The Competition between 4-Nitrophenol Reduction and BH4− Hydrolysis on Metal Nanoparticle Catalysts
Abstract
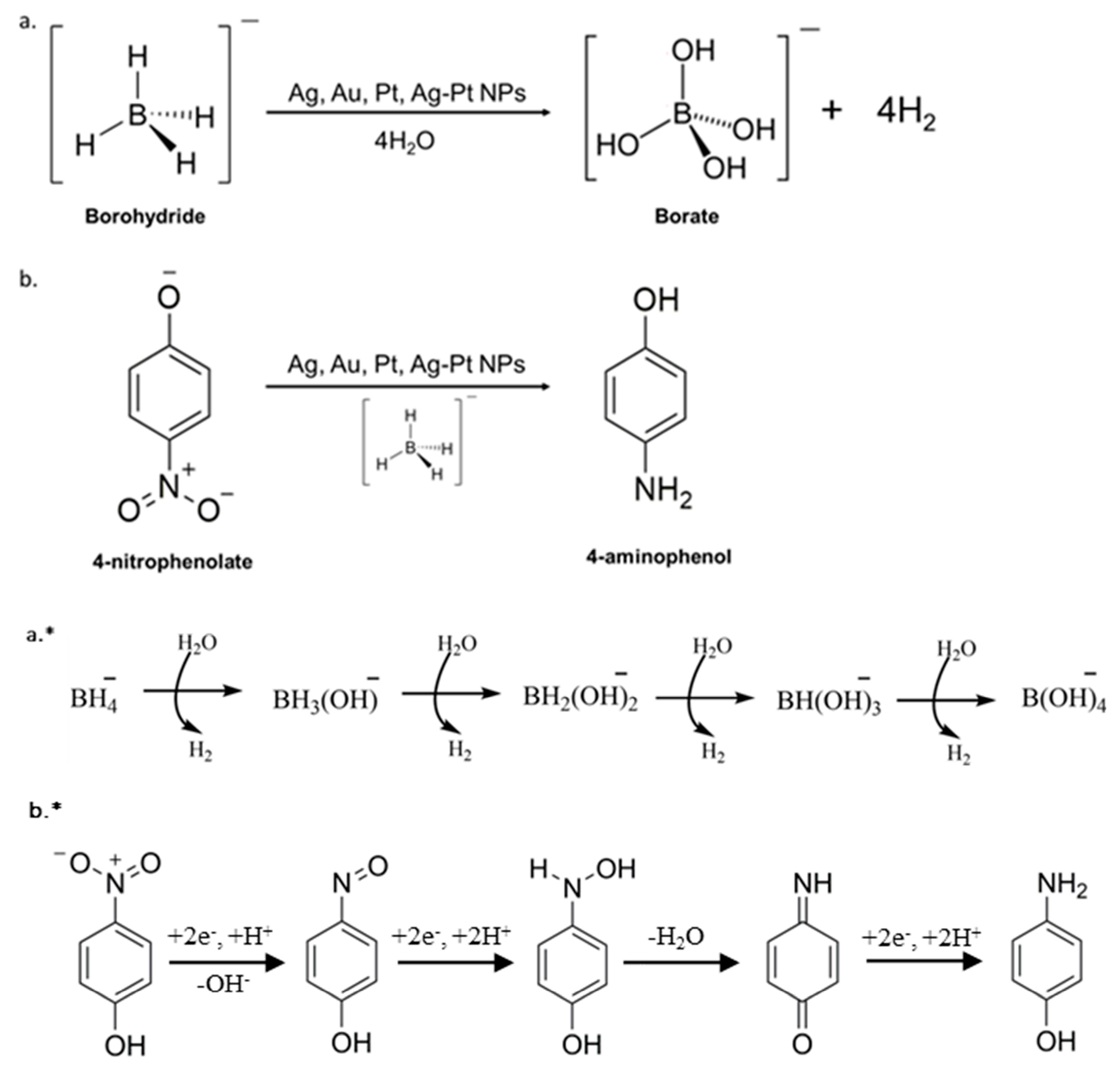
:1. Introduction
- (i)
- Reduction via adsorbed (hydrogen atoms)/hydrides formed during BHR on the surfaces of M0-NPs. This mechanism explains the large excess of BH4− required for these reductions.
- (ii)
- A direct reaction between an adsorbed BH4− and an adsorbed substrate. This mechanism can, in principle, involve an H. atom transfer, which would leave a BH3- radical anion that is expected to react with water to form the BH4− radical [25]. Alternatively, this reduction step might involve a hydride transfer, leaving a BH3 adsorbed to the surface.
