Sulforaphane’s Multifaceted Potential: From Neuroprotection to Anticancer Action
Abstract
1. Origin and Discovery
2. SFN as a Neuroprotective Agent
3. SFN as an Anticancer Agent
4. Chemoprotectant Properties of SFN
5. Effects of SFN on Tumors, Chemotherapy, Radiation Therapy, and Cardiotoxicity
6. SFN on Metastasis
7. SFN Bioavailability and Pharmacokinetics
8. Ongoing and Completed Clinical Trials on SFN
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fahey, J.W.; Talalay, P. Antioxidant functions of sulforaphane: A potent inducer of Phase II detoxication enzymes. Food Chem. Toxicol. 1999, 37, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.K.; Elmore, S.; Methven, L. Flavour Development, Analysis and Perception in Food and Beverages; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Zhang, Y.; Tang, L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol. Sin. 2007, 28, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Kushad, M.M.; Brown, A.F.; Kurilich, A.C.; Juvik, J.A.; Klein, B.P.; Wallig, M.A.; Jeffery, E.H. Variation of glucosinolates in vegetable crops of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1541–1548. [Google Scholar] [CrossRef]
- Shapiro, T.A.; Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: Metabolism and excretion in humans. Cancer Epidemiol. Biomark. Prev. 2001, 10, 501–508. [Google Scholar]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane: Translational research from laboratory bench to clinic. Nutr. Rev. 2013, 71, 709–726. [Google Scholar] [CrossRef]
- Johansson, N.L.; Pavia, C.S.; Chiao, J.W. Growth inhibition of a spectrum of bacterial and fungal pathogens by sulforaphane, an isothiocyanate product found in broccoli and other cruciferous vegetables. Planta Med. 2008, 74, 747–750. [Google Scholar] [CrossRef]
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a Potential Protective Phytochemical against Neurodegenerative Diseases. Oxidative Med. Cell. Longev. 2013, 415078. [Google Scholar] [CrossRef]
- Amjad, A.I.; Parikh, R.A.; Appleman, L.J.; Hahm, E.R.; Singh, K.; Singh, S.V. Broccoli-Derived Sulforaphane and Chemoprevention of Prostate Cancer: From Bench to Bedside. Curr. Pharmacol. Rep. 2015, 1, 382–390. [Google Scholar] [CrossRef]
- Greaney, A.J.; Maier, N.K.; Leppla, S.H.; Moayeri, M. Sulforaphane inhibits multiple inflammasomes through an Nrf2-independent mechanism. J. Leukoc. Biol. 2016, 99, 189–199. [Google Scholar] [CrossRef]
- Sikdar, S.; Papadopoulou, M.; Dubois, J. What Do We Know About Sulforaphane Protection against Photoaging? J. Cosmet. Dermatol. 2016, 15, 72–77. [Google Scholar] [CrossRef][Green Version]
- Bhat, S.A.; Kamal, M.A.; Yarla, N.S.; Ashraf, G.M. Synopsis on Managment Strategies for Neurodegenerative Disorders: Challenges from Bench to Bedside in Successful Drug Discovery and Development. Curr. Top. Med. Chem. 2017, 17, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Ladak, Z.; Garcia, E.; Yoon, J.; Landry, T.; Armstrong, E.A.; Yager, J.Y.; Persad, S. Sulforaphane (SFA) protects neuronal cells from oxygen & glucose deprivation (OGD). PLoS ONE 2021, 16, e0248777. [Google Scholar] [CrossRef]
- Kraft, A.D.; Johnson, D.A.; Johnson, J.A. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J. Neurosci. 2004, 24, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Sandouka, S.; Shekh-Ahmad, T. Induction of the Nrf2 Pathway by Sulforaphane Is Neuroprotective in a Rat Temporal Lobe Epilepsy Model. Antioxidants 2021, 10, 1702. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, B.; Wang, X.; Wu, L.; Yang, Y.; Cheng, X.; Hu, Z.; Cai, X.; Yang, J.; Sun, X.; et al. Sulforaphane protects against rotenone-induced neurotoxicity in vivo: Involvement of the mTOR, Nrf2, and autophagy pathways. Sci. Rep. 2016, 6, 32206. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, L.; Li, X.; Zhang, L.; Lv, J.; Guo, X.; Chen, H.; Zhao, T. Neuroprotective effects of an Nrf2 agonist on high glucose-induced damage in HT22 cells. Biol. Res. 2019, 52, 53. [Google Scholar] [CrossRef]
