Abstract
Benzophenanthridine alkaloids are a class of isoquinoline compounds, which are widely found in the plants of papaveraceae, corydalis, and rutaceae. Biological activities and clinical studies have shown that benzophenanthridine alkaloids have inhibitory effects on many cancers. Considering that the anticancer activities and mechanisms of many natural benzophenanthridine alkaloids have been discovered in succession, the purpose of this paper is to review the anticancer effects of benzophenanthridine alkaloids and explore the application potential of these natural products in the development of antitumor drugs. A literature survey was carried out using Scopus, Pubmed, Reaxys, and Google Scholar databases. This review summarizes and analyzes the current status of research on the antitumor activity and antitumor mechanism of natural products of benzophenanthridine from different sources. The research progress of the antitumor activity of natural products of benzophenanthridine from 1983 to 2023 was reviewed. The antitumor activities of 90 natural products of benzophenanthridine and their related analogues were summarized, and the results directly or indirectly showed that natural products of benzophenanthridine had the effects of antidrug-resistant tumor cell lines, antitumor stem cells, and inducing ferroptosis. In conclusion, benzophenanthridine alkaloids have inhibitory effects on a variety of cancers and have the potential to counteract tumor resistance, and they have great application potential in the development of antitumor drugs.
1. Introduction
Tumor refers to a disease with the highest mortality rate. Under the action of a wide variety of factors, cells in local tissues lose their normal regulation of growth at the gene level, thus causing abnormal cell proliferation [1]. Over 60% of anticancer drugs have been derived from natural products and natural product derivatives [2].
Figure 1 presents the structure of the benzophenanthridine compound, containing a non-aromatic heterocycle (B ring). It is primarily distributed in papaveraceae and rutaceae [3]; pertains to isoquinoline alkaloids; and exhibits antitumor, antifungal, antiviral, anti-inflammatory, immune regulation, and other pharmacological activities [4].
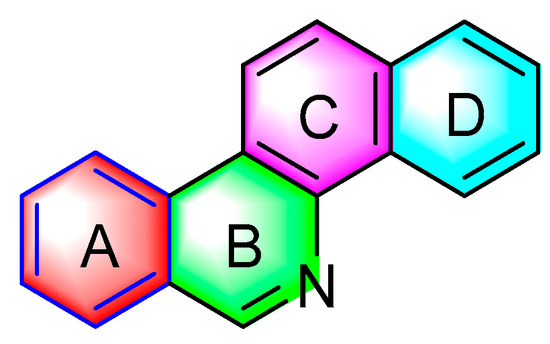
Figure 1.
Chemical structure of benzophenanthridine alkaloids.
Indeed, benzophenanthridine alkaloids play an anticancer role via different mechanisms. Benzophenanthridine alkaloids are capable of affecting the activity of DNA topoisomerase I and topoisomerase II, suppressing the rapid proliferation of tumor cells [5], inducing cancer cells ferroptosis [6], inhibiting the growth of tumor stem cells [7], and so forth. Benzophenanthridine natural products combine with negatively charged membrane surfaces and proteins, and react with sulfhydryl residues of amino acids, thus interfering with collagenase, tubulin assembly, Na+/K+ATP ase, as well as other functions [8]. The antitumor activities of benzophenanthridine alkaloids are reviewed to lay a theoretical basis for the development of novel antitumor drugs with natural benzophenanthridine alkaloids as the lead compounds.
2. Antitumor Activities of Benzophenanthridine Alkaloids from Natural Products
2.1. Antitumor Activities of Benzophenanthridine Alkaloids from Papaveraceae Plants
2.1.1. Antitumor Activities of Benzophenanthridine Alkaloids from Papaver SPP
Quaternary benzophenanthridine alkaloids (QBAs) primarily originate from plants of the genus Papaver. Representative compounds comprise sanguinarine (1), chelerythrine (2), sanguilutine (3), sanguirubine (4), chelirubine (5), chelilutine (6), and macarpine (7) (Figure 2).
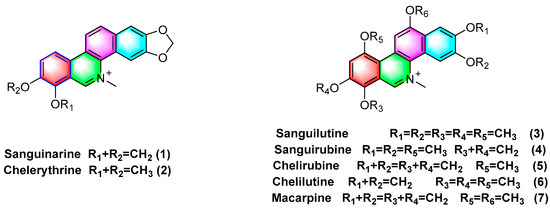
Figure 2.
Benzophenanthridine alkaloids from Papaver spp.
The presence of moderate or high levels of ROS in tumor cells affects the initiation and proliferation of cancer to a certain extent. Benzophenanthridine natural products are capable of inducing, or activating different molecular signal transduction, activating proteins of related pathways, or making them apoptosis by interfering with the signal pathway of ROS, and treating and eliminating tumor cells by regulating dysfunctional proteins [9]. Sanguinarine (1), one of the most famous benzophenanthridine alkaloids, can act on ROS-dependent mitochondria to induce autophagy and apoptosis, or inhibit the mitosis of cancer cells by changing the acidic conditions of lysosomes and interfering with the formation of autophagosomes lysosomes, such that liver cancer [10] and MDA-MB-231 human breast cancer [11] can be inhibited. Sanguinarine (1) is capable of significantly targeting ephrin type-B receptor 4 (EphB4) and hypoxia inducible factor-1α (HIF-1α) in breast cancer, inhibiting the activation of the downstream protein signal transducer and activator of transcription 3 (STAT3) in cells, blocking hypoxia-induced HIF-1α or STAT3 interacts, and downregulating the mRNA levels of its target genes, thus inhibiting breast cancer cell hyperplasia [12]. In addition, in the breast cancer model, sanguinarine (1) has been proven to have the effects of inhibiting the metastasis of breast cancer and anti epithelial mesenchymal transformation (EMT) [13]. A recent review article also showed that sanguinarine (1) is a very promising therapeutic option for breast cancer [14]. Sanguinarine (1) facilitates apoptosis in HeLa cells as a treatment for cervical cancer with an IC50 value of 3.5 μM [15]. Sanguinarine (1) exhibits anti-microtubule activity while inhibiting the binding of colchicine and podophyllotoxin to tubulin with IC50 values of 32 μM and 46 μM. The IC50 values for chelerythrine (2) have been obtained as 55 μM and 60 μM [16]. Sanguinarine (1) induces apoptosis in HeLa cells by upregulating the expression of the proapoptotic protein Bax and inhibiting the antiapoptotic protein BcI-2, and 0.5 μM sanguinarine treatment leads to a significantly reduced number of colonies formed by HeLa cells [15]. Sanguinarine (1) is cytotoxic to different resistant cancer cell lines, and the main mechanisms of action are the inhibition of P-glycoprotein transporters, NF-kB activation, and so forth. For CCRF-CEM, CEM/ADR5000, U87MG, U87ΔEGFR, MDA231, MDA-BCRP, p53+/+, p53−/−, HEK293, and HEK293/ABCB5 cell lines are significantly inhibited with IC50 values of 0.3–4.1 μM [17].
Moreover, sanguinarine (1) exhibits strong cytotoxicity against non-small cell lung cancer (NSCLC) with an IC50 value of 2.19 μM. Its mechanism of action is likely to be correlated with blocking NF-κB, and Akt and ERK1 signaling pathways have a correlation with the inhibition of cancer cell migration [18]. Sanguinarine (1) inhibits the proliferation of BGC-823 gastric cancer cells by downregulating the expression of miR-96-5p and miR-29c-3p and upregulating the expression of MAP4K4, pMEK4, and pJNK1 protein in gastric cancer cells BGC-823 [19]. Sanguinarine (1) inhibits human prostate cancer cells by inducing ROS-dependent Par-4 cleavage and increasing ROS concentrations in cancer cells. Moreover, it induces growth arrest and apoptosis of human prostate cancer cells PC3 and DU145 with active caspases, which are activated at 2 μM concentration, such that their colony formation can be inhibited [20]. The existing research has also suggested that long-term treatment with sanguinarine (1) causes telomere attrition and cell growth retardation, such that cancer cells become senescent. The main mechanisms are associated with the downregulation of the reverse transcriptase hTERT gene expression and inhibition of telomerase activity [21]. Sanguinarine (1) induces apoptosis in human HT-29 cells, demonstrating potential therapeutic applications in the treatment of colon cancer [22].
Sanguinarine (1) overexpresses inducing long non-coding RNA casc2 in SKOV3 cells or inhibits the NF-kB signaling pathway, thus inhibiting SKOV3 cell growth, proliferation, migration, invasion, and so forth, while ultimately facilitating apoptosis [23]. Moreover, it enhances the sensitivity of cisplatin-resistant cells’ ovarian cancer A2780 to cisplatin [24]. Sanguinarine (1) also has potential therapeutic effects against several cell lines with leukemia (e.g., HL-60, drug-resistant HL-60/MX1, drug-resistant HL-60/MX2 (acute promyelocytic leukemia), J45.01 (acute T cell leukemia), U266B1 (myeloma), CCRF/CEM, and CEM/C1 (acute lymphoblastic leukemia)). To be specific, the most potent activity is observed against drug-resistant HL-60/MX2 with IC50 values of 0.10 ± 0.05 [25]. Sanguinarine (1) can inhibit the migration of 786-O cells in vitro and in vivo, and reverse the epithelial−mesenchymal transition with IC50 values of 0.5959 μM [26].
Moreover, recent research has confirmed that sanguinarine (1) is capable of inducing H2O2-dependent cellular ferroptosis in human cervical cancer (Hela) based on a major mechanism that is correlated with the downregulation of SLC7A11 and the depletion of GSH [6]. Besides the above cancer cells, existing studies have found that sanguinarine (1), which specifically targets lung cancer stem cells, is a natural anti-lung cancer drug-resistant compound. Furthermore, sanguinarine (1) inhibits pancreatic cancer stem cells by inhibiting the sonic hedgehog signaling pathway [27]. The therapeutic effect of sanguinarine on various tumors has been verified at the animal level [28,29,30]. Overall, sanguinarine (1) has anticancer potential and is expected to become a leading compound of anticancer natural products [31].
Extensive research has confirmed that chelerythrine (2) is capable of affecting estrogen signaling pathways (e.g., the estrogen receptor ER- α 36, ER- α 66, ER-β1, and Src expression), inhibiting gastric cancer cell (AGS) growth proliferation, and facilitating their apoptosis [32]. Chelerythrine (2) can act on tgfb1-erk1/2/Smad2/3-snail/ZEB1 signaling to inhibit the progression of cell lines U251 and T98G of glioblastoma (GBM) (e.g., proliferation, migration, stemness, and invasion [33]). In addition, chelerythrine (2) also promotes Drp1 mitochondrial translocation to enhance glioma cell lines necroptosis [34].
In human hepatocellular carcinoma (HCC), chelerythrine (2) can inhibit human hepatocellular carcinoma Hep3B cells by downregulating the expression of p-FAK and MMP-2/9. Moreover, the main mechanism of action is correlated with the alteration of phosphoinositide 3-kinase (PI3K), Akt, and the mammalian target of rapamycin (mTOR) signaling pathways [35]. Chelerythrine (2) has exhibited anticancer activity in vivo and in vitro, and considerable existing research has confirmed that chelerythrine can act on different pathways (e.g., DNA, MAPK, apoptosis, ROS, cell cycle, autophagy, tumor metastasis, and PKC) to inhibit or facilitate apoptosis in a variety of cancer cells (e.g., non-small cell lung cancer [36], prostate cancer [37], lung adenocarcinoma [38], renal cancer [39], and melanoma cells [40], colorectal cancer [41]), thus suggesting that the benzophenanthridine alkaloids exhibit high anticancer activity. Previous studies have proven that chelerythrine (2) exhibits antitumor stem cell properties, which are mediated by the downregulation of β- Catenin expression, thus inhibiting non-small cell lung cancer stem cells [42]. Both sanguinarine (1) and chelerythrine (2) have anticancer activities on human breast cancer cells, but sanguinarine (1) has more potential [43]. However, chelerythrine (2) has been reported as a promoter that can regulate c-MYC oncogenes, which has become a new strategy to develop anticancer molecules [44].
Existing research has confirmed that the hydroxymethyl group at the C-6 position of benzophenanthridine alkaloids takes on a critical significance to cellular activity, and the introduction of different groups at the C-6 position can change their activity (Figure 3 and Figure 4) [45]. For instance, the introduction of malonate, dialkylphosphite, and nitroalkanes significantly enhances their cytotoxicity. Furthermore, derivatives (8–12) (Table 1) obtained after the insertion of the electron-donating group at the C-6 position by sanguinarine exhibit higher cellular activity than those of chelerythrine (13–16) (Table 2).
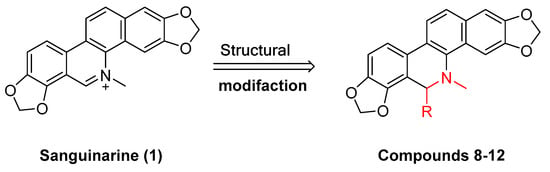
Figure 3.
