Flavones and Related Compounds: Synthesis and Biological Activity
Abstract
1. Introduction
2. Biological Activity of Flavones, Flavonols, and Aurones
2.1. Anticancer Activity
| Entry | Chemical Structure | Cancer Cell Lines against the Tested Compounds Present Cytotoxic Activity | Ref. |
|---|---|---|---|
| 1 | 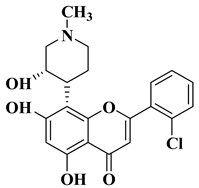 Flavopiridol Flavopiridol |
| [44] |
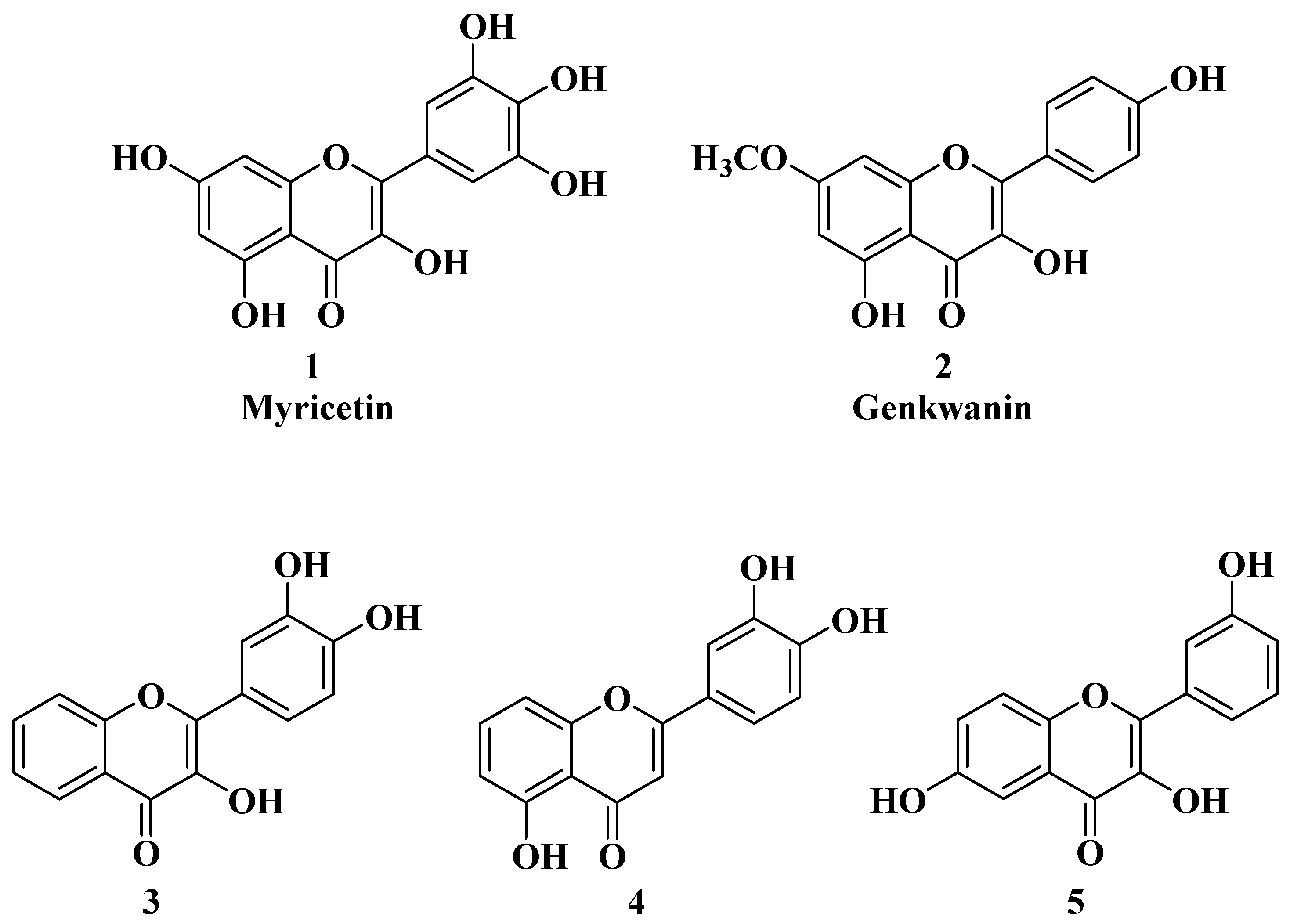
| 2 | 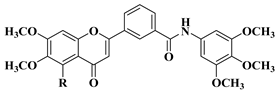 | R = OCH3
| [30] |
R = OH
| |||
| 3 | 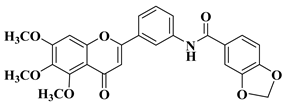 |
| [30] |
| 4 | 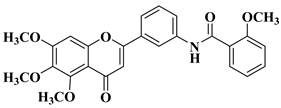 |
| [30] |
| 5 |  |
| [31] |
| 6 | 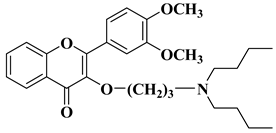 |
| [32] |
| 7 | 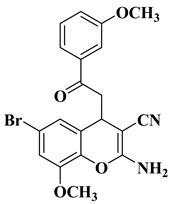 |
| [10] |
| 8 | 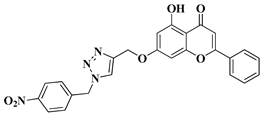 |
| [33] |
| 9 | 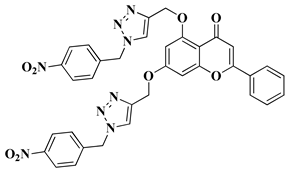 |
| [33] |
| 10 | 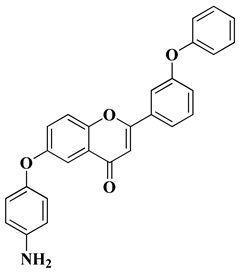 |
| [34] |
| 11 | 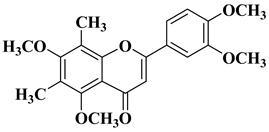 |
| [35] |
| 12 | 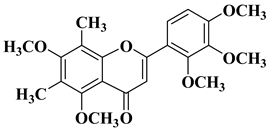 |
| [35] |
| 13 | 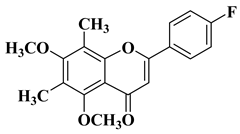 |
| [35] |
| 14 | 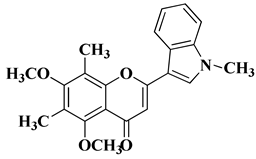 |
| [35] |
| 15 | 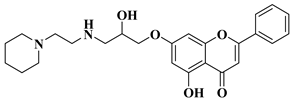 |
| [45] |
| 16 | 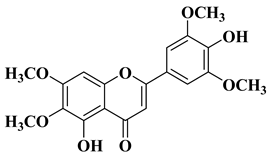 |
| [46] |
| 17 | 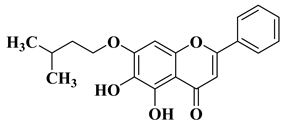 |
| [11] |
| 18 | 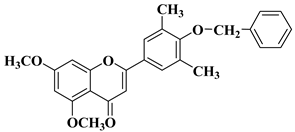 |
| [47] |
| 19 | 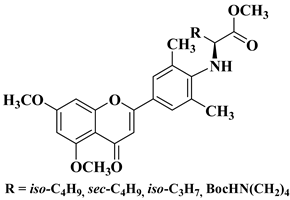 |
| [47] |
| 20 | 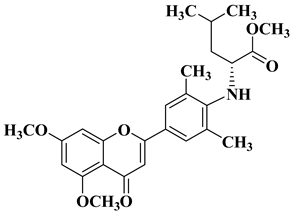 |
| [47] |
| 21 | 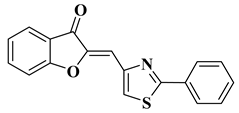 |
| [43] |
| 22 | 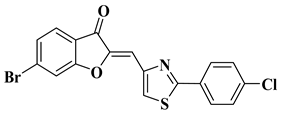 |
| [43] |
| 23 | 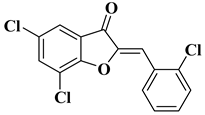 |
| [48] |
| 24 |  |
| [48] |
| 25 |  |
| [49] |
| 26 | 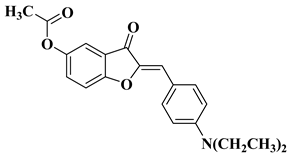 |
| [49] |
| 27 |  |
| [50] |
| 28 | 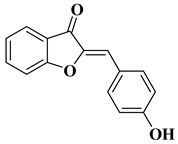 |
| [51] |
| 29 | 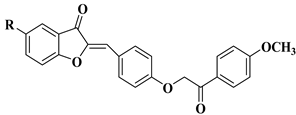 | R = Cl: leukemia cell lines MOLT-4 (−17.79% mean growth percentage), and SR (−22.38% mean growth percentage). R = H: renal cancer cell line UO-31 (−44.36% mean growth percentage). The mean growth percentages were determined for five concentrations ranging from 10−4 to 10−8 M. | [52] |
| 30 | 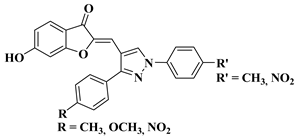 |
R′ = NO2 and R = NO2 (IC50 = 25.1 µM). | [53] |
| 31 | 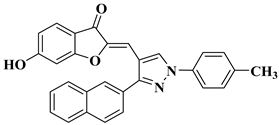 |
| [53] |
2.2. Antibacterial and Antifungal Activity
2.3. Antiviral Activity
| Entry | Chemical Structure | Microbial Strains against the Tested Compounds Present Antimicrobial Activity | Ref. |
|---|---|---|---|
| 1 | 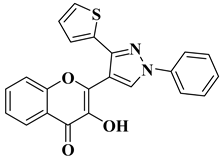 | Antifungal activity (inhibition zone for 50 μg/mL solution): Aspergillus niger (IZ = 16 mm) Penicillium italicum (IZ = 20 mm) Fusarium oxysporum (IZ = 31 mm) | [55] |
| 2 | 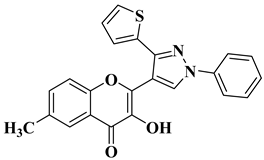 | Antibacterial activity (inhibition zone for 50 μg/mL solution): Staphylococcus aureus (IZ = 31 mm) Pseudomonas aeruginosa (IZ = 11 mm) Escherichia coli (IZ = 30 mm) | [55] |
| 3 | 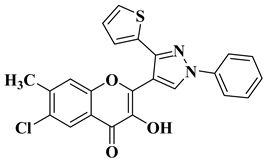 | Antibacterial activity (inhibition zone for 50 μg/mL solution): Staphylococcus aureus (IZ = 30 mm) Bacillus subtilis (IZ = 11 mm) Escherichia coli (IZ = 31 mm) Antifungal activity (inhibition zone for 50 μg/mL solution): Aspergillus niger (IZ = 13 mm) Penicillium italicum (IZ = 24 mm) Fusarium oxysporum (IZ = 25 mm) | [55] |
| 4 |  | Antibacterial activity (inhibition zone for 50 μg/mL solution): Staphylococcus aureus (IZ = 33 mm) Bacillus subtilis (IZ = 17 mm) Escherichia coli (IZ = 33 mm) Antifungal activity (inhibition zone for 50 μg/mL solution): Aspergillus niger (IZ = 14 mm) Penicillium italicum (IZ = 26 mm) Fusarium oxysporum (IZ = 27 mm) | [55] |
| 5 | 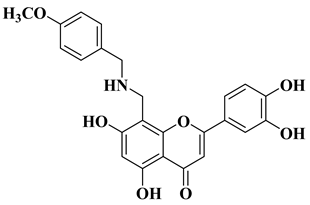 | Antibacterial activity: Staphylococcus aureus (MIC = 2 mg/L) Escherichia coli (MIC = 4 mg/L) Salmonella gallinarum (MIC = 0.125 mg/L) | [56] |
| 6 | 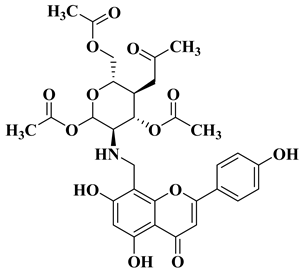 | Antibacterial activity: Staphylococcus aureus (MIC = 1 mg/L) Escherichia coli (MIC = 2 mg/L) Salmonella gallinarum (MIC = 0.05 mg/L) Listeria monocytogenes (MIC = 0.5 mg/L) | [56] |
| 7 | 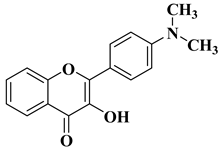 | Antifungal activity (percentage inhibition at 0.25 mg/mL and, respectively 0.5 mg/mL concentration): Acremonium strictum (81.33%; 100%) Penicillium expansum (60.87%; 100%) Aspergillus flavus (41.02%; 65.64%) | [57] |
| 8 |  | Antifungal activity (percentage inhibition at 0.25 mg/mL and, respectively 0.5 mg/mL concentration): Acremonium strictum (70%; 100%) Penicillium expansum (42.15%; 100%) Aspergillus flavus (6.41%; 46.15%) | [57] |
| 9 | 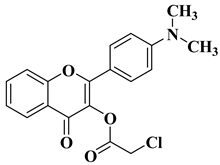 | Antifungal activity (percentage inhibition at 0.25 mg/mL and, respectively 0.5 mg/mL concentration): Acremonium strictum (76.88%; 100%) Aspergillus flavus (15.38%; 60.51%) | [57] |
| 10 | 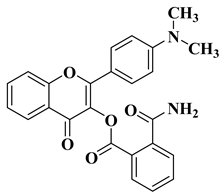 | Antifungal activity (percentage inhibition at 0.25 mg/mL and, respectively 0.5 mg/mL concentration): Acremonium strictum (73.33%; 100%) | [57] |
| 11 |  | Antibacterial activity: Staphylococcus aureus (MIC = 1.25 mg/mL) Bacillus subtilis (MIC = 0.02 mg/mL) Mycobacterium smegmatis (MIC = 0.625 mg/mL) Antifungal activity: Fusarium oxysporum (MIC = 0.625 mg/mL) | [58] |
| 12 | 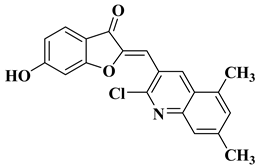 | Antibacterial activity: Staphylococcus aureus (MIC = 2.5 mg/mL) Bacillus subtilis (MIC = 0.156 mg/mL) Mycobacterium smegmatis (MIC = 0.078 mg/mL) Anti biofilm and anti quorum sensing activity (100 μg/mL) Antifungal activity: Fusarium oxysporum (MIC = 0.313 mg/mL) Candida albicans (MIC = 0.078 mg/mL) | [58] |
