Preparation and Application of Amino-Terminated Hyperbranched Magnetic Composites in High-Turbidity Water Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Fe3O4/HBPN
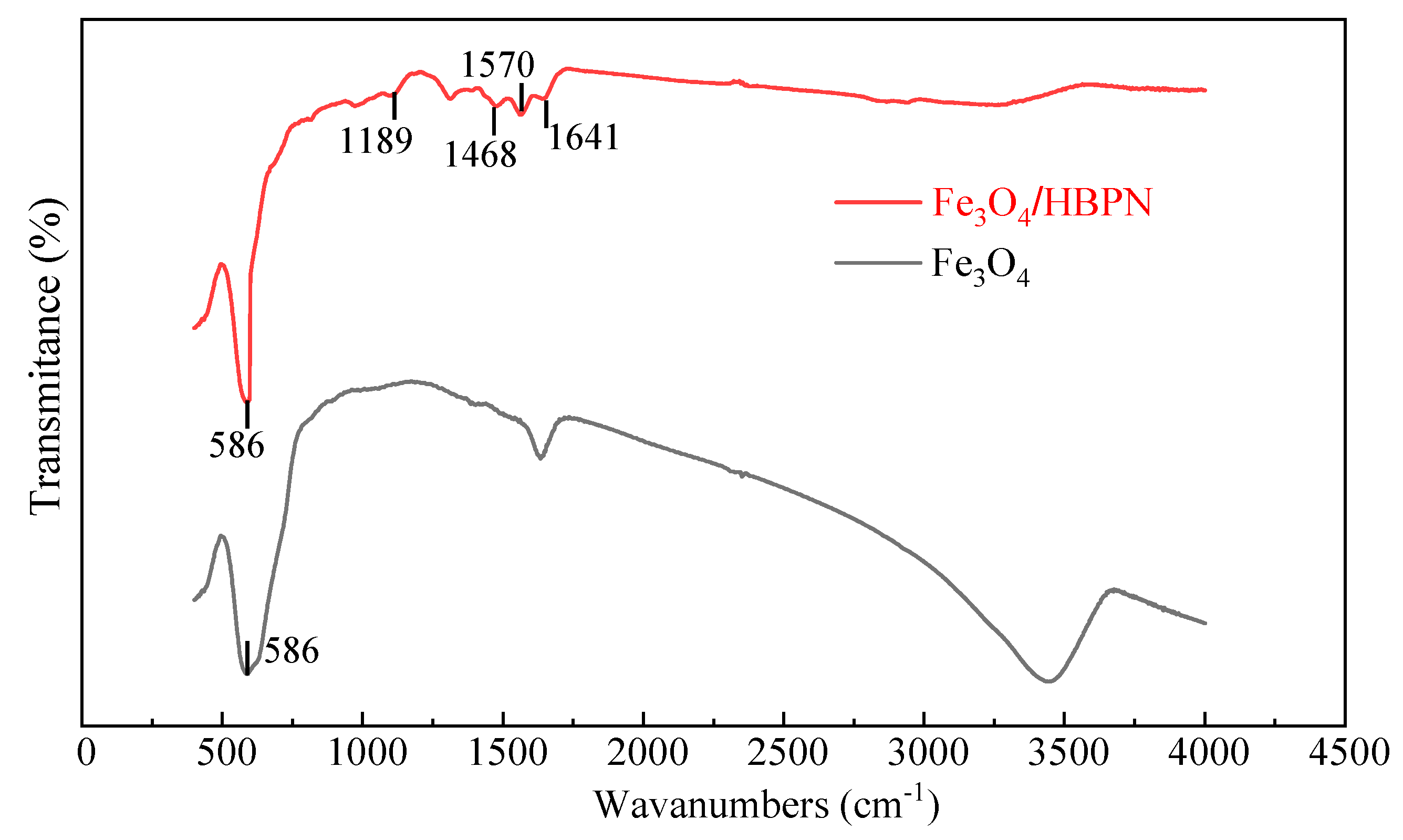
2.1.1. FTIR
2.1.2. XRD
2.1.3. XPS
2.1.4. SEM and EDS
2.1.5. TGA
2.1.6. VSM
2.1.7. Zeta Potential
2.2. Application Performance of Fe3O4/HBPN
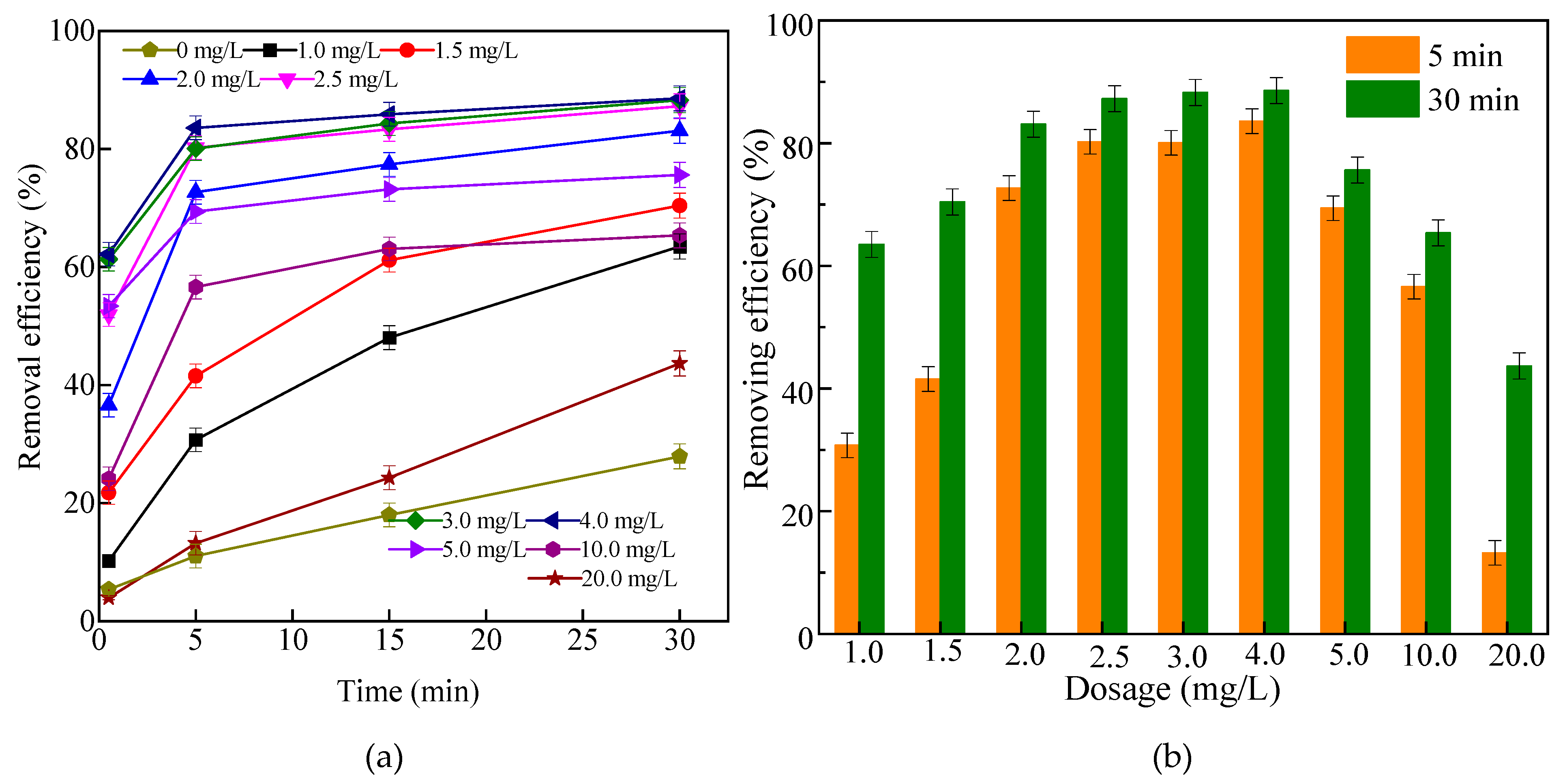
2.2.1. Effects of Dosage
2.2.2. Effects of pH
2.2.3. Effects of Concentration of Kaolin Suspensions
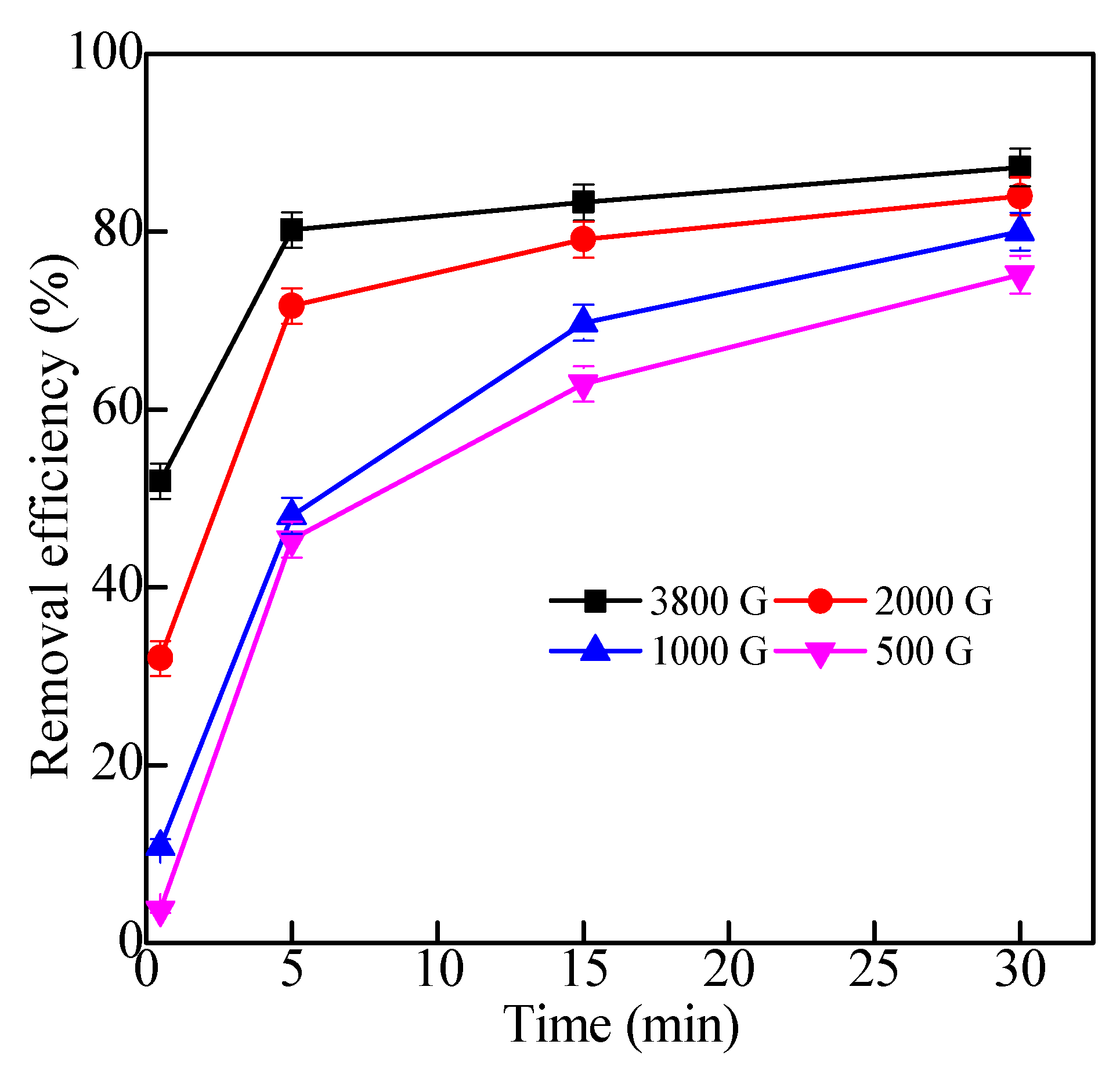
2.2.4. Effects of Magnetic Fields
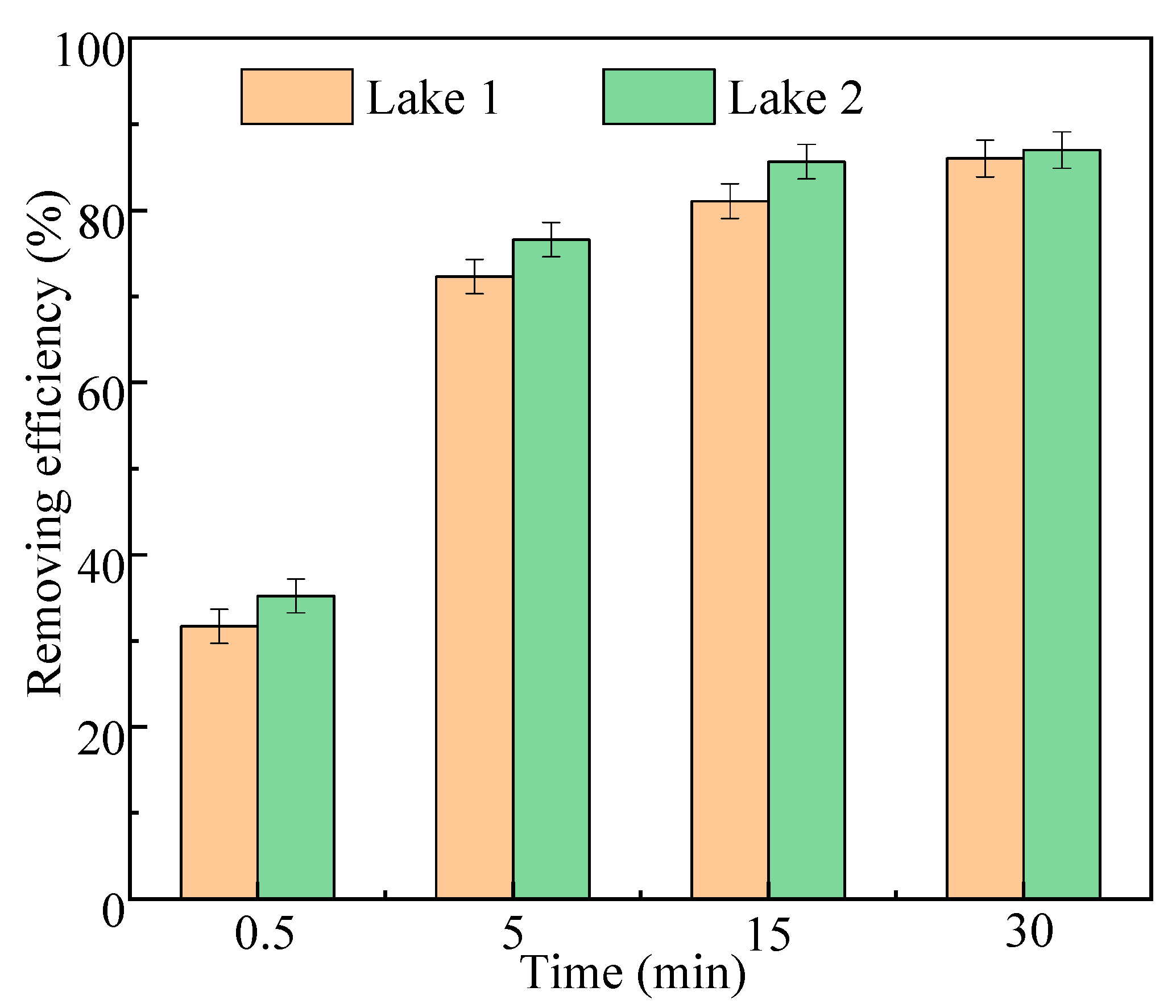
2.2.5. The Actual Water Application
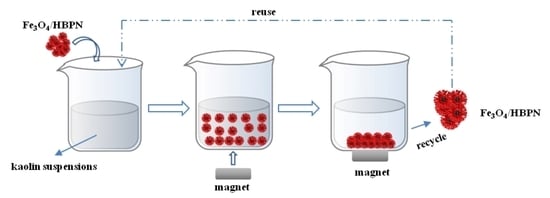
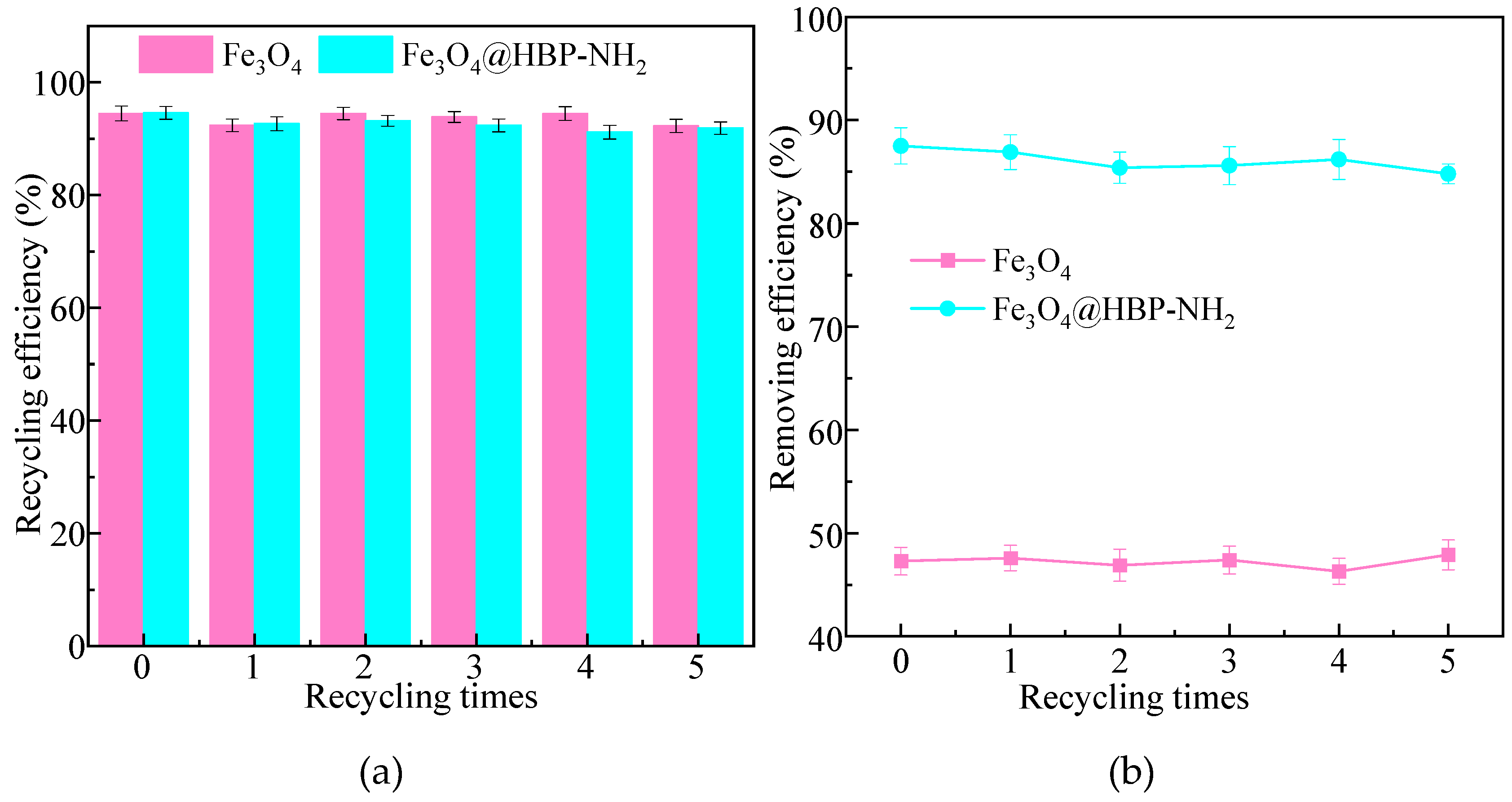
2.3. Recycling and Reusing
3. Materials and Methods
3.1. Materials
3.2. Synthesis of magnetic Fe3O4 nanoparticles
3.3. Preparation of Magnetic Fe3O4/HBPN Composites
3.4. Characterization of Fe3O4/HBPN
3.5. The Magnetic Separation Experiment
3.6. Recycle and Reuse of Fe3O4/HBPN
3.7. Analytical Methods
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xia, X.; Lan, S.; Li, X.; Xie, Y.; Liang, Y.; Yan, P.; Chen, Z.; Xing, Y. Characterization and coagulation-flocculation performance of a composite flocculant in high-turbidity drinking water treatment. Chemosphere 2018, 206, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Wang, Z.; Zhang, R.; Ma, J.; Zhang, H.; Li, S.; Li, J. Aspergillus oryzae, a novel eco-friendly fungal bioflocculant for turbid drinking water treatment. Sep. Purif. Technol. 2021, 279, 119669. [Google Scholar] [CrossRef]
- Shabanizadeh, H.; Taghavijeloudar, M. A sustainable approach for industrial wastewater treatment using pomegranate seeds in flocculation-coagulation process: Optimization of COD and turbidity removal by response surface methodology (RSM). J. Water Process Eng. 2023, 53, 103651. [Google Scholar] [CrossRef]
- Liu, X.; Xia, J.; Zu, J.; Zeng, Z.; Li, Y.; Li, J.; Wang, Q.; Liu, Z.; Cai, W. Spatiotemporal variations and gradient functions of water turbidity in shallow lakes. Ecol. Indic. 2023, 147, 109928. [Google Scholar] [CrossRef]
- Song, S.; Le-Clech, P.; Shen, Y. Microscale fluid and particle dynamics in filtration processes in water treatment: A review. Water Res. 2023, 233, 119746. [Google Scholar] [CrossRef]
- Zhao, K.; Wei, Y.; Dong, J.; Zhao, P.; Wang, Y.; Pan, X.; Wang, J. Separation and characterization of microplastic and nanoplastic particles in marine environment. Environ. Pollut. 2022, 297, 118773. [Google Scholar] [CrossRef]
- Bokhari, T.H.; Sultana, H.; Usman, M. Adsorptive micellar flocculation (surfactant-based phase separation technique): Theory and applications. J. Mol. Liq. 2020, 323, 115001. [Google Scholar]
