Effect of Extraction Methods on the Antioxidant Potential and Cytotoxicity of the Combined Ethanolic Extracts of Daucus carota L., Beta vulgaris L., Phyllanthus emblica L. and Lycopersicon esculentum against Gastric Adenocarcinoma Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Drying Techniques on Crude Fiber Content
2.2. Total Phenolic Content (TPC) and Flavonoid Content
2.3. Ascorbic Acid Content and Carotenoids Content
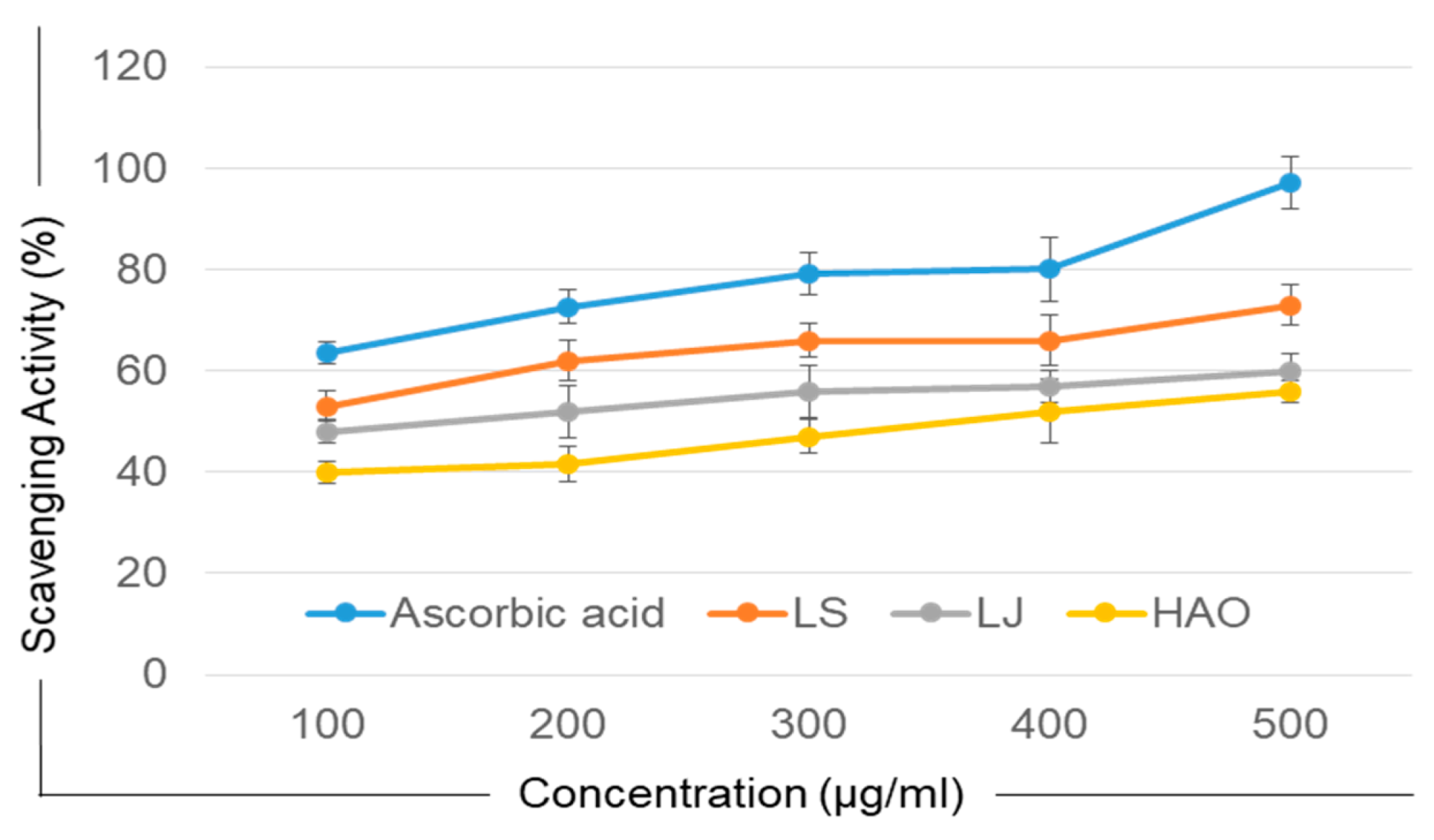
2.4. Antioxidant Activity
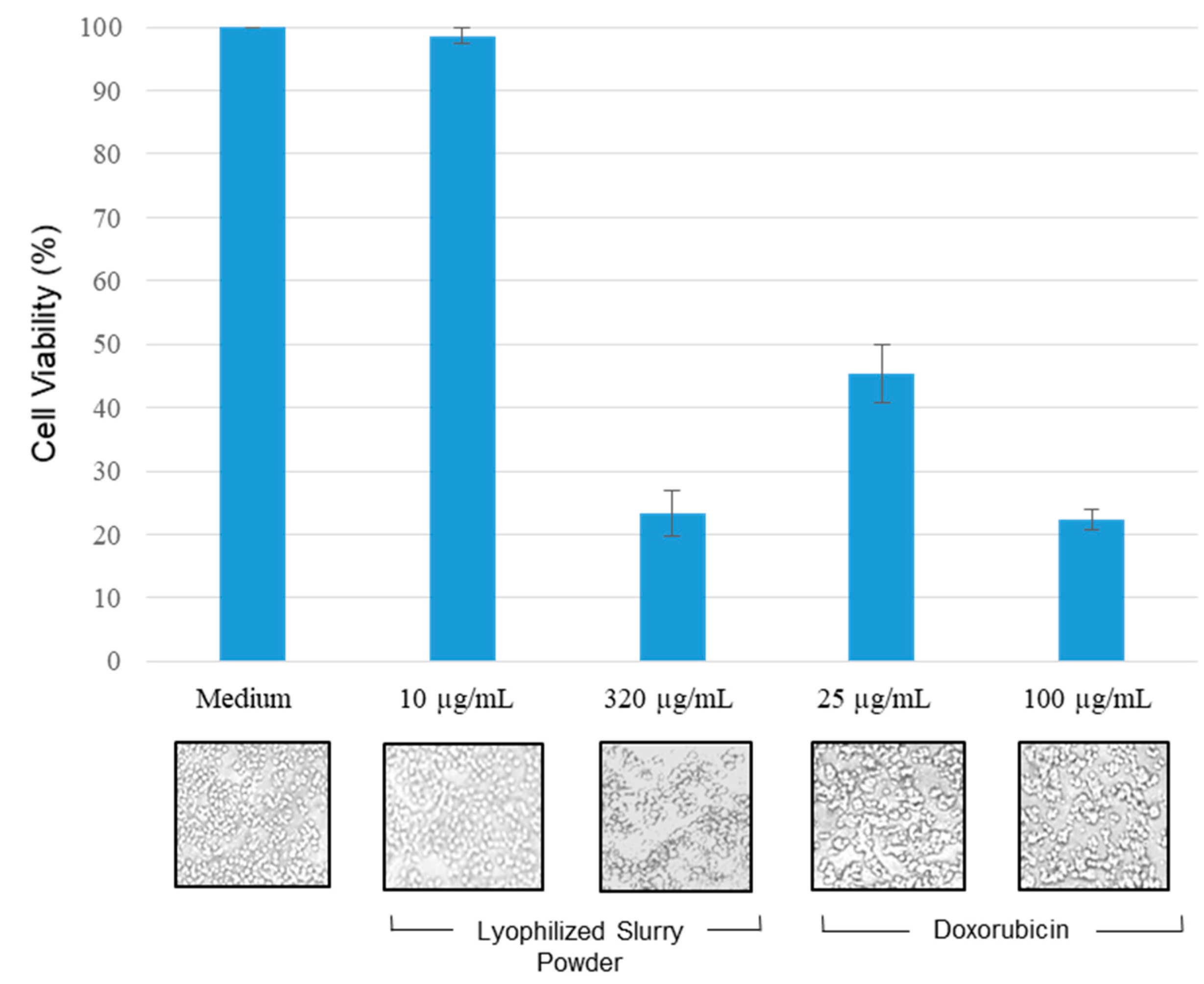
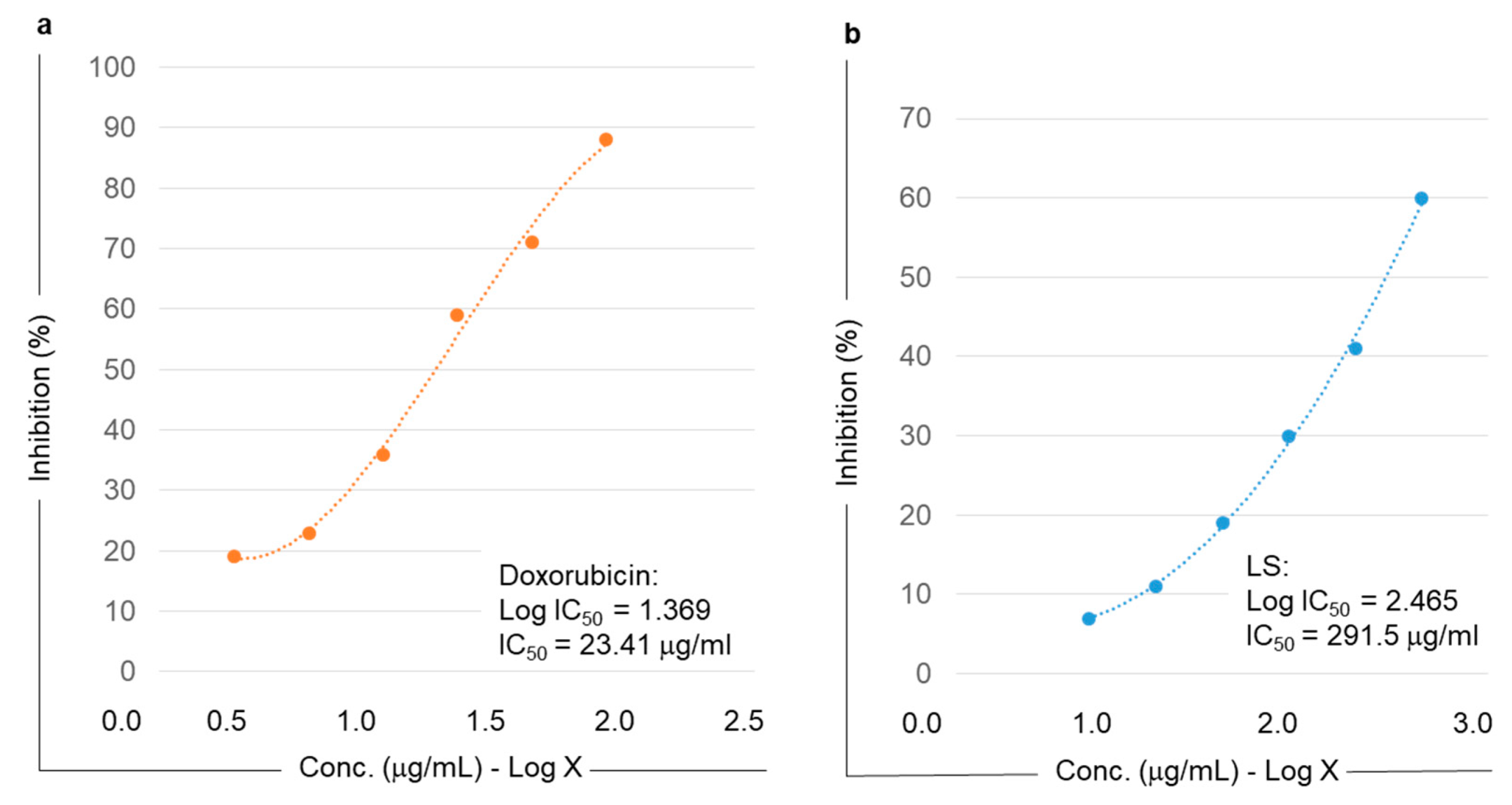
2.5. Anti-Proliferative Activity of Lyophilized Slurry (LS) Extract Assessed by MTT Assay
3. Materials and Methods
3.1. Sample Collection
3.2. Reagents
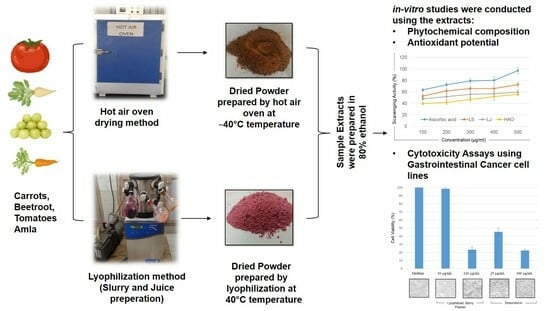
3.3. Drying Processes
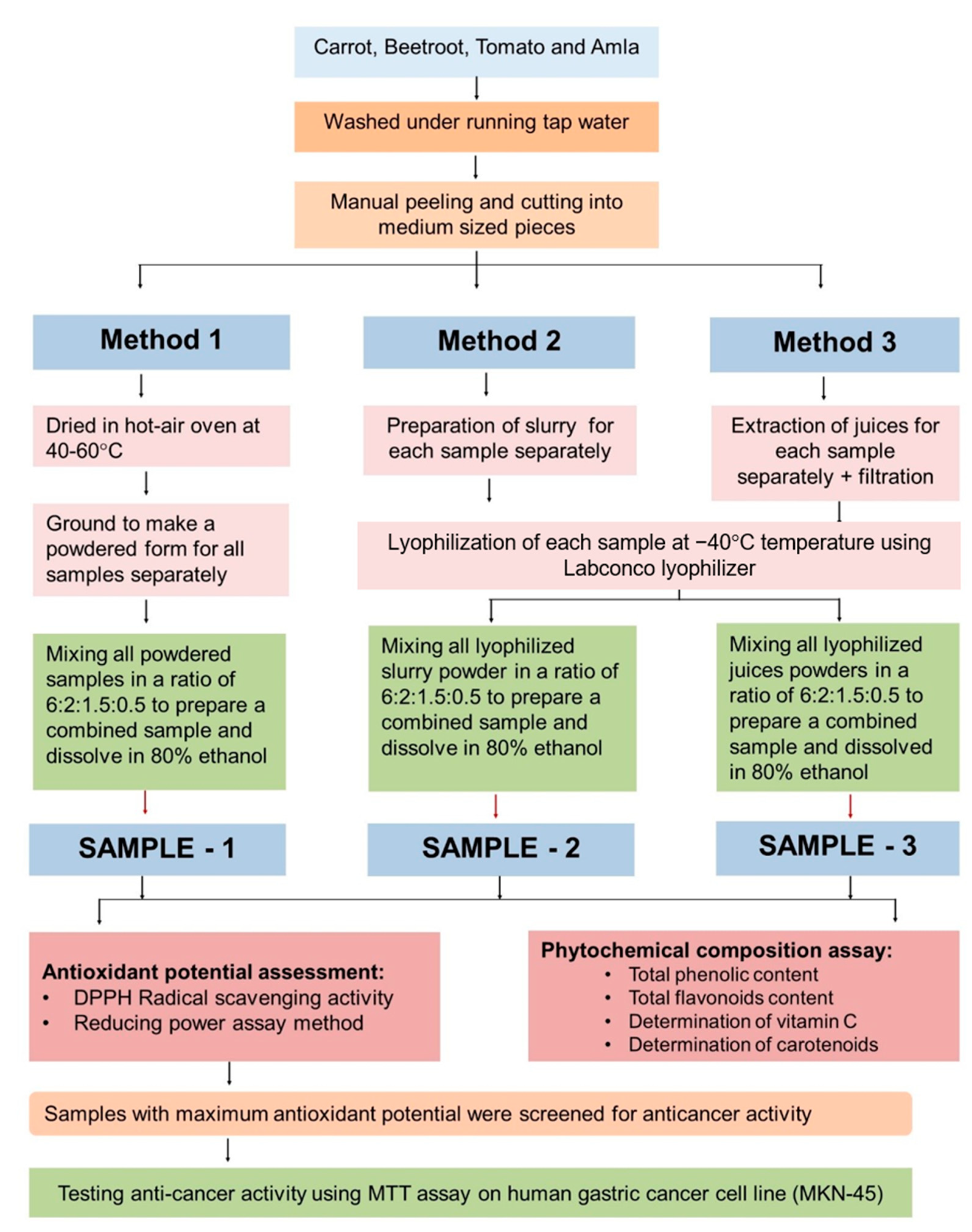
3.4. Preparation of Samples
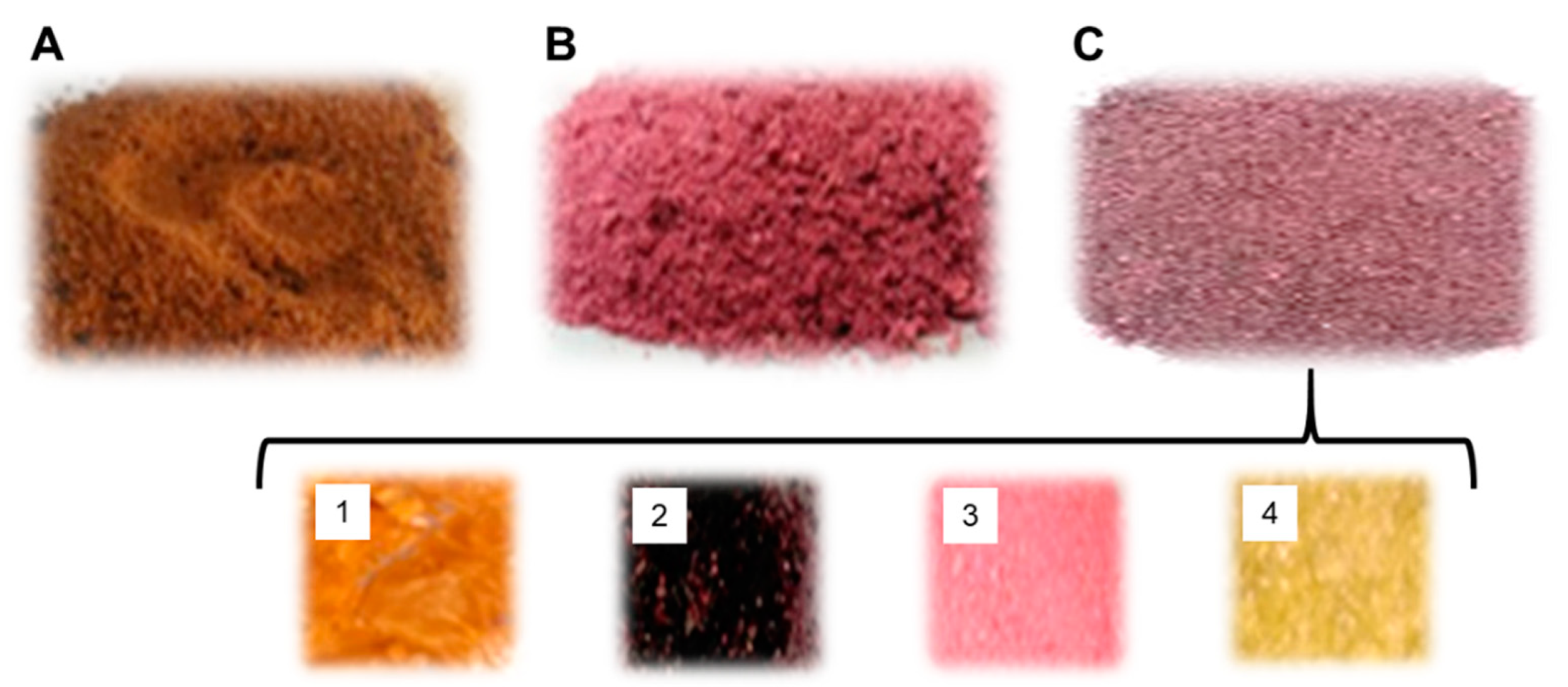
3.4.1. Method 1 (Hot-Air Oven-Dried Powder Sample)
3.4.2. Method 2 (Lyophilized Slurry Powder Sample)
3.4.3. Method 3 (Lyophilized Juice Powder Sample)
3.5. Extraction
3.6. Determination of Crude Fiber Content
3.7. Determination of Total Phenolic Content
3.8. Determination of Total Flavonoid Content
3.9. Determination of Total Ascorbic Acid
3.10. Extraction and Determination of Carotenoids
3.11. 2,2-Diphenyl-1-Picrylhydrazyl Radical Scavenging Activity
3.12. Reducing Power Assay
3.13. Evaluation of the Cytotoxic Activity of the LS Extract in Gastric Adenocarcinoma (MKN-45) Cell Lines by MTT Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Lin, T.M.; Durance, T.D.; Scaman, C.H. Characterization of vacuum microwave, air and freeze dried carrot slices. Int. Food Res. J. 1998, 31, 111–117. [Google Scholar] [CrossRef]
- Maleki, M.; Shahidi, F.; Varidi, M.J.; Azarpazhooh, E. Hot-air drying kinetics of novel functional carrot snack: Impregnated using polyphenolic rich osmotic solution with ultrasound pretreatment. J. Food Process. Eng. 2020, 43, e13331. [Google Scholar] [CrossRef]
- Pandey, P.; Grover, K.; Dhillon, T.S.; Kaur, A.; Javed, M. Evaluation of polyphenols enriched dairy products developed by incorporating black carrot (Daucus carota L.) concentrate. Heliyon 2021, 7, e06880. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhang, L.; Hou, X. Potential roles and molecular mechanisms of phytochemicals against cancer. Food Funct. 2022, 13, 9208–9225. [Google Scholar] [CrossRef]
