CCSD(T) Rotational Constants for Highly Challenging C5H2 Isomers—A Comparison between Theory and Experiment
Abstract
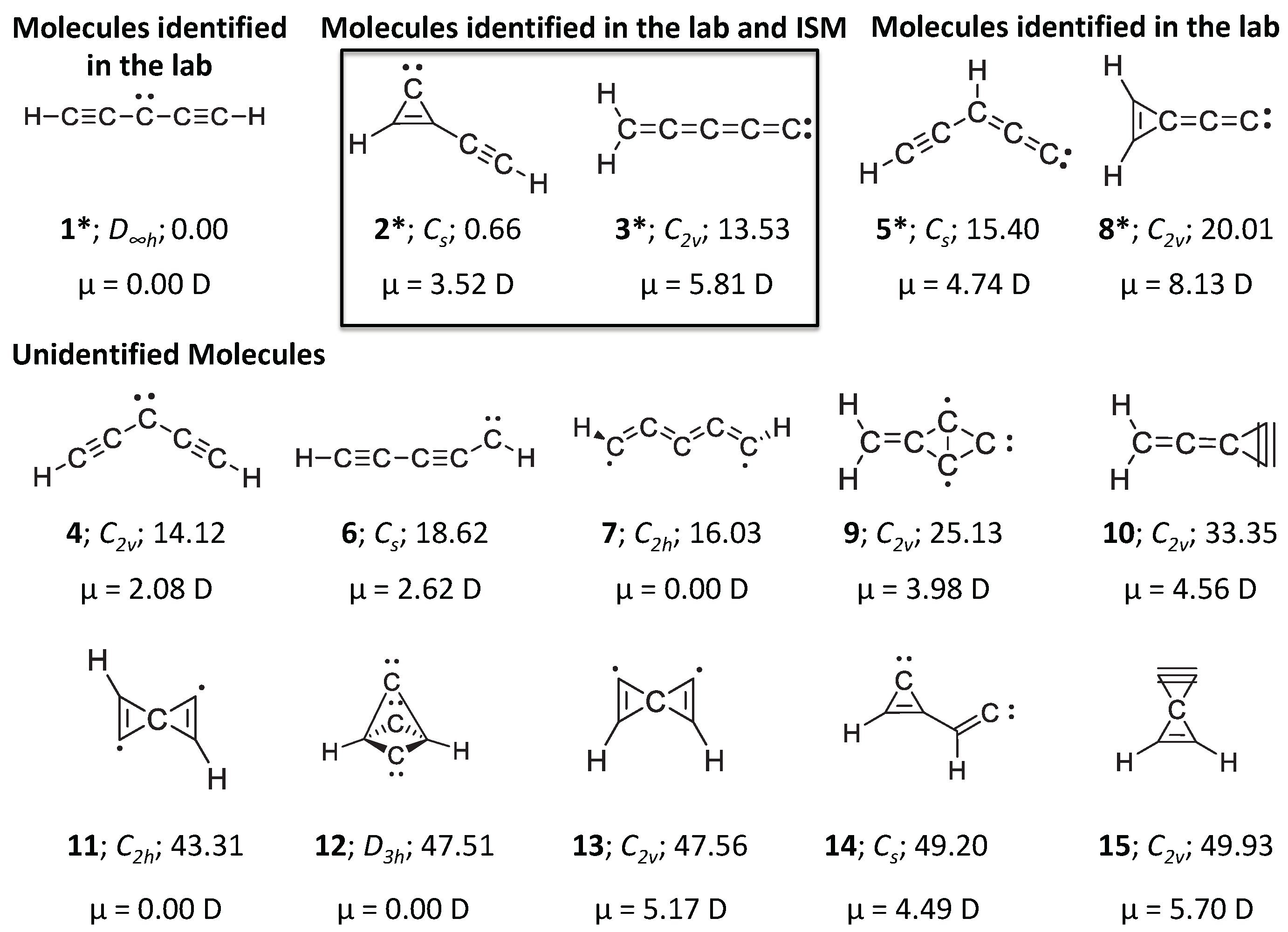
1. Introduction
2. Computational Details
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| DFT | Density functional theory |
| CC | CCSD(T), coupled-cluster singles, doubles including perturbative triples |
| ptC | Planar tetracoordinate carbon |
References
- Ekkers, J.; Flygare, W.H. Pulsed microwave Fourier transform spectrometer. Rev. Sci. Instruments 1976, 47, 448–454. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Travers, M.J.; Kovács, A.; Chen, W.; Novick, S.E.; Gottlieb, C.A.; Thaddeus, P. Detection and Characterization of the Cumulene Carbenes H2C5 and H2C6. Science 1997, 275, 518–520. [Google Scholar] [CrossRef]
- Seburg, R.A.; McMahon, R.J.; Stanton, J.F.; Gauss, J. Structures and Stabilities of C5H2 Isomers: Quantum Chemical Studies. J. Am. Chem. Soc. 1997, 119, 10838–10845. [Google Scholar] [CrossRef]
- Sattelmeyer, K.W.; Stanton, J.F. Computational Studies of C6H2 Isomers. J. Am. Chem. Soc. 2000, 122, 8220–8227. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Thaddeus, P. Laboratory Detection of a Bent-Chain Carbene Isomer of C6H2. Astrophys. J. Lett. 2002, 569, L55. [Google Scholar] [CrossRef]
- Travers, M.J.; McCarthy, M.C.; Gottlieb, C.A.; Thaddeus, P. Laboratory Detection of the Ring-Chain Molecule C5H2. Astrophys. J. 1997, 483, L135–L138. [Google Scholar] [CrossRef]
- Thirumoorthy, K.; Viji, M.; Pandey, A.P.; Netke, T.G.; Sekar, B.; Yadav, G.; Deshpande, S.; Thimmakondu, V.S. Many Unknowns Below or Close to the Experimentally Known Cumulene Carbene—A Case Study of C9H2 Isomers. Chem. Phys. 2019, 527, 110496. [Google Scholar] [CrossRef]
- Thirumoorthy, K.; Cooksy, A.; Thimmakondu, V.S. Si2C5H2 Isomers—Search Algorithms Versus Chemical Intuition. Phys. Chem. Chem. Phys. 2020, 22, 5865–5872. [Google Scholar] [CrossRef]
- Watrous, A.G.; Westbrook, B.R.; Fortenberry, R.C. Theoretical spectra and energetics for c-C3HC2H, l-C5H2, and bipyramidal D3h C5H2. Front. Astron. Space Sci. 2022, 9, 1051535. [Google Scholar] [CrossRef]
- Roy, T.; Ghosal, S.; Thimmakondu, V.S. Six Low-Lying Isomers of C11H8 Are Unidentified in the Laboratory—A Theoretical Study. J. Phys. Chem. A 2021, 125, 4352–4364. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.S.; Peeters, E.; Cami, J.; Schmidt, T.W. Open questions on carbon-based molecules in space. Commun. Chem. 2022, 5, 94. [Google Scholar] [CrossRef]
- Vega-Vega, A.; Largo, A.; Redondo, P.; Barrientos, C. Structure and Spectroscopic Properties of [Mg,C,N,O] Isomers: Plausible Astronomical Molecules. ACS Earth Space Chem. 2017, 1, 158–167. [Google Scholar] [CrossRef]
- Tuli, L.B.; Goettl, S.J.; Turner, A.M.; Howlader, A.H.; Hemberger, P.; Wnuk, S.F.; Guo, T.; Mebel, A.M.; Kaiser, R.I. Gas phase synthesis of the C40 nano bowl C40H10. Nat. Commun. 2023, 14, 1527. [Google Scholar] [CrossRef]
- Thimmakondu, V.S.; Karton, A. Energetic and Spectroscopic Properties of the Low-Lying C7H2 Isomers: A High-Level Ab Initio Perspective. Phys. Chem. Chem. Phys. 2017, 19, 17685–17697. [Google Scholar] [CrossRef]