- Xu, S.; Shi, J.; Deng, J.; Sun, H.; Wu, J.; Ye, Z. Flocculation and dewatering of the Kaolin slurry treated by single- and dual-polymer flocculants. Chemosphere 2023, 328, 138445. [Google Scholar] [CrossRef]
- Cruz, D.; Pimentel, M.; Russo, A.; Cabral, W. Charge neutralization mechanism efficiency in water with high color turbidity ratio using aluminium sulfate and flocculation index. Water 2020, 12, 572. [Google Scholar] [CrossRef]
- Zhou, L.; Han, Y.; Li, W.; Zhu, Y. Study on polymer-bridging flocculation performance of ultrafine specular hematite ore and its high gradient magnetic separation behavior: Description of floc microstructure and flocculation mechanism. Sep. Purif. Technol. 2021, 276, 119304. [Google Scholar] [CrossRef]
- Housni, S.; Abramson, S.; Guigner, J.M.; Levitz, P.; Michot, L. Flocculation and magnetically-assisted sedimentation of size-sorted beidellite platelets mixed with maghemite nanoparticles. Nano Res. 2020, 13, 3001–3011. [Google Scholar] [CrossRef]
- Lee, H.K.; Kim, J.H.; Kim, I.; Jeon, H. Efficient separation performance of suspended soil and strontium from aqueous solution using magnetic flocculant. J. Environ. Chem. Eng. 2021, 9, 106810. [Google Scholar] [CrossRef]
- Ma, J.; Fu, X.; Jiang, L.; Zhu, G.; Shi, J. Magnetic flocculants synthesized by Fe3O4 coated with cationic polyacrylamide for high turbid water flocculation. Environ. Sci. Pollut. Res. 2018, 25, 25955–25966. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Xu, H.; Fan, Q.; Zhu, C.; Liu, J.; Zhu, M.; Wang, X.; Niu, A. Preparation and application of magnetic composites using controllable assembly for use in water treatment: A review. Molecules 2023, 28, 5799. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, X.; Qin, L.; Li, H.; Liang, W. Magnetic coagulation and flocculation of a kaolin suspension using Fe3O4 coated with SiO2. J. Environ. Chem. Eng. 2021, 9, 105980. [Google Scholar] [CrossRef]
- Wang, T.; Yang, W.; Hong, Y.; Hou, Y. Magnetic nanoparticles grafted with amino-riched dendrimer as magnetic flocculant for efficient harvesting of oleaginous microalgae. Chem. Eng. J. 2016, 297, 304–314. [Google Scholar] [CrossRef]
- Perez, T.; Pasquini, D.; Lima, A.d.F.; Rosa, E.V.; Sousa, M.H.; Cerqueira, D.A.; de Morais, L.C. Efficient removal of lead ions from water by magnetic nanosorbents based on manganese ferrite nanoparticles capped with thin layers of modified biopolymers. J. Environ. Chem. Eng. 2019, 7, 102892. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, W.; Liu, L.; Li, F.; Fan, Q.; Sun, X. Harvesting Chlorella vulgaris by magnetic flocculation using Fe3O4 coating with polyaluminium chloride and polyacrylamide. Bioresour. Technol. 2015, 198, 789–796. