A Turn-On Quinazolinone-Based Fluorescence Probe for Selective Detection of Carbon Monoxide
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural and Property Changes in NPQ in Reaction to CO
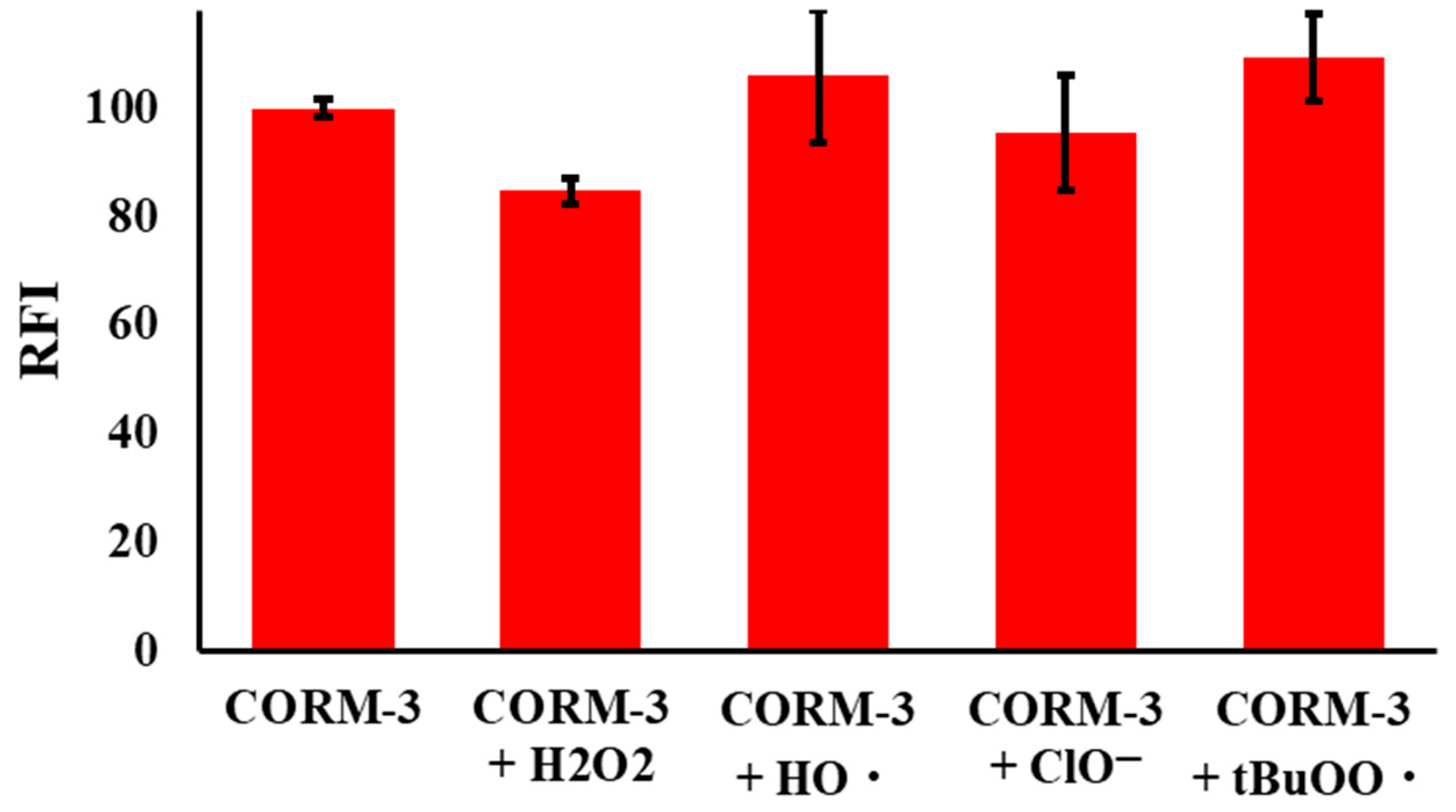
2.2. Selectivity of NPQ to CO
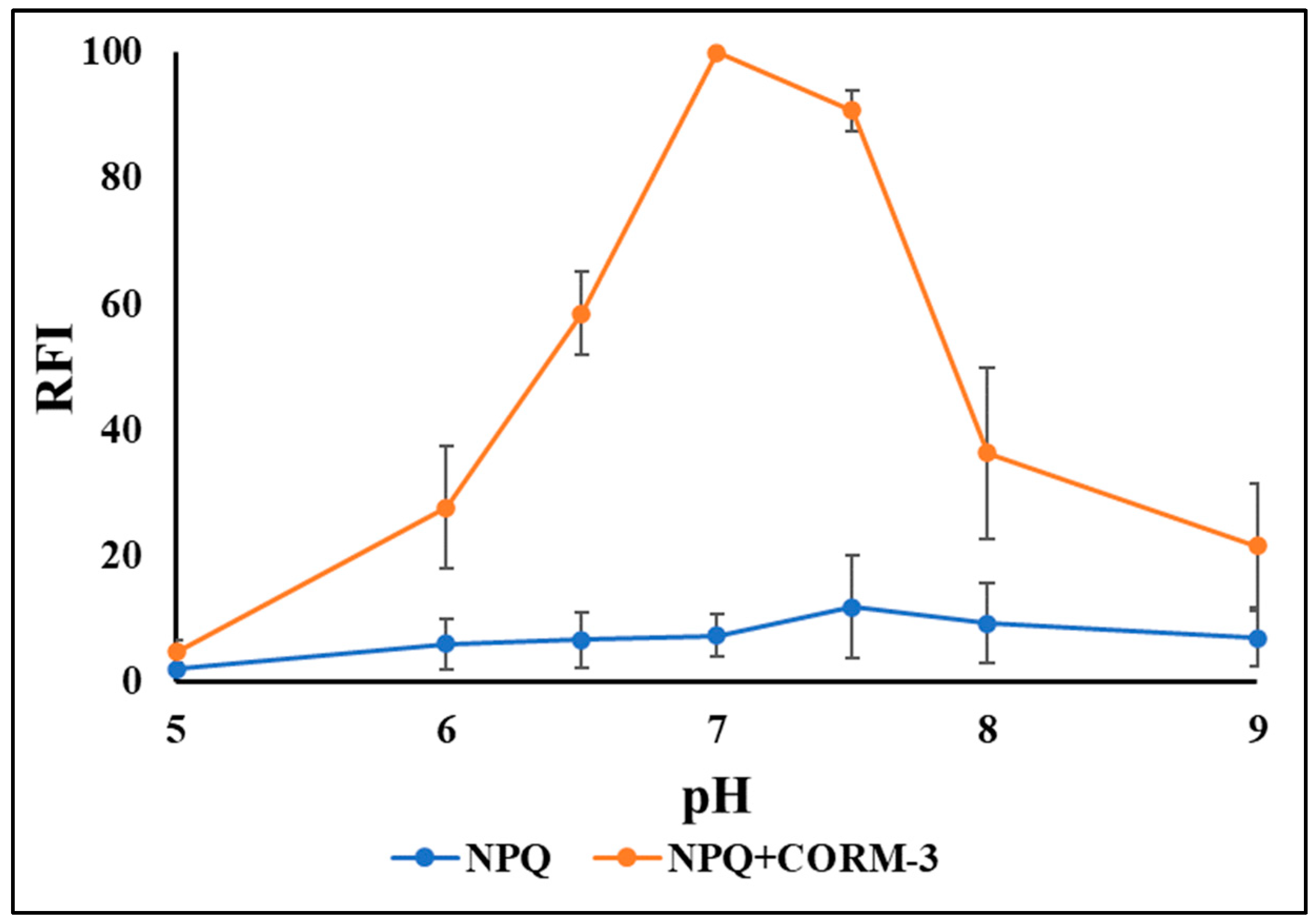
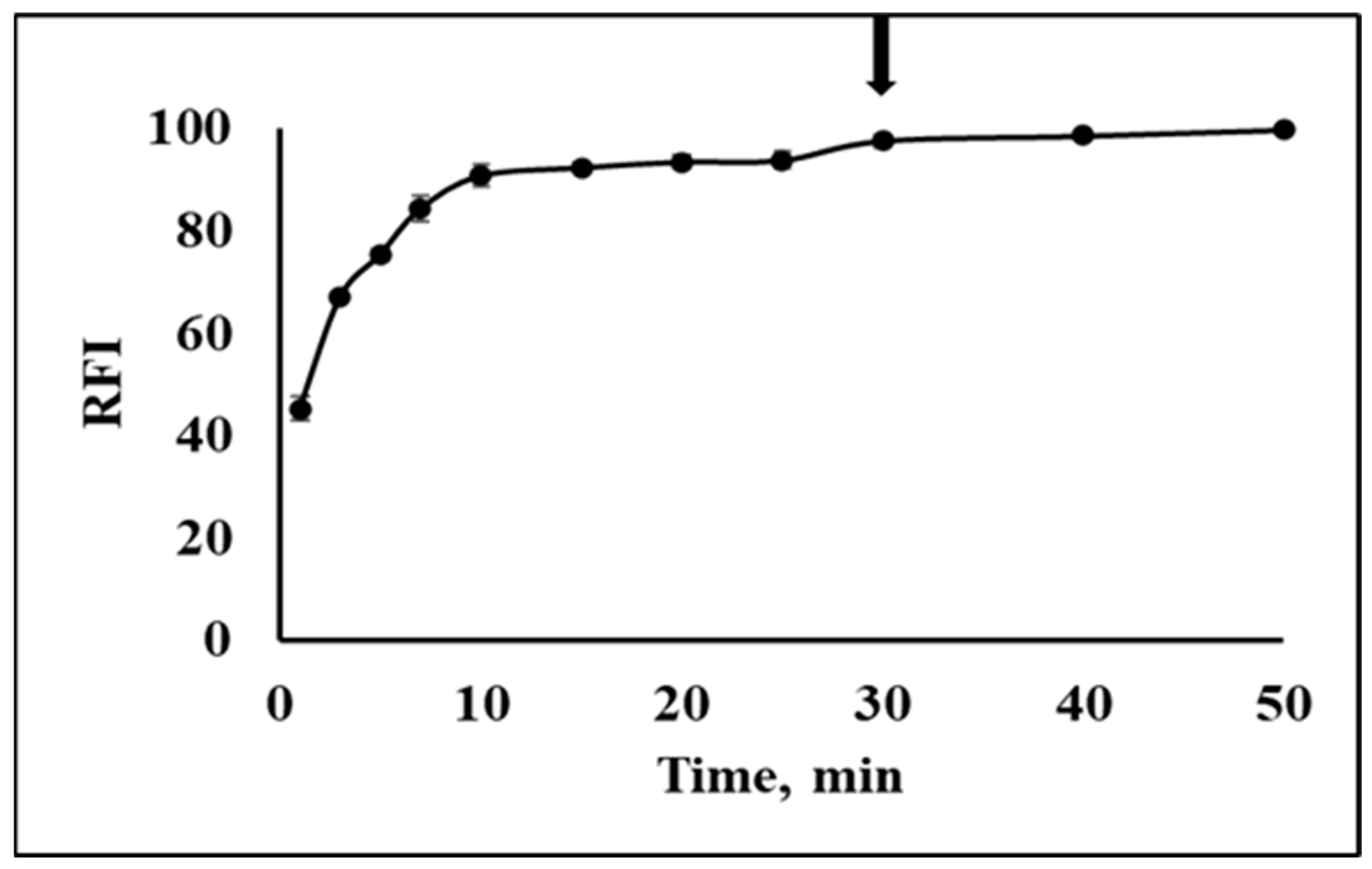
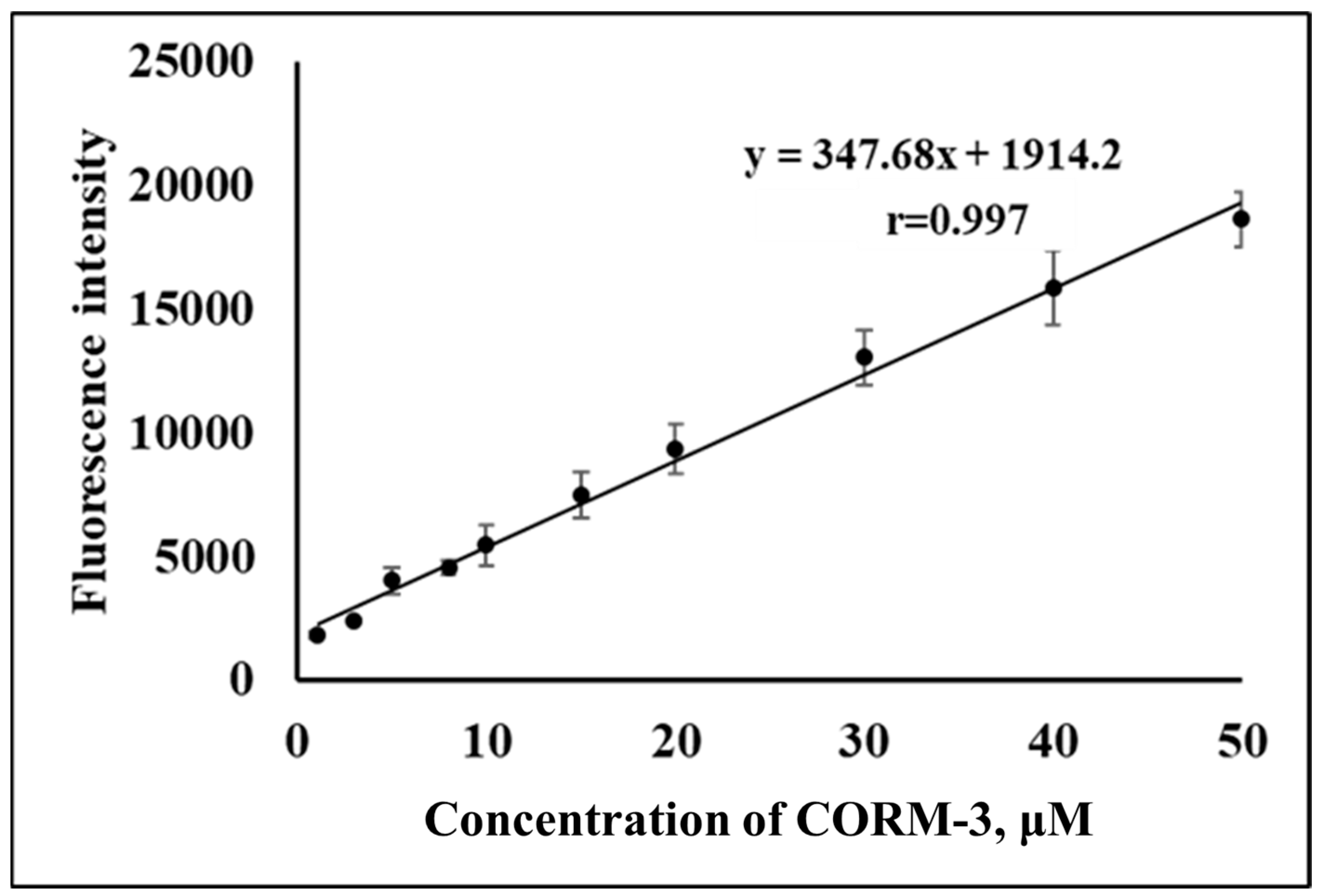
2.3. Optimization of Reaction Condition and Validation of CO Determination
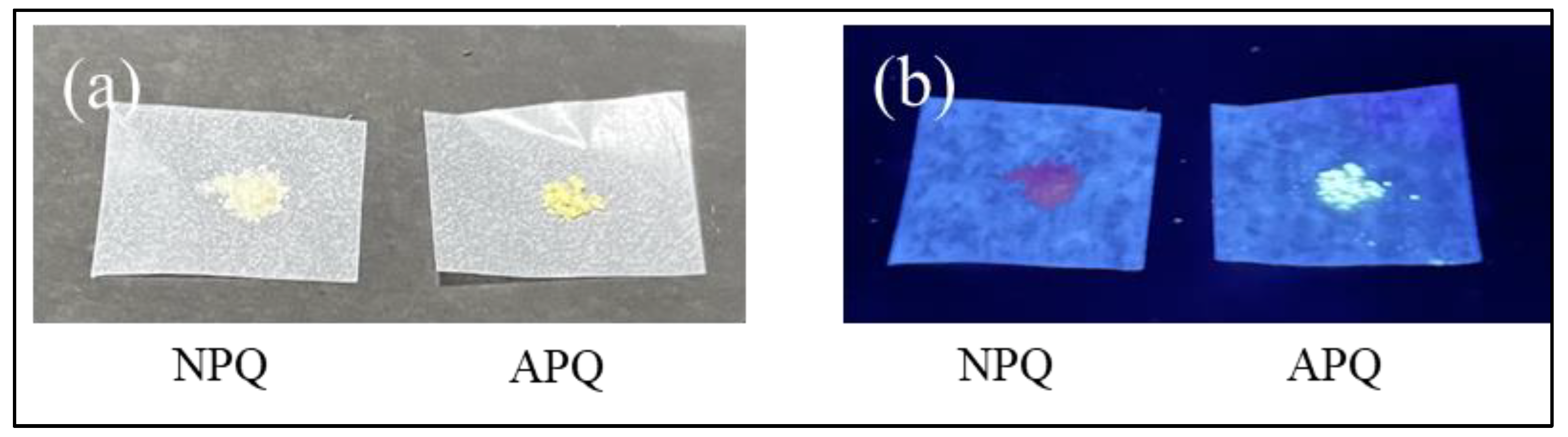
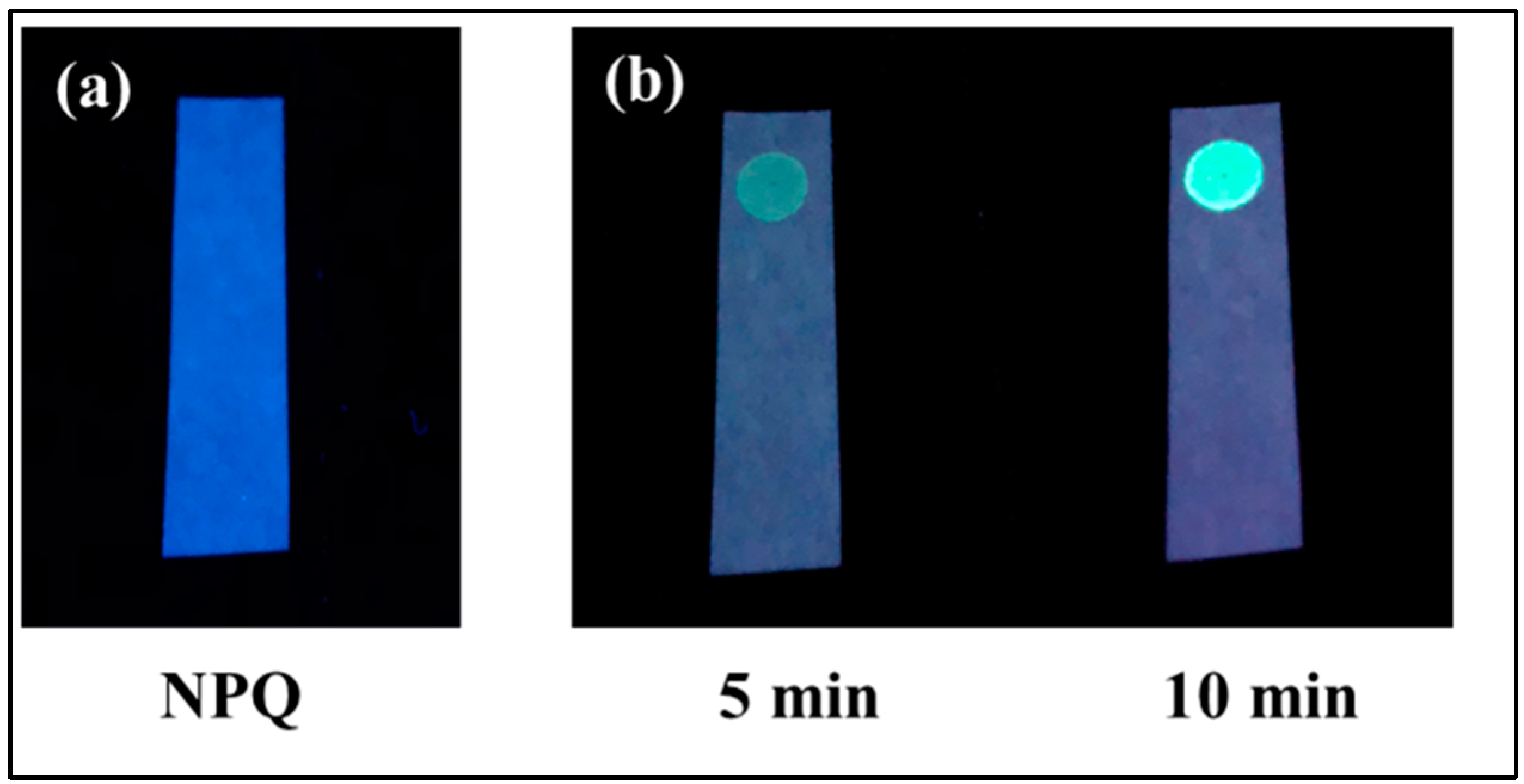
2.4. NPQ Test Paper for Visual Detection of CO
3. Experimental
3.1. Material and Instruments
3.2. Synthesis of NPQ
3.3. Synthesis of APQ
3.4. General Procedure for Fluorescence Measurement of CO Released from CORM-3
3.5. CO Sensing with NPQ Test Paper
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Golob, N.; Grenc, D.; Brvar, M. Carbon Monoxide Poisoning in Wood Pellet Storerooms. Occup. Med. (Chic. Ill) 2018, 68, 143–145. [Google Scholar] [CrossRef]
- Motterlini, R.; Otterbein, L.E. The Therapeutic Potential of Carbon Monoxide. Nat. Rev. Drug Discov. 2010, 9, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Roderique, J.D.; Josef, C.S.; Feldman, M.J.; Spiess, B.D. A Modern Literature Review of Carbon Monoxide Poisoning Theories, Therapies, and Potential Targets for Therapy Advancement. Toxicology 2015, 334, 45–58. [Google Scholar] [CrossRef]
- Raub, J.A.; Mathieu-Nolf, M.; Hampson, N.B.; Thom, S.R. Carbon Monoxide Poisoning — a Public Health Perspective. Toxicology 2000, 145, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Türkan, H.; Vucinic, S.; Naqvi, S.; Bedair, R.; Rezaee, R.; Tsatsakis, A. Carbon Monoxide Poisoning. Toxicol. Rep. 2020, 7, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Prockop, L.D.; Chichkova, R.I. Carbon Monoxide Intoxication: An Updated Review. J. Neurol. Sci. 2007, 262, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Stucki, D.; Stahl, W. Carbon Monoxide – beyond Toxicity? Toxicol. Lett. 2020, 333, 251–260. [Google Scholar] [CrossRef]