- Thirumoorthy, K.; Karton, A.; Thimmakondu, V.S. From High-Energy C7H2 Isomers with A Planar Tetracoordinate Carbon Atom to An Experimentally Known Carbene. J. Phys. Chem. A 2018, 122, 9054–9064. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Sivadasan, D.; Job, N.; Sinjari, A.; Thirumoorthy, K.; Anoop, A.; Thimmakondu, V.S. Why Are MgC3H Isomers Missing in the Interstellar Medium? J. Phys. Chem. A 2022, 126, 4465–4475. [Google Scholar] [CrossRef]
- Puzzarini, C.; Heckert, M.; Gauss, J. The Accuracy of Rotational Constants Predicted by High-Level Quantum-Chemical Calculations. I. Molecules Containing First-Row Atoms. J. Chem. Phys. 2008, 128, 194108. [Google Scholar] [CrossRef] [PubMed]
- Puzzarini, C.; Stanton, J.F.; Gauss, J. Quantum-Chemical Calculation of Spectroscopic Parameters for Rotational Spectroscopy. Int. Rev. Phys. Chem. 2010, 29, 273–367. [Google Scholar] [CrossRef]
- Puzzarini, C.; Stanton, J.F. Connections between the accuracy of rotational constants and equilibrium molecular structures. Phys. Chem. Chem. Phys. 2023, 25, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Fulara, J.; Freivogel, P.; Forney, D.; Maier, J.P. Electronic Absorption Spectra of Linear Carbon Chains in Neon Matrices. III. HC2n+1H. J. Chem. Phys. 1995, 103, 8805–8810. [Google Scholar] [CrossRef]
- Blanksby, S.J.; Dua, S.; Bowie, J.H.; Schröder, D.; Schwarz, H. Gas-Phase Syntheses of Three Isomeric C5H2 Radical Anions and Their Elusive Neutrals. A Joint Experimental and Theoretical Study. J. Phys. Chem. A 1998, 102, 9949–9956. [Google Scholar] [CrossRef]
- Bowling, N.P.; Halter, R.J.; Hodges, J.A.; Seburg, R.A.; Thomas, P.S.; Simmons, C.S.; Stanton, J.F.; McMahon, R.J. Reactive Carbon-Chain Molecules: Synthesis of 1-Diazo-2,4-pentadiyne and Spectroscopic Characterization of Triplet Pentadiynylidene (H-C-C--C-C-H). J. Am. Chem. Soc. 2006, 128, 3291–3302. [Google Scholar] [CrossRef]
- Sun, Y.L.; Huang, W.J.; Lee, S.H. Formation of C3H2, C5H2, C7H2, and C9H2 From Reactions of CH, C3H, C5H, and C7H Radicals with C2H2. Phys. Chem. Chem. Phys. 2016, 18, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Steglich, M.; Fulara, J.; Maity, S.; Nagy, A.; Maier, J.P. Electronic Spectra of Linear HC5H and Cumulene Carbene H2C5. J. Chem. Phys. 2015, 142, 244311. [Google Scholar] [CrossRef]
- Reusch, E.; Kaiser, D.; Schleier, D.; Buschmann, R.; Krueger, A.; Hermann, T.; Engels, B.; Fischer, I.; Hemberger, P. Pentadiynylidene and Its Methyl-Substituted Derivates: Threshold Photoelectron Spectroscopy of R1-C5-R2 Triplet Carbon Chains. J. Phys. Chem. A 2019, 123, 2008–2017. [Google Scholar] [CrossRef]
- He, C.; Galimova, G.R.; Luo, Y.; Zhao, L.; Eckhardt, A.K.; Sun, R.; Mebel, A.M.; Kaiser, R.I. A Chemical Dynamics Study on the Gas-Phase Formation of Triplet and Singlet C5H2 Carbenes. Proc. Natl. Acad. Sci. USA 2020, 117, 30142–30150. [Google Scholar] [CrossRef]
- Gottlieb, C.A.; McCarthy, M.C.; Gordon, V.D.; Chakan, J.M.; Apponi, A.J.; Thaddeus, P. Laboratory Detection of Two New C5H2 Isomers. Astrophys. J. 1998, 509, L141. [Google Scholar] [CrossRef]
- Thaddeus, P.; Vrtilek, J.M.; Gottlieb, C.A. Laboratory and Astronomical Identification of Cyclopropenylidene, C3H2. Astrophys. J. 1985, 299, L63–L66. [Google Scholar] [CrossRef]
