Stereospecificity of Ginsenoside AD-1 and AD-2 Showed Anticancer Activity via Inducing Mitochondrial Dysfunction and Reactive Oxygen Species Mediate Cell Apoptosis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of 20(R)-AD-1, 20(S)-AD-1, 20(R)-AD-2 and 20(S)-AD-2 on Cell Proliferation
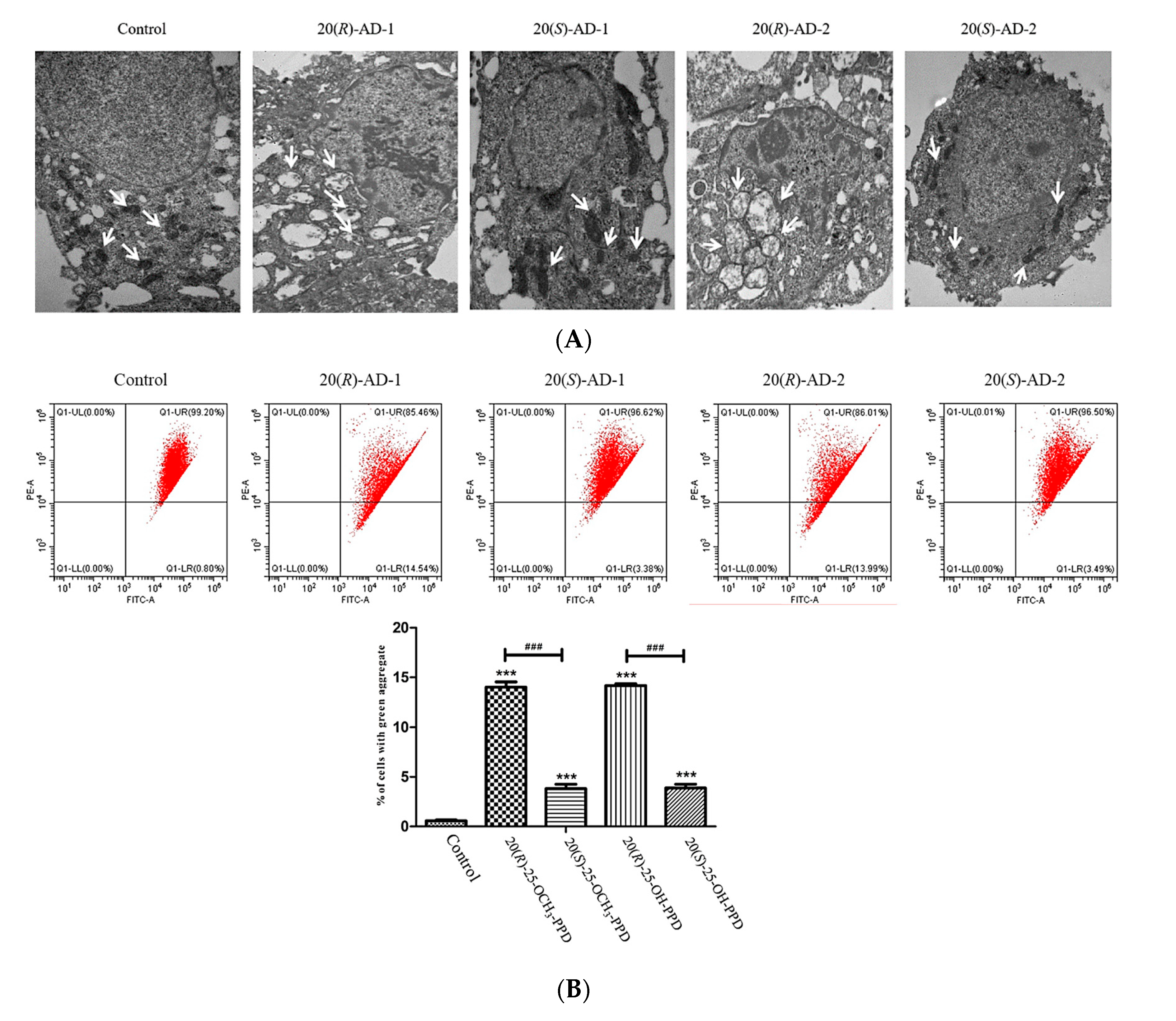
2.2. Effects of 20(R)-AD-1, 20(S)-AD-1, 20(R)-AD-2 and 20(S)-AD-2 on Apoptosis in BGC-803 Cells
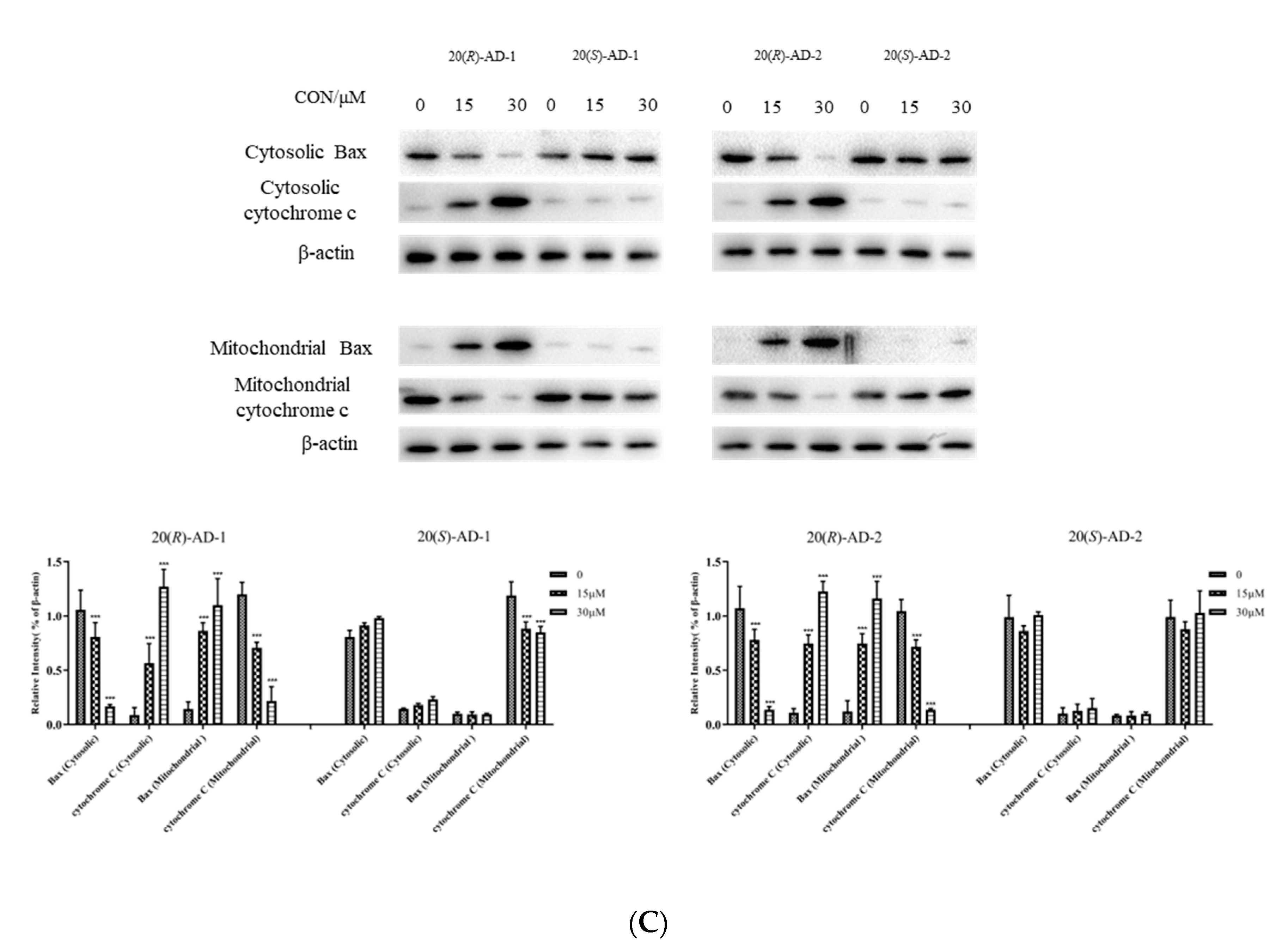
2.3. 20(R)-AD-1 and 20(R)-AD-2 Induce Apoptosis through Mitochondrial Pathway
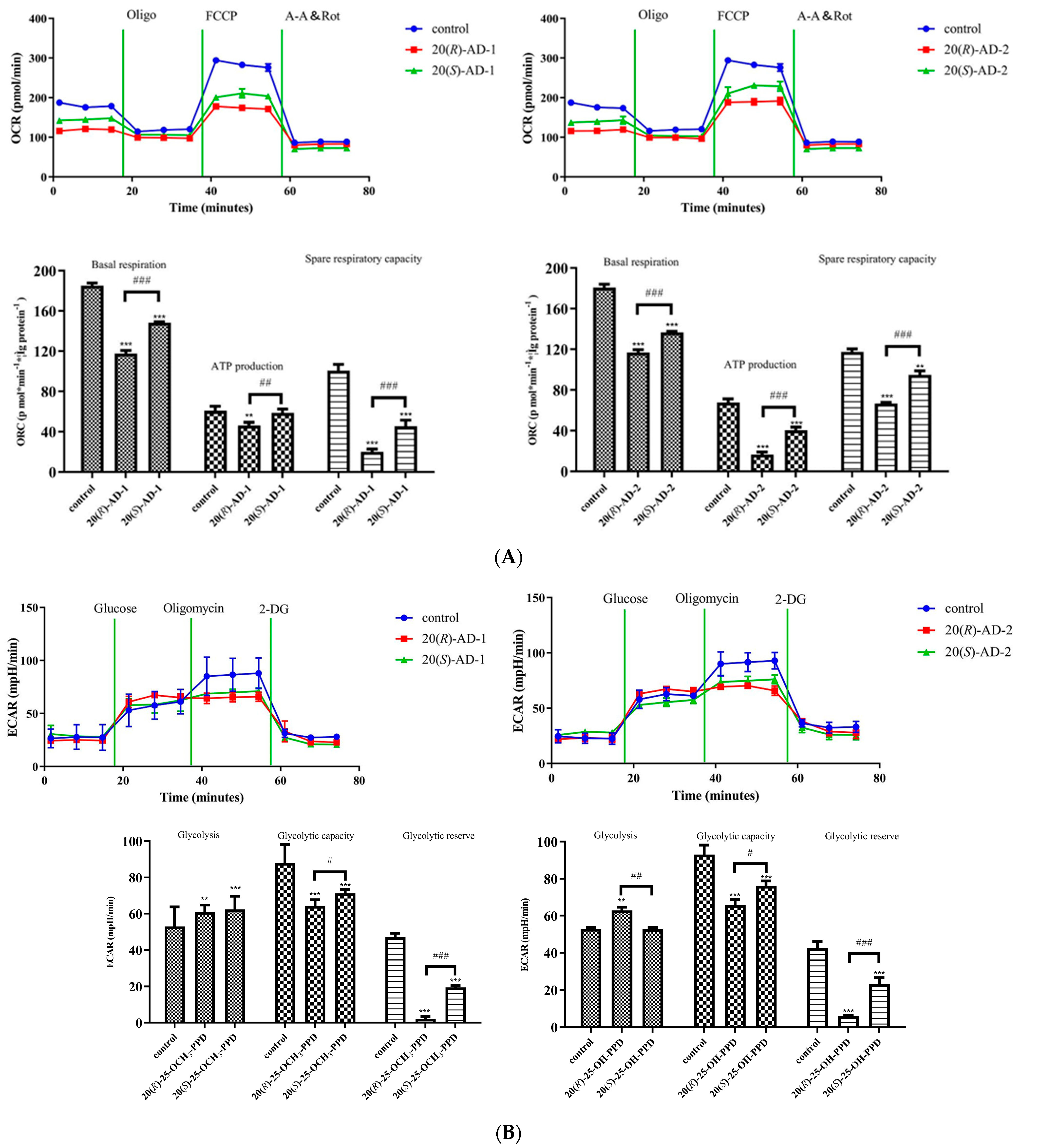
2.4. Effects of 20(R)-AD-1, 20(S)-AD-1, 20(R)-AD-2 and 20(S)-AD-2 on Oxidative Phosphorylation and Glycolysis
2.5. 20(R)-AD-1 and 20(R)-AD-2 Triggered Reactive Oxygen Species Generation, Which Was Involved in 20(R)-AD-1- and 20(R)-AD-2-Induced Apoptosis
3. Materials and Methods
3.1. Reagents
3.2. Cell Culture
3.3. Cell Viability Assay
3.4. Morphological Observation and DAPI Staining
3.5. Transmission Electron Microscopy
3.6. Flow Cytometry
3.7. Metabolic Profiling
3.8. Western Blot Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, X.; Sun, Y.Y.; Zhao, C.; Qu, F.Z.; Zhao, Y.Q. 12-Chloracetyl-PPD, a novel dammarane derivative, shows anti-cancer activity via delay the progression of cell cycle G2/M phase and reactive oxygen species-mediate cell apoptosis. Eur. J. Pharmacol. 2017, 798, 49–56. [Google Scholar] [CrossRef]
- Moreno-Sanchez, R.; Rodriguez-Enriquez, S.; Marin-Hernandez, A.; Saavedra, E. Energy metabolism in tumor cells. FEBS J. 2007, 274, 1393–1418. [Google Scholar] [CrossRef]