| 13 | 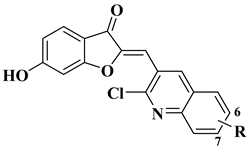 | Antibacterial activity (R=6-OCH3, 7-Cl) Staphylococcus aureus (MIC = 1.25 mg/mL) Bacillus subtilis (MIC = 1.25 mg/mL) Klebsiella pneumoniae (MIC = 0.625 mg/mL) Anti biofilm activity (R=6-OCH3, 100 μg/mL) Antifungal activity (R=7-Cl): Candida albicans (MIC = 0.156 mg/mL) | [58] |
| 14 | 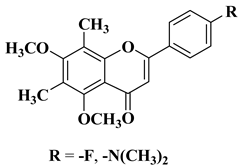 | Antibacterial activity Mycobacterium tuberculosis H37Rv (MIC = 6.25 µg/mL) | [35] |
| 15 | 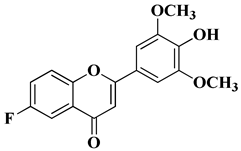 | Antiviral activity Human cytomegalovirus (EC50 = 0.126 nM) | [60] |
| 16 | 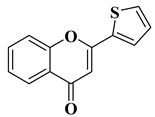 | Antiviral activity Chikungunya Virus (IC50 = 0.44 µM) | [61] |
| 17 |  | Antiviral activity Chikungunya Virus (IC50 = 0.45 µM) | [61] |
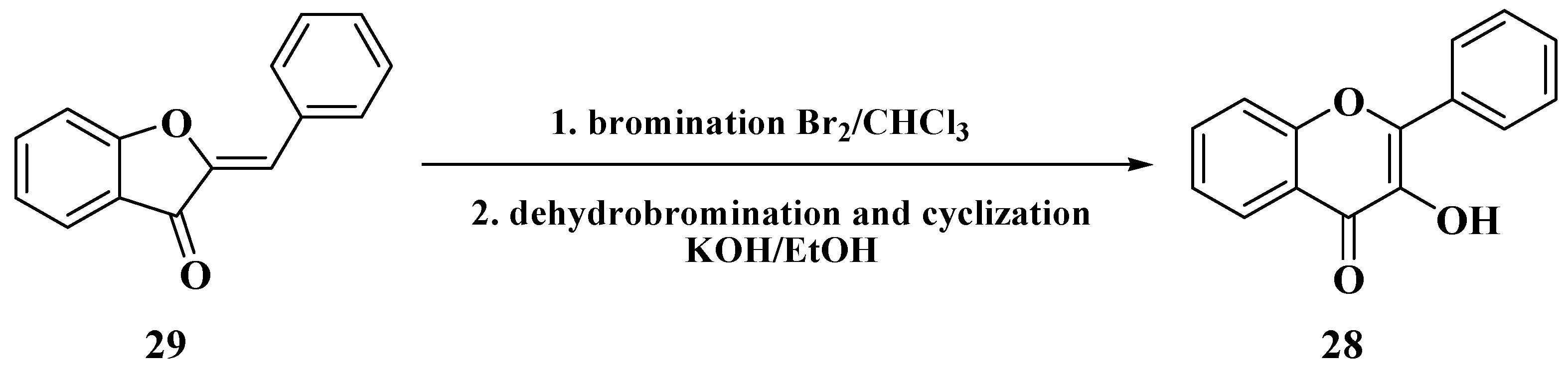
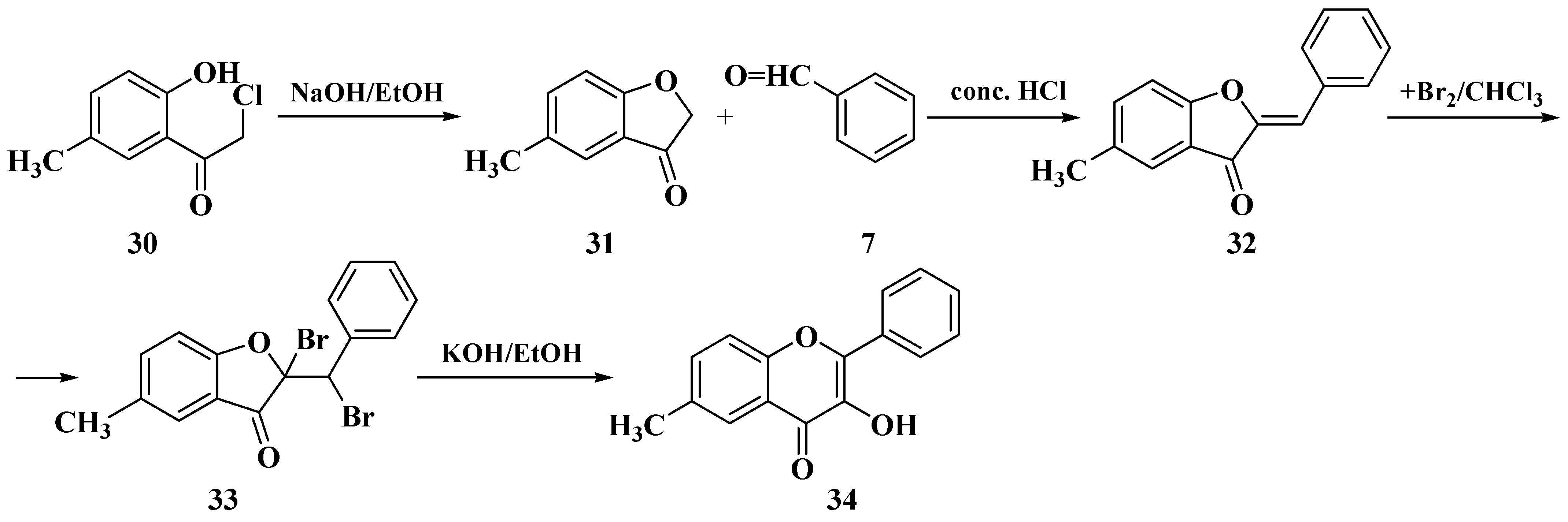
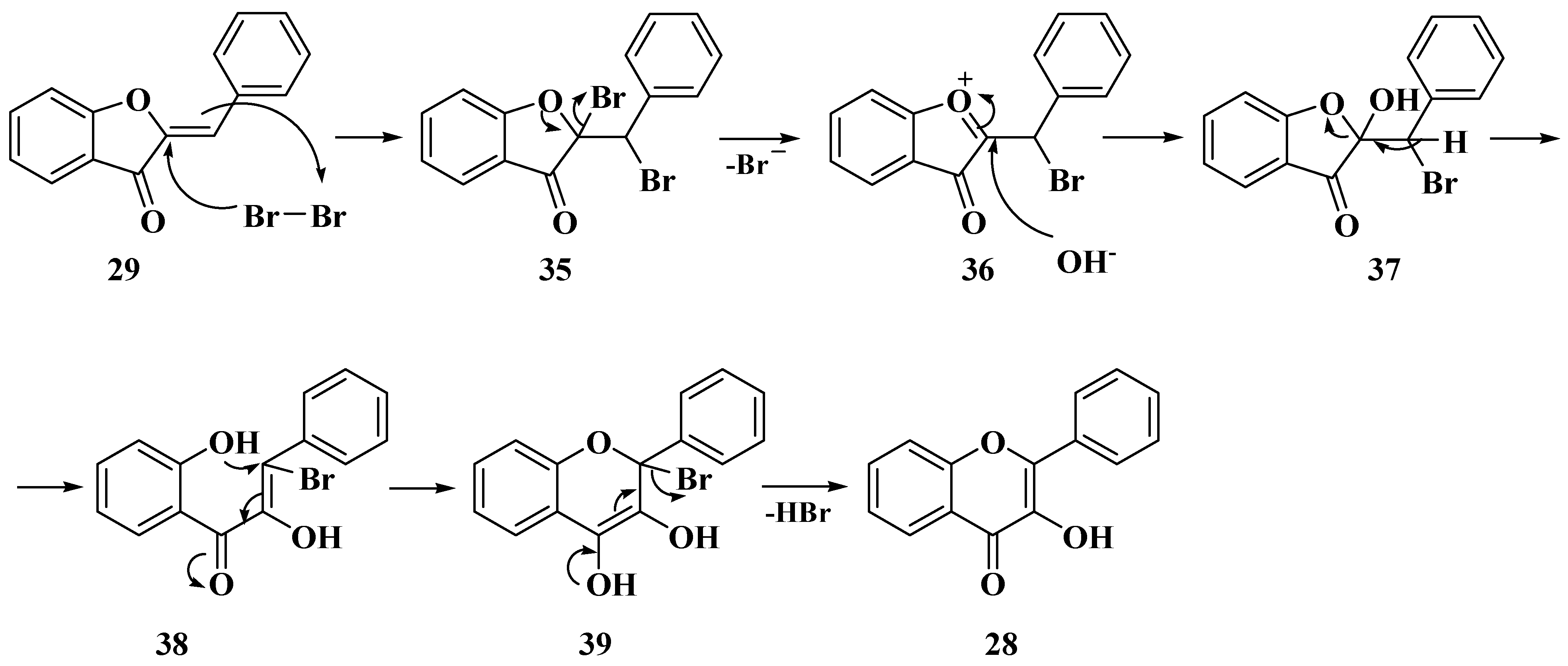
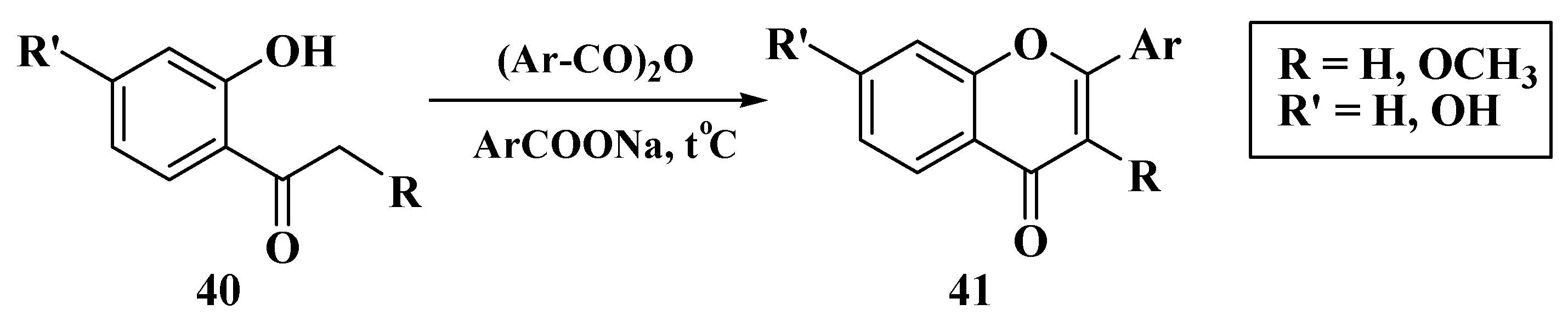
3. Chemical Synthesis of Flavones
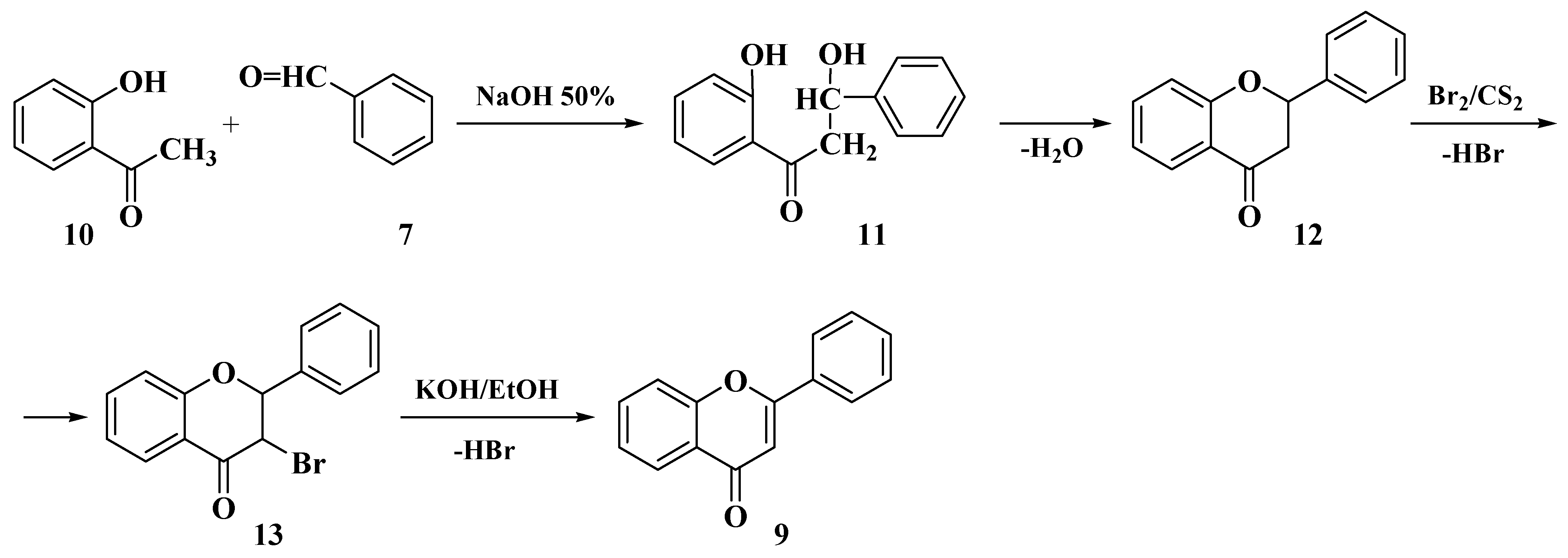
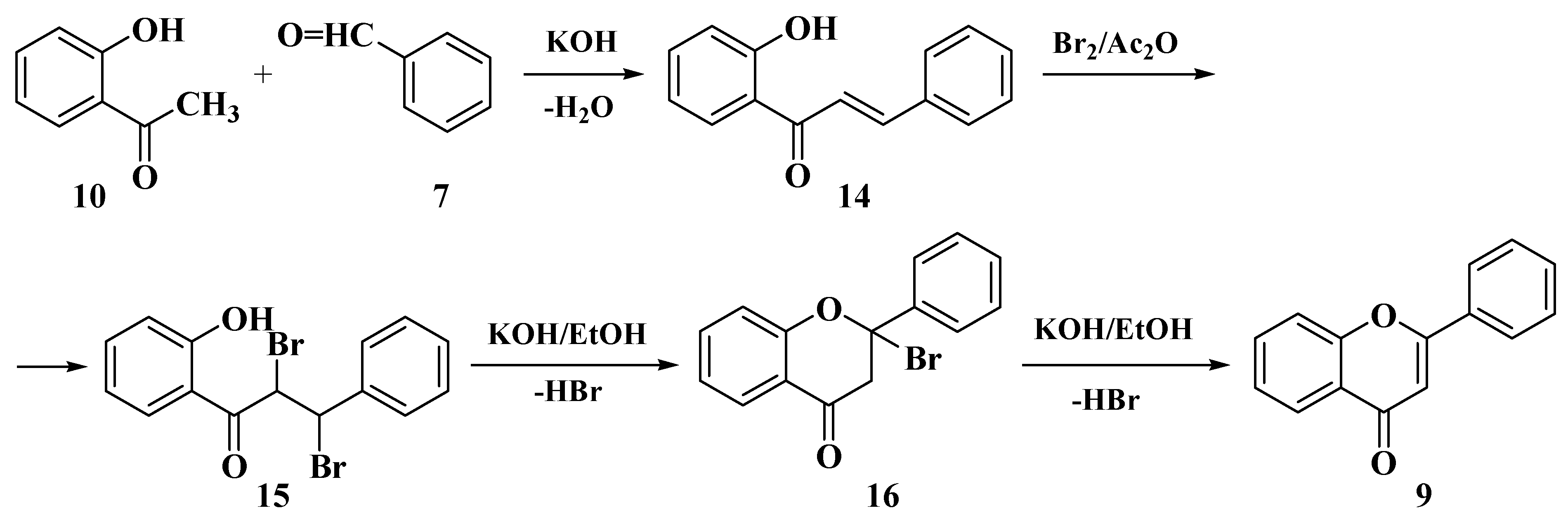
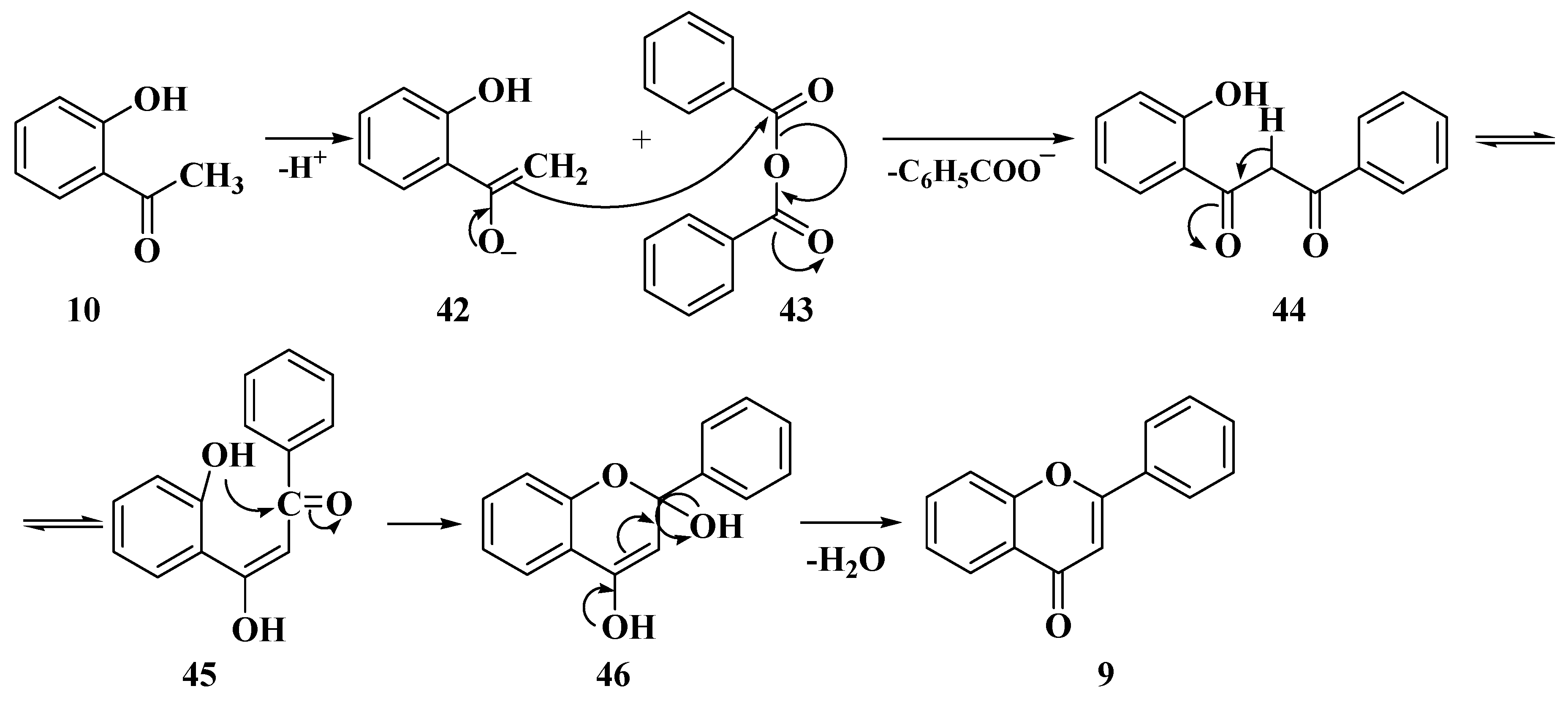
3.1. Von Kostanecki Method
3.2. Von Auwers–Müller Method
3.3. Allan–Robinson Method
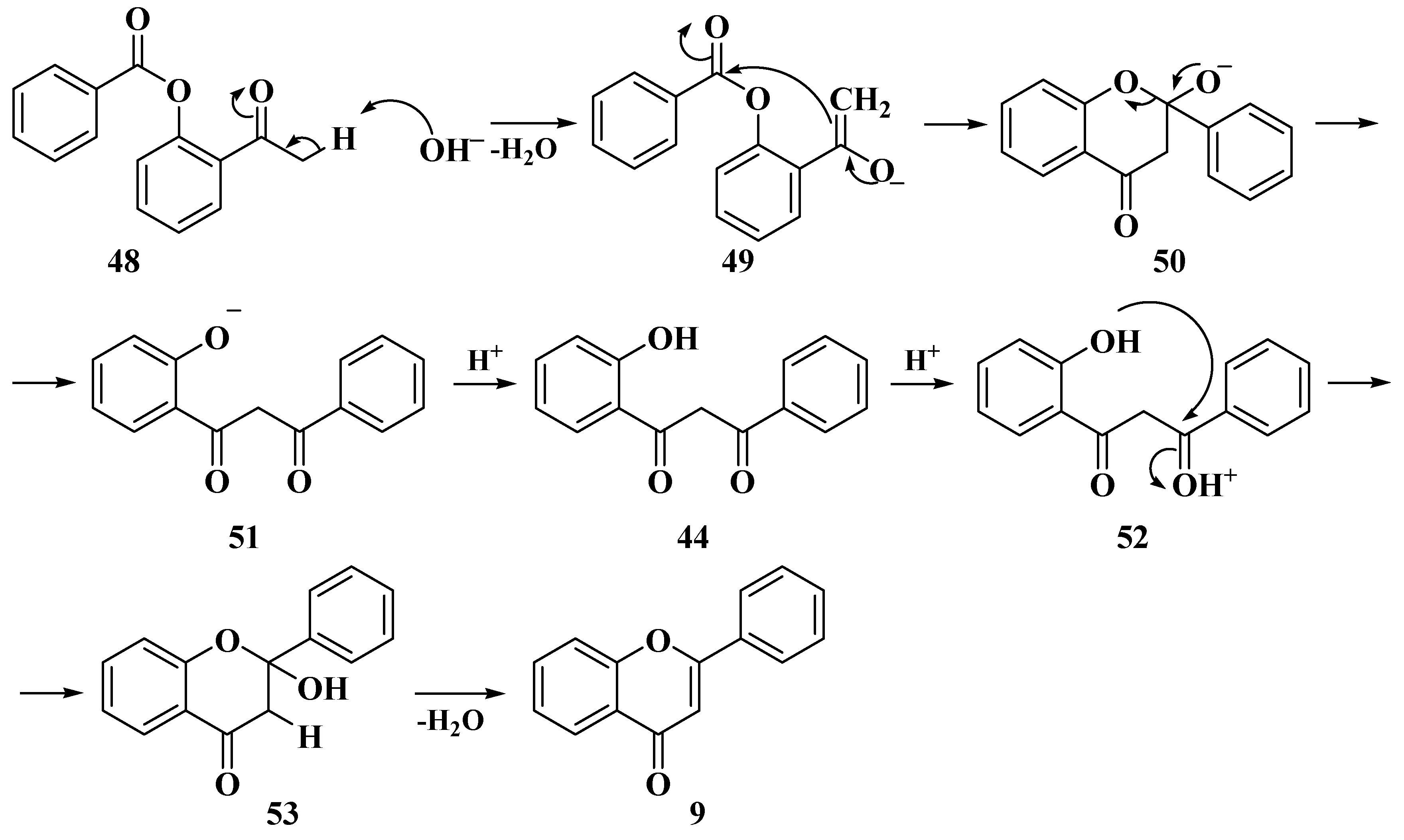
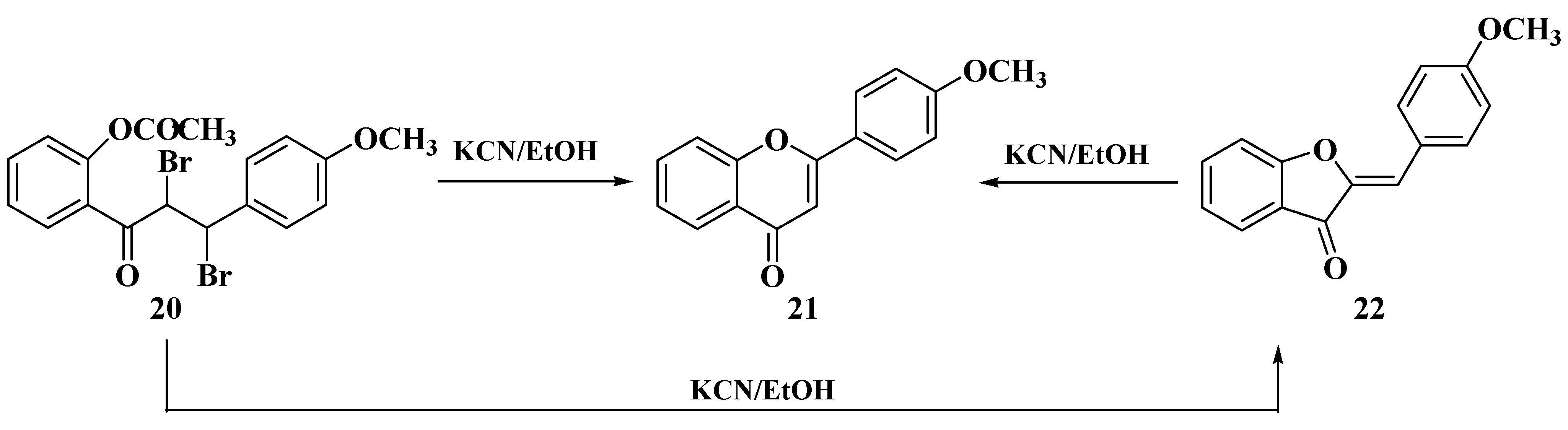
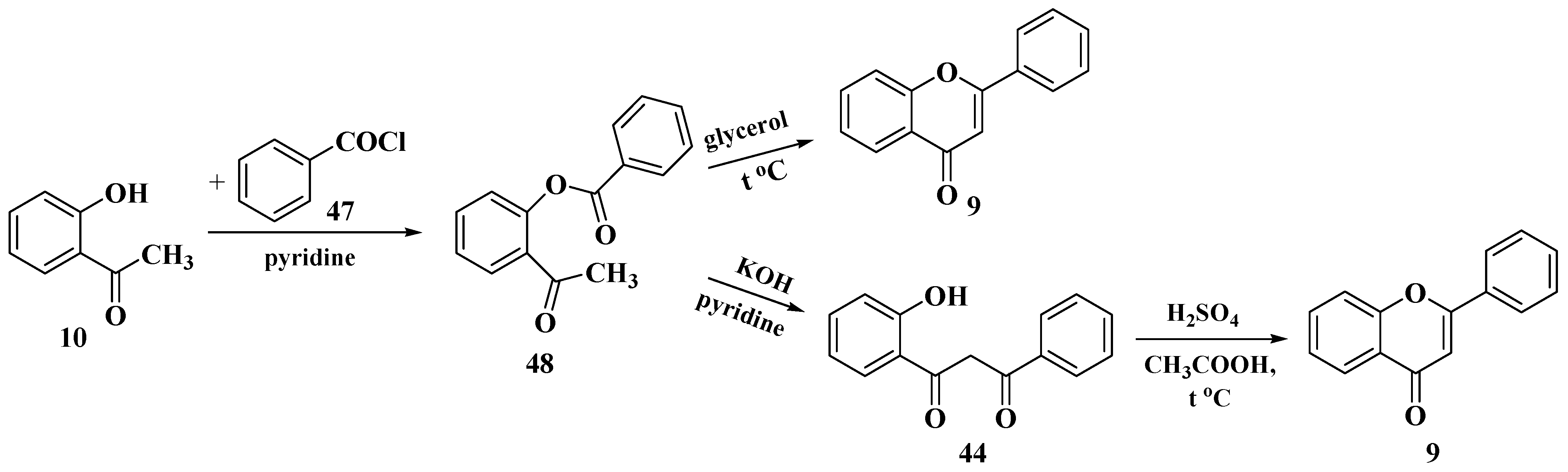
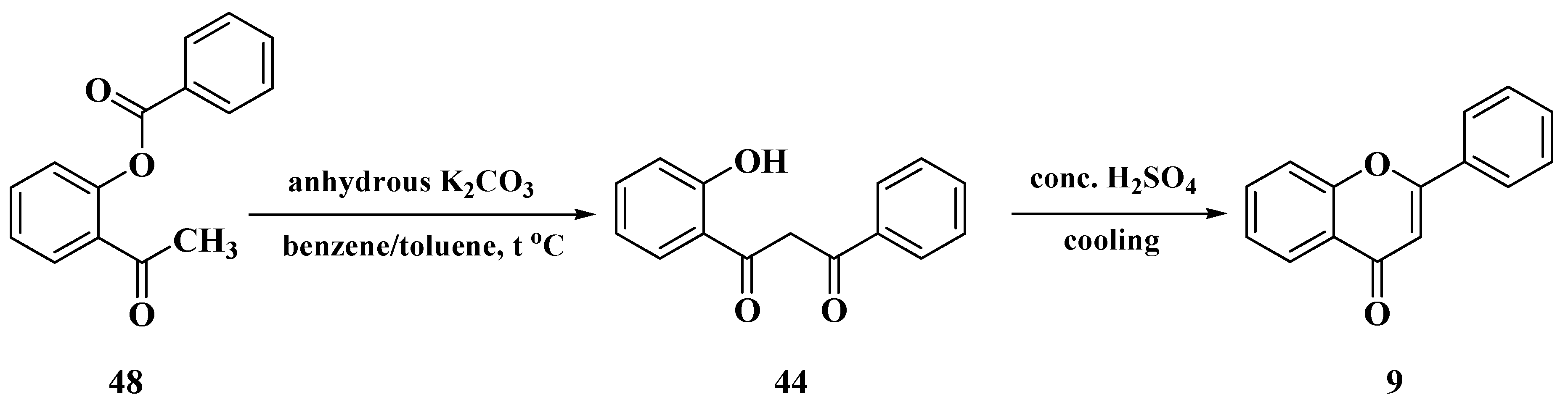
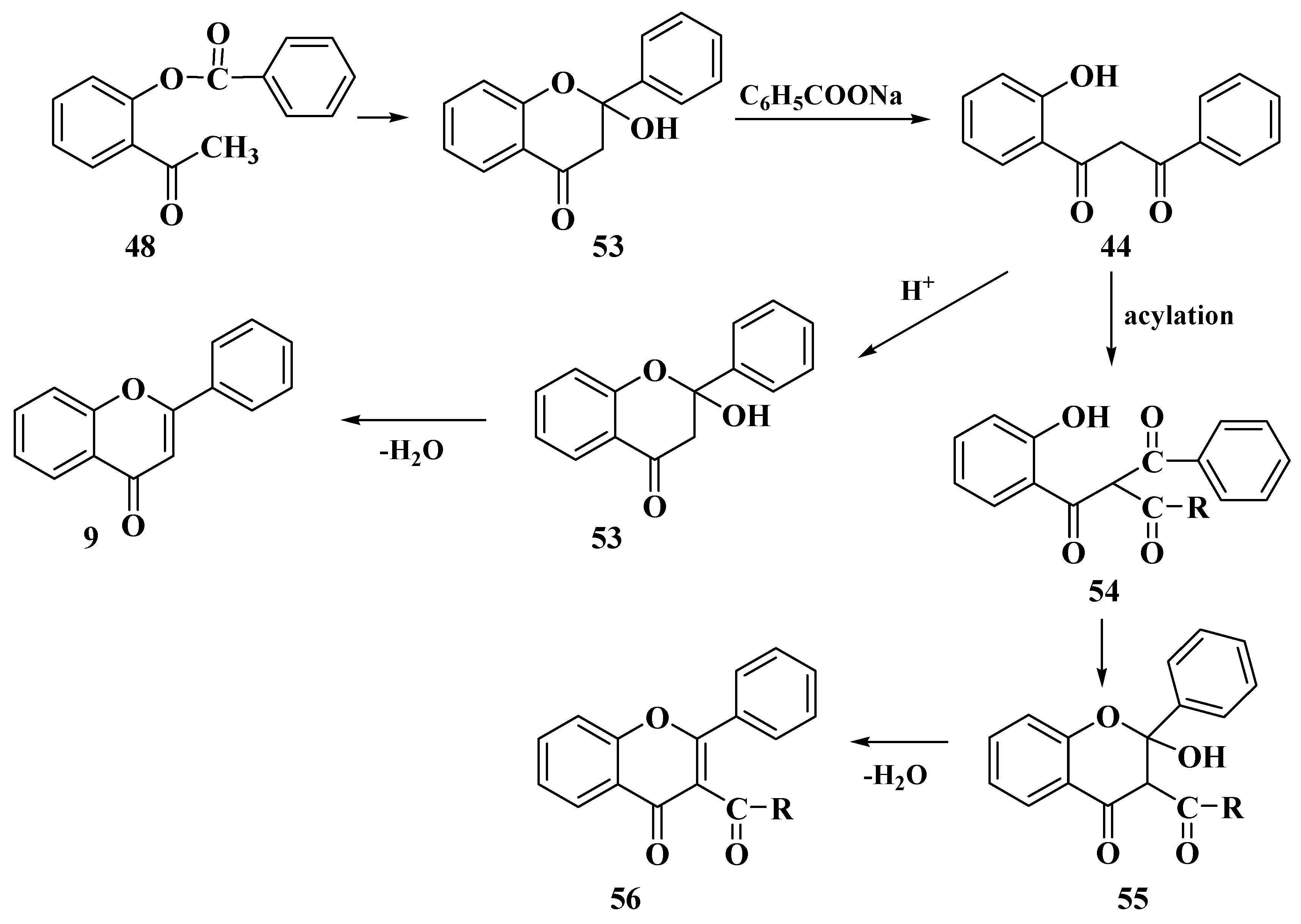
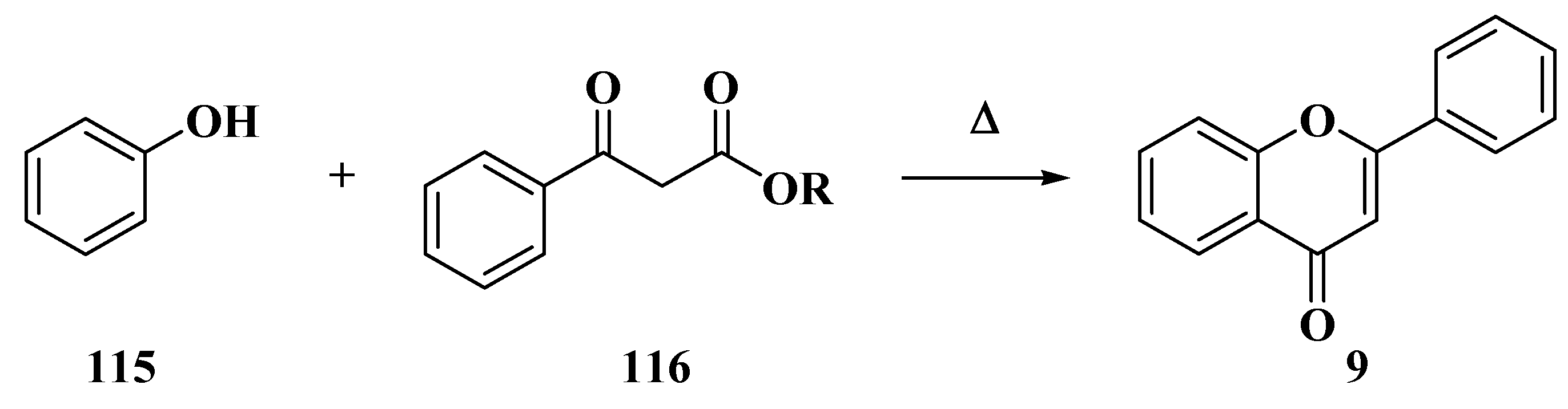
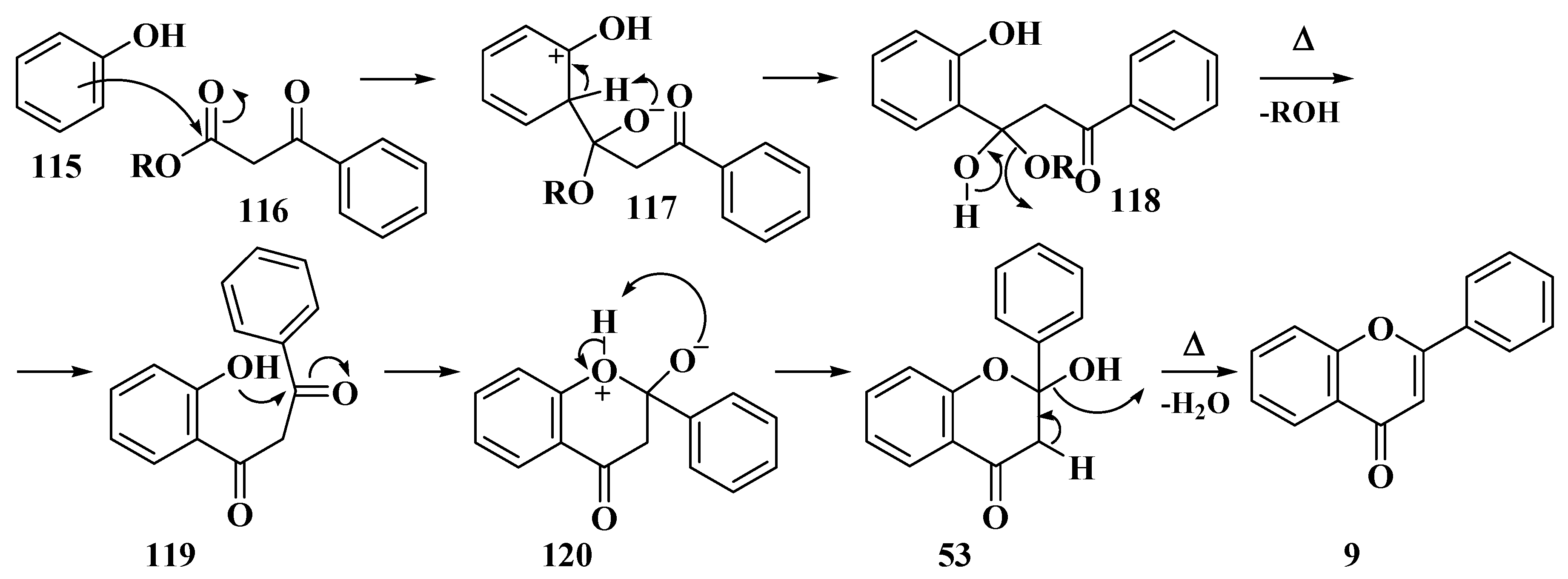
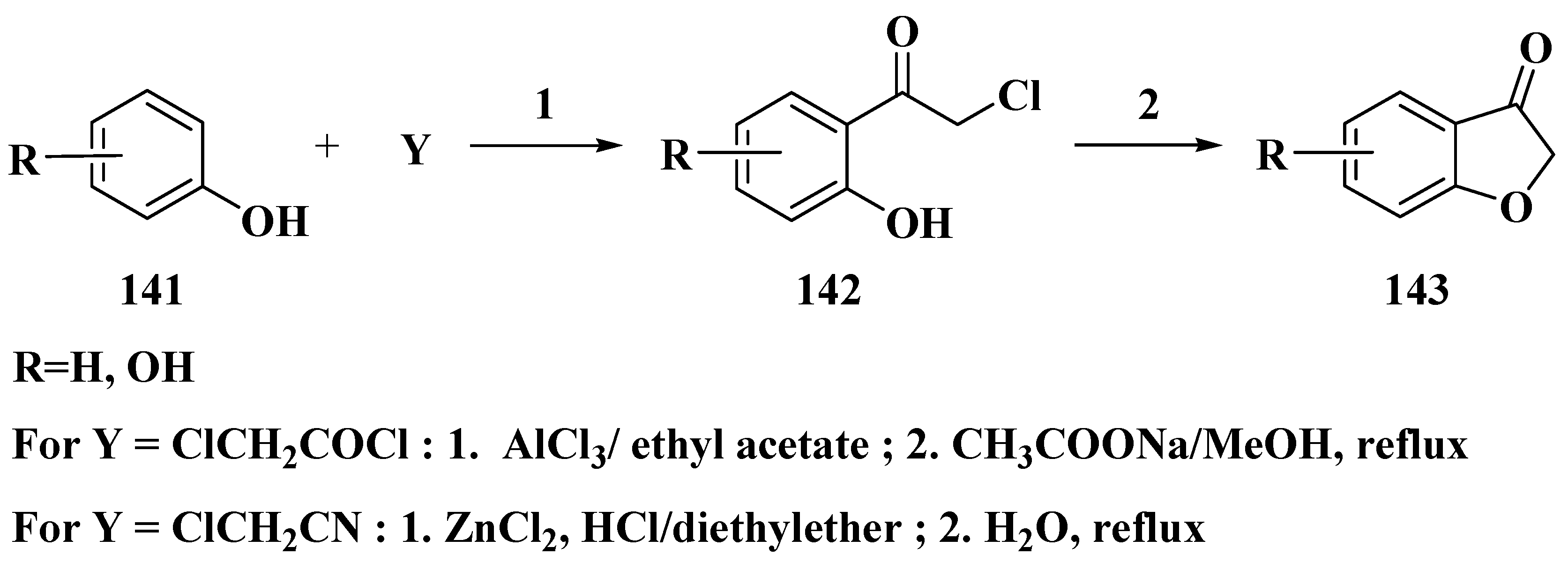
3.4. Baker–Verkataraman Method
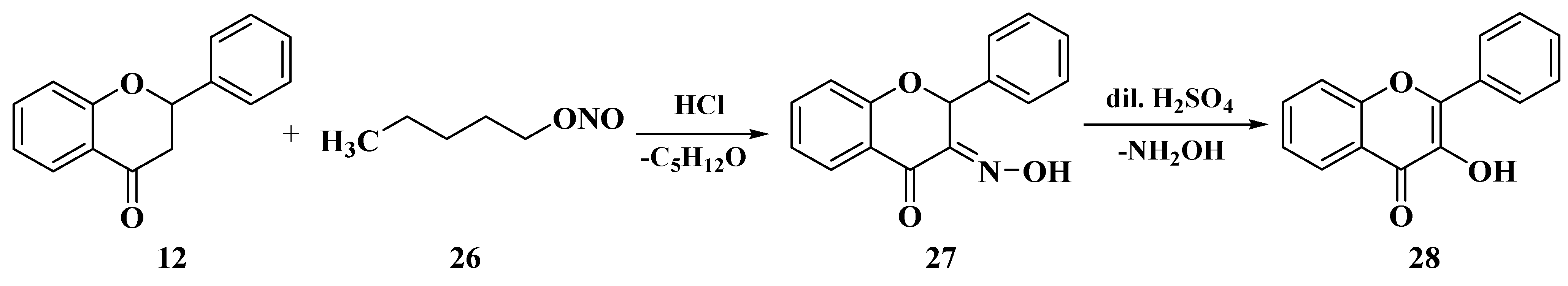
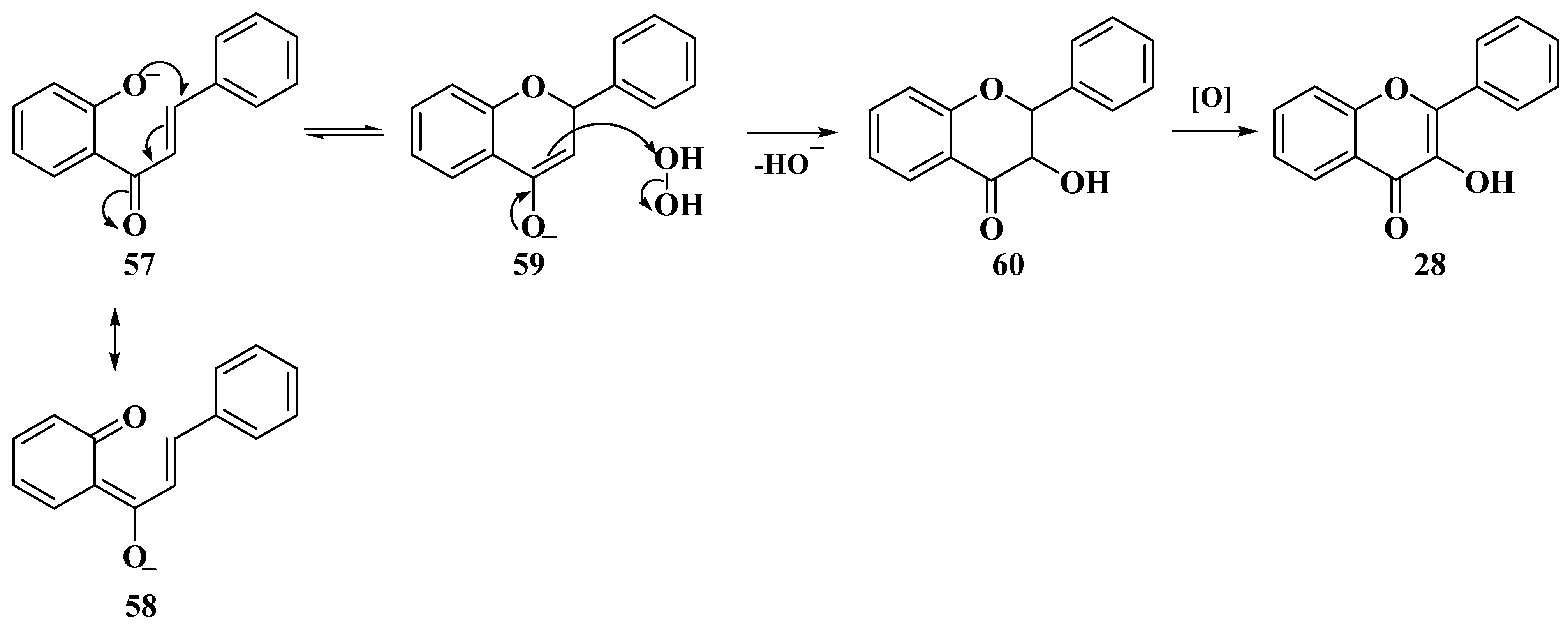
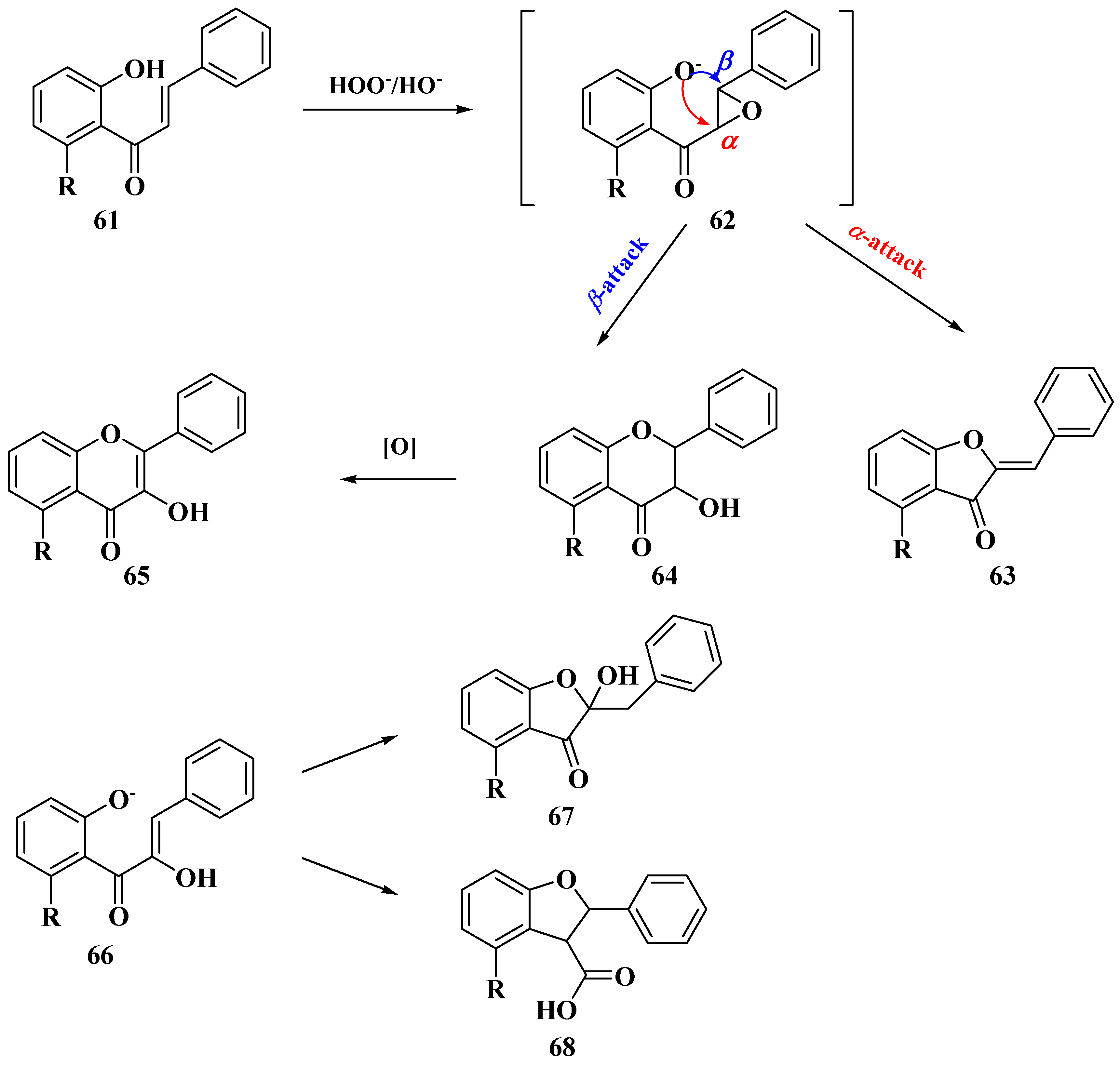
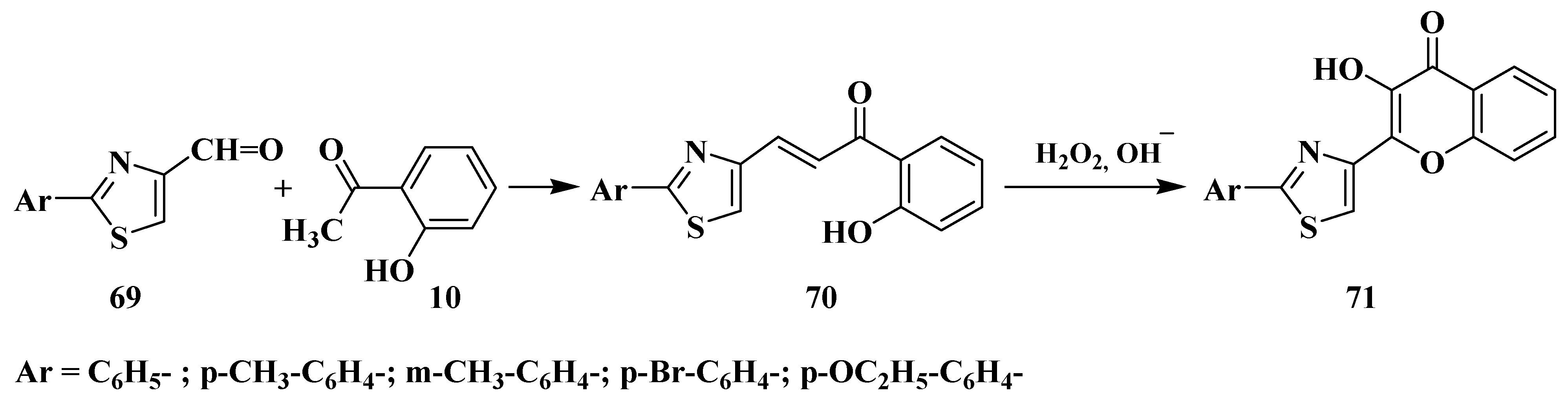
3.5. Algar–Flynn–Oyamada Method
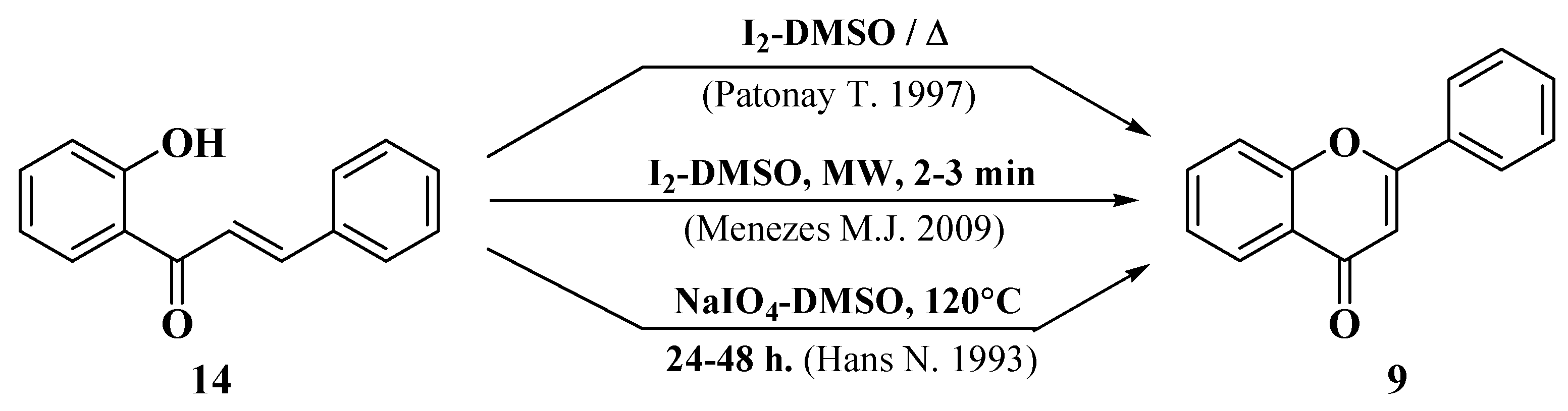
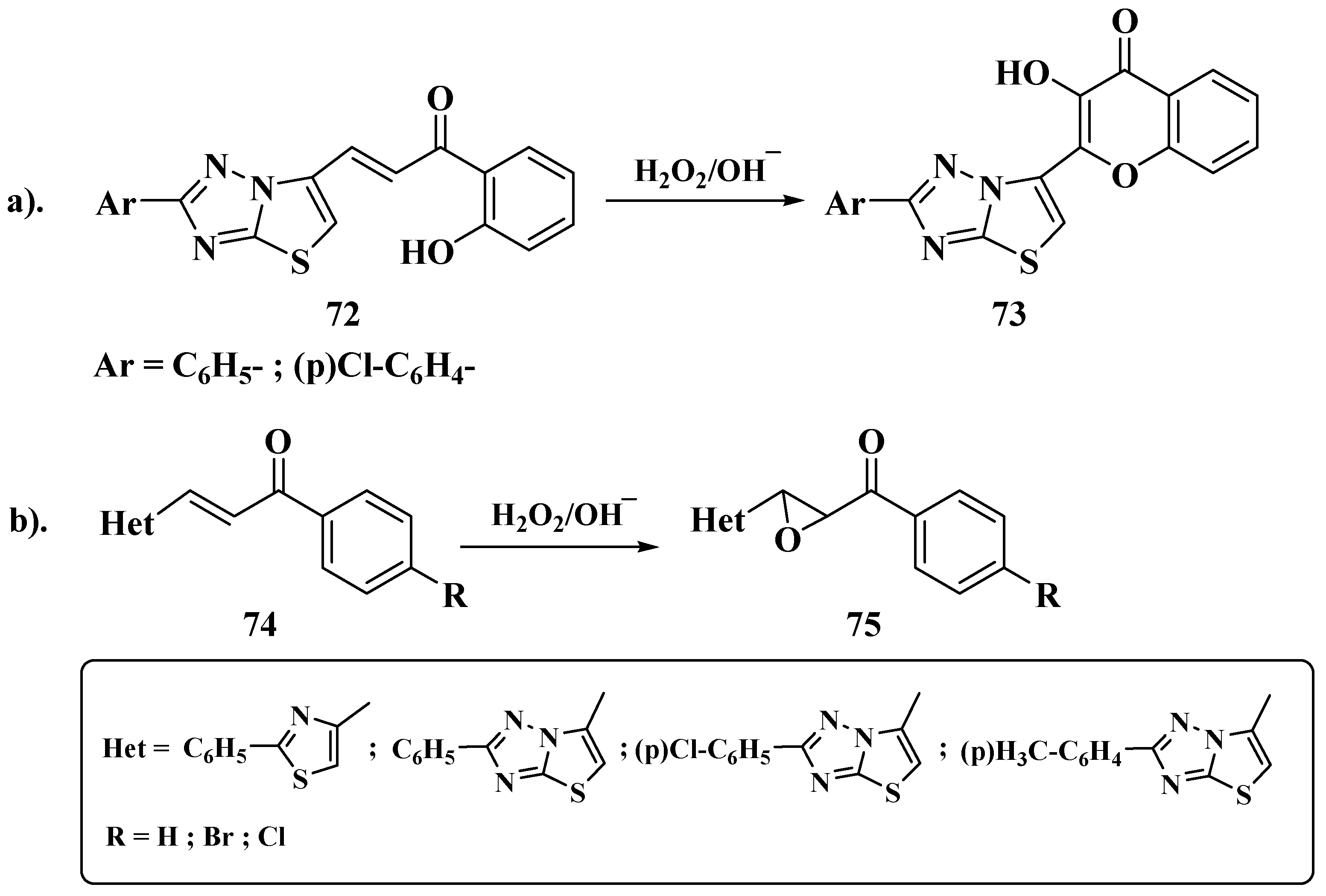
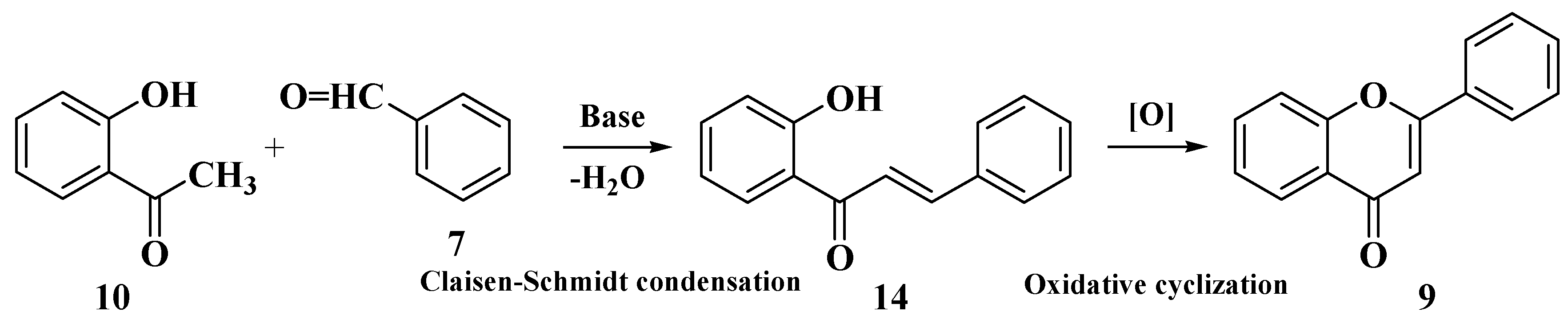
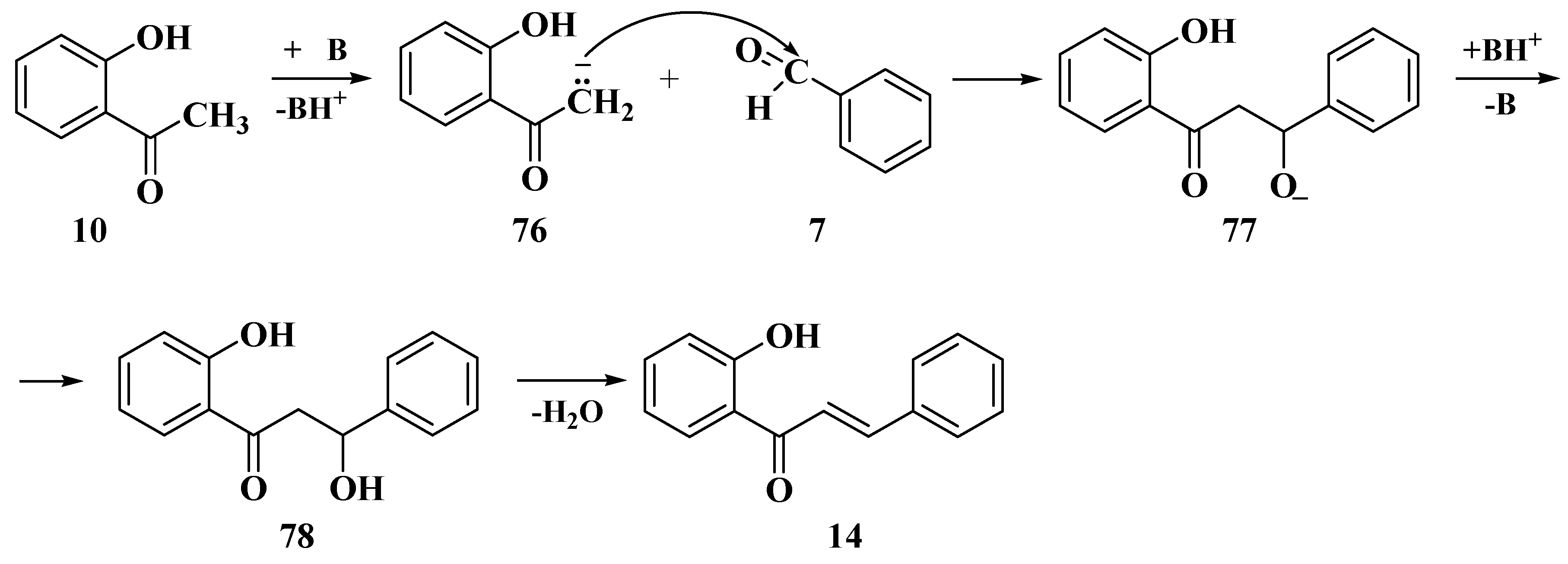
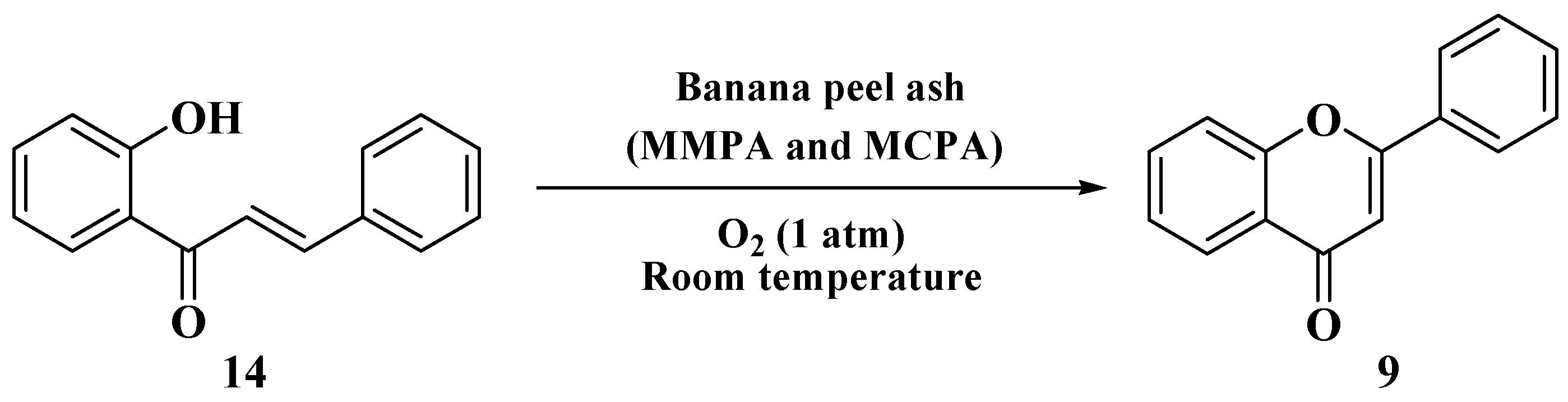
3.6. Claisen–Schmidt Method
3.7. Mentzer Method
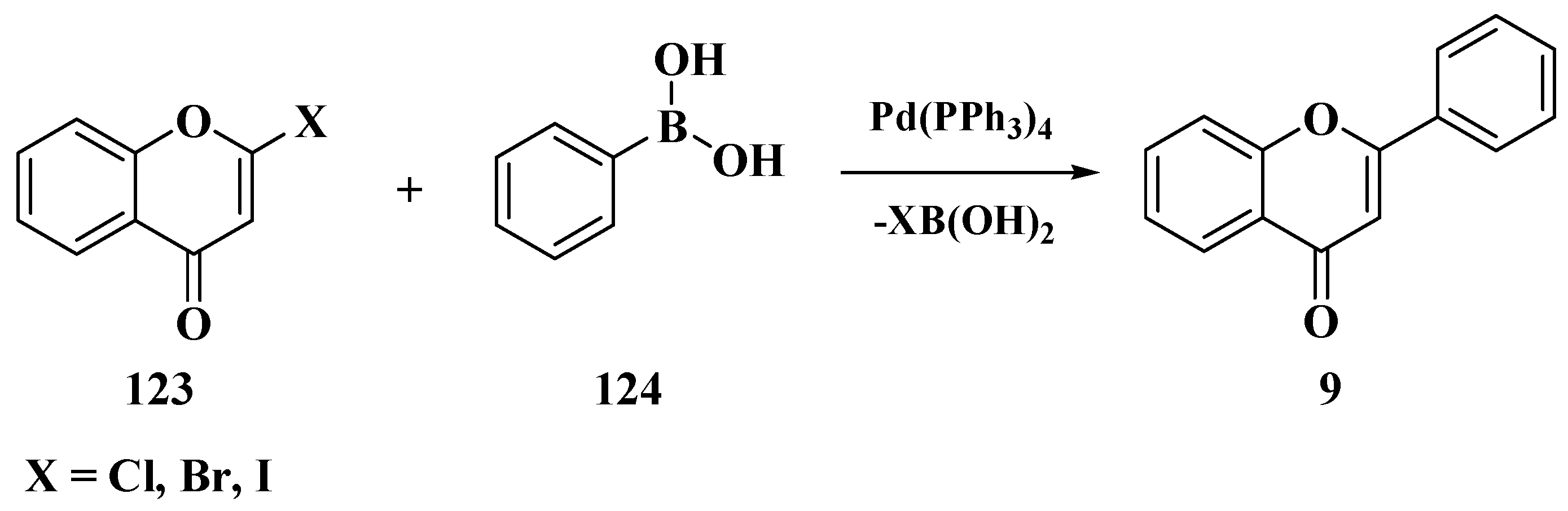
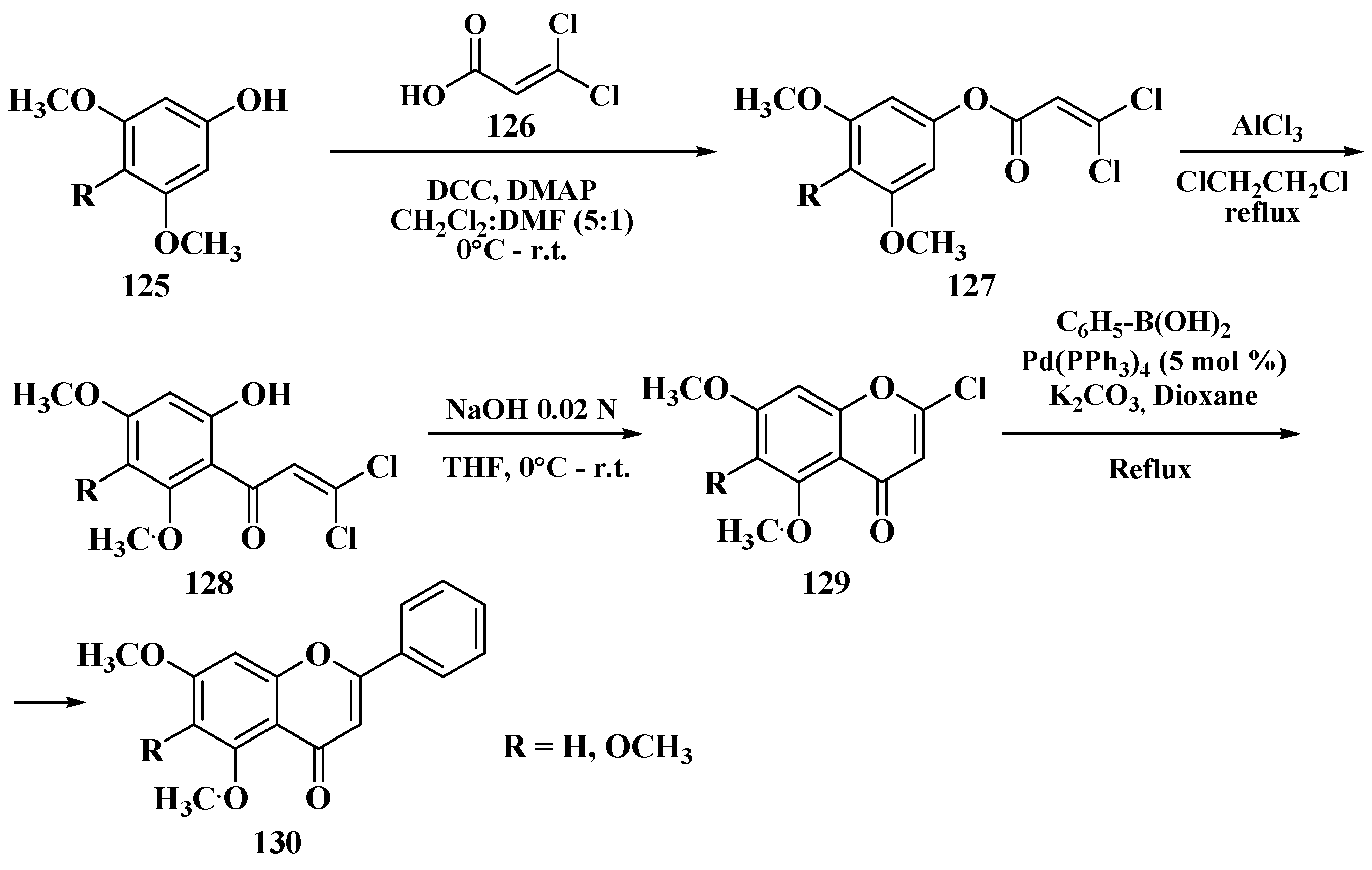
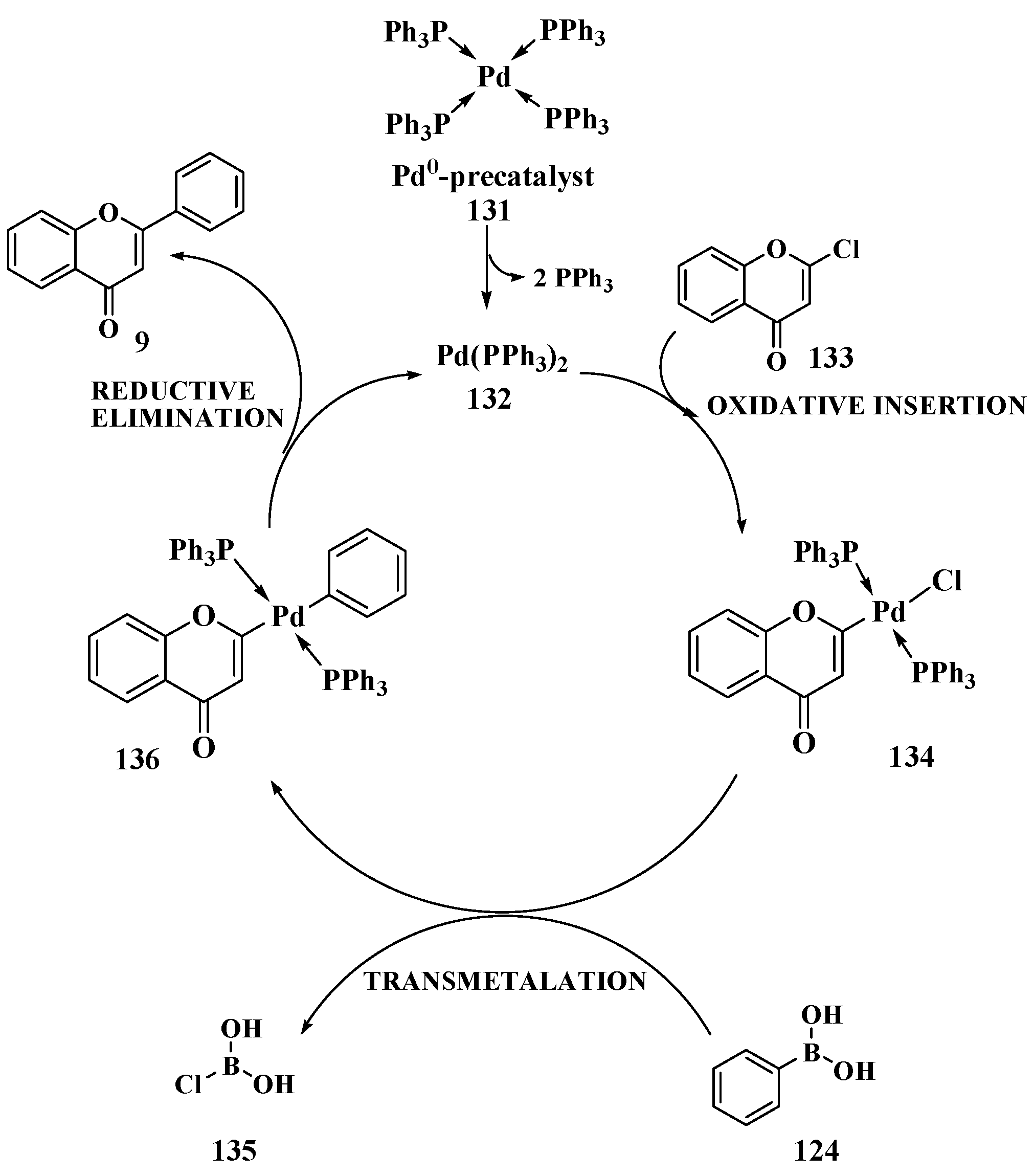
3.8. Suzuki–Miyaura Method
4. Chemical Synthesis of Aurones
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berim, A.; Gang, D.R. Methoxylated flavones: Occurrence, importance, biosynthesis. Phytochem. Rev. 2016, 15, 363–390. [Google Scholar] [CrossRef]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol. J. 2007, 2, 1214–1234. [Google Scholar] [CrossRef] [PubMed]
- Kshatriya, R.; Jejurkar, V.P.; Saha, S. In memory of Prof. Venkataraman: Recent advances in the synthetic methodologies of flavones. Tetrahedron 2018, 74, 811–833. [Google Scholar] [CrossRef]
- Tóth, S.; Szepesi, Á.; Tran-Nguyen, V.-K.; Sarkadi, B.; Német, K.; Falson, P.; Di Pietro, A.; Szakács, G.; Boumendjel, A. Synthesis and anticancer cytotoxicity of azaaurones overcoming multidrug resistance. Molecules 2020, 25, 764. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.X.; Kumamoto, T. Flavonoids as protein kinase inhibitors for cancer chemoprevention: Direct binding and molecular modeling. Antioxid. Redox Signal. 2010, 13, 691–719. [Google Scholar] [CrossRef] [PubMed]
- Polier, G.; Ding, J.; Konkimalla, B.V.; Eick, D.; Ribeiro, N.; Köhler, R.; Giaisi, M.; Efferth, T.; Desaubry, L.; Krammer, P.H.; et al. Wogonin and related natural flavones are inhibitors of CDK9 that induce apoptosis in cancer cells by transcriptional suppression of Mcl-1. Cell Death Dis. 2011, 2, e182. [Google Scholar] [CrossRef]
- Golub, A.G.; Bdzhola, V.G.; Ostrynska, O.V.; Kyshenia, I.V.; Sapelkin, V.M.; Prykhod’ko, A.O.; Kukharenko, O.P.; Yarmoluk, S.M. Discovery and characterization of synthetic 4′-hydroxyflavones—New CK2 inhibitors from flavone family. Bioorg. Med. Chem. 2013, 21, 6681–6689. [Google Scholar] [CrossRef]
- Chao, S.W.; Su, M.Y.; Chiou, L.C.; Chen, L.C.; Chang, C.I.; Huang, W.J. Total Synthesis of Hispidulin and the Structural Basis for Its Inhibition of Proto-oncogene Kinase Pim-1. J. Nat. Prod. 2015, 78, 1969–1976. [Google Scholar] [CrossRef]
- Sedlacek, H.; Czech, J.; Naik, R.; Kaur, G.; Worland, P.; Losiewicz, M.; Parker, B.; Carlson, B.; Smith, A.; Senderowicz, A.; et al. Flavopiridol (L86 8275; NSC 649890), a new kinase inhibitor for tumor therapy. Int. J. Oncol. 1996, 9, 1143–1168. [Google Scholar] [CrossRef]
- Pontes, O.; Costa, M.; Santos, F.; Sampaio-Marques, B.; Dias, T.; Ludovico, P.; Baltazar, F.; Proença, F. Exploitation of new chalcones and 4H-chromenes as agents for cancer treatment. Eur. J. Med. Chem. 2018, 157, 101–114. [Google Scholar] [CrossRef]
- Monasterio, A.; Urdaci, M.; Pinchuk, I.; Moratalla, N.; Martínez, I. Flavonoids induce apoptosis in human leukemia U937 cells through caspase-and caspase-calpain-dependent pathways. Nutr. Cancer 2004, 50, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Ribeiro, D.; Silva, P.M.A.; Nazareth, N.; Monteiro, M.; Palmeira, A.; Saraiva, L.; Pinto, M.; Bousbaa, H.; Cidade, H. New alkoxy flavone derivatives targeting caspases: Synthesis and antitumor activity evaluation. Molecules 2019, 24, 129. [Google Scholar] [CrossRef] [PubMed]
- Kariagina, A.; Doseff, A.I. Anti-Inflammatory Mechanisms of Dietary Flavones: Tapping into Nature to Control Chronic Inflammation in Obesity and Cancer. Int. J. Mol. Sci. 2022, 23, 15753. [Google Scholar] [CrossRef] [PubMed]
- Occhiuto, C.J.; Moerland, J.A.; Leal, A.S.; Gallo, K.A.; Liby, K.T. The Multi-Faceted Consequences of NRF2 Activation throughout Carcinogenesis. Mol. Cells 2023, 46, 176–186. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, M.; Wang, L.; Yu, S. Anti-tumor effects and associated molecular mechanisms of myricetin. Biomed. Pharmacother. 2019, 120, 109506. [Google Scholar] [CrossRef]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef]