- Lim, H.M.; Park, S.-H. Regulation of reactive oxygen species by phytochemicals for the management of cancer and diabetes. Crit. Rev. Food Sci. Nutr. 2022, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Manosso, L.M.; Arent, C.O.; Borba, L.A.; Abelaira, H.M.; Réus, G.Z. Natural Phytochemicals for the Treatment of Major Depressive Disorder: A Mini-Review of Pre-and Clinical Studies. CNS Neurol. Disord.-Drug Targets 2023, 22, 237–254. [Google Scholar] [PubMed]
- Das, R.; Lami, M.S.; Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Idroes, R.; Mohamed, A.A.R.; Hossain, M.J.; Dhama, K.; Mostafa-Hedeab, G.; et al. Ginkgo biloba: A treasure of functional phytochemicals with multimedicinal applications. Evid.-Based Complement. Altern. Med. 2022, 2022, 8288818. [Google Scholar]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef]
- Gaikwad, S.B.; Krishna Mohan, G.; Sandhya Rani, M. Phytochemicals for diabetes Management. Pharm. Crops 2014, 5, 11–28. [Google Scholar] [CrossRef]
- Ghildiyal, R.; Prakash, V.; Chaudhary, V.K.; Gupta, V.; Gabrani, R. Phyochemical as antiviral agents: Recent updates. Plant Based Bioact. 2020, 279–295. [Google Scholar] [CrossRef]
- Owen, L.; Laird, K.; Shivkumar, M. Antiviral plant-derived natural products to combat RNA viruses: Targets throughout the viral life cycle. Lett. Appl. Microbiol. 2022, 75, 476–499. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.M.; Wilcox, S.; Frongillo, E.A.; Murphy, E.A.; Hutto, B.; Wilson, M.; Davey, M.; Bernhart, J.A.; Okpara, N.; Bailey, S.; et al. Effect of a Plant-Based vs Omnivorous Soul Food Diet on Weight and Lipid Levels Among African American Adults: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2250626. [Google Scholar] [CrossRef]
- Tomar, B.S.; Jat, G.S.; Singh, J. Organic Vegetable Production: Needs, Challenges, and Strategies. In Organic Crop Production Management: Focus on India, with Global Implications; Apple Academic Press: Waretown, NJ, USA, 2023. [Google Scholar]