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, X.; Cui, X.; Zheng, A.; Li, J.; Bai, Y.; Zheng, Y. Facile synthesis of hyperbranched magnetic nanomaterials for selective adsorption of proteins. Talanta 2023, 252, 123895. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Q.; Yan, C.; Li, J.; Zhou, W.; Gao, H.; Zhang, S.; Lu, R. Hyperbranched aromatic polyamide modified magnetic nanoparticles for the extraction of benzoylurea insecticides. J. Sep. Sci. 2021, 44, 1931–1938. [Google Scholar] [CrossRef]
- Huo, L.; Zhang, Z.; Shi, X. Latest research and developing tendency of hyperbranched polymers fabrication. J. Polym. Res. 2021, 28, 355. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Z.; Zhang, D. Synthesis and application of epoxy-ended hyperbranched polymers. Chem. Eng. J. 2018, 343, 283–302. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Kim, K.H. Hyperbranched polymers as superior adsorbent for the treatment of dyes in water. Adv. Colloid Interface Sci. 2022, 302, 102633. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, M.L.; Ganjaee, S.M.; Bahram, R. Polyester-amide hyperbranched polymer as an interfacial modifier for graphene oxide nanosheets: Mechanistic approach in an epoxy nanocomposite coating. Prog. Org. Coat. 2020, 142, 105573. [Google Scholar] [CrossRef]
- Wan, W.; Hui, O.; Jiang, Z.; Cui, Y.; Li, J.; He, M.; Yang, S.; Zhang, X.; Feng, Y.; Wei, Y. Synthesis and intracellular drug delivery applications of hyperbranched polymers functionalized β-cyclodextrin. Colloid Interface Sci. Commun. 2021, 42, 100425. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, X.; Liu, X.; Li, J.; Zhou, W.; Gao, H.; Zhang, S.; Lu, R. Magnetic nanoparticles modified with hyperbranched polyamidoamine for the extraction of benzoylurea insecticides prior to their quantitation by HPLC. Microchim. Acta 2019, 186, 351. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, J.; Chen, P.; Zeng, Q.; Wen, X.; Wen, W.; Liu, Y.; Chen, A.; Guan, J.; Liu, X.; et al. A new metallic composite cathode originated from hyperbranched polymer coated MOF for High-performance Lithium-Sulfur batteries. Chem. Eng. J. 2022, 435, 135125. [Google Scholar] [CrossRef]
- Malekzadeh, A.M.; Ramazani, A.; Rezaei, S.J.T.; Niknejad, H. Design and construction of multifunctional hyperbranched polymers coated magnetite nanoparticles for both targeting magnetic resonance imaging and cancer therapy. J. Colloid Interface Sci. 2017, 490, 64–73. [Google Scholar] [CrossRef]
- Tian, W.; Li, X.; Wang, J. Supramolecular hyperbranched polymers. Chem. Commun. 2017, 53, 2531–2542. [Google Scholar] [CrossRef]
- Belgaonkar, M.S.; Balasubramanian, K. Hyperbranched polymer-based nanocomposites: Synthesis, progress, and applications. Eur. Polym. J. 2021, 147, 110301. [Google Scholar] [CrossRef]