Study on structure–activity relationship of sanguinarine.
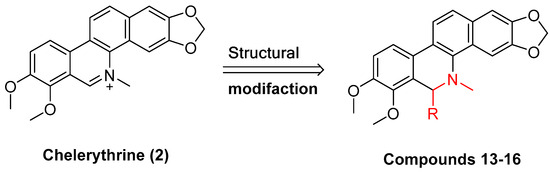
Figure 4.
Study on structure–activity relationship of chelerythrine.

Table 1.
The IC50 value of compounds 8–12.

Table 2.
The IC50 values of compounds 13–16.
The compounds chelerythrine (2), structurally modified to yield compounds 13–16, sanguinarine (1), and derivatives 8–12, exhibit potent activity against Jurkat clone e6-1 and THP-1 leukemia cell lines, with IC50 values from 0.18 to 7.94 μM. Notably, most of the activities of the above compounds are higher than those of chelerythrine (2) and sanguinarine (1)’s original activities, in which the IC50 values of compound 12 against the above two leukemia cell lines reach 0.53 ± 0.05 and 0.18 ± 0.03 μM, respectively [46].
Sanguinarine (1), chelerythrine (2), sanguilutine (3), sanguirubine (4), chelirubine (5), and macarpine (7) (Figure 1) exhibit antitumor activity against various cancer cell lines, including human leukemia (e.g., HL-60, THP-1, MT-4, CEM, and U937), human prostate cancer (e.g., DU-145, LNCaP, and PC3), human epidermoid carcinoma (e.g., A431, nheks,), human pancreatic cancer (e.g., AsPC-1 and BXPC-3), human melanoma (e.g., M4Beu, A372, OCM-1), human non-small cell lung cancer (A549), human breast cancer (e.g., MCF-7 and MDA-MB-231), human ovarian adenocarcinoma (OVCAR-3), cervical cancer (e.g., hen-16-2 and HeLa), human colon cancer (e.g., HCT-116, SW480), and human gastric cancer cells (BGC-823) with IC50 values < 10 μM [47].
Sanguinarine (1), chelerythrine (2), chelirubine (5), macarpine (7), and sanguirubine (4) inhibit HL-60, KF-II, A431, and HeLa activity with IC50 values ≤ 0.7 (μg/mL). Of these, macarpine (7) shows the optimal activity, with IC50 values against four cell lines (μg/mL) followed by 0.012, 0.013, 0.024, and 0.015 [48].
Sanguinaria canadensis L. can extract and purify sanguilutine (3). Some research has suggested that the antiproliferative activities of sanguilutine (3) and chelilutine (6) are related to the induction of oxidative stress. As indicated by the results, against three different cancer cell lines, HeLa, A2780, and HL-60, the IC50 values of sanguilutine range from 0.04 to 0.46 (μg/mL), and the IC50 values of chelilutine (μg/mL) range from 0.16 to 0.84 [49].
Hammerová, Jindřiš Ka et al. [50] further elucidated the mechanism of sanguilutine (3) in inducing apoptosis in melanoma cells. As a result, sanguinarine caused a decrease in the mitochondrial membrane potential and levels of antiapoptotic proteins of the bcl-2 protein family, BCL XL, and myeloid cell leukemia protein 1 (Mcl-1), as well as downregulated levels of the X-linked inhibitor of apoptosis protein (XIAP) to facilitate melanoma cell apoptosis.
2.1.2. Antitumor Activities of Benzophenanthridine Alkaloids from Corydalis saxicola Bunting
The 6-acetyl-5,6-dihydrosanguinarine (17), 8-acetyldihydrochelerythrine (18), and dihydrochelerythrine (19) (Figure 5) are isolated from Corydalis saxicola Bunting, and the above alkaloids have some antitumor effects on squamous cell carcinoma, lung cancer, and liver cancer of the tongue. To be specific, the mechanism of action against human tongue squamous cell carcinoma may be inhibiting NF-κB activation, downregulating BcI-2 protein expression on mRNA, and reducing telomerase activity; inhibiting non-small cell lung cancer A549 cell proliferation, migration, and inducing apoptosis; inhibiting proliferation and migration and upregulating the intracellular NF-κB p65 expression of the subunit [51].
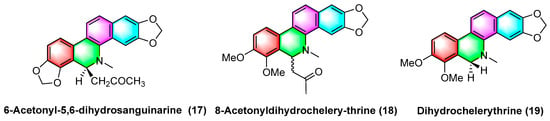
Figure 5.
Benzophenanthridine alkaloids from Corydalis saxicola Bunting.
Feng Qin et al. [52] extracted and isolated two novel dimeric benzophenanthridine alkaloids (20, 21) (Figure 6) from Corydalis saxicola, which comprised a mixture of benzophenanthridine and protoberberine passing between the 6, 12 C-C σ Bond direct coupling generation. Compounds 20 and 21 inhibited T24 cells with IC50 values of 13.26 μM and 9.45 μM.
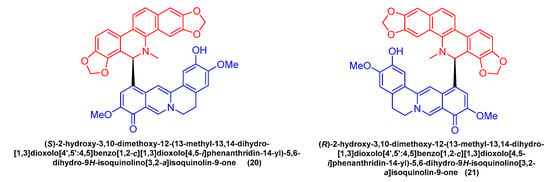
Figure 6.
Benzophenanthridine alkaloids from Corydalis saxicola.
2.1.3. Antitumor Activities of Benzophenanthridine Alkaloids from Chelidonium
Sakineh Kazemi noureini et al. [53] have indicated that chelidonine (22) (Figure 7) can inhibit MCF-7 in a dose-dependent manner, including cell senescence, apoptosis of autophagic syncytial cells by inhibiting telomerase activity, and chelidonine (22) at a 0.05 μM concentration, thus inducing senescence in MCF-7 cells with an LD50 value of 8 μM. Chelidonine (22) is capable of inhibiting the pharmacological activity of the NRAS activator stk19 kinase. It inhibits arginine, lysine, and leucine (Q61R, Q61K, and Q61L) in NRAS mutant melanoma at glutamate 61 (Q61), thus inhibiting the downstream signaling pathways of RAS proteins (e.g., Raf/MEK and P13K-AKT). As a result, cellular senescence and apoptosis are caused. Chelidonine inhibits STK19 kinase with an IC50 value of 123.5 ± 19.3 Nm [54]. Csomós, I., et al. [55] have indicated that chelidonine (22) arrests the G2/S phase of melanoma cells by inhibiting the phosphorylation of complexine and serine in the STAT3 signaling pathway in human melanoma cells, thus inhibiting melanoma. The inhibition effect is significant at 1 μg/mL concentration. It has also been documented that chelidonine (22) can inhibit growth, invasion, angiogenesis, and suppress gene expression in head and neck cancer cell lines. At 10 μg/mL, it significantly inhibits FADU, HLaC78, HlaC79, and HLaC79-Tax cell lines [56]. Radim havelek et al. [57] indicated that chelidonine (22) can inhibit the cell cycle of leukemic T cells in different p53 states while suppressing tubulin polymerization in A549 cells. The IC50 value of different tumor suppressor proteins of the p53 gene for MOLT-4, HL-60, U-937, Raji, Jurkat, and others ranges from 2.2 to 5.0 μM. For non-small cell lung cancer cells (NSCLC), chelidonine (22) has strong inhibitory effects, which are achieved primarily through the ability to selectively inhibit the EGFR phosphorylation and inhibit mitochondrial function in EGFR double mutant cells. The IC50 of chelidonine after 72 h treatment of various NSCLC cell lines (e.g., H1975, PC9, H460, and H358) ranges from 2.58 to 12.77 μM. Against A549, CCD19 is less active with IC50 value > 20 μM [58]. Chelidonine (22) can induce cell death in T98G cells through two apoptotic pathways: caspase-dependent and caspase-independent. As a result, cell mitosis is arrested, thus causing cell death, and inhibiting human glioblastoma. 0.6 μM of chelidonine (22) can significantly inhibit the G2/M phase of mitosis in T98G cells [59]. Lenvatinib is capable of enhancing the apoptosis of HCC cells by chelidonine (22), thus inhibiting the epithelial mesenchymal transition (EMT)-related factor of HCC cells based on the possible mechanism. Moreover, chelidonine inhibits HCC cells MHCC97-H and LM-3 with IC50 values of 7.72 μM and 6.34 μM [60]. Chelidonine (22) can inhibit the cell cycle of leukemic T cells in different p53 states while suppressing tubulin polymerization in A549 cells, and the IC50 values of different tumor suppressor proteins’ p53 gene for MOLT-4, HL-60, U-937, and Raji range from 4.8 to 8.3 μM [51].
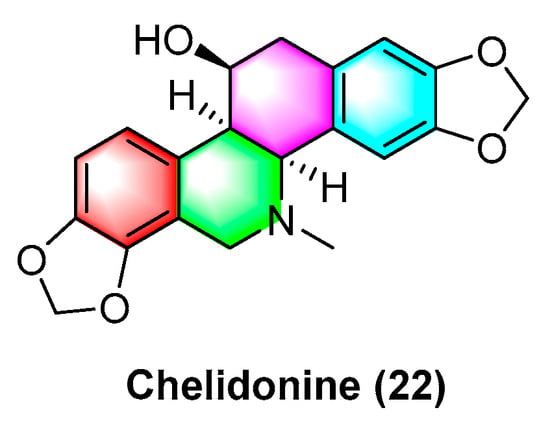
Figure 7.
Benzophenanthridine alkaloid from Chelidonium.
Havelek R. et al. [61] have indicated that homochelidonine (23) (Figure 8) induces apoptosis and arrests the G2 phase mitotic cell cycle in cancer cells, and 20 µM homochelidonine (23) inhibits the cell growth of SK-BR-3, HepG2, and MCF-7 by over 50%.
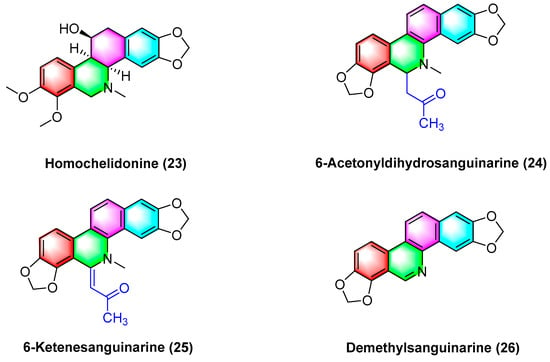
Figure 8.
Benzophenanthridine alkaloids from Corydalis bungeana Turcz.
Acetyldihydrosanguinarine (24), 6-ketenesanguinarine (25), and demethylsanguinarine (26) (Figure 8) are isolated from Corydalis bungeana Turcz. Xiyun Ye et al. [62] determined the cytotoxic activity of the above two compounds against A549, HT-29, kb16, and P-388 cell lines, respectively, and their ED50 (μg/mL) values reach 1.840, 1.600, 0.340, and 0.051, respectively.
2.1.4. Antitumor Activities of Benzophenanthridine Alkaloids from Corydalis
Corynoline (27) (Figure 9) is a natural product derived from the traditional Chinese medicine Corydalis. It significantly inhibits the cell cycle and induces apoptosis in melanoma cells B16F10 and A375 in vivo, with an IC50 value of 6.16 μM. The IC50 value of A375 reaches 5.56 μM. The mechanism between them is correlated with the upregulated gene expression of Bax and cleavage of Caspase-3 [63].
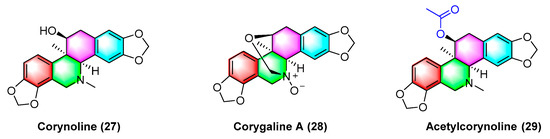
Figure 9.
Benzophenanthridine alkaloids from corydalis.
Corygaline A (28) (Figure 9), isolated from Corydalis bungeana Turcz, refers to a hexahydrobenzophenanthridine alkaloid with an unusual carbon skeleton. Corygaline A (28) is capable of inhibiting the NO production in LPS-activated RAW264.7 macrophages with an IC50 value of 2.9 μM. Moreover, it is independent of dose [64].
Acetylcorynoline (29) (Figure 9), originating from the rhizome of the natural plant Corydalis incisa, inhibits the mitotic process of cancer cells by affecting chromosomes, spindles, and the cytoplasm during mitosis, which eventually arrests the mitotic process and induces apoptosis. The mitotic process of the cells is significantly inhibited by 10 μM acetylcorynoline. Acetylcorynoline (29) potently inhibits human colon carcinoma HCT-116, lung adenocarcinoma cell NCI-H23, lung carcinoma H460, as well as cervical carcinoma TuWi with EC50 values < 20 μg/mL [65].
As shown in Figure 10, dehydroambiguanine A (30) and (6R, 13S, 14S)-ambinine (31) are extracted from Corydalis ambigua subsp. Amurensis. As indicated by the result, both alkaloids can inhibit the proliferation of tumor cells during activity tests. They exhibit strong activity against the human colon cancer cell line HCT-116, dehydroambiguanine A (30) with an IC50 value of 49.8 ± 4.79 μM. Furthermore, (6R, 13S, 14S)-ambinine (31) is less active (IC50 values > 200 μM) [66].