- Menon, A.; Stojceska, V.; Tassou, S.A. A systematic review on the recent advances of the energy efficiency improvements in non-conventional food drying technologies. Trends Food Sci. Technol. 2020, 100, 67–76. [Google Scholar] [CrossRef]
- Vidinamo, F.; Fawzia, S.; Karim, M.A. Effect of drying methods and storage with agro-ecological conditions on phytochemicals and antioxidant activity of fruits: A review. Crit. Rev. Food Sci. Nutr. 2020, 62, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Figiel, A.; Legua, P.; Lech, K.; Carbonell-Barrachina, Á.A.; Hernández, F. Chemical composition, antioxidant capacity, and sensory quality of dried jujube fruits as affected by cultivar and drying method. Food Chem. 2016, 207, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Hall, R.D.; Capanoglu, E. A review on the effect of drying on antioxidant potential of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. S1), S110–S129. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.R.; Marques, C.; de Moraes, A.C.S.; Masson, M.L. Trends in quality assessment and drying methods used for fruits and vegetables. Food Control 2022, 142, 109254. [Google Scholar] [CrossRef]
- Ma, Y.; Yi, J.; Jin, X.; Li, X.; Feng, S.; Bi, J. Freeze-Drying of Fruits and Vegetables in Food Industry: Effects on Phytochemicals and Bioactive Properties Attributes—A Comprehensive Review. Food Rev. Int. 2022, 1–19. [Google Scholar] [CrossRef]
- Bhatta, S.; Janezic, T.S.; Ratti, C. Freeze-drying of plant-based foods. Foods 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Nagy, S.A.; Karim, M.A. Transport of cellular water during drying: An understanding of cell rupturing mechanism in apple tissue. Int. Food Res. J. 2018, 105, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.; Karim, A.; Joardder, M.U.; Miller, G. Modeling heat and mass transfer process during convection drying of fruit. In Proceedings of the 4th International Conference on Computational Methods, Gold Coast, Australia, 25–27 November 2012; pp. 1–8. [Google Scholar]
- Singh, M.N.; Srivastava, R.; Yadav, I. Study of different varietis of carrot and its benefits for human health: A review. J. Pharmacogn. Phytochem. 2021, 10, 1293–1299. [Google Scholar] [CrossRef]
- Kar, S.; Kundu, S.; Mal, D. Nutritional Quality of Colored Vegetables: A. Nutraceuticals Food 2021, 6, 37. [Google Scholar]
