PEGA-BA@Ce6@PFCE Micelles as Oxygen Nanoshuttles for Tumor Hypoxia Relief and Enhanced Photodynamic Therapy
Abstract
:1. Introduction
2. Results and Discussion
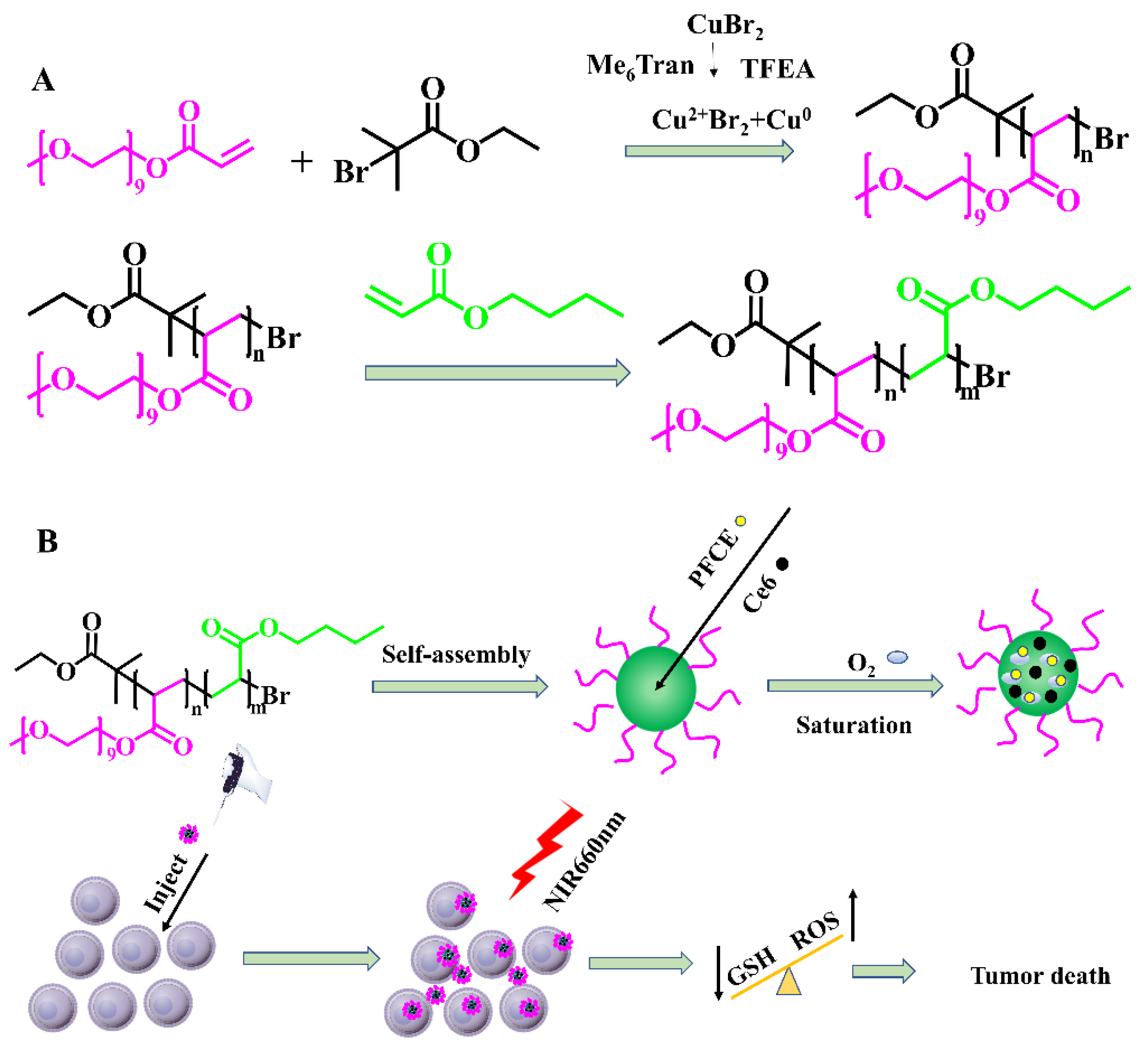
2.1. Composition of PEGA-BA
2.2. Physicochemical Characterizations of PEGA-BA Micelles

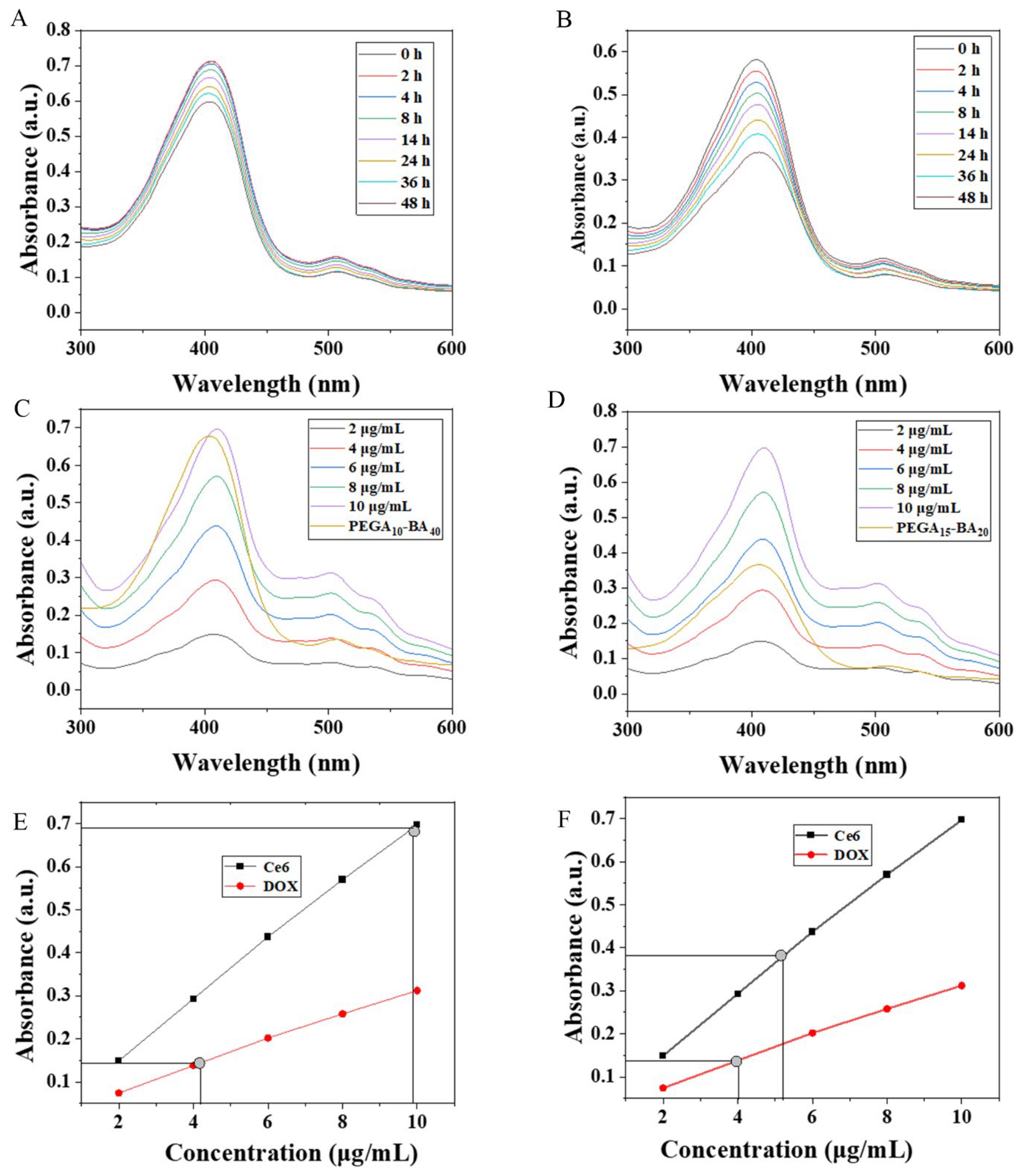
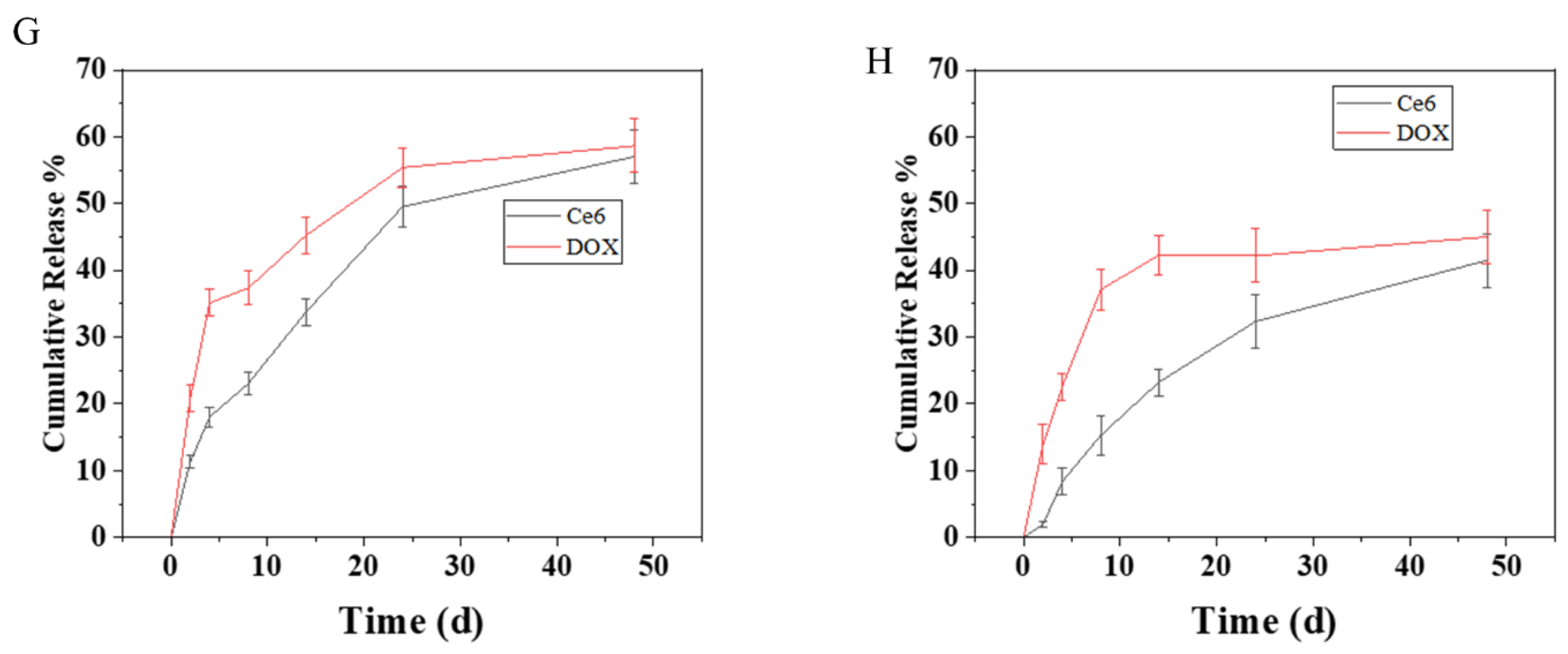
2.3. Drug Loading and Release
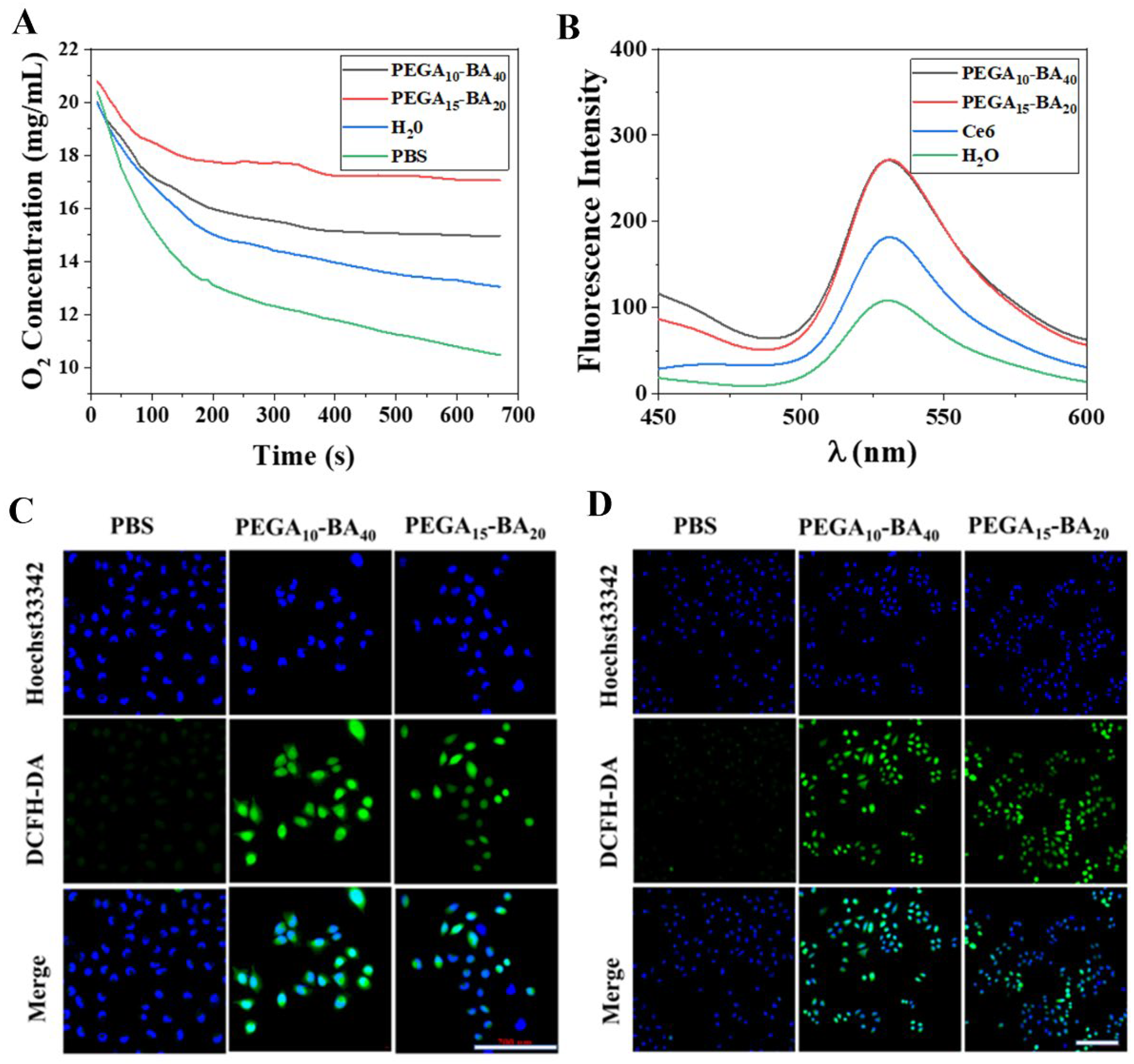
2.4. Measurement of O2 Release
2.5. Measurement of ROS Generation
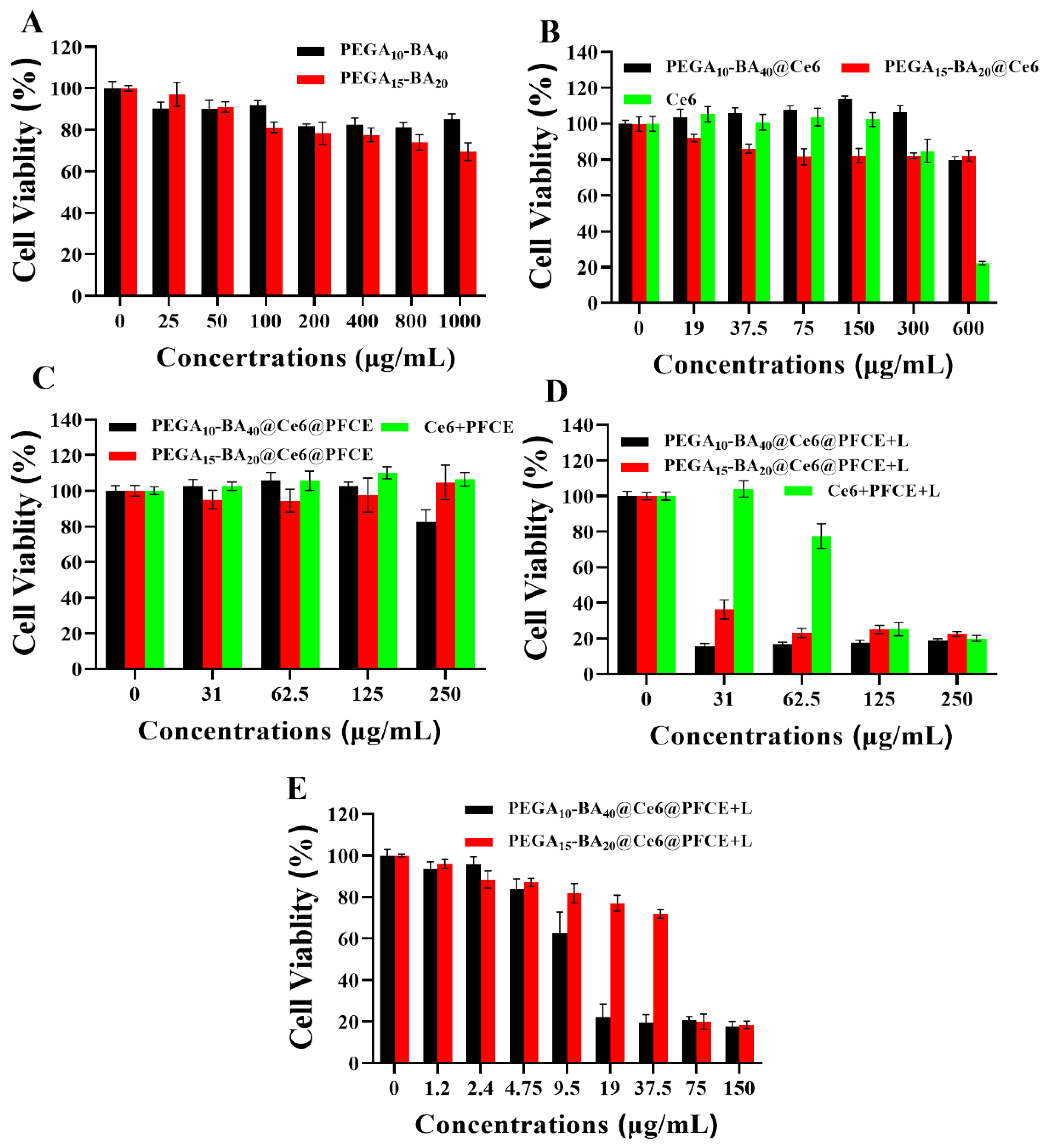
2.6. In Vitro Photo-Toxicity Efficacy
3. Materials and Methods
3.1. Materials
3.2. Cell
3.3. Synthesis of PEGA-BA
3.4. Preparation of PEGA-BA@Ce6@PFCE Micelles
3.5. Physicochemical Characterization of Drug Loaded PEGA-BA Micelles
3.6. Drug Release of PEGA-BA Micelles
3.7. Measurement of O2 Release
3.8. In Vitro ROS Generation
3.9. In Vitro Photo-Toxicity Efficacy
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Park, J.H.; Kim, H.J.; Kim, C.W.; Kim, H.C.; Jung, Y.; Lee, H.S.; Lee, Y.; Ju, Y.S.; Oh, J.E.; Park, S.H.; et al. Tumor hypoxia represses gammadelta T cell-mediated antitumor immunity against brain tumors. Nat. Immunol. 2021, 22, 336–346. [Google Scholar] [CrossRef]
