In Situ Decoration of Bi2S3 Nanosheets on Zinc Oxide/Cellulose Acetate Composite Films for Photodegradation of Dyes under Visible Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
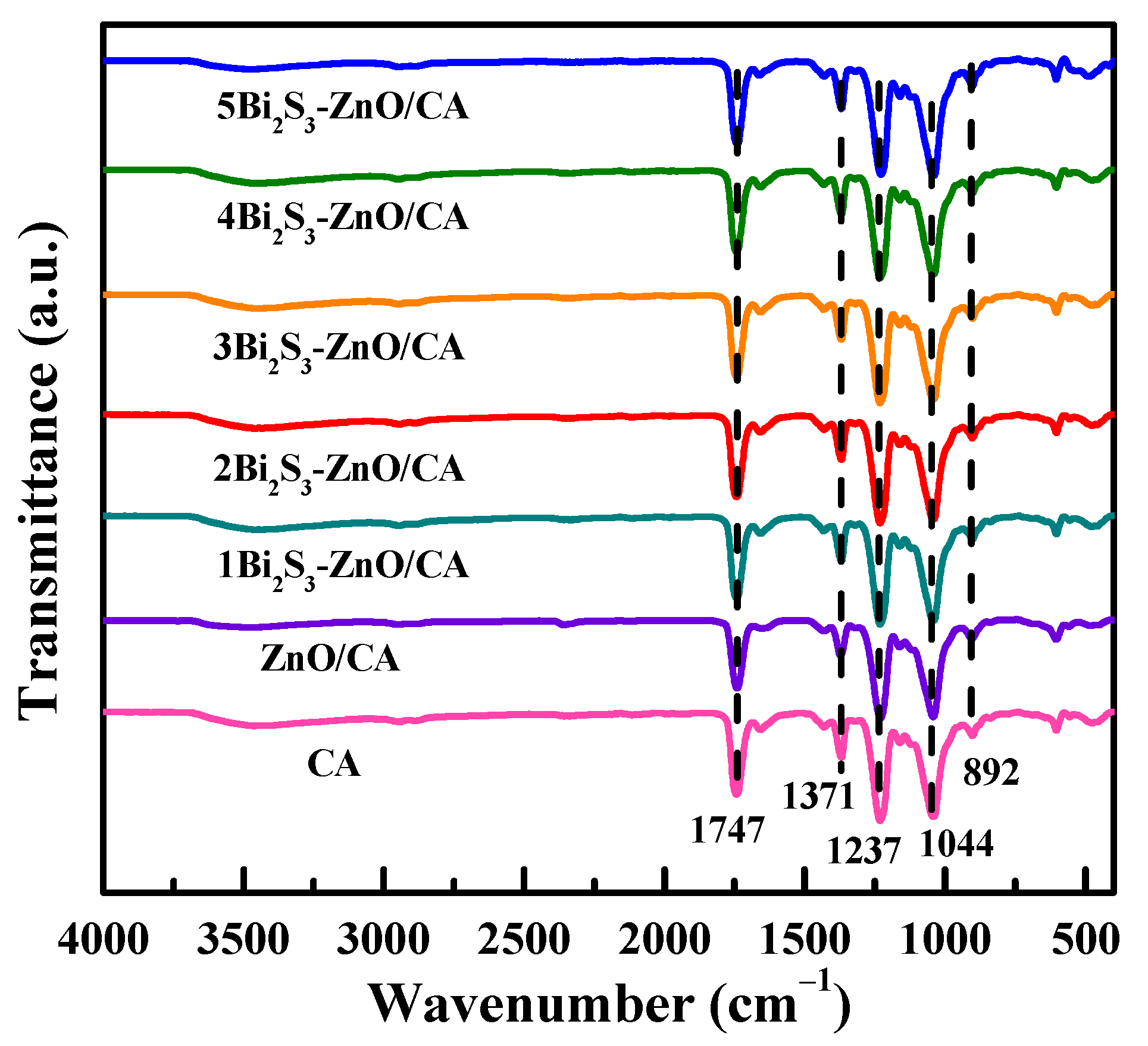
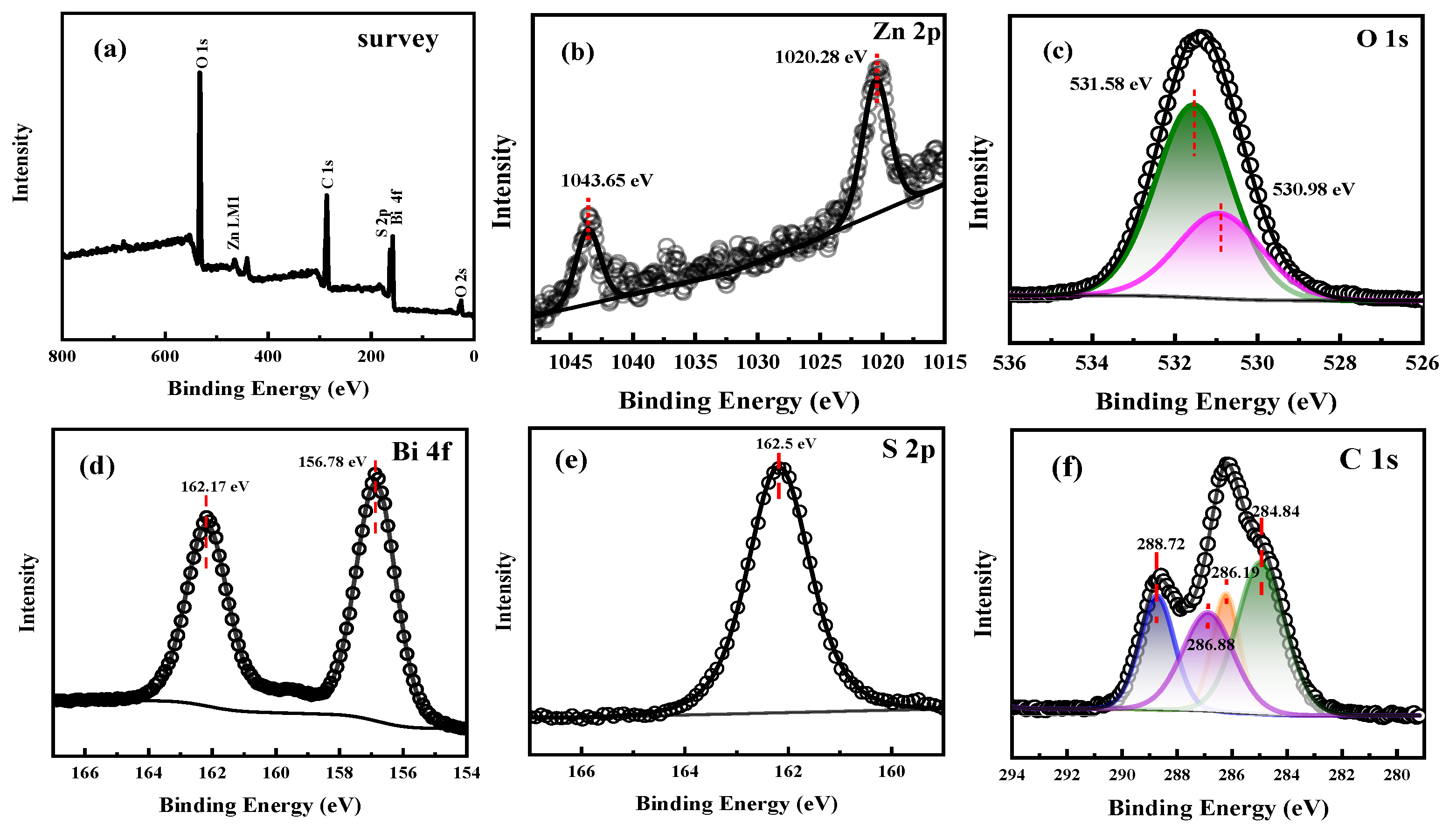
2.1. Structural Characterization
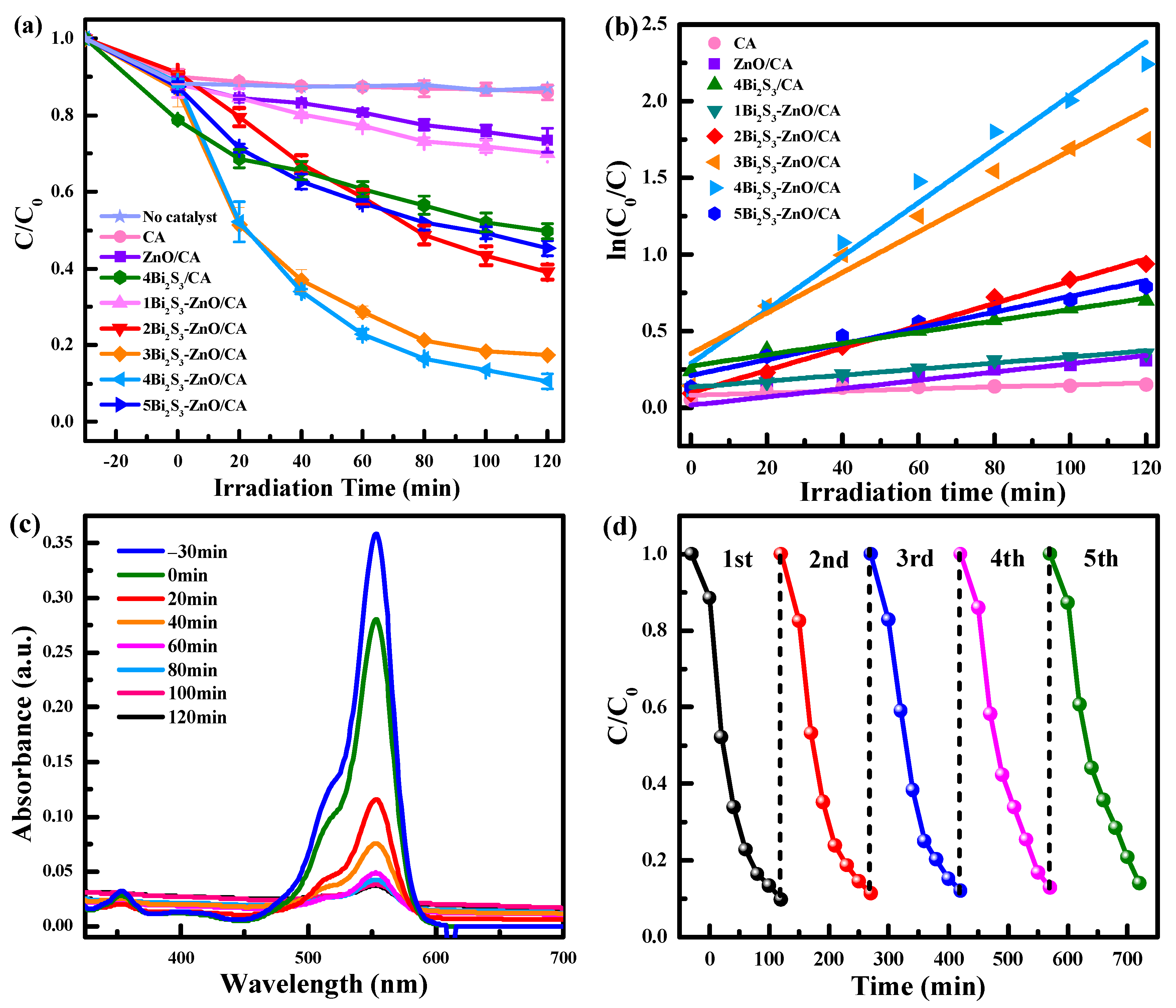
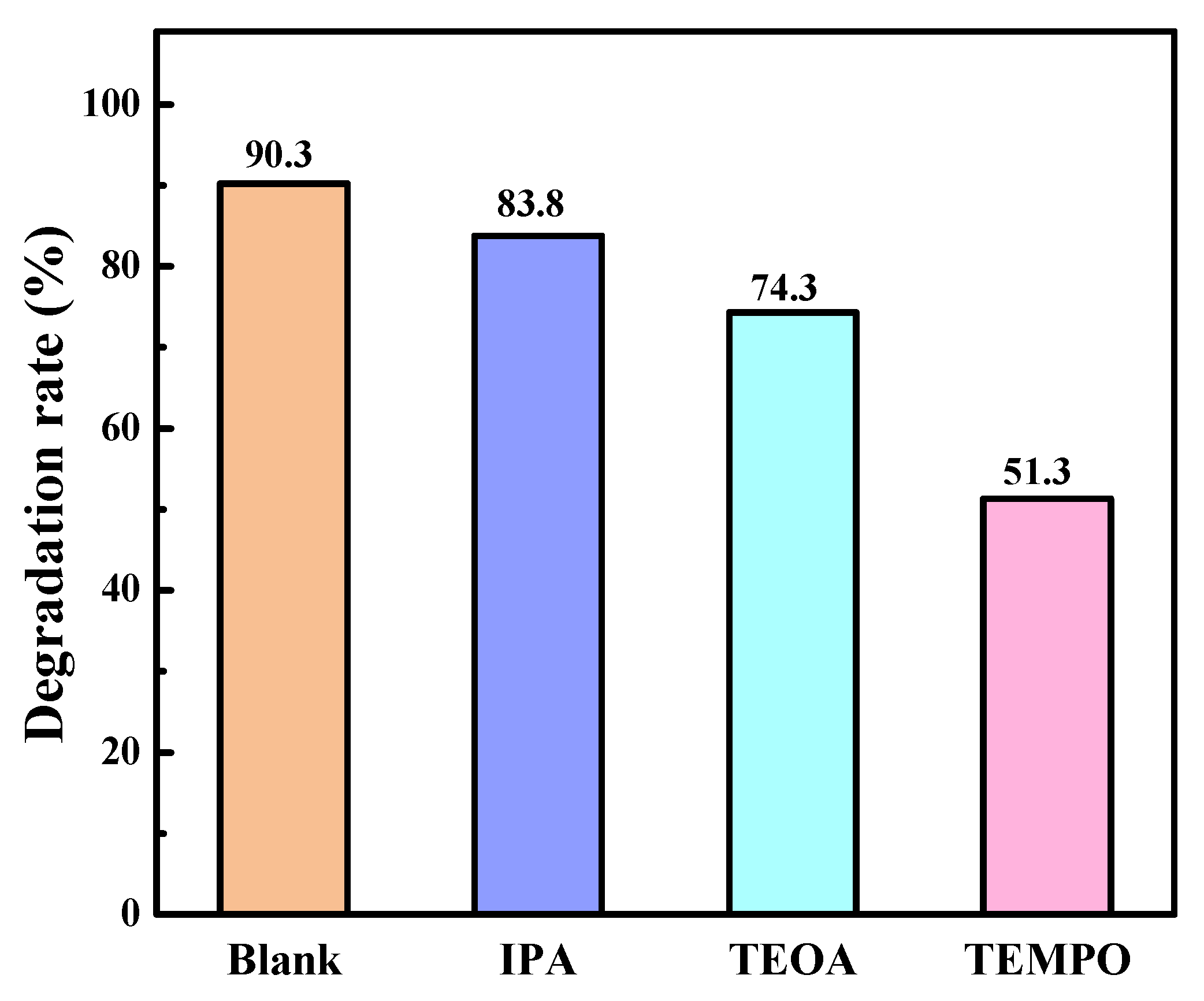
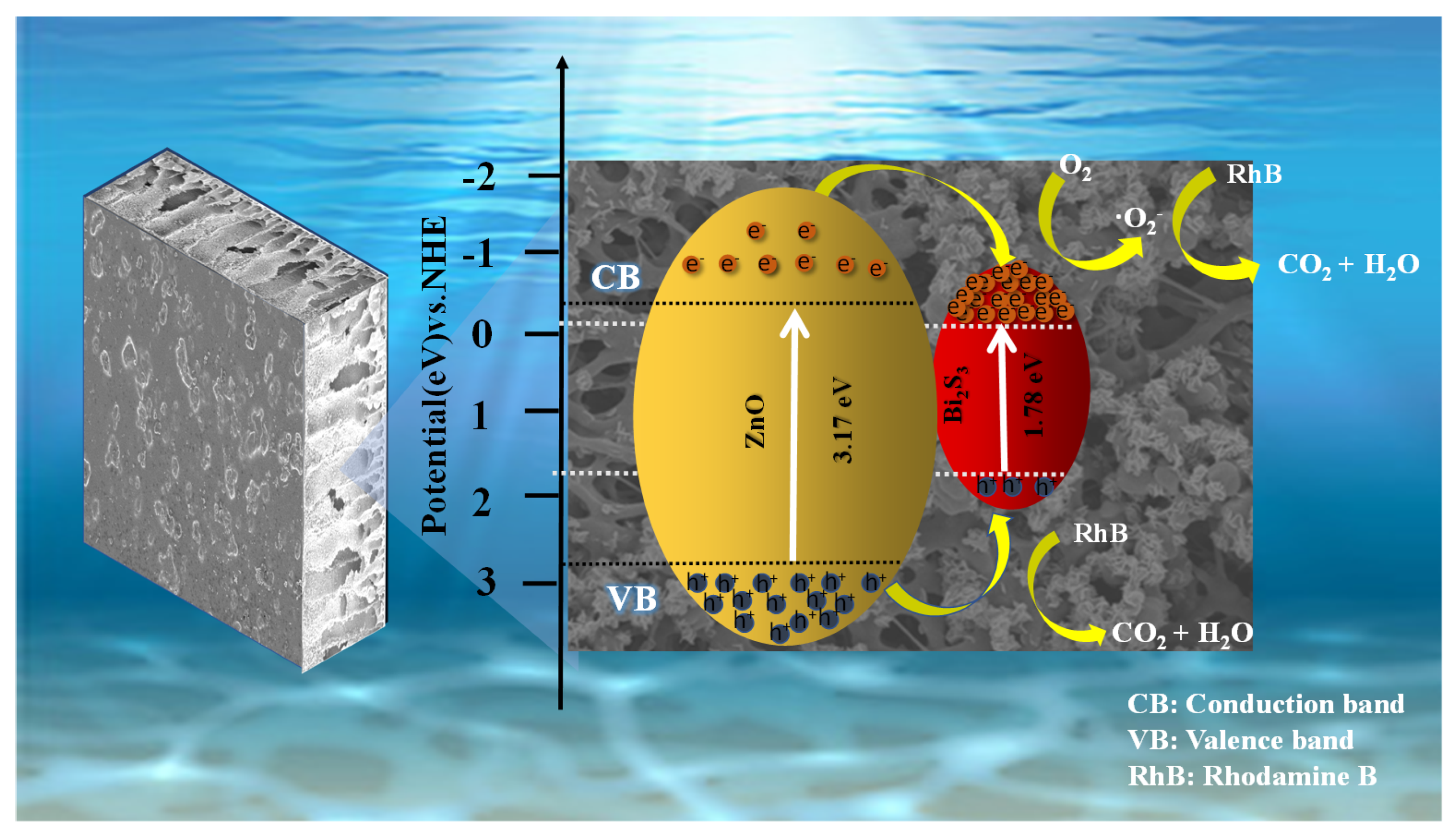
2.2. Photocatalytic Efficiency
3. Experimental Section
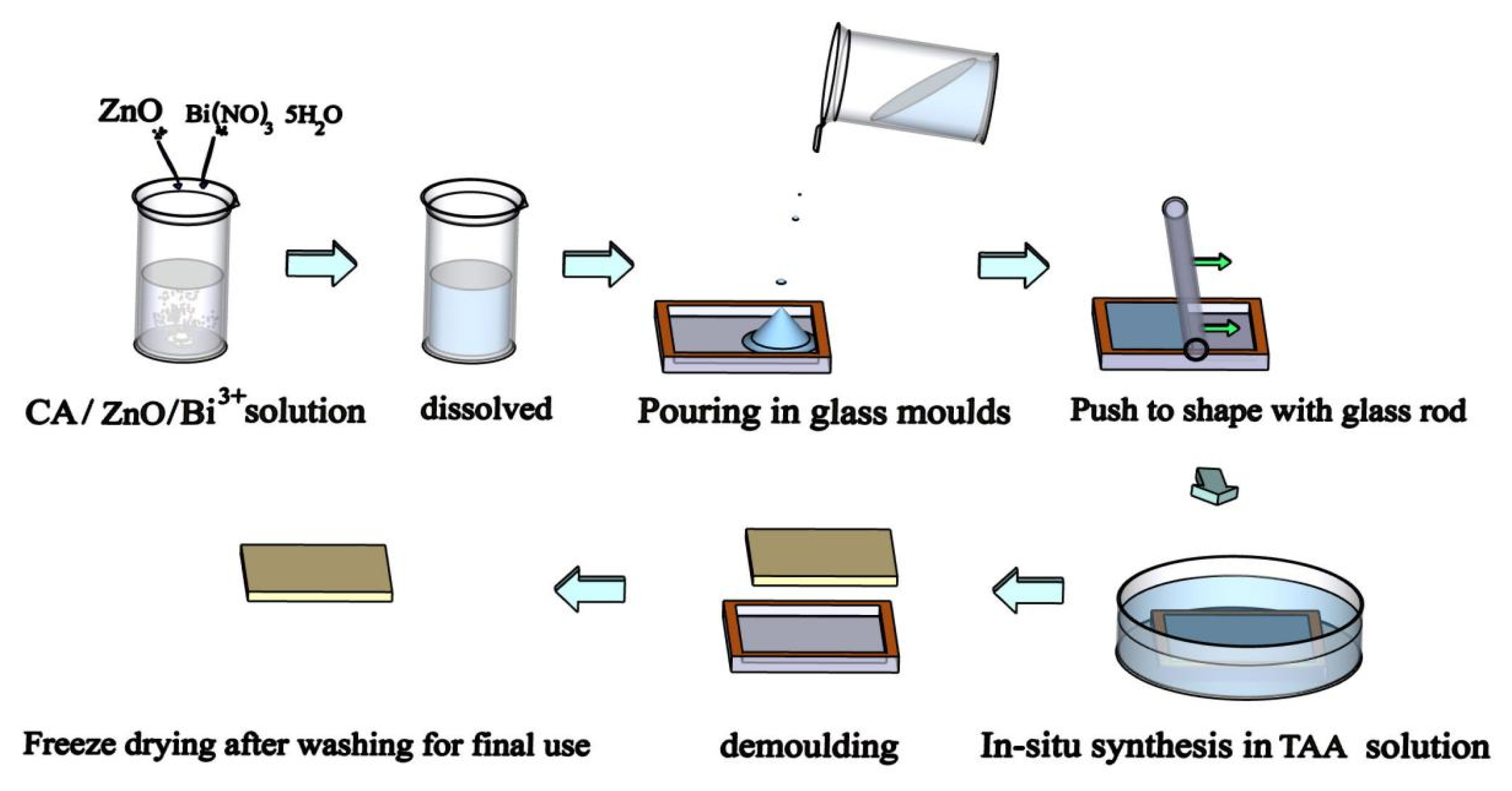
3.1. Synthesis of Bi2S3-ZnO/CA Composite Films
3.2. Characterization
3.3. Evaluation of Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Uddin, F. Environmental hazard in textile dyeing wastewater from local textile industry. Cellulose 2021, 28, 10715–10739. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, Y.; Xie, B.; Ni, Z.; Xia, S. Br doping promotes the transform of Cu2O (100) to Cu2O (111) and facilitates efficient photocatalytic degradation of tetracycline. Mol. Catal. 2023, 548, 113431. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Xia, Y.; Liang, Z.; Huang, R.; Liang, R.; Yan, G. Modification of polymeric carbon nitride with Au–CeO2 hybrids to improve photocatalytic activity for hydrogen evolution. Molecules 2022, 27, 7489. [Google Scholar] [CrossRef] [PubMed]
- Kesraoui, A.; Selmi, T.; Seffen, M.; Brouers, F. Influence of alternating current on the adsorption of indigo carmine. Environ. Sci. Pollut. Res. 2017, 24, 9940–9950. [Google Scholar] [CrossRef]
- Sarma, G.K.; Gupta, S.S.; Bhattacharyya, K.G. RETRACTED: Adsorption of Crystal violet on raw and acid-treated montmorillonite, K10, in aqueous suspension. J. Environ. Manag. 2016, 171, 1–10. [Google Scholar] [CrossRef]
- Barisci, S.; Inan, H.; Turkay, O.; Dimoglo, A.; Erol, D. Degradation of toxic indigo carmine dye by electrosynthesized ferrate (VI). J. Appl. Solut. Chem. Model. 2017, 6, 75–83. [Google Scholar] [CrossRef]