- Bagheri, F.; Rashedi, V. Simultaneous Exposure to Noise and Carbon Monoxide Increases the Risk of Alzheimer’s Disease: A Literature Review. Med. Gas Res. 2020, 10, 85. [Google Scholar] [CrossRef]
- Bernardi, C.; Chiesa, L.M.; Soncin, S.; Passero, E.; Biondi, P.A. Determination of Carbon Monoxide in Tuna by Gas Chromatography with Micro-Thermal Conductivity Detector. J. Chromatogr. Sci. 2008, 46, 392–394. [Google Scholar] [CrossRef]
- Oliverio, S.; Varlet, V. Carbon Monoxide Analysis Method in Human Blood by Airtight Gas Syringe – Gas Chromatography – Mass Spectrometry (AGS-GC-MS): Relevance for Postmortem Poisoning Diagnosis. J. Chromatogr. B 2018, 1090, 81–89. [Google Scholar] [CrossRef]
- Esler, M.B.; Griffith, D.W.T.; Wilson, S.R.; Steele, L.P. Precision Trace Gas Analysis by FT-IR Spectroscopy. 1. Simultaneous Analysis of CO2, CH4, N2O, and CO in Air. Anal. Chem. 2000, 72, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Kim, J.; Lee, Y. Improved Electrochemical Microsensor for the Real-Time Simultaneous Analysis of Endogenous Nitric Oxide and Carbon Monoxide Generation. Anal. Chem. 2012, 84, 1792–1796. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sun, L.; Zeng, F.; Wu, S. Activatable Fluorescent Probe Based on Aggregation-Induced Emission for Detecting Hypoxia-Related Pathological Conditions. Anal. Chim. Acta 2020, 1125, 152–161. [Google Scholar] [CrossRef]
- Chan, J.; Dodani, S.C.; Chang, C.J. Reaction-Based Small-Molecule Fluorescent Probes for Chemoselective Bioimaging. Nat. Chem. 2012, 4, 973–984. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent Chemosensors: The Past, Present and Future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef]
- Yang, M.; Fan, J.; Du, J.; Peng, X. Small-Molecule Fluorescent Probes for Imaging Gaseous Signaling Molecules: Current Progress and Future Implications. Chem. Sci. 2020, 11, 5127–5141. [Google Scholar] [CrossRef]
- Amilan Jose, D.; Sharma, N.; Sakla, R.; Kaushik, R.; Gadiyaram, S. Fluorescent Nanoprobes for the Sensing of Gasotransmitters Hydrogen Sulfide (H2S), Nitric Oxide (NO) and Carbon Monoxide (CO). Methods 2019, 168, 62–75. [Google Scholar] [CrossRef]
