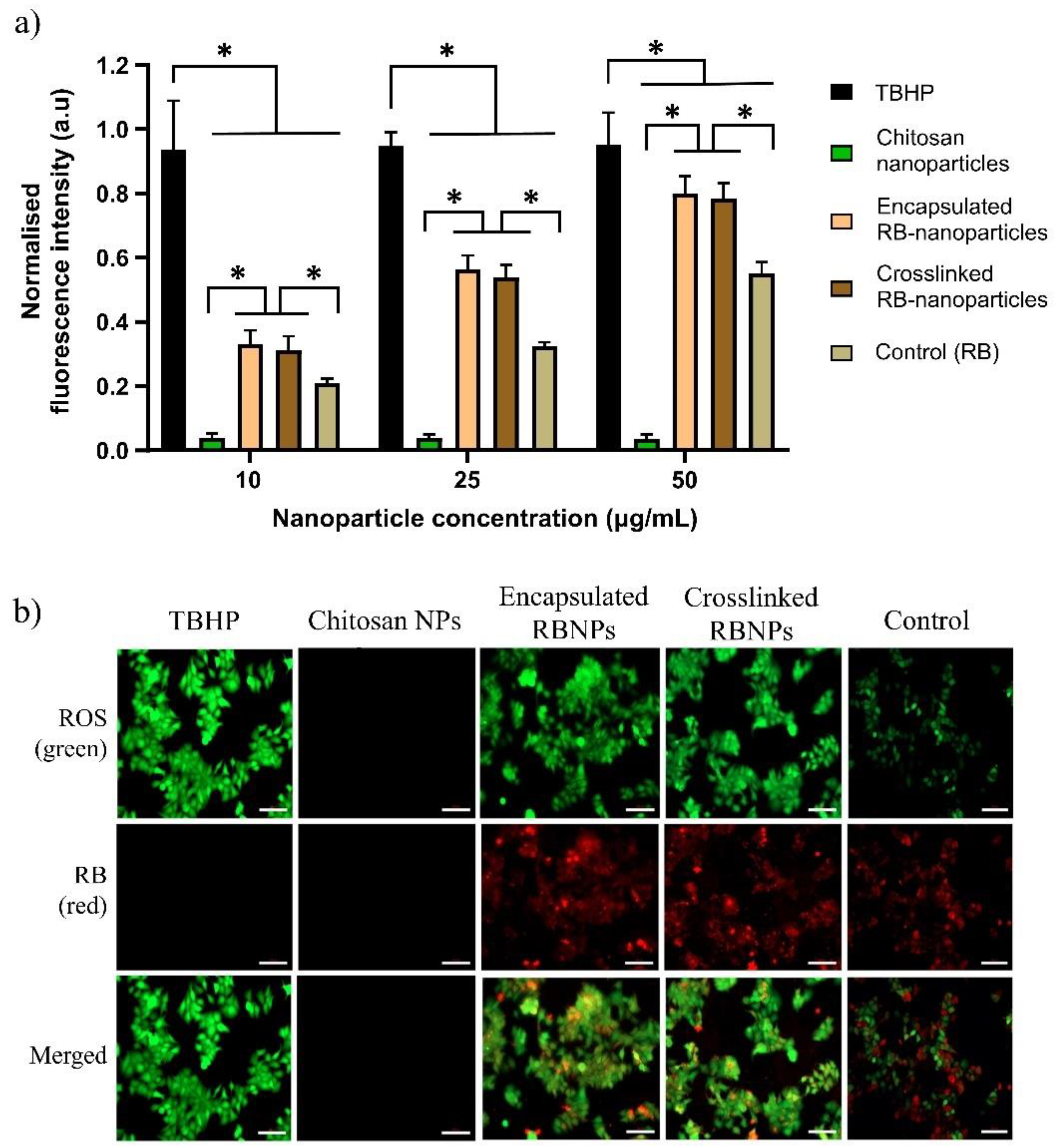
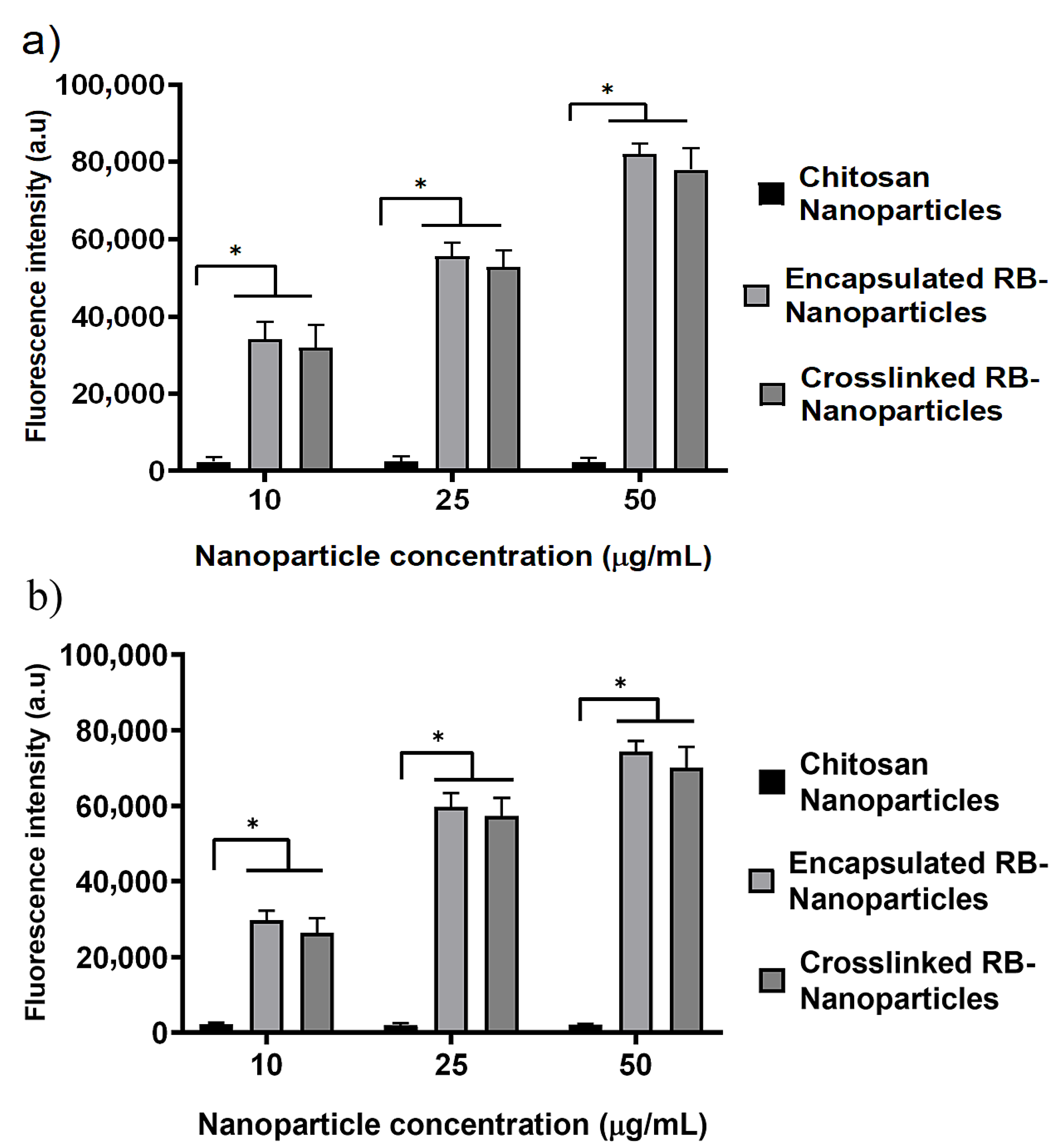
2.4. ROS Measurement and Intracellular Nanoparticle Uptake
2.5. Dark Toxicity in Noncancerous Human Breast and Prostate Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Rose Bengal-Encapsulated Chitosan Nanoparticles
4.3. Synthesis of Rose Bengal-Crosslinked Chitosan Nanoparticles
4.4. Purification and Characterisation of Nanoparticles
4.4.1. RB Encapsulation and Crosslinking Efficiency
4.4.2. Dynamic Light Scattering (DLS) and Scanning Electron Microscopy (SEM)
4.5. Cell Culture
4.6. Photodynamic Treatment of Breast and Prostate Cancer Cells
4.6.1. Laser System
4.6.2. Dosage Regimes
- Fifty μg/mL nanoparticles and 90 mW laser irradiation for ten minutes (Fluence ~228 J/cm2, Irradiance ~0.38 W/cm2);
- Twenty-five μg/mL nanoparticles and 90 mW laser irradiation for ten minutes (Fluence ~228 J/cm2, Irradiance ~0.38 W/cm2);
- Twenty-five μg/mL nanoparticles and 50 mW laser irradiation for ten minutes (Fluence ~126 J/cm2, Irradiance ~0.21 W/cm2).
4.6.3. Treatment Groups
- PDT + RBNPcrosslinked (+RBNPcrosslinked + L): the cancer cells were incubated for one hour with RB-crosslinked chitosan nanoparticles, and, then, laser irradiated;
- PDT + RBNPencap (+RBNPencap + L): the cancer cells were incubated for one hour with RB-encapsulated chitosan nanoparticles, and, then, laser irradiated;
- Laser + CSNP (+CSNP + L): the cancer cells were incubated for one hour with blank chitosan nanoparticles, and, then, laser irradiated;
- RB-crosslinked chitosan nanoparticles only (+RBNPcrosslinked − L): the cancer cells were incubated for one hour with RB-crosslinked chitosan nanoparticles, but not laser irradiated;
- RB-encapsulated chitosan nanoparticles only (+RBNPencap − L): the cancer cells were incubated for one hour with RB-encapsulated chitosan nanoparticles, but not laser irradiated;
- Blank chitosan nanoparticles only (+CSNP − L): the cancer cells were incubated for one hour with blank chitosan nanoparticles, but not laser irradiated;