- Mizuno, K.; Kume, T.; Muto, C.; Takada-Takatori, Y.; Izumi, Y.; Sugimoto, H.; Akaike, A. Glutathione biosynthesis via activation of the nuclear factor E2-related factor 2 (Nrf2)—Antioxidant-response element (ARE) pathway is essential for neuroprotective effects of sulforaphane and 6-(methylsulfinyl) hexyl isothiocyanate. J. Pharmacol. Sci. 2011, 115, 320–328. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, J.; Chang, N. Epigenetic modification of Nrf2 by sulforaphane increases the antioxidative and anti-inflammatory capacity in a cellular model of Alzheimer’s disease. Eur. J. Pharmacol. 2018, 824, 1–10. [Google Scholar] [CrossRef]
- Zhao, X.; Wen, L.; Dong, M.; Lu, X. Sulforaphane activates the cerebral vascular Nrf2-ARE pathway and suppresses inflammation to attenuate cerebral vasospasm in rat with subarachnoid hemorrhage. Brain Res. 2016, 1653, 1–7. [Google Scholar] [CrossRef]
- Jazwa, A.; Rojo, A.I.; Innamorato, N.G.; Hesse, M.; Fernández-Ruiz, J.; Cuadrado, A. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid. Redox Signal. 2011, 14, 2347–2360. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Yan, W.; Chen, S.; Sun, C.R.; Zhang, J.M. The role of Nrf2 signaling in the regulation of antioxidants and detoxifying enzymes after traumatic brain injury in rats and mice. Acta Pharmacol. Sin. 2010, 31, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Morroni, F.; Sita, G.; Djemil, A.; D’Amico, M.; Pruccoli, L.; Cantelli-Forti, G.; Hrelia, P.; Tarozzi, A. Comparison of Adaptive Neuroprotective Mechanisms of Sulforaphane and its Interconversion Product Erucin in in Vitro and in Vivo Models of Parkinson’s Disease. J. Agric. Food Chem. 2018, 66, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Benedict, A.L.; Mountney, A.; Hurtado, A.; Bryan, K.E.; Schnaar, R.L.; Dinkova-Kostova, A.T.; Talalay, P. Neuroprotective effects of sulforaphane after contusive spinal cord injury. J. Neurotrauma 2012, 29, 2576–2586. [Google Scholar] [CrossRef]
- Dash, P.K.; Zhao, J.; Orsi, S.A.; Zhang, M.; Moore, A.N. Sulforaphane improves cognitive function administered following traumatic brain injury. Neurosci. Lett. 2009, 460, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Trio, P.Z.; Fujisaki, S.; Tanigawa, S.; Hisanaga, A.; Sakao, K.; Hou, D.X. DNA Microarray Highlights Nrf2-Mediated Neuron Protection Targeted by Wasabi-Derived Isothiocyanates in IMR-32 Cells. Gene Regul. Syst. Biol. 2016, 10, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Kobori, N.; Aronowski, J.; Dash, P.K. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci. Lett. 2006, 393, 108–112. [Google Scholar] [CrossRef]
- Ma, L.L.; Xing, G.P.; Yu, Y.; Liang, H.; Yu, T.X.; Zheng, W.H.; Lai, T.B. Sulforaphane exerts neuroprotective effects via suppression of the inflammatory response in a rat model of focal cerebral ischemia. Int. J. Clin. Exp. Med. 2015, 8, 17811–17817. [Google Scholar]
- Imai, T.; Matsubara, H.; Hara, H. Potential therapeutic effects of Nrf2 activators on intracranial hemorrhage. J. Cereb. Blood Flow. Metab. 2021, 41, 1483–1500. [Google Scholar] [CrossRef]
- Pan, J.; Wang, R.; Pei, Y.; Wang, D.; Wu, N.; Ji, Y.; Tang, Q.; Liu, L.; Cheng, K.; Liu, Q.; et al. Sulforaphane alleviated vascular remodeling in hypoxic pulmonary hypertension via inhibiting inflammation and oxidative stress. J. Nutr. Biochem. 2023, 111, 109182. [Google Scholar] [CrossRef]
- Park, H.M.; Kim, J.A.; Kwak, M.K. Protection against amyloid beta cytotoxicity by sulforaphane: Role of the proteasome. Arch. Pharm. Res. 2009, 32, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Malaguti, M.; Rizzo, B.; Barbalace, M.C.; Fabbri, D.; Hrelia, S. Neuroprotective effect of sulforaphane against methylglyoxal cytotoxicity. Chem. Res. Toxicol. 2015, 28, 1234–1245. [Google Scholar] [CrossRef]
- Qin, S.; Yang, C.; Huang, W.; Du, S.; Mai, H.; Xiao, J.; Lü, T. Sulforaphane attenuates microglia-mediated neuronal necroptosis through down-regulation of MAPK/NF-κB signaling pathways in LPS-activated BV-2 microglia. Pharmacol. Res. 2018, 133, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rabaza, V.; Cabrera-Pastor, A.; Taoro-Gonzalez, L.; Gonzalez-Usano, A.; Agusti, A.; Balzano, T.; Llansola, M.; Felipo, V. Neuroinflammation increases GABAergic tone and impairs cognitive and motor function in hyperammonemia by increasing GAT-3 membrane expression. Reversal by sulforaphane by promoting M2 polarization of microglia. J. Neuroinflammation 2016, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, L.; Chen, B.; Zheng, P.; He, Y.; Ding, Y.; Deng, Y.; Lu, X.; Guo, X.; Zhang, Y.; et al. DNA Demethylation Upregulated Nrf2 Expression in Alzheimer’s Disease Cellular Model. Front. Aging Neurosci. 2015, 7, 244. [Google Scholar] [CrossRef] [PubMed]