- Pandey, P.; Grover, K. Characterization of black carrot (Daucus carota L.) polyphenols; role in health promotion and disease prevention: An overview. J. Pharmacogn. Phytochem. 2020, 9, 2784–2792. [Google Scholar] [CrossRef]
- Pandey, G.; Pandey, V.; Pandey, P.R.; Thomas, G. Effect of extraction solvent temperature on betalain content, phenolic content, antioxidant activity and stability of beetroot (Beta vulgaris L.) powder under different storage conditions. Plant Arch. 2018, 18, 1623–1627. [Google Scholar]
- Hadipour, E.; Taleghani, A.; Tayarani-Najaran, N.; Tayarani-Najaran, Z. Biological effects of red beetroot and betalains: A review. Phytother. Res. 2020, 34, 1847–1867. [Google Scholar] [CrossRef] [PubMed]
- Shejawal, K.P.; Randive, D.S.; Bhinge, S.D.; Bhutkar, M.A.; Todkar, S.S.; Mulla, A.S.; Jadhav, N.R. Green synthesis of silver, iron and gold nanoparticles of lycopene extracted from tomato: Their characterization and cytotoxicity against COLO320DM, HT29 and Hella cell. J. Mater. Sci. Mater. Med. 2021, 32, 19. [Google Scholar] [CrossRef]
- Chauhan, M.; Garg, V.; Zia, G.; Dutt, R. Potential Role of Phytochemicals of Fruits and Vegetables in Human Diet. Res. J. Pharm. Technol. 2020, 13, 1587–1591. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New insights of the application of water or ethanol-water plant extract rich in active compounds in food. Front. Nutr. 2023, 10, 431. [Google Scholar] [CrossRef]
- Dar, A.H.; Kumar, N.; Shah, S.; Shams, R. Processing of fruits and vegetables. In Agro-Processing and Food Engineering: Operational and Application Aspects; Springer: Berlin/Heidelberg, Germany, 2022; pp. 535–579. [Google Scholar]
- Kayacan, S.; Karasu, S.; Akman, P.K.; Goktas, H.; Doymaz, I.; Sagdic, O. Effect of different drying methods on total bioactive compounds, phenolic profile, in vitro bioaccessibility of phenolic and HMF formation of persimmon. LWT 2020, 118, 108830. [Google Scholar] [CrossRef]
- Figiel, A.; Michalska, A. Overall quality of fruits and vegetables products affected by the drying processes with the assistance of vacuum-microwaves. Int. J. Mol. Sci. 2017, 18, 71. [Google Scholar] [CrossRef] [PubMed]