2. Results and Discussions
2.1. Characterization
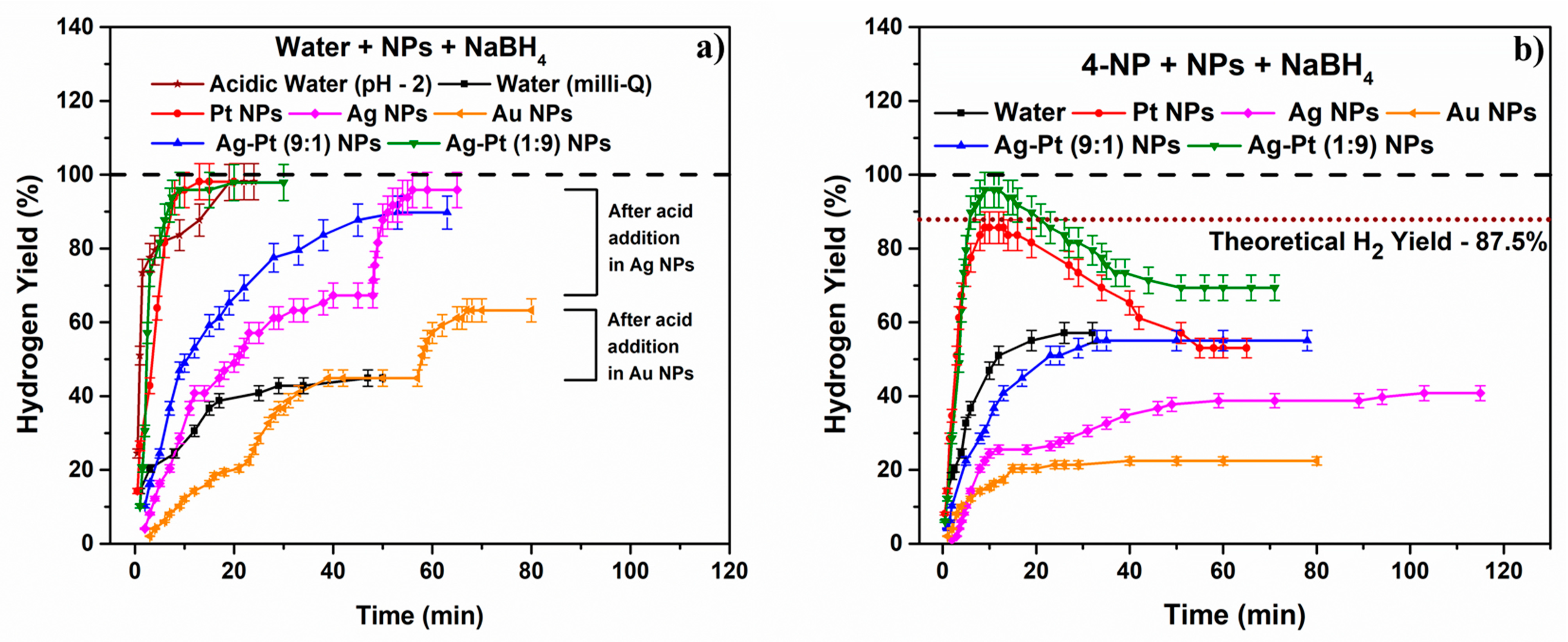
2.2. Sodium Borohydride Hydrolysis
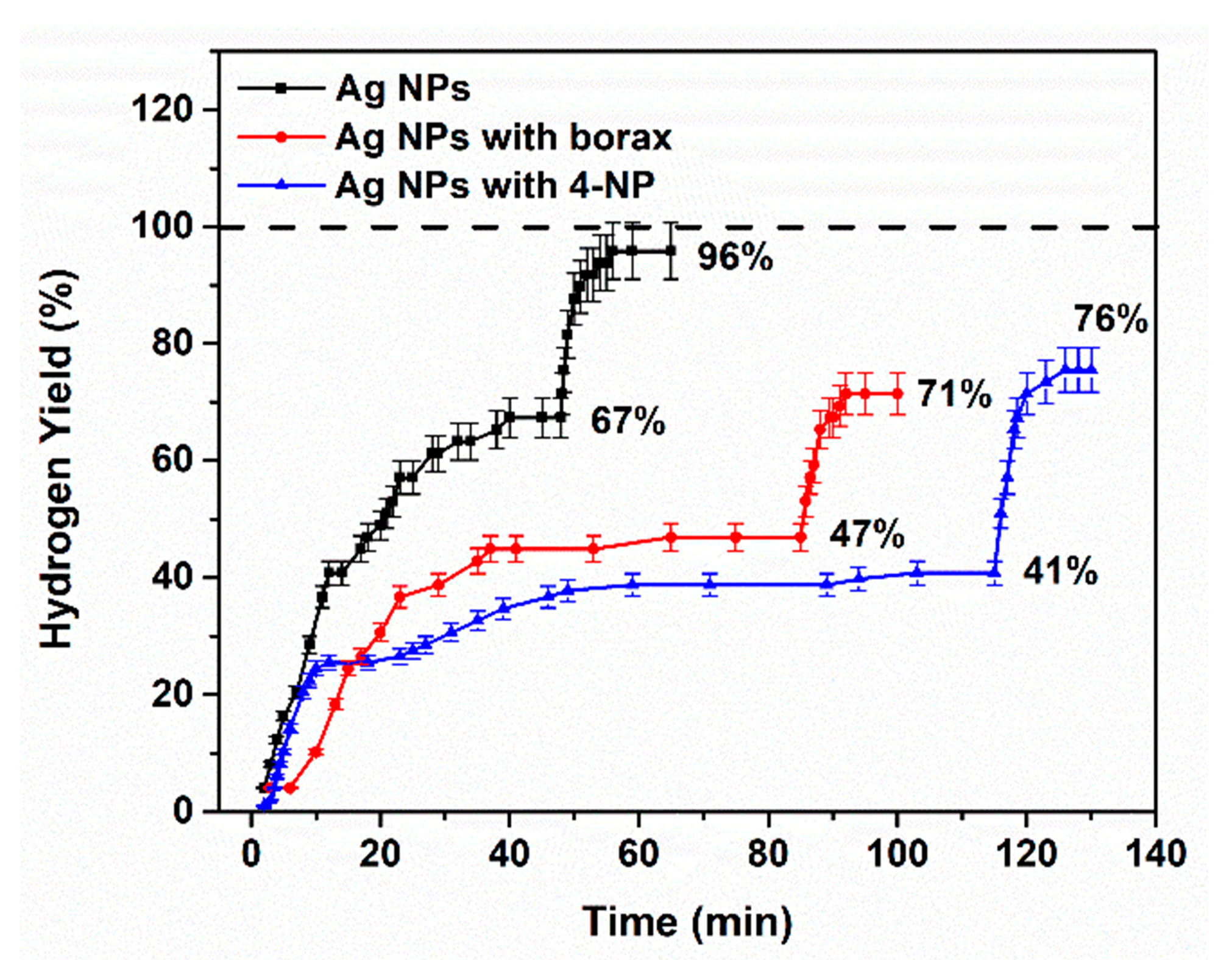
2.2.1. The Effect of pH
2.2.2. The Competitive Effect of 4-Nitrophenol on the Catalytic BHR at Various M0-NP and BM-NP Catalyst Surfaces
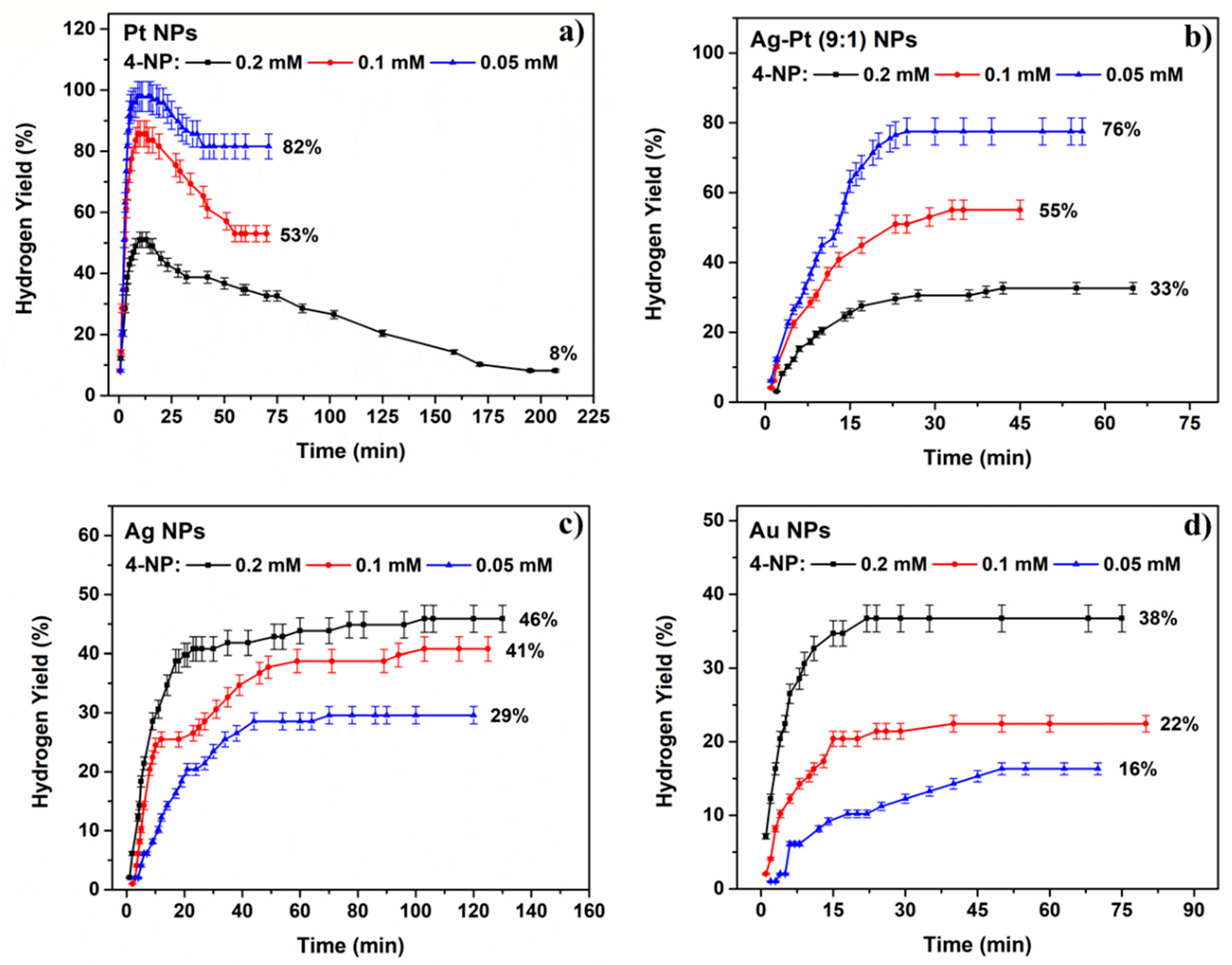
2.2.3. The Effect of 4-Nitrophenol Concentration
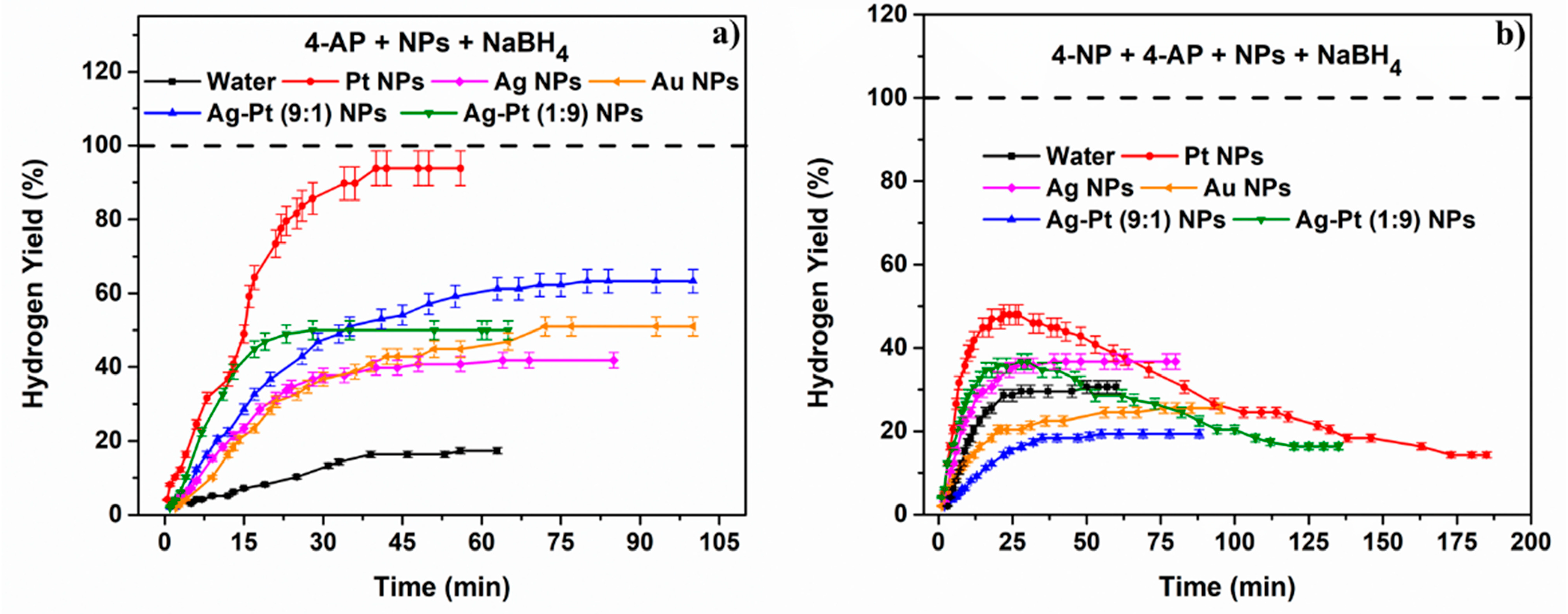
2.2.4. The Effect of the 4-Aminophenol Product
3. Materials and Methods
3.1. Materials
3.2. Methods and Instrumentation
3.3. Catalyst Preparation
3.4. Hydrogen Evolution Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Schlesinger, H.I.; Brown, H.C.; Finholt, A.E.; Gilbreath, J.R.; Hoekstra, H.R.; Hyde, E.K. Sodium Borohydride, Its Hydrolysis and Its Use as a Reducing Agent and in the Generation of Hydrogen. J. Am. Chem. Soc. 1953, 75, 215–219. [Google Scholar] [CrossRef]
- Karimi, B.; Shafiee Afarani, M.; Arabi, A.M. Hydrothermal Synthesis of Cadmium Selenide Quantum Dots: Effect of Reducing Agent. Appl. Phys. A Mater. Sci. Process. 2020, 126, 1–9. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, S.; Hosmane, N.S. Boron-Enriched Advanced Energy Materials. Inorganica Chim. Acta 2018, 471, 577–586. [Google Scholar] [CrossRef]
- Luo, C.; Fu, F.; Yang, X.; Wei, J.; Wang, C.; Zhu, J.; Huang, D.; Astruc, D.; Zhao, P. Highly Efficient and Selective Co@ZIF-8 Nanocatalyst for Hydrogen Release from Sodium Borohydride Hydrolysis. ChemCatChem 2019, 11, 1643–1649. [Google Scholar] [CrossRef]
- Lai, Q.; Alligier, D.; Aguey-Zinsou, K.F.; Demirci, U.B. Hydrogen Generation from a Sodium Borohydride-Nickel Core@shell Structure under Hydrolytic Conditions. Nanoscale Adv. 2019, 1, 2707–2717. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Fu, F.; Astruc, D. Hydrogen Generation upon Nanocatalyzed Hydrolysis of Hydrogen-Rich Boron Derivatives: Recent Developments. Acc. Chem. Res. 2020, 53, 2483–2493. [Google Scholar] [CrossRef]
