Abstract
In order to study the importance of the diffusion mechanism of CH4 and CO2 in coal for the development of coalbed methane, the aim of this paper is to reveal the influence mechanism of pressure, temperature, water content and other factors on the molecular diffusion behavior of gas at the molecular level. In this paper, non-sticky coal in Chicheng Coal Mine is taken as the research object. Based on the molecular dynamics method (MD) and Monte Carlo (GCMC) method, the diffusion characteristics and microscopic mechanism of CH4 and CO2 in coal under different pressures (100 kPa–10 MPa), temperatures (293.15–313.15 K) and water contents (1–5%) were analyzed in order to lay a theoretical foundation for revealing the diffusion characteristics of CBM in coal, and provide technical support for further improving CBM extraction. The results show that high temperature is conducive to gas diffusion, while high pressure and water are not conducive to gas diffusion in the coal macromolecular model.
1. Introduction
The gas diffusion performance in coal is an important parameter that affects the gas production rate of coalbed methane wells and determines the final production of coalbed methane [1]. Gas diffusion in coal is due to the slow movement of gas from high concentration to low concentration under the condition of concentration difference, and it presents a process of uniform distribution after a long time [2]. Gas diffusion is an inefficient means of material transport. Diffusion coefficient is commonly used to characterize the diffusion degree of gas in coal seam. The diffusion coefficient of gas in coal can be obtained via the molecular dynamics simulation method. So far, many scholars have studied the diffusion properties of CH4 and CO2 gases in coal. Diffusion is an important part of gas migration in coal, which is usually related to gas type, moisture, gas pressure and temperature [3].
Li Bin [4], taking anthracite, coking coal and long-flame coal as research objects, carried out experiments on adsorption–deformation–seepage of CO2, CH4 and CH4 in coal of different rank under stress, and studied molecular simulation of adsorption and diffusion behavior of CO2, CH4 and CH4 in different coal macromolecular models. Miao Zhang et al. [5] carried out an adsorption–desorption diffusion test of CO2 in coal particles under different temperature and pressure conditions, and used different adsorption and diffusion models to fit and analyze the test results. Dai Xuanyan [6] studied the adsorption and diffusion states of single and mixed components of (1:1) CH4 and CO2 of three minerals (illite, montmorillonite and calcite), and found that the self-diffusion coefficients of CH4 and CO2 first decreased and then increased with an increase in buried depth. Junlin Liu et al. [7] studied the diffusion behavior of CO2 and CH4 gases in the CO2-ECBM process by taking the pore characteristics of coal reservoirs of 13 coals in Liuzhuang Mine and 7 coals in Qidong Mine in the low-permeability coal-bearing area of Lianghuai, China, as the research object. Kaiyuan Li [8], based on coal gangue samples of different particle-size groups as research objects, simulated the characteristics of CO2 diffusion over time in porous media samples by using laboratory isothermal adsorption experiments, equation fitting, software simulation and other methods. You et al. [9] used molecular simulation to replace a lignite model with graphite surface containing OH, -COOH and carbonyl groups. By analyzing the radial distribution function and diffusion coefficient of H2O molecules, it was concluded that -COOH was the preferred adsorption site. Hu et al. [10] compared the diffusion characteristics of CO2 and CH4 in coal, and found that the CO2 diffusion coefficient was about 10−9 m2/s. An Fenghua [11] studied the diffusion coefficient under different stress, concentration gradient, temperature and gas type conditions with the direct steady-state method based on Fick’s law, and the results show that the diffusion coefficient of gas has a negative linear relationship with stress. Xu et al. [12] proposed a new laboratory measurement method for methane diffusion coefficient in coal matrix, using coal matrix flakes instead of coal particles as measurement samples. By means of molecular simulation, Yu Song [13] et al. studied the diffusion characteristics of CO2 and CH4 molecules using a Wise bituminous coal macromolecular structure model, indicating that CO2 and CH4 are mainly diffused via micropores in the coal model. Liu et al. [14] studied the effect of coal type size on gas diffusion of pulverized coal and lump coal under unconstrained conditions, and the results showed that there was a scale effect on gas diffusion in coal. Keshavarz et al. [15] studied the effects of maceral composition and coal rank on the diffusion rate of CO2 and CH4 in 18 Australian bituminous and subbituminous coals. Hu et al. [16] further established a simplified numerical method for a dual-dispersion diffusion model and compared it with the experimental results. Hu et al. [17] studied the self-diffusion and mutual diffusion of CO2-CH4 mixture via molecular simulation, and the results showed that the self-diffusion coefficient decreased with an increase in gas concentration and increased with an increase in temperature.
In the article “Simulation study on molecular adsorption of coal in Chicheng Coal Mine”, I studied the adsorption characteristics of coal and obtained the following results: In the macromolecular structure model of dry coal, under the same conditions, the adsorption capacity, interaction energy and adsorption heat of CO2 were all greater than that of CH4, and CO2 was more sensitive to temperature changes. The equivalent adsorption heat of CO2 and CH4 adsorbed in wet coal with different water content decreased with an increase in pressure and increased with an increase in water content [18]. The diffusion mechanism of CH4 and CO2 in coal is analyzed in this paper. The effects of temperature, pressure and water content on the diffusion of CO2 and CH4 on the macromolecular structure of non-stick coal are mainly studied, which is very important for the development of coalbed methane.
2. Results and Discussion
2.1. Structural Characterization and Construction of Macromolecular Structure of Coal
This study selected the non-caking coal of Chicheng Coal Mine as the research object, and industrial/elemental analysis, such as FTIR, XPS and solid [15] C NMR (Ceshigou Research Service, Beijing, China), was used to characterize and analyze the organic matter in the coal sample, including the aromatic structures, oxygen-containing functional groups, fatty carton structure, occurrence state of sulfur element and other parameter characteristics, on the basis of which a coal macromolecular structure model was constructed. This analysis provided support for the construction ideas and methods of the coal macromolecular structure model [18].
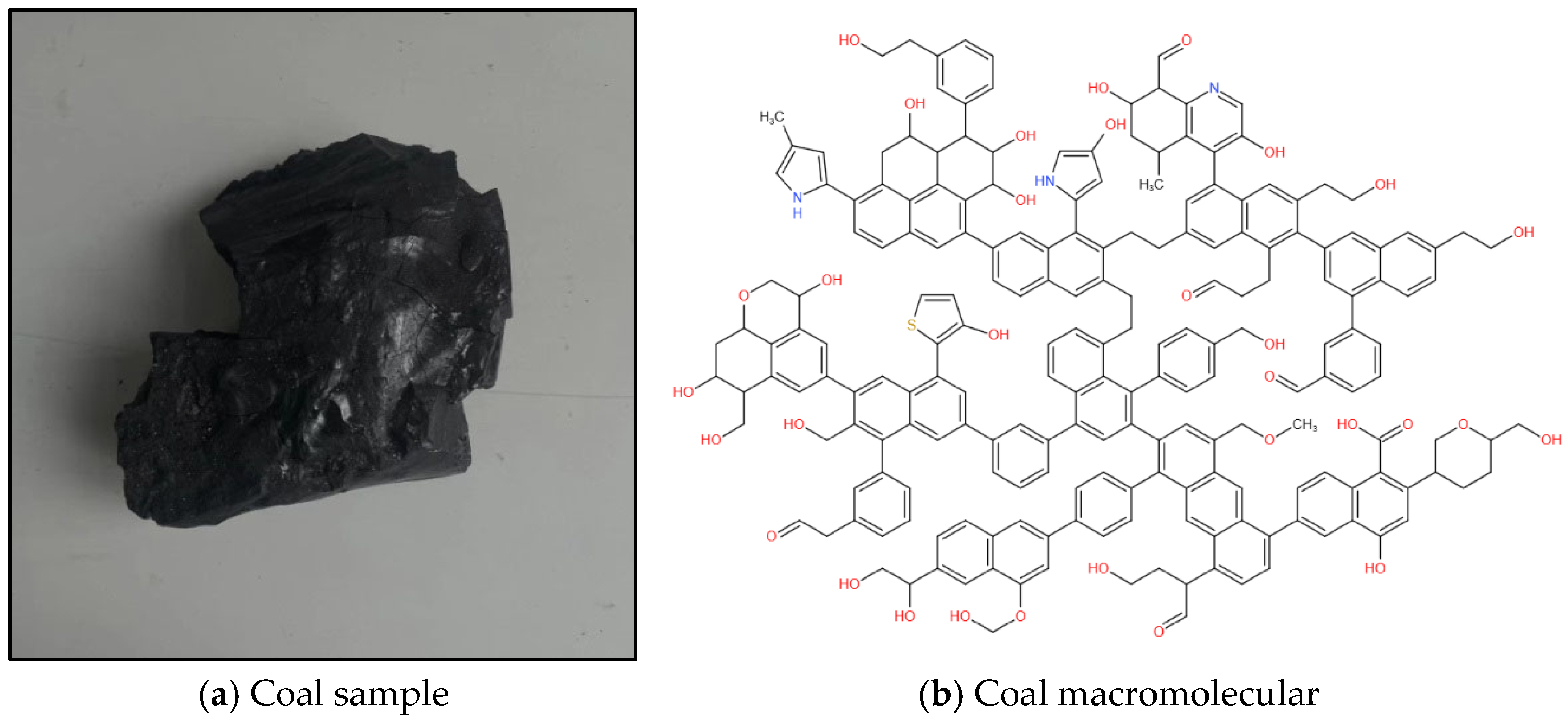
The study of the physicochemical structure of coal can enable a complete understanding of the adsorption performance of coal for gas [19]. In this study, fresh coal samples (density 1.16 g/cm3, R°max 0.665%) from the 1502−2 working face of Chicheng Coal Mine were selected. The coal samples were crushed, screened and divided using a crusher and a vibrating screen machine to produce analytical samples with a particle size below 200 mesh. Based on the results of the elemental analysis, Fourier-transform infrared spectroscopy (FT−IR), X-ray photoelectron spectroscopy (XPS) and carbon-13 nuclear magnetic resonance (13C NMR) experimental characterization, the molecular formula of non-sticky coal in Chicheng Coal Mine was determined to be C207H181O32N3S (C: 76.39%, N: 1.29%, O: 15.73%, H: 5.61%, S: 0.99). The coal macromolecular model is shown in Figure 1 [18].