- Shen, Z.; Xia, J.; Ma, Q.; Zhu, W.; Gao, Z.; Han, S.; Liang, Y.; Cao, J.; Sun, Y. Tumor Microenvironment-triggered Nanosystems as dual-relief Tumor Hypoxia Immunomodulators for enhanced Phototherapy. Theranostics 2020, 10, 9132–9152. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Ma, S.; Song, W.; Xu, Y.; Si, X.; Lv, S.; Zhang, Y.; Tang, Z.; Chen, X. Rationally Designed Polymer Conjugate for Tumor-Specific Amplification of Oxidative Stress and Boosting Antitumor Immunity. Nano Lett. 2020, 20, 2514–2521. [Google Scholar] [CrossRef]
- Liu, J.; Wu, M.; Pan, Y.; Duan, Y.; Dong, Z.; Chao, Y.; Liu, Z.; Liu, B. Biodegradable Nanoscale Coordination Polymers for Targeted Tumor Combination Therapy with Oxidative Stress Amplification. Adv. Funct. Mater. 2020, 30, 1908865. [Google Scholar] [CrossRef]
- Du, J.; Shi, T.; Long, S.; Chen, P.; Sun, W.; Fan, J.; Peng, X. Enhanced photodynamic therapy for overcoming tumor hypoxia: From microenvironment regulation to photosensitizer innovation. Coord. Chem. Rev. 2021, 427, 213604. [Google Scholar] [CrossRef]
- Xu, M.; Wang, P.; Sun, S.; Gao, L.; Sun, L.; Zhang, L.; Zhang, J.; Wang, S.; Liang, X. Smart strategies to overcome tumor hypoxia toward the enhancement of cancer therapy. Nanoscale 2020, 12, 21519–21533. [Google Scholar] [CrossRef]
- Emami Nejad, A.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Haghjooy Javanmard, S.; Taherian, M.; Ahmadlou, M.; et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: A novel approach to developing treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar]