- Zang, W.; Wang, T.; Wang, Y.; Li, M.; Xuan, X.; Ma, Y.; Zhao, G. Myricetin exerts anti-proliferative, anti-invasive, and pro-apoptotic effects on esophageal carcinoma EC9706 and KYSE30 cells via RSK2. Tumor Biol. 2014, 35, 12583–12592. [Google Scholar] [CrossRef]
- Chen, Y.C.; He, X.L.; Qi, L.; Shi, W.; Yuan, L.-W.; Huang, M.-Y.; Xu, Y.-L.; Chen, X.; Gu, L.; Zhang, L.-L.; et al. Myricetin inhibits interferon-γ-induced PD-L1 and IDO1 expression in lung cancer cells. Biochem. Pharmacol. 2022, 197, 114940. [Google Scholar] [CrossRef]
- Han, S.-H.; Lee, J.-H.; Woo, J.-S.; Jung, G.-H.; Jung, S.-H.; Han, E.-J.; Kim, B.; Cho, S.-D.; Nam, J.-S.; Che, J.H.; et al. Myricetin induces apoptosis and autophagy in human gastric cancer cells through inhibition of the PI3K/Akt/mTOR pathway. Heliyon 2022, 8, e09309. [Google Scholar] [CrossRef]
- Singh, S.S.; Yap, W.N.; Arfuso, F.; Kar, S.; Wang, C.; Cai, W.; Dharmarajan, A.M.; Sethi, G.; Kumar, A.P. Targeting the PI3K/Akt signaling pathway in gastric carcinoma: A reality for personalized medicine? World J. Gastroenterol. 2015, 21, 12261–12273. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, H.; Li, D.; Shu, Q.; Wang, T.; Song, X.; Xu, H. Myricetin alleviates the formaldehyde-enhanced Warburg effect in tumor cells through inhibition of HIF-1α. Toxicology and Appl. Pharmacol. 2022, 454, 116246. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Li, Z.; Zhang, X.; Chen, M.; Zhang, X. Identification of MAP Kinase Kinase 3 as a protein target of myricetin in non-small cell lung cancer cells. Biomed. Pharmacother. 2023, 161, 114460. [Google Scholar] [CrossRef]
- El Menyiy, N.; Aboulaghras, S.; Bakrim, S.; Moubachir, R.; Taha, D.; Khalid, A.; Abdalla, A.N.; Algarni, A.S.; Hermansyah, A.; Ming, L.C.; et al. Genkwanin: An emerging natural compound with multifaceted pharmacological effects. Biomed. Pharmacother. 2023, 165, 115159. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hong, J.; Li, H.; Qi, X.; Guo, Y.; Han, M.; Wang, X. Genkwanin nanosuspensions: A novel and potential antitumor drug in breast carcinoma therapy. Drug Deliv. 2017, 24, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, M.; Andruniów, T.; Sroka, Z. Flavones’ and Flavonols’ Antiradical Structure–Activity Relationship—A Quantum Chemical Study. Antioxidants 2020, 9, 461. [Google Scholar] [CrossRef]
- Grigalius, I.; Petrikaite, V. Relationship between antioxidant and anticancer activity of trihydroxyflavones. Molecules 2017, 22, 2169. [Google Scholar] [CrossRef]
- Zhao, L.; Yuan, X.; Wang, J.; Feng, Y.; Ji, F.; Li, Z.; Bian, J. A review on flavones targeting serine/threonine protein kinases for potential anticancer drugs. Bioorg. Med. Chem. 2019, 27, 677–685. [Google Scholar] [CrossRef]
- Chao, S.H.; Price, D.H. Flavopiridol Inactivates P-TEFb and Blocks Most RNA Polymerase II Transcription in Vivo. J. Biol. Chem. 2001, 276, 31793–31799. [Google Scholar] [CrossRef]
- Hassan, A.H.; Choi, E.; Yoon, Y.M.; Lee, K.W.; Yoo, S.Y.; Cho, M.C.; Yang, J.S.; Kim, H.I.; Hong, J.Y.; Shin, J.-S.; et al. Natural products hybrids: 3,5,4′-Trimethoxystilbene-5,6,7-trimethoxyflavone chimeric analogs as potential cytotoxic agents against diverse human cancer cells. Eur. J. Med. Chem. 2019, 161, 559–580. [Google Scholar] [CrossRef]
- Hassan, A.H.E.; Lee, K.T.; Lee, Y.S. Flavone-based arylamides as potential anticancers: Design, synthesis and in vitro cell-based/cell-free evaluations. Eur. J. Med. Chem. 2020, 187, 111965. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Guo, S.; Rajaram, P.; Lee, M.; Chen, G.; Fong, R.; Gonzalez, A.; Zhang, Q.; Zheng, S.; et al. Structure-activity relationship and pharmacokinetic studies of 3-O-substitutedflavonols as anti-prostate cancer agents. Eur. J. Med. Chem. 2018, 157, 978–993. [Google Scholar] [CrossRef] [PubMed]
- Németh-Rieder, A.; Keglevich, P.; Hunyadi, A.; Latif, A.D.; Zupkó, I.; Hazai, L. Synthesis and In Vitro Anticancer Evaluation of Flavone—1,2,3-Triazole Hybrids. Molecules 2023, 28, 626. [Google Scholar] [CrossRef] [PubMed]
- Mobbili, G.; Romaldi, B.; Sabbatini, G.; Amici, A.; Marcaccio, M.; Galeazzi, R.; Laudadio, E.; Armeni, T.; Minnelli, C. Identification of Flavone Derivative Displaying a 4-Aminophenoxy Moiety as Potential Selective Anticancer Agent in NSCLC Tumor Cells. Molecules 2023, 28, 3239. [Google Scholar] [CrossRef] [PubMed]
- Bollikolla, H.B.; Anandam, R.; Chinnam, S.; Varala, R.; Khandapu, B.M.K.; Kapavarapu, R.; Syed, K.S.; Dubasi, N.; Syed, M.A. C-Dimethylated Flavones as Possible Potential Anti-Tubercular and Anticancer Agents. Chem. Biodivers. 2023, 20, e202201201. [Google Scholar] [CrossRef]
- Schoepfer, J.; Fretz, H.; Chaudhuri, B.; Muller, L.; Seeber, E.; Meijer, L.; Lozach, O.; Vangrevelinghe, E.; Furet, P. Structure-based design and synthesis of 2-benzylidene-benzofuran-3-ones as flavopiridol mimics. J. Med. Chem. 2002, 45, 1741–1747. [Google Scholar] [CrossRef]
- Priyadarshani, G.; Nayak, A.; Amrutkar, S.M.; Das, S.; Guchhait, S.K.; Kundu, C.N.; Banerjee, U.C. Scaffold-Hopping of Aurones: 2-Arylideneimidazo[1,2-a]pyridinones as Topoisomerase IIα-Inhibiting Anticancer Agents. ACS Med. Chem. Lett. 2016, 7, 1056–1061. [Google Scholar] [CrossRef]
- French, K.J.; Schrecengost, R.S.; Lee, B.D.; Zhuang, Y.; Smith, S.N.; Eberly, J.L.; Yun, J.; Smith, C.D. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003, 63, 5962–5969. [Google Scholar]
- Lawrence, N.J.; Rennison, D.; McGown, A.T.; Hadfield, J.A. The total synthesis of an aurone isolated from Uvaria hamiltonii: Aurones and flavones as anticancer agents. Bioorg. Med. Chem. Lett. 2003, 13, 3759–3763. [Google Scholar] [CrossRef]