- Laser only (–NP + L): the cancer cells were irradiated without using nanoparticles;
- Control (–NP − L): this consisted of MCF-7 and PC3 cancer cells without treatment.
4.7. Cell Viability Assay
4.8. Dark toxicity Measurement
4.9. Measurement and Detection of Intracellular Reactive Oxygen Species (ROS)
4.10. Cytotoxicity Assay
4.10.1. Live–Dead Cell Imaging
4.10.2. Cell Viability Assay
4.11. Cellular Uptake and Fluorescence Intensity Quantification of Nanoparticles
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Trimble, E.L.; Ungerleider, R.S.; Abrams, J.A.; Kaplan, R.S.; Feigal, E.G.; Smith, M.A.; Carter, C.L.; Friedman, M.A. Neoadjuvant therapy in cancer treatment. Cancer 1993, 72, 3515–3524. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Grumezescu, A.M. Photodynamic Therapy—An Up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Panzarini, E.; Inguscio, V.; Dini, L. Overview of Cell Death Mechanisms Induced by Rose Bengal Acetate-Photodynamic Therapy. Int. J. Photoenergy 2011, 2011, 713726. [Google Scholar] [CrossRef]
- Li, W.; Ma, Q.; Wu, E. Perspectives on the Role of Photodynamic Therapy in the Treatment of Pancreatic Cancer. Int. J. Photoenergy 2012, 2012, 637429. [Google Scholar] [CrossRef]
- Valenzeno, D.P. Photomodification of Biological-Membranes with Emphasis on Singlet Oxygen Mechanisms. Photochem. Photobiol. 1987, 46, 147–160. [Google Scholar] [CrossRef]
- Gannon, M.J.; Brown, S.B. Photodynamic therapy and its applications in gynaecology. Br. J. Obstet. Gynaecol. 1999, 106, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liang, M.; Lei, Q.; Li, G.; Wu, S. The Current Status of Photodynamic Therapy in Cancer Treatment. Cancers 2023, 15, 585. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, Y.; Song, W.; Jiang, X.; Deng, Z.; Xiong, W.; Shen, J. Metabolic reprogramming mediated PD-L1 depression and hypoxia reversion to reactivate tumor therapy. J. Control Release. 2022, 352, 793–812. [Google Scholar] [CrossRef] [PubMed]