- Schachtele, S.J.; Hu, S.; Lokensgard, J.R. Modulation of experimental herpes encephalitis-associated neurotoxicity through sulforaphane treatment. PLoS ONE 2012, 7, e36216. [Google Scholar] [CrossRef] [PubMed]
- Klomparens, E.A.; Ding, Y. The neuroprotective mechanisms and effects of sulforaphane. Brain Circ. 2019, 5, 74–83. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Wu, Y.; Sarkissyan, M.; Vadgama, J.V. Epigenetics in breast and prostate cancer. Methods Mol. Biol. 2015, 1238, 425–466. [Google Scholar] [CrossRef]
- Chu, D.T.; Ngo, A.D.; Wu, C.C. Epigenetics in cancer development, diagnosis and therapy. Prog. Mol. Biol. Transl. Sci. 2023, 198, 73–92. [Google Scholar] [CrossRef]
- Meeran, S.M.; Ahmed, A.; Tollefsbol, T.O. Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin. Epigenetics 2010, 1, 101–116. [Google Scholar] [CrossRef]
- Paul, B.; Li, Y.; Tollefsbol, T.O. The Effects of Combinatorial Genistein and Sulforaphane in Breast Tumor Inhibition: Role in Epigenetic Regulation. Int. J. Mol. Sci. 2018, 19, 1754. [Google Scholar] [CrossRef] [PubMed]
- Cao, C. HDAC5-LSD1 axis regulates antineoplastic effect of natural HDAC inhibitor sulforaphane in human breast cancer cells. Int. J. Cancer 2018, 143, 1388–1401. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Liu, Z.; Wood, R.J. Association between histone deacetylase activity and vitamin D-dependent gene expressions in relation to sulforaphane in human colorectal cancer cells. J. Sci. Food Agric. 2021, 101, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.D.; Hsu, A.; Yu, Z.; Dashwood, R.H.; Ho, E. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells. Mol. Nutr. Food Res. 2011, 55, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Liu, Z.; Wood, R.J. Histone deacetylase activity and vitamin D-dependent gene expressions in relation to sulforaphane in human breast cancer cells. J. Food Biochem. 2020, 44, e13114. [Google Scholar] [CrossRef]
- Smith, W.S. Pathophysiology of focal cerebral ischemia: A therapeutic perspective. J. Vasc. Interv. Radiol. 2004, 15, S3–S12. [Google Scholar] [CrossRef]
- Traystman, R.J. Animal models of focal and global cerebral ischemia. ILAR J. 2003, 44, 85–95. [Google Scholar] [CrossRef]
- Li, Q.; Fadoul, G.; Ikonomovic, M.; Yang, T.; Zhang, F. Sulforaphane promotes white matter plasticity and improves long-term neurological outcomes after ischemic stroke via the Nrf2 pathway. Free Radic. Biol. Med. 2022, 193, 292–303. [Google Scholar] [CrossRef]
- Franke, M.; Bieber, M.; Kraft, P.; Weber, A.N.R.; Stoll, G.; Schuhmann, M.K. The NLRP3 inflammasome drives inflammation in ischemia/reperfusion injury after transient middle cerebral artery occlusion in mice. Brain Behav. Immun. 2021, 92, 223–233. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-Inflammatory Effect of Sulforaphane on LPS-Activated Microglia Potentially through JNK/AP-1/NF-κB Inhibition and Nrf2/HO-1 Activation. Cells 2019, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Lee, J.H.; Gaire, B.P.; Kim, S.Y. Sulforaphane Inhibits MGO-AGE-Mediated Neuroinflammation by Suppressing NF-κB, MAPK, and AGE-RAGE Signaling Pathways in Microglial Cells. Antioxidants 2020, 9, 792. [Google Scholar] [CrossRef]
- Wang, Z.C.; Chen, Q.; Wang, J.; Yu, L.S.; Chen, L.W. Sulforaphane mitigates LPS-induced neuroinflammation through modulation of Cezanne/NF-κB signalling. Life Sci. 2020, 262, 118519. [Google Scholar] [CrossRef]
- Kapoor, K.; Bhandare, A.M.; Farnham, M.M.; Pilowsky, P.M. Alerted microglia and the sympathetic nervous system: A novel form of microglia in the development of hypertension. Respir. Physiol. Neurobiol. 2016, 226, 51–62. [Google Scholar] [CrossRef]
