Lignosulfonate-Assisted In Situ Deposition of Palladium Nanoparticles on Carbon Nanotubes for the Electrocatalytic Sensing of Hydrazine
Abstract
1. Introduction
2. Results and Discussion
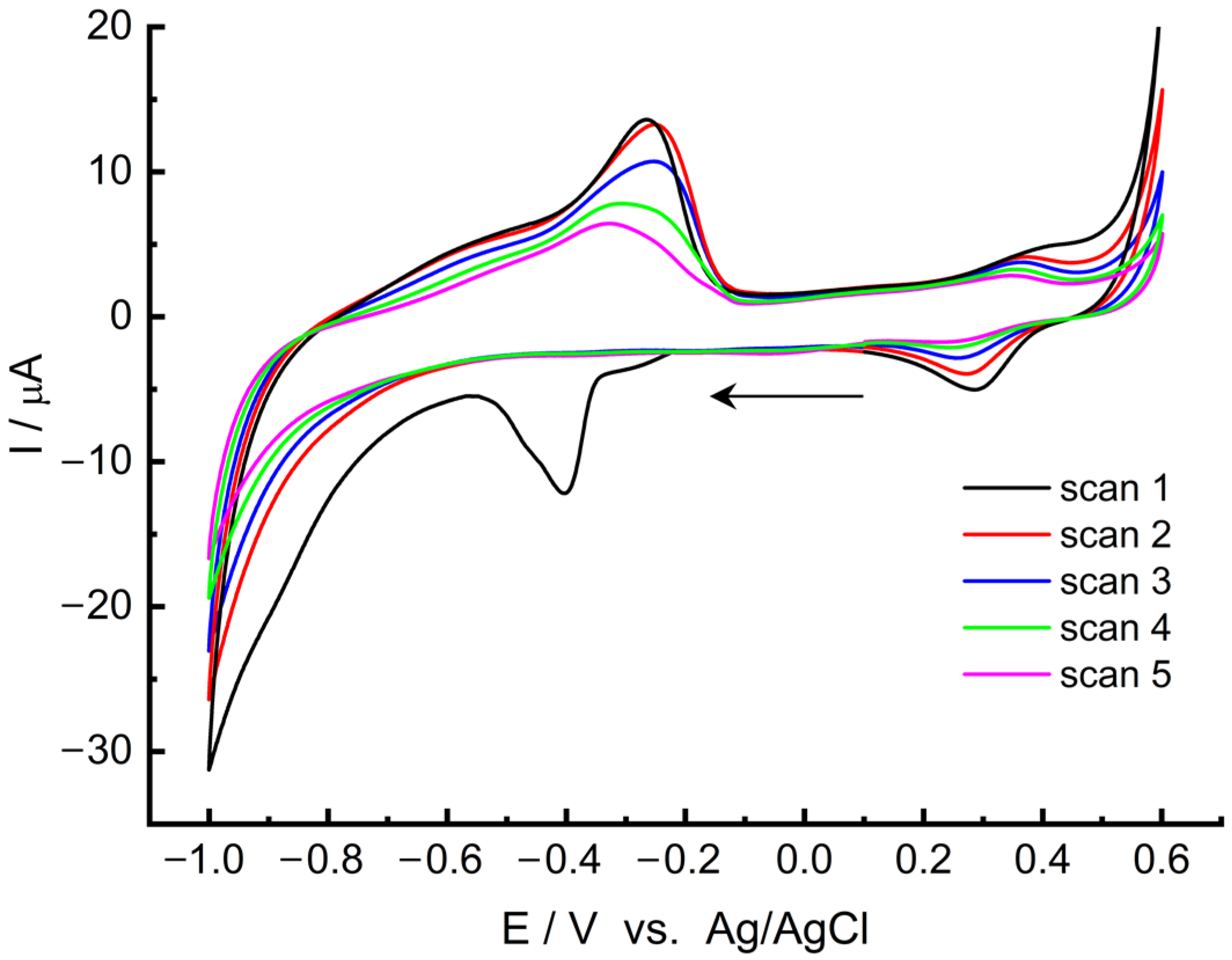
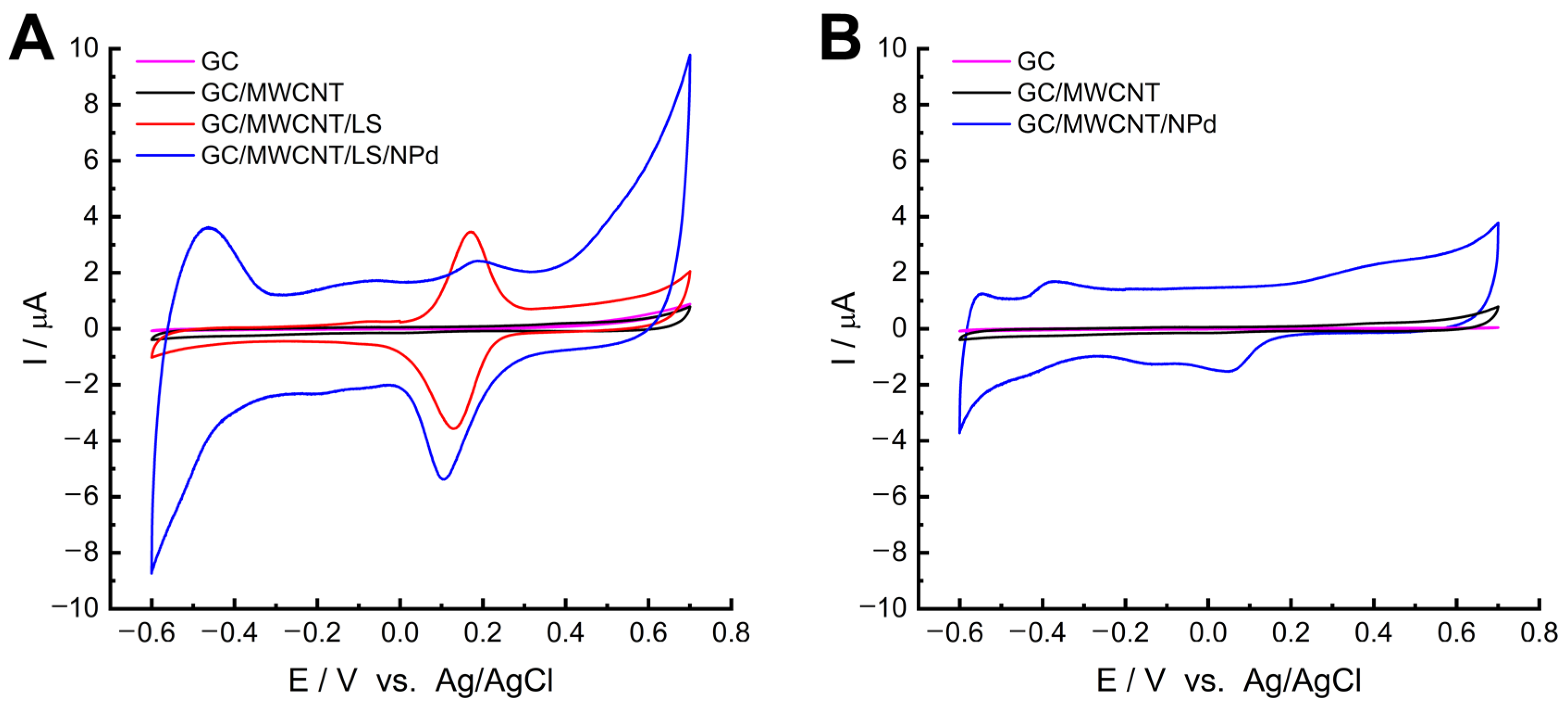
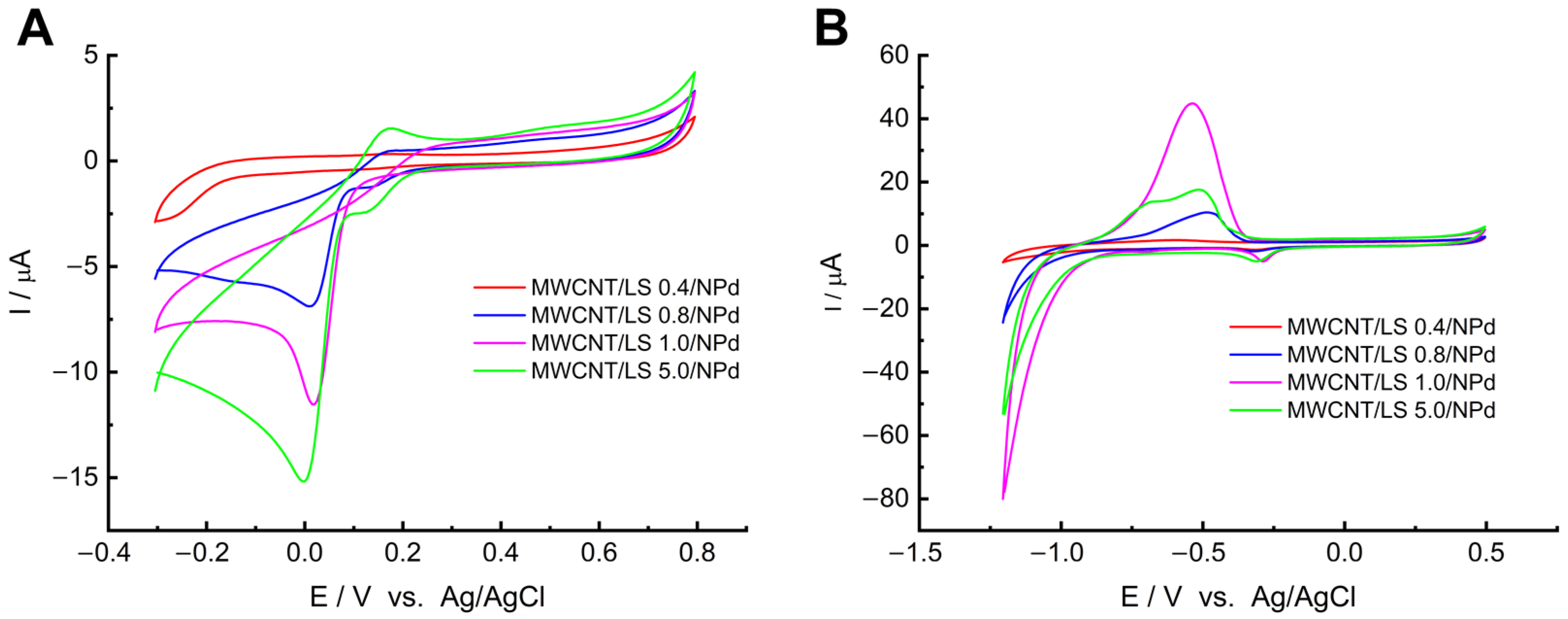
2.1. Coordination of Palladium on MWCNT/LS and Electrochemical Formation of MWCNT/LS/NPd
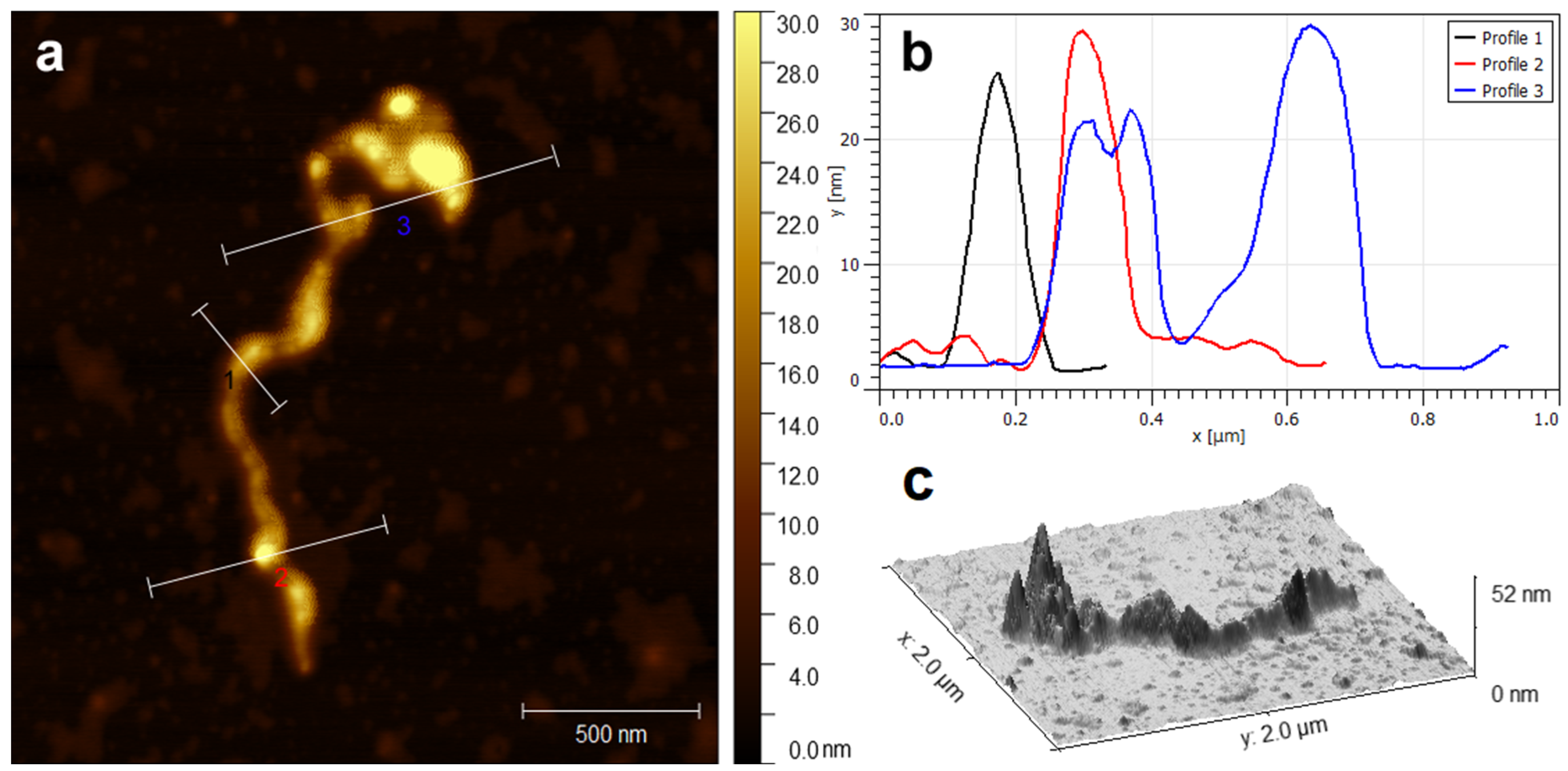
2.2. Physicochemical Analysis of MWCNT/LS/NPd
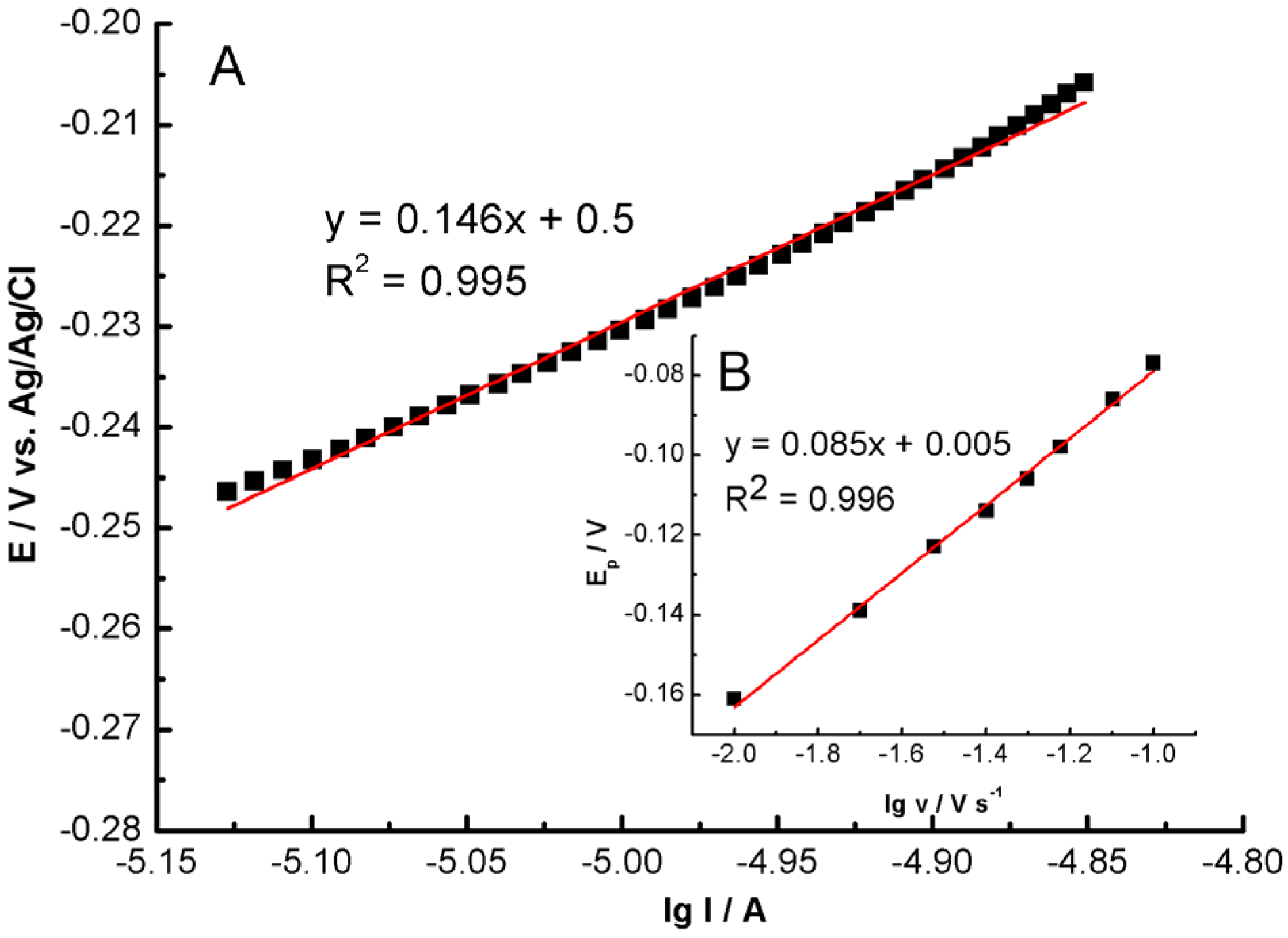
2.3. Electrochemical Study of GC/MWCNT/LS/NPd
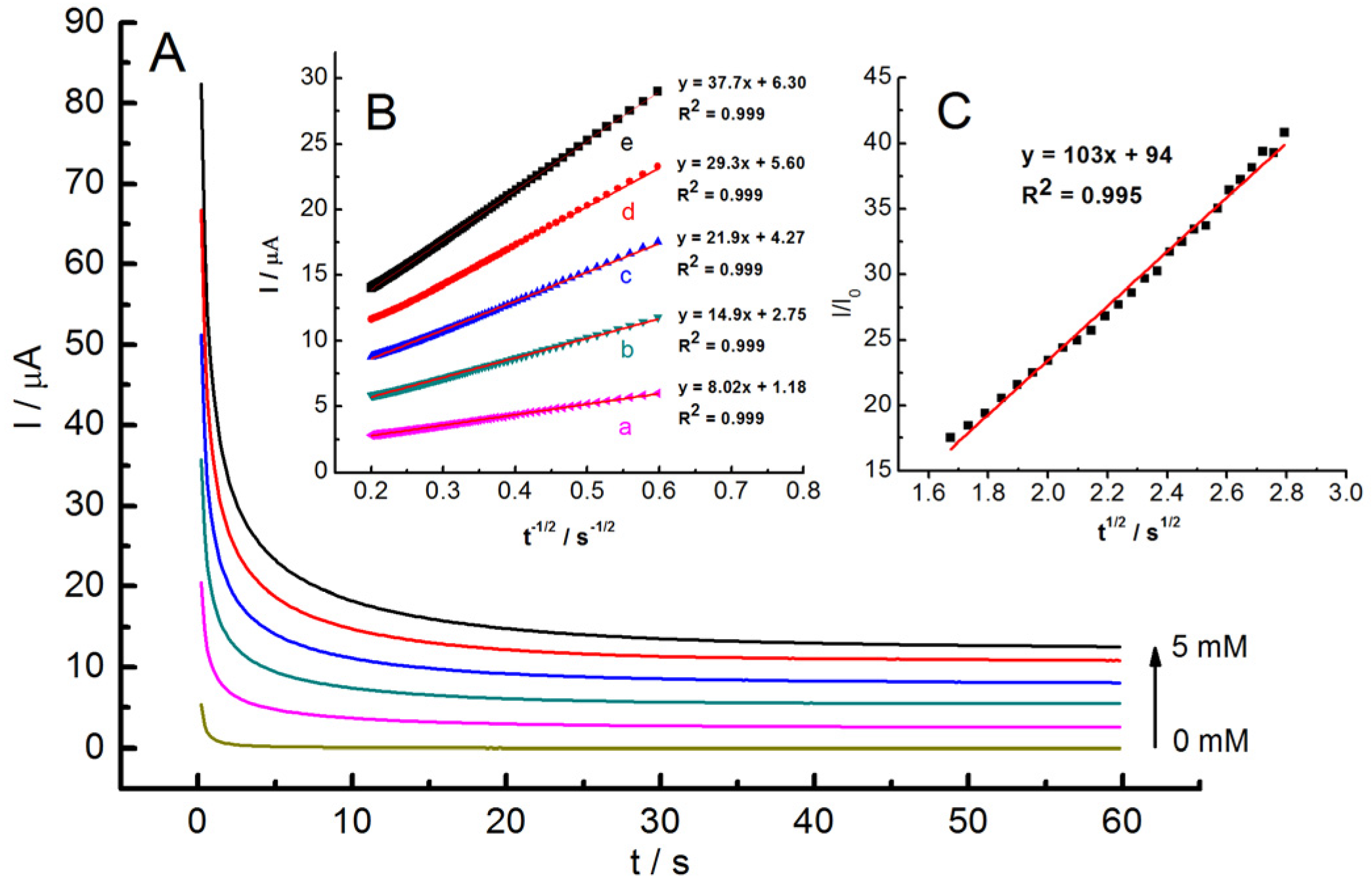
2.4. Electrocatalytic Hydrazine Oxidation at GC/MWCNT/LS/NPd
3. Experimental Section
3.1. Materials and Reagents
3.2. Apparatus
3.3. Preparation of MWCNT/LS Hybrid Material
3.4. Preparation of MWCNT/LS/NPd Electrode Material
3.5. Preparation of GC/MWCNT, GC/MWCNT/LS, and GC/MWCNT/LS/NPd Modified Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, X.; Zheng, J. Amperometric hydrazine sensor based on the use of a gold nanoparticle-modified nanocomposite consisting of porous polydopamine, multiwalled carbon nanotubes and reduced graphene oxide. Microchim. Acta 2020, 187, 89. [Google Scholar] [CrossRef] [PubMed]
- Avanes, A.; Hasanzadeh-Karamjavan, M.; Shokri-Jarcheloo, G. Electrocatalytic oxidation and amperometric determination of hydrazine using a carbon paste electrode modified with β-nickel hydroxide nanoplatelets. Microchim. Acta 2019, 186, 441. [Google Scholar] [CrossRef]
- Deroco, P.; Melo, I.G.; Silva, L.S.; Eguiluz, K.; Salazar-Banda, G.R.; Fatibello-Filho, O. Carbon black supported Au–Pd core-shell nanoparticles within a dihexadecylphosphate film for the development of hydrazine electrochemical sensor. Sens. Actuators B 2018, 256, 535–542. [Google Scholar] [CrossRef]
- Zhao, J.; Yue, P.; Tricard, S.; Pang, T.; Yang, Y.; Fang, J. Prussian blue (PB)/carbon nanopolyhedra/polypyrrole composite as electrode: A high performance sensor to detect hydrazine with long linear range. Sens. Actuators B Chem. 2017, 251, 706–712. [Google Scholar] [CrossRef]
- Tang, Y.-Y.; Kao, C.-L.; Chen, P.-Y. Electrochemical detection of hydrazine using a highly sensitive nanoporous gold electrode. Anal. Chim. Acta 2012, 711, 32–39. [Google Scholar] [CrossRef]
- Lin, H.; Yang, J.; Liu, J.; Huang, Y.; Xiao, J.; Zhang, X. Properties of Pd nanoparticles-embedded polyaniline multilayer film and its electrocatalytic activity for hydrazine oxidation. Electrochim. Acta 2013, 90, 382–392. [Google Scholar] [CrossRef]
- Panchompoo, J.; Aldous, L.; Downing, C.; Crossley, A.; Compton, R.G. Facile Synthesis of Pd Nanoparticle Modified Carbon Black for Electroanalysis: Application to the Detection of Hydrazine. Electroanalysis 2011, 23, 1568–1578. [Google Scholar] [CrossRef]
- Lu, Z.; Fan, W.; Shi, X.; Lu, Y.; Fan, C. Two Distinctly Separated Emission Colorimetric NIR Fluorescent Probe for Fast Hydrazine Detection in Living Cells and Mice upon Independent Excitations. Anal. Chem. 2017, 89, 9918–9925. [Google Scholar] [CrossRef]
- Zhai, Q.; Feng, W.; Feng, G. Rapid detection of hydrazine in almost wholly water solution and in living cells with a new colorimetric and fluorescent turn-on probe. Anal. Methods 2016, 8, 5832–5837. [Google Scholar] [CrossRef]
- Garrod, S.; Bollard, M.E.; Nicholls, A.W.; Connor, S.C.; Connelly, J.; Nicholson, J.K.; Holmes, E. Integrated Metabonomic Analysis of the Multiorgan Effects of Hydrazine Toxicity in the Rat. Chem. Res. Toxicol. 2005, 18, 115–122. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, M.; Zheng, M.; Tang, Y.; Chen, Y.; Lu, T. Electrocatalytic oxidation and detection of hydrazine at carbon nanotube-supported palladium nanoparticles in strong acidic solution conditions. Electrochim. Acta 2011, 56, 4930–4936. [Google Scholar] [CrossRef]