- Nakonieczna, J.; Wolnikowska, K.; Ogonowska, P.; Neubauer, D.; Bernat, A.; Kamysz, W. Rose bengal-mediated photoinactivation of multidrug resistant pseudomonas aeruginosa is enhanced in the presence of antimicrobial peptides. Front. Microbiol. 2018, 9, 1949. [Google Scholar] [CrossRef] [PubMed]
- Amescua, G.; Arboleda, A.; Nikpoor, N.; Durkee, H.; Relhan, N.; Aguilar, M.C.; Flynn, H.W.; Miller, D.; Parel, J.-M. Rose bengal photodynamic antimicrobial therapy: A novel treatment for resistant Fusarium keratitis. Cornea 2017, 36, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Houang, J.; Halliday, C.; Chen, S.; Ho, C.-H.; Bekmukhametova, A.; Lauto, A. Effective photodynamic treatment of Trichophyton species with Rose Bengal. J. Biophotonics 2021, 14, e202000340. [Google Scholar] [CrossRef] [PubMed]
- Atenco-Cuautle, J.C.; Delgado-López, M.G.; Ramos-Garcia, R.; Ramirez-San-Juan, J.C.; Ramírez-Ramírez, J.; Spezzia-Mazzocco, T. Rose bengal as a photosensitizer in the photodynamic therapy of breast cancer cell lines. In Proceedings of the 17th International Photodynamic Association World Congress, Cambridge, MA, USA, 28 June–4 July 2019; Volume 11070, pp. 364–367. [Google Scholar] [CrossRef]
- Lauto, A.; Mawad, D.; Barton, M.; Gupta, A.; Piller, S.C.; Hook, J. Photochemical tissue bonding with chitosan adhesive films. Biomed. Eng. Online 2010, 9, 47. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Rubio, V.; Qi, J.; Xia, R.; Shi, Z.-Z.; Peterson, L.; Tung, C.-H.; O’Neill, B.E. Cancer treatment using an optically inert Rose Bengal derivative combined with pulsed focused ultrasound. J. Control. Release 2011, 156, 315–322. [Google Scholar] [CrossRef]
- Orphan Drug Status for PV-10 for HCC. Oncol. Times 2011, 33, 32. [CrossRef]