- Wang, J.; Karpus, J.; Zhao, B.S.; Luo, Z.; Chen, P.R.; He, C. A Selective Fluorescent Probe for Carbon Monoxide Imaging in Living Cells. Angew. Chem. Int. Ed. 2012, 51, 9652–9656. [Google Scholar] [CrossRef]
- Michel, B.W.; Lippert, A.R.; Chang, C.J. A Reaction-Based Fluorescent Probe for Selective Imaging of Carbon Monoxide in Living Cells Using a Palladium-Mediated Carbonylation. J. Am. Chem. Soc. 2012, 134, 15668–15671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kong, X.; Tang, Y.; Li, M.; Yin, Y.; Lin, W. The Development of a Hemicyanine-Based Ratiometric CO Fluorescent Probe with a Long Emission Wavelength and Its Applications for Imaging CO in Vitro and in Vivo. New J. Chem. 2020, 44, 12107–12112. [Google Scholar] [CrossRef]
- Dhara, K.; Lohar, S.; Patra, A.; Roy, P.; Saha, S.K.; Sadhukhan, G.C.; Chattopadhyay, P. A New Lysosome-Targetable Turn-on Fluorogenic Probe for Carbon Monoxide Imaging in Living Cells. Anal. Chem. 2018, 90, 2933–2938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Mu, X.; Zhu, J.; Yan, L. A Metal-Free Coumarin-Based Fluorescent Probe for the Turn-on Monitoring of Carbon Monoxide in an Aqueous Solution and Living Cells. Analyst 2021, 146, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.-Q.; Xia, Y.-S.; Jiang, W.-L.; Wang, W.-X.; Tan, Z.-K.; Guo, K.-Y.; Mao, G.-J.; Li, C.-Y. A Novel Precipitating-Fluorochrome-Based Fluorescent Probe for Monitoring Carbon Monoxide during Drug-Induced Liver Injury. Talanta 2022, 243, 123398. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.-L.; Wang, W.-X.; Mao, G.-J.; Yan, L.; Du, Y.; Li, Y.; Li, C.-Y. Construction of NIR and Ratiometric Fluorescent Probe for Monitoring Carbon Monoxide under Oxidative Stress in Zebrafish. Anal. Chem. 2021, 93, 2510–2518. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W.-X.; Tian, Y.; Tan, M.; Du, Y.; Jie, J.; Li, C.-Y. A Near-Infrared Fluorescence Probe with a Large Stokes Shift for Detecting Carbon Monoxide in Living Cells and Mice. Dye. Pigm. 2020, 180, 108517. [Google Scholar] [CrossRef]