- Huang, H.; Li, T.; Sun, Y.; Yu, L.; Wang, C.; Shen, R.; Ye, W.; Wang, D.; Li, Y. Amperometric sensing of hydrazine in environmental and biological samples by using CeO2-encapsulated gold nanoparticles on reduced graphene oxide. Microchim. Acta 2019, 186, 46. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, Z.; Guo, Z.; Wang, Q. Flow-injection electrogenerated chemiluminescence detection of hydrazine based on its in-situ electrochemical modification at a pre-anodized platinum electrode. Analyst 2002, 127, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Nagaraja, K.S.; Balasubramanian, N. Spectrophotometric determination of hydrazine. Talanta 2008, 75, 27–31. [Google Scholar] [CrossRef]
- Sun, M.; Bai, L.; Liu, D.Q. A generic approach for the determination of trace hydrazine in drug substances using in situ derivatization-headspace GC-MS. J. Pharm. Biomed. Anal. 2009, 49, 529–533. [Google Scholar] [CrossRef]
- Kosyakov, D.S.; Amosov, A.S.; Ul’yanovskii, N.V.; Ladesov, A.V.; Khabarov, Y.G.; Shpigun, O.A. Spectrophotometric determination of hydrazine, methylhydrazine, and 1,1-dimethylhydrazine with preliminary derivatization by 5-nitro-2-furaldehyde. J. Anal. Chem. 2017, 72, 171–177. [Google Scholar] [CrossRef]
- Kato, T.; Sugahara, S.; Kajitani, T.; Senga, Y.; Egawa, M.; Kamiya, H.; Seike, Y. Determination of Trace Hydrazine in Environmental Water Samples by in situ Solid Phase Extraction. Anal. Sci. 2017, 33, 487–491. [Google Scholar] [CrossRef][Green Version]
- Koreshkova, A.N.; Gupta, V.; Peristyy, A.; Nesterenko, P.N.; Rodemann, T.; Paull, B. Ion chromatographic determination of hydrazine in excess ammonia for monitoring graphene oxide reduction reaction. Talanta 2019, 205, 120081. [Google Scholar] [CrossRef]
- Kosyakov, D.S.; Pikovskoi, I.I.; Ul’yanovskii, N.V.; Kozhevnikov, A.Y. Direct determination of hydrazine, methylhydrazine, and 1,1-dimethylhydrazine by zwitterionic hydrophilic interaction liquid chromatography with amperometric detection. Int. J. Environ. Anal. Chem. 2017, 97, 313–329. [Google Scholar] [CrossRef]
- McAdam, K.; Kimpton, H.; Essen, S.; Davis, P.; Vas, C.; Wright, C.; Porter, A.; Rodu, B. Analysis of hydrazine in smokeless tobacco products by gas chromatography–mass spectrometry. Chem. Cent. J. 2015, 9, 13. [Google Scholar] [CrossRef]
- Mohammadi, S.Z.; Beitollahi, H.; Asadi, E.B. Electrochemical determination of hydrazine using a ZrO2 nanoparticles-modified carbon paste electrode. Environ. Monit. Assess. 2015, 187, 122. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, A.K.; Ahmed, M.S.; Roy, N.; Jeon, S. Electrochemical determination of hydrazine in surface water on Co(OH)2 nanoparticles immobilized on functionalized graphene interface. Appl. Surf. Sci. 2021, 540, 148346. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Hosseinzadeh, R.; Afshar, A.A.; Varma, R.S.; Jang, H.W.; Shokouhimehr, M. Electrochemical Detection of Hydrazine by Carbon Paste Electrode Modified with Ferrocene Derivatives, Ionic Liquid, and CoS2-Carbon Nanotube Nanocomposite. ACS Omega 2021, 6, 4641–4648. [Google Scholar] [CrossRef] [PubMed]