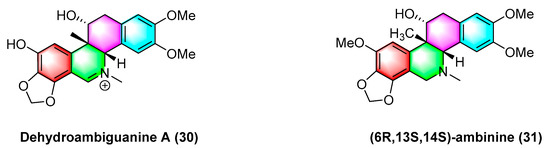
Figure 10.
Benzophenanthridine alkaloids from Corydalis ambigua subsp. Amurensis.
2.1.5. Antitumor Activities of Benzophenanthridine Alkaloids from Macleaya cordata
The benzophenanthridine alkaloids cordatine (32) and 6-methoxyldihydrochelerythrine (33) in Figure 11 are extracted from the fruits of Macleaya cordata. Hui Liang Zou et al. [67] determined the cytotoxic activity of the above two benzophenanthridine alkaloids against MCF-7 and SF-268 through the MTT assay. As revealed by the results, the IC50 value of cordatine (32) against MCF-7 cells is 34.78 mM, and that against SF-268 cells reaches 11.79 mM. Furthermore, the IC50 value of 6-methoxydihydrochelerythrine (33) reaches 21.45 mM and 4.28 mM, respectively.
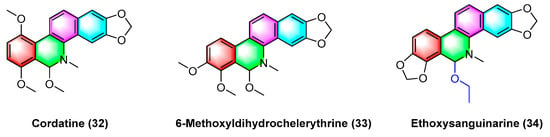
Figure 11.
Benzophenanthridine alkaloids from the fruits of Macleaya cordata.
Ethoxysanguinarine (34) is derived from Macleaya cordata (Willd) r. br., and ethoxysanguinarine (34) inhibits the anchorage-dependent and anchorage-independent growth of breast cancer cells by inducing cell autophagy by upregulating the activity of AMP-activated protein kinase (AMPK). Ethoxysanguinarine (34) exhibits strong activity against seven breast cancer cell lines (including MCF-7, sk-br3, MDA-MB-231, MDA-MB-436, MDA-MB-468, MDA-MB-453, and MDA-MB-435S) with IC50 values from 2.63 to 9.15 μM [68]. Recently, a study showed that it inhibits the viability of MCF-7 and MDA-MB-231 human breast cancer cells and induces apoptosis via a mechanism related to a Hakai-related signaling pathway [69].
Five dihydrodibenzophenanthridine alkaloids, termed maclekarpine A–E (35–39) (Figure 12), are isolated from the fall back roots of Macleaya cordata. The alkaloids maclekarpine A–E (35–39) exhibit high antitumor activity and have an inhibitory effect on several human cancer cells (e.g., human colon cancer cell line HCT-8, human hepatoma cell line BEL-7402, human gastric cancer cell line BGC-823, human ovarian cancer cell line A2780, as well as human lung cancer cell line A549). Maclekarpine A (35) exhibits excellent activity against BGC-823 with an IC50 value of 0.7 μM, except that maclekarpine B (36) is inactive. The IC50 value of maclekarpine C (37) ranges from 1.6 to 3.4 μM. Maclekarpine D (38) IC50 values range from 0.2 to 2.0 μM. Maclekarpine E (39) has a significant inhibitory activity against BGC-823 with the IC50 value of 0.1 μM [70].
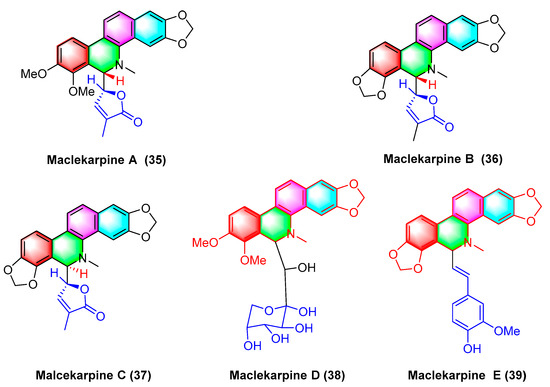
Figure 12.
Benzophenanthridine alkaloids from the fall back roots of Macleaya cordata.
(±)-macleayin A (40, 41) and (±)-macleayin B (42, 43) in Figure 13 originating from Macleaya cordata (Willd.) r. br. are enantiomeric natural dimeric alkaloids. (±)-macleayin A (40, 41) are prepared by coupling dihydrosanguinarine with allocryptamine through the 6,13′-C-C bond, and (±)-macleayin B (42, 43) are synthesized by coupling dihydrosanguinarine with proto opioid through a 6,13′-C-C bond. In the in vitro activity test, (±)-macleayin A and (±)-macleayin B have significant inhibitory effects on cancer cell HL-60 with IC50 values from 3.51 to 9.64 μM. Macleayin A exhibits the optimal activity, while the IC50 values of sanguinarine and allocryptophylline reach 7.71 and 7.18 μM, respectively [71].
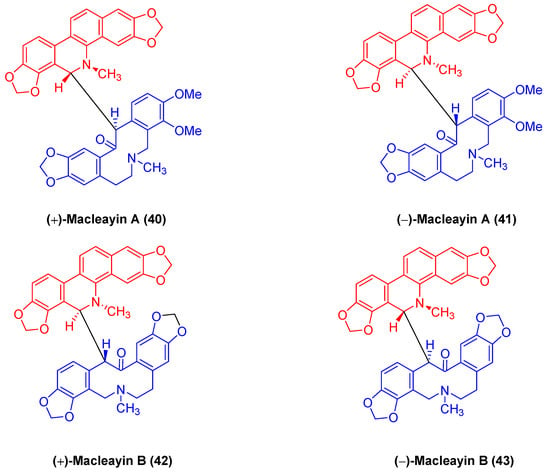
Figure 13.
Benzophenanthridine alkaloids from Macleaya cordata (Willd.) r. br.
The natural benzophenanthridine alkaloids (−)-macleayin C (44), (+)-macleayin C (45), (−)-macleayin D (46), (+)-macleayin D (47), (−)-macleayin E (48), and (+)-macleayin E (49) (Figure 14) with enantiomers originate from Macleaya cordata. They are novel compounds comprising dihydrophenanthridine alkaloids with phenylpropane. Among the cytotoxic activities, (−)-macleayin C (44) and (+)-macleayin C (45) are more active against HL-60 cancer cells with IC50 values of 12.13 and 10.15 μM, respectively. (−)-macleayin D (46), (+)-macleayin D (47), (−)-macleayin E (48), and (+)-macleayin E (49) significantly inhibit HL-60 and A549 cancer cells with IC50 values from 18.46 to 35.26 μM [72].
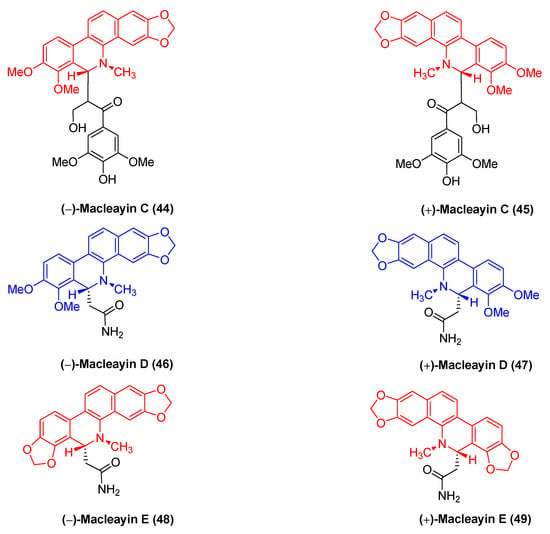
Figure 14.
Benzophenanthridine alkaloids from Macleaya cordata.
Bis-[6-(5,6dihydrochelerythrinyl)] ether (50) (Figure 15) refers to a benzophenanthridine benzophenanthridine alkaloid dimer originating from the roots of M. microcarpa. It exhibits favorable cytotoxic activity against human cancer cell lines (e.g., HCT-8, BEL-7402, BGC-823, and A2780) with IC50 values of 1.6, 2.1, 0.1, and 1.6 μM, respectively [43]. Moreover, existing research has indicated that the natural benzophenanthridine dimeric alkaloid (+)-1,3-bis (8-hydro) (51) exhibits antitumor activity [73].
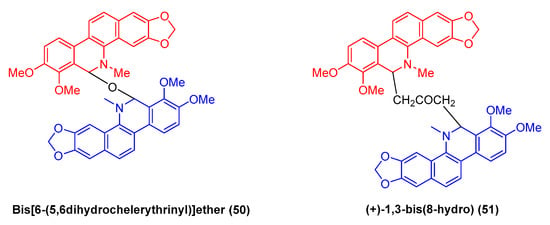
Figure 15.
Benzophenanthridine alkaloids from M. microcarpa.
2.2. Antitumor Activities of Benzophenanthridine Alkaloids from Plants of the Genus Tanneraceae
Jie Jiang et al. [74] isolated and extracted two benzophenanthridine alkaloids with anticancer activity dihydrosanguinarine (52) and dihydrochelilutine (53) (Figure 16) from Thalictrum microgynum Lecoy ex Oliv. Dihydrosanguinarine (52) inhibits pancreatic cancer cells by downregulating mut-p53 or wt-p53 protein expression and regulating the RAS/Raf/MEK/ERK pathway, and the mechanism may be correlated with inducing apoptosis in PANC-1 and SW1990 cells and triggering cell cycle arrest in PANC-1 cells [75]. Dihydrosanguinarine (52) at 5 μM concentration significantly inhibits human promyelocytic leukemia HL-60 cells with p < 0.001 while reducing cell survival [76].
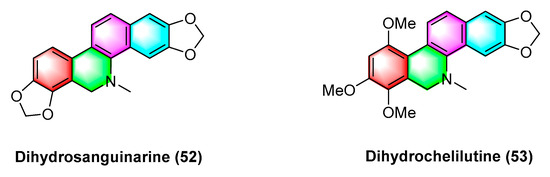
Figure 16.
Benzophenanthridine alkaloids from Thalictrum microgynum Lecoy ex Oliv.
2.3. Antitumor Activities of Benzophenanthridine Alkaloids from Rutaceae
2.3.1. Antitumor Activities of Benzophenanthridine Alkaloids from Zanthoxylum rhoifolium
Nitidine chloride (54), a herb derived from the root of Zanthoxylum avicennae, exhibits anti-inflammatory, antifungal, anti-HIV, and antimalarial biological activities and shows high activity against tumor cancer cells [77], as indicated by the IC50 values (μM) against four cancer cell lines (including HepG2, A549, NCI-H460, and CNE1) that reach 1.40 ± 0.16, 1.88 ± 0.24, 2.35 ± 0.35, and 1.85 ± 0.08, respectively. Pan X et al. [78] have confirmed that nitidine chloride (54) can inhibit the protrusion formation and partial proteolytic activity of MMP-9 and MMP-2 in a dose-dependent manner; reduce the PDGF-induced phosphorylation of c-Src, FAK, and MAPKs; and decrease AP-1 transcriptional activity to inhibit the human breast cancer MDA-MB-231 cell line. Nitidine chloride (54) can inhibit HepG2, HCCLM3, and Huh7 growth, arrest G1/s cell cycle, inhibit proliferation, induce apoptosis, and suppress the expression of cegf-a and VEGFR2 in HCC cells in vitro and in vivo by activating the mitochondria-dependent pathway [79]. Huaping Mou et al. [80] have suggested that nitidine chloride (54) can inactivate S-phase kinase-associated protein 2 (Skp2) to inhibit ovarian cancer, mainly downregulating Skp2 expression and enhancing the sensitivity of ovarian cancer cells to nitidine chloride, in which p < 0.05. Nitidine chloride (54) is capable of inhibiting renal cancer cell 786-O and A498 cells, as confirmed by cell viability and flow cytometric apoptosis analysis. Its mechanism is primarily induced by downregulating the signaling process of p53, BcI-2, caspase-3, and inhibiting ERK [81]. Nitidine chloride (54) can induce autophagy and apoptosis of ovarian cancer cells through various signaling pathways, such as Akt/mTOR. It may become a potential target for ovarian cancer chemotherapy [82].