- Chang, C.-C.; Yang, Y.-T.; Yang, J.-C.; Wu, H.-D.; Tsai, T. Absorption and emission spectral shifts of rose bengal associated with DMPC liposomes. Dye. Pigment. 2008, 79, 170–175. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.-H.; Liu, Q.; Huang, H.; Chen, M.; Li, K.; Li, C.; Yu, X.-F.; Chu, P.K. Rose-bengal-conjugated gold nanorods for in vivo photodynamic and photothermal oral cancer therapies. Biomaterials 2014, 35, 1954–1966. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.; Varga, F. Hepatic storage and biliary excretion of rose bengal in the rat. Acta Physiol. Acad. Sci. Hung. 1979, 54, 89–94. [Google Scholar] [PubMed]
- Gianotti, E.; Martins Estevão, B.; Cucinotta, F.; Hioka, N.; Rizzi, M.; Renò, F.; Marchese, L. An efficient rose bengal based nanoplatform for photodynamic therapy. Chemistry 2014, 20, 10921–10925. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Samia, A.C.; Meyers, J.D.; Panagopoulos, I.; Fei, B.; Burda, C. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J. Am. Chem. Soc. 2008, 130, 10643–10647. [Google Scholar] [CrossRef] [PubMed]
- Kale, M.B. Rose Bengal-Conjugated Gold Nanoparticles: Quantification of Singlet Oxygen Generation in Photodynamic Therapy. Master’s Thesis, Macquarie University, Sydney, Australia, 2022. [Google Scholar]
- Cheng, Y.; Doane, T.L.; Chuang, C.-H.; Ziady, A.; Burda, C. Near infrared light-triggered drug generation and release from gold nanoparticle carriers for photodynamic therapy. Small 2014, 10, 1799–1804. [Google Scholar] [CrossRef]
- Borodziuk, A.; Kowalik, P.; Duda, M.; Wojciechowski, T.; Minikayev, R.; Kalinowska, D.; Klepka, M.; Sobczak, K.; Kłopotowski, Ł.; Sikora, B. Unmodified Rose Bengal photosensitizer conjugated with NaYF4:Yb,Er upconverting nanoparticles for efficient photodynamic therapy. Nanotechnology 2020, 31, 465101. [Google Scholar] [CrossRef]
- Sabri, T.; Pawelek, P.D.; Capobianco, J.A. Dual Activity of Rose Bengal Functionalized to Albumin-Coated Lanthanide-Doped Upconverting Nanoparticles: Targeting and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 26947–26953. [Google Scholar] [CrossRef]
- Jain, A.; Koyani, R.; Muñoz, C.; Sengar, P.; Contreras, O.E.; Juárez, P.; Hirata, G.A. Magnetic-luminescent cerium-doped gadolinium aluminum garnet nanoparticles for simultaneous imaging and photodynamic therapy of cancer cells. J. Colloid Interface Sci. 2018, 526, 220–229. [Google Scholar] [CrossRef]