- Murugadoss, G.; Manibalan, G.; Hazra, S.; Kumar, M.R.; Rajabathar, J.R.; Yadav, H.M. Electrochemical determination of hydrazine using facilely synthesized Sn-decorated α-Fe2O3 nanoparticles modified electrode. J. Mater. Sci. Mater. Electron. 2022, 33, 13593–13603. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Morawski, F.M.; Pessanha, E.C.; de Lima, S.L.S.; da Costa, D.S.; Ribeiro, G.A.C.; Vaz, J.; Mouta, R.; Tanaka, A.A.; Liu, L.; et al. Facile Gram-Scale Synthesis of NiO Nanoflowers for Highly Selective and Sensitive Electrocatalytic Detection of Hydrazine. ACS Omega 2023, 8, 11978–11986. [Google Scholar] [CrossRef]
- Neto, A.G.S.; Santos, A.C.F.; Junior, J.A.O.; Jost, C.L.; de Sousa, C.S.; Goulart, M.O.F.; Santana, A.E.G.; Gonzalez, S.Y.G.; da Silva, E.E.; Fonsaca, J.E.S.; et al. Exploiting synergistic effects: Cheap ultra-sensitive electrochemical rutin detection using WO3/rGO nanocomposite in combination with multiwalled carbon nanotubes. Microchem. J. 2023, 193, 109090. [Google Scholar] [CrossRef]
- Batchelor-McAuley, C.; Banks, C.E.; Simm, A.O.; Jones, T.G.J.; Compton, R.G. The electroanalytical detection of hydrazine: A comparison of the use of palladium nanoparticles supported on boron-doped diamond and palladium plated BDD microdisc array. Analyst 2006, 131, 106–110. [Google Scholar] [CrossRef]
- Granot, E.; Filanovsky, B.; Presman, I.; Kuras, I.; Patolsky, F. Hydrazine/air direct-liquid fuel cell based on nanostructured copper anodes. J. Power Source 2012, 204, 116–121. [Google Scholar] [CrossRef]
- Roy, C.; Bertin, E.; Martin, M.H.; Garbarino, S.; Guay, D. Hydrazine Oxidation at Porous and Preferentially Oriented {100} Pt Thin Films. Electrocatalysis 2013, 4, 76–84. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Bai, J.; Jiang, X.; Fan, G. High sensitivity hydrogen peroxide and hydrazine sensor based on silver nanocubes with rich {100} facets as an enhanced electrochemical sensing platform. Biosens. Bioelectron. 2013, 43, 180–185. [Google Scholar] [CrossRef]
- Koçak, S.; Aslışen, B. Hydrazine oxidation at gold nanoparticles and poly(bromocresol purple) carbon nanotube modified glassy carbon electrode. Sens. Actuators B Chem. 2014, 196, 610–618. [Google Scholar] [CrossRef]
- Serov, A.; Padilla, M.; Roy, A.J.; Atanassov, P.; Sakamoto, T.; Asazawa, K.; Tanaka, H. Anode catalysts for direct hydrazine fuel cells: From laboratory test to an electric vehicle. Angew. Chem. 2017, 126, 10504. [Google Scholar] [CrossRef]
- Rajkumar, M.; Hong, C.P.; Chen, S.-M. Electrochemical Synthesis of Palladium Nano Urchins Decorated Multi Walled Carbon Nanotubes for Electrocatalytic Oxidation of Hydrazine and Reduction of Hydrogen peroxide. Int. J. Electrochem. Sci. 2013, 8, 5262–5274. [Google Scholar] [CrossRef]
- Promsuwan, K.; Kachatong, N.; Limbut, W. Simple flow injection system for non-enzymatic glucose sensing based on an electrode modified with palladium nanoparticles-graphene nanoplatelets/mullti-walled carbon nanotubes. Electrochim. Acta 2019, 320, 134621. [Google Scholar] [CrossRef]
- Kongkaew, S.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. A preparation of homogeneous distribution of palladium nanoparticle on poly (acrylic acid)-functionalized graphene oxide modified electrode for formalin oxidation. Electrochim. Acta 2017, 247, 229–240. [Google Scholar] [CrossRef]
- Miao, R.; Yang, M.; Compton, R.G. The Electro-oxidation of Hydrazine with Palladium Nanoparticle Modified Electrodes: Dissecting Chemical and Physical Effects: Catalysis, Surface Roughness, or Porosity? J. Phys. Chem. Lett. 2021, 12, 6661–6666. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Kubendhiran, S.; Chen, S.M.; Sireesha, P.; Karuppiah, C.; Su, C. Functionalization of reduced graphene oxide with β-cyclodextrin modified palladium nanoparticles for the detection of hydrazine in environmental water samples. Electroanalysis 2017, 29, 587–594. [Google Scholar] [CrossRef]