- Ibrahim, Y.O.; Gondal, M. Visible-light-driven photocatalytic performance of a Z-scheme based TiO2/WO3/g-C3N4 ternary heterojunctions. Mol. Catal. 2021, 505, 111494. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Mittal, A.; Saleh, T.A.; Nayak, A.; Agarwal, S.; Sikarwar, S. Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater. Sci. Eng. C 2012, 32, 12–17. [Google Scholar] [CrossRef]
- Yu, Z.; Qian, L.; Zhong, T.; Ran, Q.; Huang, J.; Hou, Y.; Li, F.; Li, M.; Sun, Q.; Zhang, H. Enhanced visible light photocatalytic activity of CdS through controllable self-assembly compositing with ZIF-67. Mol. Catal. 2020, 485, 110797. [Google Scholar] [CrossRef]
- Wu, G.; Liang, R.; Ge, M.; Sun, G.; Zhang, Y.; Xing, G. Surface passivation using 2D perovskites toward efficient and stable perovskite solar cells. Adv. Mater. 2022, 34, 2105635. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.-Q.; Al-Deyab, S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Li, Y.; Yang, X.; Zhao, L.; Peng, J. Boosting Photocatalytic Performance of ZnO Nanowires via Building Heterojunction with g-C3N4. Molecules 2023, 28, 5563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, Y.; Hu, W.; Fan, Y. Mesoporous CuO Prepared in a Natural Deep Eutectic Solvent Medium for Effective Photodegradation of Rhodamine B. Molecules 2023, 28, 5554. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, A.W.; Khatri, Z.; Ahmed, F.; Memon, M.H. Effect of silicone nano, nano/micro and nano/macro-emulsion softeners on color yield and physical characteristics of dyed cotton fabric. J. Surfactants Deterg. 2015, 18, 205–211. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Li, F.; Dou, L.; Li, Y.; Zhao, J.; Hao, Y. A chelation strategy for in-situ constructing surface oxygen vacancy on {001} facets exposed BiOBr nanosheets. Sci. Rep. 2016, 6, 24918. [Google Scholar] [CrossRef]
- Wang, M.; Ge, R.; Zhao, P.; Williams, G.R.; Yu, D.; Bligh, S.A. Exploring wettability difference-driven wetting by utilizing electrospun chimeric Janus microfiber comprising cellulose acetate and polyvinylpyrrolidone. Mater. Des. 2023, 226, 111652. [Google Scholar] [CrossRef]