- Shi, G.; Yoon, T.; Cha, S.; Kim, S.; Yousuf, M.; Ahmed, N.; Kim, D.; Kang, H.-W.; Kim, K.S. Turn-on and Turn-off Fluorescent Probes for Carbon Monoxide Detection and Blood Carboxyhemoglobin Determination. ACS Sens. 2018, 3, 1102–1108. [Google Scholar] [CrossRef]
- Gong, S.; Hong, J.; Zhou, E.; Feng, G. A Near-Infrared Fluorescent Probe for Imaging Endogenous Carbon Monoxide in Living Systems with a Large Stokes Shift. Talanta 2019, 201, 40–45. [Google Scholar] [CrossRef] [PubMed]
- El-Maghrabey, M.H.; Watanabe, R.; Kishikawa, N.; Kuroda, N. Detection of Hydrogen Sulfide in Water Samples with 2-(4-Hydroxyphenyl)-4,5-Di(2-Pyridyl)Imidazole-Copper(II) Complex Using Environmentally Green Microplate Fluorescence Assay Method. Anal. Chim. Acta 2019, 1057, 123–131. [Google Scholar] [CrossRef]
- El-Shaheny, R.; Belal, F.; El-Shabrawy, Y.; El-Maghrabey, M. Nanostructures-Based Sensing Strategies for Hydrogen Sulfide. Trends Environ. Anal. Chem. 2021, 31, e00133. [Google Scholar] [CrossRef]
- Tange, A.; Higashi, A.; Kishikawa, N.; Kuroda, N. Simple Fluorescence Assay for Triethylamine Based on the Palladium Catalytic Dimerization of Benzofuran-2-Boronic Acid. Anal. Sci. 2021, 37, 1465–1467. [Google Scholar] [CrossRef]
- El-Maghrabey, M.; Mine, M.; Kishikawa, N.; Ohyama, K.; Kuroda, N. A Novel Dual Labeling Approach Enables Converting Fluorescence Labeling Reagents into Fluorogenic Ones via Introduction of Purification Tags. Application to Determination of Glyoxylic Acid in Serum. Talanta 2018, 180, 323–328. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Zhou, J.; Yang, J.; Zhong, C.; Zhang, Y.; Luo, Y.; Fu, Y.; Huang, J.; Song, Z.; et al. Design of a Quinazolinone-Based Environment-Sensitive Fluorescent Dye: Solvatochromic Fluorescence and Application for One-Photon and Two-Photon Bioimaging. Dye. Pigm. 2019, 165, 58–64. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Peng, Z.; Wang, Z.; Wang, Y.; Lu, P. Preparation and Photophysical Properties of Quinazoline-Based Fluorophores. RSC Adv. 2020, 10, 30297–30303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, Y.; Kong, X.; Lin, W. An Endoplasmic Reticulum Targetable Turn-on Fluorescence Probe for Imaging Application of Carbon Monoxide in Living Cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 247, 119150. [Google Scholar] [CrossRef] [PubMed]