- von Bernhardi, R.; Heredia, F.; Salgado, N.; Muñoz, P. Microglia Function in the Normal Brain. Adv. Exp. Med. Biol. 2016, 949, 67–92. [Google Scholar] [CrossRef]
- Caceres, J.A.; Goldstein, J.N. Intracranial hemorrhage. Emerg. Med. Clin. N. Am. 2012, 30, 771–794. [Google Scholar] [CrossRef]
- Yin, X.P.; Chen, Z.Y.; Zhou, J.; Wu, D.; Bao, B. Mechanisms underlying the perifocal neuroprotective effect of the Nrf2-ARE signaling pathway after intracranial hemorrhage. Drug Des. Devel Ther. 2015, 9, 5973–5986. [Google Scholar] [CrossRef]
- Zeng, H.; Trujillo, O.N.; Moyer, M.P.; Botnen, J.H. Prolonged sulforaphane treatment activates survival signaling in nontumorigenic NCM460 colon cells but apoptotic signaling in tumorigenic HCT116 colon cells. Nutr. Cancer 2011, 63, 248–255. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Q.; Li, N.; Xu, M.; Miyamoto, T.; Liu, J. Sulforaphane suppresses metastasis of triple-negative breast cancer cells by targeting the RAF/MEK/ERK pathway. NPJ Breast Cancer 2022, 8, 40. [Google Scholar] [CrossRef]
- Myzak, M.C.; Karplus, P.A.; Chung, F.L.; Dashwood, R.H. A novel mechanism of chemoprotection by sulforaphane: Inhibition of histone deacetylase. Cancer Res. 2004, 64, 5767–5774. [Google Scholar] [CrossRef] [PubMed]
- Cornblatt, B.S.; Ye, L.; Dinkova-Kostova, A.T.; Erb, M.; Fahey, J.W.; Singh, N.K.; Chen, M.S.; Stierer, T.; Garrett-Mayer, E.; Argani, P.; et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis 2007, 28, 1485–1490. [Google Scholar] [CrossRef]
- Janssen-Heininger, Y.M.; Mossman, B.T.; Heintz, N.H.; Forman, H.J.; Kalyanaraman, B.; Finkel, T.; Stamler, J.S.; Rhee, S.G.; van der Vliet, A. Redox-based regulation of signal transduction: Principles, pitfalls, and promises. Free Radic. Biol. Med. 2008, 45, 1–17. [Google Scholar] [CrossRef]
- Mimura, J.; Ema, M.; Sogawa, K.; Fujii-Kuriyama, Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes. Dev. 1999, 13, 20–25. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Zhang, R.; Miao, Q.W.; Zhu, C.X.; Zhao, Y.; Liu, L.; Yang, J.; An, L. Sulforaphane ameliorates neurobehavioral deficits and protects the brain from amyloid β deposits and peroxidation in mice with Alzheimer-like lesions. Am. J. Alzheimers Dis. Other Demen 2015, 30, 183–191. [Google Scholar] [CrossRef]
- Lee, S.; Choi, B.R.; Kim, J.; LaFerla, F.M.; Park, J.H.Y.; Han, J.S.; Lee, K.W.; Kim, J. Sulforaphane Upregulates the Heat Shock Protein Co-Chaperone CHIP and Clears Amyloid-β and Tau in a Mouse Model. of Alzheimer’s Disease. Mol. Nutr. Food Res. 2018, 62, e1800240. [Google Scholar] [CrossRef]
- Livingstone, T.L.; Saha, S.; Bernuzzi, F.; Savva, G.M.; Troncoso-Rey, P.; Traka, M.H.; Mills, R.D.; Ball, R.Y.; Mithen, R.F. Accumulation of Sulforaphane and Alliin in Human Prostate Tissue. Nutrients 2022, 14, 3263. [Google Scholar] [CrossRef]
- Singh, K.B.; Hahm, E.R.; Alumkal, J.J.; Foley, L.M.; Hitchens, T.K.; Shiva, S.S.; Parikh, R.A.; Jacobs, B.L.; Singh, S.V. Reversal of the Warburg phenomenon in chemoprevention of prostate cancer by sulforaphane. Carcinogenesis 2019, 40, 1545–1556. [Google Scholar] [CrossRef]
- He, C.; Buongiorno, L.P.; Wang, W.; Tang, J.C.Y.; Miceli, N.; Taviano, M.F.; Shan, Y.; Bao, Y. The Inhibitory Effect of Sulforaphane on Bladder Cancer Cell Depends on GSH Depletion-Induced by Nrf2 Translocation. Molecules 2021, 26, 4919. [Google Scholar] [CrossRef]
- Royston, K.J.; Paul, B.; Nozell, S.; Rajbhandari, R.; Tollefsbol, T.O. Withaferin A and sulforaphane regulate breast cancer cell cycle progression through epigenetic mechanisms. Exp. Cell Res. 2018, 368, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, Z.; Zhou, C.; Chen, K.; Li, X.; Wang, Z.; Wu, Z.; Ma, J.; Ma, Q.; Duan, W. Activation of Nrf2 by Sulforaphane Inhibits High Glucose-Induced Progression of Pancreatic Cancer via AMPK Dependent Signaling. Cell Physiol. Biochem. 2018, 50, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Tsaur, I.; Thomas, A.; Taskiran, E.; Rutz, J.; Chun, F.K.; Haferkamp, A.; Juengel, E.; Blaheta, R.A. Concomitant Use of Sulforaphane Enhances Antitumor Efficacy of Sunitinib in Renal Cell Carcinoma In Vitro. Cancers 2022, 14, 4643. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.T.; Liu, X.D.; Zhan, Z.P.; Wu, Q.J. Sulforaphane enhances the cisplatin sensitivity through regulating DNA repair and accumulation of intracellular cisplatin in ovarian cancer cells. Exp. Cell Res. 2020, 393, 112061. [Google Scholar] [CrossRef]