In cancer cell proliferation, nitidine chloride (54) can serve as a potent inhibitor while inhibiting cell growth, apoptosis, migration, and invasion in glioblastoma cell lines by downregulating the expression of calmodulin-dependent protein kinase III, which may be correlated with the activation of Cdc20 oncoprotein expression [83]. Moreover, osteosarcoma cells can be inhibited by nitidine chloride (54), and Hui Xu et al. [84] have indicated that nitidine chloride (54) inhibits the growth, migration, and invasion and induces the apoptosis of osteosarcoma cells through an MTT assay and flow cytometric analysis. The mechanism may be correlated with the inhibition of sin1 expression in osteosarcoma cells. As revealed by the result, nitidine chloride (54) inhibits rectal cancer HCT-116 cells by inhibiting the phosphorylation pathway of ERK, and the mechanism is the upregulation of the expression of Bax, p53, and the downregulation of the expression of caspase-3, caspase-9, and BcI-2 [85]. Hyoung Yang et al. [86] have found that nitidine chloride (54) inhibits human oral squamous cell carcinoma (OSCC) by inhibiting the signal transducer and activator of transcription 3 (STAT3), and the main mechanism is the downregulation of myeloid cell leukemia-1 (MCl-1) protein in HSC-3 and HSC-4 by inhibiting the STAT3 pathway. Furthermore, acute myeloid leukemia (AML) can be inhibited by nitidine chloride (54) by inhibiting the phosphorylation of Akt and ERK. Its main mechanism is to downregulate cyclin B1, CDK1, and BCl; upregulate p27 and Bax in AML cells; inactivate PARP; and activate caspase-3-related signaling pathways [87]. Existing research on the anticancer effect of nitidine chloride (54) has suggested that it is capable of inhibiting leukemia (CML) by downregulating the expression of proto oncogene c-myc, and the possible mechanism of action of nitidine chloride (54) is to induce apoptosis and upregulate caspase-3 and PARP-1 in K562 cells, thus enhancing the effect of imatinib on K562 cells [88]. Nitidine chloride (54) is also active against BRCA1-deficient cancer cells. Existing research has suggested that bicarbonate chloride (DSBs) is also active at 0.2 µM, thus significantly inhibiting MDA-436BRCA1-KO cells (p ≤ 0.001), and 0.4 µ m is significantly (p ≤ 0.001) resistant to HCC1937-BRCA1−/− inhibition [89].
Some studies have indicated that bicarbonate chloride (54) exhibits antitumor stem cell properties (e.g., inhibiting the epithelial mesenchymal transition (EMT) and inhibiting glioma stem cell properties through the JAK2/STAT3 signaling pathway [90]). Bicarbonate chloride (54) also exhibited dose-dependent anti-liver cancer stem cell activity, which was confirmed in nude mice experiments [91]. Furthermore, nitidine chloride (54) inhibits the epithelial mesenchymal transition process while suppressing tumor stem cell properties in breast cancer cells through the hedgehog signaling pathway [92]. In the in vitro activity study, nitidine chloride (54) inhibited bladder cancer cells by downregulating the expression of Lymphocyte antigen 75 (LY75) [93].
Avicine (55) and nitidine chloride (54) (Figure 17) are two types of benzophenanthridine alkaloids derived from the roots of Zanthoxylum avicennae that serve as cholinesterases (AChE), monoamine oxidases A (Maos), and A β 1–42 targeted inhibitors. Erika Plaza et al. [79] analyzed the IC50 values for inhibiting EeAChEH, rAChE, EqAChE, MAO A, and MAO B using avicine (55) and nitidine chloride (54). The IC50 values (μM) of avicine-inhibited AChEW as 0.15 ± 0.01, 0.52 ± 0.05, and 0.88 ± 0.08 reach 0.41 ± 0.02, and the inhibition Maos IC50 values (>100 μM). However, the IC50 values (μM) of nitidine chloride-inhibited AChE reach 0.65 ± 0.09, 1.25 ± 0.09, and 5.73 ± 0.60, and those of inhibition MAOs IC50 values reach 1.89 ± 0.17 and >300 μM.
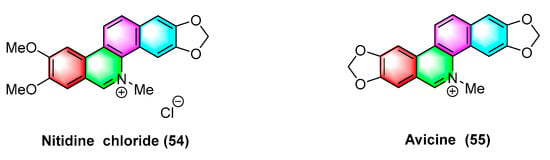
Figure 17.
Benzophenanthridine alkaloids from Zanthoxylum rhoifolium.
Buegenine (56) (Figure 18), derived from Zanthoxylum buesgenii, is cytotoxic in nine cancer cell lines, including leukemic cancer cells CCRF-CEM and CEM/ADR5000, breast cancer cell MDA-MB231 and its resistant subline MDA-MB231/BCRP, colon cancer cell HCT-116p53+/+ and its resistant subline HCT-116p53−/−, glioblastoma U87MG and its resistant subline U87MGΔEGFR, HepG2 hepatoma cells, and AML12 normal liver cells. It exhibits high activity with IC50 values less than 65 μM. Notably, buegenine (56) exhibits better activity against drug-resistant CEM/ADR5000 cell lines than doxorubicin [94].
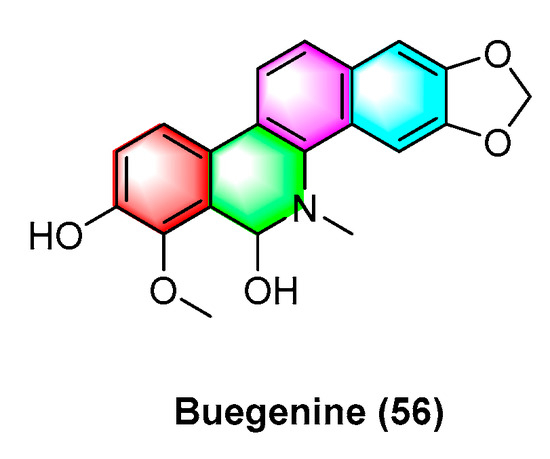
Figure 18.
Benzophenanthridine alkaloids from Zanthoxylum buesgenii.
A wide variety of benzophenanthridine alkaloids (Figure 19) have been extracted by Deng, Y’s group [95] in Zanthoxylum avicennae from Yunnan Province, and their antileukemic cell line HLa activity in vitro is determined, with better activity of bocconoline (60), zanthoxyline (61), nitidine chloride (54), chelerythrine (2), and their IC50 values (μM) in the order of 7.65 ± 0.11, 24.94 ± 1.99, 3.59 ± 0.82, and 15.52 ± 0.26. Nitidine chloride (54) results in significant S-phase arrest and induces apoptosis in HEL cells, thus becoming a promising potential antileukemic candidate. Furthermore, other alkaloids 2-(5,6-dihydrochelerythrine-6-ylethyl acetate) (57), 6-acetonydihy–drochelerythrine (58), the activities of 6 β-hydroxymethyldihydronitidine (59), o-methylzanthoxyline (62), rhoifoline B (63), and n-nornitidine (64) are less weak with IC50 values higher than 30 μM.
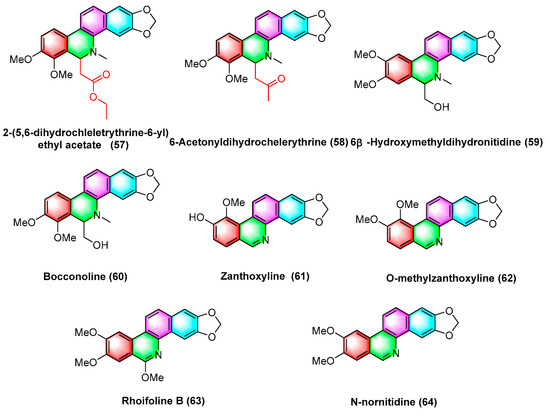
Figure 19.
Benzophenanthridine alkaloids from Zanthoxylum avicennae.
The natural product rhoifoline B (63) is structurally modified to obtain compound 65 (Figure 20) with the hydroxy substitutions at the 11-position. Compound 66 (Figure 20), originating from compound 65 through structural modification, enhances the inhibition selectivity of tyrosine DNA phosphodiesterase 1 (TDP1), with an IC50 value of 1.7 ± 0.24 μM. It is also capable of inhibiting the enhancement of DNA topoisomerase IB (TOP1) activity, thus effectively inhibiting MCF7, A549, H460, and HepG2 cells. The structure–activity relationship study indicates that the 11 hydroxyl groups of compound 65 are replaced by different chemical structures. For instance, compounds with triazole branched chains exhibit poor activity, which may be correlated with poor cell permeability [96].
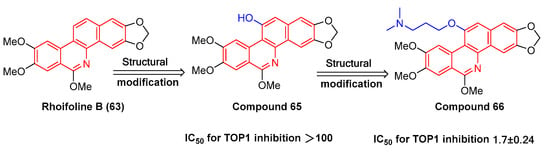
Figure 20.
Study on structure–activity relationship of rhoifoline B.
In Figure 21, the benzophenanthridine alkaloids 8-acetyldihydrocheleryrhtine (67), arnottianamide (68), and 8-oxochelerythrine (69) with anticancer effects are extracted and isolated from Zanthoxylum paracanthum kokwaro. Several studies have found that 8-acetyldihydrocheleryrhtine (67), arnottianamide (68), and 8-oxochelerythrine (69) can inhibit human breast cancer cell line HCC-1395 and human prostate cancer cell DU 145. The IC50 value for 8-acetyldihydrochelerythrine (67) reaches 9.99 μg/mL and 66.82 μg/mL. To be specific, the IC50 value of zanthoxylamide (68) reaches 38.34 μg/mL and 84.31 μg/mL. The IC50 value of 8-oxovalerythrine (69) reaches 14.09 μg/mL and 63.41 μg/mL [97].
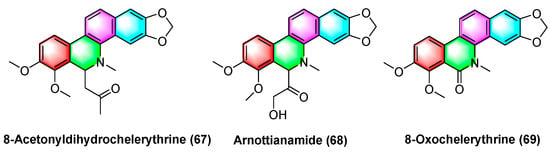
Figure 21.
Benzophenanthridine alkaloids from Zanthoxylum paracanthum Kokwaro.
Two benzophenanthridine alkaloids with cytotoxic activity against a variety of human cancer cell strains, zanthocadinanine C (70) and 7-methoxy-8-demethoxynitidine (71) (Figure 22), are isolated from the traditional drug Zanthoxylum nitidum (Roxb.) DC. Zanthocadinanine C (70) and 7-methoxy-8-demethoxynitidine (71) exhibit cytotoxic activity against five human cancer cell lines KB, MCF-7, LNCaP, HepG-2, and lu-1, whereas the alkaloid 7-methoxy-8-demethoxynitidine (71) has high activity with IC50 values from 10.3 to 12.6 μM. Furthermore, zanthocadinanine C (70) is less active with IC50 > 100 μM [98].
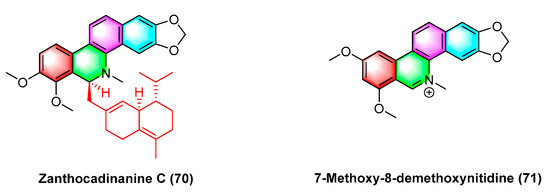
Figure 22.
Benzophenanthridine alkaloids from Zanthoxylum nitidum (Roxb.) DC.
Dihydronitidine (72) (Figure 23), a benzophenanthridine alkaloid, is isolated from Zanthoxylum. Dihydronitidine exhibits high antitumor activity, and its mechanism may be the regulation of cell cycle-related genes CDK2 and CCNE in tumor cells and the upregulation of the expression of relevant genes of apoptosis. For six different cancer cell lines, including lung cancer cell A549, colon adenocarcinoma cell COLO-201, pancreatic cancer cell MIA-PaCa2, epidermoid carcinoma cell A431, gastric cancer cell KATO III, and breast cancer cell SKBR-3, the ED50 values of dihydrobifrontal bases range from 0.19 to 4.60 μg/mL [99].
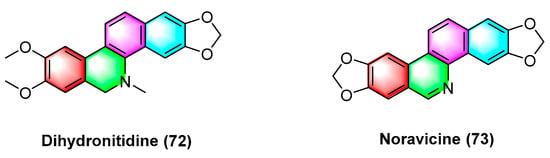
Figure 23.
Benzophenanthridine alkaloids from Zanthoxylum.
Noravicine (73) (Figure 23), a benzophenanthridine alkaloid isolated from the roots of nitidum needles of Zanthoxylum avicennae, exhibits strong cytotoxicity against Ball-1 cancer cells with IC50 values of 74.8 ± 5.93 μM [89].
The isolated benzophenanthridine alkaloids extracted from Zanthoxylum integrifoliolum are isodecarine (74), 8-Demethyloxychelerythrine (75), Norchelerythrine (76), Oxychelerythrine (77), Decarine (78), Dihydrocherythrinylacetaldehyde (79), and 6-Acetonyldihydrochelerythrine (80) (Figure 24). Compounds 74–80 exhibit potent toxicity against P-388 and HT-29 cancer cell lines with ED50 values from 1.15 to 10.30 μg/mL, where dihydrocherythrinylacetaldehyde (79) exhibits the optimal activity, with ED50 values of 1.15 and 4.08 μg/mL, respectively [100].
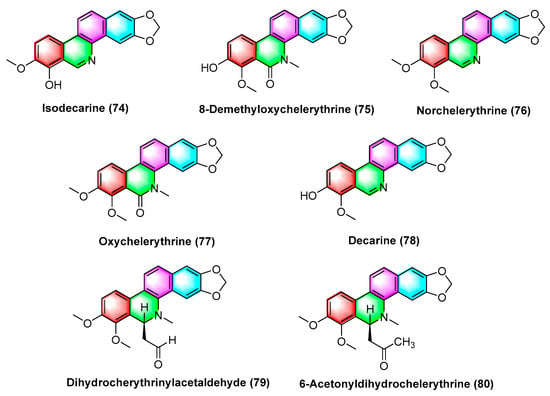
Figure 24.