- Patel, N.; Miotello, A. Progress in Co-B Related Catalyst for Hydrogen Production by Hydrolysis of Boron-Hydrides: A Review and the Perspectives to Substitute Noble Metals. Int. J. Hydrogen Energy 2015, 40, 1429–1464. [Google Scholar] [CrossRef]
- Guella, G.; Patton, B.; Miotello, A. Kinetic Features of the Platinum Catalyzed Hydrolysis of Sodium Borohydride from 11B NMR Measurements. J. Phys. Chem. C 2007, 111, 18744–18750. [Google Scholar] [CrossRef]
- Grzeschik, R.; Schäfer, D.; Holtum, T.; Küpper, S.; Hoffmann, A.; Schlücker, S. On the Overlooked Critical Role of the PH Value on the Kinetics of the 4-Nitrophenol NaBH4-Reduction Catalyzed by Noble-Metal Nanoparticles (Pt, Pd, and Au). J. Phys. Chem. C 2020, 124, 50. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X. Catalytic Hydrolysis of Sodium Borohydride for Hydrogen Production Using Magnetic Recyclable CoFe2O4-Modified Transition-Metal Nanoparticles. ACS Appl. Nano Mater. 2021, 4, 11312–11320. [Google Scholar] [CrossRef]
- Honnappa, B.; Mohan, S.; Shanmugam, M.; Augustin, A.; Sagayaraj, P.J.J.; Chuaicham, C.; Rajendran, S.; Hoang, T.K.A.; Sasaki, K.; Sekar, K. Transition Metal Quantum Dots for the Electrocatalytic Hydrogen Evolution Reaction: Recent Progresses and Challenges. Energy Adv. 2022, 1, 738. [Google Scholar] [CrossRef]
- Khan, S.B.; Khan, M.S.J.; Kamal, T.; Asiri, A.M.; Bakhsh, E.M. Polymer Supported Metallic Nanoparticles as a Solid Catalyst for the Removal of Organic Pollutants. Cellulose 2020, 27, 5907–5921. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic Concepts and Recent Advances in Nitrophenol Reduction by Gold- and Other Transition Metal Nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Visentin, C.; da Trentin, A.W.S.; Braun, A.B.; Thomé, A. Life Cycle Sustainability Assessment of the Nanoscale Zero-Valent Iron Synthesis Process for Application in Contaminated Site Remediation. Environ. Pollut. 2021, 268, 115915. [Google Scholar] [CrossRef] [PubMed]