- Hao, Q.; Wang, M.; Sun, N.X.; Zhu, C.; Lin, Y.M.; Li, C.; Liu, F.; Zhu, W.W. Sulforaphane suppresses carcinogenesis of colorectal cancer through the ERK/Nrf2-UDP glucuronosyltransferase 1A metabolic axis activation. Oncol. Rep. 2020, 43, 1067–1080. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Dong, N.; Su, X.; Duan, M.; Wei, Y.; Wei, J.; Liu, G.; Peng, Q.; Zhao, Y. Sulforaphane induces S-phase arrest and apoptosis via p53-dependent manner in gastric cancer cells. Sci. Rep. 2021, 11, 2504. [Google Scholar] [CrossRef]
- Jin, J.; Xie, Y.; Zhang, J.S.; Wang, J.Q.; Dai, S.J.; He, W.F.; Li, S.Y.; Ashby, C.R., Jr.; Chen, Z.S.; He, Q. Sunitinib resistance in renal cell carcinoma: From molecular mechanisms to predictive biomarkers. Drug Resist. Updat. 2023, 67, 100929. [Google Scholar] [CrossRef]
- Linsalata, M.; Orlando, A.; Russo, F. Pharmacological and dietary agents for colorectal cancer chemoprevention: Effects on polyamine metabolism (review). Int. J. Oncol. 2014, 45, 1802–1812. [Google Scholar] [CrossRef]
- Chain, N.G. N-and O-Glycosylation; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Tsai, J.Y.; Tsai, S.H.; Wu, C.C. The chemopreventive isothiocyanate sulforaphane reduces anoikis resistance and anchorage-independent growth in non-small cell human lung cancer cells. Toxicol. Appl. Pharmacol. 2019, 362, 116–124. [Google Scholar] [CrossRef]
- Kallifatidis, G.; Labsch, S.; Rausch, V.; Mattern, J.; Gladkich, J.; Moldenhauer, G.; Büchler, M.W.; Salnikov, A.V.; Herr, I. Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Mol. Ther. 2011, 19, 188–195. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Y.; Ma, L.; Ji, R.; Qu, Y.; Xin, Y.; Lv, G. Chemopreventive activity of sulforaphane. Drug Des. Devel Ther. 2018, 12, 2905–2913. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.F.; Chen, P.C.; Lin, Y.C.; Chou, K.Y.; Chen, H.E.; Ho, C.Y.; Lin, J.F.; Hwang, T.I. Miconazole Contributes to NRF2 Activation by Noncanonical P62-KEAP1 Pathway in Bladder Cancer Cells. Drug Des. Devel Ther. 2020, 14, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jiang, D.; Wu, K. p62 promotes bladder cancer cell growth by activating KEAP1/NRF2-dependent antioxidative response. Cancer Sci. 2020, 111, 1156–1164. [Google Scholar] [CrossRef]
- Sompakdee, V.; Prawan, A.; Senggunprai, L.; Kukongviriyapan, U.; Samathiwat, P.; Wandee, J.; Kukongviriyapan, V. Suppression of Nrf2 confers chemosensitizing effect through enhanced oxidant-mediated mitochondrial dysfunction. Biomed. Pharmacother. 2018, 101, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Račkauskas, R.; Zhou, D.; Ūselis, S.; Strupas, K.; Herr, I.; Schemmer, P. Sulforaphane sensitizes human cholangiocarcinoma to cisplatin via the downregulation of anti-apoptotic proteins. Oncol. Rep. 2017, 37, 3660–3666. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Kostov, R.V.; Kensler, T.W. KEAP1 and Done? Targeting the NRF2 Pathway with Sulforaphane. Trends Food Sci. Technol. 2017, 69, 257–269. [Google Scholar] [CrossRef]
- Su, X.; Jiang, X.; Meng, L.; Dong, X.; Shen, Y.; Xin, Y. Anticancer Activity of Sulforaphane: The Epigenetic Mechanisms and the Nrf2 Signaling Pathway. Oxid. Med. Cell Longev. 2018, 2018, 5438179. [Google Scholar] [CrossRef]
- Pouremamali, F.; Pouremamali, A.; Dadashpour, M.; Soozangar, N.; Jeddi, F. An update of Nrf2 activators and inhibitors in cancer prevention/promotion. Cell. Commun. Signal 2022, 20, 100. [Google Scholar] [CrossRef]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef]
- Sishi, B.J.N. Autophagy Upregulation Reduces Doxorubicin-Induced Cardiotoxicity. In Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging; Academic Press: Cambridge, MA, USA, 2015; pp. 157–173. [Google Scholar]