- Detsi, A.; Majdalani, M.; Kontogiorgis, C.A.; Hadjipavlou-Litina, D.; Kefalas, P. Natural and synthetic 2′-hydroxy-chalcones and aurones: Synthesis, characterization and evaluation of the antioxidant and soybean lipoxygenase inhibitory activity. Bioorg. Med. Chem. 2009, 17, 8073–8085. [Google Scholar] [CrossRef]
- Hadjeri, M.; Barbier, M.; Ronot, X.; Mariotte, A.M.; Boumendjel, A.; Boutonnat, J. Modulation of P-glycoprotein-mediated multidrug resistance by flavonoid derivatives and analogues. J. Med. Chem. 2003, 46, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.M.; Wu, C.P.; Ambudkar, S.V.; Go, M.L. In vitro and in vivo modulation of ABCG2 by functionalized aurones and structurally related analogs. Biochem. Pharmacol. 2011, 82, 1562–1571. [Google Scholar] [CrossRef]
- Coman, F.M.; Mbaveng, A.T.; Marc, G.; Leonte, D.; Brém, B.; Vlase, L.; Imre, S.; Kuete, V.; Zaharia, V. Heterocycles 47. Synthesis, Characterization and Biological Evaluation of some New Thiazole Aurones as Antiproliferative Agents. Farmacia 2020, 68, 492–506. [Google Scholar] [CrossRef]
- Semenov, I.; Akyuz, C.; Roginskaya, V.; Chauhan, D.; Corey, S.J. Growth inhibition and apoptosis of myeloma cells by the CDK inhibitor flavopiridol. Leuk. Res. 2002, 26, 271–280. [Google Scholar] [CrossRef]
- Jeon, K.-H.; Park, S.; Shin, J.-H.; Jung, A.-R.; Hwang, S.-Y.; Seo, S.H.; Jo, H.; Na, Y.; Kwon, Y. Synthesis and evaluation of 7-(3-aminopropyloxy)-substituted flavone analogue as a topoisomerase IIα catalytic inhibitor and its sensitizing effect to enzalutamide in castration-resistant prostate cancer cells. Eur. J. Med. Chem. 2023, 246, 114999. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Li, W.; Liu, K.; Wang, Q. Synthesis and anti-proliferative activities of 5,6,7-trimethoxyflavones and their derivatives. Nat. Prod. Res. 2022, 36, 4070–4075. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, J.; Li, K.; Luo, J.; Yang, S.; Song, J.-R.; Chen, C.; Pan, W.-D. Synthesis of flavone derivatives via N-amination and evaluation of their anticancer activities. Molecules 2019, 24, 2723. [Google Scholar] [CrossRef]
- Elhadi, A.A.; Osman, H.; Iqbal, M.A.; Rajeswari, S.K.; Ahamed, M.B.K.; Majid, A.M.A.; Rosli, M.M.; Razak, I.A.; Majid, A.S.A. Synthesis and structural elucidation of two new series of aurone derivatives as potent inhibitors against the proliferation of human cancer cells. Med. Chem. Res. 2015, 24, 3504–3515. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, L.; Liu, Y.; Chen, S.; Cheng, H.; Lu, X.; Zheng, Z.; Zhou, G.-C. Design, synthesis and discovery of 5-hydroxyaurone derivatives as growth inhibitors against HUVEC and some cancer cell lines. Eur. J. Med. Chem. 2010, 45, 5950–5957. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, H.; Liu, Y.-M.; Yao, X.; Tong, M.; Wang, Y.-H.; Liao, D.-F. Synthesis, Characterization, and Anticancer Effect of Trifluoromethylated Aurone Derivatives. J. Heterocycl. Chem. 2014, 6, 1098–1107. [Google Scholar] [CrossRef]
- Uesawa, Y.; Sakagami, H.; Ikezoe, N.; Takao, K.; Kagaya, H.; Sugita, Y. Quantitative structure-cytotoxicity relationship of aurones. Anticancer Res. 2017, 37, 6169–6176. [Google Scholar] [CrossRef]
- Demirayak, S.; Yurttas, L.; Gundogdu-Karaburun, N.; Karaburun, A.C.; Kayagil, I. Synthesis and anticancer activity evaluation of new aurone derivatives. J. Enzym. Inhib. Med. Chem. 2015, 30, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Lathwal, E.; Kumar, S.; Sahoo, P.K.; Ghosh, S.; Mahata, S.; Nasare, V.D.; Kumar, S. Synthesis, cytotoxic evaluation and structure activity relationship of pyrazole hybrid aurones on gastric cancer (AGS) cell lines. Results Chem. 2022, 4, 100590. [Google Scholar] [CrossRef]
- Gao, L.; Tang, Z.; Li, T.; Wang, J. Myricetin exerts anti-biofilm activity and attenuates osteomyelitis by inhibiting the TLR2/MAPK pathway in experimental mice. Microb. Pathog. 2023, 182, 106165. [Google Scholar] [CrossRef] [PubMed]
- Ashok, D.; Kifah, M.A.; Lakshmi, B.V.; Sarasija, M.; Adam, S. Microwave-assisted one-pot synthesis of some new flavonols by modified Algar–Flynn–Oyamada reaction and their antimicrobial activity. Chem. Heterocycl. Compd. 2016, 52, 172–176. [Google Scholar] [CrossRef]
- Lv, X.H.; Liu, H.; Ren, Z.L.; Wang, W.; Tang, F.; Cao, H.Q. Design, synthesis and biological evaluation of novel flavone Mannich base derivatives as potential antibacterial agents. Mol. Divers. 2019, 23, 299–306. [Google Scholar] [CrossRef]
- Khdera, H.A.; Saad, S.Y.; Moustapha, A.; Kandil, F. Synthesis of new flavonoid derivatives based on 3-hydroxy-4′-dimethylamino flavone and study the activity of some of them as antifungal. Heliyon 2022, 8, e12062. [Google Scholar] [CrossRef]
- Kumar, G.; Lathwal, E.; Saroha, B.; Kumar, S.; Kumar, S.; Chauhan, N.S.; Kumar, T. Synthesis and Biological Evaluation of Quinoline-Based Novel Aurones. ChemistrySelect 2020, 5, 3539–3543. [Google Scholar] [CrossRef]
- Pan, H.; He, J.; Yang, Z.; Yao, X.; Zhang, H.; Li, R.; Xiao, Y.; Zhao, C.; Jiang, H.; Liu, Y.; et al. Myricetin possesses the potency against SARS-CoV-2 infection through blocking viral-entry facilitators and suppressing inflammation in rats and mice. Phytomedicine 2023, 116, 154858. [Google Scholar] [CrossRef]
- Fujimoto, K.J.; Nema, D.; Ninomiya, M.; Koketsu, M.; Sadanari, H.; Takemoto, M.; Daikoku, T.; Murayama, T. An in silico-designed flavone derivative, 6-fluoro-4′-hydroxy-3′,5′-dimetoxyflavone, has a greater anti-human cytomegalovirus effect than ganciclovir in infected cells. Antivir. Res. 2018, 154, 10–16. [Google Scholar] [CrossRef]