- Fu, F.; Li, L.; Liu, L.; Cai, J.; Zhang, Y.; Zhou, J.; Zhang, L. Construction of cellulose based ZnO nanocomposite films with antibacterial properties through one-step coagulation. ACS Appl. Mater. Interfaces 2015, 7, 2597–2606. [Google Scholar] [CrossRef]
- Abad, S.N.K.; Mozammel, M.; Moghaddam, J.; Mostafaei, A.; Chmielus, M. Highly porous, flexible and robust cellulose acetate/Au/ZnO as a hybrid photocatalyst. Appl. Surf. Sci. 2020, 526, 146237. [Google Scholar] [CrossRef]
- Mahapatra, A.D.; Basak, D. Enhanced ultraviolet photosensing properties in Bi2S3 nanoparticles decorated ZnO nanorods’ heterostructure. J. Alloys Compd. 2019, 797, 766–774. [Google Scholar] [CrossRef]
- Luo, W.; Li, F.; Li, Q.; Wang, X.; Yang, W.; Zhou, L.; Mai, L. Heterostructured Bi2S3-Bi2O3 nanosheets with a built-in electric field for improved sodium storage. ACS Appl. Mater. Interfaces 2018, 10, 7201–7207. [Google Scholar] [CrossRef]
- Ju, P.; Zhang, Y.; Hao, L.; Cao, J.; Zhai, X.; Dou, K.; Jiang, F.; Sun, C. 1D Bi2S3 nanorods modified 2D BiOI nanoplates for highly efficient photocatalytic activity: Pivotal roles of oxygen vacancies and Z-scheme heterojunction. J. Mater. Sci. Technol. 2023, 142, 45–59. [Google Scholar] [CrossRef]
- Huang, W.; Xing, C.; Wang, Y.; Li, Z.; Wu, L.; Ma, D.; Dai, X.; Xiang, Y.; Li, J.; Fan, D. Facile fabrication and characterization of two-dimensional bismuth (III) sulfide nanosheets for high-performance photodetector applications under ambient conditions. Nanoscale 2018, 10, 2404–2412. [Google Scholar] [CrossRef]
- Fenelon, E.; Bui, D.; Tran, H.H.; You, S.; Wang, Y.; Cao, T.; Van Pham, V. Straightforward synthesis of SnO2/Bi2S3/BiOCl-Bi24O31Cl10 composites for drastically enhancing rhodamine B photocatalytic degradation under visible light. ACS Omega 2020, 5, 20438–20449. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Cao, X.; Dai, G.; Wang, L.; Peng, Y.; Geng, B. Facile one-pot synthesis of novel hierarchical Bi2O3/Bi2S3 nanoflower photocatalyst with intrinsic pn junction for efficient photocatalytic removals of RhB and Cr (VI). J. Hazard. Mater. 2020, 381, 120942. [Google Scholar] [CrossRef]