Benzophenanthridine alkaloids from Zanthoxylum integrifoliolum.
The alkaloid zanthoisobutylamide A (81) (Figure 25), isolated from Zanthoxylum nitidum roots by extraction, refers to a dihydrobenzophenanthridine alkaloid dimer linked with an unsaturated alkylamide through a C-6 bond. Zanthoisobutylamide A (81) potently inhibits human epidermal carcinoma A431 cells with the IC50 value of 29.75 μg/mL [101].
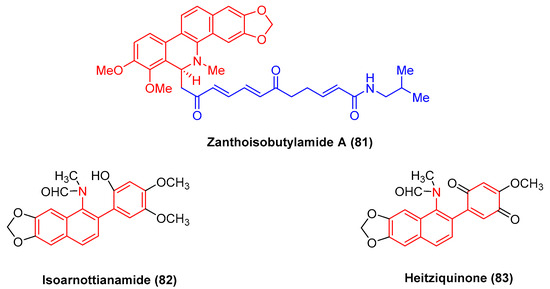
Figure 25.
Benzophenanthridine alkaloids from Zanthoxylum nitidum.
Isoarnottianamide (82) and heitziquinone (83) (Figure 25) refer to a new class of benzophenanthridine alkaloids. To be specific, heitziquinone (83) was isolated from the subfractions of isoarnottianamide (82), and its toxic activity against RAW 264.7 cells was examined [102].
2.3.2. Antitumor Activities of Benzophenanthridine Alkaloids from Fagaropsis
Fagaridine chloride (84) (Figure 26), a benzophenanthridinium natural product isolated from Fagara tessmannii, has shown promising antitumor effects, and the mechanism is correlated with the activation of caspases, the alteration of MMPs, and the secretion amount of ROS. Fagaridine chloride (84) exhibits potent cytotoxicity against several cancer cell lines (e.g., CCRF-CEM cells, CEM/ADR5000 cells, MDA-MB-231-pcDNA3 cells, MDA-MB-231-BCRP clone 23 cells, HCT-116p53 +/+, HCT-116p53−/− cells, U87.MG cells and counterparts U87.MGΔEGFR, and HepG2 cells). The IC50 values range from 1.69 to 63.38 μM. Furthermore, it exhibits strong cytotoxicity against CCRF-CEM cells, CEM/ADR5000, and U87.MG cells [103].
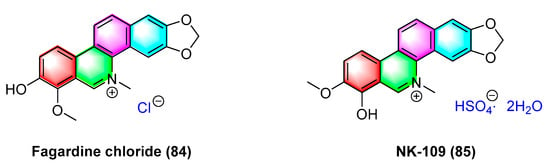
Figure 26.
Benzophenanthridine alkaloids from Fagara tessmannii.
NK-109 (85) (Figure 26) refers to a benzophenanthridinium salt alkaloid that is structurally similar to fagaridine but contains a phenolic hydroxyl substitution at C-7. It is a topoisomerase II inhibitor with better activity. Its metabolites are less toxic to host cells and exhibit significant antitumor activity in clinical trials [104], and NK-109 (85) exhibits significant inhibitory activity against a mixture of HeLa S3 cells and human liver S-9 with IC50 values of 0.08 μg/mL [105] and 0.32 μM [104]. The IC50 values for K562/ADM cells, adrr MCF7 cells, PC-9/CDDP cells, and SKOV3/VP cells are obtained as 0.045, 0.42, 0.19, and 0.21 μg/mL, respectively [106]. Research has shown that 6-substituted derivatives of NK-109 will affect their antitumor activity. The 8-O-substituted derivatives partially inhibit biological reduction. Bulky hydrophobic substituents will weaken activity, while hydrophilic substituents show similar activity as NK-109. The 5-substituted derivatives show strong activity [104].
The natural product fagaridine chloride (84) is structurally modified to compounds dihydrofagaridin (86), 5,6,7,10-tetrahydro-8-methoxy-5-methyl-2,3-methyl-enedioxy-7,10-dioxobenzophenanthridine (87), and 10- hydroxyfagaridinetosylate (88) (Figure 27). The compounds dihydrofagaridin (86), 5,6,7,10-Tetrahyd ro-8-methoxy-5-methyl-2,3-meth- yl-enedioxy-7,10-dioxobenzo[c]phenanthridine (87), and 10-hydroxyfagaridinetosylate (88) significantly inhibit non-small cell lung cancer cell line A-549, ovarian cancer cell line SKOV-3, colon cancer cell line HCT-15, colon cancer cell line XF-498, and melanoma cell line SK-Mel-2 [107].
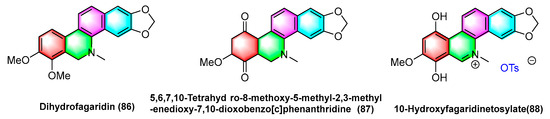
Figure 27.
Benzophenanthridine alkaloids from fagaropsis.
2.3.3. Antitumor Activities of Benzopheridine Alkaloid from Toddalia
Benzophenanthridine alkaloid, 8-acetonyldihydronitidine (89), extracted from Toddalia asiatica, can induce p53 expression, enhance caspase-3 activity, and exhibits good antitumor activity. It is capable of significantly inhibiting colorectal cancer HCT-116 and tumor cell proliferation and inducing apoptosis in vivo, with an IC50 value of 12.91 μM [108].
Benzophenanthridine alkaloids 8-methoxynorchelerythrine (90), 11-demethylrhoifoline B (91), 8-methoxynitidine (92), 8-acetylnorchelerythrine (93), 8,9,10,12-tetramethoxynorchelerythrine (94), isointegriamide (95), pancorine (96), 8-methoxychelerythrine (97), oxynitidine (98), and oxysanguinarine (99) (Figure 28) are extracted from Toddalia asiatica. Studies have shown that the above compounds could inhibit eight types of human tumor cell lines in vitro, including A549 (human lung cancer), BGC-823 (human gastric cancer), HCT-15 (human colon cancer), Hela (human cervical cancer), HepG2 (human liver cancer), MCF-7 (human breast cancer), SK-MEL-2 (human skin cancer), and SGC-7901 (human gastric cancer). Their IC50 values range from 1.3 to 17.8 μg/mL [109].
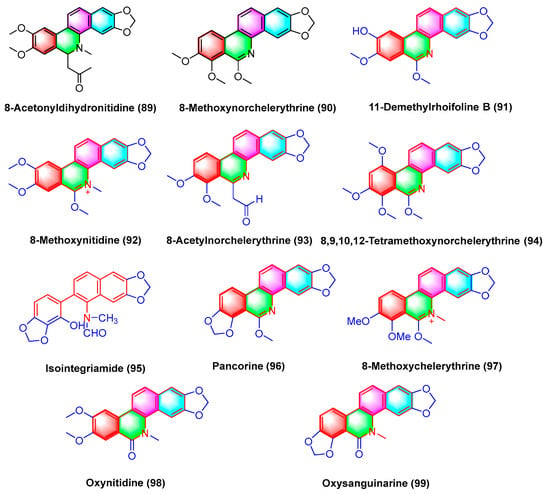
Figure 28.
Benzophenanthridine alkaloids from Toddalia asiatica.
Some derivatives (100, 101, 102) (Figure 29) originate from the benzophenanthridine natural product oxynitidine (98) after semi synthetic structure modification. W.-L. Tang et al. [110] had suggested that the derivatives of oxynidine are capable of inhibiting the growth and proliferation of human cancer cells and inducing apoptosis by inhibiting the activity of DNA topoisomerase IB (TOP1). For HCT-116, MCF-7, DU145, and A549 cancer cell lines, the GI50 ± SD of camptothecin (CPT) (μM) reaches 0.009, 0.012, 0.21, and 0.099, respectively. As the derivatives of topoisomerase IB inhibitors 100, 101, and 102, they inhibit the GI50 ± SD of HCT-116, and MCF-7, DU145, and A549 (μM), respectively, reach 0.29, 0.10, 0.014, and 0.98; 0.036, 0.090, 0.002, and 0.97; 0.076, 0.34, 0.018, and 0.79. Compared with camptothecin, compounds 100, 101, and 102 have better efficacy in the treatment for DU145 cancer cells.
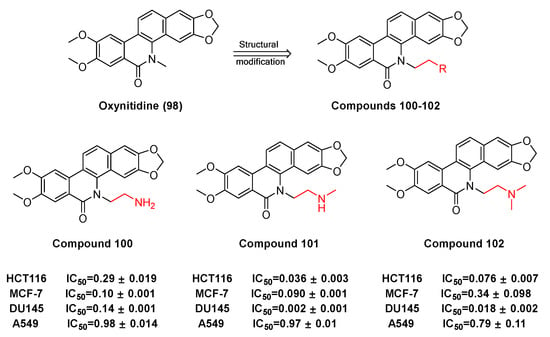
Figure 29.
Study on structure–activity relationship of oxynitidine.
3. Conclusions and Perspectives
The natural benzophenanthridine alkaloids have aroused increasing attention for their extensive and significant biological activities and favorable therapeutic effects in many aspects (e.g., anti-inflammatory, antitumor, antibacterial, and antifungal). Natural benzophenanthridine alkaloids (e.g., sanguinarine, chelerythrine, and nitidine) have achieved a high potential application value and bright application prospect. Studies have shown the potential therapeutic effects of 90 natural benzophenanthridine alkaloids on tumor cells, including human leukemia (e.g., HL-60, THP-1, MT-4, CEM, and U937), human prostate cancer (e.g., DU-145, LNCaP, and PC3), human epidermoid cancer (e.g., A431 and nheks), human pancreatic cancer (e.g., AsPC-1 and BXPC-3), human melanoma (e.g., M4Beu, A372, and OCM-1), human non-small cell lung cancer (A549), human breast cancer (e.g., MCF-7 and MDA-MB-231), human ovarian adenocarcinoma (OVCAR-3), cervical cancer (hen-16-2, HeLa), human colon cancer (HCT-116, SW480), human gastric cancer cell (BGC-823), etc. Moreover, the anticancer activity of various natural benzophenanthridine alkaloids has been verified in animal anticancer experiments. For instance, sanguinarine (1) exhibits antitumor properties against human and rodent breast cancer cells in vitro and in vivo. Nitidine chloride reduced the tumor volume and weight by inhibiting ERK, STAT3, and SHH pathways and regulating Bcl-2, Bax, CDK1, and VEGF pathways in mouse experiments [96]. Moreover, the activity of a few natural benzophenanthridine alkaloids is better than that of marketed drugs. For instance, burgenine (56) is more active than Doxorubicin in drug-resistant CEM/ADR5000 cell lines. Oxynitidine derivatives 100, 101, and 102 exhibit better inhibitory activity on DU145 cells than camptothecin (CPT). Existing research has suggested that natural benzophenanthridine alkaloids exhibit extensive anticancer activity.
Natural benzophenanthridine alkaloids have promising applications in the fight against the drug resistance of tumors. For instance, NK-109 (85) exhibits strong cytotoxicity to drug-resistant cell lines K562/ADM, AdrR MCF7, PC-9/CDDP, and SKOV3/V, with IC50 values of 0.045, 0.42, 0.19, and 0.21 μg/mL, respectively, and the drug molecule has entered the clinical research stage. In addition, the natural products of benzophenanthridine exhibit significant cell activity against cancer stem cells. Sanguinarine (1) exerts a specific targeting effect on lung cancer stem cells, and it is a natural drug-resistant compound against lung cancer. In addition, sanguinarine (1) inhibits pancreatic cancer stem cells by inhibiting the sonic hedgehog signaling pathway. Chelerythrine (2) downregulates β- Catenin, thus inhibiting non-small cell lung cancer stem cells. Nitidine chloride (54) inhibits epithelial mesenchymal transformation (EMT) and glioma stem cell characteristics by the JAK2/STAT3 signaling pathway. Furthermore, it exhibits a dose-dependent antihepatoma stem cell activity, as confirmed in nude mouse experiments. Moreover, it can inhibit the epithelial mesenchymal transformation of breast cancer cells and suppress the characteristics of tumor stem cells through the hedgehog signaling pathway. Ferroptosis takes on critical significance in the treatment of cancer stem cells [111]. Recent research has revealed that sanguinarine (1) can induce H2O2-dependent cell ferroptosis in human cervical cancer (HeLa) by downregulating SLC7A11 and GSH [6].