- Hasan, K.; Joseph, R.G.; Patole, S.P.; Al-Qawasmeh, R.A. Development of magnetic Fe3O4-chitosan immobilized Cu(II) Schiff base catalyst: An efficient and reusable catalyst for microwave assisted one-pot synthesis of propargylamines via A3 coupling. Catal. Commun. 2023, 174, 106588. [Google Scholar] [CrossRef]
- Yeamsuksawat, T.; Zhao, H.; Liang, J. Characterization and antimicrobial performance of magnetic Fe3O4@Chitosan@Ag nanoparticles synthesized via suspension technique. Mater. Today Commun. 2021, 28, 102481. [Google Scholar] [CrossRef]
- Calahorra-Rio, L.; Guadano-Sanchez, M.; Moya-Cavas, T.; Urraca, J.L. Magnetic core-shell nanoparticles using molecularly imprinted polymers for zearalenone determination. Molecules 2022, 27, 8166. [Google Scholar] [CrossRef] [PubMed]
- Bilkan, M.T.; Yurdakul, Ş.; Demircioğlu, Z.; Büyükgüngör, O. Crystal structure, FT-IR, FT-Raman and DFT studies on a novel compound [C10H9N3]4AgNO3. J. Organomet. Chem. 2016, 805, 108–116. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Jiang, X.; Fan, Q.; Li, X.; Jiao, L.; Liang, W. Harvesting of Chlorella vulgaris using Fe3O4 coated with modified plant polyphenol. Environ. Sci. Pollut. Res. 2018, 25, 26246–26258. [Google Scholar] [CrossRef]
- Balkanloo, P.G.; Mahmoudian, M.; Hosseinzadeh, M.T. A comparative study between MMT-Fe3O4/PES, MMT-HBE/PES, and MMT-acid activated/PES mixed matrix membranes. Chem. Eng. J. 2020, 396, 125188. [Google Scholar] [CrossRef]
- Tariq, A.; Aamir, M.; Farhat Mehmood, R.; Akhtar, J.; Sher, M. Nanostructured Fe3O4@SiO2 shell-coated APTES/AEAPS as an efficient and recyclable catalyst for selective N-alkylation of amines using alcohol. Mater. Today: Proc. 2022, 53, 361–368. [Google Scholar] [CrossRef]
- Seal, P.; Alam, A.; Borgohain, C.; Paul, N.; Babu, P.D.; Borah, J.P. Optimization of self heating properties of Fe3O4 using PEG and amine functionalized MWCNT. J. Alloys Compd. 2021, 882, 160653. [Google Scholar] [CrossRef]
- Wu, G.; Tu, H.; Niu, F.; Lu, S.; Liu, Y.; Gao, K.; Chen, Z.; Wang, P.; Li, Z. Synthesis of polymer-functionalized β-cyclodextrin, Mg2+ doped, coating magnetic Fe3O4 nanoparticle carriers for penicillin G acylase immobilization. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130609. [Google Scholar] [CrossRef]
- Lu, M.; Liu, M.; Wang, L.; Xu, S.; Zhao, J.; Li, H. Structural and magnetic properties of CoFe2O4 /CoFe2/SiO2 nanocomposites with exchange coupling behavior. J. Alloys Compd. 2017, 690, 27–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, T.; Xu, Y.; Yang, Y.; Sheng, D.; Ma, Q. Quaternized salicylaldehyde Schiff base side-chain polymer-grafted magnetic Fe3O4 nanoparticles for the removal and detection of Cu2+ ions in water. Appl. Surf. Sci. 2023, 611, 155632. [Google Scholar] [CrossRef]