- Pelicano, H.; Lu, W.; Zhou, Y.; Zhang, W.; Chen, Z.; Hu, Y.; Huang, P. Mitochondrial Dysfunction and Reactive Oxygen Species Imbalance Promote Breast Cancer Cell Motility through a CXCL14-Mediated Mechanism. Cancer Res. 2009, 69, 2375–2383. [Google Scholar] [CrossRef]
- Gu, X.; Hao, D.; Xiao, P. Research progress of Chinese herbal medicine compounds and their bioactivities: Fruitful 2020. Chin. Herb. Med. 2022, 14, 171–186. [Google Scholar] [CrossRef]
- Lei, M.; Chen, N.; Xu, Y.; Gong, Q.; Gao, J. Lithocarpus polystachyus (Sweet Tea) water extract promotes human hepatocytes HL7702 proliferation through activation of HGF/AKT/ERK signaling pathway. Chin. Herb. Med. 2022, 14, 576–582. [Google Scholar] [CrossRef]
- Yaqoob, M.D.; Xu, L.; Li, C.; Leong, M.M.L.; Xu, D.D. Targeting mitochondria for cancer photodynamic therapy. Photodiagnosis Photodyn. Ther. 2022, 38, 102830. [Google Scholar] [CrossRef]
- Zhang, K.; Ying, H.; Zhao, R.; Chen, Y.; Deng, Q. Capilliposide from Lysimachia capillipes promotes terminal differentiations and reverses paclitaxel resistance in A2780T cells of human ovarian cancer by regulating Fos/Jun pathway. Chin. Herb. Med. 2022, 14, 111–116. [Google Scholar] [CrossRef]
- Gong, X.; Cui, H.T.; Bian, Y.H.; Li, Y.T.; Wang, Y.X.; Peng, Y.F.; Wen, W.B.; Li, K.; Wang, H.W.; Zhang, Z.Y.; et al. Ethanol extract of Ardisiae Japonicae Herba inhibits hepatoma carcinoma cell proliferation in vitro through regulating lipid metabolism. Chin. Herb. Med. 2021, 13, 410–415. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, L.; Li, Y.Y.; He, D.; Zheng, L.F. Chrysophanol localizes in mitochondria to promote cell death through upregulation of mitochondrial cyclophilin D in HepG2 cells. Chin. Herb. Med. 2021, 13, 221–227. [Google Scholar] [CrossRef]
- Helms, S. Cancer prevention and therapeutics: Panax ginseng. Altern. Med. Rev. A J. Clin. Ther. 2004, 9, 259–274. [Google Scholar]
- Wang, X.J.; Xie, Q.; Liu, Y.; Jiang, S.; Li, W.; Li, B.; Wang, W.; Liu, C.X. Panax japonicus and chikusetsusaponins: A review of diverse biological activities and pharmacology mechanism. Chin. Herb. Med. 2021, 13, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Yun, T.K. Experimental and epidemiological evidence on non-organ specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutat. Res. 2003, 523–524, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.T.; Wang, C.Z.; Wang, A.B.; Wu, J.; Basila, D.; Yuan, C.S. Antihyperglycemic effects of total ginsenosides from leaves and stem of Panax ginseng. Acta Pharmacol. Sin. 2005, 26, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J. Korean Med. Sci. 2001, 16, S28–S37. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.; Hu, C. Efficacy and safety of ginseng. Public Health Nutr. 2000, 3, 473–485. [Google Scholar] [CrossRef]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Xie, X.; Eberding, A.; Madera, C.; Fazli, L.; Jia, W.; Goldenberg, L.; Gleave, M.; Guns, E.S. Rh2 synergistically enhances paclitaxel or mitoxantrone in prostate cancer models. J. Urol. 2006, 175, 1926–1931. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Q.; Liu, K.; Li, G.; Zheng, R. Ginsenoside Rh(2) enhances antitumour activity and decreases genotoxic effect of cyclophosphamide. Basic Clin. Pharmacol. Toxicol. 2006, 98, 411–415. [Google Scholar] [CrossRef]
- Sato, K.; Mochizuki, M.; Saiki, I.; Yoo, Y.C.; Samukawa, K.; Azuma, I. Inhibition of tumor angiogenesis and metastasis by a saponin of Panax ginseng, ginsenoside-Rb2. Biol. Pharm. Bull. 1994, 17, 635–639. [Google Scholar] [CrossRef]
- Nakata, H.; Kikuchi, Y.; Tode, T.; Hirata, J.; Kita, T.; Ishii, K.; Kudoh, K.; Nagata, I.; Shinomiya, N. Inhibitory effects of ginsenoside Rh2 on tumor growth in nude mice bearing human ovarian cancer cells. Jpn. J. Cancer Res. 1998, 89, 733–740. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.; Rayburn, E.R.; Hill, D.L.; Wang, H.; Zhang, R. In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother. Pharmacol. 2007, 59, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.M.; Li, N.; Zhang, H.; Wu, C.F.; Piao, H.R.; Zhao, Y.Q. Novel dammarane-type sapogenins from Panax ginseng berry and their biological activities. Bioorg. Med. Chem. Lett. 2011, 21, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, W.; Han, L.; Rayburn, E.R.; Hill, D.L.; Wang, H.; Zhang, R. Isolation, structural determination, and evaluation of the biological activity of 20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol [20(S)-25-OCH3-PPD], a novel natural product from Panax notoginseng. Med. Chem. 2007, 3, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Zhao, Y.; Fang, W.; Yang, W. Anticancer activity of Panax notoginseng extract 20(S)-25-OCH3-PPD: Targetting beta-catenin signalling. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1074–1078. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, G.T.; Roh, S.H.; Song, J.S.; Kim, H.J.; Hong, S.S.; Kwon, S.W.; Park, J.H. Proteomic analysis of the anti-cancer effect of 20S-ginsenoside Rg3 in human colon cancer cell lines. Biosci. Biotechnol. Biochem. 2009, 73, 811–816. [Google Scholar] [CrossRef]
- Hao, M.; Wang, W.; Zhao, Y.; Zhang, R.; Wang, H. Pharmacokinetics and tissue distribution of 25-hydroxyprotopanaxadiol, an anti-cancer compound isolated from Panax ginseng, in athymic mice bearing xenografts of human pancreatic tumors. Eur. J. Drug Metab. Pharmacokinet. 2011, 35, 109–113. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, Y.; Cao, J.; Zhao, C.; Zhao, Y. Crystallization-induced dynamic resolution R-epimer from 25-OCH3-PPD epimeric mixture. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 1005, 39–46. [Google Scholar] [CrossRef]
- Green, D.R. Apoptotic pathways: Paper wraps stone blunts scissors. Cell 2000, 102, 1–4. [Google Scholar] [CrossRef]
- Pop, C.; Salvesen, G.S. Human caspases: Activation, specificity, and regulation. J. Biol. Chem. 2009, 284, 21777–21781. [Google Scholar] [CrossRef]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, W.; Kuang, X.; Hou, S.; Liu, H. Nanopreparations for mitochondria