- Cernicharo, J.; Gottlieb, C.A.; Guelin, M.; Killian, T.C.; Paubert, G.; Thaddeus, P.; Vrtilek, J.M. Astronomical Detection of H2CCC. Astrophys. J. 1991, 368, L39–L41. [Google Scholar] [CrossRef]
- Vrtilek, J.M.; Gottlieb, A.; Gottlieb, E.W.; Killian, T.C.; Thaddeus, P. Laboratory Detection of Propadienylidene, H2CCC. Astrophys. J. 1990, 364, L53–L56. [Google Scholar] [CrossRef]
- Spezzano, S.; Brünken, S.; Schilke, P.; Caselli, P.; Menten, K.M.; McCarthy, M.C.; Bizzocchi, L.; Trevinõ-Morales, S.P.; Aikawa, Y.; Schlemmer, S. Interstellar Detection of c-C3D2. Astrophys. J. 2013, 769, L19. [Google Scholar] [CrossRef]
- Sipilä, O.; Spezzano, S.; Caselli, P. Understanding the C3H2 Cyclic-to-Linear Ratio in L1544. Astron. Astrophys. 2016, 591, L1. [Google Scholar] [CrossRef]
- Spezzano, S.; Caselli, P.; Bizzocchi, L.; Giuliano, B.M.; Lattanzi, V. The Observed Chemical Structure of L1544. Astron. Astrophys. 2017, 606, A82. [Google Scholar] [CrossRef]
- Chantzos, J.; Spezzano, S.; Caselli, P.; Chacón-Tanarro, A.; Bizzocchi, L.; Sipilä, O.; Giuliano, B.M. A Study of the c-C3HD/c-C3H2 Ratio in Low-mass Star-forming Regions. Astrophys. J. 2018, 863, 126. [Google Scholar] [CrossRef]
- Cabezas, C.; Tercero, B.; Agúndez, M.; Marcelino, N.; Pardo, J.R.; de Vicente, P.; Cernicharo, J. Cumulene Carbenes in TMC-1: Astronomical Discovery of l-H2C5. Astron. Astrophys. 2021, 650, L9. [Google Scholar] [CrossRef]
- Cernicharo, J.; Agúndez, M.; Cabezas, C.; Tercero, B.; Marcelino, N.; Pardo, J.R.; de Vicente, P. Pure Hydrocarbon Cycles in TMC-1: Discovery of Ethynyl Cyclopropenylidene, Cyclopentadiene, and Indene. Astron. Astrophys. 2021, 649, L15. [Google Scholar] [CrossRef]
- Mebel, A.M.; Kim, G.S.; Kislov, V.V.; Kaiser, R.I. The Reaction of Tricarbon with Acetylene: An Ab Initio/RRKM Study of the Potential Energy Surface and Product Branching Ratios. J. Phys. Chem. A 2007, 111, 6704–6712. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Guo, Y.; Mebel, A.M.; Kaiser, R.I. A Crossed Beam Investigation of the Reactions of Tricarbon Molecules, C3(), with Acetylene, C2H2(), Ethylene, C2H4(Ag), and Benzene, C6H6(A1g). Chem. Phys. Lett. 2007, 449, 44–52. [Google Scholar] [CrossRef]
- Sun, B.J.; Huang, C.Y.; Kuo, H.H.; Chen, K.T.; Sun, H.L.; Huang, C.H.; Tsai, M.F.; Kao, C.H.; Wang, Y.S.; Gao, L.G.; et al. Formation of Interstellar 2,4-pentadiynylidyne, HCCCCC(X2Π), via the Neutral-Neutral Reaction of Ground State Carbon Atom, C(3P), with Diacetylene, HCCCCH(). J. Chem. Phys. 2008, 128, 244303. [Google Scholar] [CrossRef]
- Hansen, N.; Klippenstein, S.J.; Miller, J.A.; Wang, J.; Cool, T.A.; Law, M.E.; Westmoreland, P.R.; Kasper, T.; Kohse-Höinghaus, K. Identification of C5Hx Isomers in Fuel-Rich Flames by Photoionization Mass Spectrometry and Electronic Structure Calculations. J. Phys. Chem. A 2006, 110, 4376–4388. [Google Scholar] [CrossRef] [PubMed]
- Thimmakondu, V.S.; Karton, A. The Quest for the Carbene Bent-pentadiynylidene Isomer of C5H2. Chem. Phys. 2018, 515, 411–417. [Google Scholar] [CrossRef]