- Ejaz, A.; Ahmed, M.S.; Jeon, S. Highly efficient benzylamine functionalized graphene supported palladium for electrocatalytic hydrazine determination. Sens. Actuators B Chem. 2015, 221, 1256–1263. [Google Scholar] [CrossRef]
- Tang, X.; Haddad, P.-A.; Mager, N.; Gen, X.; Reckinger, N.; Hermans, S.; Debliquy, M.; Raskin, J.-P. Chemically deposited palladium nanoparticles on graphene for hydrogen sensor applications. Sci. Rep. 2019, 9, 3653. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Z.; Xiao, J.; Chen, C.; Xiao, F.; Wang, S.; Liu, Y. Facile and Green Synthesis of Palladium Nanoparticles-Graphene-Carbon Nanotube Material with High Catalytic Activity. Sci. Rep. 2013, 3, 2527. [Google Scholar] [CrossRef]
- Chen, X.; Liu, W.; Tang, L.; Wang, J.; Pan, H.; Du, M. Electrochemical sensor for detection of hydrazine based on Au@Pd core–shell nanoparticles supported on amino-functionalized TiO2 nanotubes. Mater. Sci. Eng. C 2014, 34, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Gioia, D.; Casella, I.G. Pulsed electrodeposition of palladium nano-particles on coated multi-walled carbon nanotubes/nafion composite substrates: Electrocatalytic oxidation of hydrazine and propranolol in acid conditions. Sens. Actuators B Chem. 2016, 237, 400–407. [Google Scholar] [CrossRef]
- Wildgoose, G.; Banks, C.E.; Compton, R.G. Metal nanoparticles and related materials supported on carbon nanotubes: Methods and applications. Small 2006, 2, 182–193. [Google Scholar] [CrossRef]
- Cornelio, B.; Rance, G.A.; Laronze-Cochard, M.; Fontana, A.; Sapi, J.; Khlobystov, A.N. Palladium nanoparticles on carbon nanotubes as catalysts of cross-coupling reactions. J. Mater. Chem. A 2013, 1, 8737–8744. [Google Scholar] [CrossRef]
- Wang, W.; Yang, G.; Wang, Q.; Cao, Y.; Wang, H.; Yu, H. Modifying carbon nanotubes supported palladium nanoparticles via regulating the electronic metal–carbon interaction for phenol hydrogenation. Chem. Eng. J. 2022, 436, 131758. [Google Scholar] [CrossRef]
- Kondratiev, V.V.; Babkova, T.A.; Tolstopjatova, E.G. PEDOT-supported Pd nanoparticles as a catalyst for hydrazine oxidation. J. Solid State Electrochem. 2013, 17, 1621–1630. [Google Scholar] [CrossRef]
- Ilieva, M.; Nakova, A.; Tsakova, V. Pd-modified PEDOT layers obtained through electroless metal deposition—electrooxidation of glycerol. J. Solid State Electrochem. 2016, 20, 3015–3023. [Google Scholar] [CrossRef]
- Nakova, A.; Ilieva, M.; Boiadjieva-Scherzer, T.; Tsakova, V. High-density Pd nanoparticles distribution on PEDOT obtained through electroless metal deposition on pre-reduced polymer layers. Electrochim. Acta 2017, 253, 128–133. [Google Scholar] [CrossRef]
- Promsuwan, K.; Thongtawat, J.; Limbut, W. Porous palladium-poly(3,4-ethylenedioxythiophene)–coated carbon microspheres/graphene nanoplatelet–modified electrode for flow-based-amperometric hydrazine sensor. Microchim. Acta 2020, 187, 539. [Google Scholar] [CrossRef]
- Ghasemi, S.; Hosseini, S.R.; Hasanpoor, F.; Nabipour, S. Amperometric hydrazine sensor based on the use of Pt-Pd nanoparticles placed on reduced graphene oxide nanosheets. Microchim. Acta 2019, 186, 601. [Google Scholar] [CrossRef]
- Coccia, F.; Tonucci, L.; Bosco, D.; Bressan, M.; d’Alessandro, N. One-pot synthesis of lignin-stabilised platinum and palladium nanoparticles and their catalytic behaviour in oxidation and reduction reactions. Green Chem. 2012, 14, 1073–1078. [Google Scholar] [CrossRef]