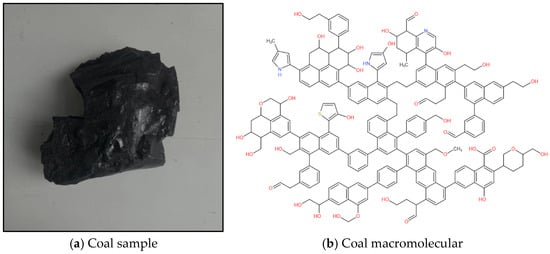
Figure 1.
Test coal sample and plane model of coal macromolecular structure.
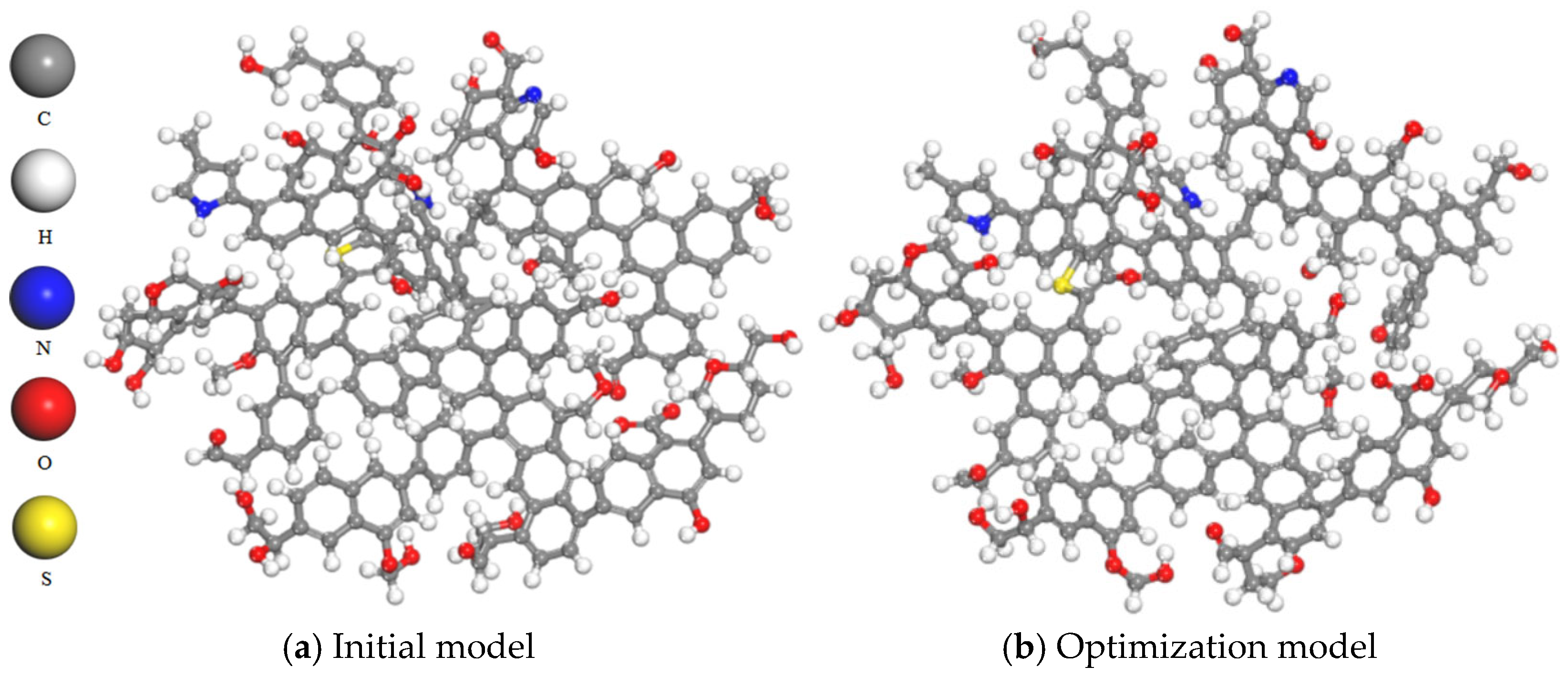
The two-dimensional plane model of coal macromolecules shown in Figure 1 was imported into the MS molecular simulation software to construct an initial three-dimensional structure, as shown in Figure 2a. The Forcite module for geometric optimization of the model was used and the COMPASS force field was selected for geometric optimization. The selection of the COMPASS force field is justified by its ability to provide a unified approach for modeling both organic and inorganic molecular systems. This force field can be applied to various types of molecules, including organics, polymers, gases and inorganics, utilizing a classified treatment approach that employs different models for different systems. Furthermore, it allows an accurate description even when mixing these two types of systems together. In comparison to the commonly used Dreiding force field, the COMPASS force field yields more precise results in terms of structure and binding energy calculations. The parameters are derived from ab initio parameterization and empirical optimization [20]. The forcefield was set to charge, the calculation accuracy was set to Fine, and the iteration step was set to 5000. The model was then subjected to annealing and the NVT ensemble was selected. The temperature of Nose was set to 300–600 K, and the number of cycles was set to five. Pneumatic parameters were specified accordingly. The model structure after dynamic optimization is shown in Figure 2b [18].
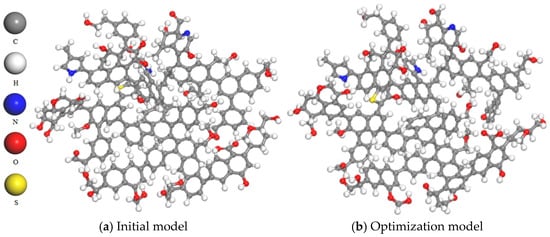
Figure 2.
Comparison before and after model optimization.