- Thimmakondu, V.S.; Ulusoy, I.; Wilson, A.K.; Karton, A. Theoretical Studies of Two Key Low-Lying Carbenes of C5H2 Missing in the Laboratory. J. Phys. Chem. A 2019, 123, 6618–6627. [Google Scholar] [CrossRef] [PubMed]
- Karton, A.; Thimmakondu, V.S. From Molecules with a Planar Tetracoordinate Carbon to an Astronomically Known C5H2 Carbene. J. Phys. Chem. A 2022, 126, 2561–2568. [Google Scholar] [CrossRef]
- Fortenberry, R.C. The Formation of Astromolecule Ethynyl Cyclopropenylidene (c-C3HCCH) from C2H and c-C3H2. Astrophys. J. 2021, 921, 132. [Google Scholar] [CrossRef]
- Thimmakondu, V.S.; Thirumoorthy, K. Si3C2H2 Isomers with A Planar Tetracoordinate Carbon or Silicon Atom(s). Comput. Theor. Chem. 2019, 1157, 40–46. [Google Scholar] [CrossRef]
- Thirumoorthy, K.; Chandrasekaran, V.; Cooksy, A.L.; Thimmakondu, V.S. Kinetic Stability of Si2C5H2 Isomer with a Planar Tetracoordinate Carbon Atom. Chemistry 2021, 3, 13–27. [Google Scholar] [CrossRef]
- Thirumoorthy, K.; Thimmakondu, V.S. Flat Crown Ethers with Planar Tetracoordinate Carbon Atoms. Int. J. Quantum Chem. 2021, 121, e26479. [Google Scholar] [CrossRef]
- Job, N.; Khatun, M.; Thirumoorthy, K.; CH, S.S.R.; Chandrasekaran, V.; Anoop, A.; Thimmakondu, V.S. CAl4Mg0/-: Global Minima with a Planar Tetracoordinate Carbon Atom. Atoms 2021, 9, 24. [Google Scholar] [CrossRef]
- Satpati, S.; Roy, T.; Giri, S.; Anoop, A.; Thimmakondu, V.S.; Ghosal, S. Structure and Bonding Patterns in C5H4 Isomers: Pyramidane, Planar Tetracoordinate Carbon, and Spiro Molecules. Atoms 2023, 11, 96. [Google Scholar] [CrossRef]
- Raghavachari, K.; Trucks, G.W.; Pople, J.A.; Head-Gordon, M. A Fifth-Order Perturbation Comparison of Electron Correlation Theories. Chem. Phys. Lett. 1989, 157, 479–483. [Google Scholar] [CrossRef]
- Bartlett, R.J.; Watts, J.; Kucharski, S.; Noga, J. Non-Iterative Fifth-Order Triple and Quadruple Excitation Energy Corrections in Correlated Methods. Chem. Phys. Lett. 1990, 165, 513–522. [Google Scholar] [CrossRef]
- Stanton, J.F. Why CCSD(T) Works: A Different Perspective. Chem. Phys. Lett. 1997, 281, 130–134. [Google Scholar] [CrossRef]
- Raghavachari, K. Historical perspective on: A fifthorder perturbation comparison of electron correlation theories [Volume 157, Issue 6, 26 May 1989, Pages 479–483]. Chem. Phys. Lett. 2013, 589, 35. [Google Scholar] [CrossRef]
- Karton, A. Quantum mechanical thermochemical predictions 100 years after the Schrodinger equation. Annu. Rep. Comput. Chem. 2022, 18, 123–166. [Google Scholar]
- Dunning, T.H. Gaussian Basis Sets for Use In Correlated Molecular Calculations. I. The Atoms Boron Through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula Into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys. 2006, 125, 194101. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theo. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Boese, A.D.; Handy, N.C. New exchange-correlation density functionals: The role of the kinetic-energy density. J. Chem. Phys. 2002, 116, 9559–9569. [Google Scholar] [CrossRef]
- Staroverov, V.N.; Scuseria, G.E.; Tao, J.; Perdew, J.P. Comparative assessment of a new nonempirical density functional: Molecules and hydrogen-bonded complexes. J. Chem. Phys. 2003, 119, 12129–12137. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Boese, A.D.; Martin, J.M.L. Development of density functionals for thermochemical kinetics. J. Chem. Phys. 2004, 121, 3405–3416. [Google Scholar] [CrossRef]