- Chen, G.; Yang, Y.; Xu, Q.; Ling, M.; Lin, H.; Ma, W.; Sun, R.; Xu, Y.; Liu, X.; Li, N.; et al. Self-Amplification of Tumor Oxidative Stress with Degradable Metallic Complexes for Synergistic Cascade Tumor Therapy. Nano Lett. 2020, 20, 8141–8150. [Google Scholar] [CrossRef]
- Risson, E.; Nobre, A.R.; Maguer-Satta, V.; Aguirre-Ghiso, J.A. The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat. Cancer 2020, 1, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huan, L.; Guo, T.; Wu, Y.; Liu, Y.; Wang, Q.; Huang, S.; Xu, Y.; Liang, L.; He, X. LncRNA SNHG11 facilitates tumor metastasis by interacting with and stabilizing HIF-1alpha. Oncogene 2020, 39, 7005–7018. [Google Scholar] [CrossRef]
- Kanamori, A.; Matsubara, D.; Saitoh, Y.; Fukui, Y.; Gotoh, N.; Kaneko, S.; Seiki, M.; Murakami, Y.; Inoue, J.I.; Sakamoto, T. Mint3 depletion restricts tumor malignancy of pancreatic cancer cells by decreasing SKP2 expression via HIF-1. Oncogene 2020, 39, 6218–6230. [Google Scholar] [CrossRef]
- Kishimoto, S.; Brender, J.R.; Chandramouli, G.V.R.; Saida, Y.; Yamamoto, K.; Mitchell, J.B.; Krishna, M.C. Hypoxia-Activated Prodrug Evofosfamide Treatment in Pancreatic Ductal Adenocarcinoma Xenografts Alters the Tumor Redox Status to Potentiate Radiotherapy. Antioxid. Redox Signal 2021, 35, 904–915. [Google Scholar] [CrossRef]