- Badavath, V.N.; Jadav, S.; Pastorino, B.; de Lamballerie, X.; Sinha, N.B.; Jayaprakash, V. Synthesis and Antiviral Activity of 2-aryl-4H-chromen-4-one Derivatives Against Chikungunya Virus. Lett. Drug Des. Discov. 2016, 13, 1019–1024. [Google Scholar] [CrossRef]
- Zemplén, G.; Bognár, R. Umwandlung des Hesperetins in Diosmetin, des Hesperidins in Diosmin und des Isosakuranetins in Acacetin. Berichte Dtsch. Chem. Ges. 1943, 76, 452–457. [Google Scholar] [CrossRef]
- Kostanecki, S.V.; Levi, R.; Tambor, J. Synthese des 2-Oxyflavons. Berichte Dtsch. Chem. Ges. 1899, 32, 326–332. [Google Scholar] [CrossRef]
- Donnelly, J.A.; Acton, J.P.; Donnelly, D.J.; Philbin, E.M. Steric and Electronic Effects in the Emilewiczvon Kostanecki Cyclization of Chalcone Dihalides. Proc. R. Ir. Acad. B. 1983, 83B, 49–56. [Google Scholar]
- Bate-Smith, E.C.; Geissman, T.A. Benzalcoumaranones. Nature 1951, 167, 688. [Google Scholar] [CrossRef]
- Narasimhachari, N.; Seshadri, T.R. A new synthesis of flavones. Proc. Indian Acad. Sci. Sect. A 1949, 30, 151–162. [Google Scholar] [CrossRef]
- Donnelly, D.J.; Donnelly, J.A.; Murphy, J.J.; Philbin, E.M.; Wheeler, T.S. Steric effects on the cyclisation of chalcone dibromides. Chem. Commun. 1966, 12, 351–352. [Google Scholar] [CrossRef]
- Hutchins, W.A.; Wheeler, T.S. 17. Chalkones: A new synthesis of chrysin, apigenin, and luteolin. J. Chem. Soc. 1939, 83, 91. [Google Scholar] [CrossRef]
- Fitzgerald, D.M.; O’Sullivan, J.F.; Philbin, E.M.; Wheeler, T.S. Ring expansion of 2-benzylidenecoumaran-3-ones—A synthesis of flavones. J. Chem. Soc. 1955, 860, 860–862. [Google Scholar] [CrossRef]
- Donnelly, J.A.; Doran, H.J. Chalcone dihalides-VII. The course of the cyclization of 2′-hydroxy-6′-methoxyl derivatives. Tetrahedron 1975, 31, 1565–1569. [Google Scholar] [CrossRef]
- Donnelly, J.A.; Doran, H.J. Chalcone dihalides-VI. Generalisation of their use in the synthesis of naturally occurring flavones. Tetrahedron Lett. 1974, 15, 4083–4084. [Google Scholar] [CrossRef]
- Mahal, H.S.; Rai, H.S.; Venkataraman, K. Synthetical experiments in the chromone group. Part XVI. Chalkones and flavanones and their oxidation to flavones by means of selenium dioxide. J. Chem. Soc. 1935, 866, 866–868. [Google Scholar] [CrossRef]
- Kostanecki, V.S.; Szabranski, W. Synthese des Flavonols. Berichte Dtsch. Chem. Ges. 1904, 37, 2819–2820. [Google Scholar] [CrossRef]
- Auwers, K.; Müller, K. Umwandlung von Benzal-cumaranonen in Flavonole. Berichte Dtsch. Chem. Ges. 1908, 41, 4233–4241. [Google Scholar] [CrossRef]
- Stoermer, R.; Bartsch, F. Synthesen des Cumaranons (Ketocumarans) und seiner Homologen aus Phenoxyessigsäuren. Berichte Dtsch. Chem. Ges. 1900, 33, 3175–3181. [Google Scholar] [CrossRef]
- Auwers, K.V.; Pohl, P. Über die Umwandlung von Benzalcumaranonen in Flavonole. Justus Liebig’s Ann. Chem. 1914, 405, 243–294. [Google Scholar] [CrossRef]
- Auwers, K.V. Zur Bildung von Flavonolen aus Benzal-cumaranonen. Berichte Dtsch. Chem. Ges. 1916, 49, 809–819. [Google Scholar] [CrossRef]
- Allan, J.; Robinson, R. CCXC.—An accessible derivative of chromonol. J. Chem. Soc. 1924, 125, 2192–2195. [Google Scholar] [CrossRef]
- Wheeler, T.S. Flavone. Org. Synth. 1952, 32, 72. [Google Scholar]
- Allan, J.; Robinson, R. CCCIX.—A new synthesis of fisetin and of quercetin. J. Chem. Soc. 1926, 129, 2334–2336. [Google Scholar] [CrossRef]
- Kalff, J.; Robinson, R. CCLXIV.—A synthesis of datiscetin. J. Chem. Soc. Trans. 1925, 127, 1968–1973. [Google Scholar] [CrossRef]
- Kalff, J.; Robinson, R. XXVIII.—A synthesis of myricetin and of a galangin monomethyl ether occurring in galanga root. J. Chem. Soc. Trans. 1925, 127, 181–184. [Google Scholar] [CrossRef]
- Dreyer, D.L.; Tabata, S.; Horowitz, R.M. Flavonoids of citrus—VIII. Tetrahedron 1964, 20, 2977–2983. [Google Scholar] [CrossRef]
- Robinson, R.; Shinoda, J. CCLXV.—The synthesis of certain 2-styrylchromonol derivatives. J. Chem. Soc. Trans. 1925, 127, 1973–1980. [Google Scholar] [CrossRef]
- Fukui, K.; Nakayama, M.; Horie, T. Synthetic Studies of the Flavone Derivatives. IX. The Syntheses of Axillarin and Its Related Compounds. Bull. Chem. Soc. Jpn. 1969, 42, 1649–1652. [Google Scholar] [CrossRef]
- Fukui, K.; Matsumoto, T.; Nakamura, S.; Nakayama, M.; Horie, T. Synthetic Studies of the Flavone Derivatives. VII. The Synthesis of Jaceidin. Bull. Chem. Soc. Jpn. 1968, 41, 1413–1417. [Google Scholar] [CrossRef]
- Baker, W. Molecular Rearrangement of Some o-Acyloxyacetophenones. J. Chem. Soc. 1933, 1933, 1381–1389. [Google Scholar] [CrossRef]
- Mahal, H.S.; Venkataraman, K. A Synthesis of Flavones at Room Temperature. Curr. Sci. 1933, 2, 214–215. [Google Scholar]
- Chadha, T.C.; Venkataraman, K. 256. Synthetical experiments in the chromone group. Part VIII. Derivatives of o-hydroxy-,2:5-dihydroxy-, and 2:4:5-trihydroxy-acetophenone. J. Chem. Soc. 1933, 1933, 1073. [Google Scholar] [CrossRef]
- Mahal, H.S.; Venkataraman, K. 387. Synthetical experiments in the chromone group. Part XIV. The action of sodamide on 1-acyloxy-2-acetonaphthones. J. Chem. Soc. 1934, 1934, 1767. [Google Scholar] [CrossRef]
- Ameen, D.; Snape, T.J. Mechanism and application of Baker-Venkataraman O→C acyl migration reactions. Synthesis 2015, 47, 141–158. [Google Scholar] [CrossRef]
- Cramer, F.; Elschnig, G.H. Über Einschluß-verbindungen, IX. Mitteil.: Die blauen Jodverbindungen der Flavone. Chem. Ber. 1956, 89, 1–12. [Google Scholar] [CrossRef]
- Ares, J.J.; Outt, P.E.; Kakodkar, S.V.; Buss, R.C.; Geiger, J.C. A Convenient Large-Scale Synthesis of 5-Methoxyflavone and Its Application to Analog Preparation. J. Org. Chem. 1993, 58, 7903–7905. [Google Scholar] [CrossRef]