targeting drug delivery system: Current strategies and future prospective. Asian J. Pharm. Sci. 2017, 12, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Rehm, M.; Huber, H.J.; Hellwig, C.T.; Anguissola, S.; Dussmann, H.; Prehn, J.H. Dynamics of outer mitochondrial membrane permeabilization during apoptosis. Cell Death Differ. 2009, 16, 613–623. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Zu, X.L.; Guppy, M. Cancer metabolism: Facts, fantasy, and fiction. Biochem. Biophys. Res. Commun. 2004, 313, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Guppy, M.; Leedman, P.; Zu, X.; Russell, V. Contribution by different fuels and metabolic pathways to the total ATP turnover of proliferating MCF-7 breast cancer cells. Biochem. J. 2002, 364, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Moungjaroen, J.; Nimmannit, U.; Callery, P.S.; Wang, L.; Azad, N.; Lipipun, V.; Chanvorachote, P.; Rojanasakul, Y. Reactive oxygen species mediate caspase activation and apoptosis induced by lipoic acid in human lung epithelial cancer cells through Bcl-2 down-regulation. J. Pharmacol. Exp. Ther. 2006, 319, 1062–1069. [Google Scholar] [CrossRef]
- Yang, E.S.; Woo, S.M.; Choi, K.S.; Kwon, T.K. Acrolein sensitizes human renal cancer Caki cells to TRAIL-induced apoptosis via ROS-mediated up-regulation of death receptor-5 (DR5) and down-regulation of Bcl-2. Exp. Cell Res. 2011, 317, 2592–2601. [Google Scholar] [CrossRef]