- Hu, J.; Liu, S. Modulating intracellular oxidative stress via engineered nanotherapeutics. J. Control. Release 2020, 319, 333–343. [Google Scholar] [CrossRef]
- Tang, W.; Yang, Z.; He, L.; Deng, L.; Fathi, P.; Zhu, S.; Li, L.; Shen, B.; Wang, Z.; Jacobson, O.; et al. A hybrid semiconducting organosilica-based O(2) nanoeconomizer for on-demand synergistic photothermally boosted radiotherapy. Nat. Commun. 2021, 12, 523. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, S.; Wu, Y.; Zhu, L.; Xu, J.; Hu, T.; Wei, B.; Tang, R. Cisplatin-Cross-Linked and Oxygen-Resupply Hyaluronic Acid-Based Nanocarriers for Chemo-photodynamic Therapy. ACS Appl. Nano Mater. 2021, 4, 10194–10208. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhang, W.; Xuan, Q.; Ma, T.; Zhang, Q.; Chen, C.; Wang, P. Oxygen-Abundant and pH/NIR Dual-Responsive Nanocarriers for Tumor Hypoxia Reduction Therapy. ACS Appl. Nano Mater. 2021, 4, 11480–11492. [Google Scholar] [CrossRef]
- Wang, D.; Wu, H.; Phua, S.Z.F.; Yang, G.; Qi Lim, W.; Gu, L.; Qian, C.; Wang, H.; Guo, Z.; Chen, H.; et al. Self-assembled single-atom nanozyme for enhanced photodynamic therapy treatment of tumor. Nat. Commun. 2020, 11, 357. [Google Scholar] [CrossRef]
- Chen, W.H.; Yu, K.J.; Jhou, J.W.; Pang, H.H.; Weng, W.H.; Lin, W.S.; Yang, H.W. Glucose/Glutathione Co-triggered Tumor Hypoxia Relief and Chemodynamic Therapy to Enhance Photothermal Therapy in Bladder Cancer. ACS Appl. Bio. Mater. 2021, 4, 7485–7496. [Google Scholar] [CrossRef]