- Choi, S.; Lew, K.L.; Xiao, H.; Herman-Antosiewicz, A.; Xiao, D.; Brown, C.K.; Singh, S.V. D,L-Sulforaphane-induced cell death in human prostate cancer cells is regulated by inhibitor of apoptosis family proteins and Apaf-1. Carcinogenesis 2007, 28, 151–162. [Google Scholar] [CrossRef]
- Jo, G.H.; Kim, G.Y.; Kim, W.J.; Park, K.Y.; Choi, Y.H. Sulforaphane induces apoptosis in T24 human urinary bladder cancer cells through a reactive oxygen species-mediated mitochondrial pathway: The involvement of endoplasmic reticulum stress and the Nrf2 signaling pathway. Int. J. Oncol. 2014, 45, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, P.; Kim, J.S. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef] [PubMed]
- Talalay, P.; Fahey, J.W.; Healy, Z.R.; Wehage, S.L.; Benedict, A.L.; Min, C.; Dinkova-Kostova, A.T. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc. Natl. Acad. Sci. USA 2007, 104, 17500–17505. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, Q.; Zhang, Y.; Shi, W.; Wang, H.; Zheng, Z.; Meng, L.; Xin, Y.; Jiang, X. Sulforaphane-Mediated Nrf2 Activation Prevents Radiation-Induced Skin Injury through Inhibiting the Oxidative-Stress-Activated DNA Damage and NLRP3 Inflammasome. Antioxidants 2021, 10, 1850. [Google Scholar] [CrossRef]
- Ullah, M.F. Sulforaphane (SFN): An Isothiocyanate in a Cancer Chemoprevention Paradigm. Medicines 2015, 2, 141–156. [Google Scholar] [CrossRef]
- Bose, C.; Awasthi, S.; Sharma, R.; Beneš, H.; Hauer-Jensen, M.; Boerma, M.; Singh, S.P. Sulforaphane potentiates anticancer effects of doxorubicin and attenuates its cardiotoxicity in a breast cancer model. PLoS ONE 2018, 13, e0193918. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, Q.; Sun, Y.P.; Wang, X.; Lv, L.; Zhang, L.P.; Liu, J.S.; Zhao, S.; Wang, X.L. Sulforaphane protection against the development of doxorubicin-induced chronic heart failure is associated with Nrf2 Upregulation. Cardiovasc. Ther. 2017, 35, e12277. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, R.; McElhanon, K.; Allen, C.D.; Megyesi, J.K.; Beneš, H.; Singh, S.P. Sulforaphane protects the heart from doxorubicin-induced toxicity. Free Radic. Biol. Med. 2015, 86, 90–101. [Google Scholar] [CrossRef]
- Li, B.; Kim, D.S.; Yadav, R.K.; Kim, H.R.; Chae, H.J. Sulforaphane prevents doxorubicin-induced oxidative stress and cell death in rat H9c2 cells. Int. J. Mol. Med. 2015, 36, 53–64. [Google Scholar] [CrossRef]
- Tomlinson, L.; Lu, Z.Q.; Bentley, R.A.; Colley, H.E.; Murdoch, C.; Webb, S.D.; Cross, M.J.; Copple, I.M.; Sharma, P. Attenuation of doxorubicin-induced cardiotoxicity in a human in vitro cardiac model by the induction of the NRF-2 pathway. Biomed. Pharmacother. 2019, 112, 108637. [Google Scholar] [CrossRef]
- Traka, M.H.; Melchini, A.; Mithen, R.F. Sulforaphane and prostate cancer interception. Drug Discov. Today 2014, 19, 1488–1492. [Google Scholar] [CrossRef]
- Jiang, L.L.; Zhou, S.J.; Zhang, X.M.; Chen, H.Q.; Liu, W. Sulforaphane suppresses in vitro and in vivo lung tumorigenesis through downregulation of HDAC activity. Biomed. Pharmacother. 2016, 78, 74–80. [Google Scholar] [CrossRef]
- Atwell, L.L.; Beaver, L.M.; Shannon, J.; Williams, D.E.; Dashwood, R.H.; Ho, E. Epigenetic Regulation by Sulforaphane: Opportunities for Breast and Prostate Cancer Chemoprevention. Curr. Pharmacol. Rep. 2015, 1, 102–111. [Google Scholar] [CrossRef]
- Jabbarzadeh Kaboli, P.; Afzalipour Khoshkbejari, M.; Mohammadi, M.; Abiri, A.; Mokhtarian, R.; Vazifemand, R.; Amanollahi, S.; Yazdi Sani, S.; Li, M.; Zhao, Y.; et al. Targets and mechanisms of sulforaphane derivatives obtained from cruciferous plants with special focus on breast cancer—Contradictory effects and future perspectives. Biomed. Pharmacother. 2020, 121, 109635. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.X.; Zou, Y.J.; Zhuang, X.B.; Chen, S.X.; Lin, Y.; Li, W.L.; Lin, J.J.; Lin, Z.Q. Sulforaphane suppresses EMT and metastasis in human lung cancer through miR-616-5p-mediated GSK3beta/beta-catenin signaling pathways. Acta Pharmacol. Sin. 2017, 38, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Clarke, J.D.; Dashwood, R.H. Dietary sulforaphane, a histone deacetylase inhibitor for cancer prevention. J. Nutr. 2009, 139, 2393–2396. [Google Scholar] [CrossRef]