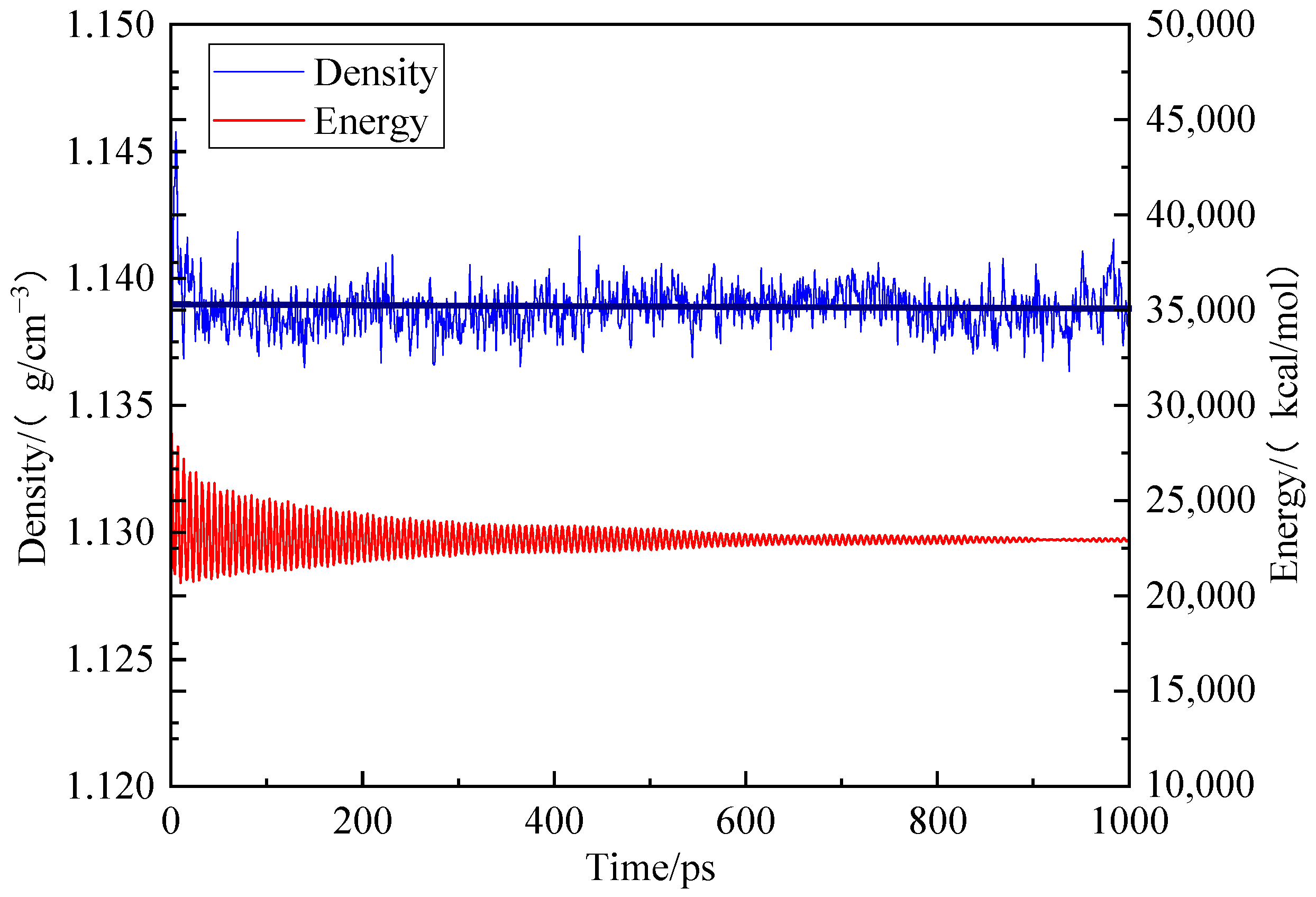
To establish the periodic boundary condition, the Amorphous Cell module was employed to put 10 optimized coal molecular models into the periodic cell. Firstly, the geometric optimization was conducted with predefined mechanical parameters. Subsequently, the model underwent annealing through a series of NPT cycles at temperatures ranging from 300 K to 600 K. A total of five cycles using the COMPASS force field, atom-based method for van der Waals term and Ewald method for electrostatic action term was performed. Finally, dynamic optimization was applied to the model with unchanged mechanical parameters as before. After kinetic treatment for 1000 ps, the total energy of the coal crystal cell model decreased and stabilized at its lowest value of 22,985.040 kcal/mol while maintaining a density of 1.138 g/cm3, which closely approximates that of real coal, as shown in Figure 3 [18].
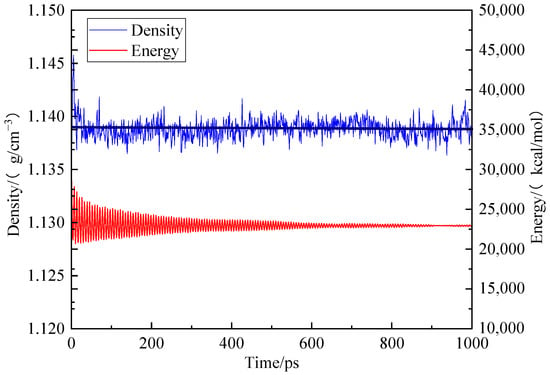
Figure 3.
Trends of energy and concentration of different coal samples during kinetic optimization.
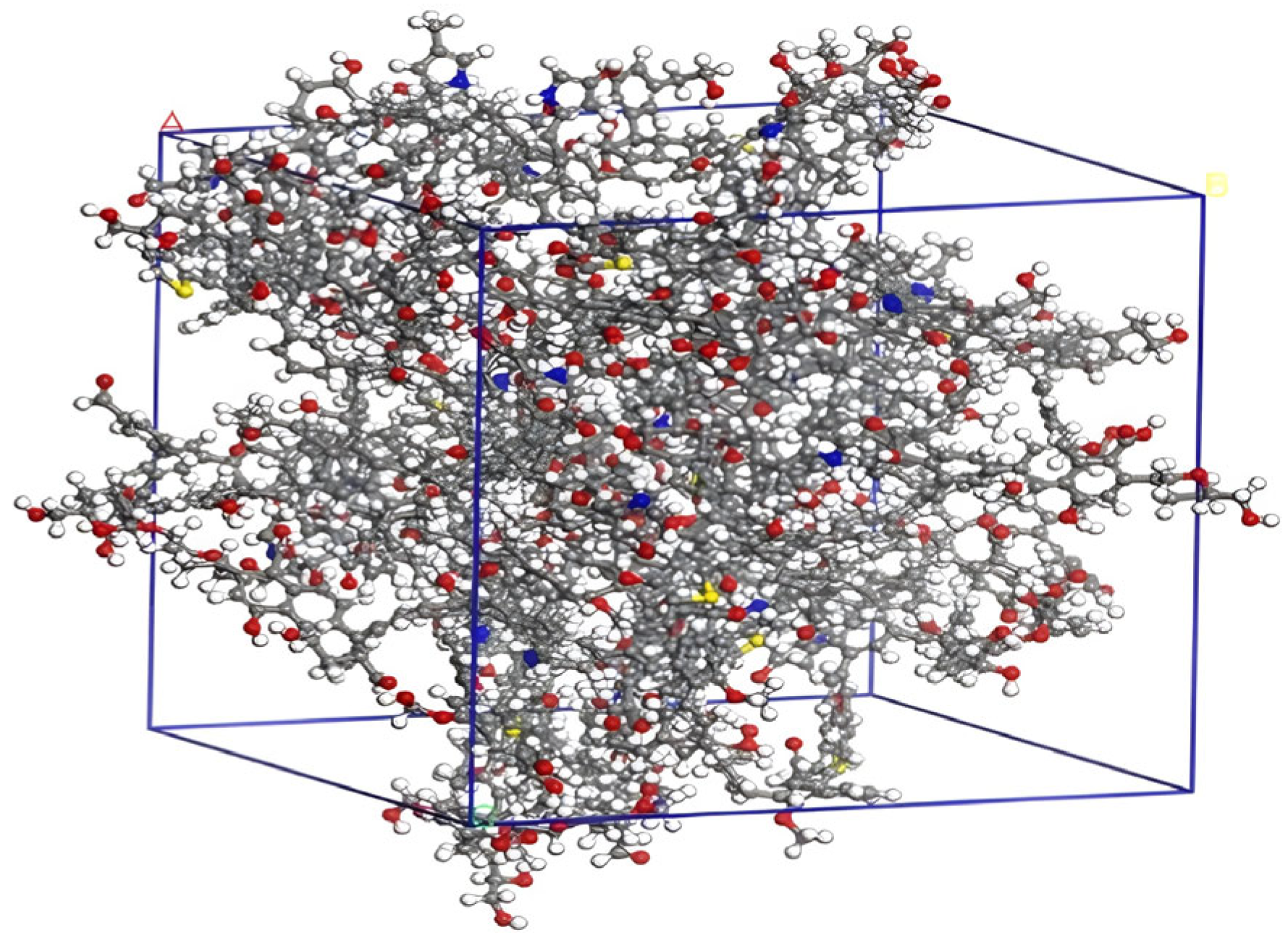
The optimized coal macromolecular structure model is shown in Figure 4 (the structural model size of coal is A = B = C = 3.94357 nm), whose molecular formula is C2070H1810N30O320S10 [18].
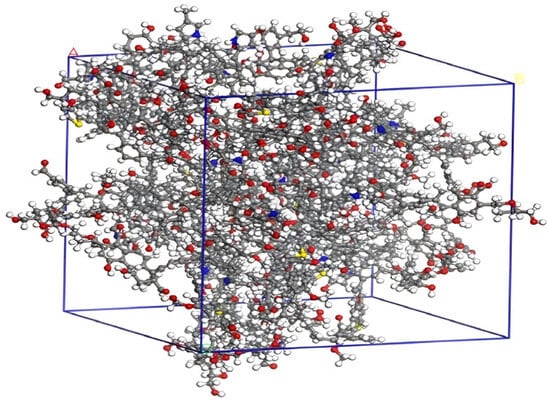
Figure 4.
Coal macromolecular structure cell model.
2.2. Theoretical Formula of Gas Diffusion Characteristics of Coal
The Focite module was used to calculate the molecular dynamics of gas diffusion, and the mean azimuth shift curve of CO2 and CH4 gases in the non-stick coal large molecular structure was obtained. The root mean square shift (MSD) formula is as follows [21]:
where ri(t) and ri(0) are the position vectors at t time and initial time of the i-th gas molecule, respectively, in ps; Nt is the number of molecular dynamics steps; and t0 is the initial time.
The diffusion coefficient of gas molecules in a coal macromolecular model can be obtained via the root-mean-square displacement curve and Einstein method, in which the formula of the Einstein method is as follows [22]:
where D is the gas diffusion coefficient, in m2/s.