- Ji, B.; Wei, M.; Yang, B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics 2022, 12, 434–458. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy-Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Y.; Choi, W.; Jangili, P.; Ge, Y.; Xu, Y.; Kang, J.; Liu, L.; Zhang, B.; Xie, Z.; et al. Overcoming barriers in photodynamic therapy harnessing nano-formulation strategies. Chem. Soc. Rev. 2021, 50, 9152–9201. [Google Scholar] [CrossRef]
- Mali, A.; Kaijzel, E.L.; Lamb, H.J.; Cruz, L.J. (19)F-nanoparticles: Platform for in vivo delivery of fluorinated biomaterials for (19)F-MRI. J. Control Release 2021, 338, 870–889. [Google Scholar] [CrossRef]
- Joseph, J.M.; Gigliobianco, M.R.; Firouzabadi, B.M.; Censi, R.; Di Martino, P. Nanotechnology as a Versatile Tool for (19)F-MRI Agent’s Formulation: A Glimpse into the Use of Perfluorinated and Fluorinated Compounds in Nanoparticles. Pharmaceutics 2022, 14, 382. [Google Scholar] [CrossRef]
- Xing, L.; Gong, J.H.; Wang, Y.; Zhu, Y.; Huang, Z.J.; Zhao, J.; Li, F.; Wang, J.H.; Wen, H.; Jiang, H.L. Hypoxia alleviation-triggered enhanced photodynamic therapy in combination with IDO inhibitor for preferable cancer therapy. Biomaterials 2019, 206, 170–182. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.M.; Yu, H.; Deng, K.; Zhou, W.; Wang, C.X.; Zhang, Y.; Li, K.H.; Zhuo, R.X.; Huang, S.W. Fluorinated polymeric micelles to overcome hypoxia and enhance photodynamic cancer therapy. Biomater. Sci. 2018, 6, 3096–3107. [Google Scholar] [CrossRef]