- Al-Sehemi, A.G.; Pannipara, M.; Kalam, A. Quinazolinone Derivative: Model Compound for Determination of Dipole Moment, Solvatochromism and Metal Ion Sensing. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 171, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, L.M.; Solntsev, K.M. Excited-State Proton Transfer: From Constrained Systems to “Super” Photoacids to Superfast Proton Transfer. Acc. Chem. Res. 2002, 35, 19–27. [Google Scholar] [CrossRef]
- Catalan, J.; Fabero, F.; Soledad Guijarro, M.; Claramunt, R.M.; Santa Maria, M.D.; Foces-Foces, M. de la C.; Hernandez Cano, F.; Elguero, J.; Sastre, R. Photoinduced Intramolecular Proton Transfer as the Mechanism of Ultraviolet Stabilizers: A Reappraisal. J. Am. Chem. Soc. 1990, 112, 747–759. [Google Scholar] [CrossRef]
- Zeng, F.; Bao, G.; Zhou, B.; Han, Y. A New Fluorescent Probe for Ultrasensitive Detection of Phosgene in Solution and the Gas Phase. New J. Chem. 2021, 45, 5631–5636. [Google Scholar] [CrossRef]
- Gao, M.; Li, S.; Lin, Y.; Geng, Y.; Ling, X.; Wang, L.; Qin, A.; Tang, B.Z. Fluorescent Light-up Detection of Amine Vapors Based on Aggregation-Induced Emission. ACS Sens. 2016, 1, 179–184. [Google Scholar] [CrossRef]
- Santos-Silva, T.; Mukhopadhyay, A.; Seixas, J.D.; Bernardes, G.J.L.; Romão, C.C.; Romão, M.J. CORM-3 Reactivity toward Proteins: The Crystal Structure of a Ru(II) Dicarbonyl−Lysozyme Complex. J. Am. Chem. Soc. 2011, 133, 1192–1195. [Google Scholar] [CrossRef]
- Pannipara, M.; Asiri, A.M.; Alamry, K.A.; Arshad, M.N.; El-Daly, S.A. Synthesis, Spectral Behaviour and Photophysics of Donor–Acceptor Kind of Chalcones: Excited State Intramolecular Charge Transfer and Fluorescence Quenching Studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 1893–1902. [Google Scholar] [CrossRef]
- Hariharasubramanian, A.; Ravichandran, Y.D. Synthesis and Studies of Electrochemical Properties of Lophine Derivatives. RSC Adv. 2014, 4, 54740–54746. [Google Scholar] [CrossRef]