- Gabr, S.A.; Alghadir, A.H. Evaluation of the biological effects of lyophilized hydrophilic extract of Rhus coriaria on myeloperoxidase (MPO) activity, wound healing, and microbial infections of skin wound tissues. Evid.-Based Complement. Altern. Med. 2019, 2019, 5861537. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, J.; Lu, S.; Tang, W.; Zhou, Y.; Quek, S.Y. Effects of different drying methods on phenolic components and in vitro hypoglycemic activities of pulp extracts from two Chinese bayberry (Myrica rubra Sieb. et Zucc.) cultivars. Food Sci. Hum. Wellness 2022, 11, 366–373. [Google Scholar] [CrossRef]
- Pathania, S.; Kaur, N. Utilization of fruits and vegetable by-products for isolation of dietary fibres and its potential application as functional ingredients. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100295. [Google Scholar] [CrossRef]
- Lai, C.; Liang, Y.; Zhang, L.; Huang, J.; Kaliaperumal, K.; Jiang, Y.; Zhang, J. Variations of Bioactive Phytochemicals and Antioxidant Capacity of Navel Orange Peel in Response to Different Drying Methods. Antioxidants 2022, 11, 1543. [Google Scholar] [CrossRef]
- Fathi, F.; Ebrahimi, S.N.; Matos, L.C.; Oliveira, M.B.P.P.; Alves, R.C. Emerging drying techniques for food safety and quality: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1125–1160. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.; Aydın, O.; Kolaylı, S. Effect of different drying conditions on the vitamin C (ascorbic acid) content of Hayward kiwifruits (Actinidia deliciosa Planch). Food Bioprod. Process. 2010, 88, 165–173. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimization of the Extraction Parameters for the Isolation of Bioactive Compounds from Orange Peel Waste. Sustainability 2022, 14, 13926. [Google Scholar] [CrossRef]
- Ray, A.; Dubey, K.K.; Marathe, S.J.; Singhal, R. Supercritical fluid extraction of bioactives from fruit waste and its therapeutic potential. Food Biosci. 2023, 52, 102418. [Google Scholar] [CrossRef]
- Vidya, R.; Kalaivani, K.; Amudha, P. Therapeutic Potential of Cucumis melo (L.) Fruit Extract and Its Silver Nanopartciles Against DEN-Induced Hepatocellular Cancer in Rats. Appl. Biochem. Biotechnol. 2022, 194, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Tran, T.G.; Tran, N.L. Phytochemical compound yield and antioxidant activity of cocoa pod husk (Theobroma cacao L.) as influenced by different dehydration conditions. Dry. Technol. 2022, 40, 2021–2033. [Google Scholar] [CrossRef]