- Shen, H.; Chen, Z.; Li, Z.; Hu, M.; Dong, X.; Xia, Q. Controlled synthesis of 2,4,6-trichlorophenol-imprinted amino-functionalized nano-Fe3O4-polymer magnetic composite for highly selective adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 439–450. [Google Scholar] [CrossRef]
- Deng, Z.; Luo, Y.; Bian, M.; Guo, X.; Zhang, N. Synthesis of easily renewable and recoverable magnetic PEI-modified Fe3O4 nanoparticles and its application for adsorption and enrichment of tungsten from aqueous solutions. Environ. Pollut. 2023, 330, 121703. [Google Scholar] [CrossRef]
- Sharifi, M.J.; Nouralishahi, A.; Hallajisani, A. Fe3O4-chitosan nanocomposite as a magnetic biosorbent for removal of nickel and cobalt heavy metals from polluted water. Int. J. Biol. Macromol. 2023, 248, 125984. [Google Scholar] [CrossRef]
- Nagappan, S.; Duraivel, M.; Muthuchamy, N.; Han, S.H.; Mohan, B.; Park, S.; Prabakar, K.; Lee, J.M.; Park, K.H. Straightforward engineering of porous C3N4/Fe3O4 electrocatalyst for oxygen reduction reaction in alkaline medium. Mater. Today Chem. 2023, 30, 101534. [Google Scholar] [CrossRef]
- Ghobeira, R.; Tabaei, P.S.E.; Morent, R.; Geyter, N.D. Chemical characterization of plasma-activated polymeric surfaces via XPS analyses: A review. Surf. Interfaces 2022, 31, 102087. [Google Scholar] [CrossRef]
- Ma, B.; Li, S.; Wang, S.; Gao, M.; Guo, L.; She, Z.; Zhao, Y.; Jin, C.; Yu, N.; Zhao, C. Effect of Fe3O4 nanoparticles on composition and spectroscopic characteristics of extracellular polymeric substances from activated sludge. Process Biochem. 2018, 75, 212–220. [Google Scholar] [CrossRef]
- Maslakov, K.I.; Teterin, Y.A.; Popel, A.J.; Teterin, A.Y.; Ivanov, K.E.; Kalmykov, S.N.; Petrov, V.G.; Springell, R.; Scott, T.B.; Farnan, I. XPS study of the surface chemistry of UO2 (111) single crystal film. Appl. Surf. Sci. 2017, 433, 582–588. [Google Scholar] [CrossRef]
- Sitthichai, S.; Pilapong, C.; Thongtem, T.; Thongtem, S. CMC-coated Fe3O4 nanoparticles as new MRI probes for hepatocellular carcinoma. Appl. Surf. Sci. 2015, 356, 972–977. [Google Scholar] [CrossRef]
- Lu, J.; Fu, F.; Ding, Z.; Na, L.; Bing, T. Removal mechanism of selenite by Fe3O4-precipitated mesoporous magnetic carbon microspheres. J. Hazard. Mater. 2017, 330, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.U.; Shin, J.R.; Han, J.S.; Kim, Y.H.; An, G.S. Self-assembled core-shell Fe3O4-Pt nanoparticles via silylation/polymerization-based amino-functionalization. Colloid Interface Sci. Commun. 2022, 50, 100655. [Google Scholar] [CrossRef]