- Jain, P.K.; Makrandi, J.K.; Grover, S.K. A Facile Baker-Venkataraman Synthesis of Flavones using Phase Transfer Catalysis. Synthesis 1982, 1982, 221–222. [Google Scholar] [CrossRef]
- Saxena, S.; Makrandi, J.; Grover, S. Synthesis of 5- and/or 7-Hydroxyflavones using A Modified Phase Transfer-Catalysed Baker-Venkataraman Transformation. Synthesis 1985, 1985, 697. [Google Scholar] [CrossRef]
- Song, G.-Y.; Ahn, B.-Z. Synthesis of dibenzoylmethanes as intermediates for flavone synthesis by a modified Baker-Venkataraman rearrangement. Arch. Pharm. Res. 1994, 17, 434–437. [Google Scholar] [CrossRef]
- Stanek, F.; Stodulski, M. Mild and efficient organocatalytic method for the synthesis of flavones. Tetrahedron Lett. 2016, 57, 3841–3843. [Google Scholar] [CrossRef]
- Pinto, D.C.G.A.; Silva, A.M.S.; Cavaleiro, J.A.S. Baker-Venkataraman rearrangement under microwave irradiation: A new strategy for the synthesis of 3-aroyl-5-hydroxyflavones. Synthesis 2007, 12, 1897–1900. [Google Scholar] [CrossRef]
- Sarda, S.R.; Pathan, M.Y.; Paike, V.V.; Pachmase, P.R.; Jadhav, W.N.; Pawar, R.P. A facile synthesis of flavones using recyclable ionic liquid under microwave irradiation. Arkivoc 2006, 2006, 43–48. [Google Scholar] [CrossRef]
- Miyake, H.; Nishino, S.; Nishimura, A.; Sasaki, M. New synthesis of 3-bromoflavones via bromination of 1-(2-hydroxyphenyl)-3- arylpropane-1,3-dione by CuBr2, and conversion into 3-aminoflavones. Chem. Lett. 2007, 36, 522–523. [Google Scholar] [CrossRef]
- Zubaidha, P.K.; Hashmi, A.M.; Bhosale, R.S. FeCl3 catalyzed dehydrative cyclisation of 1, 3-(diaryl diketones) to flavones. Heterocycl. Commun. 2005, 11, 97–100. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Mereddy, A.R. Microwave-assisted synthesis of functionalized flavones and chromones. Tetrahedron Lett. 2005, 46, 6315–6317. [Google Scholar] [CrossRef]
- Nishinaga, A.; Ando, H.; Maruyama, K.; Mashino, T. A New Metal Complex Promoted System for Highly Selective Synthesis of 4 H -Chromen-4-ones (Chromones). Synthesis 1992, 1992, 839–841. [Google Scholar] [CrossRef]
- Varma, R.S.; Saini, R.K.; Kumar, D. An Expeditious Synthesis of Flavones on Montmorillonite K 10 Clay with Microwaves. J. Chem. Res. Synop. 1998, 6, 348–349. [Google Scholar] [CrossRef]
- Hoshino, Y.; Takeno, N. A Facile Preparation of Flavones Using Nonaqueous Cation-Exchange Resin. Bull. Chem. Soc. Jpn. 1987, 60, 1919–1920. [Google Scholar] [CrossRef]
- Pérez, M.E.; Ruiz, D.M.; Autino, J.C.; Blanco, M.N.; Pizzio, L.R.; Romanelli, G.P. Mesoporous titania/tungstophosphoric acid composites: Suitable synthesis of flavones. J. Porous Mater. 2013, 20, 1433–1440. [Google Scholar] [CrossRef]
- Bennardi, D.O.; Romanelli, G.P.; Autino, J.C.; Pizzio, L.R. Supported trifluoromethanesulfonic acid as catalyst in the synthesis of flavone and chromone derivatives. Appl. Catal. A Gen. 2007, 324, 62–68. [Google Scholar] [CrossRef]
- Jorge, L.; Autino, C. Synthesis of substituted flavones and chromones using a Wells-Dawson heteropolyacid as catalyst. Arkivoc 2008, 11, 123. [Google Scholar]
- Bennardi, D.O.; Romanelli, G.P.; Jios, J.L.; Vázquez, P.G.; Cáceres, C.V.; Autino, C. Synthesis of substituted flavones and arylchromones using ρ and si keggin heteropolyacids as catalysts. Heterocycl. Commun. 2007, 13, 77–82. [Google Scholar] [CrossRef]
- Kucukislamoglu, M.; Nebioglu, M.; Zengin, M.; Arslan, M.; Yayli, N. An environmentally benign synthesis of flavones from 1,3-diketones using silica gel supported NaHSO4 catalyst. J. Chem. Res. 2005, 9, 556–557. [Google Scholar] [CrossRef]
- Oyamada, T. A new general method for the synthesis of the derivates of flavonol. Bull. Chem. Soc. Jpn. 1935, 10, 182–186. [Google Scholar] [CrossRef]
- Algar, J.; Flynn, J. A New Method for the Synthesis of Flavonols. Proc. R. Ir. Acad. B 1934, 42, 1–8. [Google Scholar]
- Serdiuk, I.E.; Roshal, A.D.; Błażejowski, J. Quantum-chemical analysis of the Algar-Flynn-Oyamada reaction mechanism. Chem. Heterocycl. Compd. 2014, 50, 396–403. [Google Scholar] [CrossRef]
- Dean, F.M.; Podimuang, V. 737. The course of the Algar–Flynn–Oyamada (A.F.O.) reaction. J. Chem. Soc. 1965, 3978, 3978–3987. [Google Scholar] [CrossRef]
- Adams, C.J.; Main, L. Synthesis of 2′-hydroxychalcone epoxides. Tetrahedron 1991, 47, 4959–4978. [Google Scholar] [CrossRef]
- Bennett, M.; Burke, A.J.; Ivo O’Sullivan, W. Aspects of the Algar-Flynn-Oyamada (AFO) reaction. Tetrahedron 1996, 52, 7163–7178. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Hatua, K. Computational insight of the mechanism of Algar-Flynn-Oyamada (AFO) reaction. RSC Adv. 2014, 4, 18702–18709. [Google Scholar] [CrossRef]
- Fougerousse, A.; Gonzalez, E.; Brouillard, R. A convenient method for synthesizing 2-aryl-3-hydroxy-4-oxo-4H-1- benzopyrans or flavonols. J. Org. Chem. 2000, 65, 583–586. [Google Scholar] [CrossRef]
- Cummins, B.; Donnelly, D.; Eades, J.; Fletcher, H.; Cinnéide, F.; Philbin, E.; Swirski, J.; Wheeler, T.; Wilson, R. Oxidation of chalcones (AFO reaction). Tetrahedron 1963, 19, 499–512. [Google Scholar] [CrossRef]
- Shen, X.; Zhou, Q.; Xiong, W.; Pu, W.; Zhang, W.; Zhang, G.; Wang, C. Synthesis of 5-subsituted flavonols via the Algar-Flynn-Oyamada (AFO) reaction: The mechanistic implication. Tetrahedron 2017, 73, 4822–4829. [Google Scholar] [CrossRef]
- Nhu, D.; Hawkins, B.C.; Burns, C.J. Phase transfer catalysis extends the scope of the Algar-Flynn-Oyamada synthesis of 3-Hydroxyflavones. Aust. J. Chem. 2015, 68, 1102–1107. [Google Scholar] [CrossRef]
- Jain, A.C.; Gupta, S.M.; Sharma, A. Synthesis of 2,2-Dimethyl-2 H-pyran-fused Flavonols Using the Modified Algar-Flynn-Oyamada Reaction. Bull. Chem. Soc. Jpn. 1983, 56, 1267–1268. [Google Scholar] [CrossRef]