- Pasechny, D.S.; Smotraeva, I.V.; Balanov, P.E. Lyophilization effect on rosehip extract physico-chemical properties. AIP Conf. Proc. 2022, 2767, 020012. [Google Scholar]
- Kumnerdkhonkaen, P.; Saenglee, S.; Asgar, M.A.; Senawong, G.; Khongsukwiwat, K.; Senawong, T. Antiproliferative activities and phenolic acid content of water and ethanolic extracts of the powdered formula of Houttuynia cordata Thunb. fermented broth and Phyllanthus emblica Linn. fruit. BMC Complement. Altern. Med. 2018, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Tamboli, A.M.; Wadkar, K.A. Comparative cytotoxic activity of Convolvulus pluricaulis against human hepatoma cell line (HepG2) and normal cell line (L929) via apoptosis pathways by flow cytometry analysis. Bull. Natl. Res. Cent. 2022, 46, 145. [Google Scholar] [CrossRef]
- Sophia, A.; Faiyazuddin, M.; Alam, P.; Hussain, M.T.; Shakeel, F. GC–MS characterization and evaluation of antimicrobial, anticancer and wound healing efficiency of combined ethanolic extract of Tridax procumbens and Acalypha indica. J. Mol. Struct. 2022, 1250, 131678. [Google Scholar] [CrossRef]
- Purnamasari, R.; Winarni, D.; Permanasari, A.A.; Agustina, E.; Hayaza, S.; Darmanto, W. Anticancer activity of methanol extract of Ficus carica leaves and fruits against proliferation, apoptosis, and necrosis in Huh7it cells. Cancer Inform. 2019, 18, 1176935119842576. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Zafar, T.A. Indian herbal medicine and their functional components in cancer therapy and prevention. In Functional Foods in Cancer Prevention and Therapy; Academic Press: Cambridge, MA, USA, 2020; pp. 169–194. [Google Scholar]
- Jongrungraungchok, S.; Madaka, F.; Wunnakup, T.; Sudsai, T.; Pongphaew, C.; Songsak, T.; Pradubyat, N. In vitro antioxidant, anti-inflammatory, and anticancer activities of mixture Thai medicinal plants. BMC Complement. Med. Ther. 2023, 23, 43. [Google Scholar] [CrossRef]