- Chai, J.D.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom–Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical hybrid density functional with perturbative second-order correlation. J. Chem. Phys. 2006, 124, 034108. [Google Scholar] [CrossRef]
- Kozuch, S.; Martin, J.M.L. DSD-PBEP86: In search of the best double-hybrid DFT with spin-component scaled MP2 and dispersion corrections. Phys. Chem. Chem. Phys. 2011, 13, 20104–20107. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Density functional theory with London dispersion corrections. WIREs Comput. Mol. Sci. 2011, 1, 211–228. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D.; Johnson, E.R. Exchange-Hole Dipole Moment and the Dispersion Interaction. J. Chem. Phys. 2005, 122, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Purvis, G.D.; Bartlett, R.J. A Full Coupled-Cluster Singles and Doubles Model: The Inclusion of Disconnected Triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Stanton, J.F.; Gauss, J.; Watts, J.D.; Bartlett, R.J. A Direct Product Decomposition Approach for Symmetry Exploitation in Many-Body Methods. I. Energy Calculations. J. Chem. Phys. 1991, 94, 4334–4345. [Google Scholar] [CrossRef]
- Prascher, B.P.; Woon, D.E.; Peterson, K.A.; Dunning, T.H.; Wilson, A.K. Gaussian Basis Sets for Use in Correlated Molecular Calculations. VII. Valence, Core-valence, and Scalar Relativistic Basis sets for Li, Be, Na, and Mg. Theor. Chem. Acc. 2011, 128, 69–82. [Google Scholar] [CrossRef]
- Peterson, K.A.; Dunning, T.H. Accurate Correlation Consistent Basis Sets for Molecular Core-Valence Correlation Effects: The Second Row Atoms Al-Ar, and the First Row Atoms B-Ne Revisited. J. Chem. Phys. 2002, 117, 10548–10560. [Google Scholar] [CrossRef]
- Spackman, P.R.; Jayatilaka, D.; Karton, A. Basis Set Convergence of CCSD(T) Equilibrium Geometries Using a Large and Diverse Set of Molecular Structures. J. Chem. Phys. 2016, 145, 104101. [Google Scholar] [CrossRef]
- Gauss, J.; Stanton, J.F. Analytic CCSD(T) Second Derivatives. Chem. Phys. Lett. 1997, 276, 70–77. [Google Scholar] [CrossRef]
- Stanton, J.F.; Gauss, J.; Cheng, L.; Harding, M.E.; Matthews, D.A.; Szalay, P.G. CFOUR, Coupled-Cluster Techniques for Computational Chemistry, a Quantum-Chemical Program Package. With Contributions from A.A. Auer, R.J. Bartlett, U. Benedikt, C. Berger, D.E. Bernholdt, Y.J. Bomble, O. Christiansen, F. Engel, R. Faber, M. Heckert, O. Heun, M. Hilgenberg, C. Huber, T.-C. Jagau, D. Jonsson, J. Jusélius, T. Kirsch, K. Klein, W.J. Lauderdale, F. Lipparini, T. Metzroth, L.A. Mück, D.P. O’Neill, D.R. Price, E. Prochnow, C. Puzzarini, K. Ruud, F. Schiffmann, W. Schwalbach, C. Simmons, S. Stopkowicz, A. Tajti, J. Vázquez, F. Wang, J.D. Watts and the Integral Packages MOLECULE (J. Almlöf and P.R. Taylor), PROPS (P.R. Taylor), ABACUS (T. Helgaker, H.J. Aa. Jensen, P. Jørgensen, and J. Olsen), and ECP Routines by A. V. Mitin and C. van Wüllen. Available online: http://www.cfour.de (accessed on 15 July 2023).