- Liu, Z.; Xue, Y.; Wu, M.; Yang, G.; Lan, M.; Zhang, W. Sensitization of Hypoxic Tumor to Photodynamic Therapy via Oxygen Self-Supply of Fluorinated Photosensitizers. Biomacromolecules 2019, 20, 4563–4573. [Google Scholar] [CrossRef]
- Li, J.; Xue, Y.; Tian, J.; Liu, Z.; Zhuang, A.; Gu, P.; Zhou, H.; Zhang, W.; Fan, X. Fluorinated-functionalized hyaluronic acid nanoparticles for enhanced photodynamic therapy of ocular choroidal melanoma by ameliorating hypoxia. Carbohydr. Polym. 2020, 237, 116119. [Google Scholar] [CrossRef]
- Sosnowski, S.; Szymanski, R.; Lorandi, F.; Olszewski, M.; Sobieski, J.; Yin, R.; Bockstaller, M.R.; Matyjaszewski, K. Distribution of Alternating Sequences in Methyl Methacrylate/n-Butyl Acrylate Copolymers Prepared by Atom Transfer Radical Polymerization. Macromolecules 2021, 54, 9837–9849. [Google Scholar] [CrossRef]
- Whitfield, R.; Parkatzidis, K.; Bradford, K.G.E.; Truong, N.P.; Konkolewicz, D.; Anastasaki, A. Low ppm CuBr-Triggered Atom Transfer Radical Polymerization under Mild Conditions. Macromolecules 2021, 54, 3075–3083. [Google Scholar] [CrossRef]