- Moldovan, C.; Frumuzachi, O.; Babotă, M.; Menghini, L.; Cesa, S.; Gavan, A.; Sisea, C.R.; Tanase, C.; Dias, M.I.; Pereira, C.; et al. Development of an Optimized Drying Process for the Recovery of Bioactive Compounds from the Autumn Fruits of Berberis vulgaris L. and Crataegus monogyna Jacq. Antioxidants 2021, 10, 1579. [Google Scholar] [CrossRef]
- Indian Pharmacopoeia Commision. Herbs and Herbal Products. In Indian Pharmacopoeia; Indian Pharmacopoeia Commision: Ghaziabad, India, 2018; Volume 3, pp. 2491–2497. [Google Scholar]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Hung, P.V.; Duy, T.L. Effects of drying methods on bioactive compounds of vegetables and correlation between bioactive compounds and their antioxidants. Int. Food Res. J. 2012, 19, 327. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total Phenolics and Total Flavonoids in Bulgarian Fruits and Vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Pathy, K. Process for preparation of vitamin C and method for determination of vitamin C in tablets. SF J. Chem. Res. 2018, 2, 2. [Google Scholar] [CrossRef]
- Ali, M.A.; Yusof, Y.A.; Chin, N.L.; Ibrahim, M.N. Effect of different drying treatments on colour quality and ascorbic acid concentration of guava fruit. Int. Food Res. J. 2016, 23, S155–S161. [Google Scholar]
- Rebecca, L.J.; Sharmila, S.; Das, M.P.; Seshiah, C. Extraction and purification of carotenoids from vegetables. J. Chem. Pharm. Res. 2014, 6, 594–598. [Google Scholar]
- Oyaizu, M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. J. Acad. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Verma, A.R.; Vijayakumar, M.; Mathela, C.S.; Rao, C.V. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem. Toxicol. 2009, 47, 2196–2201. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.J.; Tarloff, J.B. Evaluation of hepatic subcellular fractions for Alamar blue and MTT reductase activity. Toxicol. Vitr. 2001, 15, 257–259. [Google Scholar] [CrossRef]
| Dried Samples | Weight (g) | Crude Fiber (%) |
|---|---|---|
| Hot-air oven-dried sample (HAO) | 2.0 | 2.0 |
| Lyophilized slurry (LS) | 2.0 | 4.2 |
| Lyophilized juices (LJ) | 2.0 | 1.4 |
| Dried Samples | Total Phenolic Content (mg Gallic Acid/100 g) | Total Flavonoid Content (mg Quercetin/100 g) |
|---|---|---|
| Hot-air oven-dried sample (HAO) | 72.05 ± 0.01 | 18.635 ± 0.005 |
| Lyophilized slurry (LS) | 171.20 ± 0.02 | 23.635 ± 0.003 |