Through linear fitting of the gas mean azimuth shift curve in the coal molecular model, the slope can be obtained as , as shown in Equation (3), and the diffusion coefficient can be simplified as shown in Equation (4) [21]:
3. Materials and Methods
3.1. Influence of Temperature on Diffusion Performance
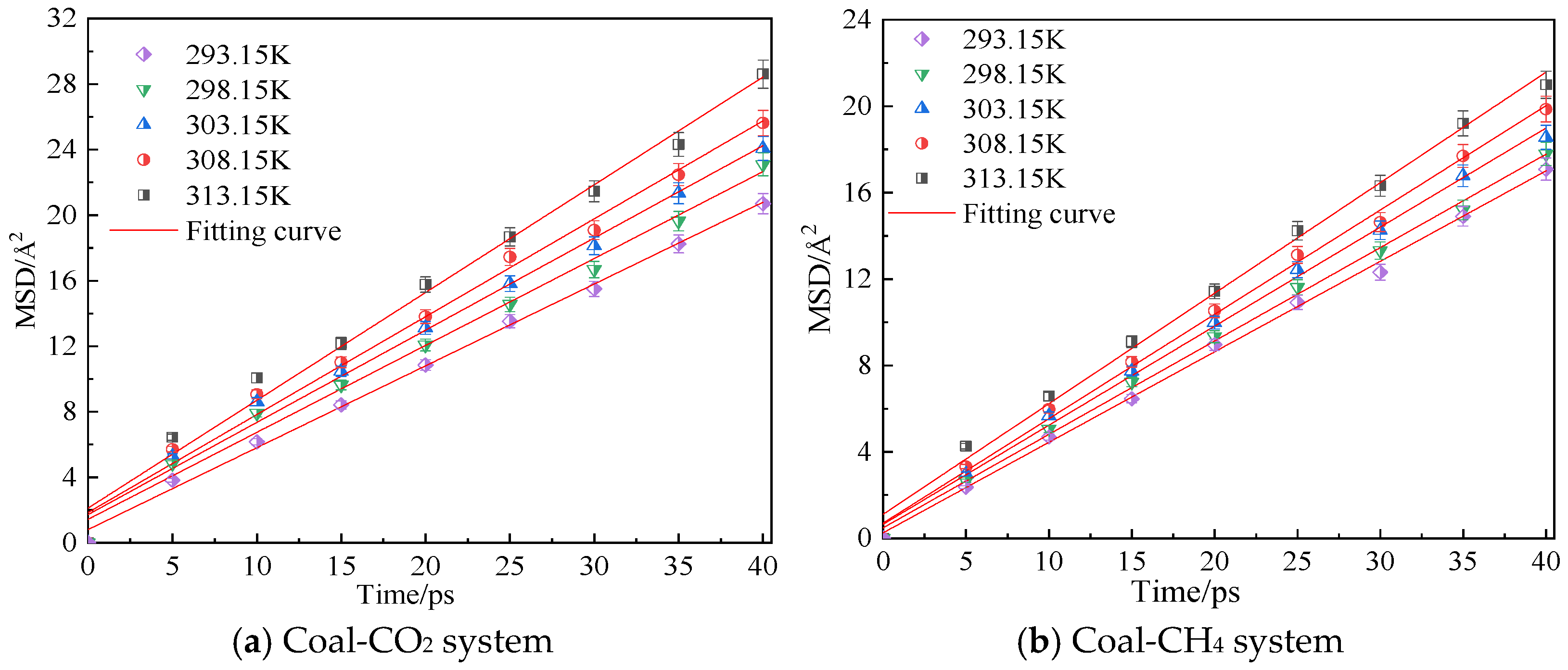
Figure 5 shows the mean azimuth shift curves of CO2 and CH4 at an adsorption pressure of 5 MPa and temperatures of 293.15 K, 298.15 K, 303.15 K, 308.15 K and 313.15 K. Through linear fitting of the mean azimuth shift curve, the diffusion coefficients of CO2 and CH4 at different temperatures can be obtained, as shown in Table 1. The simulation results show that the diffusion coefficients of CO2 and CH4 in the coal samples gradually increase with an increase in temperature. Under the same conditions, the diffusion coefficient of CH4 is smaller than that of CO2. The reason is that the kinetic energy of gas molecules increases with an increase in temperature, so the movement rate in the pores of coal body increases, which is conducive to the diffusion of gas molecules. The resistance to diffusion is smaller, so a high temperature can promote the diffusion rate of gas in coal.
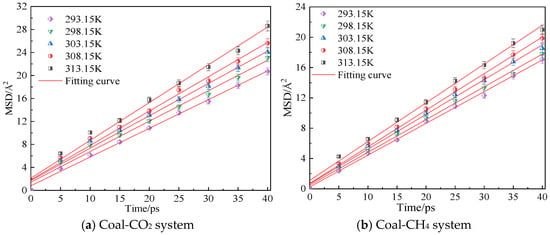
Figure 5.
Mean square displacement curve of CO2/CH4 at different temperatures.

Table 1.
Diffusivity of CO2/CH4 at different temperatures of coal samples.
To study the effect of temperature on gas diffusion, the diffusion activation energy of gas was calculated according to the Arrhenius equation, which is expressed as follows [22]:
where D0 refers to the pre-factor, in m2/s, and ED is the diffusion activation energy, in kJ/mol.
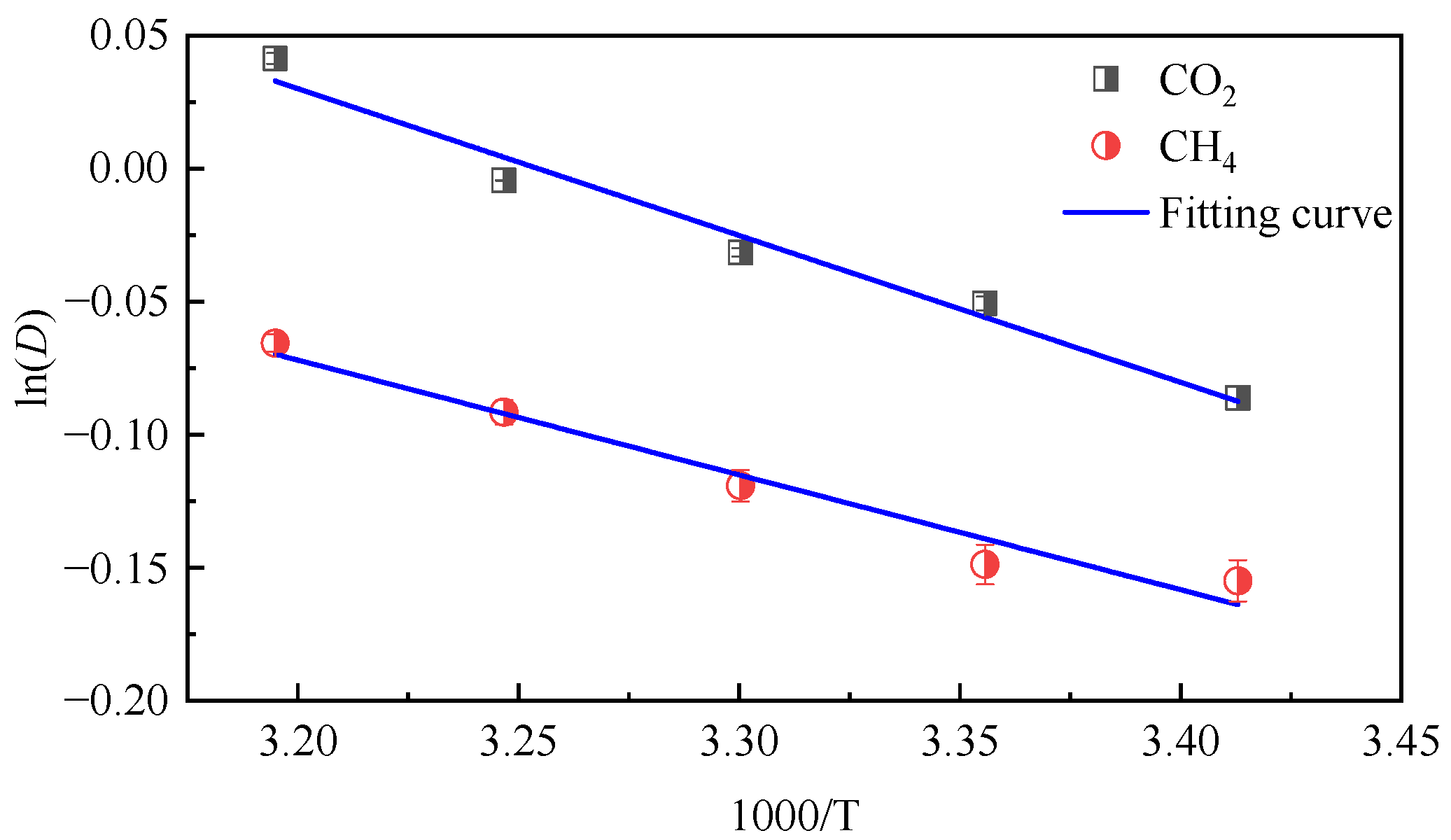
Using the diffusion coefficient calculated above, logarithm was taken on both sides of Equation (5), lnD and 1000/T curves were drawn, and the value of diffusion activation energy could be calculated through fitting. The fitting graph is shown in Figure 6.