- Dhaini, B.; Wagner, L.; Moinard, M.; Daouk, J.; Arnoux, P.; Schohn, H.; Schneller, P.; Acherar, S.; Hamieh, T.; Frochot, C. Importance of Rose Bengal Loaded with Nanoparticles for Anti-Cancer Photodynamic Therapy. Pharmaceuticals 2022, 15, 1093. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Hou, L.; Li, H.-L.; Wang, X.; Cao, Y.; Zhang, B.-Y.; Wang, J.-T.; Wei, S.-J.; Dang, H.-W.; Ran, H.-T. A nanosystem loaded with perfluorohexane and rose bengal coupled upconversion nanoparticles for multimodal imaging and synergetic chemo-photodynamic therapy of cancer. Biomater. Sci. 2020, 8, 2488–2506. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Lin, R.; Li, H.-J.; He, W.-L.; Du, J.-Z.; Wang, J. Strategies to improve tumor penetration of nanomedicines through nanoparticle design. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1519. [Google Scholar] [CrossRef] [PubMed]
- Rawal, T.; Parmar, R.; Tyagi, R.K.; Butani, S. Rifampicin loaded chitosan nanoparticle dry powder presents an improved therapeutic approach for alveolar tuberculosis. Colloids Surf. B Biointerfaces 2017, 154, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Virmani, T.; Kumar, G.; Sharma, A.; Pathak, K.; Akhtar, M.S.; Afzal, O.; Altamimi, A.S.A. Amelioration of Cancer Employing Chitosan, Its Derivatives, and Chitosan-Based Nanoparticles: Recent Updates. Polymers 2023, 15, 2928. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, Y.; Jiang, X.; Zheng, C.; Luo, W.; Xiang, X.; Qi, X.; Shen, J. Metformin modified chitosan as a multi-functional adjuvant to enhance cisplatin-based tumor chemotherapy efficacy. Int. J. Biol. Macromol. 2023, 224, 797–809. [Google Scholar] [CrossRef]