- Dharia, J.R.; Johnson, K.F.; Schlenoff, J.B. Synthesis and Characterization of Wavelength-Shifting Monomers and Polymers Based on 3-Hydroxyflavone. Macromolecules 1994, 27, 5167–5172. [Google Scholar] [CrossRef]
- Tong, J.; Liu, C.; Wang, B. Improved Synthesis of Icaritin and Total Synthesis of β-Anhydroicaritin. Chem. Res. Chin. Univ. 2019, 35, 616–620. [Google Scholar] [CrossRef]
- Ashraf, J.; Mughal, E.U.; Sadiq, A.; Bibi, M.; Naeem, N.; Ali, A.; Massadaq, A.; Fatima, N.; Javid, A.; Zafar, M.N.; et al. Exploring 3-hydroxyflavone scaffolds as mushroom tyrosinase inhibitors: Synthesis, X-ray crystallography, antimicrobial, fluorescence behaviour, structure-activity relationship and molecular modelling studies. J. Biomol. Struct. Dyn. 2020, 39, 7107–7122. [Google Scholar] [CrossRef]
- Simiti, I.; Zaharia, V.; Mager, S.; Horn, M.; Koteles-Popa, T. Heterocyclen 67. Mitt.: Darstellung und charakterisierung einiger 2-(2-aryl-thiazol-4-yl)-3-hydroxy-chromone. Arch. Pharm. 1991, 324, 913–915. [Google Scholar] [CrossRef]
- Zaharia, V.; Imre, S.; Palibroda, N. Heterocycles. Obtaining and physico-chemical characterization of some thiazolo and thiazolo [3,2-b][1,2,4] triazolic hydroxy-heterochalcones. Rev. Chim. 2009, 4, 391–397. [Google Scholar]
- Enchev, V.; Mehandzhiyski, A.Y. Computational insight on the chalcone formation mechanism by the Claisen–Schmidt reaction. Int. J. Quantum Chem. 2017, 117, e25365. [Google Scholar] [CrossRef]
- Patonay, T.; Cavaleiro, J.A.S.; Lévai, A.; Silva, A.M.S. Dehydrogenation by iodine-dimethylsulfoxide system: A general route to susbtituted chromones and thiochromones. Heterocycl. Commun. 1997, 3, 223–229. [Google Scholar] [CrossRef]
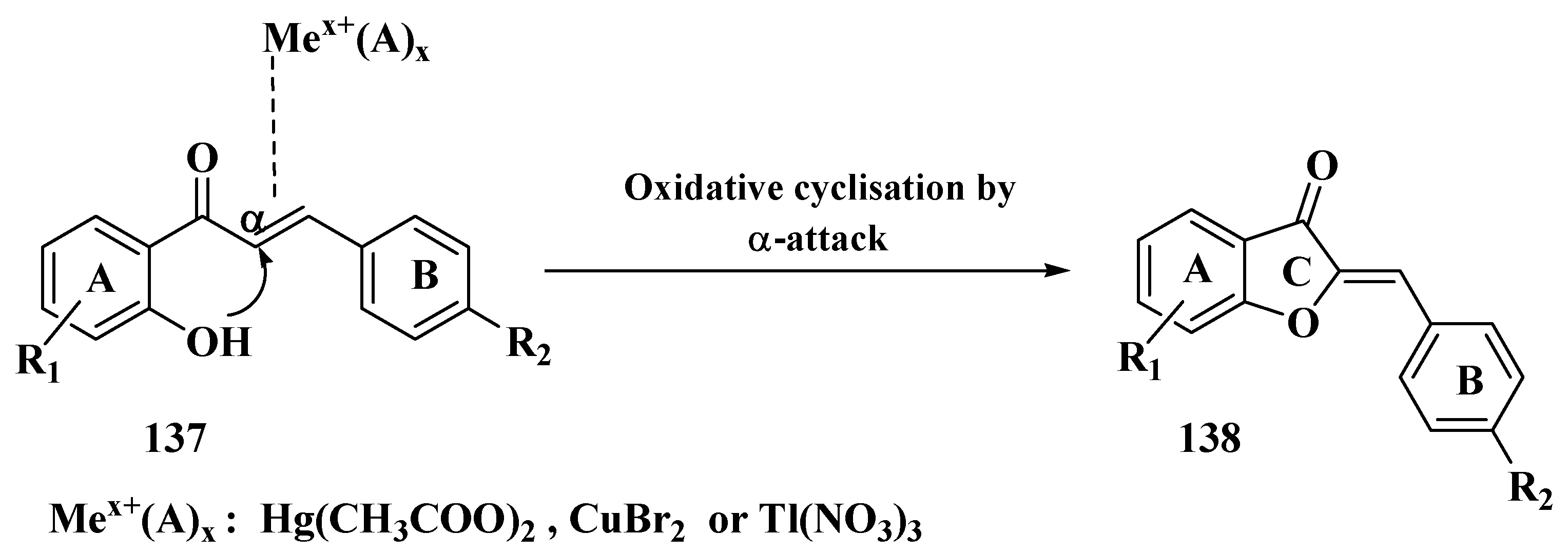
- Masesane, I.B. A comprehensive review of the oxidative cyclisation of 2′-hydroxychalcones to aurones and flavones. Int. J. Chem. Stud. 2015, 3, 53–59. [Google Scholar]
- Menezes, M.J.; Manjrekar, S.; Pai, V.; Patre, R.E.; Tilve, S.G. A facile microwave assisted synthesis of flavones. Indian J. Chem Sect. B Org. Med. Chem. 2009, 48, 1311–1314. [Google Scholar]
- Hans, N.; Grover, S.K. An efficient conversion of 2′-hydroxychalcones to flavones. Synth. Commun. 1993, 23, 1021–1023. [Google Scholar] [CrossRef]
- Constantinescu, T.; Leonte, D.; Bencze, L.C.; Vlase, L.; Imre, S.; Hanganu, D.; Zaharia, V. Heterocycles 43. Synthesis, characterization and antioxidant activity of some thiazole hydroxychalcones and their flavonoidic derivatives. Farmacia 2018, 66, 663–673. [Google Scholar] [CrossRef]
- Litkei, G.; Gulácsi, K.; Antus, S.; Blaskó, G. Cyclodehydrogenation of 2′-hydroxychalcones with hypervalent iodine reagent: A new synthesis of flavones. Liebigs Ann. 1995, 1995, 1711–1715. [Google Scholar] [CrossRef]
- Gulácsi, K.; Litkei, G.; Antus, S.; Gunda, T.E. A short and facile synthetic route to prenylated flavones. Cyclodehydrogenation of prenylated 2′-hydroxychalcones by a hypervalent iodine reagent. Tetrahedron 1998, 54, 13867–13876. [Google Scholar] [CrossRef]
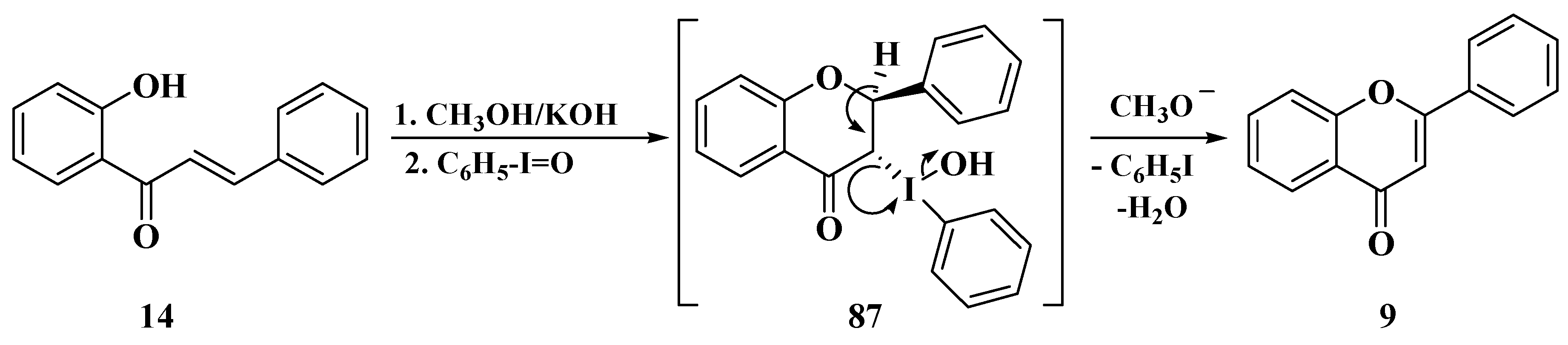
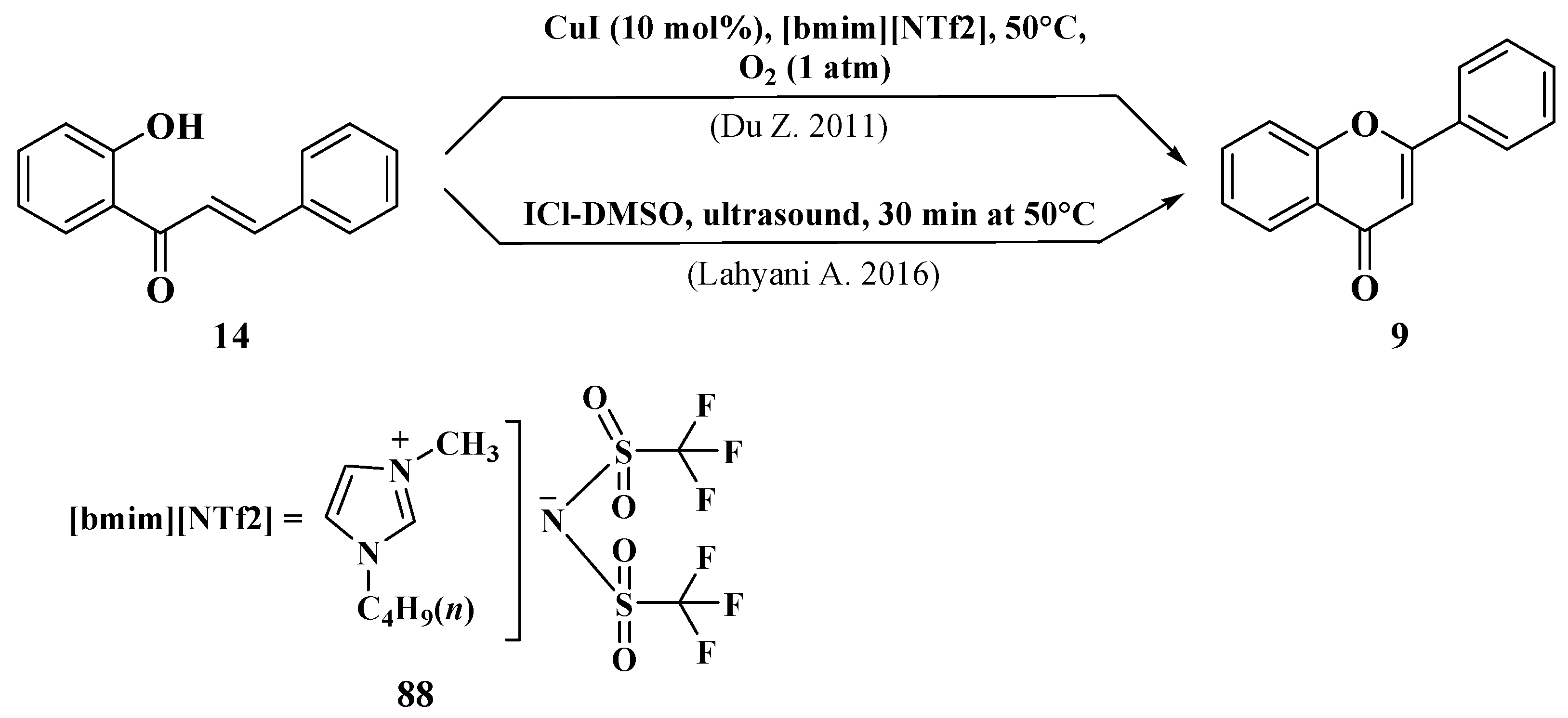
- Du, Z.; Ng, H.; Zhang, K.; Zeng, H.; Wang, J. Ionic liquid mediated Cu-catalyzed cascade oxa-Michael-oxidation: Efficient synthesis of flavones under mild reaction conditions. Org. Biomol. Chem. 2011, 9, 6930–6933. [Google Scholar] [CrossRef]
- Lahyani, A.; Trabelsi, M. Ultrasonic-assisted synthesis of flavones by oxidative cyclization of 2′-hydroxychalcones using iodine monochloride. Ultrason. Sonochem. 2016, 31, 626–630. [Google Scholar] [CrossRef]
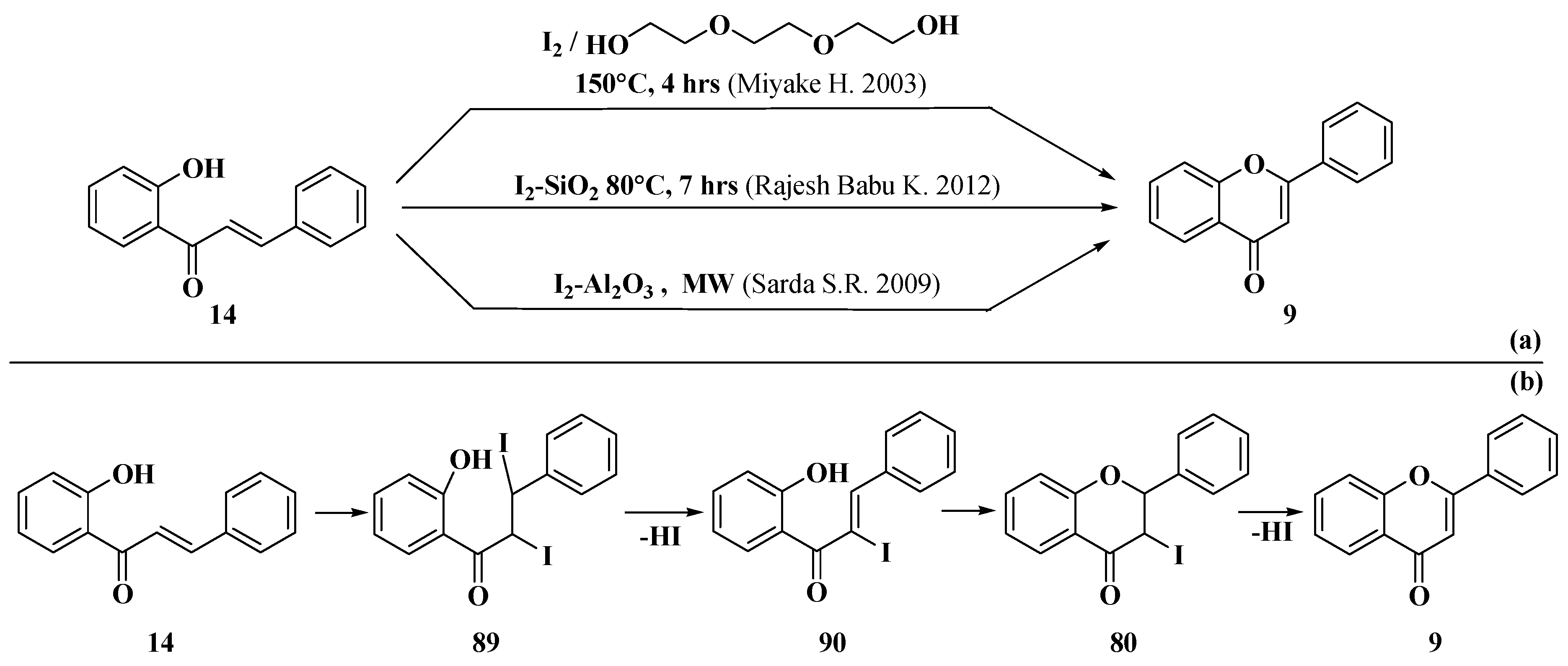
- Miyake, H.; Takizawa, E.; Sasaki, M. Syntheses of flavones via the iodine-mediated oxidative cyclization of 1,3-diphenylprop-2-en-1-ones. Bull. Chem. Soc. Jpn. 2003, 76, 835–836. [Google Scholar] [CrossRef]
- Rajesh Babu, K.; Vijaya Kumar, K.; Vijaya, M.; Madhavarao, V. A novel solid supported synthesis of flavones. Int. J. Pharm. Technol. 2012, 4, 3943–3950. [Google Scholar]
- Sarda, S.R.; Jadhav, W.N.; Pawar, R.P. I2-Al2O3: A suitable heterogeneous catalyst for the synthesis of flavones under microwave irradiation. Int. J. ChemTech Res. 2009, 1, 539–543. [Google Scholar]
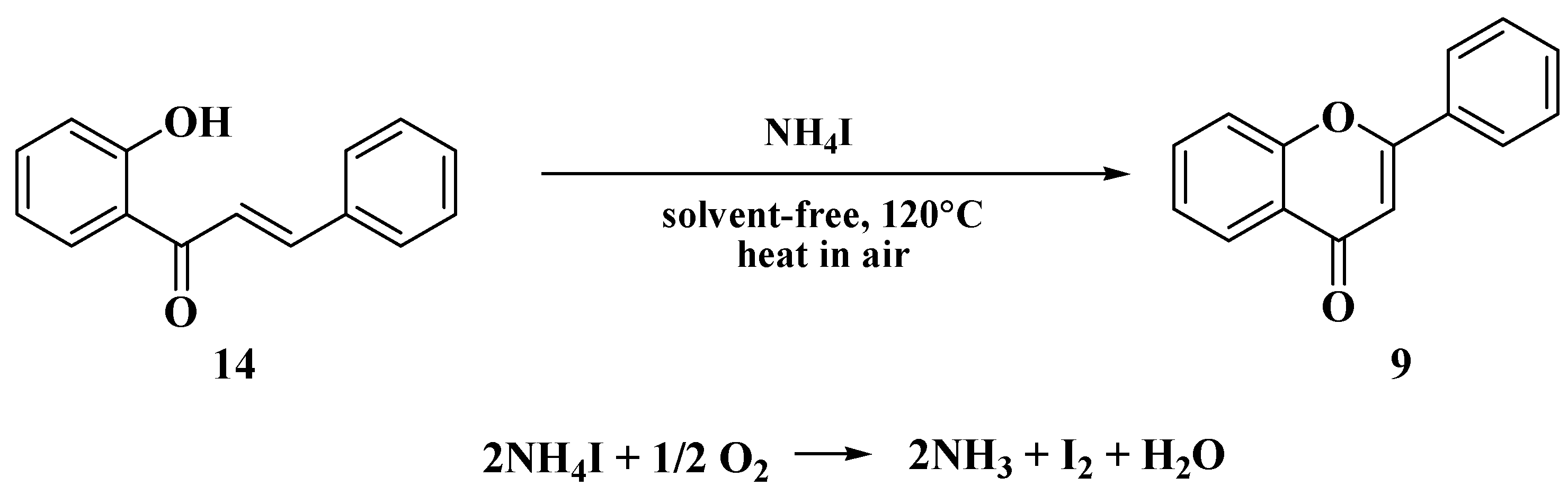
- Kulkarni, P.S.; Kondhare, D.D.; Varala, R.; Zubaidha, P.K. Cyclization of 2′-hydroxychalcones to flavones using ammonium iodide as an iodine source—An eco-friendly approach. J. Serbian Chem. Soc. 2013, 78, 909–916. [Google Scholar] [CrossRef]
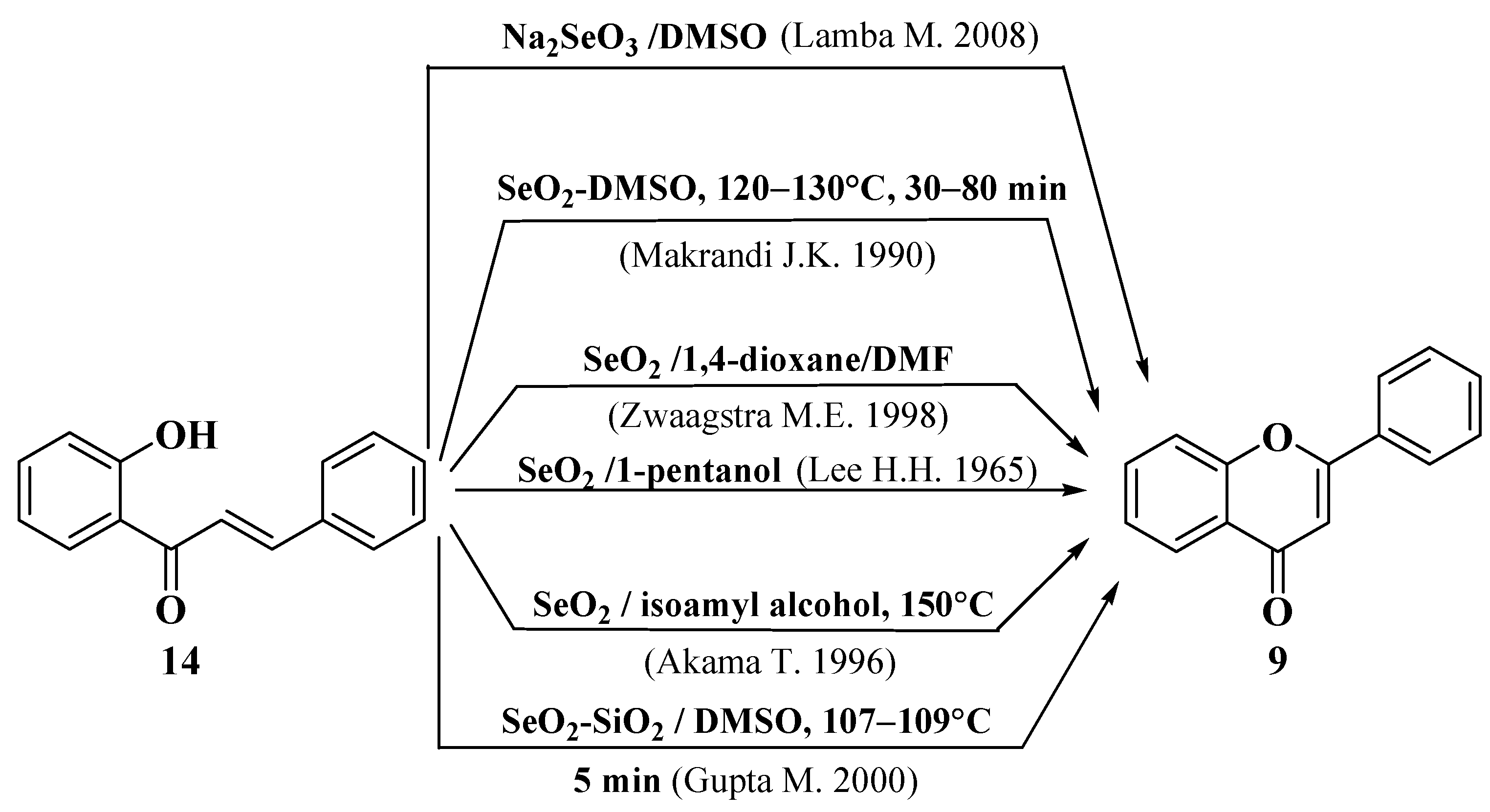
- Lee, H.H.; Tan, C.H. 496. Syntheses of flavones from Lindera lucida. J. Chem. Soc. 1965, 1965, 2743–2749. [Google Scholar] [CrossRef]
- Zwaagstra, M.E.; Timmerman, H.; van de Stolpe, A.C.; de Kanter, F.J.; Tamura, M.; Wada, Y.; Zhang, M.Q. Synthesis and structure—Activity relationships of carboxyflavones as structurally rigid CysLT1 (LTD4) receptor antagonists. J. Med. Chem. 1998, 41, 1428–1438. [Google Scholar] [CrossRef]
- Akama, T.; Shida, Y.; Sugaya, T.; Ishida, H.; Gomi, K.; Kasai, M. Novel 5-aminoflavone derivatives as specific antitumor agents in breast cancer. J. Med. Chem. 1996, 39, 3461–3469. [Google Scholar] [CrossRef] [PubMed]
- Makrandi, J.K.; Seema, S. ChemInform Abstract: An Efficient Procedure for Cyclization of 2′-Hydroxychalcones into Flavones. ChemInform 1990, 21, 181. [Google Scholar] [CrossRef]
- Gupta, M.; Paul, S.; Gupta, R.; Loupy, A. A rapid method for the cyclization of 2′-hydroxychalcones into flavones. Org. Prep. Proced. Int. 2000, 32, 280–283. [Google Scholar] [CrossRef]
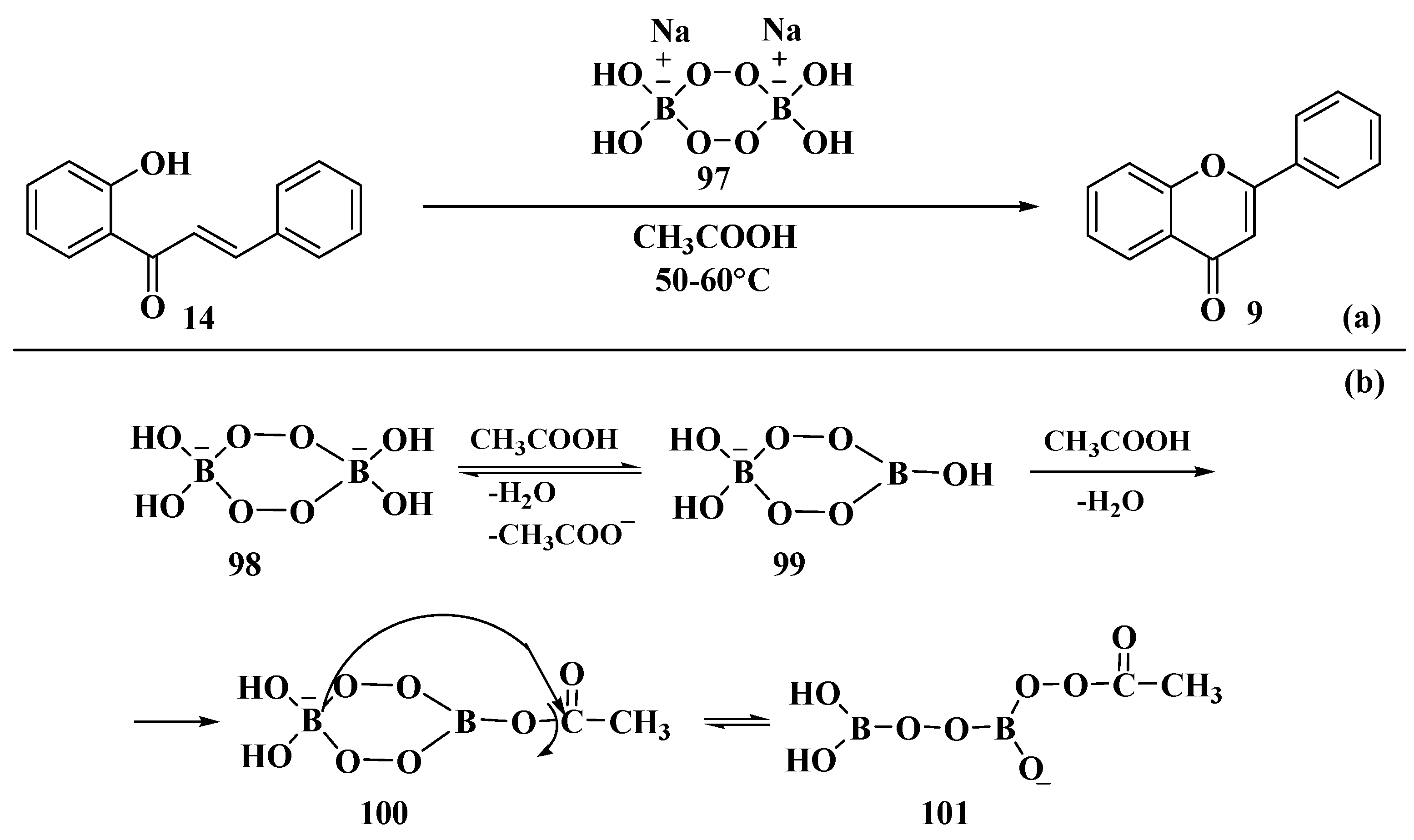