- Perdew, J.P.; Schmidt, K. Jacob’s ladder of density functional approximations for the exchange-correlation energy. AIP Conf. Proc. 2000, 577, 1. [Google Scholar]
- Ceselin, G.; Salta, Z.; Bloino, J.; Tasinato, N.; Barone, V. Accurate Quantum Chemical Spectroscopic Characterization of Glycolic Acid: A Route Toward its Astrophysical Detection. J. Phys. Chem. A 2022, 126, 2373–2387. [Google Scholar] [CrossRef]
- Ceselin, G.; Barone, V.; Tasinato, N. Accurate Biomolecular Structures by the Nano-LEGO Approach: Pick the Bricks and Build Your Geometry. J. Chem. Theory Comput. 2021, 17, 7290–7311. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Ceselin, G.; Fuse, M.; Tasinato, N. Accuracy Meets Interpretability for Computational Spectroscopy by Means of Hybrid and Double-Hybrid Functionals. Front. Chem. 2020, 8, 584203. [Google Scholar] [CrossRef]
- Boussessi, R.; Ceselin, G.; Tasinato, N.; Barone, V. DFT meets the segmented polarization consistent basis sets: Performances in the computation of molecular structures, rotational and vibrational spectroscopic properties. J. Mol. Struct. 2020, 1208, 127886. [Google Scholar] [CrossRef]
- Karton, A.; Spackman, P.R. Evaluation of Density Functional Theory for A Large and Diverse Set of Organic and Inorganic Equilibrium Structures. J. Comput. Chem. 2021, 42, 1590–1601. [Google Scholar] [CrossRef]
- Martin, J.; Santra, G. Empirical Double-Hybrid Density Functional Theory: A ’Third Way’ In Between WFT and DFT. Isr. J. Chem. 2020, 60, 787–804. [Google Scholar] [CrossRef]
- DePinto, J.T.; McMahon, R.J. Structure and rearrangements of 1,3-diphenylpropynylidene. J. Am. Chem. Soc. 1993, 115, 12573–12574. [Google Scholar] [CrossRef]
- DePinto, J.T.; deProphetis, W.A.; Menke, J.L.; McMahon, R.J. Triplet 1,3-Diphenylpropynylidene (Ph-C-C-C-Ph). J. Am. Chem. Soc. 2007, 129, 2308–2315. [Google Scholar] [CrossRef] [PubMed]
- Sateesh, B.; Srinivas Reddy, A.; Narahari Sastry, G. Towards Design of the Smallest Planar Tetracoordinate Carbon and Boron Systems. J. Comput. Chem. 2007, 28, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Priyakumar, U.; Reddy, A.; Sastry, G. The Design of Molecules Containing Planar Tetracoordinate Carbon. Tetrahedron Lett. 2004, 45, 2495–2498. [Google Scholar] [CrossRef]
| Rotational Constants | % Error | ||||||
|---|---|---|---|---|---|---|---|
| Isomer | Level of the Theory | ||||||
| 2 | Semi-Experimental | 36,972.92 | 3448.64 | 3154.31 | - | - | - |
| fc-CC/VDZ | 33,109.37 | 3309.59 | 3008.83 | −10.45 | −4.03 | −4.61 | |
| fc-CC/VTZ | 34,266.70 | 3394.14 | 3088.25 | −7.32 | −1.58 | −2.09 | |
| fc-CC/VQZ | 34,547.62 | 3409.12 | 3102.93 | −6.56 | −1.15 | −1.63 | |
| ae-CC/VDZ | 33,184.61 | 3315.35 | 3014.21 | −10.25 | −3.87 | −4.44 | |
| ae-CC/VTZ | 34,640.92 | 3429.06 | 3120.20 | −6.31 | −0.57 | −1.08 | |
| ae-CC/CVTZ | 34,465.55 | 3408.64 | 3101.86 | −6.78 | −1.16 | −1.66 | |
| ae-CC/CVTZ | 34,544.69 | 3414.01 | 3106.95 | −6.57 | −1.00 | −1.50 | |
| 3 | Semi-Experimental | 231,551.05 e | 2216.88 | 2283.64 | - | - | - |
| fc-CC/VDZ | 282,533.83 | 2219.13 | 2201.84 | N/A | 0.10 | −3.58 | |
| fc-CC/VTZ | 289,957.56 | 2280.08 | 2262.29 | N/A | 2.85 | −0.93 | |
| fc-CC/VQZ | 290,263.96 | 2289.74 | 2271.82 | N/A | 3.29 | −0.52 | |
| ae-CC/VDZ | 283,066.03 | 2223.09 | 2205.77 | N/A | 0.28 | −3.41 | |
| ae-CC/VTZ | 292,400.34 | 2302.87 | 2284.87 | N/A | 3.88 | 0.05 | |