- Luo, M.; Liu, X.; Zu, Y.; Fu, Y.; Zhang, S.; Yao, L.; Efferth, T. Cajanol, a novel anticancer agent from Pigeonpea [Cajanus cajan (L.) Millsp.] roots, induces apoptosis in human breast cancer cells through a ROS-mediated mitochondrial pathway. Chem. Biol. Interact. 2010, 188, 151–160. [Google Scholar] [CrossRef]
- Zhang, L.H.; Jia, Y.L.; Lin, X.X.; Zhang, H.Q.; Dong, X.W.; Zhao, J.M.; Shen, J.; Shen, H.J.; Li, F.F.; Yan, X.F.; et al. AD-1, a novel ginsenoside derivative, shows anti-lung cancer activity via activation of p38 MAPK pathway and generation of reactive oxygen species. Biochim. Biophys. Acta 2013, 1830, 4148–4159. [Google Scholar] [CrossRef]
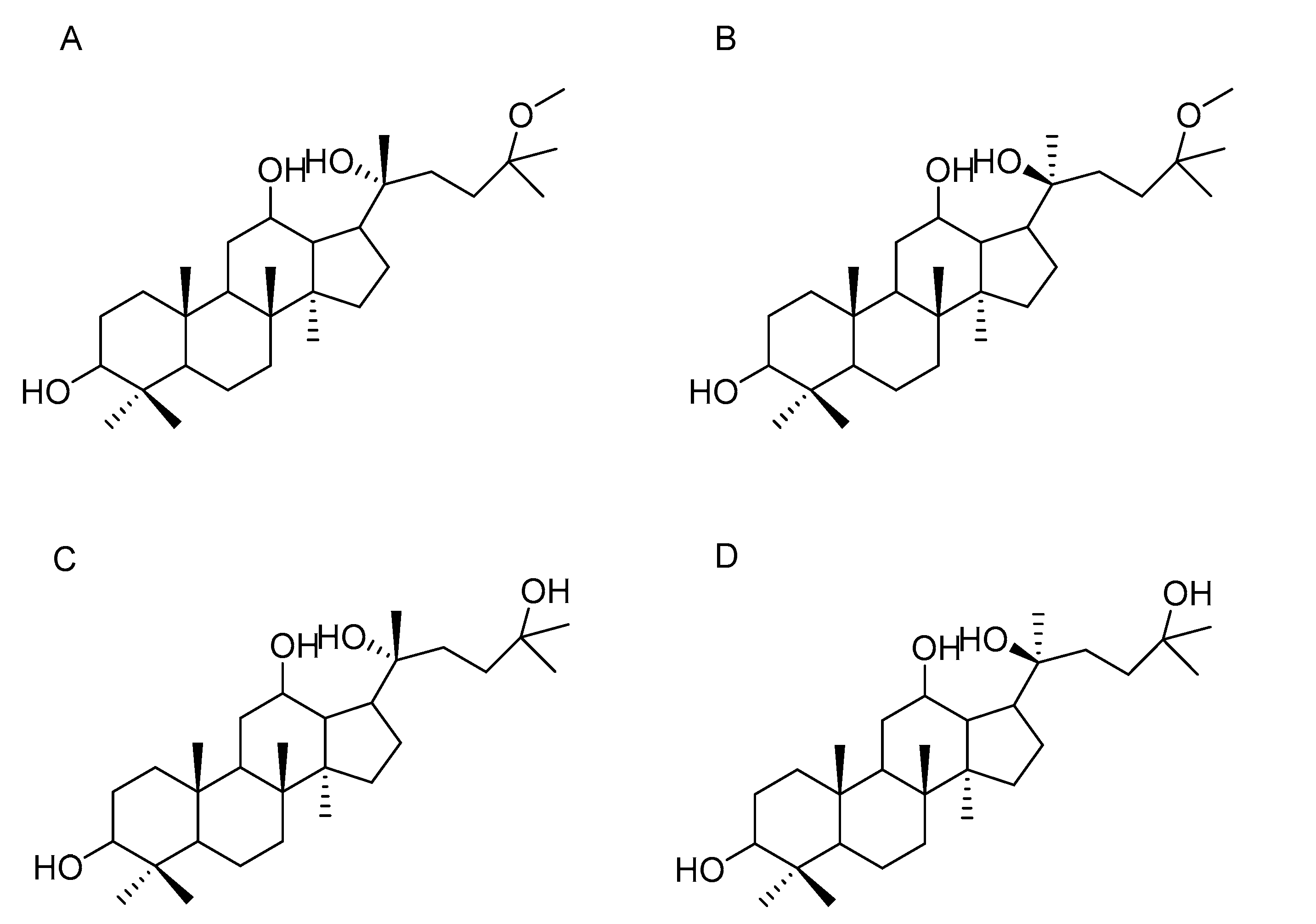



| Cell Type | Cell Line | IC50 (μM) 48 h | |||
|---|---|---|---|---|---|
| 20(R)-AD-1 | 20(S)-AD-1 | 20(R)-AD-2 | 20(S)-AD-2 | ||
| Gastric | BGC-803 | 8.11 ± 0.36 | 57.731 ± 1.23 | 15.519 ± 0.68 | 55.959 ± 1.26 |
| BGC-823 | 12.995 ± 0.62 | 44.617 ± 0.79 | 16.120 ± 1.36 | 61.836 ± 2.18 | |
| SGC-7901 | 17.109 ± 1.31 | 51.666 ± 0.94 | 20.814 ± 1.65 | 69.522 ± 2.36 | |
| MKN-28 | 18.558 ± 0.43 | 45.978 ± 0.69 | 22.275 ± 0.54 | 42.583 ± 0.63 | |
| Lung | A549 | 19.618 ± 0.32 | 46.554 ± 1.38 | 23.937 ± 0.65 | 55.341 ± 0.84 |
| Colon | LoVo | 21.72 ± 0.12 | 55.244 ± 0.59 | 25.713 ± 0.74 | 54.647 ± 1.63 |
| HCT-116 | 20.76 ± 0.53 | 46.005 ± 1.03 | 25.099 ± 0.63 | 49.464 ± 1.54 | |
| Liver | HepG-2 | 19.167 ± 0.18 | 49.587 ± 0.65 | 28.179 ± 1.26 | 59.533 ± 0.87 |
| Prostate | C4-2B | 17.11 ± 0.36 | 38.731 ± 1.23 | 22.519 ± 0.68 | 49.959 ± 1.26 |
| PC3 | 27.995 ± 0.62 | 43.667 ± 0.79 | 35.12 ± 1.36 | 60.826 ± 2.18 | |