- Coccia, F.; Tonucci, L.; d’Alessandro, N.; D’Ambrosio, P.; Bressan, M. Palladium nanoparticles, stabilized by lignin, as catalyst for cross-coupling reactions in water. Inorganica Chim. Acta 2013, 399, 12–18. [Google Scholar] [CrossRef]
- Mohazzab, B.F.; Jaleh, B.; Nasrollahzadeh, M.; Khazalpour, S.; Sajjadi, M.; Varma, R.S. Upgraded Valorization of Biowaste: Laser-Assisted Synthesis of Pd/Calcium Lignosulfonate Nanocomposite for Hydrogen Storage andEnvironmental Remediation. ACS Omega 2020, 5, 5888–5899. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Cao, M.; Chang, Z.; Tan, J.; Wang, X.; Zhang, P.; Lin, S.; Liu, J.; Li, A. Superoxide Radical-Mediated Self-Synthesized Au/MoO3–x Hybrids with Enhanced Peroxidase-like Activity and Photothermal Effect for Anti-MRSA Therapy. ACS Appl. Mater. Interfaces 2022, 14, 13025–13037. [Google Scholar] [CrossRef]
- Płócienniczak, P.; Rębiś, T.; Nowicki, M.; Milczarek, G. A green approach for hybrid material preparation based on carbon nanotubes/lignosulfonate decorated with silver nanostructures for electrocatalytic sensing of H2O2. J. Electroanal. Chem. 2021, 880, 114896. [Google Scholar] [CrossRef]
- Płócienniczak, P.; Rębiś, T.; Leda, A.; Milczarek, G. Lignosulfonate-assisted synthesis of platinum nanoparticles deposited on multi-walled carbon nanotubes for biosensing of glucose. Colloids Surf. B—Biointerfaces 2022, 210, 112222. [Google Scholar] [CrossRef]
- Martyanov, K.; Kuropatov, V. Functionalized o-Quinones: Concepts, Achievements and Prospects. Inorganics 2018, 6, 48. [Google Scholar] [CrossRef]
- Pierpont, C.G. Unique properties of transition metal quinone complexes of the MQ3 series. Coord. Chem. Rev. 2001, 219–221, 415–433. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Méndez-Rojas, M.A.; Garnovskii, A.D.; Ivakhnenko, E.P.; Ortiz-Méndez, U. Coordination Chemistry of O-Quinone Complexes. J. Coord. Chem. 2002, 55, 745–770. [Google Scholar] [CrossRef]
- Velmurugan, M.; Thirumalraj, B.; Chen, S.-M. Development of electrochemical sensor for the determination of palladium ions (Pd2+) using flexible screen printed un-modified carbon electrode. J. Colloid Interface Sci. 2017, 485, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Karuppiah, C.; Velmurugan, M.; Chen, S.-M.; Devasenathipathy, R.; Krthik, R.; Wang, S.-F. Electrochemical Activation of Graphite Nanosheets Decorated with Palladium Nanoparticles for High Performance Amperometric Hydrazine Sensor. Electroanalysis 2016, 28, 808–816. [Google Scholar] [CrossRef]
- Wen, C.; Li, Z.; Cao, C.; Wang, Y.; Guo, P.; Zhao, X.S. Structural evolution of palladium nanoparticles and their electrocatalytic activity toward ethanol oxidation in alkaline solution. RSC Adv. 2016, 6, 91991–91998. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Zhao, D.; Xu, C. Facile fabrication of nanoporous PdFe alloy for nonenzymatic electrochemical sensing of hydrogen peroxide and glucose. Anal. Chim. Acta 2014, 832, 34–43. [Google Scholar] [CrossRef]
- McConnell, C.; Kanakaraj, S.N.; Dugre, J.; Malik, R.; Zhang, G.; Haase, M.R.; Hsieh, Y.-Y.; Fang, Y.; Mast, D.; Shanov, V. Hydrogen Sensors Based on Flexible Carbon Nanotube-Palladium Composite Sheets Integrated with Ripstop Fabric. ACS Omega 2020, 5, 487–497. [Google Scholar] [CrossRef]
- Daoush, W.M.; Imae, T. Syntheses and characterizations of multiwalled carbon nanotubes-supported palladium nanocomposites. J. Mater. Res. 2012, 27, 1680–1687. [Google Scholar] [CrossRef]