Author Contributions
Conceptualization, R.P., M.X. and B.X.; writing—review and editing, R.P., M.X., S.H., Z.D., Y.L. and X.C.; supervision, Q.M., W.Y., S.W., G.Y., Z.B., X.X. and S.Q.; funding acquisition, X.X. and S.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 82204250); China Postdoctoral Science Foundation (No. 2021M693961); Young and Middle-Aged Talent Project of Hubei Provincial Department of Education (No. Q20222808); Youth Talent Project of Health Commission of Hubei Province (No. ZY2021Q026); Hubei University of Science and Technology Doctoral Startup Fund Project (No. BK202029); Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515010151, No. 2022A1515140138); Outstanding Young and Middle-Aged Scientific and Technological Innovation Team in Colleges and Universities in Hubei Province (No. T2021022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Feng, S.; Chen, X.M.; Wang, J.F.; Xu, X.Q. 4032-4040-Th17 Cells Associated Cytokines and Cancer. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4032–4040. [Google Scholar] [PubMed]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Molecular Targets and Anticancer Potential of Sanguinarine—A Benzophenanthridine Alkaloid. Phytomedicine 2017, 34, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Menéndez-Perdomo, I.M.; Facchini, P.J. Benzylisoquinoline Alkaloid Biosynthesis in Opium Poppy: An Update. Phytochem. Rev. 2019, 18, 1457–1482. [Google Scholar] [CrossRef]
- Fu, C.; Guan, G.; Wang, H. The Anticancer Effect of Sanguinarine: A Review. Curr. Pharm. Des. 2018, 24, 2760–2764. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Yang, Z.; Liu, Z.; Liu, H.; Yin, J. Research Progress on Natural Benzophenanthridine Alkaloids and Their Pharmacological Functions: A Review. Nat. Prod. Commun. 2016, 11, 1181–1188. [Google Scholar] [CrossRef]
- Alakkal, A.; Thayyullathil, F.; Pallichankandy, S.; Subburayan, K.; Cheratta, A.R.; Galadari, S. Sanguinarine Induces H2O2-Dependent Apoptosis and Ferroptosis in Human Cervical Cancer. Biomedicines 2022, 10, 1795. [Google Scholar] [CrossRef]
- Yang, J.; Fang, Z.; Wu, J.; Yin, X.; Fang, Y.; Zhao, F.; Zhu, S.; Li, Y. Construction and Application of a Lung Cancer Stem Cell Model: Antitumor Drug Screening and Molecular Mechanism of the Inhibitory Effects of Sanguinarine. Tumor Biol. 2016, 37, 13871–13883. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, B. Toxicological Effects of Berberine and Sanguinarine. Front. Mol. Biosci. 2018, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Rashid, K.; AlAmodi, A.A.; Agha, M.V.; Akhtar, S.; Hakeem, I.; Raza, S.S.; Uddin, S. Reactive Oxygen Species (ROS) in Cancer Pathogenesis and Therapy: An Update on the Role of ROS in Anticancer Action of Benzophenanthridine Alkaloids. Biomed. Pharmacother. 2021, 143, 112142. [Google Scholar] [CrossRef]
- Wang, J.; Su, Q.; Wu, Q.; Chen, K.; Ullah, A.; Ghauri, M.A.; Zhang, Y. Sanguinarine Impairs Lysosomal Function and Induces ROS-Dependent Mitophagy and Apoptosis in Human Hepatocellular Carcinoma Cells. Arch. Pharm. Res. 2021, 44, 1025–1036. [Google Scholar] [CrossRef]
- Choi, W.Y.; Kim, G.-Y.; Lee, W.H.; Choi, Y.H. Sanguinarine, a Benzophenanthridine Alkaloid, Induces Apoptosis in MDA-MB-231 Human Breast Carcinoma Cells through a Reactive Oxygen Species-Mediated Mitochondrial Pathway. Chemotherapy 2008, 54, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Wang, J.; Wu, Q.; Ullah, A.; Ghauri, M.A.; Sarwar, A.; Chen, L.; Liu, F.; Zhang, Y. Sanguinarine Combats Hypoxia-Induced Activation of EphB4 and HIF-1α Pathways in Breast Cancer. Phytomedicine 2021, 84, 153503. [Google Scholar] [CrossRef] [PubMed]
- Ghauri, M.A.; Su, Q.; Ullah, A.; Wang, J.; Sarwar, A.; Wu, Q.; Zhang, D.; Zhang, Y. Sanguinarine Impedes Metastasis and Causes Inversion of Epithelial to Mesenchymal Transition in Breast Cancer. Phytomedicine 2021, 84, 153500. [Google Scholar] [CrossRef] [PubMed]
- Malla, R.R.; Bhamidipati, P.; Adem, M. Insights into the Potential of Sanguinarine as a Promising Therapeutic Option for Breast Cancer. Biochem. Pharmacol. 2023, 212, 115565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Venkat, P.S.; Gu, C.; Meng, Y. Sanguinarine Exhibits Potent Efficacy against Cervical Cancer Cells through Inhibiting the STAT3 Pathway in Vitro and in Vivo. Cancer Manag. Res. 2019, 11, 7557–7566. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.J.; Post, C.B.; Liu, Y.; Rhyu, G.I. Structural Changes at the Metal Ion Binding Site during the Phosphoglucomutase Reaction. Biochemistry 1993, 32, 48–57. [Google Scholar] [CrossRef]
- Saeed, M.E.M.; Mahmoud, N.; Sugimoto, Y.; Efferth, T.; Abdel-Aziz, H. Molecular Determinants of Sensitivity or Resistance of Cancer Cells Toward Sanguinarine. Front. Pharmacol. 2018, 9, 136. [Google Scholar] [CrossRef]
- Hałas-Wiśniewska, M.; Zielińska, W.; Izdebska, M.; Grzanka, A. The Synergistic Effect of Piperlongumine and Sanguinarine on the Non-Small Lung Cancer. Molecules 2020, 25, 3045. [Google Scholar] [CrossRef]
- Dong, X.-Z.; Song, Y.; Lu, Y.-P.; Hu, Y.; Liu, P.; Zhang, L. Sanguinarine Inhibits the Proliferation of BGC-823 Gastric Cancer Cells via Regulating MiR-96-5p/MiR-29c-3p and the MAPK/JNK Signaling Pathway. J. Nat. Med. 2019, 73, 777–788. [Google Scholar] [CrossRef]
- Rahman, A.; Pallichankandy, S.; Thayyullathil, F.; Galadari, S. Critical Role of H2O2 in Mediating Sanguinarine-Induced Apoptosis in Prostate Cancer Cells via Facilitating Ceramide Generation, ERK1/2 Phosphorylation, and Par-4 Cleavage. Free Radic. Biol. Med. 2019, 134, 527–544. [Google Scholar] [CrossRef]
- Yan, S.; Lin, S.; Chen, K.; Yin, S.; Peng, H.; Cai, N.; Ma, W.; Songyang, Z.; Huang, Y. Natural Product Library Screens Identify Sanguinarine Chloride as a Potent Inhibitor of Telomerase Expression and Activity. Cells 2022, 11, 1485. [Google Scholar] [CrossRef]
- Lee, J.S.; Jung, W.-K.; Jeong, M.H.; Yoon, T.R.; Kim, H.K. Sanguinarine Induces Apoptosis of HT-29 Human Colon Cancer Cells via the Regulation of Bax/Bcl-2 Ratio and Caspase-9-Dependent Pathway. Int. J. Toxicol. 2012, 31, 70–77. [Google Scholar] [CrossRef]
- Zhang, S.; Leng, T.; Zhang, Q.; Zhao, Q.; Nie, X.; Yang, L. Sanguinarine Inhibits Epithelial Ovarian Cancer Development via Regulating Long Non-Coding RNA CASC2-EIF4A3 Axis and/or Inhibiting NF-ΚB Signaling or PI3K/AKT/MTOR Pathway. Biomed. Pharmacother. 2018, 102, 302–308. [Google Scholar] [CrossRef]
- Sarkhosh-Inanlou, R.; Molaparast, M.; Mohammadzadeh, A.; Shafiei-Irannejad, V. Sanguinarine Enhances Cisplatin Sensitivity via Glutathione Depletion in Cisplatin-resistant Ovarian Cancer (A2780) Cells. Chem. Biol. Drug Des. 2020, 95, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Och, A.; Zalewski, D.; Komsta, Ł.; Kołodziej, P.; Kocki, J.; Bogucka-Kocka, A. Cytotoxic and Proapoptotic Activity of Sanguinarine, Berberine, and Extracts of Chelidonium majus L. and Berberis thunbergii DC. toward Hematopoietic Cancer Cell Lines. Toxins 2019, 11, 485. [Google Scholar] [CrossRef]
- Wen, Y.; Song, Y.; Ma, Y.; Wen, J.; Yang, J. Sanguinarine Targets BRD4 to Suppress Cell Proliferation and Migration in Clear Cell Renal Cell Carcinoma. J. Biochem. Mol. Toxicol. 2023, 2, e23451. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yu, W.; Shrivastava, A.; Alemi, F.; Lankachandra, K.; Srivastava, R.K.; Shankar, S. Sanguinarine Inhibits Pancreatic Cancer Stem Cell Characteristics by Inducing Oxidative Stress and Suppressing Sonic Hedgehog-Gli-Nanog Pathway. Carcinogenesis 2017, 38, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Pica, F.; Balestrieri, E.; Serafino, A.; Sorrentino, R.; Gaziano, R.; Moroni, G.; Moroni, N.; Palmieri, G.; Mattei, M.; Garaci, E.; et al. Antitumor Effects of the Benzophenanthridine Alkaloid Sanguinarine in a Rat Syngeneic Model of Colorectal Cancer. Anticancer. Drugs 2012, 23, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Kalogris, C.; Garulli, C.; Pietrella, L.; Gambini, V.; Pucciarelli, S.; Lucci, C.; Tilio, M.; Zabaleta, M.E.; Bartolacci, C.; Andreani, C.; et al. Sanguinarine Suppresses Basal-like Breast Cancer Growth through Dihydrofolate Reductase Inhibition. Biochem. Pharmacol. 2014, 90, 226–234. [Google Scholar] [CrossRef]
- De Stefano, I.; Raspaglio, G.; Zannoni, G.F.; Travaglia, D.; Prisco, M.G.; Mosca, M.; Ferlini, C.; Scambia, G.; Gallo, D. Antiproliferative and Antiangiogenic Effects of the Benzophenanthridine Alkaloid Sanguinarine in Melanoma. Biochem. Pharmacol. 2009, 78, 1374–1381. [Google Scholar] [CrossRef]
- Gaziano, R. Antitumor Effects of the Benzophenanthridine Alkaloid Sanguinarine: Evidence and Perspectives. World J. Gastrointest. Oncol. 2016, 8, 30. [Google Scholar] [CrossRef]
- Tian, Y.-Q.; Hu, D.; Zhang, Y.-L.; Zou, J.; Chen, G.-L.; Guo, M.-Q. Inhibitors Targeting Multiple Janus Kinases from Zanthoxylum Simulans Mediate Inhibition and Apoptosis against Gastric Cancer Cells via the Estrogen Pathway. Front. Chem. 2022, 10, 922110. [Google Scholar] [CrossRef]
- Zhu, M.; Niu, J.; Jiang, J.; Dong, T.; Chen, Y.; Yang, X.; Liu, P. Chelerythrine Inhibits the Progression of Glioblastoma by Suppressing the TGFB1-ERK1/2/Smad2/3-Snail/ZEB1 Signaling Pathway. Life Sci. 2022, 293, 120358. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zheng, S.-Y.; Jiang, R.-L.; Wu, H.-D.; Li, Y.-A.; Lu, J.-L.; Xiong, Y.; Han, B.; Lin, L. Necroptosis Signaling and Mitochondrial Dysfunction Cross-Talking Facilitate Cell Death Mediated by Chelerythrine in Glioma. Free Radic. Biol. Med. 2023, 202, 76–96. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pan, Y.; Zhang, G.; Wu, Y.; Zhong, W.; Chu, C.; Qian, Y.; Zhu, G. Chelerythrine Inhibits Human Hepatocellular Carcinoma Metastasis in Vitro. Biol. Pharm. Bull. 2018, 41, 36–46. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Zhang, N.; Li, N.; Liu, C.; Wang, B. Using Liposomes to Alleviate the Toxicity of Chelerythrine, a Natural PKC Inhibitor, in Treating Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 658543. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, D.; Qian, J.; Cheng, Y. Chelerythrine Suppresses Proliferation and Metastasis of Human Prostate Cancer Cells via Modulating MMP/TIMP/NF-ΚB System. Mol. Cell. Biochem. 2020, 474, 199–208. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, L.; Xue, Y.; Zhao, Z.; Li, H.; Niu, Z.; Wang, X.; Chen, P.; Zhang, J.; Zhang, X. Benzophenanthridine Alkaloids Suppress Lung Adenocarcinoma by Blocking TMEM16A Ca2+-Activated Cl− Channels. Pflüg. Arch. Eur. J. Physiol. 2020, 472, 1457–1467. [Google Scholar] [CrossRef]