- Dutta, D.P.; Roy, M.; Mishra, R.K.; Meena, S.S.; Yadav, A.; Kaushik, C.P.; Tyagi, A.K. Structural investigations on Mo, Cs and Ba ions-loaded iron phosphate glass for nuclear waste storage application. J. Alloys Compd. 2021, 850, 156715. [Google Scholar] [CrossRef]
- Sai, D.L.; Lee, J.; Nguyen, D.L.; Kim, Y.P. Tailoring photosensitive ROS for advanced photodynamic therapy. Exp. Mol. Med. 2021, 53, 495–504. [Google Scholar] [CrossRef]
- Ming, L.; Cheng, K.; Chen, Y.; Yang, R.; Chen, D. Enhancement of tumor lethality of ROS in photodynamic therapy. Cancer Med. 2021, 10, 257–268. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Li, Y.; Fei, Y.; Xue, C.; Yao, X.; Zhao, Y.; Wang, X.; Li, M.; Luo, Z. An ROS-Activatable Nanoassembly Remodulates Tumor Cell Metabolism for Enhanced Ferroptosis Therapy. Adv. Healthc. Mater. 2022, 11, e2101702. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, S.; Zhang, L.; Xu, S.; Dai, C.; Gan, S.; Xie, G.; Feng, G.; Tang, B.Z. Cationization to boost both type I and type II ROS generation for photodynamic therapy. Biomaterials 2022, 280, 121255. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Huo, Z.; Ge, S.; He, Y.; Pang, X. Preparation of holmium doped paramagnetic photoluminescence composite colloidal polymer particles through coordination induced self-assembly. Compos. Commun. 2022, 31, 101118. [Google Scholar] [CrossRef]