- Lu, J.; Han, Q.; Wang, Z. Synthesis of TiO2/Bi2S3 heterojunction with a nuclear-shell structure and its high photocatalytic activity. Mater. Res. Bull. 2012, 47, 1621–1624. [Google Scholar] [CrossRef]
- Ouyang, W.; Teng, F.; Jiang, M.; Fang, X. ZnO film UV photodetector with enhanced performance: Heterojunction with CdMoO4 microplates and the hot electron injection effect of Au nanoparticles. Small 2017, 13, 1702177. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, Q.; Wu, A.; Jiang, M.; Liang, Z.; Jiang, B.; Fu, H. Cost-effective large-scale synthesis of ZnO photocatalyst with excellent performance for dye photodegradation. Chem. Commun. 2012, 48, 2858–2860. [Google Scholar] [CrossRef]
- Bai, Z.; Fu, M.; Zhang, Y. Vertically aligned and ordered ZnO/CdS nanowire arrays for self-powered UV-visible photosensing. J. Mater. Sci. 2017, 52, 1308–1317. [Google Scholar] [CrossRef]
- Yi, S.; Zhao, F.; Yue, X.; Wang, D.; Lin, Y. Enhanced solar light-driven photocatalytic activity of BiOBr-ZnO heterojunctions with effective separation and transfer properties of photo-generated chargers. New J. Chem. 2015, 39, 6659–6666. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, X.; Feng, Z.; Jia, W.; Zheng, X.; Li, C. Facile synthesis of heterojunctioned ZnO/Bi2S3 nanocomposites for enhanced photocatalytic reduction of aqueous Cr (VI) under visible-light irradiation. Catalysts 2019, 9, 624. [Google Scholar] [CrossRef]
- Zhou, Z.; Peng, X.; Zhong, L.; Wu, L.; Cao, X.; Sun, R.C. Electrospun cellulose acetate supported Ag@AgCl composites with facet-dependent photocatalytic properties on degradation of organic dyes under visible-light irradiation. Carbohydr. Polym. 2016, 136, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.; Ho, W.; Lee, S. Efficient visible light photocatalytic removal of NO with BiOBr-graphene nanocomposites. J. Phys. Chem. C 2011, 115, 25330–25337. [Google Scholar] [CrossRef]