- Ishag, A.; Li, Y.; Zhang, N.; Wang, H.; Guo, H.; Mei, P.; Sun, Y. Environmental Application of Emerging Zero-Valent Iron-Based Materials on Removal of Radionuclides from the Wastewater: A Review. Environ. Res. 2020, 188, 109855. [Google Scholar] [CrossRef] [PubMed]
- Varshney, S.; Bar-Ziv, R.; Zidki, T. On the Remarkable Performance of Silver-based Alloy Nanoparticles in 4-nitrophenol Catalytic Reduction. ChemCatChem 2020, 12, 4680–4688. [Google Scholar] [CrossRef]
- Khan, S.B. Metal Nanoparticles Containing Chitosan Wrapped Cellulose Nanocomposites for Catalytic Hydrogen Production and Reduction of Environmental Pollutants. Carbohydr. Polym. 2020, 242, 116286. [Google Scholar] [CrossRef]
- Manno, R.; Sebastian, V.; Irusta, S.; Mallada, R.; Santamaria, J. Ultra-Small Silver Nanoparticles Immobilized in Mesoporous SBA-15. Microwave-Assisted Synthesis and Catalytic Activity in the 4-Nitrophenol Reduction. Catal. Today 2021, 362, 81–89. [Google Scholar] [CrossRef]
- Bogireddy, N.K.R.; Ghafour El Hachimi, A.; Muñiz, J.; Elías, A.L.; Lei, Y.; Terrones, M.; Agarwal, V. Integration of Nitrogen-Doped Graphene Oxide Dots with Au Nanoparticles for Enhanced Electrocatalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2021, 4, 11513–11525. [Google Scholar] [CrossRef]
- Jin, Q.; Lu, B.; Pan, Y.; Tao, X.; Himmelhaver, C.; Shen, Y.; Gu, S.; Zeng, Y.; Li, X.J. Novel Porous Ceramic Sheet Supported Metal Reactors for Continuous-Flow Catalysis. Catal. Today 2020, 358, 324–332. [Google Scholar] [CrossRef]
- Liu, G.F.; Qiao, X.X.; Cai, Y.L.; Xu, J.Y.; Yan, Y.; Karadeniz, B.; Lü, J.; Cao, R. Aluminum Metal-Organic Framework-Silver Nanoparticle Composites for Catalytic Reduction of Nitrophenols. ACS Appl. Nano Mater. 2020, 3, 11426–11433. [Google Scholar] [CrossRef]