- He, H.; Zhuo, R.; Dai, J.; Wang, X.; Huang, X.; Wang, H.; Xu, D. Chelerythrine Induces Apoptosis via ROS-mediated Endoplasmic Reticulum Stress and STAT3 Pathways in Human Renal Cell Carcinoma. J. Cell. Mol. Med. 2020, 24, 50–60. [Google Scholar] [CrossRef]
- Cho, O.; Lee, J.-W.; Kim, H.-S.; Jeong, Y.-J.; Heo, T.-H. Chelerythrine, a Novel Small Molecule Targeting IL-2, Inhibits Melanoma Progression by Blocking the Interaction between IL-2 and Its Receptor. Life Sci. 2023, 320, 121559. [Google Scholar] [CrossRef]
- Liang, D.; Liu, L.; Zheng, Q.; Zhao, M.; Zhang, G.; Tang, S.; Tang, J.; Chen, N. Chelerythrine Chloride Inhibits the Progression of Colorectal Cancer by Targeting Cancer-associated Fibroblasts through Intervention with WNT10B/B-catenin and TGFB2/Smad2/3 Axis. Phytother. Res. 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Heng, W.S.; Cheah, S.-C. Chelerythrine Chloride Downregulates β-Catenin and Inhibits Stem Cell Properties of Non-Small Cell Lung Carcinoma. Molecules 2020, 25, 224. [Google Scholar] [CrossRef]
- Almeida, I.V.; Fernandes, L.M.; Biazi, B.I.; Vicentini, V.E.P. Evaluation of the Anticancer Activities of the Plant Alkaloids Sanguinarine and Chelerythrine in Human Breast Adenocarcinoma Cells. Anticancer. Agents Med. Chem. 2017, 17, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Bhat, J.; Chatterjee, S. Skeleton Selectivity in Complexation of Chelerythrine and Chelerythrine-like Natural Plant Alkaloids with the G-Quadruplex Formed at the Promoter of c-MYC Oncogene: In Silico Exploration. RSC Adv. 2016, 6, 36667–36680. [Google Scholar] [CrossRef]
- Romo-Pérez, A.; Miranda, L.D.; Chávez-Blanco, A.D.; Dueñas-González, A.; Camacho-Corona, M.d.R.; Acosta-Huerta, A.; García, A. Mild C(Sp)–H Functionalization of Dihydrosanguinarine and Dihydrochelerythrine for Development of Highly Cytotoxic Derivatives. Eur. J. Med. Chem. 2017, 138, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xu, X.; Li, J.; Deng, L.; Mu, S. Synthesis and Antileukemia Activity Evaluation of Benzophenanthridine Alkaloid Derivatives. Molecules 2022, 27, 3934. [Google Scholar] [CrossRef] [PubMed]
- Slaninová, I.; Pěnčíková, K.; Urbanová, J.; Slanina, J.; Táborská, E. Antitumour Activities of Sanguinarine and Related Alkaloids. Phytochem. Rev. 2014, 13, 51–68. [Google Scholar] [CrossRef]
- Slaninová, I.; Slunská, Z.; Šinkora, J.; Vlková, M.; Táborská, E. Screening of Minor Benzo(c.)Phenanthridine Alkaloids for Antiproliferative and Apoptotic Activities. Pharm. Biol. 2007, 45, 131–139. [Google Scholar] [CrossRef]
- Slunská, Z.; Gelnarová, E.; Hammerová, J.; Táborská, E.; Slaninová, I. Effect of Quaternary Benzo[c]Phenanthridine Alkaloids Sanguilutine and Chelilutine on Normal and Cancer Cells. Toxicol. Vitr. 2010, 24, 697–706. [Google Scholar] [CrossRef]
- Hammerová, J.; Uldrijan, S.; Táborská, E.; Vaculová, A.H.; Slaninová, I. Necroptosis Modulated by Autophagy Is a Predominant Form of Melanoma Cell Death Induced by Sanguilutine. Biol. Chem. 2012, 393, 647–658. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, L.; Chang, B.; Yu, J.; Bao, J.; Yao, Q.; Luo, J. The Traditional Uses, Phytochemistry, Pharmacokinetics, Pharmacology, Toxicity, and Applications of Corydalis Saxicola Bunting: A Review. Front. Pharmacol. 2022, 13, 822792. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Dai, L.; Zhang, B.; Huang, R.; Wang, F.; Qin, J.; Liang, D.; Wang, H. (±)-Corysaxicolaine A: A Pair of Antitumor Enantiomeric Alkaloid Dimers from Corydalis Saxicola. Org. Biomol. Chem. 2022, 20, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Kazemi Noureini, S.; Fatemi, L.; Wink, M. Telomere Shortening in Breast Cancer Cells (MCF7) under Treatment with Low Doses of the Benzylisoquinoline Alkaloid Chelidonine. PLoS ONE 2018, 13, e0204901. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Chen, K.; Wang, C.; Chen, Z.; Meng, Z.; Wang, P. Targeting NRAS-Mutant Cancers with the Selective STK19 Kinase Inhibitor Chelidonine. Clin. Cancer Res. 2020, 26, 3408–3419. [Google Scholar] [CrossRef]
- Csomós, I.; Nagy, P.; Filep, C.; Rebenku, I.; Nizsalóczki, E.; Kovács, T.; Vámosi, G.; Mátyus, L.; Bodnár, A. Opposing Effects of Chelidonine on Tyrosine and Serine Phosphorylation of STAT3 in Human Uveal Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 12974. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, R.; Roller, J.; Polednik, C.; Schmidt, M. Effect of Chelidonine on Growth, Invasion, Angiogenesis and Gene Expression in Head and Neck Cancer Cell Lines. Oncol. Lett. 2018, 16, 3108–3116. [Google Scholar] [CrossRef]
- Havelek, R.; Seifrtova, M.; Kralovec, K.; Krocova, E.; Tejkalova, V.; Novotny, I.; Cahlikova, L.; Safratova, M.; Opletal, L.; Bilkova, Z.; et al. Comparative Cytotoxicity of Chelidonine and Homochelidonine, the Dimethoxy Analogues Isolated from Chelidonium majus L. (Papaveraceae), against Human Leukemic and Lung Carcinoma Cells. Phytomedicine 2016, 23, 253–266. [Google Scholar] [CrossRef]
- Xie, Y.-J.; Gao, W.-N.; Wu, Q.-B.; Yao, X.-J.; Jiang, Z.-B.; Wang, Y.-W.; Wang, W.-J.; Li, W.; Hussain, S.; Liu, L.; et al. Chelidonine Selectively Inhibits the Growth of Gefitinib-Resistant Non-Small Cell Lung Cancer Cells through the EGFR-AMPK Pathway. Pharmacol. Res. 2020, 159, 104934. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Lee, K.W.; Kim, M.; Lee, Y.; Yoo, J.; Hwangbo, C.; Park, K.H.; Kim, K.D. Chelidonine Induces Caspase-Dependent and Caspase-Independent Cell Death through G 2/M Arrest in the T98G Human Glioblastoma Cell Line. Evid. Based Complement. Alternat. Med. 2019, 2019, 6318179. [Google Scholar] [CrossRef]
- Hou, F.; Guo, L.; Zheng, K.; Song, J.; Wang, Q.; Zheng, Y. Chelidonine Enhances the Antitumor Effect of Lenvatinib on Hepatocellular Carcinoma Cells. OncoTargets Ther. 2019, 12, 6685–6697. [Google Scholar] [CrossRef]
- Havelek, R.; Seifrtova, M.; Kralovec, K.; Habartova, K.; Cahlikova, L.; Rezacova, M. Chelidonine and Homochelidonine Induce Cell Death through Cell Cycle Checkpoints and MAP Kinase Pathways. Nat. Prod. Commun. 2017, 12, 1934578X1701200910. [Google Scholar] [CrossRef]
- Yi, C.; Li, X.; Chen, S.; Liu, M.; Lu, W.; Ye, X. Natural Product Corynoline Suppresses Melanoma Cell Growth through Inducing Oxidative Stress. Phytother. Res. 2020, 34, 2766–2777. [Google Scholar] [CrossRef]
- Chen, J.-J.; Duh, C.-Y.; Chen, I.-S. New Tetrahydroprotoberberine N-Oxide Alkaloids and Cytotoxic Constituents of Corydalis Tashiroi. Planta Med. 1999, 65, 643–647. [Google Scholar] [CrossRef]
- Gao, C.; Gu, X.; Wang, X.; Cao, H.; Lin, B.; Liu, Y.; Di, X. Corygaline A, a Hexahydrobenzophenanthridine Alkaloid with an Unusual Carbon Skeleton from Corydalis Bungeana Turcz. Org. Biomol. Chem. 2018, 16, 8710–8714. [Google Scholar] [CrossRef]
- Li, J.; Yan, Z.; Li, H.; Shi, Q.; Huang, L.; Nimishetti, N.; Allen, T.D.; Yang, D.; Zhang, J. A High-Content Screen for Anti-Mitosis and Polyploidy-Induction Identifies an Unknown Activity of Two Benzophenanthridine Alkaloids from Corydalis longicalcarata. Phytochem. Lett. 2021, 41, 180–185. [Google Scholar] [CrossRef]
- Liu, Z.; Mi, Z.; Wang, P.; Chang, S.; Han, N.; Yin, J. Two New Alkaloids from the Tubers of Corydalis Ambigua Subsp. Amurensis and Their Anti-Proliferative Activity. Nat. Prod. Res. 2020, 34, 3305–3312. [Google Scholar] [CrossRef]
- Zou, H.-L.; Li, H.-Y.; Liu, B.-L.; Zhou, G.-X. A New Cytotoxic Benzophenanthridine Isoquinoline Alkaloid from the Fruits of Macleaya cordata. J. Asian Nat. Prod. Res. 2015, 17, 856–860. [Google Scholar] [CrossRef]
- Si, Y.; Wang, J.; Liu, X.; Zhou, T.; Xiang, Y.; Zhang, T.; Wang, X.; Feng, T.; Xu, L.; Yu, Q.; et al. Ethoxysanguinarine, a Novel Direct Activator of AMP-Activated Protein Kinase, Induces Autophagy and Exhibits Therapeutic Potential in Breast Cancer Cells. Front. Pharmacol. 2020, 10, 1503. [Google Scholar] [CrossRef]
- Ma, L.; Xuan, X.; Chen, X.; Fan, M.; Liu, J.; Huang, G.; Liu, Z. Ethoxysanguinarine Induces Apoptosis, Inhibits Metastasis and Sensitizes Cells to Docetaxel in Breast Cancer Cells through Inhibition of Hakai. Chem. Biodivers. 2023, 20, e202200284. [Google Scholar] [CrossRef]
- Deng, A.; Qin, H. Cytotoxic Dihydrobenzophenanthridine Alkaloids from the Roots of Macleaya microcarpa. Phytochemistry 2010, 71, 816–822. [Google Scholar] [CrossRef]
- Sai, C.-M.; Li, D.-H.; Xue, C.-M.; Wang, K.-B.; Hu, P.; Pei, Y.-H.; Bai, J.; Jing, Y.-K.; Li, Z.-L.; Hua, H.-M. Two Pairs of Enantiomeric Alkaloid Dimers from Macleaya Cordata. Org. Lett. 2015, 17, 4102–4105. [Google Scholar] [CrossRef]
- Sai, C.-M.; Li, D.-H.; Li, S.-G.; Han, T.; Guo, Y.-Z.; Pei, Y.-H.; Bai, J.; Jing, Y.-K.; Li, Z.-L.; Hua, H.-M. Racemic Alkaloids from Macleaya cordata: Structural Elucidation, Chiral Resolution, and Cytotoxic, Antibacterial Activities. RSC Adv. 2016, 6, 41173–41180. [Google Scholar] [CrossRef]
- Phillips, S.D.; Castle, R.N. A Review of the Chemistry of the Antitumor Benzo[c]Phenanthridine Alkaloids Nitidine and Fagaronine and of the Related Antitumor Alkaloid Coralyne. J. Heterocycl. Chem. 1981, 18, 223–232. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, H.; Hu, S.; Cheng, L.; Wang, F.; Zhang, G. Benzophenanthridine Alkaloids from the Roots of Thalictrum Microgynum Lecoy.Ex Oliv. Nat. Prod. Res. 2019, 33, 2964–2969. [Google Scholar] [CrossRef]