- Qaiser, A.A.; Hyland, M.M.; Patterson, D.A. Surface and charge transport characterization of polyaniline-cellulose acetate composite membranes. J. Phys. Chem. B 2011, 115, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Naeem, H.; Tofil, H.M.; Soliman, M.; Hai, A.; Zaidi, S.H.H.; Kizilbash, N.; Alruwaili, D.; Ajmal, M.; Siddiq, M. Reduced graphene oxide-zinc sulfide nanocomposite decorated with silver nanoparticles for wastewater treatment by adsorption, photocatalysis and antimicrobial action. Molecules 2023, 28, 926. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, P.; Wang, Q.; Wu, Y.; Cao, D.; Qiao, Q.A. Construction of Bi2S3-BiOBr nanosheets on TiO2 NTA as the effective photocatalysts: Pollutant removal, photoelectric conversion and hydrogen generation. J. Colloid Inter. Sci. 2021, 585, 459–469. [Google Scholar] [CrossRef]
- Pan, L.; Yao, L.; Mei, H.; Liu, H.; Jin, Z.; Zhou, S.; Zhang, M.; Zhu, G.; Cheng, L.; Zhang, L. Structurally designable Bi2S3/P-doped ZnO S-scheme photothermal metamaterial enhanced CO2 reduction. Sep. Purif. Technol. 2023, 312, 123365. [Google Scholar] [CrossRef]
- Fang, L.; Zhang, B.; Li, W.; Li, X.; Xin, T.; Zhang, Q. Controllable synthesis of ZnO hierarchical architectures and their photocatalytic property. Superlattices Microstruct. 2014, 75, 324–333. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, H.; Zhu, Y.; Chen, S.; Zhang, Y.; Wei, D.; Sun, J.; Fan, H. A one-pot synthesis of multifunctional Bi2S3 nanoparticles and the construction of core–shell Bi2S3@ Ce6-CeO2 nanocomposites for NIR-triggered phototherapy. J. Mater. Chem. B 2020, 8, 4093–4105. [Google Scholar] [CrossRef]
- Wang, X.; Lv, R.; Wang, K. Synthesis of ZnO@ZnS-Bi2S3 core-shell nanorod grown on reduced graphene oxide sheets and its enhanced photocatalytic performance. J. Mater. Chem. A 2014, 2, 8304–8313. [Google Scholar]