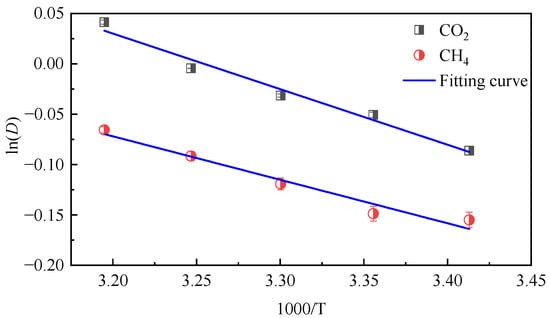
Figure 6.
lnD of CO2/CH4 gas in coal molecules as a function of 1/T.
The fitting results show that the diffusion activation energy of CO2 is 4.57 kJ·mol−1 and that of CH4 is 3.56 kJ·mol−1, and the activation energy of CO2 is greater than that of CH4 because the molecular diameter of CO2 is smaller than that of CH4, which is more favorable for diffusion in micropores. It also shows that the system of CO2 and coal molecules is more dependent on temperature.
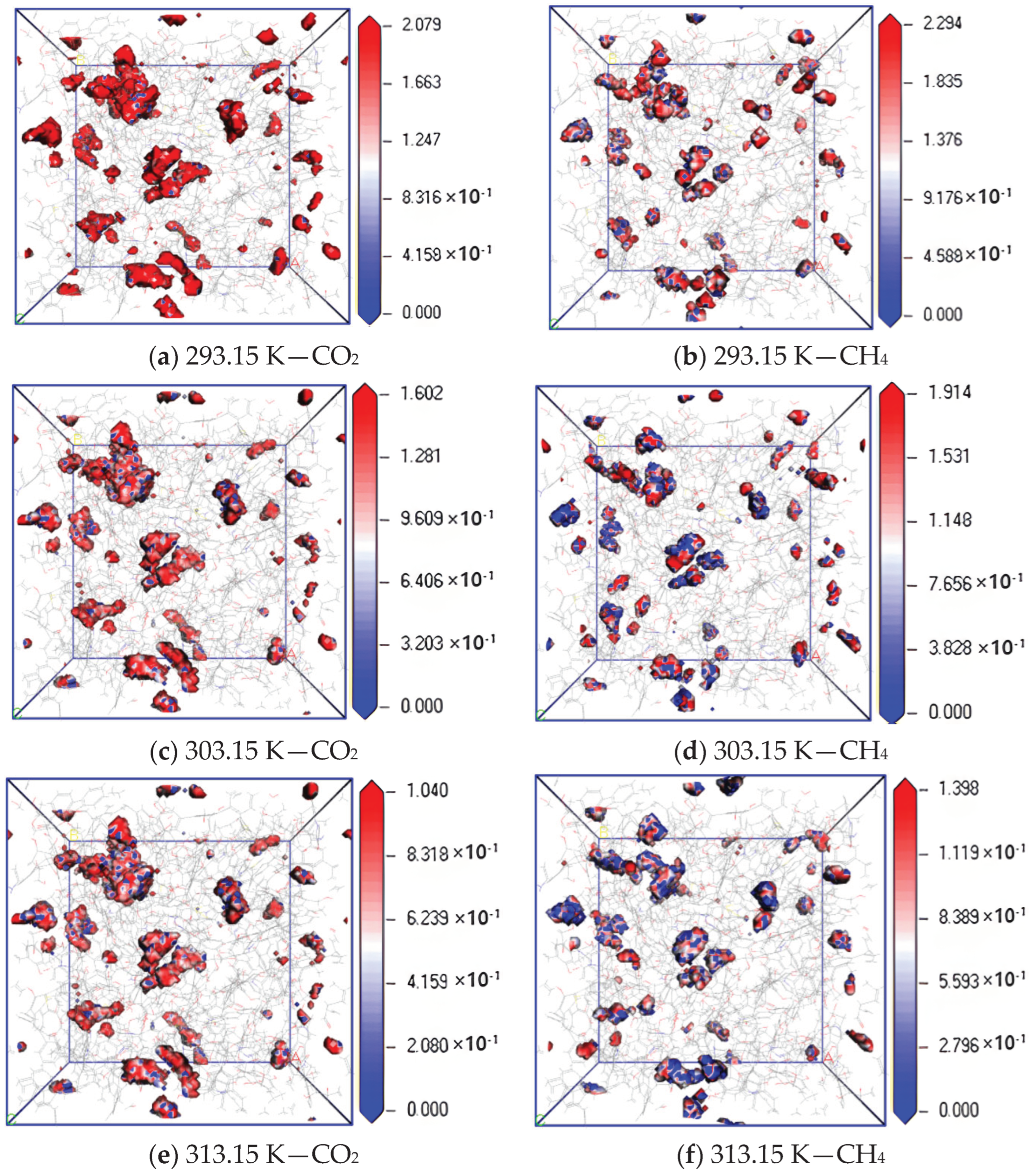
To further study the diffusion mechanism of CO2 and CH4 in coal, the equipotential surface diagram of CO2 and CH4 at different temperatures with pressure of 5 MPa was obtained according to the trajectory file obtained via simulation calculation, as shown in Figure 7. The absolute value of equipotential value reflects the density of molecular distribution, and a lower equipotential value indicates a wider probability distribution, that is, the diffusion effect is better [22,23].
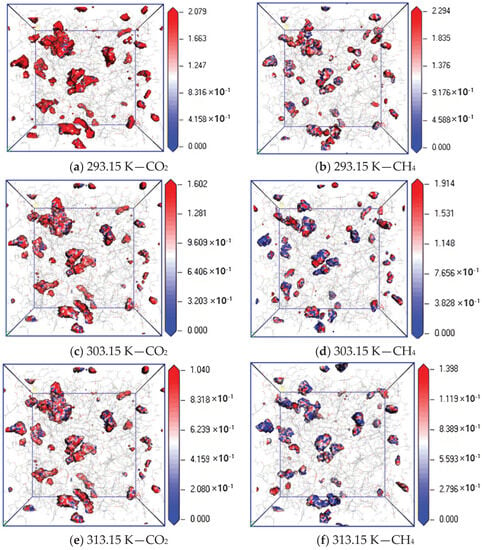
Figure 7.
Isopotential value distribution of CO2 and CH4 at different temperatures.
The simulation results show that when the adsorption temperature is 293.15 K, 303.15 K and 313.15 K, the maximum equipotential value of CO2 gas is 2.079, 1.602 and 1.040, and that of CH4 is 2.294, 1.914 and 1.398, respectively. The equipotential value of CH4 is greater than that of CO2. The maximum equipotential values of CO2 and CH4 gases in coal gradually decrease with an increase in temperature, which indicates that the two gases can be more widely and evenly distributed in the pores on the surface of coal molecules. The increase in temperature increases the movement frequency of nuclei and electrons inside gas molecules, which leads to the acceleration of gas diffusion rate in coal.