- Ibrahim, F.; El-Enany, N.; El-Shaheny, R.; Mikhail, I. Study of Micelle and Metal Complexation-Mediated Fluorescence Enhancements of Raloxifene Hydrochloride. Application to Pharmaceutical Analysis. J. Mol. Liq. 2018, 252, 408–415. [Google Scholar] [CrossRef]
- Alty, I.G.; Cheek, D.W.; Chen, T.; Smith, D.B.; Walhout, E.Q.; Abelt, C.J. Intramolecular Hydrogen-Bonding Effects on the Fluorescence of PRODAN Derivatives. J. Phys. Chem. A 2016, 120, 3518–3523. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Lazarus, L.S.; Benninghoff, A.D.; Berreau, L.M. CO Sense and Release Flavonols: Progress toward the Development of an Analyte Replacement PhotoCORM for Use in Living Cells. ACS Omega 2020, 5, 10021–10033. [Google Scholar] [CrossRef]
- Morstein, J.; Höfler, D.; Ueno, K.; Jurss, J.W.; Walvoord, R.R.; Bruemmer, K.J.; Rezgui, S.P.; Brewer, T.F.; Saitoe, M.; Michel, B.W.; et al. Ligand-Directed Approach to Activity-Based Sensing: Developing Palladacycle Fluorescent Probes That Enable Endogenous Carbon Monoxide Detection. J. Am. Chem. Soc. 2020, 142, 15917–15930. [Google Scholar] [CrossRef] [PubMed]
- Zong, P.; Chen, Y.; Liu, K.; Bi, J.; Ren, M.; Wang, S.; Kong, F. Construction of a Unique Two-Photon Fluorescent Probe and the Application for Endogenous CO Detection in Live Organisms. Talanta 2022, 240, 123194. [Google Scholar] [CrossRef]
- Kumar Saha, S.; Chandra Saha, N. A New Naphthalimide-Pd(II) Complex as a Light-up Fluorescent Chemosensor for Selective Detection of Carbon Monoxide in Aqueous Medium. Inorg. Chim. Acta 2021, 517, 120204. [Google Scholar] [CrossRef]
- Reddy, K.R.; Maheswari, C.U.; Venkateshwar, M.; Kantam, M.L. Selective Oxidation of Aromatic Amines to Nitro Derivatives Using Potassium Iodide-Tert-Butyl Hydroperoxide Catalytic System. Adv. Synth. Catal. 2009, 351, 93–96. [Google Scholar] [CrossRef]