- Xiao, S.; Shen, Z.; Song, S.; Han, S.; Du, Y.; Wang, H. Enhanced sulfadiazine degradation in a multi-electrode paralleling DBD plasma system coupled with ZnO/cellulose acetate films. J. Environ. Chem. Eng. 2023, 11, 109063. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, L.; Qu, K.; Qin, L.; Zhuang, L.; Yang, H.; Xu, Z. Novel Ag-AgBr decorated composite membrane for dye rejection and photodegradation under visible light. Front. Chem. 2021, 15, 892–901. [Google Scholar] [CrossRef]
- Jian, J.; Kuang, D.; Wang, X.; Zhou, H.; Gao, H.; Sun, W.; Yuan, Z.; Zeng, J.; You, K.; Luo, H.A. Highly dispersed Co/SBA-15 mesoporous materials as efficient and stable catalyst for partial oxidation of cyclohexane with molecular oxygen. Mater. Chem. Phys. 2020, 246, 122814. [Google Scholar] [CrossRef]
- Jian, J.; Yang, D.; Liu, P.; You, K.; Sun, W.; Zhou, H.; Yuan, Z.; Ai, Q.; Luo, H. Solvent-free partial oxidation of cyclohexane to KA oil over hydrotalcite-derived Cu-MgAlO mixed metal oxides. Chin. J. Chem. Eng. 2022, 42, 269–276. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Preparation of antimicrobial ultrafine cellulose acetate fibers with silver nanoparticles. Macromol. Rapid Commun. 2004, 25, 1632–1637. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Azzam, A.B.; Geioushy, R.A.; Farida, M.; Salah, B.A. Visible-light-driven 3D hierarchical Bi2S3/BiOBr hybrid structure for superior photocatalytic Cr(VI) reduction. J. Alloys Compd. 2021, 857, 157513. [Google Scholar] [CrossRef]
- Jatoi, A.W.; Kim, I.S.; Ni, Q. Cellulose acetate nanofibers embedded with AgNPs anchored TiO2 nanoparticles for long term excellent antibacterial applications. Carbohydr. Polym. 2019, 207, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Shukrullah, S.; Naz, M.Y.; Bhatti, H.N. Dual S-scheme ZnO-gC3N4-CuO heterosystem: A potential photocatalyst for H2 evolution and wastewater treatment. React. Chem. Eng. 2023, 8, 1159–1175. [Google Scholar] [CrossRef]