- El-Aal, M.A.; Said, A.E.-A.A.; Goda, M.N.; Abo Zeid, E.F.; Ibrahim, S.M. Fe3O4@CMC-Cu magnetic nanocomposite as an efficient catalyst for reduction of toxic pollutants in water. J. Mol. Liq. 2023, 385, 122317. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, J.; Jia, H.; Guo, X.; Yue, Y.; Yuan, Y.; Yue, T. Synthesis of silver/Fe3O4@chitosan@polyvinyl alcohol magnetic nanoparticles as an antibacterial agent for accelerating wound healing. Int. J. Biol. Macromol. 2022, 221, 1404–1414. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, X.; Wang, X.; Wang, Q.; Liang, W. Magnetic polyphenol nanocomposite of Fe3O4/SiO2/PP for Cd(II) adsorption from aqueous solution. Environ. Technol. 2020, 43, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Xiong, L.; Liu, F.; Li, C. Grafting hyperbranched polymers onto TiO2 nanoparticles via thiol-yne click chemistry and its effect on the mechanical, thermal and surface properties of polyurethane coating. Materials 2019, 12, 2817. [Google Scholar] [CrossRef] [PubMed]
- Kalbasi, R.J.; Zamani, F. Synthesis and characterization of Ni nanoparticles incorporated into hyperbranched polyamidoamine–polyvinylamine/SBA-15 catalyst for simple reduction of nitro aromatic compounds. RSC Adv. 2014, 4, 7444–7453. [Google Scholar] [CrossRef]
- Oliva, F.S.N.; Sahihi, M.; Lenglet, L.; Ospina, A.; Guenin, E.; Jaramillo-Botero, A.; Goddard, W.A.; Bedoui, F. Nanoparticle size and surface chemistry effects on mechanical and physical properties of nano-reinforced polymers: The case of PVDF-Fe3O4 nano-composites. Polym. Test. 2023, 117, 107851. [Google Scholar] [CrossRef]
- Vattathurvalappil, S.H.; Kundurthi, S.; Drzal, L.T.; Haq, M. Thermo-mechanical degradation in ABS-Fe3O4 polymer nanocomposite due to repeated electromagnetic heating. Compos. Part B Eng. 2020, 201, 108374. [Google Scholar] [CrossRef]
- Bahadur, A.; Saeed, A.; Shoaib, M.; Iqbal, S.; Bashir, M.I.; Waqas, M.; Hussain, M.N.; Abbas, N. Eco-friendly synthesis of magnetite (Fe3O4) nanoparticles with tunable size: Dielectric, magnetic, thermal and optical studies. Mater. Chem. Phys. 2017, 198, 229–235. [Google Scholar] [CrossRef]
- Xia, Z.; Singh, A.; Kiratitanavit, W.; Mosurkal, R.; Kumar, J.; Nagarajan, R. Unraveling the mechanism of thermal and thermo-oxidative degradation of tannic acid. Thermochim. Acta 2015, 605, 77–85. [Google Scholar] [CrossRef]
- Thébault, M.; Pizzi, A.; Essawy, H.A.; Barhoum, A.; Assche, G.V. Isocyanate free condensed tannin-based polyurethanes. Eur. Polym. J. 2015, 67, 513–526. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Kumar, S.H.; Jyothi, N.V.V. Rapid removal of Ni(II) from aqueous solution using 3-Mercaptopropionic acid functionalized bio magnetite nanoparticles. Water Resour. Ind. 2015, 12, 1–7. [Google Scholar] [CrossRef]