- Guo, H.; Li, F.; Qiu, H.; Liu, J.; Qin, S.; Hou, Y.; Wang, C. Preparation and Characterization of Chitosan Nanoparticles for Chemotherapy of Melanoma through Enhancing Tumor Penetration. Front. Pharmacol. 2020, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.M.; Muchtaridi, M. Chitosan-Based Nanoparticles of Targeted Drug Delivery System in Breast Cancer Treatment. Polymers 2021, 13, 1717. [Google Scholar] [CrossRef]
- Shrestha, A.; Hamblin, M.R.; Kishen, A. Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 491–501. [Google Scholar] [CrossRef]
- de Freitas, L.M.; Calixto, G.M.F.; Chorilli, M.; Giusti, J.S.M.; Bagnato, V.S.; Soukos, N.S.; Amiji, M.M.; Fontana, C.R. Polymeric Nanoparticle-Based Photodynamic Therapy for Chronic Periodontitis In Vivo. Int. J. Mol. Sci. 2016, 17, 769. [Google Scholar] [CrossRef]
- Sakima, V.T.; Barbugli, P.A.; Cerri, P.S.; Chorilli, M.; Carmello, J.C.; Pavarina, A.C.; Mima, E.G. de O. Antimicrobial Photodynamic Therapy Mediated by Curcumin-Loaded Polymeric Nanoparticles in a Murine Model of Oral Candidiasis. Molecules 2018, 23, 2075. [Google Scholar] [CrossRef]
- Hermanson, G.T. (Ed.) Microparticles and Nanoparticles. In Bioconjugate Techniques, 3rd ed.; Academic Press: Boston, MA, USA, 2013; Chapter 14; pp. 549–587. ISBN 978-0-12-382239-0. [Google Scholar]
- Bekmukhametova, A.; Uddin, M.M.N.; Houang, J.; Malladi, C.; George, L.; Wuhrer, R.; Barman, S.K.; Wu, M.J.; Mawad, D.; Lauto, A. Fabrication and characterization of chitosan nanoparticles using the coffee-ring effect for photodynamic therapy. Lasers Surg. Med. 2022, 54, 758–766. [Google Scholar] [CrossRef]
- Uppal, A.; Jain, B.; Gupta, P.K.; Das, K. Photodynamic action of Rose Bengal silica nanoparticle complex on breast and oral cancer cell lines. Photochem. Photobiol. 2011, 87, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Cai, X.; Sun, C.; Liang, S.; Shao, S.; Huang, S.; Cheng, Z.; Pang, M.; Xing, B.; Al Kheraif, A.A.; et al. O2-Loaded pH-Responsive Multifunctional Nanodrug Carrier for Overcoming Hypoxia and Highly Efficient Chemo-Photodynamic Cancer Therapy. Chem. Mater. 2019, 31, 483–490. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Babu, A.; Kim, S.