- Murray, R.W.; Singh, M.; Rath, N. Stereochemistry in the Oxidation of Primary Amines to Nitro Compounds by Dimethyldioxirane. Tetrahedron Asymmetry 1996, 7, 1611–1619. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.; Wang, X.; Duan, Q.; Jia, P.; Zhu, H.; Li, Z.; Zhang, X.; Ren, X.; Zhu, B.; et al. A Metal-Free near-Infrared Fluorescent Probe for Tracking the Glucose-Induced Fluctuations of Carbon Monoxide in Living Cells and Zebrafish. Sens. Actuators B Chem. 2019, 291, 329–336. [Google Scholar] [CrossRef]
- Das, B.; Lohar, S.; Patra, A.; Ahmmed, E.; Mandal, S.K.; Bhakta, J.N.; Dhara, K.; Chattopadhyay, P. A Naphthalimide-Based Fluorescence ‘“Turn-on”’ Chemosensor for Highly Selective Detection of Carbon Monoxide: Imaging Applications in Living Cells. New J. Chem. 2018, 42, 13497–13502. [Google Scholar] [CrossRef]
- Sayahi, M.H.; Bahadorikhalili, S.; Mahdavi, M.; Baghshirin, L. Synthesis of Quinazolin-4(3H)-Ones via the Reaction of Isatoic Anhydride with Benzyl Azides in the Presence of Potassium Tert-Butoxide in DMSO. Chem. Heterocycl. Compd. (N. Y.) 2019, 55, 964–967. [Google Scholar] [CrossRef]
- Saravanan, G.; Alagarsamy, V.; Prakash, C.R. Synthesis, Analgesic, Anti-Inflammatory, and in Vitro Antimicrobial Activities of Some Novel Quinazolin-4(3H)-One Derivatives. Med. Chem. Res. 2013, 22, 340–350. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Satyanarayana, M.; Murthy, G.N.; Siddaiah, V. Cyclisation of 2-(2-Aminophenyl)Quinazolin-4(3H)-One Reexamined: Formation of Isomeric Angular Fused Quinazolinoquinazolinones and Their Spectroscopic Identification. Tetrahedron Lett. 2012, 53, 2643–2646. [Google Scholar] [CrossRef]

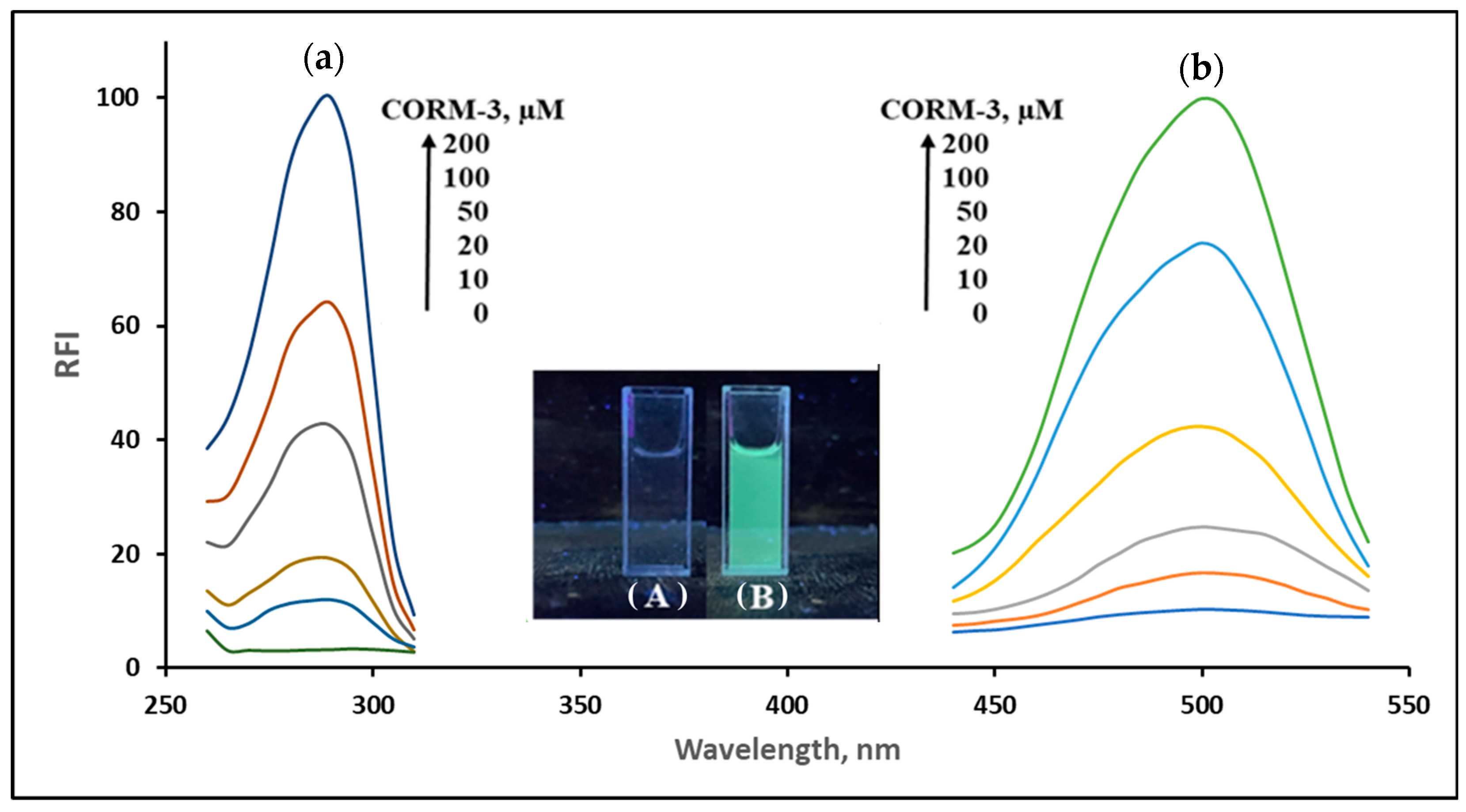
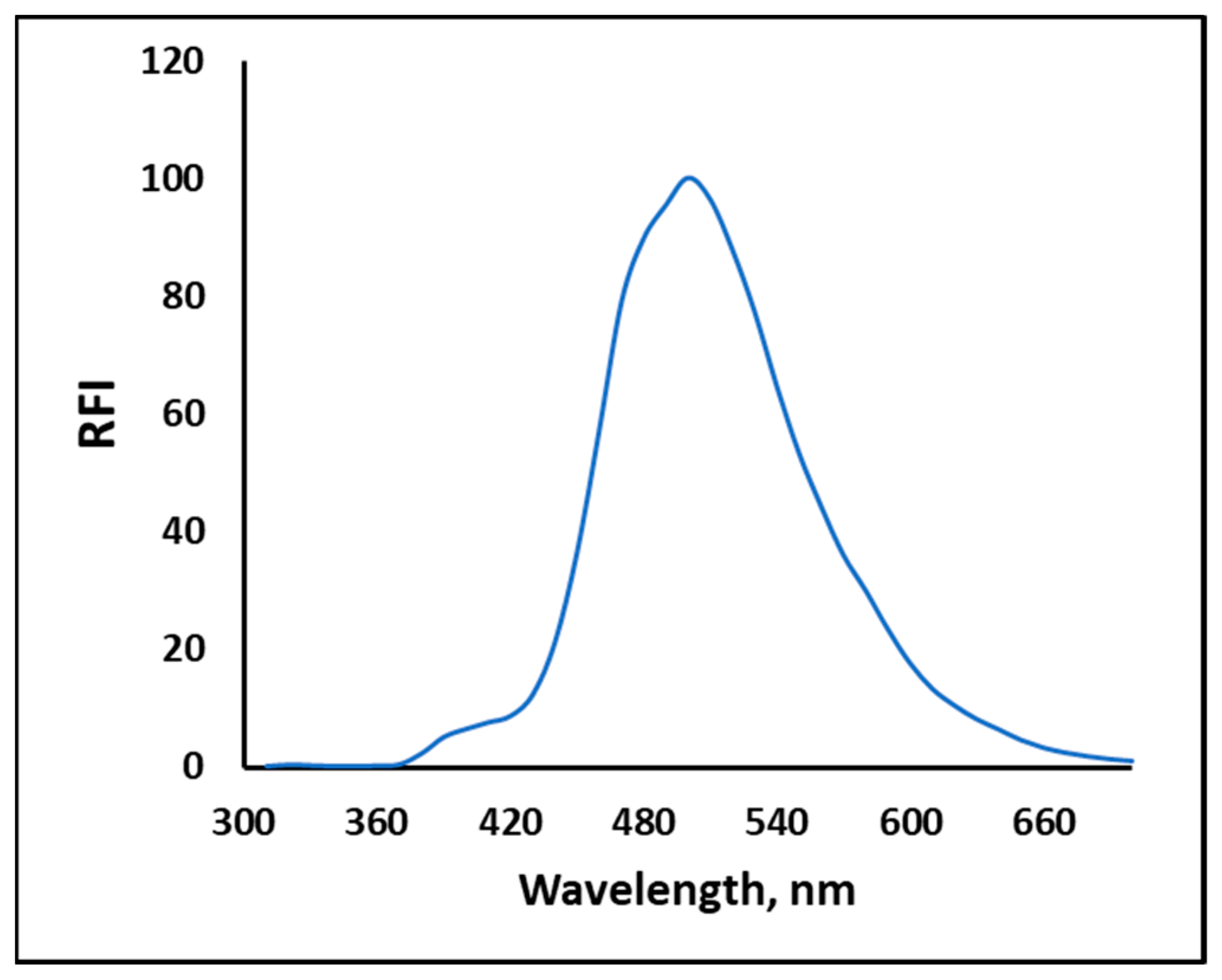
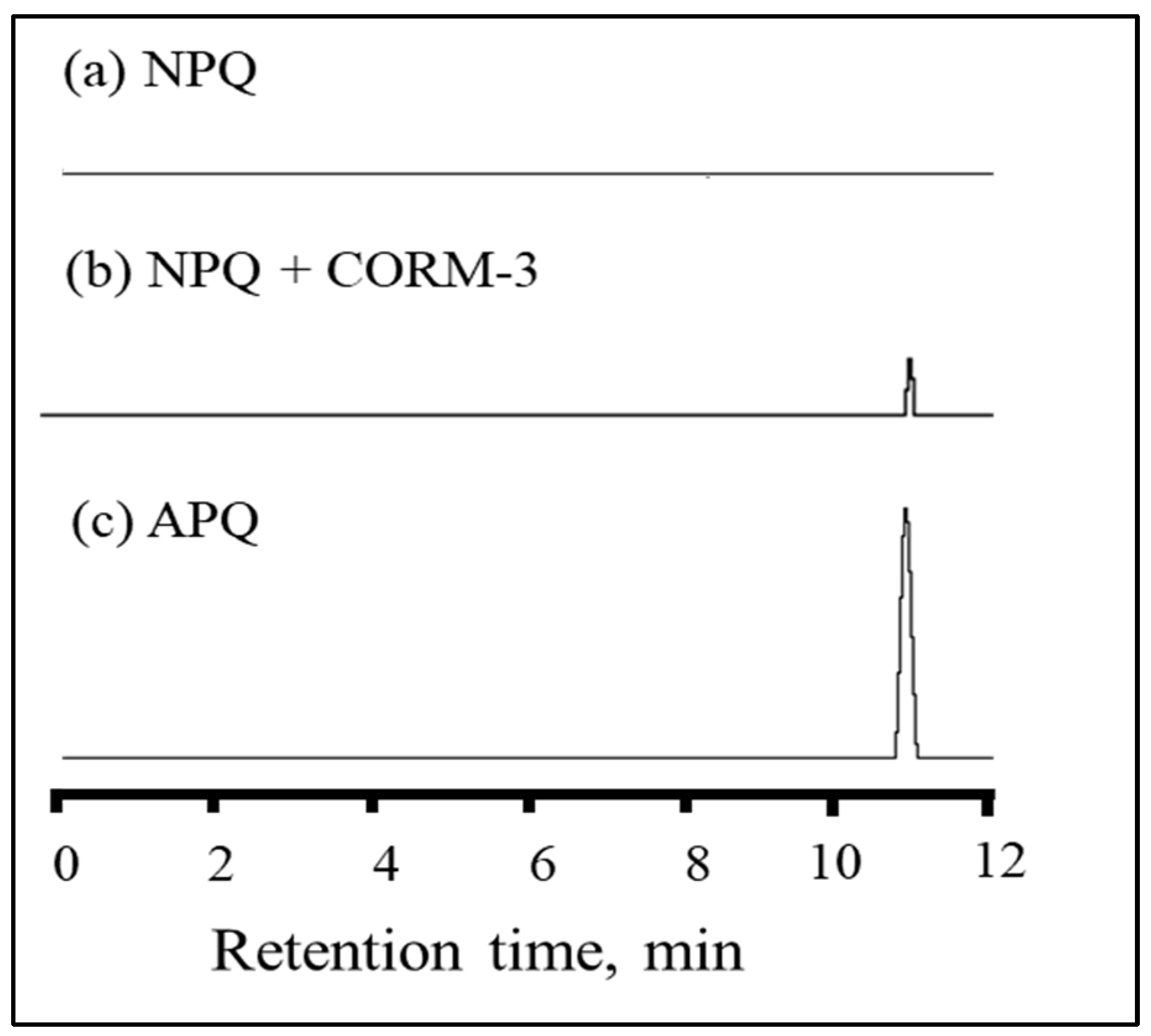

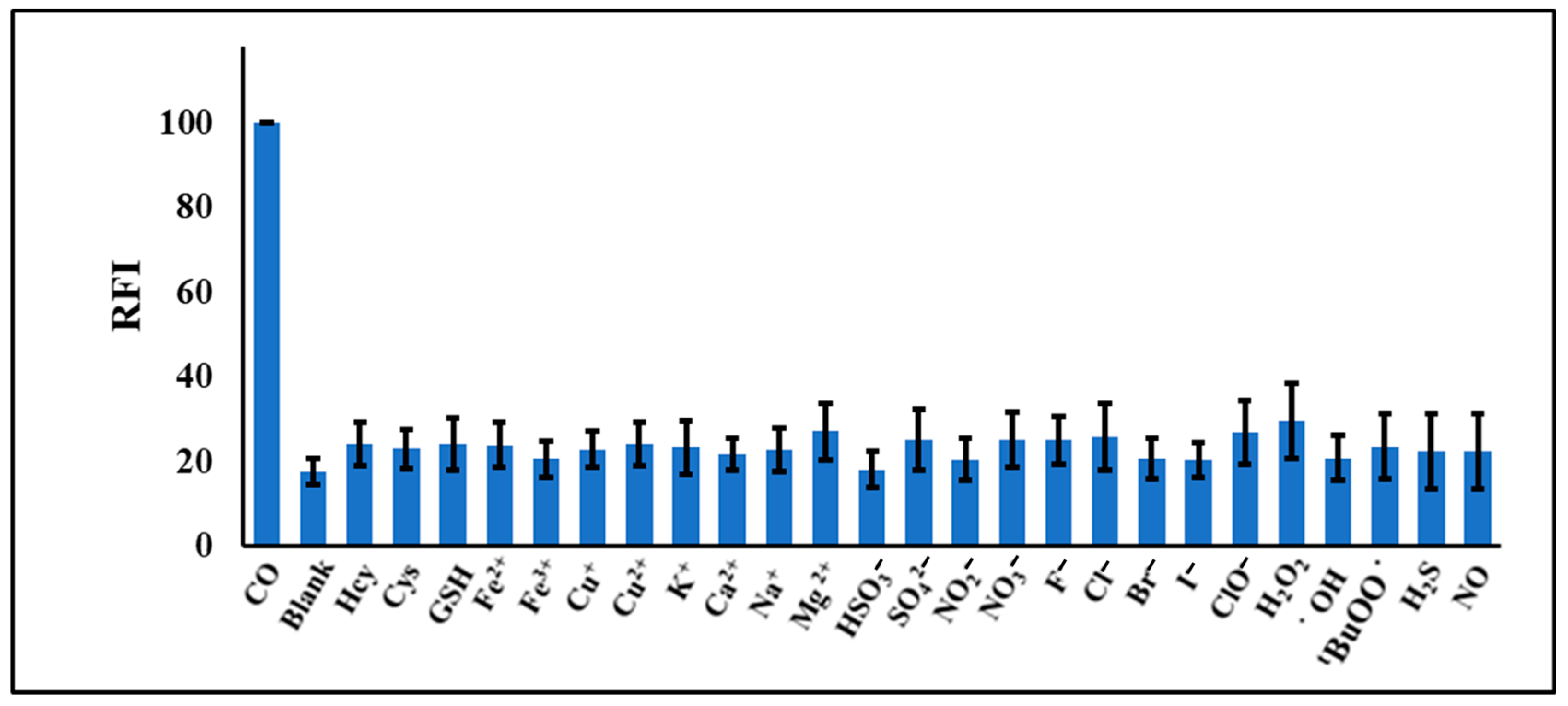


| Solvent | Absorbance λ-max (nm) | Fluorescence λ-max (nm) | Stokes Shift | Molar Absorption Coefficients | PLQY (%) a | Brightness b |
|---|---|---|---|---|---|---|
| Toluene | 296 | 420 | 124 | 22,233 | 0.3 | 6,670 |
| CH2Cl2 | 287 | 430 | 143 | 24,467 | 1.3 | 31,807 |
| CHCl3 | 287 | 440 | 153 | 14,100 | 3.9 | 54,990 |
| Ethyl acetate | 293 | 440 | 147 | 25,667 | 1.8 | 46,201 |
| ACN | 286 | 450 | 164 | 28,467 | 2.2 | 62,627 |
| Ethanol | 286 | 460 | 174 | 26,300 | 2.9 | 76,270 |
| Methanol | 285 | 470 | 185 | 18,833 | 2.6 | 48,966 |