- Cui, K.; Yuan, L.; Zhao, Z. Magnetic properties of Ni3Si/Fe3O4@PVDF composites with different Fe3O4 nanoparticles content based on lamellar Ni3Si template. Mater. Sci. Eng. B 2023, 290, 116330. [Google Scholar] [CrossRef]
- Mou, F.Z.; Guan, J.G.; Ma, H.R.; Xu, L.L.; Shi, W.D. Magnetic iron oxide chestnutlike hierarchical nanostructures: Preparation and their excellent arsenic removal capabilities. ACS Appl. Mater. Interfaces 2012, 4, 3987–3993. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.H.A.; Lananan, F.; Wan, N.S.D.; Su, S.L.; Khatoon, H.; Endut, A.; Jusoh, A. Harvesting microalgae, Chlorella sp. by bio-flocculation of Moringa oleifera seed derivatives from aquaculture wastewater phytoremediation. Int. Biodeterior. Biodegrad. 2014, 95, 270–275. [Google Scholar] [CrossRef]
- Liu, P.; Wang, T.; Yang, Z.; Hong, Y.; Hou, Y. Long-chain poly-arginine functionalized porous Fe3O4 microspheres as magnetic flocculant for efficient harvesting of oleaginous microalgae. Algal Res. 2017, 27, 99–108. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, Q.; Wang, X.; Jiang, X.; Jiao, L.; Liang, W. Application of Fe3O4 coated with modified plant polyphenol to harvest oleaginous microalgae. Algal Res. 2019, 38, 101417. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, Y.; Yu, W.; Shen, H.Y.; Zhang, H.Q.; Gu, N. Preparation and characterization of magnetite nanoparticles coated by amino silane. Colloids Surf. A Physicochem. Eng. Asp. 2003, 212, 219–226. [Google Scholar] [CrossRef]
- Toh, P.Y.; Ng, B.W.; Chong, C.; Ahmad, A.L.; Yang, J.W.; Derek, C.J.C.; Lim, J.K. Magnetophoretic separation of microalgae: The role of nanoparticles and polymer binder in harvesting biofuel. RSC Adv. 2014, 4, 4114–4121. [Google Scholar] [CrossRef]
- Wang, S.; Stiles, A.R.; Guo, C.; Liu, C. Harvesting microalgae by magnetic separation: A review. Algal Res. 2015, 9, 178–185. [Google Scholar] [CrossRef]
- Li, Z.; Gong, W.; Chen, X.; Liu, L.; Meng, R.; Ding, Y.; Yao, J. Sustainable cationic cellulose for highly efficient flocculation of Kaolin suspension. Cellulose 2021, 28, 11097–11108. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, H.; Wang, Y.; Chen, X.; Zhao, C.; An, Y.; Tang, X. A novel carboxyl-rich chitosan-based polymer and its application for clay flocculation and cationic dye removal. Sci. Total Environ. 2018, 640–641, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gui, X.; Cao, Y.; Gao, L.; Li, S. Effect of pH on the flocculation behaviors of kaolin using a pH-sensitive copolymer. Water Sci. Technol. 