| DU145 | 39.109 ± 1.31 | 60.666 ± 0.94 | 46.814 ± 1.65 | 88.582 ± 2.36 | |
| LnCaP | 20.558 ± 0.43 | 35.978 ± 0.69 | 26.275 ± 0.54 | 41.593 ± 0.63 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Ding, M.; Zhao, H.; Zhou, M.; Lu, X.; Sun, Y.; Zhang, Q.; Zhao, Y.; Wang, R. Stereospecificity of Ginsenoside AD-1 and AD-2 Showed Anticancer Activity via Inducing Mitochondrial Dysfunction and Reactive Oxygen Species Mediate Cell Apoptosis. Molecules 2023, 28, 6698. https://doi.org/10.3390/molecules28186698
Wang X, Ding M, Zhao H, Zhou M, Lu X, Sun Y, Zhang Q, Zhao Y, Wang R. Stereospecificity of Ginsenoside AD-1 and AD-2 Showed Anticancer Activity via Inducing Mitochondrial Dysfunction and Reactive Oxygen Species Mediate Cell Apoptosis. Molecules. 2023; 28(18):6698. https://doi.org/10.3390/molecules28186698
Chicago/Turabian StyleWang, Xude, Meng Ding, Hong Zhao, Mengru Zhou, Xuan Lu, Yuanyuan Sun, Qinggao Zhang, Yuqing Zhao, and Ruoyu Wang. 2023. "Stereospecificity of Ginsenoside AD-1 and AD-2 Showed Anticancer Activity via Inducing Mitochondrial Dysfunction and Reactive Oxygen Species Mediate Cell Apoptosis" Molecules 28, no. 18: 6698. https://doi.org/10.3390/molecules28186698
APA StyleWang, X., Ding, M., Zhao, H., Zhou, M., Lu, X., Sun, Y., Zhang, Q., Zhao, Y., & Wang, R. (2023). Stereospecificity of Ginsenoside AD-1 and AD-2 Showed Anticancer Activity via Inducing Mitochondrial Dysfunction and Reactive Oxygen Species Mediate Cell Apoptosis. Molecules, 28(18), 6698. https://doi.org/10.3390/molecules28186698