- García-Trenco, A.; Regoutz, A.; White, E.R.; Payne, D.J.; Shaffer, M.S.P.; Williams, C.K. PdIn intermetallic nanoparticles for the Hydrogenation of CO2 to Methanol. Appl. Catal. B Environ. 2018, 220, 9–18. [Google Scholar] [CrossRef]
- Xue, N.; Yu, R.-J.; Yuan, C.-Z.; Xie, X.; Jiang, Y.-F.; Zhou, H.-Y.; Cheang, T.-Y.; Xu, A.-W. In situ redox deposition of palladium nanoparticles on oxygen-deficient tungsten oxide as efficient hydrogenation catalysts. RSC Adv. 2017, 7, 2351–2357. [Google Scholar] [CrossRef]
- Sun, S.; Dummer, N.F.; Bere, T.; Barnes, A.J.; Shaw, G.; Douthwaite, M.; Pattisson, S.; Lewis, R.J.; Richards, N.; Morgan, D.J.; et al. Selective oxidation of methane to methanol and methyl hydroperoxide over palladium modified MoO3 photocatalyst under ambient conditions. Catal. Sci. Technol. 2022, 12, 3727–3736. [Google Scholar] [CrossRef]
- Enneiymy, M.; Fioux, P.; Drian, C.L.; Ghimbeu, C.M.; Becht, J.-M. Palladium nanoparticles embedded in mesoporous carbons as efficient, green and reusable catalysts for mild hydrogenations of nitroarenes. RSC Adv. 2020, 10, 36741–36750. [Google Scholar] [CrossRef]
- Lesiak, B.; Kövér, L.; Tóth, J.; Zemek, J.; Jiricek, P.; Kromka, A.; Rangama, N. C sp2/sp3 hybridisations in carbon nanomaterials – XPS and (X)AES study. Appl. Surf. Sci. 2018, 452, 223–231. [Google Scholar] [CrossRef]
- Gurzęda, B.; Florczak, P.; Kempiński, M.; Peplińska, B.; Krawczyk, P.; Jurga, S. Synthesis of graphite oxide by electrochemical oxidation in aqueous perchloric acid. Carbon 2016, 100, 540–545. [Google Scholar] [CrossRef]
- Tang, P.-D.; Du, Q.-S.; Li, D.-P.; Dai, J. Fabrication and Characterization of Graphene Microcrystal Prepared from Lignin Refined from Sugarcane Bagasse. Nanomaterials 2018, 8, 565. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Sun, E.; Lü, W.; Li, S.; Yang, B.; Zhang, R.; Cao, W. Dynamic characteristics of defect dipoles in Mn-doped 0.24Pb(In1/2Nb1/2)O3–0.47Pb(Mg1/3Nb2/3)O3–0.29PbTiO3 single crystal. CrystEngComm 2019, 21, 348–355. [Google Scholar] [CrossRef]
- Chen, H.; Wei, G.; Ispas, A.; Hickey, S.G.; Eychmüller, A. Synthesis of Palladium Nanoparticles and Their Applications for Surface-Enhanced Raman Scattering and Electrocatalysis. J. Phys. Chem. C 2010, 114, 21976–21981. [Google Scholar] [CrossRef]
- Ruano, D.; Pabón, B.M.; Azenha, C.; Mateos-Pedrero, C.; Mendes, A.; Pérez-Dieste, V.; Concepción, P. Influence of the ZrO2 Crystalline Phases on the Nature of Active Sites in PdCu/ZrO2 Catalysts for the Methanol Steam Reforming Reaction—An In Situ Spectroscopic Study. Catalysts 2020, 10, 1005. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.T.; El-Chaghaby, G.A.; Helal, F.S. Individual and competitive adsorption of phenol and nickel onto multiwalled carbon nanotubes. J. Adv. Res. 2015, 6, 405–514. [Google Scholar] [CrossRef]
- Xu, J.; Lv, X.; Li, J.; Li, Y.; Shen, L.; Zhou, H.; Xu, X. Simultaneous adsorption and dechlorination of 2,4-dichlorophenol by Pd/Fe nanoparticles with multi-walled carbon nanotube support. J. Hazard. Mater. 2012, 225–226, 36–45. [Google Scholar] [CrossRef]
- Hubkowska, K.; Soszko, M.; Symonowicz, M.; Łukaszewski, M.; Czerwiński, A. Electrochemical Behavior of a Pd Thin Film Electrode in Concentrated Alkaline Media. Electrocatalysis 2017, 8, 295–300. [Google Scholar] [CrossRef]
- Hara, M.; Linke, U.; Wandlowski, T. Preparation and electrochemical characterization of palladium single crystal electrodes in 0.1 M H2SO4 and HClO4: Part I. Low-index phases. Electrochim. Acta 2007, 52, 5733–5748. [Google Scholar] [CrossRef]