- Kim, D.H.; Sung, B.; Kang, Y.J.; Hwang, S.Y.; Kim, M.J.; Yoon, J.H.; Im, E.; Kim, N.D. Sulforaphane inhibits hypoxia-induced HIF-1alpha and VEGF expression and migration of human colon cancer cells. Int. J. Oncol. 2015, 47, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Hu, R.; Hebbar, V.; Kim, B.R.; Chen, C.; Winnik, B.; Buckley, B.; Soteropoulos, P.; Tolias, P.; Hart, R.P.; Kong, A.N. In vivo pharmacokinetics and regulation of gene expression profiles by isothiocyanate sulforaphane in the rat. J. Pharmacol. Exp. Ther. 2004, 310, 263–271. [Google Scholar] [CrossRef]
- Hanlon, N.; Coldham, N.; Gielbert, A.; Kuhnert, N.; Sauer, M.J.; King, L.J.; Ioannides, C. Absolute bioavailability and dose-dependent pharmacokinetic behaviour of dietary doses of the chemopreventive isothiocyanate sulforaphane in rat. Br. J. Nutr. 2008, 99, 559–564. [Google Scholar] [CrossRef]
- Hu, R.; Khor, T.O.; Shen, G.; Jeong, W.S.; Hebbar, V.; Chen, C.; Xu, C.; Reddy, B.; Chada, K.; Kong, A.N. Cancer chemoprevention of intestinal polyposis in ApcMin/+ mice by sulforaphane, a natural product derived from cruciferous vegetable. Carcinogenesis 2006, 27, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- Atwell, L.L.; Hsu, A.; Wong, C.P.; Stevens, J.F.; Bella, D.; Yu, T.W.; Pereira, C.B.; Löhr, C.V.; Christensen, J.M.; Dashwood, R.H.; et al. Absorption and chemopreventive targets of sulforaphane in humans following consumption of broccoli sprouts or a myrosinase-treated broccoli sprout extract. Mol. Nutr. Food Res. 2015, 59, 424–433. [Google Scholar] [CrossRef] [PubMed]
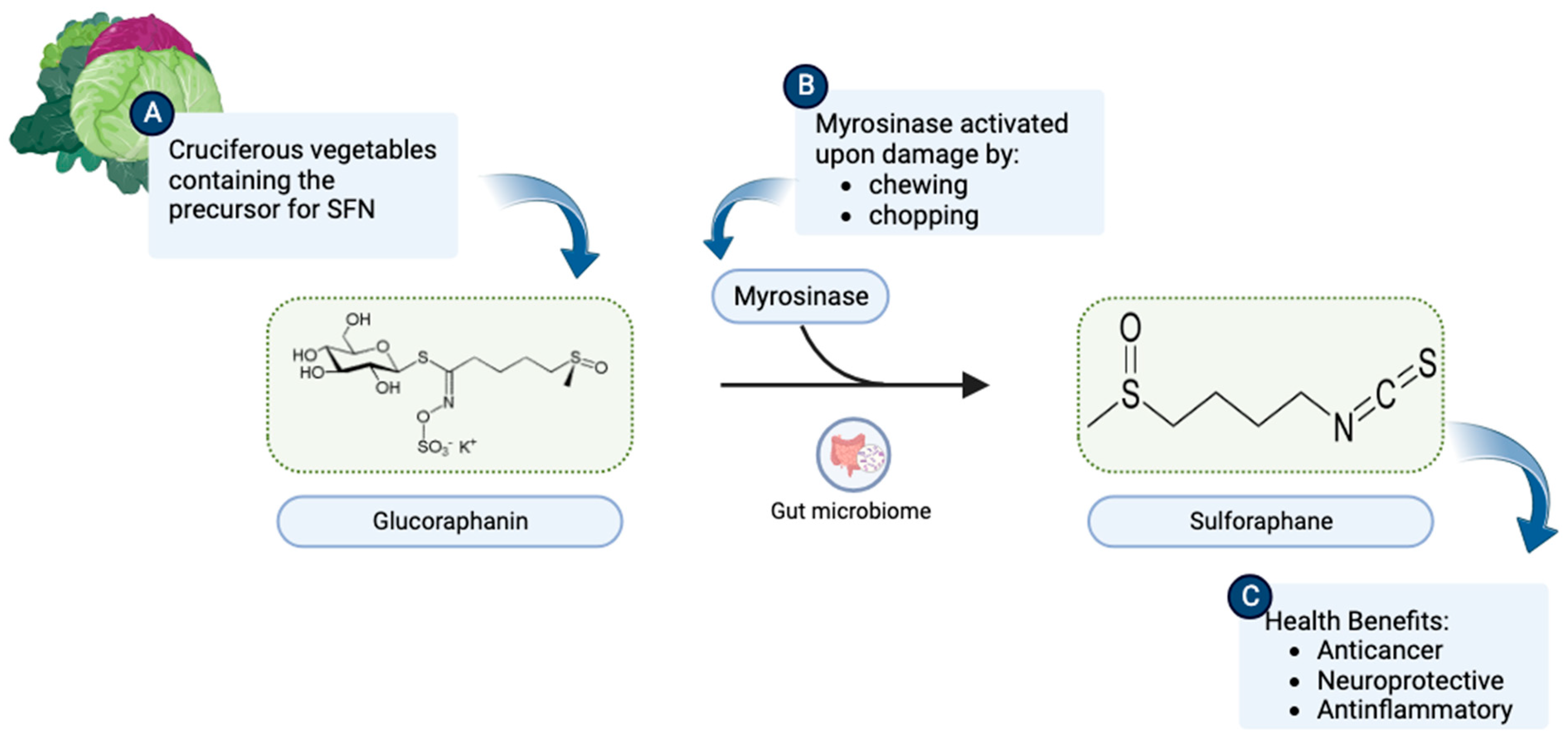
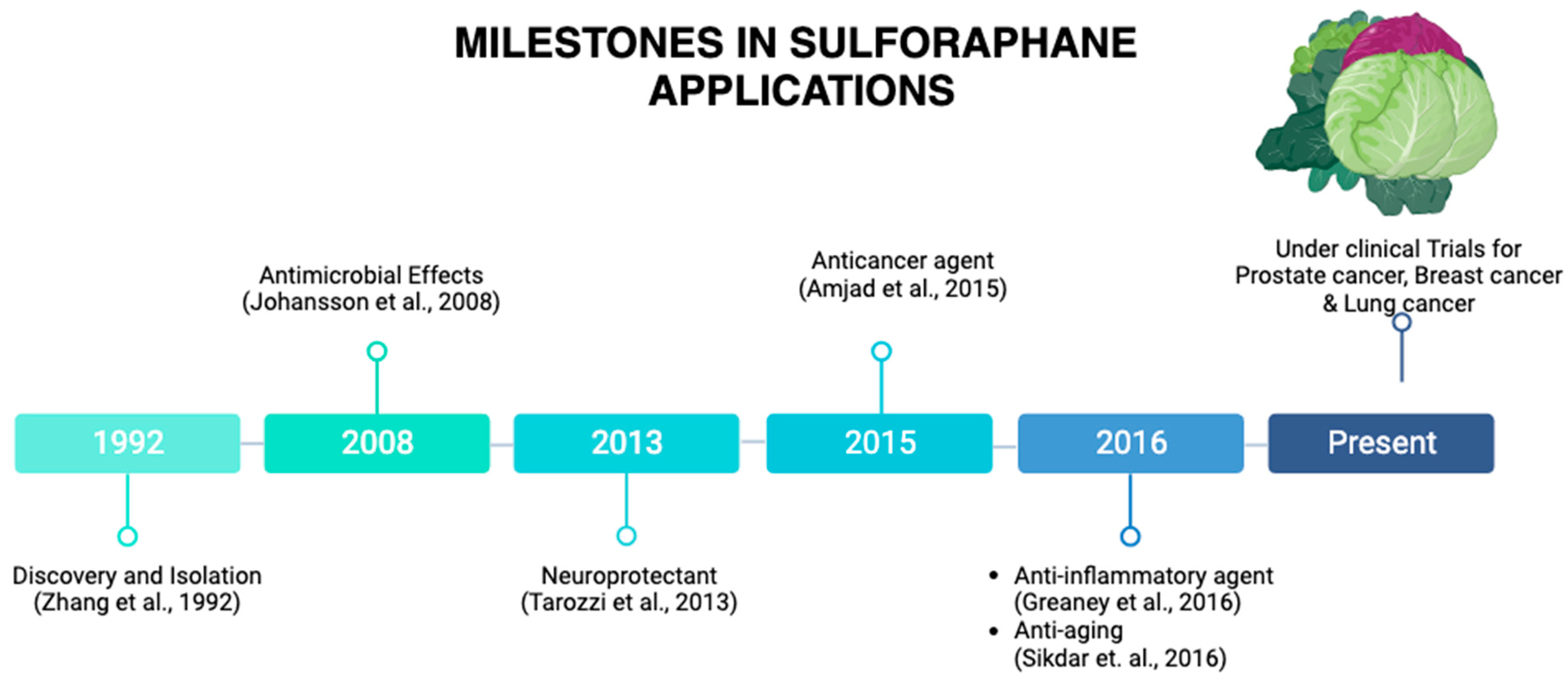
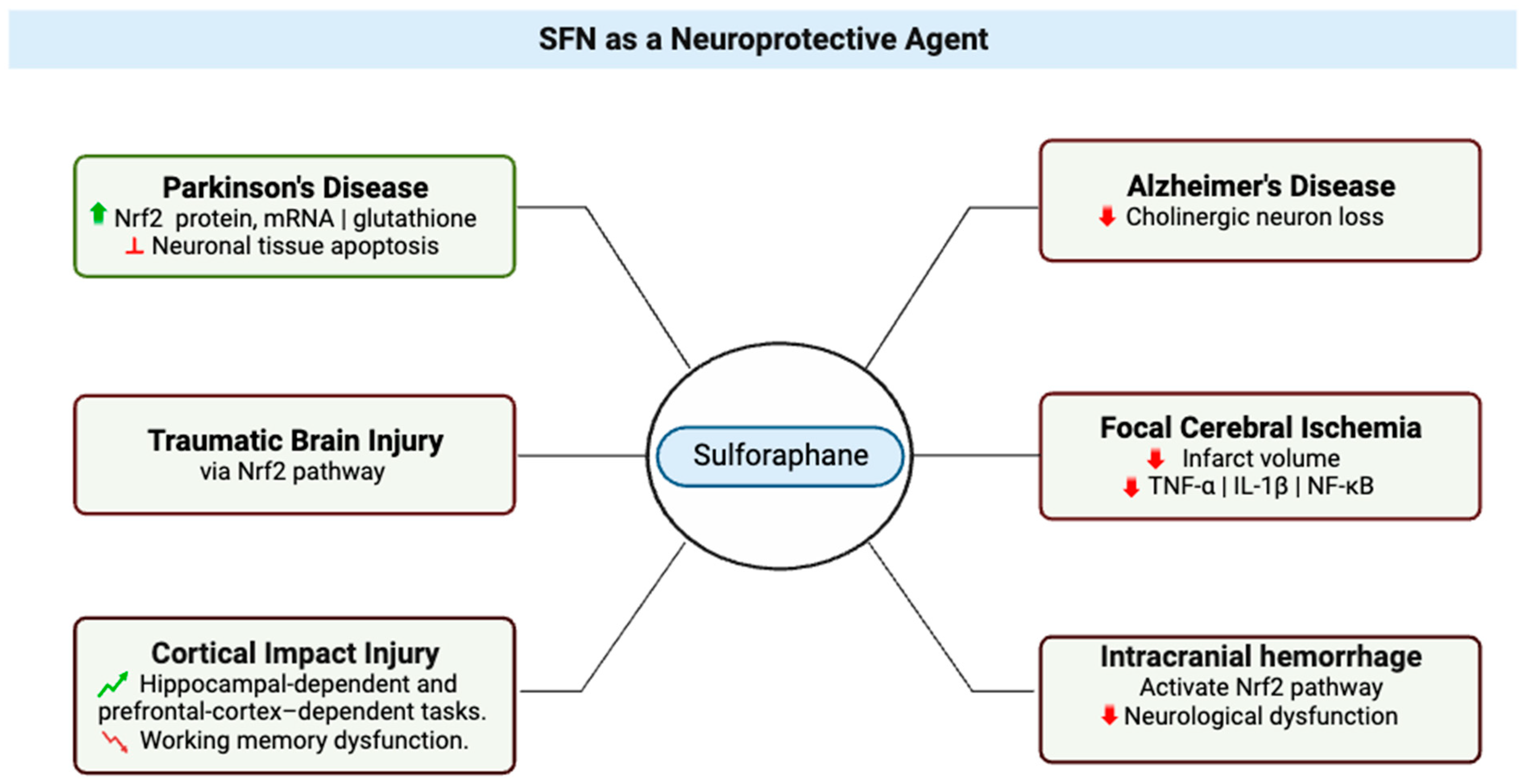
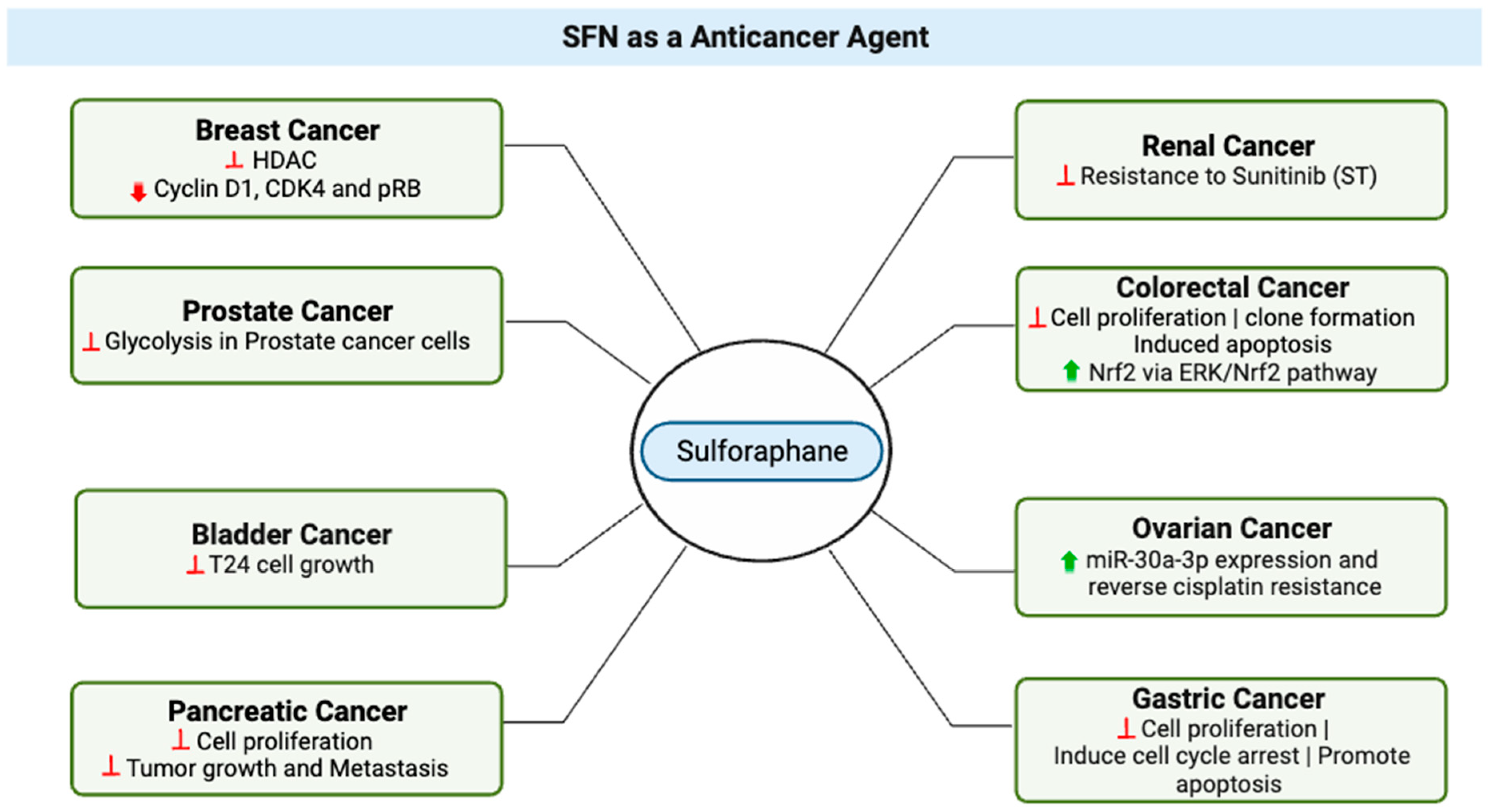

| Topic | Article | Model | Effect |
|---|---|---|---|
| Neuroprotectant | Ladak et al., 2021 [14] | in vitro, cultured neuronal cells | Low doses of SFN in neuronal, astrocytes, and cocultures was neuroprotective |
| Sandouka et al., 2021 [16] | in vitro, cortical cell cultures | SFN reduced neuronal cell death | |
| in vivo, temporal lobe epilepsy rat model | SFN exerted neuroprotective effects by increasing Nrf2 expression and related antioxidant genes, improved oxidative stress markers, and increased the total antioxidant capacity in both the plasma and hippocampus | ||
| Zhao et al., 2019 [18] | in vitro, cultured HT22 mouse hippocampal cells | SFN protected HT22 cells against high glucose-induced injury | |
| Morroni et al., 2018 [24] | in vitro, cultured SH-SY5Y cells | SFN reduced neuronal apoptosis induced by 6-OHDA in SH-SY5Y cells | |
| Royston et al., 2018 [72] | in vitro, cultured breast cancer cell lines MCF-7 [ERα (+)] and the ERα (−) MDA-MB-231 | SFN in combination with Withaferin A reactivated tumor suppressor gene p21 | |
| Zhao et al., 2018 [20] | in vitro, cellular model of AD | SFN upregulated Nrf2 expression promoted the nuclear translocation of Nrf2 by decreasing DNA levels of the Nrf2 promoter, thus leading to antioxidative and anti-inflammatory properties | |
| Zhao et al., 2016 [21] | in vivo, animal model of SAH male Sprague–Dawley rats | Nrf2–ARE signaling pathway was activated in the basilar artery after SAH | |
| Benedict et al.,2012 [25] | in vivo, rat model of contusion SCI | SFN upregulated the phase 2 antioxidant response, decreased mRNA levels of inflammatory cytokines, and enhanced hindlimb locomotor function at the injury site | |
| Jazwa et al., 2011 [22] | in vivo, Nrf2-knockout mice and their wild type | SFN protected against MPTP-induced death of nigral dopaminergic neurons | |
| Mizuno et al., 2011 [19] | in vitro, primary neuronal cultures of rat striatum | SFN protected against H2O2- and paraquat-induced cytotoxicity | |
| Dash et al., 2009 [26] | in vivo, mouse model of TBI | SFN improved working memory, decreased oxidative damage in the brain | |
| Park et al., 2009 [32] | in vitro, cultured neurons with Aβ | SFN protected cells from Aβ1–42-mediated cell death in Neuro2A and N1E 115 cells | |
| Chemoprotectant | Kallifatidis, G. et al., 2011 [82] | in vivo, BALBc male mice | SFN effectively inhibited tumor growth and increased the sensitivity of cancer cells |