- Mondal, T.; Sermiagin, A.; Meyerstein, D.; Zidki, T.; Kornweitz, H. On the Mechanism of Reduction of M(H2O)mN+ by Borohydride: The Case of Ag(H2O)2+. Nanoscale 2020, 12, 1657–1672. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, N.; Ameta, R.; Meena, R.K. Biogenic Silver Nanoparticles from Trachyspermum Ammi (Ajwain) Seeds Extract for Catalytic Reduction of p-Nitrophenol to p-Aminophenol in Excess of NaBH4. J. Mol. Liq. 2017, 230, 74–84. [Google Scholar] [CrossRef]
- Sermiagin, A.; Meyerstein, D.; Bar-Ziv, R.; Zidki, T. The Chemical Properties of Hydrogen Atoms Adsorbed on Mo-Nanoparticles Suspended in Aqueous Solutions: The Case of Ago-NPs and Auo-NPs Reduced by BD4−. Angew. Chemie Int. Ed. 2018, 57, 16525–16528. [Google Scholar] [CrossRef] [PubMed]
- Karimadom, B.R.; Varshney, S.; Zidki, T.; Meyerstein, D.; Kornweitz, H. DFT Study of the BH4− Hydrolysis on Au(111) Surface. ChemPhysChem 2022, 23, e202200069. [Google Scholar] [CrossRef]
- Xia, M.; Mao, C.; Guo, Y.; Pu, L.; Zhang, Y. Successive Degradation of Organophosphate Nerve Agent by Integrating the Merits of Artificial Enzyme and Metal Nanoparticle Catalyst. Colloids Interface Sci. Commun. 2021, 41, 100382. [Google Scholar] [CrossRef]
- Chinnappan, A.; Eshkalak, S.K.; Baskar, C.; Khatibzadeh, M.; Kowsari, E.; Ramakrishna, S. Flower-like 3-Dimensional Hierarchical Co3O4/NiO Microspheres for 4-Nitrophenol Reduction Reaction. Nanoscale Adv. 2019, 1, 305–313. [Google Scholar] [CrossRef]
- Hervés, P.; Pérez-Lorenzo, M.; Liz-Marzán, L.M.; Dzubiella, J.; Lu, Y.; Ballauff, M. Catalysis by Metallic Nanoparticles in Aqueous Solution: Model Reactions. Chem. Soc. Rev. 2012, 41, 5577–5587. [Google Scholar] [CrossRef]