3.2. Influence of Pressure on Diffusion Performance
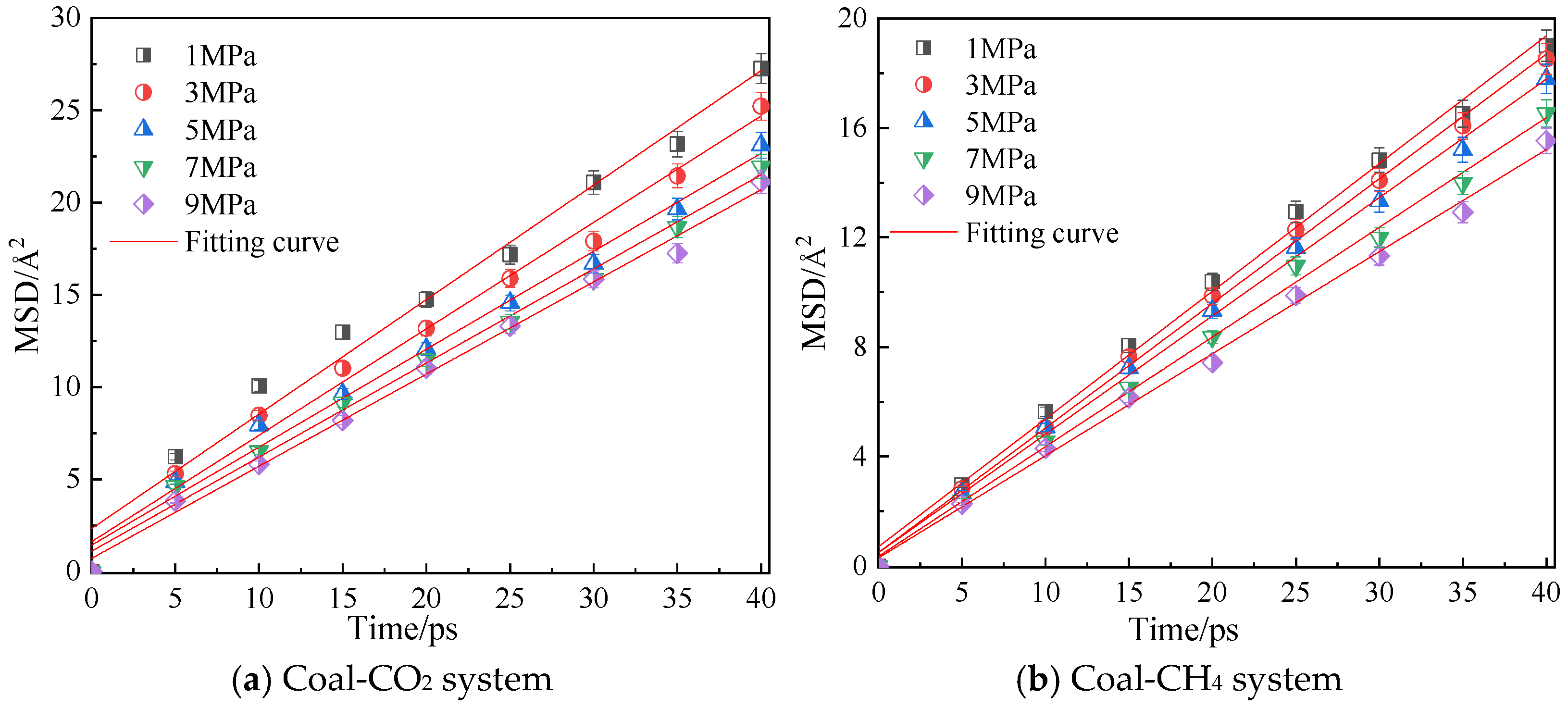
Figure 8 shows the mean azimuth shift curves of CO2 and CH4 when the adsorption temperature is 298.15 K and the adsorption pressure is 1 MPa, 3 MPa, 5 MPa, 7 MPa and 9 MPa. Through linear fitting of the mean azimuth shift curve, the diffusion coefficients of CO2 and CH4 under different pressures are shown in Table 2. The simulation results show that the diffusion coefficients of CO2 and CH4 gradually decrease with an increase in pressure, indicating that a high pressure is not conducive to the diffusion of gas in coal, because with an increase in pressure, the average free path of the two gas molecules decreases [22], and they are more likely to collide with the surface of coal, thus inhibiting the diffusion of gas in coal. The diffusion coefficient of CO2 is always greater than that of CH4 under the same pressure.
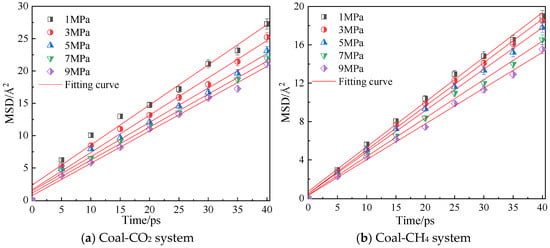
Figure 8.
Mean square displacement curve of CO2/CH4 at different pressure.

Table 2.
Diffusivity of CO2/CH4 under different pressures of coal samples.
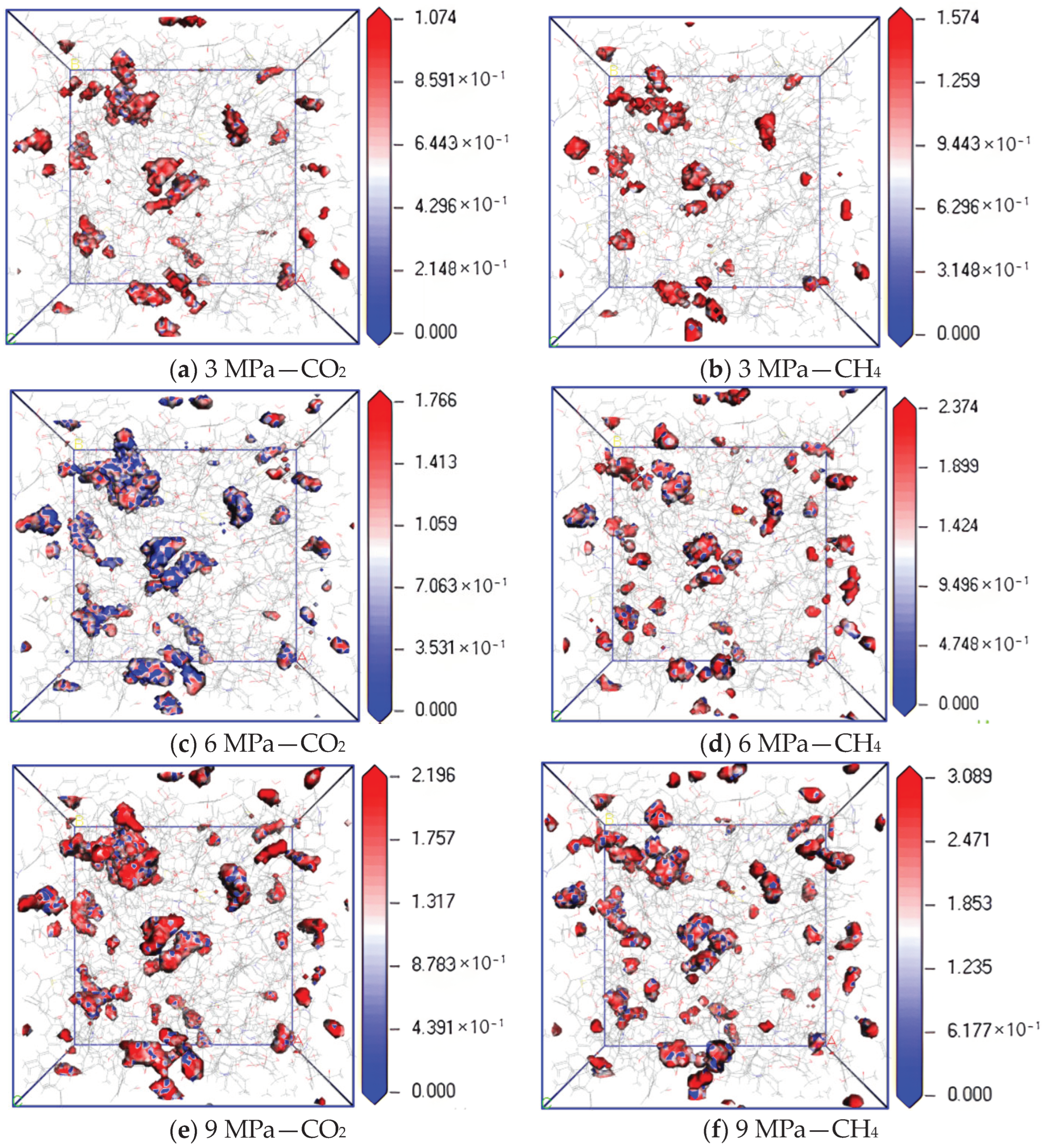
Figure 9 shows the isopotential surface diagram of CO2 and CH4 changing with adsorption pressure at a temperature of 298.15 K and pressures of 3 MPa, 6 MPa and 9 MPa. It can be found from the simulation results that the equipotential values of CO2 and CH4 gradually increase with an increase in pressure. The maximum equipotential values of CO2 at 3 MPa, 6 MPa and 9 MPa are 1.074, 1.766 and 2.196, respectively, and the maximum equipotential values of CH4 are 1.574, 2.374 and 3.089, respectively, indicating that the higher the pressure, the higher the maximum equipotential value. The greater the adsorption capacity of two gases, the stronger the interaction energy between molecules, and the greater the binding degree of gas molecular diffusion. Under the same pressure condition, the equipotential value of CH4 is higher than that of CO2, indicating that with an increase in pressure, the filling ability of CH4 in the micropores on the coal surface is stronger than that of CO2, which makes the interaction force between CH4 molecules in the micropores stronger, resulting in a greater degree of diffusion obstruction.
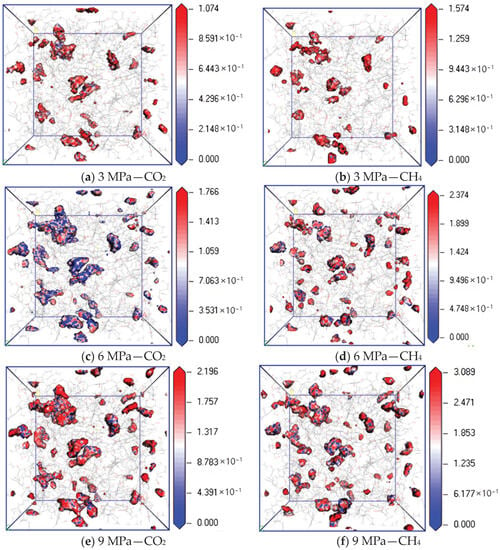
Figure 9.
Isopotential value distribution of CO2 and CH4 at different pressure.