- Ellacott, S.H.; Sanchez-Cano, C.; Mansfield, E.D.H.; Rho, J.Y.; Song, J.I.; Peltier, R.; Perrier, S. Comparative Study of the Cellular Uptake and Intracellular Behavior of a Library of Cyclic Peptide-Polymer Nanotubes with Different Self-Assembling Properties. Biomacromolecules 2021, 22, 710–722. [Google Scholar] [CrossRef]
- Langenfeld, N.J.; Bugbee, B. Evaluation of Three Electrochemical Dissolved Oxygen Meters. HortTechnology 2021, 31, 428–431. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Jiang, X.; Luo, W.; Mo, Y.; Dai, C.; Zhu, L. PEGA-BA@Ce6@PFCE Micelles as Oxygen Nanoshuttles for Tumor Hypoxia Relief and Enhanced Photodynamic Therapy. Molecules 2023, 28, 6697. https://doi.org/10.3390/molecules28186697
Zhang J, Jiang X, Luo W, Mo Y, Dai C, Zhu L. PEGA-BA@Ce6@PFCE Micelles as Oxygen Nanoshuttles for Tumor Hypoxia Relief and Enhanced Photodynamic Therapy. Molecules. 2023; 28(18):6697. https://doi.org/10.3390/molecules28186697
Chicago/Turabian StyleZhang, Junan, Xiaoyun Jiang, Wenyue Luo, Yongjie Mo, Chunyan Dai, and Linhua Zhu. 2023. "PEGA-BA@Ce6@PFCE Micelles as Oxygen Nanoshuttles for Tumor Hypoxia Relief and Enhanced Photodynamic Therapy" Molecules 28, no. 18: 6697. https://doi.org/10.3390/molecules28186697
APA StyleZhang, J., Jiang, X., Luo, W., Mo, Y., Dai, C., & Zhu, L. (2023). PEGA-BA@Ce6@PFCE Micelles as Oxygen Nanoshuttles for Tumor Hypoxia Relief and Enhanced Photodynamic Therapy. Molecules, 28(18), 6697. https://doi.org/10.3390/molecules28186697