- Gu, S.; Wunder, S.; Lu, Y.; Ballauff, M.; Fenger, R.; Rademann, K.; Jaquet, B.; Zaccone, A. Kinetic Analysis of the Catalytic Reduction of 4-Nitrophenol by Metallic Nanoparticles. J. Phys. Chem. C 2014, 118, 18618–18625. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, S.; Zhang, Y.; Wang, L.; Liu, Y.; Duan, Q. One-Pot Facile Synthesis of Noble Metal Nanoparticles Supported on RGO with Enhanced Catalytic Performance for 4-Nitrophenol Reduction. Molecules 2021, 26, 7261. [Google Scholar] [CrossRef]
- Zidki, T.; Hänel, A.; Bar-Ziv, R. Reactions of Methyl Radicals with Silica Supported Silver Nanoparticles in Aqueous Solutions. Radiat. Phys. Chem. 2016, 124, 41–45. [Google Scholar] [CrossRef]
- Rolly, G.S.; Meyerstein, D.; Yardeni, G.; Bar-Ziv, R.; Zidki, T. New Insights into HER Catalysis: The Effect of Nano-Silica Support on Catalysis by Silver Nanoparticles. Phys. Chem. Chem. Phys. 2020, 22, 6401–6405. [Google Scholar] [CrossRef] [PubMed]
- Alegria, E.C.B.A.; Ribeiro, A.P.C.; Mendes, M.; Ferraria, A.M.; Botelho do Rego, A.M.; Pombeiro, A.J.L. Effect of Phenolic Compounds on the Synthesis of Gold Nanoparticles and Its Catalytic Activity in the Reduction of Nitro Compounds. Nanomaterials 2018, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Akhade, S.A.; Chen, X.; Liu, Y.; Lee, M.-S.; Glezakou, V.-A.; Rousseau, R.; Lercher, J.A. The Nature of Hydrogen Adsorption on Platinum in the Aqueous Phase. Angew. Chem. Int. Ed. 2019, 58, 3527–3532. [Google Scholar] [CrossRef]
- Haynes, W.; Lide, D.; Bruno, T. Handbook of Chemistry and Physics, 97th ed.; CRC: Boca Raton, FL, USA, 2017; Volume 2016–2017. [Google Scholar]
- Wang, Q.; Fu, F.; Yang, S.; Martinez Moro, M.; Ramirez, M.D.L.A.; Moya, S.; Salmon, L.; Ruiz, J.; Astruc, D. Dramatic Synergy in CoPt Nanocatalysts Stabilized by “Click” Dendrimers for Evolution of Hydrogen from Hydrolysis of Ammonia Borane. ACS Catal. 2019, 9, 1110–1119. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, K.; Wang, D.; Meng, W.; Qi, N.; Cao, Z.; Zhang, K.; Chen, H.; Li, G. Highly Efficient Hydrogen Evolution from the Hydrolysis of Ammonia Borane Solution with the Co–Mo–B/NF Nanocatalyst. Renew. Energy 2020, 154, 453–460. [Google Scholar] [CrossRef]