3.3. Influence of Moisture Content on Diffusion Performance
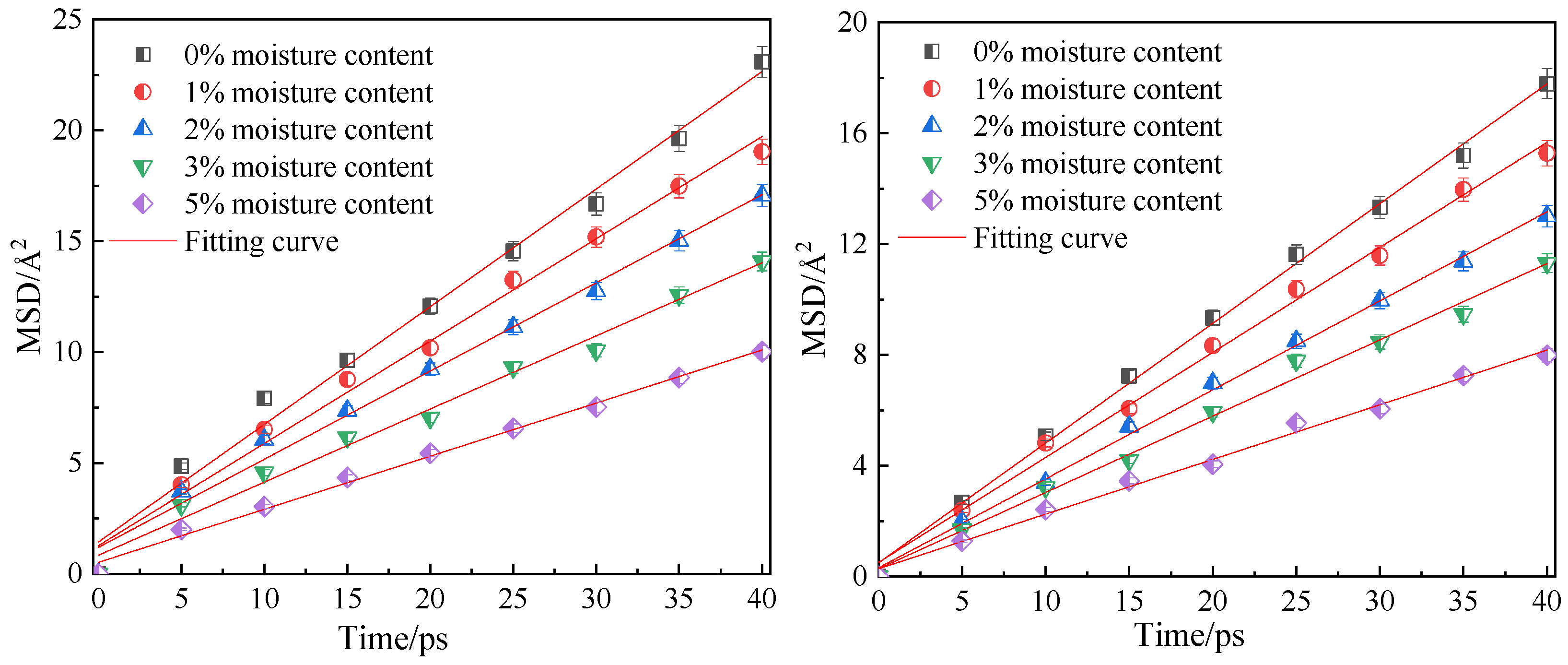
Figure 10 shows the fitting curves of the mean azimuth shift of CO2 and CH4 when the adsorption temperature is 298.15 K; the adsorption pressure is 5 MPa; and the water content is 0%, 1%, 2%, 3% and 5%. Through linear fitting of the mean azimuth shift curve, the diffusion coefficients of CO2 and CH4 under different water content conditions were obtained, as shown in Table 3. The simulation results show that with an increase in water content in the coal molecular model, the diffusion coefficients of the two gas molecules CO2 and CH4 decrease significantly, indicating that water is not conducive to the diffusion of gas in the coal seam. This is because the increase in water content not only occupies the pore space and blocks the diffusion channel of gas in the coal, but also the coal matrix will expand and deform after absorbing water. The effective channel is narrowed and the collision chance between the gas and the hole wall increases, so diffusion is blocked. In addition, water will form ice-like clusters when adsorbed at the adsorption sites on the coal surface [22,24], which can make the micropores clogged.
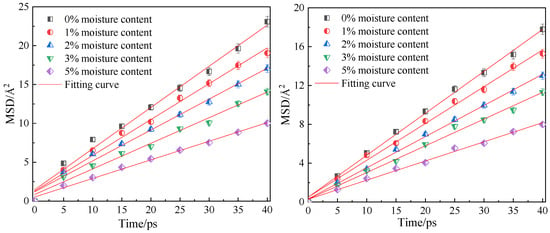
Figure 10.
Mean square displacement curve of CO2/CH4 at different moisture content.

Table 3.
Diffusivity of CO2/CH4 under different water content of coal samples.
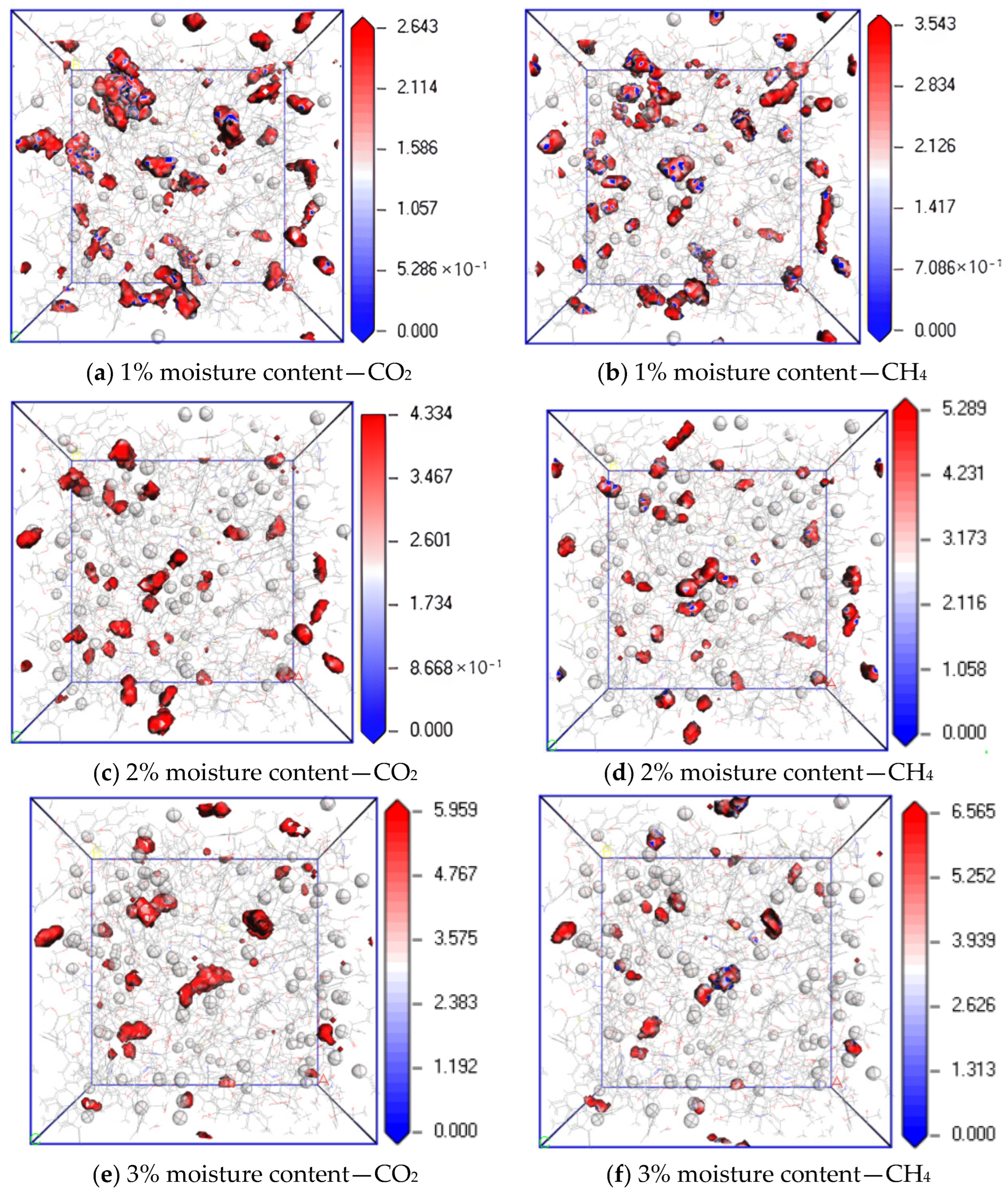
Figure 11 shows the equipotential surface diagram of CO2 and CH4 when the temperature is 298.15 K; the pressure is 5 MPa; and the water content is 1%, 2% and 4%. It can be found from the simulation results that when the water content is 1%, 3% and 5%, the maximum equipotential values of CO2 are 1.074, 1.766 and 2.196, and the maximum equipotential values of CH4 are 1.574, 2.374 and 3.089, respectively. The equipotential values of CO2 and CH4 gradually increase with an increase in water content. The reason is that an increase in water content in coal makes the H bond between water molecules stronger, which promotes the interaction force between water molecules and the induction force on CO2 and CH4 molecules. As a result, the potential energy of the system increases with an increase in water content, resulting in the obstruction of gas diffusion. Under the same water condition, the equipotential value of CH4 is higher than that of CO2 because of the hydration of CH4 by H2O [25].