- Sivaranjini, B.; Mangaiyarkarasi, R.; Ganesh, V.; Umadevi, S. Vertical alignment of liquid crystals over a functionalized flexible substrate. Sci. Rep. 2018, 8, 8891. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, L.; Cheng, H.; Zhu, Y. Significantly enhanced photocatalytic performance of ZnO via graphene hybridization and the mechanism study. Appl. Catal. B 2011, 101, 382–387. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, S.; Umar, A.; Kansal, S.K. Bismuth sulphide (Bi2S3) nanotubes as an efficient photocatalyst for methylene blue dye degradation. Nanosci. Nanotechnol. Lett. 2016, 8, 266–272. [Google Scholar] [CrossRef]
- Wang, X.; Sui, Y.; Jian, J.; Yuan, Z.; Zeng, J.; Zhang, L.; Wang, T.; Zhou, H. Ag@AgCl nanoparticles in-situ deposited cellulose acetate/silk fibroin composite film for photocatalytic and antibacterial applications. Cellulose 2020, 27, 7721–7737. [Google Scholar] [CrossRef]
- Ji, X.; Li, C.; Liu, J.; Zhang, T.; Yang, Y.; Yu, R.; Luo, X. Controlled Synthesis and Visible-Light-Driven Photocatalytic Activity of BiOBr Particles for Ultrafast Degradation of Pollutants. Molecules 2023, 28, 5558. [Google Scholar] [CrossRef]
- Xu, J.; Jian, J.; Dan, Y.; Song, J.; Meng, L.; Deng, P.; Sun, W.; Zhang, Y.; Xiong, J.; Yuan, Z.; et al. Durable and recyclable BiOBr/silk fibroin-cellulose acetate composite film for efficient photodegradation of dyes under visible light irradiation. Front. Chem. Sci. Eng. 2023, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Tang, Y.; Ng, Y.H.; Li, L.; Jiang, F.; Wang, J.; Chen, W.; Li, L. Understanding photoelectrocatalytic degradation of tetracycline over three-dimensional coral-like ZnO/BiVO4 nanocomposite. Mater. Chem. Phys. 2021, 271, 124871. [Google Scholar] [CrossRef]
- Murugadoss, G.; Salla, S.; Kumar, M.R.; Kandhasamy, N.; Al Garalleh, H.; Garaleh, M.; Brindhadevi, K.; Pugazhendhi, A. Decoration of ZnO surface with tiny sulfide-based nanoparticles for improve photocatalytic degradation efficiency. Environ. Res. 2023, 220, 115171. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Tan, C.; Yang, H.; Kuang, D.; Nie, Z.; Zhou, H. Facile preparation of recyclable and flexible BiOBr@TiO2/PU-SF composite porous membrane for efficient photocatalytic degradation of mineral flotation wastewater. J. Water Process Eng. 2022, 50, 103127. [Google Scholar] [CrossRef]