- Coşkuner Filiz, B.; Kantürk Figen, A.; Pişkin, S. The Remarkable Role of Metal Promoters on the Catalytic Activity of Co-Cu Based Nanoparticles for Boosting Hydrogen Evolution: Ammonia Borane Hydrolysis. Appl. Catal. B Environ. 2018, 238, 365–380. [Google Scholar] [CrossRef]
- Yao, K.; Zhao, C.; Wang, N.; Li, T.; Lu, W.; Wang, J. An Aqueous Synthesis of Porous PtPd Nanoparticles with Reversed Bimetallic Structures for Highly Efficient Hydrogen Generation from Ammonia Borane Hydrolysis †. Nanoscale 2019, 12, 638–647. [Google Scholar] [CrossRef]
- Mehdi, S.; Liu, Y.; Wei, H.; Wen, H.; Shen, R.; Peng, Z.; Zhang, H.; Wu, X.; Wang, C.; Guan, S.; et al. Co-Based Nanoparticles Fabricated on Ni Foams for Efficient Hydrogen Generation from Ammonia Borane. ACS Appl. Nano Mater. 2022, 5, 5064–5074. [Google Scholar] [CrossRef]
- Yao, W.; Wang, J.; Zhong, A.; Wang, S.; Shao, Y. Transition-Metal-Free Catalytic Hydroboration Reduction of Amides to Amines. Org. Chem. Front. 2020, 7, 3515–3520. [Google Scholar] [CrossRef]
- Yao, W.; He, L.; Han, D.; Zhong, A. Sodium Triethylborohydride-Catalyzed Controlled Reduction of Unactivated Amides to Secondary or Tertiary Amines. J. Org. Chem. 2019, 84, 14627–14635. [Google Scholar] [CrossRef]
- Yao, W.; Wang, J.; Zhong, A.; Li, J.; Yang, J. Combined KOH/BEt3 Catalyst for Selective Deaminative Hydroboration of Aromatic Carboxamides for Construction of Luminophores. Org. Lett. 2020, 22, 8086–8090. [Google Scholar] [CrossRef]
- Creighton, J.A.; Blatchford, C.G.; Albrecht, M.G. Plasma Resonance Enhancement of Raman Scattering by Pyridine Adsorbed on Silver or Gold Sol Particles of Size Comparable to the Excitation Wavelength. J. Chem. Soc. 1979, 75, 790–798. [Google Scholar] [CrossRef]