- Amorim, F.M.L.; Crisafulli, R.; Linares, J.J. An Alkaline-Acid Glycerol Electrochemical Reformer for Simultaneous Production of Hydrogen and Electricity. Nanomaterials 2022, 12, 1315. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Buttry, D.A. Influence of Halide Ions on Anodic Oxidation of Ethanol on Palladium. Electrocatalysis 2016, 7, 201–206. [Google Scholar] [CrossRef]
- Duncan, H.; Lasia, A. Mechanism of hydrogen adsorption/absorption at thin Pd layers on Au(1 1 1). Electrochim. Acta 2007, 52, 6195–6205. [Google Scholar] [CrossRef]
- Daubinger, P.; Kieninger, J.; Unmüssig, T.; Urban, G.A. Electrochemical characteristics of nanostructured platinum electrodes – a cyclic voltammetry study. Phys. Chem. Chem. Phys. 2014, 16, 8392–8399. [Google Scholar] [CrossRef] [PubMed]
- Swetha, P.; Devi, K.S.; Kumar, A.S. In-situ trapping and confining of highly redox active quinoline quinones on MWCNT modified glassy carbon electrode and its selective electrocatalytic oxidation and sensing of hydrazine. Electrochim. Acta 2014, 147, 62–72. [Google Scholar] [CrossRef]
- Rębiś, T.; Sobkowiak, M.; Milczarek, G. Electrocatalytic oxidation and detection of hydrazine at conducting polymer/lignosulfonate composite modified electrodes. J. Electroanal. Chem. 2016, 780, 257–263. [Google Scholar] [CrossRef]
- Miao, R.; Compton, R.G. Mechanism of hydrazine oxidation at Palladium electrodes: Long-lived radical di-cation formation. Electrochim. Acta 2021, 388, 138655. [Google Scholar] [CrossRef]
- Miao, R.; Compton, R.G. The Electro-Oxidation of Hydrazine: A Self-Inhibiting Reaction. J. Phys. Chem. Lett. 2021, 12, 1601–1605. [Google Scholar] [CrossRef]
- Guidelli, R.; Compton, R.G.; Feliu, J.M.; Gileadi, E.; Lipkowski, J.; Schmickler, W.; Trasatti, S. Defining the transfer coefficient in electrochemistry: An assessment (IUPAC Technical Report). Pure Appl. Chem. 2014, 86, 245–258. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, L.; Wang, T.; Han, Q.; Xu, S. A high-performance hydrazine electrochemical sensor based on gold nanoparticles/single-walled carbon nanohorns composite film. Appl. Surf. Sci. 2016, 369, 36–42. [Google Scholar] [CrossRef]
- Abbaspour, A.; Kamyabi, M.A. Electrocatalytic oxidation of hydrazine on a carbon paste electrode modified by hybrid hexacyanoferrates of copper and cobalt films. J. Electroanal. Chem. 2005, 576, 73–83. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, C.; He, X.; Li, Z.; Zhang, X.; Wang, L.; Fang, B. Detection of hydrazine based on Nano-Au deposited on Porous-TiO2 film. Electrochim. Acta 2010, 55, 7204–7210. [Google Scholar] [CrossRef]
- Zare, H.R.; Nasirizadeh, N. Electrocatalytic Characteristics of Hydrazine and Hydroxylamine Oxidation at Coumestan Modified Carbon Paste Electrode. Electroanalysis 2006, 18, 507–512. [Google Scholar] [CrossRef]
- Li, J.; Xie, H.; Chen, L. A sensitive hydrazine electrochemical sensor based on electrodeposition of gold nanoparticles on choline film modified glassy carbon electrode. Sens. Actuators B 2011, 153, 239–245. [Google Scholar] [CrossRef]
- Kim, S.P.; Lee, S.G.; Choi, M.Y.; Choi, H.C. Highly Sensitive Hydrazine Chemical Sensor Based on CNT-PdPt Nanocomposites. J. Nanomater. 2016, 16, 298. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, G.; Ji, Y.; Liu, M.; Feng, Y.; Zhang, Z.; Fang, B. Enhancement in analytical hydrazine based on gold nanoparticles deposited on ZnO-MWCNTs films. Sens. Actuators B Chem. 2010, 150, 247–253. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, J.; Hou, H.; You, T. Electrochemical Detection of Hydrazine Based on Electrospun Palladium Nanoparticle/Carbon Nanofibers. Electroanalysis 2009, 21, 1869–1874. [Google Scholar] [CrossRef]
