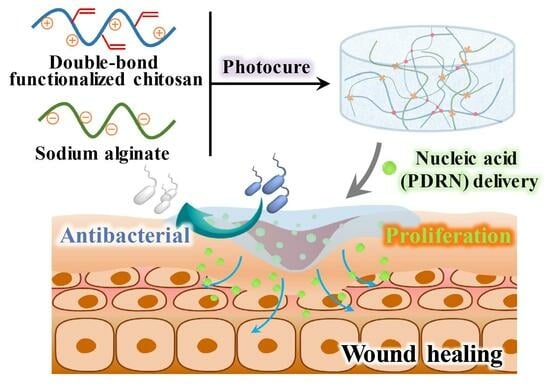
A Photocurable Polysaccharide-Based Hydrogel Delivery of Polydeoxyribonucleotide-Loaded Vectors for Wound Treatment
Abstract
:1. Introduction
2. Results and Discussion
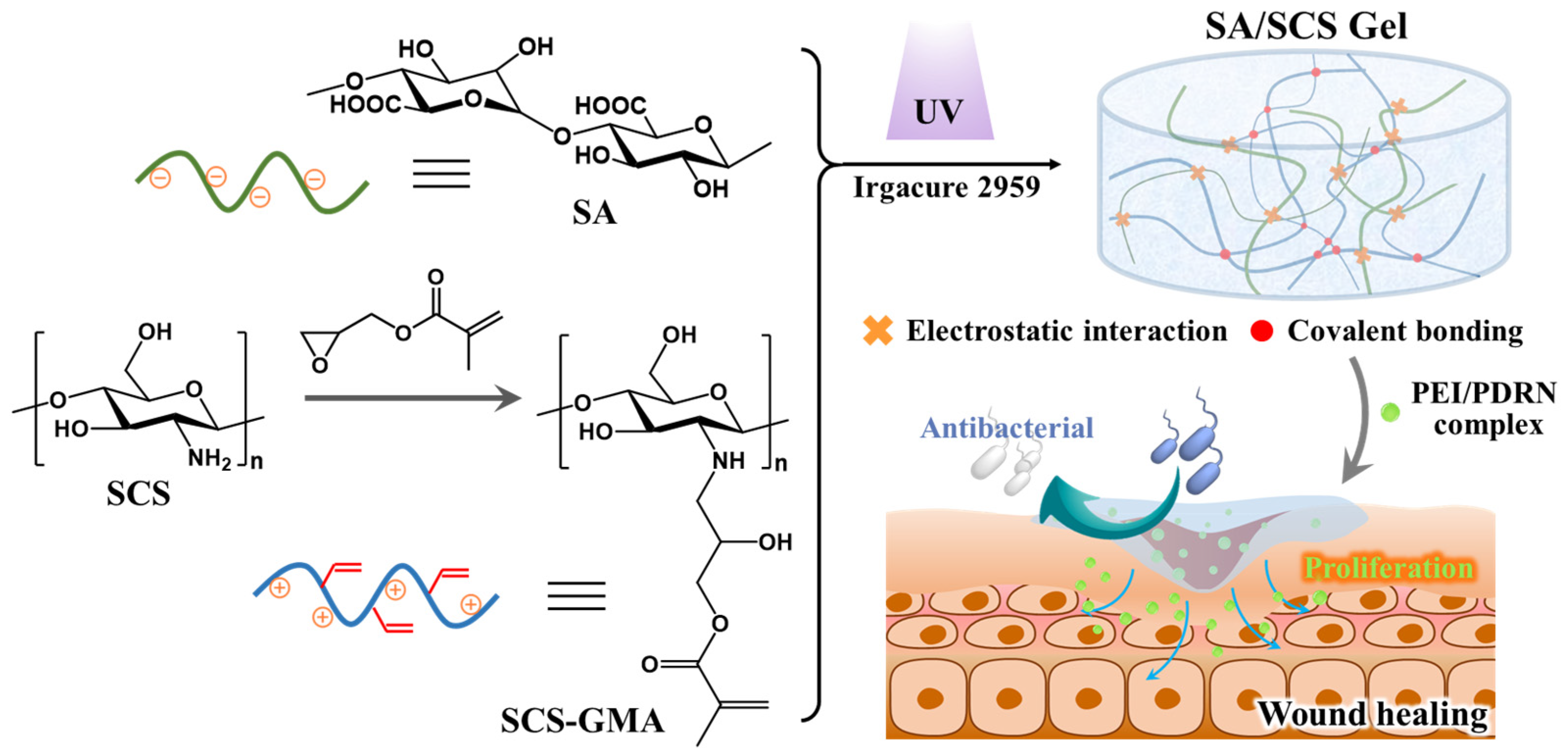
2.1. Preparation and Characterization of the SCS-GMA
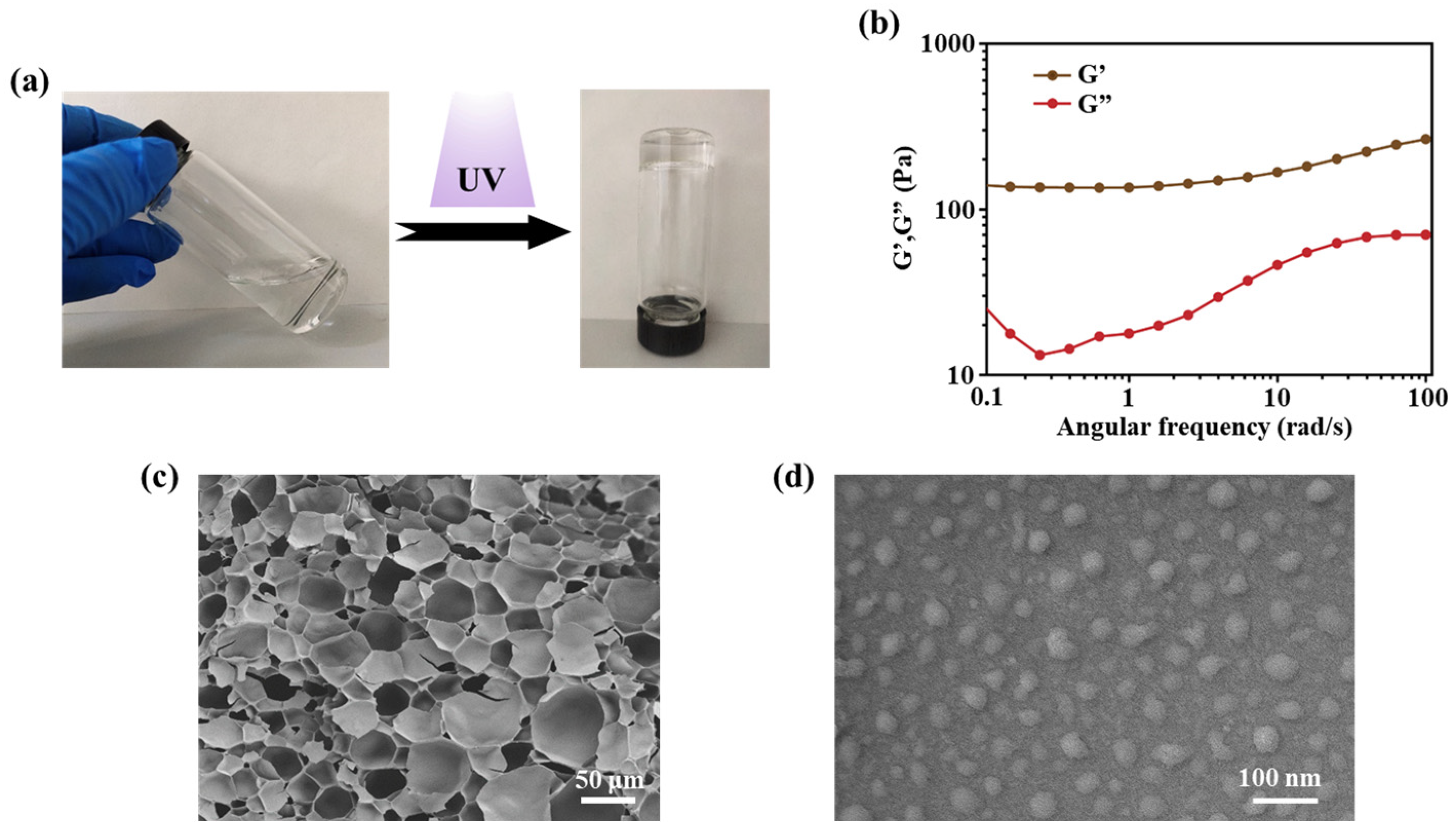
2.2. Preparation and Characterization of the SA/SCS Gel and PEI/PDRN Complexes
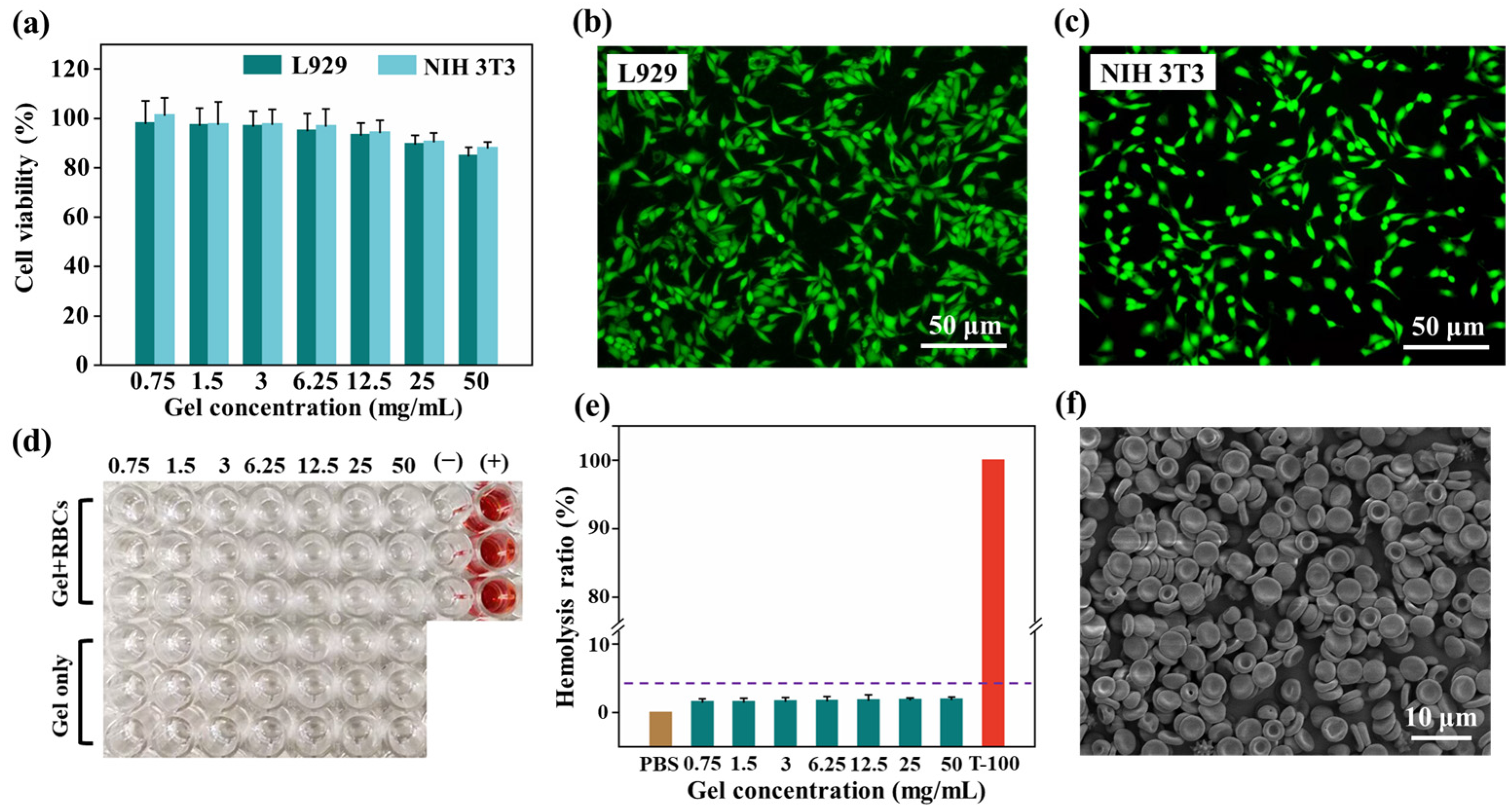
2.3. In Vitro Biocompatibility
2.4. PDRN Release Profile and Cell Proliferation
2.5. Antibacterial Ability
2.6. In Vivo Wound Healing Efficacy
3. Materials and Methods
3.1. Materials
3.2. Preparation of SCS-GMA
3.3. Preparation of SA/SCS Gel and PEI/PDRN Complexes
3.4. Cell Viability Evaluation
3.5. Hemolysis Test
3.6. PDRN Release Profile
3.7. Cell Proliferation Test
3.8. Antibacterial Activity Assay
3.9. Wound Healing Study
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dong, R.; Guo, B. Smart wound dressings for wound healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Minsart, M.; Van Vlierberghe, S.; Dubruel, P.; Mignon, A. Commercial wound dressings for the treatment of exuding wounds: An in-depth physico-chemical comparative study. Burn. Trauma 2022, 10, tkac024. [Google Scholar] [CrossRef]
- Farahani, M.; Shafiee, A. Wound healing: From passive to smart dressings. Adv. Healthc. Mater. 2021, 10, 2100477. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.P.; He, J.H.; Guo, B.L. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Wang, H.N.; Xu, Z.J.; Zhao, M.; Liu, G.T.; Wu, J. Advances of hydrogel dressings in diabetic wounds. Biomater. Sci. 2021, 9, 1530–1546. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Peter, S.; Mbese, Z.; Aderibigbe, B.A. Polymer-based wound dressing materials loaded with bioactive agents: Potential materials for the treatment of diabetic wounds. Polymers 2022, 14, 724. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, D.; Dai, K.L.; Wang, Y.X.; Song, P.; Li, H.R.; Tang, P.; Zhang, Z.Y.; Li, Z.Y.; Zhou, Y.C.; et al. Recent progress of collagen, chitosan, alginate and other hydrogels in skin repair and wound dressing applications. Int. J. Biol. Macromol. 2022, 208, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Y.; Xu, F.J. Versatile functionalization of polysaccharides via polymer grafts: From design to biomedical applications. Acc. Chem. Res. 2017, 50, 281–292. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Zhang, H.T.; Cheng, J.Q.; Ao, Q. Preparation of alginate-based biomaterials and their applications in biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef]
- Ji, D.; Park, J.M.; Oh, M.S.; Nguyen, T.L.; Shin, H.; Kim, J.S.; Kim, D.; Park, H.S.; Kim, J. Superstrong, superstiff, and conductive alginate hydrogels. Nat. Commun. 2022, 13, 3019. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Rivas-Garcia, L.; Baptista, P.V.; Fernandes, A.R. Gene therapy in cancer treatment: Why go nano? Pharmaceutics 2020, 12, 233. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, Y.F.; Wong, Y.S.; Liew, M.W.J.; Venkatraman, S. Recent advances in chitosan-based carriers for gene delivery. Mar. Drugs 2019, 17, 381. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Jing, X.D.; Liu, Y.; Yu, B.; Hu, H.; Cong, H.; Shen, Y.Q. A chitosan derivative-crosslinked hydrogel with controllable release of polydeoxyribonucleotides for wound treatment. Carbohydr. Polym. 2023, 300, 120298. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xiu, K.M.; Xu, S.L.; Yang, W.T.; Xu, F.J. Functionalized layered double hydroxide nanoparticles conjugated with disulfide-linked polycation brushes for advanced gene delivery. Bioconjugate Chem. 2013, 24, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, R.Y.; Zhao, X.; Zhang, Y.H.; Tam, A.; Yan, Y.F.; Shen, H.K.; Zhang, Y.S.; Qi, J.; Feng, Y.; et al. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019, 11, 3. [Google Scholar] [CrossRef]
- Rinoldi, C.; Ziai, Y.; Zargarian, S.S.; Nakielski, P.; Zembrzycki, K.; Bayan, M.A.H.; Zakrzewska, A.B.; Fiorelli, R.; Lanzi, M.; Kostrzewska-Ksiezyk, A.; et al. In vivo chronic brain cortex signal recording based on a soft conductive hydrogel biointerface. ACS Appl. Mater. Interfaces 2023, 15, 6283–6296. [Google Scholar] [CrossRef]
- Ziai, Y.; Rinoldi, C.; Nakielski, P.; De Sio, L.; Pierini, F. Smart plasmonic hydrogels based on gold and silver nanoparticles for biosensing application. Curr. Opin. Biomed. Eng. 2022, 24, 100413. [Google Scholar] [CrossRef]
- Han, J.; Wang, K.M.; Yang, D.Z.; Nie, J. Photopolymerization of methacrylated chitosan/PNIPAAm hybrid dual-sensitive hydrogels as carrier for drug delivery. Int. J. Biol. Macromol. 2009, 44, 229–235. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wang, J.K.; Yu, B.R.; Xu, F.J. Biomedical cationic polymers based on the amino-epoxide ring-opening reaction. Acta Polym. Sin. 2022, 53, 828–841. [Google Scholar]