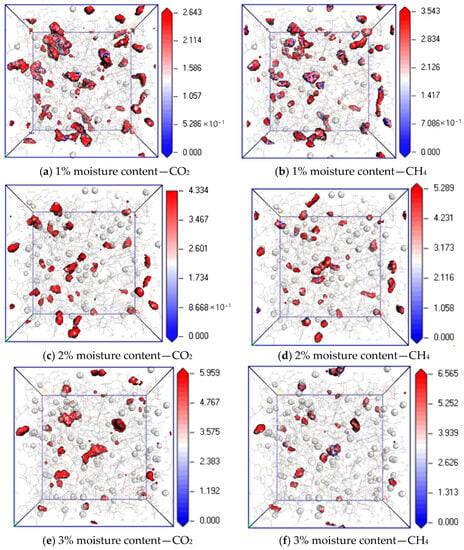
Figure 11.
Isopotential value distribution of CO2 and CH4 at different moisture content.
4. Conclusions
By means of molecular simulation, this paper studies the pore structure characteristics of CO2 and CH4 gas using a macromolecular structure model of non-cohesive coal in Chicheng Coal Mine, and investigates the influence of different temperatures, pressures and water contents on the diffusion performance of CO2 and CH4 gas adsorbed by coal and the microscopic mechanism. The main conclusions are as follows:
- (1)
- In the dry-mode macromolecular model, the diffusion coefficients of CO2 and CH4 gradually increase with an increase in temperature, and a high temperature is conducive to gas diffusion. Under the same conditions, the diffusion coefficient of CH4 is lower than that of CO2, and the diffusion activation energy of CO2 is 4.57 kJ·mol−1, while that of CH4 is 3.56 kJ·mol−1.
- (2)
- In the dry-mode macromolecular model, with an increase in pressure, the diffusion coefficients of CO2 and CH4 gradually decrease, and the equipotential values of CO2 and CH4 gradually increase, and a high pressure is not conducive to the diffusion of gas in the coal macromolecular model.
- (3)
- In the water-containing coal macromolecular model, with an increase in water content, the diffusion coefficients of CO2 and CH4 significantly decrease, and the equipotential values of CO2 and CH4 gradually increase, and water is not conducive to the diffusion of gas in the coal macromolecular model.
Author Contributions
B.J. and J.Y. led the drafting of the manuscript, production of figures and incorporation of co-author comments. B.L. and J.Z. contributed to the text and commented on the revisions. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by the Natural Science Foundation of Liaoning Province, No. 2020−MS−304. All parties included in this section have consented to the acknowledgement.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, D. Study on Adsorption and Diffusion Behavior of Soft and Hard Coal Gas Based on Molecular Simulation. Master’s Thesis, Henan Polytechnic University, Jiaozuo, China, January 2020. [Google Scholar]
- Zhang, K. Molecular Simulation of Methane Desorption and Diffusion during Coalbed Methane Drainage and Production. Master’s Thesis, North University of China, Taiyuan, China, 2020. [Google Scholar]
- Clarkson, C.R.; Bustin, R.M.J.F. The effect of pore structure and gas pressure upon the transport properties of coal: A laboratory and modeling study. 2. Adsorption rate modeling. Fuel 1999, 78, 1345–1362. [Google Scholar] [CrossRef]
- Li, B. Study on Adsorption-Deformation-Percolation-Diffusion Characteristics of CO2/CH4/N2 in Coal of Different Rank. Ph.D. Thesis, Liaoning Technical University, Fuxin, China, May 2022. [Google Scholar]
- Zhang, M.; Wei, C.H.; Liu, S.Y.; Liu, Z.Y.; Zhang, Z. Experiment and model study on CO2 adsorption-desorption diffusion in anthracite particles. Exp. Mech. 2021, 36, 753–761. [Google Scholar]
- Dai, X. Adsorption and Diffusion Characteristics of CH4/CO2 in Shale Minerals and Geological Risk Assessment of Shale Gas Exploitation. Master’s Thesis, Chongqing University, Chongqing, China, June 2022. [Google Scholar]
- Liu, J.; Liu, H.; Zhang, K.; Xue, S. Diffusion characteristics of CO2 and CH4 in CO2-ECBM process of low permeability coal seam. Coal Sci. Technol. 2022, 26, 1–11. [Google Scholar] [CrossRef]
- Li, K. Study on the Diffusion and Adsorption Characteristics of CO2 in Gangue Filling Materials in Mined-Out Area of Coal Mine. Master’s Thesis, China University of Mining and Technology, Xuzhou, China, June 2021. [Google Scholar]
- You, X.; He, M.; Cao, X.; Lyu, X.; Li, L. Structure and dynamics of water adsorbed on the lignite surface: Molecular dynamics simulation. Physicochem. Probl. Miner. Process. 2019, 55, 10–20. [Google Scholar]
- Hu, H.; Li, X.; Fang, Z.; Wei, N.; Li, Q. Small-molecule gas sorption and diffusion in coal: Molecular simulation. Energy 2010, 35, 2939–2944. [Google Scholar] [CrossRef]
- An, F.; Jia, H.; Feng, Y. Effect of stress, concentration and temperature on gas diffusion coefficient of coal measured through a direct method and its model application. Fuel 2022, 312, 122991. [Google Scholar] [CrossRef]
- Xu, H.; Tang, D.; Zhao, J.; Li, S.; Tao, S. A new laboratory method for accurate measurement of the methane diffusion coefficient and its influencing factors in the coal matrix. Fuel 2015, 158, 239–247. [Google Scholar] [CrossRef]
- Yu, S.; Bo, J.; Meijun, Q. Molecular Dynamic Simulation of Self- and Transport Diffusion for CO2/CH4/N2 in Low-Rank Coal Vitrinite. Fuels 2018, 32, 3085–3096. [Google Scholar] [CrossRef]
- Liu, T.; Lin, B.; Fu, X.; Gao, Y.; Kong, J.; Zhao, Y.; Song, H. Experimental study on gas diffusion dynamics in fractured coal: A better understanding of gas migration in in-situ coal seam. Energy 2020, 195, 10. [Google Scholar] [CrossRef]
- Keshavarz, A.; Sakurovs, R.; Grigore, M.; Sayyafzadeh, M. Effect of maceral composition and coal rank on gas diffusion in Australian coals. Int. J. Coal Geol. 2017, 173, 65–75. [Google Scholar] [CrossRef]
- Hu, S.B.; Li, X.C.; Wang, E.Y. Experimental and Numerical Study on Scale Effects of Gas Emission from Coal Particles. Transp. Porous Media 2016, 114, 133–147. [Google Scholar] [CrossRef]
- Hu, H.X.; Du, L.; Xing, Y.F.; Li, X.C. Detailed study on self- and multicomponent diffusion of CO2-CH4 gas mixture in coal by molecular simulation. Fuel 2017, 187, 220–228. [Google Scholar] [CrossRef]
- Yan, J.X.; Jia, B.S.; Liu, B.G.; Zhang, J.Y. Simulation Study on Molecular Adsorption of Coal in Chicheng Coal Mine. Molecules 2023, 28, 3302. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Microstructure Evolution of Structural Coal and Its Influence on Kinetic Characteristics of Gas Adsorption and Desorption. Ph.D. Thesis, China University of Mining and Technology, Xuzhou, China, December 2020. [Google Scholar]
- Xiang, J.H.; Zeng, F.G.; Liang, H.Z.; Sun, B.L.; Zhang, L.; Li, M.F.; Jia, J.B. Model construction of the macromolecular structure of Yanzhou Coal and its molecular simulation. J. Fuel Chem. Technol. 2011, 39, 481–488. [Google Scholar] [CrossRef]
- Li, S.; Bai, Y.; Lin, H.; Yan, M.; Long, H.; Guo, D. Effect of N2/CO2 injection pressure on methane desorption in coal rocks containing gas. Nat. Gas Ind. 2021, 41, 80–89. [Google Scholar]
- Han, J. Molecular Simulation of Gas Adsorption, Desorption and Diffusion in Water-Bearing Coal Seams. Ph.D. Thesis, Southwest Petroleum University, Chengdu, China, June 2015. [Google Scholar]
- He, P. Molecular Simulation of Prediction Models for Adsorption and Diffusion Properties of Guest Molecules in Porous Materials. Ph.D. Thesis, Beijing University of Chemical Technology, Beijing, China, June 2012. [Google Scholar]
- Zhang, B. Molecular Simulation of Adsorption and Diffusion of CH4 on Kaolinite Surface of Coal Measures. Ph.D. Thesis, Taiyuan University of Technology, Taiyuan, China, June 2018. [Google Scholar]
- Xiang, J. Structural Characteristics of Different Coal Grades and Molecular Simulation of Interactions between Coal and CH4, CO2 and H2O. Ph.D. Thesis, Taiyuan University of Technology, Taiyuan, China, June 2013. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).