-J.; Murugesan, R.; Jeyasubramanian, K. Enhanced photodynamic efficacy and efficient delivery of Rose Bengal using nanostructured poly(amidoamine) dendrimers: Potential application in photodynamic therapy of cancer. Cancer Nanotechnol. 2011, 2, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Bekmukhametova, A.; Antony, A.; Halliday, C.; Chen, S.; Ho, C.-H.; Uddin, M.M.N.; Longo, L.; Pedrinazzi, C.; George, L.; Wuhrer, R.; et al. Rose bengal–encapsulated chitosan nanoparticles for the photodynamic treatment of Trichophyton species. Photochem. Photobiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2003; Volume 91. [Google Scholar]
- Claesson-Welsh, L. Vascular permeability—The essentials. Ups. J. Med. Sci. 2015, 120, 135–143. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Su, Y.-L.; Hu, S.-H. Functional Nanoparticles for Tumor Penetration of Therapeutics. Pharmaceutics 2018, 10, 193. [Google Scholar] [CrossRef]
- Yu, W.; Liu, R.; Zhou, Y.; Gao, H. Size-Tunable Strategies for a Tumor Targeted Drug Delivery System. ACS Cent. Sci. 2020, 6, 100–116. [Google Scholar] [CrossRef]
- Wang, H.-X.; Zuo, Z.-Q.; Du, J.; Wang, Y.-C.; Sun, R.; Cao, Z.-T.; Ye, X.; Wang, J.-L.; Leong, K.; Wang, J. Surface charge critically affects tumor penetration and therapeutic efficacy of cancer nanomedicines. Nano Today 2016, 11, 133–144. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Szasz, O. Essentials of Oncothermia. Conf. Pap. Med. 2013, 2013, 159570. [Google Scholar] [CrossRef]
- Cure, J.C. Cancer an electrical phenomenon. Resonant 1991, 11. [Google Scholar]
- Shrestha, A.; Hamblin, M.R.; Kishen, A. Characterization of a conjugate between Rose Bengal and chitosan for targeted antibiofilm and tissue stabilization effects as a potential treatment of infected dentin. Antimicrob. Agents Chemother. 2012, 56, 4876–4884. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.S.; Johnson, A.J.; Petersingham, G.; Muench, G.W.; Dong, Q.; Wu, M.J. Protein kinase CK2 is involved in zinc homeostasis in breast and prostate cancer cells. BioMetals Int. J. Role Met. Ions Biol. Biochem. Med. 2019, 32, 861–873. [Google Scholar] [CrossRef]