- Wu, S.; Xu, H.; Wu, X.; Liu, P.; Shi, Y.; Pang, P.; Deng, L.; Zhou, G.; Chen, X. Dihydrosanguinarine Suppresses Pancreatic Cancer Cells via Regulation of Mut-P53/WT-P53 and the Ras/Raf/Mek/Erk Pathway. Phytomedicine 2019, 59, 152895. [Google Scholar] [CrossRef]
- Vrba, J.; Doležel, P.; Vičar, J.; Ulrichová, J. Cytotoxic Activity of Sanguinarine and Dihydrosanguinarine in Human Promyelocytic Leukemia HL-60 Cells. Toxicol. Vitr. 2009, 23, 580–588. [Google Scholar] [CrossRef]
- Qin, S.-Q.; Li, L.-C.; Song, J.-R.; Li, H.-Y.; Li, D.-P. Structurally Simple Phenanthridine Analogues Based on Nitidine and Their Antitumor Activities. Molecules 2019, 24, 437. [Google Scholar] [CrossRef]
- Pan, X.; Han, H.; Wang, L.; Yang, L.; Li, R.; Li, Z.; Liu, J.; Zhao, Q.; Qian, M.; Liu, M.; et al. Nitidine Chloride Inhibits Breast Cancer Cells Migration and Invasion by Suppressing C-Src/FAK Associated Signaling Pathway. Cancer Lett. 2011, 313, 181–191. [Google Scholar] [CrossRef]
- Lin, J.; Shen, A.; Chen, H.; Liao, J.; Xu, T.; Liu, L.; Lin, J.; Peng, J. Nitidine Chloride Inhibits Hepatic Cancer Growth via Modulation of Multiple Signaling Pathways. BMC Cancer 2014, 14, 729. [Google Scholar] [CrossRef]
- Mou, H.; Guo, P.; Li, X.; Zhang, C.; Jiang, J.; Wang, L.; Wang, Q.; Yuan, Z. Nitidine Chloride Inhibited the Expression of S Phase Kinase-Associated Protein 2 in Ovarian Cancer Cells. Cell Cycle 2017, 16, 1366–1375. [Google Scholar] [CrossRef]
- Fang, Z.; Tang, Y.; Jiao, W.; Xing, Z.; Guo, Z.; Wang, W.; Xu, Z.; Liu, Z. Nitidine Chloride Induces Apoptosis and Inhibits Tumor Cell Proliferation via Suppressing ERK Signaling Pathway in Renal Cancer. Food Chem. Toxicol. 2014, 66, 210–216. [Google Scholar] [CrossRef]
- Lian, C.; Huang, Y.; Hu, P.; Cao, Y.; Zhang, Z.; Feng, F.; Zhang, J. Nitidine Chloride Triggers Autophagy and Apoptosis of Ovarian Cancer Cellsthrough Akt/MTOR Signaling Pathway. Curr. Pharm. Des. 2023, 29, 1524–1534. [Google Scholar] [CrossRef]
- Wang, L.; Hou, Y.; Yin, X.; Su, J.; Zhao, Z.; Ye, X.; Zhou, X.; Zhou, L.; Wang, Z. Rottlerin Inhibits Cell Growth and Invasion via Down-Regulation of Cdc20 in Glioma Cells. Oncotarget 2016, 7, 69770–69782. [Google Scholar] [CrossRef]
- Xu, H.; Cao, T.; Zhang, X.; Shi, Y.; Zhang, Q.; Chai, S.; Yu, L.; Jin, G.; Ma, J.; Wang, P.; et al. Nitidine Chloride Inhibits SIN1 Expression in Osteosarcoma Cells. Mol. Ther. Oncolytics 2019, 12, 224–234. [Google Scholar] [CrossRef]
- Zhai, H.; Hu, S.; Liu, T.; Wang, F.; Wang, X.; Wu, G.; Zhang, Y.; Sui, M.; Liu, H.; Jiang, L. Nitidine Chloride Inhibits Proliferation and Induces Apoptosis in Colorectal Cancer Cells by Suppressing the ERK Signaling Pathway. Mol. Med. Rep. 2016, 13, 2536–2542. [Google Scholar] [CrossRef]
- Yang, I.; Jung, W.; Kim, L.; Shin, J.; Cho, N.; Hong, S.D.; Hong, K.; Cho, S. Nitidine Chloride Represses Mcl-1 Protein via Lysosomal Degradation in Oral Squamous Cell Carcinoma. J. Oral Pathol. Med. 2018, 47, 823–829. [Google Scholar] [CrossRef]
- Li, P.; Yan, S.; Dong, X.; Li, Z.; Qiu, Y.; Ji, C.; Zhang, J.; Ji, M.; Li, W.; Wang, H.; et al. Cell Cycle Arrest and Apoptosis Induction Activity of Nitidine Chloride on Acute Myeloid Leukemia Cells. Med. Chem. 2018, 14, 60–66. [Google Scholar] [CrossRef]
- Liu, N.; Li, P.; Zang, S.; Liu, Q.; Ma, D.; Sun, X.; Ji, C. Novel Agent Nitidine Chloride Induces Erythroid Differentiation and Apoptosis in CML Cells through C-Myc-MiRNAs Axis. PLoS ONE 2015, 10, e0116880. [Google Scholar] [CrossRef]
- Wang, C.-F.; Fan, L.; Tian, M.; Du, S.-S.; Deng, Z.-W.; Feng, J.-B.; Wang, Y.-Y.; Su, X. Cytotoxicity of Benzophenanthridine Alkaloids from the Roots of Zanthoxylum Nitidum (Roxb.) DC. Var. Fastuosum How Ex Huang. Nat. Prod. Res. 2015, 29, 1380–1383. [Google Scholar] [CrossRef]
- Jia, M.; Wang, Y.; Guo, Y.; Yu, P.; Sun, Y.; Song, Y.; Zhao, L. Nitidine Chloride Suppresses Epithelial-mesenchymal Transition and Stem Cell-like Properties in Glioblastoma by Regulating JAK2/STAT3 Signaling. Cancer Med. 2021, 10, 3113–3128. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Q.; Zhou, Y.; Zhu, H.; Li, T.; Du, F. A Novel Nitidine Chloride Nanoparticle Overcomes the Stemness of CD133+EPCAM+ Huh7 Hepatocellular Carcinoma Cells for Liver Cancer Therapy. BMC Pharmacol. Toxicol. 2022, 23, 48. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, N.; Wang, X.; Li, Y.; Qi, W.; Zhang, H.; Li, Z.; Yang, Q. Hedgehog Pathway Is Involved in Nitidine Chloride Induced Inhibition of Epithelial-Mesenchymal Transition and Cancer Stem Cells-like Properties in Breast Cancer Cells. Cell Biosci. 2016, 6, 44. [Google Scholar] [CrossRef]
- Wang, S.; Zhai, G.; Huang, Z.; Luo, J.; He, J.; Huang, J.; Yang, L.; Xiao, C.; Li, S.; Chen, K.; et al. Nitidine Chloride Regulates Cell Function of Bladder Cancer in Vitro through Downregulating Lymphocyte Antigen 75. Naunyn. Schmiedebergs Arch. Pharmacol. 2023, 396, 2071–2085. [Google Scholar] [CrossRef]
- Sandjo, L.P.; Kuete, V.; Tchangna, R.S.; Efferth, T.; Ngadjui, B.T. Cytotoxic Benzophenanthridine and Furoquinoline Alkaloids from Zanthoxylum buesgenii (Rutaceae). Chem. Cent. J. 2014, 8, 61. [Google Scholar] [CrossRef]
- Deng, Y.; Ding, T.; Deng, L.; Hao, X.; Mu, S. Active Constituents of Zanthoxylum nitidium from Yunnan Province against Leukaemia Cells in Vitro. BMC Chem. 2021, 15, 44. [Google Scholar] [CrossRef]
- Yang, H.; Wang, F.-T.; Wu, M.; Wang, W.; Agama, K.; Pommier, Y.; An, L.-K. Synthesis of 11-Aminoalkoxy Substituted Benzophenanthridine Derivatives as Tyrosyl-DNA Phosphodiesterase 1 Inhibitors and Their Anticancer Activity. Bioorganic Chem. 2022, 123, 105789. [Google Scholar] [CrossRef]
- Kaigongi, M.M.; Lukhoba, C.W.; Yaouba, S.; Makunga, N.P.; Githiomi, J.; Yenesew, A. In Vitro Antimicrobial and Antiproliferative Activities of the Root Bark Extract and Isolated Chemical Constituents of Zanthoxylum paracanthum Kokwaro (Rutaceae). Plants 2020, 9, 920. [Google Scholar] [CrossRef]
- Nguyen, T.H.V.; Tran, T.T.; Cam, T.I.; Pham, M.Q.; Pham, Q.L.; Vu, D.H.; Nguyen, X.N.; Chau, V.M.; Van, K.P. Alkaloids From Zanthoxylum Nitidum and Their Cytotoxic Activity. Nat. Prod. Commun. 2019, 14, 1934578X1984413. [Google Scholar] [CrossRef]
- Iwasaki, H.; Oku, H.; Takara, R.; Miyahira, H.; Hanashiro, K.; Yoshida, Y.; Kamada, Y.; Toyokawa, T.; Takara, K.; Inafuku, M. The Tumor Specific Cytotoxicity of Dihydronitidine from Toddalia asiatica Lam. Cancer Chemother. Pharmacol. 2006, 58, 451–459. [Google Scholar] [CrossRef]
- Chen, J.-J.; Fang, H.-Y.; Duh, C.-Y.; Chen, I.-S. New Indolopyridoquinazoline, Benzo[c]phenanthridines and Cytotoxic Constituents from Zanthoxylum integrifoliolum. Planta Med. 2005, 71, 470–475. [Google Scholar] [CrossRef]
- Phetkul, U.; Hayiawae, N.; Khunthong, S.; Daus, M.; Voravuthikunchai, S.P.; Tamvapee, P.; Watanapokasin, R.; Chakthong, S. Zanthoisobutylamides A–C: Rare Dimeric C-6 Substituent Dihydrobenzophenanthridine Alkaloids from the Roots of Zanthoxylum Nitidum. Nat. Prod. Res. 2021, 37, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Wangensteen, H.; Ho, G.T.T.; Tadesse, M.; Miles, C.O.; Moussavi, N.; Mikolo, B.; Malterud, K.E. A New Benzophenanthridine Alkaloid and Other Bioactive Constituents from the Stem Bark of Zanthoxylum heitzii. Fitoterapia 2016, 109, 196–200. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Damen, F.; Çelik, İ.; Tane, P.; Kuete, V.; Efferth, T. Cytotoxicity of the Crude Extract and Constituents of the Bark of Fagara tessmannii towards Multi-Factorial Drug Resistant Cancer Cells. J. Ethnopharmacol. 2019, 235, 28–37. [Google Scholar] [CrossRef]
- Nakanishi, T.; Masuda, A.; Suwa, M.; Akiyama, Y.; Hoshino-Abe, N.; Suzuki, M. Synthesis of Derivatives of NK109, 7-OH Benzo[c]Phenanthridine Alkaloid, and Evaluation of Their Cytotoxicities and Reduction-Resistant Properties. Bioorg. Med. Chem. Lett. 2000, 10, 2321–2323. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Suzuki, M.; Saimoto, A.; Kabasawa, T. Structural Considerations of NK109, an Antitumor Benzo[c]Phenanthridine Alkaloid. J. Nat. Prod. 1999, 62, 864–867. [Google Scholar] [CrossRef]
- Kanzawa, F.; Nishio, K.; Ishida, T.; Fukuda, M.; Kurokawa, H.; Fukumoto, H.; Nomoto, Y.; Fukuoka, K.; Bojanowski, K.; Saijo, N. Anti-Tumour Activities of a New Benzo[c]Phenanthridine Agent, 2,3-(Methylenedioxy)-5-Methyl-7-Hydroxy-8-Methoxybenzo[c]Phenanthridinium Hydrogensulphate Dihydrate (NK109), against Several Drug-Resistant Human Tumour Cell Lines. Br. J. Cancer 1997, 76, 571–581. [Google Scholar] [CrossRef]
- Cho, W.-J.; Yoo, S.-J.; Chung, B.-H.; Choi, B.-G.; Cheon, S.H.; Whang, S.-H.; Kim, S.-K.; Kang, B.-H.; Lee, C.-O. Synthesis of Benzo[c]Phenanthridine Derivatives and Theirin Vitro Antitumor Activities. Arch. Pharm. Res. 1996, 19, 321–325. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Z.; Zhang, J.; Wang, H.; Yin, S.; Du, J. 8-Acetonyldihydronitidine Inhibits the Proliferation of Human Colorectal Cancer Cells via Activation of P53. Eur. J. Pharmacol. 2019, 854, 256–264. [Google Scholar] [CrossRef]
- Hu, J.; Shi, X.; Chen, J.; Mao, X.; Zhu, L.; Yu, L.; Shi, J. Alkaloids from Toddalia asiatica and Their Cytotoxic, Antimicrobial and Antifungal Activities. Food Chem. 2014, 148, 437–444. [Google Scholar] [CrossRef]
- Tang, W.-L.; Zhang, Y.; Hu, D.-X.; Yang, H.; Yu, Q.; Chen, J.-W.; Agama, K.; Pommier, Y.; An, L.-K. Synthesis and Biological Evaluation of 5-Aminoethyl Benzophenanthridone Derivatives as DNA Topoisomerase IB Inhibitors. Eur. J. Med. Chem. 2019, 178, 81–92. [Google Scholar] [CrossRef]
- Cosialls, E.; El Hage, R.; Dos Santos, L.; Gong, C.; Mehrpour, M.; Hamaï, A. Ferroptosis: Cancer Stem Cells Rely on Iron until “to Die for” It. Cells 2021, 10, 2981. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).