| DMSO | 295 | 480 | 185 | 23,200 | 6 | 139,200 |
| 30% DMSO in PBS buffer | 280 | 500 | 220 | 22,567 | 30 | 667,010 |
| Probe Name | Detection Reaction | Wavelengths(λex/λem, nm) | Reaction Time (min) | Detection Limit (µM) | Ref. |
|---|---|---|---|---|---|
| Hcy-CO | Pd-based, Tsuji–Trost reaction | 410/515 410/600 | 15 | 3.8 | [20] |
| Flav-1 | Pd-based, Tsuji–Trost reaction | 411/603 | 15 | 3.19 | [45] |
| MPVC-1 | Pd-based, azido carbonylation reaction | 424/550 | 10 | 100 ppm (3.57 × 103 µM) | [26] |
| COP-1 | Pd-mediated carbonylation reaction | 475/507 | None | 1 | [19] |
| COP-3E-Py | Pd-based, carbonylation reaction | 521/535 | 60 | None | [46] |
| Pd-BNP-OH | Pd-based, nanostructure probe | 405/510 | 240 | 1.9 | [47] |
| MENap-Pd | Pd-based, demetallation reaction | 435/532 | 30 | 1.4 | [48] |
| Na-CM-ER | Nitro group reduction | 430/520 | 70 | 0.42 | [34] |
| LysoFP-NO2 | Nitro group reduction | 440/530 | 45 | 0.6 | [21] |
| NPQ | Nitro group reduction | 280/500 | 30 | 0.73 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tange, A.; Kishikawa, N.; Sakamoto, Y.; El-Maghrabey, M.; Wada, M.; Kuroda, N. A Turn-On Quinazolinone-Based Fluorescence Probe for Selective Detection of Carbon Monoxide. Molecules 2023, 28, 3654. https://doi.org/10.3390/molecules28093654
Tange A, Kishikawa N, Sakamoto Y, El-Maghrabey M, Wada M, Kuroda N. A Turn-On Quinazolinone-Based Fluorescence Probe for Selective Detection of Carbon Monoxide. Molecules. 2023; 28(9):3654. https://doi.org/10.3390/molecules28093654
Chicago/Turabian StyleTange, Akari, Naoya Kishikawa, Yusuke Sakamoto, Mahmoud El-Maghrabey, Mitsuhiro Wada, and Naotaka Kuroda. 2023. "A Turn-On Quinazolinone-Based Fluorescence Probe for Selective Detection of Carbon Monoxide" Molecules 28, no. 9: 3654. https://doi.org/10.3390/molecules28093654
APA StyleTange, A., Kishikawa, N., Sakamoto, Y., El-Maghrabey, M., Wada, M., & Kuroda, N. (2023). A Turn-On Quinazolinone-Based Fluorescence Probe for Selective Detection of Carbon Monoxide. Molecules, 28(9), 3654. https://doi.org/10.3390/molecules28093654