- Zidki, T.; Cohen, H.; Meyerstein, D. Photochemical Induced Growth and Aggregation of Metal Nanoparticles in Diode-Array Spectrophotometer via Excited Dimethyl-Sulfoxide. Phys. Chem. Chem. Phys. 2010, 12, 12862–12867. [Google Scholar] [CrossRef]
- Rodriguez, Y.; Mejía, M.; Kumar, N.; Bogireddy, R. Reduction of 4-Nitrophenol Using Green-Fabricated Metal Nanoparticles. RSC Adv. 2022, 12, 18661–18675. [Google Scholar] [CrossRef]
- Strachan, J.; Barnett, C.; Masters, A.F.; Maschmeyer, T. 4-Nitrophenol Reduction: Probing the Putative Mechanism of the Model Reaction. ACS Catal. 2020, 10, 5516–5521. [Google Scholar] [CrossRef]
| Absence of 4-NP a | Presence of 4-NP b | 4-NP-Red c | |
|---|---|---|---|
| Sample | k(BHR)obs × 10−3 s−1 | k(BHR)obs × 10−3 s−1 | k(Red)obs × 10−3 s−1 |
| Pt | 3.8 | 5.9 | 0.8 |
| Ag-Pt (1:9) | 8.2 | 4.5 | 2.7 |
| Ag-Pt (9:1) | 1.4 | 1.6 | 59 |
| Ag | 1.1 | 1.2 | 13.2 |
| Au | 0.46 | 2.2 | 4.9 |
| Water | 1.3 | 2.4 | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varshney, S.; Meyerstein, D.; Bar-Ziv, R.; Zidki, T. The Competition between 4-Nitrophenol Reduction and BH4− Hydrolysis on Metal Nanoparticle Catalysts. Molecules 2023, 28, 6530. https://doi.org/10.3390/molecules28186530
Varshney S, Meyerstein D, Bar-Ziv R, Zidki T. The Competition between 4-Nitrophenol Reduction and BH4− Hydrolysis on Metal Nanoparticle Catalysts. Molecules. 2023; 28(18):6530. https://doi.org/10.3390/molecules28186530
Chicago/Turabian StyleVarshney, Shalaka, Dan Meyerstein, Ronen Bar-Ziv, and Tomer Zidki. 2023. "The Competition between 4-Nitrophenol Reduction and BH4− Hydrolysis on Metal Nanoparticle Catalysts" Molecules 28, no. 18: 6530. https://doi.org/10.3390/molecules28186530
APA StyleVarshney, S., Meyerstein, D., Bar-Ziv, R., & Zidki, T. (2023). The Competition between 4-Nitrophenol Reduction and BH4− Hydrolysis on Metal Nanoparticle Catalysts. Molecules, 28(18), 6530. https://doi.org/10.3390/molecules28186530