| ae-CC/CVTZ | 290,592.49 | 2289.69 | 2271.79 | N/A | 3.28 | −0.52 | |
| ae-CC/wCVTZ | 290,836.71 | 2293.17 | 2275.23 | N/A | 3.44 | −0.37 | |
| ae-CC/CVQZ | 291,115.51 | 2300.31 | 2282.27 | N/A | 3.76 | −0.06 | |
| 5 | Semi-Experimental | 31,151.23 | 2863.13 | 2621.54 | - | - | - |
| fc-CC/VDZ | 30,282.06 | 2769.12 | 2537.12 | −2.79 | −3.28 | −3.22 | |
| fc-CC/VTZ | 31,364.94 | 2832.59 | 2597.97 | 0.69 | −1.07 | −0.90 | |
| fc-CC/VQZ | 31,248.01 | 2850.63 | 2612.32 | 0.31 | −0.44 | −0.35 | |
| ae-CC/VDZ | 30,321.18 | 2774.08 | 2541.55 | −2.66 | −3.11 | −3.05 | |
| ae-CC/VTZ | 32,467.69 | 2842.48 | 2613.66 | 4.23 | −0.72 | −0.30 | |
| ae-CC/CVTZ | 31,402.16 | 2846.75 | 2610.13 | 0.81 | −0.57 | −0.44 | |
| ae-CC/wCVTZ | 31,431.40 | 2851.69 | 2614.48 | 0.90 | −0.40 | −0.27 | |
| 8 | Semi-Experimental | 34,494.31 | 3515.75 | 3202.60 | - | - | - |
| fc-CC/VDZ | 31,020.01 | 3398.87 | 3063.23 | −10.07 | −3.32 | −4.35 | |
| fc-CC/VTZ | 31,836.35 | 3492.11 | 3146.92 | −7.71 | −0.67 | −1.74 | |
| fc-CC/VQZ | 31,978.63 | 3509.58 | 3162.50 | −7.29 | −0.18 | −1.25 | |
| ae-CC/VDZ | 31,070.60 | 3405.23 | 3068.89 | −9.93 | −3.14 | −4.18 | |
| ae-CC/VTZ | 32,080.69 | 3528.59 | 3178.94 | −7.00 | 0.37 | −0.74 | |
| ae-CC/CVTZ | 31,979.91 | 3508.69 | 3161.79 | −7.29 | −0.20 | −1.27 | |
| ae-CC/wCVTZ | 32,036.11 | 3514.77 | 3167.28 | −7.13 | −0.03 | −1.10 | |
| ae-CC/CVQZ | 32,122.26 | 3526.79 | 3177.88 | −6.88 | 0.31 | −0.77 | |
| % Error | ||||
|---|---|---|---|---|
| Isomer | Level of the Theory | |||
| 2 | fc-CC/VDZ | −4.16 | −2.92 | −3.03 |
| fc-CC/VTZ | −0.81 | −0.44 | −0.47 | |
| ae-CC/VDZ | −3.94 | −2.89 | −2.98 | |
| ae-CC/VTZ | 0.28 | 0.44 | 0.43 | |
| ae-CC/CVTZ | −0.23 | −0.16 | −0.16 | |
| 3 | fc-CC/VDZ | −2.66 | −3.08 | −3.08 |
| fc-CC/VTZ | −0.11 | −0.42 | −0.42 | |
| ae-CC/VDZ | −2.77 | −3.36 | −3.35 | |
| ae-CC/VTZ | 0.44 | 0.11 | 0.11 | |
| ae-CC/CVTZ | −0.18 | −0.46 | −0.46 | |
| ae-CC/wCVTZ | −0.10 | −0.31 | −0.31 | |
| 5 | fc-CC/VDZ | −3.09 | −2.86 | −2.88 |
| fc-CC/VTZ | 0.37 | −0.63 | −0.55 | |
| ae-CC/VDZ | −3.53 | −2.72 | −2.79 | |
| ae-CC/VTZ | 3.30 | −0.32 | −0.03 | |
| ae-CC/CVTZ | −0.09 | −0.17 | −0.17 | |
| 8 | fc-CC/VDZ | −3.00 | −3.15 | −3.14 |
| fc-CC/VTZ | −0.44 | −0.50 | −0.49 | |
| ae-CC/VDZ | −3.27 | −3.45 | −3.43 | |
| ae-CC/VTZ | −0.13 | 0.05 | 0.03 | |
| ae-CC/CVTZ | −0.44 | −0.51 | −0.51 | |
| ae-CC/wCVTZ | −0.27 | −0.34 | −0.33 | |
| Rotational Constants | % Error | ||||||
|---|---|---|---|---|---|---|---|
| Isomer | Level of the Theory | ||||||
| 2 | PBE-D3BJ | 34,776.97 | 3410.3 | 3105.75 | 0.67 | −0.11 | −0.04 |
| BLYP-D3BJ | 34,772.95 | 3406.65 | 3102.68 | 0.66 | −0.22 | −0.14 | |
| TPSS-D3BJ | 34,832.08 | 3421.18 | 3115.2 | 0.83 | 0.21 | 0.27 | |
| BMK-D3BJ | 34,305.88 | 3417.45 | 3107.86 | −0.69 | 0.10 | 0.03 | |
| B2-PLYP-D3BJ | 35,080.47 | 3437.28 | 3130.54 | 1.55 | 0.68 | 0.76 | |
| DSD-PBEP86-D3BJ | 34,906.49 | 3425.77 | 3119.61 | 1.05 | 0.34 | 0.41 | |
| 3 | PBE-D3BJ | 288,084.24 | 2289.71 | 2271.66 | −1.04 | −0.46 | −0.46 |
| BLYP-D3BJ | 290,215.02 | 2292.37 | 2274.41 | −0.31 | −0.35 | −0.34 | |
| TPSS-D3BJ | 291,046.32 | 2299.95 | 2281.92 | −0.02 | −0.02 | −0.02 | |
| BMK-D3BJ | 290,741.94 | 2324.26 | 2305.82 | −0.13 | 1.04 | 1.03 | |
| B2-PLYP-D3BJ | 293,012.38 | 2313.86 | 2295.74 | 0.65 | 0.59 | 0.59 | |
| DSD-PBEP86-D3BJ | 291,090.37 | 2305.76 | 2287.64 | −0.01 | 0.24 | 0.24 | |
| 5 | PBE-D3BJ | 31,828.92 | 2844.85 | 2611.44 | 1.26 | −0.24 | −0.12 |