- Aziz, M.A.; Kawde, A.-N. Gold nanoparticle-modified graphite pencil electrode for the high-sensitivity detection of hydrazine. Talanta 2013, 115, 214–221. [Google Scholar] [CrossRef]
- Zhou, T.; Lu, P.; Zhang, Z.; Wang, Q.; Umar, A. Perforated Co3O4 nanoneedles assembled in chrysanthemum-like Co3O4 structures for ultra-high sensitive hydrazine chemical sensor. Sens. Actuators B Chem. 2016, 235, 457–465. [Google Scholar] [CrossRef]
- Duan, C.; Dong, Y.; Sheng, Q.; Zheng, J. A high-performance non-enzymatic electrochemical hydrazine sensor based on NiCo2S4 porous sphere. Talanta 2019, 198, 23–29. [Google Scholar] [CrossRef]
- Luan, F.; Zhang, S.; Chen, D.; Zheng, K.; Zhuang, X. CoS2-decorated ionic liquid-functionalized graphene as a novel hydrazine electrochemical sensor. Talanta 2018, 182, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.K.; Ganesan, V.; Krishnamoorthi, S. Palladium nanoparticles decorated gaur gum based hybrid material for electrocatalytic hydrazine determination. Electrochim. Acta 2014, 125, 593–600. [Google Scholar] [CrossRef]





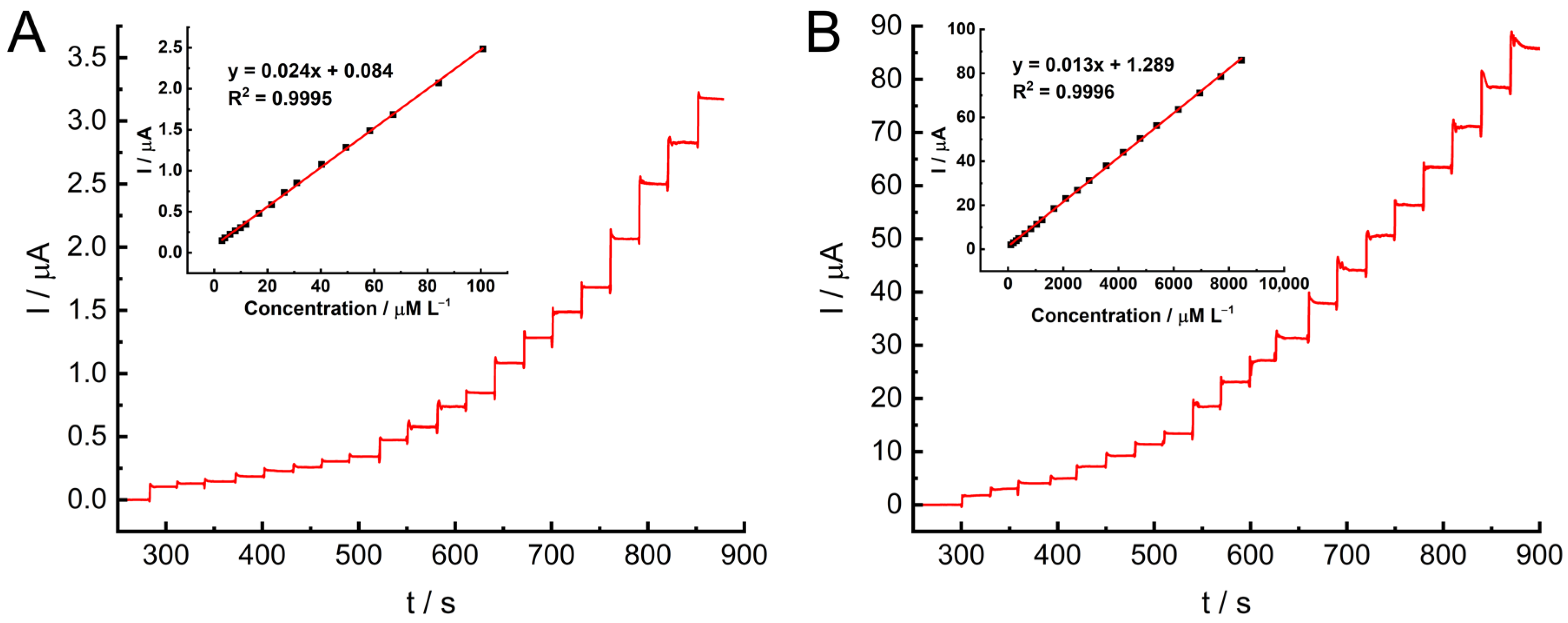
| Electrode Material | Linear Range [μM] | LOD [μM] | Reference |
|---|---|---|---|
| GC/MWCNT/LS/NPd | 3–100, 100–10,000 | 0.80 | This work |
| Pd-PEDOT@CM/GNP/GCE | 1–100, 100–5000 | 0.28 | [49] |
| Pd/MWNTs/GCE | 2.5–700 | 1.0 | [11] |
| Pd/CNF/GCE | 10–4000 | 2.9 | [97] |
| Pd/CB/GCE | 5–500 | 8.8 | [7] |
| Pd-GCM/MWCNT/GCE | 2–500 | 0.6 | [43] |
| PEDOT/Pd | 0.5–30 | 0.8 | [46] |
| PdNPs wrapped with guar gum-grafted-PAM-silica material/GCE | 50–600 | 4.1 | [102] |
| AuNP-GPE | 25–1000 | 3.07 | [98] |
| PVP-AgNCs/GCE | 5–460 | 1.1 | [30] |
| Co3O4/GCE | 50–1088 | 3.7 | [99] |
| Au@Pd/CB-DHP/GCE | 2.5–88 | 1.77 | [3] |
| NiCo2S4/GCE | 1.7–7800 | 0.6 | [100] |
| CoS2/IL-Gr/GCE | 5–100, 100–400 | 0.39 | [101] |
| GCE/PPy/LS | 2–75 | 1.65 | [86] |
| GCE/PEDOT/LS | 15–290, 380–2300 | 9.80 | [86] |
| Water Samples | Added Hydrazine [μM] | Found [μM] | Recovery [%] |
|---|---|---|---|
| S1 | 0 | N.D. | - |
| S2 | 100 | 99.6 ± 0.3 | 99.6 ± 0.3 |
| S3 | 200 | 201.2 ± 1.2 | 100.6 ± 0.6 |
| S4 | 300 | 300.4 ± 2.7 | 100.1 ± 1.0 |
| S5 | 400 | 397.3 ± 3.1 | 99.3 ± 1.0 |
| S6 | 500 | 492 ± 3.2 | 98.6 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płócienniczak-Bywalska, P.; Rębiś, T.; Leda, A.; Milczarek, G. Lignosulfonate-Assisted In Situ Deposition of Palladium Nanoparticles on Carbon Nanotubes for the Electrocatalytic Sensing of Hydrazine. Molecules 2023, 28, 7076. https://doi.org/10.3390/molecules28207076
Płócienniczak-Bywalska P, Rębiś T, Leda A, Milczarek G. Lignosulfonate-Assisted In Situ Deposition of Palladium Nanoparticles on Carbon Nanotubes for the Electrocatalytic Sensing of Hydrazine. Molecules. 2023; 28(20):7076. https://doi.org/10.3390/molecules28207076
Chicago/Turabian StylePłócienniczak-Bywalska, Patrycja, Tomasz Rębiś, Amanda Leda, and Grzegorz Milczarek. 2023. "Lignosulfonate-Assisted In Situ Deposition of Palladium Nanoparticles on Carbon Nanotubes for the Electrocatalytic Sensing of Hydrazine" Molecules 28, no. 20: 7076. https://doi.org/10.3390/molecules28207076
APA StylePłócienniczak-Bywalska, P., Rębiś, T., Leda, A., & Milczarek, G. (2023). Lignosulfonate-Assisted In Situ Deposition of Palladium Nanoparticles on Carbon Nanotubes for the Electrocatalytic Sensing of Hydrazine. Molecules, 28(20), 7076. https://doi.org/10.3390/molecules28207076