2016, 74, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yu, Y.; Sun, Y.; Zheng, X.; Chen, A. Heavy metal removal from aqueous solutions by chitosan-based magnetic composite flocculants. J. Environ. Sci. 2021, 108, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wang, C.; Wang, G.; Feng, Q. Flocculation performance and kinetics of magnetic polyacrylamide microsphere under different magnetic field strengths. J. Chem. 2020, 2020, 1579424. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Du, S.; Liang, W. Synthesis of chitosan-based grafting magnetic flocculants for flocculation of kaolin suspensions. J. Environ. Sci. 2024, 139, 193–205. [Google Scholar] [CrossRef]
- Seo, J.Y.; Praveenkumar, R.; Kim, B.; Seo, J.C.; Park, J.Y.; Na, J.G.; Sang, G.J.; Park, S.B.; Lee, K.; Oh, Y.K. Downstream integration of microalgae harvesting and cell disruption by means of cationic surfactant-decorated Fe3O4 nanoparticles. Green Chem. 2016, 18, 3981–3989. [Google Scholar] [CrossRef]
- Antarnusa, G.; Jayanti, P.D.; Denny, Y.R.; Suherman, A. Utilization of co-precipitation method on synthesis of Fe3O4 PEG. Materialia 2022, 25, 101525. [Google Scholar] [CrossRef]









| Fe3O4 | Fe3O4/HBPN | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | Fe | C | N | O | Si | Fe | |||||||
| Wt% | At% | Wt% | At% | Wt% | At% | Wt% | At% | Wt% | At% | Wt% | At% | Wt% | At% |
| 23.90 | 52.30 | 76.10 | 47.70 | 13.75 | 28.17 | 5.93 | 10.42 | 23.03 | 35.44 | 1.64 | 1.44 | 55.65 | 24.53 |
| 23.62 | 51.91 | 76.38 | 48.09 | 20.86 | 39.18 | 6.93 | 11.17 | 19.57 | 27.60 | 1.95 | 1.57 | 50.69 | 20.48 |
| 29.93 | 59.85 | 70.07 | 40.15 | 15.40 | 28.88 | 6.77 | 10.88 | 28.03 | 39.45 | 1.76 | 1.41 | 48.05 | 19.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Fan, Q.; Liu, Y.; Wang, S.; Guo, X.; Guo, L.; Zhu, M.; Wang, X. Preparation and Application of Amino-Terminated Hyperbranched Magnetic Composites in High-Turbidity Water Treatment. Molecules 2023, 28, 6787. https://doi.org/10.3390/molecules28196787
Zhao Y, Fan Q, Liu Y, Wang S, Guo X, Guo L, Zhu M, Wang X. Preparation and Application of Amino-Terminated Hyperbranched Magnetic Composites in High-Turbidity Water Treatment. Molecules. 2023; 28(19):6787. https://doi.org/10.3390/molecules28196787
Chicago/Turabian StyleZhao, Yuan, Qianlong Fan, Yinhua Liu, Shuwen Wang, Xudong Guo, Liujia Guo, Mengcheng Zhu, and Xuan Wang. 2023. "Preparation and Application of Amino-Terminated Hyperbranched Magnetic Composites in High-Turbidity Water Treatment" Molecules 28, no. 19: 6787. https://doi.org/10.3390/molecules28196787
APA StyleZhao, Y., Fan, Q., Liu, Y., Wang, S., Guo, X., Guo, L., Zhu, M., & Wang, X. (2023). Preparation and Application of Amino-Terminated Hyperbranched Magnetic Composites in High-Turbidity Water Treatment. Molecules, 28(19), 6787. https://doi.org/10.3390/molecules28196787