| Photocatalyst | Photocatalyst Form | Catalyst Dosage /(g/L) | Dye Concentration /(mg/L) | Time /min | pH Levels | Degradation Rate/min | Ref. |
|---|---|---|---|---|---|---|---|
| Bi2S3-BiOI | powder | 1.0 | 10.0-RhB | 60 | 8–9 | >99.0 | [21] |
| SnO2/Bi2S3-Bi25 | powder | 0.33 | 10.0-RhB | 180 | 8–9 | >80.0 | [23] |
| Bi2O3-Bi2S3 | powder | 0.5 | 20.0-RhB | 90 | 8–9 | >99.0 | [24] |
| TiO2-Bi2S3 | powder | 5.0 | 20.0-MO | 10 | 3–4 | >99.0 | [25] |
| Bi2S3-BiOBr/TiO2 NTA | powder | 0.05 | 20.0-RhB | 180 | 8–9 | >98.0 | [35] |
| Bi2S3-ZnO/CA | film | 1.0 | 10.0-RhB | 120 | 3–11 | >90.0 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dan, Y.; Xu, J.; Jian, J.; Meng, L.; Deng, P.; Yan, J.; Yuan, Z.; Zhang, Y.; Zhou, H. In Situ Decoration of Bi2S3 Nanosheets on Zinc Oxide/Cellulose Acetate Composite Films for Photodegradation of Dyes under Visible Light Irradiation. Molecules 2023, 28, 6882. https://doi.org/10.3390/molecules28196882
Dan Y, Xu J, Jian J, Meng L, Deng P, Yan J, Yuan Z, Zhang Y, Zhou H. In Situ Decoration of Bi2S3 Nanosheets on Zinc Oxide/Cellulose Acetate Composite Films for Photodegradation of Dyes under Visible Light Irradiation. Molecules. 2023; 28(19):6882. https://doi.org/10.3390/molecules28196882
Chicago/Turabian StyleDan, Yixiao, Jialiang Xu, Jian Jian, Lingxi Meng, Pei Deng, Jiaqi Yan, Zhengqiu Yuan, Yusheng Zhang, and Hu Zhou. 2023. "In Situ Decoration of Bi2S3 Nanosheets on Zinc Oxide/Cellulose Acetate Composite Films for Photodegradation of Dyes under Visible Light Irradiation" Molecules 28, no. 19: 6882. https://doi.org/10.3390/molecules28196882
APA StyleDan, Y., Xu, J., Jian, J., Meng, L., Deng, P., Yan, J., Yuan, Z., Zhang, Y., & Zhou, H. (2023). In Situ Decoration of Bi2S3 Nanosheets on Zinc Oxide/Cellulose Acetate Composite Films for Photodegradation of Dyes under Visible Light Irradiation. Molecules, 28(19), 6882. https://doi.org/10.3390/molecules28196882