| BLYP-D3BJ | 31,858.95 | 2844.08 | 2610.99 | 1.36 | −0.27 | −0.13 | |
| TPSS-D3BJ | 31,747.91 | 2858.87 | 2622.7 | 1.01 | 0.25 | 0.31 | |
| BMK-D3BJ | 31,631.84 | 2889.51 | 2647.65 | 0.64 | 1.33 | 1.27 | |
| B2-PLYP-D3BJ | 31,903.2 | 2873.29 | 2635.89 | 1.50 | 0.76 | 0.82 | |
| DSD-PBEP86-D3BJ | 31,573.48 | 2867.58 | 2628.82 | 0.45 | 0.56 | 0.55 | |
| 8 | PBE-D3BJ | 31,895.1 | 3495.39 | 3150.17 | −0.71 | −0.89 | −0.87 |
| BLYP-D3BJ | 31,951.11 | 3492.37 | 3148.26 | −0.53 | −0.98 | −0.93 | |
| TPSS-D3BJ | 32,043.94 | 3507.76 | 3161.66 | −0.24 | −0.54 | −0.51 | |
| BMK-D3BJ | 31,945.51 | 3514.9 | 3166.5 | −0.55 | −0.34 | −0.36 | |
| B2-PLYP-D3BJ | 32,223.32 | 3538.94 | 3188.73 | 0.31 | 0.34 | 0.34 | |
| DSD-PBEP86-D3BJ | 32,086.32 | 3531.32 | 3181.2 | −0.11 | 0.13 | 0.10 | |
| Isomer | Level of the Theory | |||
|---|---|---|---|---|
| 4 | fc-CC/VTZ | 44,468.31 | 2675.36 | 2523.54 |
| fc-CC/VQZ | 44,559.66 | 2687.42 | 2534.56 | |
| ae-CC/VTZ | 47,663.99 | 2670.19 | 2528.54 | |
| ae-CC/CVTZ | 44,964.29 | 2683.13 | 2532.04 | |
| ae-CC/wCVTZ | 45,248.67 | 2684.92 | 2534.53 | |
| ae-CC/CVQZ | 45,285.14 | 2693.43 | 2542.23 | |
| TPSS-D3BJ/VQZ | 415,092.95 | 2314.94 | 2302.10 | |
| DSD-PBEP86-D3BJ/VQZ | 48,334.30 | 2669.57 | 2529.84 | |
| 6 | fc-CC/VTZ | 575,100.98 | 2265.22 | 2256.33 |
| fc-CC/VQZ | 599,411.76 | 2273.66 | 2265.06 | |
| ae-CC/VTZ | 611,557.40 | 2287.33 | 2278.81 | |
| ae-CC/CVTZ | 572,323.22 | 2274.94 | 2265.93 | |
| ae-CC/wCVTZ | 526,553.84 | 2282.03 | 2272.19 | |
| TPSS-D3BJ/VQZ | 413,454.77 | 2315.19 | 2302.29 | |
| DSD-PBEP86-D3BJ/VQZ | 672,216.98 | 2287.43 | 2279.67 | |
| 9 | fc-CC/VTZ | 31,658.89 | 4569.62 | 3993.24 |
| fc-CC/VQZ | 31,908.98 | 4592.16 | 4014.43 | |
| ae-CC/VTZ | 32,178.66 | 4612.32 | 4034.09 | |
| ae-CC/CVTZ | 31,919.04 | 4589.89 | 4012.85 | |
| ae-CC/wCVTZ | 32,008.12 | 4598.06 | 4020.50 | |
| ae-CC/CVQZ | 32,169.05 | 4612.09 | 4033.77 | |
| TPSS-D3BJ/VQZ | 32,428.41 | 4572.42 | 4007.38 | |
| DSD-PBEP86-D3BJ/VQZ | 32,191.22 | 4616.16 | 4037.23 | |
| 13 | fc-CC/VTZ | 18,897.05 | 5445.32 | 4227.22 |
| fc-CC/VQZ | 19,024.44 | 5480.23 | 4254.63 | |
| ae-CC/VTZ | 19,101.44 | 5510.40 | 4276.67 | |
| ae-CC/CVTZ | 19,000.57 | 5475.68 | 4250.69 | |
| ae-CC/wCVTZ | 19,043.27 | 5484.32 | 4258.03 | |
| ae-CC/CVQZ | 19,130.16 | 5509.91 | 4277.81 | |
| TPSS-D3BJ/VQZ | 19,008.80 | 5482.72 | 4255.35 | |
| DSD-PBEP86-D3BJ/VQZ | 19,144.14 | 5535.55 | 4293.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thimmakondu, V.S.; Karton, A. CCSD(T) Rotational Constants for Highly Challenging C5H2 Isomers—A Comparison between Theory and Experiment. Molecules 2023, 28, 6537. https://doi.org/10.3390/molecules28186537
Thimmakondu VS, Karton A. CCSD(T) Rotational Constants for Highly Challenging C5H2 Isomers—A Comparison between Theory and Experiment. Molecules. 2023; 28(18):6537. https://doi.org/10.3390/molecules28186537
Chicago/Turabian StyleThimmakondu, Venkatesan S., and Amir Karton. 2023. "CCSD(T) Rotational Constants for Highly Challenging C5H2 Isomers—A Comparison between Theory and Experiment" Molecules 28, no. 18: 6537. https://doi.org/10.3390/molecules28186537
APA StyleThimmakondu, V. S., & Karton, A. (2023). CCSD(T) Rotational Constants for Highly Challenging C5H2 Isomers—A Comparison between Theory and Experiment. Molecules, 28(18), 6537. https://doi.org/10.3390/molecules28186537