- Jing, H.J.; Huang, X.; Du, X.J.; Mo, L.; Ma, C.Y.; Wang, H.X. Facile synthesis of pH-responsive sodium alginate/carboxymethyl chitosan hydrogel beads promoted by hydrogen bond. Carbohydr. Polym. 2022, 278, 118993. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Peng, J.R.; Xiao, H.T.; Xu, X.W.; Qian, Z.Y. Polysaccharide hydrogels: Functionalization, construction and served as scaffold for tissue engineering. Carbohydr. Polym. 2022, 278, 118952. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, J.H.; Liu, Z.Q.; Xu, F.; Zhou, J.X.; Zrinyi, M.; Osada, Y.; Chen, Y.M. Novel biocompatible polysaccharide-based self-healing hydrogel. Adv. Funct. Mater. 2015, 25, 1352–1359. [Google Scholar] [CrossRef]
- Jing, X.D.; Sun, Y.Z.; Liu, Y.; Ma, X.L.; Hu, H. Alginate/chitosan-based hydrogel loaded with gene vectors to deliver polydeoxyribonucleotide for effective wound healing. Biomater. Sci. 2021, 9, 5533–5541. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Yang, S.W.; Hao, Y.; Guo, J.Y.; Zhang, W.C.; Yu, Q.; Yin, X.Z.; Tan, Y.Q. Liquefied polysaccharides-based polymer with tunable condensed state structure for antimicrobial shield by multiple processing methods. Small Methods 2022, 6, 2200129. [Google Scholar] [CrossRef]
- Rahayu, D.P.; De Mori, A.; Yusuf, R.; Draheim, R.; Lalatsa, A.; Roldo, M. Enhancing the antibacterial effect of chitosan to combat orthopaedic implant-associated infections. Carbohydr. Polym. 2022, 289, 119385. [Google Scholar] [CrossRef] [PubMed]