| Tumors | Račkauskas et al., 2017 [87] | in vitro, culture CCC cells | Sulforaphane sensitized human cholangiocarcinoma to cisplatin |
| Chemotherapy | Choi et al., 2007 [93] | in vitro, cultured human prostate cancer cells | SFN induced cell death in human prostate cancer cells |
| Wei et al., 2021 [97] | in vivo, RISI model (C57/BL6 mice) | SFN-mediated Nrf2 activation prevents radiation-induced skin injury | |
| Radiation Therapy | Talalay et al., 2007 [96] | in vivo, human subjects and SKH-1 mice | SFN protected skin against damage by UV radiation |
| Cardiotoxicity | Bose et al., 2018 [99] | in vivo, cultured MCF 10A cells | SFN protected the heart from DOX toxicity |
| in vitro, rat breast cancer model | SFN+DOX enhanced the activity in NRCM and MCF 10A cells | ||
| Bai et al., 2017 [100] | in vivo, rat model (male Sprague–Dawley) of CHF | SFN reduced DOX-induced myocardial injury and inflammation | |
| Singh et al., 2015 [101] | in vivo, wild type 129/sv mice | SFN reduced DOX-induced cardiomyopathy mortality in mice | |
| in vitro, cultured rat H9c2 cardiomyoblast cells | SFN protected H9c2 cells from DOX cytotoxicity | ||
| Li et al., 2015 [102] | in vitro, H9c2 rat myoblasts | SFN reduced ROS production and apoptosis induced by DOX in H9c2 cells | |
| Focal Cerebral Ischemia | Li et al., 2022 [50] | in vivo, PSCI was modeled in wildtype (WT) and Nrf2 knockout (KO), male and female mice | Sulforaphane promoted white matter plasticity and improved long-term neurological outcomes after ischemic stroke |
| in vitro, primary neuronal cultures | SFN reduced neuronal death | ||
| Ma et al., 2015 [29] | in vivo, adult male Sprague–Dawley rats model of FCI | SFN inhibited cerebral ischemia-induced NF-κB pathway activation | |
| Subedi et al., 2020 [54] | in vitro, cultured BV2 microglial cells | SFN inhibited MGO-AGE-mediated neuroinflammation | |
| Neuro-Inflammation | Wang et al., 2020 [55] | in vivo, rats | SFN improved LPS-induced neurocognitive dysfunction in rats |
| in vitro, BV2 cells | SFN mitigated LPS-induced neuroinflammation through modulation of Cezanne/NF-κB signaling | ||
| Subedi et al., 2019 [53] | in vitro, cultured BV2 cells | SFN exerted an anti-neuroinflammatory effect on microglia through JNK/AP-1/NF-κB pathway inhibition and Nrf2/HO-1 pathway activation | |
| Hernandez-Rabaza et al., 2016 [35] | in vivo, hyperammonemic rats | SFN reduced neuroinflammation | |
| Pan et al., 2023 [31] | in vivo, male BALB/c mice | SFN alleviated vascular remodeling | |
| Li et al., 2015 [102] | in vitro, H9c2 rat myoblasts | SFN reduced ROS production and apoptosis induced by DOX in H9c2 cells | |
| Intracerebral Hemorrhage | Yin et al., 2015 [59] | in vivo, Sprague–Dawley rats of ICH | SFN decreased expression of Nrf2 and HO-1 in tissues surrounding hemorrhage and reduced perifocal inflammatory response |
| Anticancer | Zeng et al., 2011 [60] | in vitro, cultured colon cancer cells | SFN inhibited colon cancer cell (HCT116) proliferation |
| Zhang et al., 2022 [61] | in vitro, cultured TNBR cells | SFN suppressed metastasis of triple-negative breast cancer cells | |
| Cornblatt et al., 2007 [63] | in vivo, female Sprague–Dawley rats | SFN distributed to the breast epithelial cells in vivo and exerts a pharmacodynamic action in these target cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otoo, R.A.; Allen, A.R. Sulforaphane’s Multifaceted Potential: From Neuroprotection to Anticancer Action. Molecules 2023, 28, 6902. https://doi.org/10.3390/molecules28196902
Otoo RA, Allen AR. Sulforaphane’s Multifaceted Potential: From Neuroprotection to Anticancer Action. Molecules. 2023; 28(19):6902. https://doi.org/10.3390/molecules28196902
Chicago/Turabian StyleOtoo, Raymond A., and Antiño R. Allen. 2023. "Sulforaphane’s Multifaceted Potential: From Neuroprotection to Anticancer Action" Molecules 28, no. 19: 6902. https://doi.org/10.3390/molecules28196902
APA StyleOtoo, R. A., & Allen, A. R. (2023). Sulforaphane’s Multifaceted Potential: From Neuroprotection to Anticancer Action. Molecules, 28(19), 6902. https://doi.org/10.3390/molecules28196902