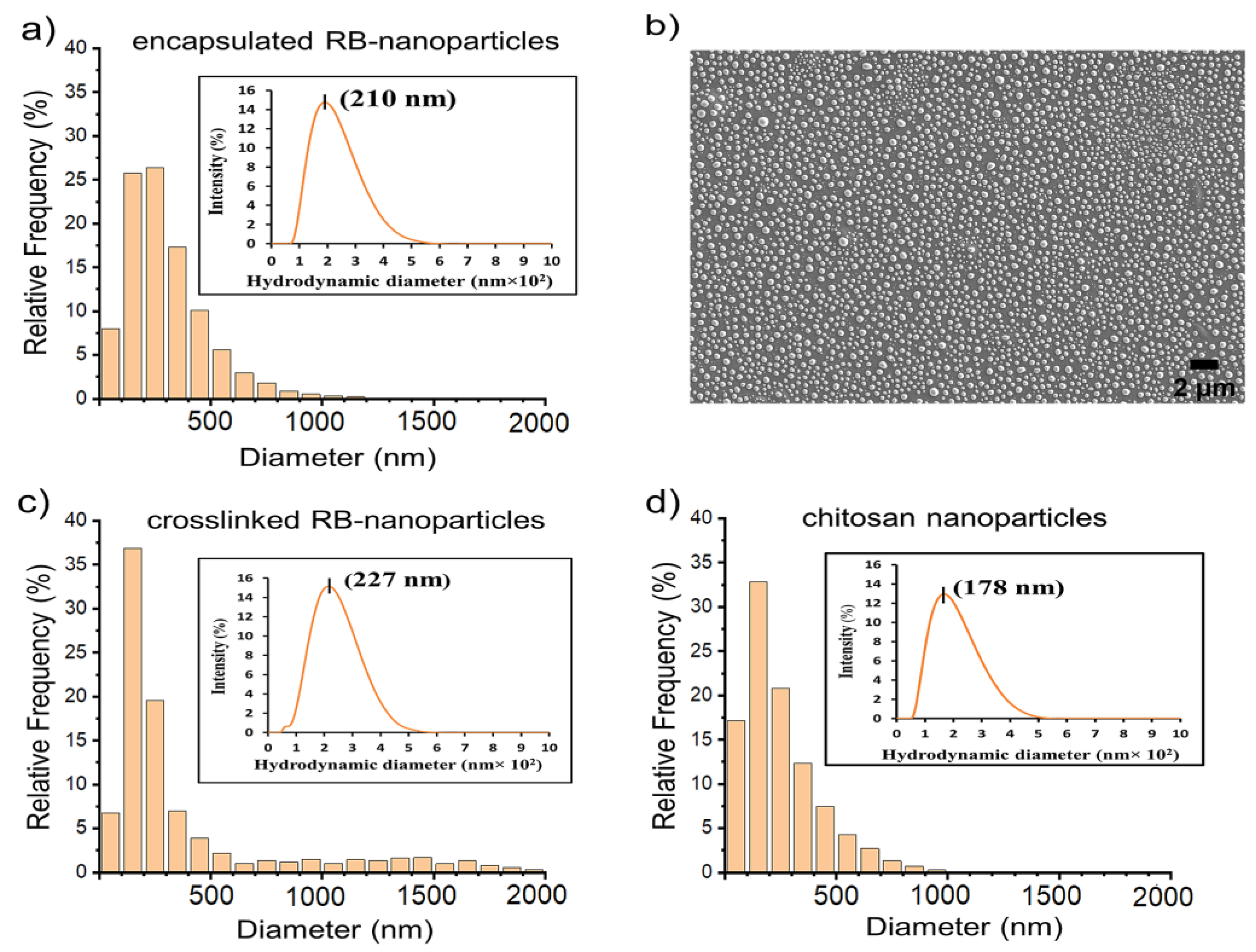

| Parameters | Rose Bengal-Encapsulated Nanoparticles | Rose Bengal-Crosslinked Nanoparticles | Chitosan Nanoparticles |
|---|---|---|---|
| pH | 5.4–5.5 | 5.5–5.6 | 5.5–5.6 |
| RB concentration (μg/mL) | 50 | 50 | n/a |
| Encapsulation and crosslinking efficiency (%) | 96 ± 3 | 95 ± 4 | n/a |
| Charge/zeta potential (mV) | 25.5 ± 0.4 | 21.1 ± 0.7 | 25.9 ± 0.9 |
| Peak maximum (nm) | 210 ± 16 | 227 ± 21 | 178 ± 17 |
| PDI | 0.21 ± 0.02 | 0.19 ± 0.01 | 0.23 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, M.M.N.; Bekmukhametova, A.; Antony, A.; Barman, S.K.; Houang, J.; Wu, M.J.; Hook, J.; George, L.; Wuhrer, R.; Mawad, D.; et al. Photodynamic Treatment of Human Breast and Prostate Cancer Cells Using Rose Bengal-Encapsulated Nanoparticles. Molecules 2023, 28, 6901. https://doi.org/10.3390/molecules28196901
Uddin MMN, Bekmukhametova A, Antony A, Barman SK, Houang J, Wu MJ, Hook J, George L, Wuhrer R, Mawad D, et al. Photodynamic Treatment of Human Breast and Prostate Cancer Cells Using Rose Bengal-Encapsulated Nanoparticles. Molecules. 2023; 28(19):6901. https://doi.org/10.3390/molecules28196901
Chicago/Turabian StyleUddin, Mir Muhammad Nasir, Alina Bekmukhametova, Anu Antony, Shital K. Barman, Jessica Houang, Ming J. Wu, James Hook, Laurel George, Richard Wuhrer, Damia Mawad, and et al. 2023. "Photodynamic Treatment of Human Breast and Prostate Cancer Cells Using Rose Bengal-Encapsulated Nanoparticles" Molecules 28, no. 19: 6901. https://doi.org/10.3390/molecules28196901
APA StyleUddin, M. M. N., Bekmukhametova, A., Antony, A., Barman, S. K., Houang, J., Wu, M. J., Hook, J., George, L., Wuhrer, R., Mawad, D., Ta, D., & Lauto, A. (2023). Photodynamic Treatment of Human Breast and Prostate Cancer Cells Using Rose Bengal-Encapsulated Nanoparticles. Molecules, 28(19), 6901. https://doi.org/10.3390/molecules28196901