- Pourbadiei, B.; Adlsadabad, S.Y.; Rahbariasr, N.; Pourjavadi, A. Synthesis and characterization of dual light/temperature-responsive supramolecular injectable hydrogel based on host-guest interaction between azobenzene and starch-grafted β-cyclodextrin: Melanoma therapy with paclitaxel. Carbohydr. Polym. 2023, 313, 120667. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Shao, Z.; Bai, Q.; Zhang, X.; He, Y.S.; Lu, J.; Zou, D.; Feng, C.; Dong, C.M. Biomimetic glycopolypeptide hydrogels with tunable adhesion and microporous structure for fast hemostasis and highly efficient wound healing. Adv. Funct. Mater. 2021, 31, 2105628. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Ma, X.; Gao, Q.; Zhang, M.; Hu, H. A Photocurable Polysaccharide-Based Hydrogel Delivery of Polydeoxyribonucleotide-Loaded Vectors for Wound Treatment. Molecules 2023, 28, 6788. https://doi.org/10.3390/molecules28196788
Li Z, Ma X, Gao Q, Zhang M, Hu H. A Photocurable Polysaccharide-Based Hydrogel Delivery of Polydeoxyribonucleotide-Loaded Vectors for Wound Treatment. Molecules. 2023; 28(19):6788. https://doi.org/10.3390/molecules28196788
Chicago/Turabian StyleLi, Zonghui, Xiaojun Ma, Qiang Gao, Mingxin Zhang, and Hao Hu. 2023. "A Photocurable Polysaccharide-Based Hydrogel Delivery of Polydeoxyribonucleotide-Loaded Vectors for Wound Treatment" Molecules 28, no. 19: 6788. https://doi.org/10.3390/molecules28196788
APA StyleLi, Z., Ma, X., Gao, Q., Zhang, M., & Hu, H. (2023). A Photocurable Polysaccharide-Based Hydrogel Delivery of Polydeoxyribonucleotide-Loaded Vectors for Wound Treatment. Molecules, 28(19), 6788. https://doi.org/10.3390/molecules28196788