| Lyophilized juices (LJ) | 120.73 ± 0.02 | 20.754 ± 0.005 |
| Different Dried Samples | Ascorbic Acid Content (mg) | Carotenoids Content (mg/100 g Sample) |
|---|---|---|
| Hot-air oven dried sample (HAO) | 2.68 | 14.00 |
| Lyophilized slurry (LS) | 6.51 | 30.25 |
| Lyophilized juices (LJ) | 2.99 | 23.25 |
| Drying Techniques | Reducing Power (Total Antioxidant Capacity) (Absorbance) |
|---|---|
| Hot-air oven-dried sample (HAO) | 0.235 |
| Lyophilized slurry (LS) | 1.827 |
| Lyophilized juices (LJ) | 1.521 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, M.; Garg, V.; Zia, G.; Dutt, R.; Alghamdi, B.S.; Zawawi, A.; Ashraf, G.M.; Farhana, A. Effect of Extraction Methods on the Antioxidant Potential and Cytotoxicity of the Combined Ethanolic Extracts of Daucus carota L., Beta vulgaris L., Phyllanthus emblica L. and Lycopersicon esculentum against Gastric Adenocarcinoma Cells. Molecules 2023, 28, 6589. https://doi.org/10.3390/molecules28186589
Chauhan M, Garg V, Zia G, Dutt R, Alghamdi BS, Zawawi A, Ashraf GM, Farhana A. Effect of Extraction Methods on the Antioxidant Potential and Cytotoxicity of the Combined Ethanolic Extracts of Daucus carota L., Beta vulgaris L., Phyllanthus emblica L. and Lycopersicon esculentum against Gastric Adenocarcinoma Cells. Molecules. 2023; 28(18):6589. https://doi.org/10.3390/molecules28186589
Chicago/Turabian StyleChauhan, Mahima, Vandana Garg, Ghazala Zia, Rohit Dutt, Badrah S. Alghamdi, Ayat Zawawi, Ghulam Md. Ashraf, and Aisha Farhana. 2023. "Effect of Extraction Methods on the Antioxidant Potential and Cytotoxicity of the Combined Ethanolic Extracts of Daucus carota L., Beta vulgaris L., Phyllanthus emblica L. and Lycopersicon esculentum against Gastric Adenocarcinoma Cells" Molecules 28, no. 18: 6589. https://doi.org/10.3390/molecules28186589
APA StyleChauhan, M., Garg, V., Zia, G., Dutt, R., Alghamdi, B. S., Zawawi, A., Ashraf, G. M., & Farhana, A. (2023). Effect of Extraction Methods on the Antioxidant Potential and Cytotoxicity of the Combined Ethanolic Extracts of Daucus carota L., Beta vulgaris L., Phyllanthus emblica L. and Lycopersicon esculentum against Gastric Adenocarcinoma Cells. Molecules, 28(18), 6589. https://doi.org/10.3390/molecules28186589