- Lamba, M.; Makrandi, J.K. Sodium selenite-dimethylsulfoxide: A highly efficient reagent for dehydrogenation. J. Chem. Res. 2008, 4, 225–226. [Google Scholar] [CrossRef]
- Kasahara, A.; Izumi, T.; Ooshima, M. A New Method of Preparing Flavones. Bull. Chem. Soc. Jpn. 1974, 47, 2526–2528. [Google Scholar] [CrossRef]
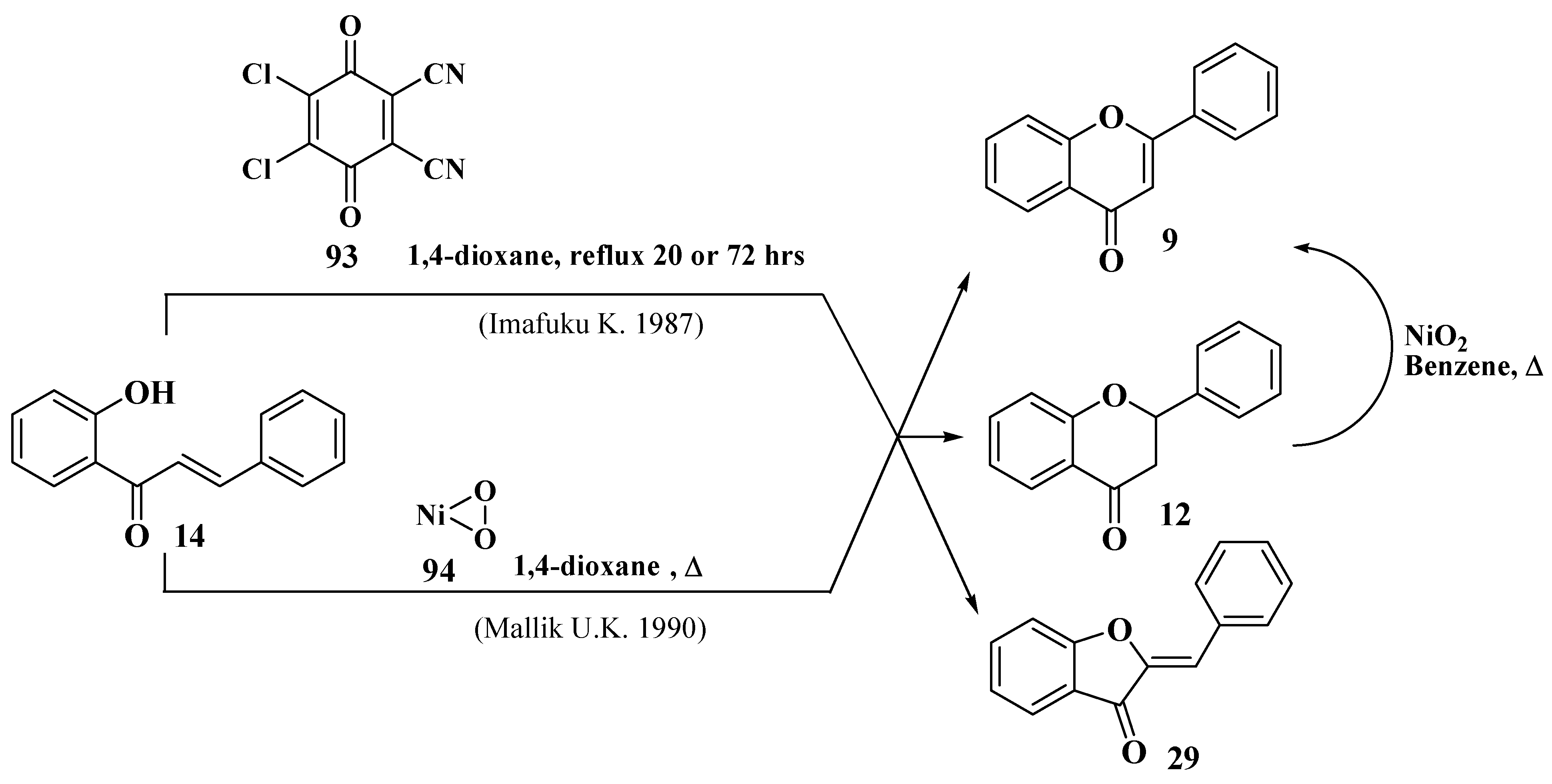
- Imafuku, K.; Honda, M.; Mcomie, J.F. Cyclodehydrogenation of 2′-Hydroxychalcones with DDQ: A Simple Route for Flavones and Aurones. Synthesis 1987, 1987, 199–201. [Google Scholar] [CrossRef]
- Mallik, U.K.; Saha, M.M.; Mallik, A.K. ChemInform Abstract: Cyclodehydrogenation of 2′-Hydroxychalcones and Dehydrogenation of Flavanones Using Nickel Peroxide. Abstract 182. ChemInform 1990, 21, 116. [Google Scholar] [CrossRef]
- Hoshino, Y.; Oohinata, T.; Takeno, N. The Direct Preparation of Flavones from 2′-Hydroxychalcones Using Disulfides. Bull. Chem. Soc. Jpn. 1986, 59, 2351–2352. [Google Scholar] [CrossRef]
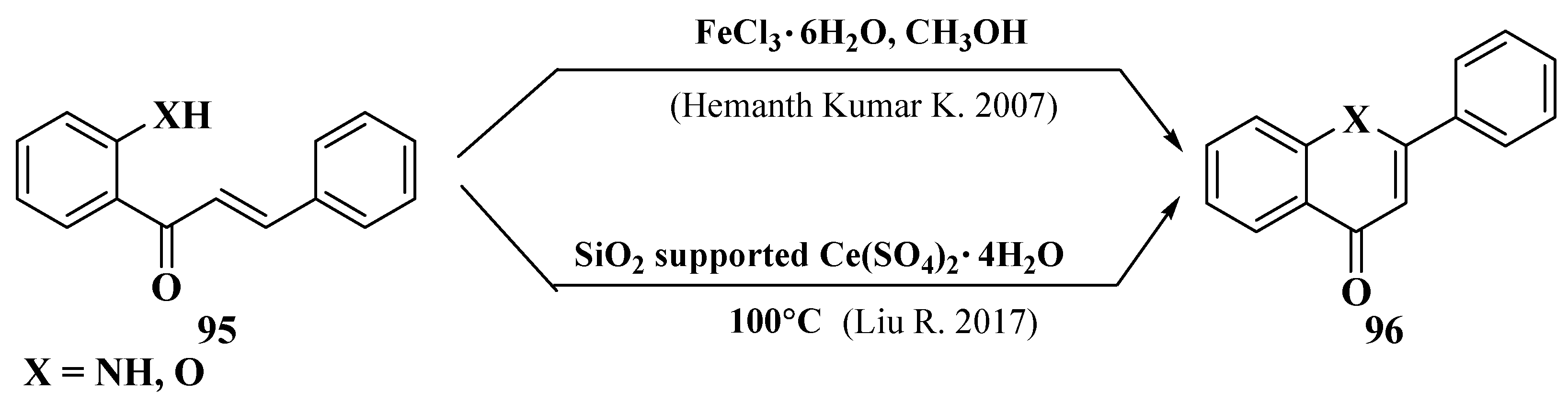
- Hemanth Kumar, K.; Perumal, P.T. A novel one-pot oxidative cyclization of 2′-amino and 2′-hydroxychalcones employing FeCl3•6H2O-methanol. Synthesis of 4-alkoxy-2-aryl-quinolines and flavones. Tetrahedron 2007, 63, 9531–9535. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Xu, K.; Tan, G. Silica-gel-supported Ce(SO4)2•4H2O-mediated cyclization of 2′-amino and 2′-hydroxychalcones under solvent-free conditions. Synth. Commun. 2017, 47, 1–9. [Google Scholar] [CrossRef]
- Ganguly, N.C.; Chandra, S.; Barik, S.K. Sodium perborate tetrahydrate-mediated transformations of 2′-hydroxychalcones to flavanones, flavones, and 3′,5′-diiodoflavone under mild, environmentally friendly conditions. Synth. Commun. 2013, 43, 1351–1361. [Google Scholar] [CrossRef]
- McKillop, A.; Kemp, D. Further functional group oxidations using sodium perborate. Tetrahedron 1989, 45, 3299–3306. [Google Scholar] [CrossRef]
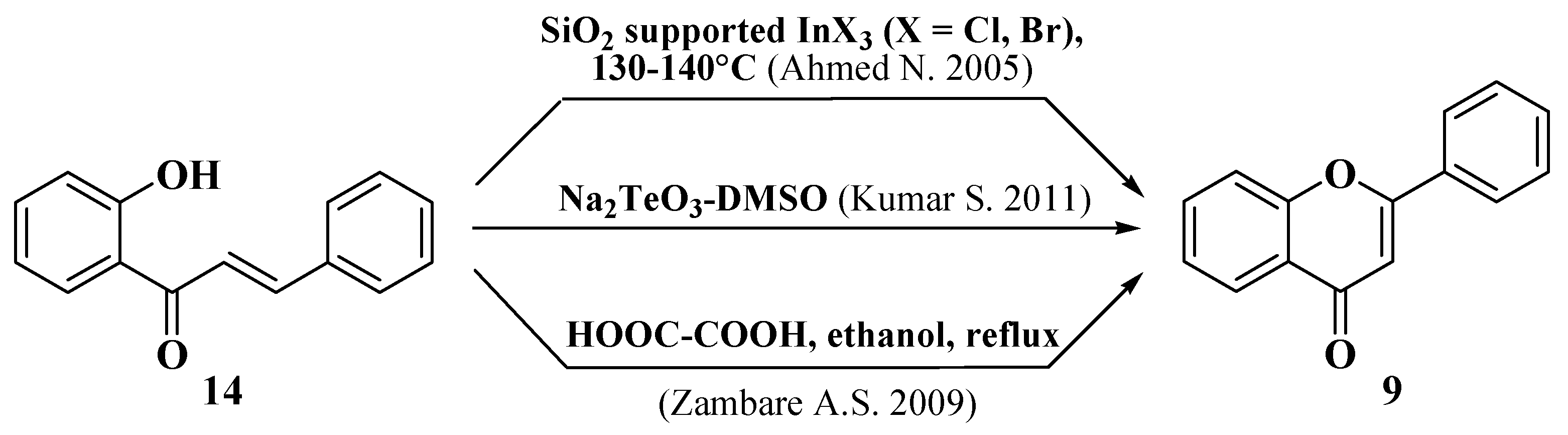
- Ahmed, N.; Ali, H.; Van Lier, J.E. Silica gel supported InBr3 and InCl3: New catalysts for the facile and rapid oxidation of 2′-hydroxychalcones and flavanones to their corresponding flavones under solvent free conditions. Tetrahedron Lett. 2005, 46, 253–256. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, D. Oxidative cyclisation of 2′-hydroxychalcones using sodium tellurite: Synthesis of flavones. Orient. J. Chem. 2011, 27, 761–763. [Google Scholar]
- Zambare, A.S.; Sangshetti, J.N.; Kokare, N.D.; Shinde, D.B. Development of mild and efficient method for synthesis of substituted flavones using oxalic acid catalyst. Chin. Chem. Lett. 2009, 20, 171–174. [Google Scholar] [CrossRef]
- Matsushima, R.; Kageyama, H. Photochemical cyclization of 2′-hydroxychalcones. J. Chem. Soc. Perkin Trans. II 1985, 53, 743–748. [Google Scholar] [CrossRef]
- Maki, Y.; Shimada, K.; Sako, M.; Hirota, K. Photo-oxidative cyclisation of 2′-hydroxychalcones leading to flavones induced by heterocycle-oxides: High efficiency of pybimido[54-]pteridine -oxide for the photochemical dehydrogenation. Tetrahedron 1988, 44, 3187–3194. [Google Scholar] [CrossRef]
- Saničanin, Z.; Tabaković, I. Electrochemical transformations of 2-hydroxychalcones into flavanoids. Tetrahedron Lett. 1986, 27, 407–408. [Google Scholar] [CrossRef]
- Tamuli, K.J.; Sahoo, R.K.; Bordoloi, M. A Biocatalytic Green Alternative to Existing Hazardous Reaction Medium: Synthesis of Chalcone and Flavone Derivatives via Claisen-Schmidt Reaction at Room Temperature. New J. Chem. 2020, 44, 20956–20965. [Google Scholar] [CrossRef]
- Nana, F.; Kuete, V.; Zaharia, V.; Ngameni, B.; Sandjo, L.P. Synthesis of Functionalized 1-Aryl-3-phenylthiazolylpropanoids and Their Potential as Anticancer Agents. ChemistrySelect 2020, 5, 7675–7678. [Google Scholar] [CrossRef]
- Wang, Z. Mentzer Pyrone Synthesis. In Compr Org Name React Reagents; Wang, Z., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2010; pp. 1901–1904. [Google Scholar]
- Von Pechmann, H.; Duisberg, C. Ueber die Verbindungen der Phenole mit Acetessigäther. Berichte Dtsch. Chem. Ges. 1883, 16, 2119–2128. [Google Scholar] [CrossRef]
- Mentzer, C.; Molho, D.; Vercier, P. Sur un nouveau mode de condensation desters beta-cetoniques et de phenols en chromones. Comptes Rendus Hebd. Seances L Acad. Sci. 1951, 232, 1488–1490. [Google Scholar]
- Seijas, J.A.; Vázquez-Tato, M.P.; Carballido-Reboredo, R. Solvent-Free Synthesis of Functionalized Flavones under Microwave Irradiation. J. Org. Chem. 2005, 70, 2855–2858. [Google Scholar] [CrossRef]
- Selepe, M.A.; Van Heerden, F.R. Application of the Suzuki-Miyaura reaction in the synthesis of flavonoids. Molecules 2013, 18, 4739–4765. [Google Scholar] [CrossRef] [PubMed]
- Kraus, G.A.; Gupta, V. Divergent approach to flavones and aurones via dihaloacrylic acids. Unexpected dependence on the halogen atom. Org. Lett. 2010, 12, 5278–5280. [Google Scholar] [CrossRef]
- Corbet, J.P.; Mignani, G. Selected patented cross-coupling reaction technologies. Chem. Rev. 2006, 106, 2651–2710. [Google Scholar] [CrossRef]
- Agrawal, N.N.; Soni, P.A. A new process for the synthesis of aurones by using mercury (II) acetate in pyridine and cupric bromide in dimethyl sulfoxide. Indian J. Chem Sect. B Org. Med. Chem. 2006, 45, 1301–1303. [Google Scholar] [CrossRef]
- Ameta, K.L.; Rathore, N.S.; Kumar, B.; Malaga, M.E.S.; Manuela Verastegui, P.; Gilman, R.H.; Verma, B.L. Synthesis and Trypanocidal Evaluation of Some Novel 2-(Substituted Benzylidene)-5,7-Dibromo-6-Hydroxy-1-Benzofuran-3(2H)-Ones. Int. J. Org. Chem. 2012, 2, 295–301. [Google Scholar] [CrossRef][Green Version]
- Thakkar, K.; Cushman, M. A Novel Oxidative Cyclization of 2′-Hydroxychalcones to 4,5-Dialkoxyaurones by Thallium(III) Nitrate. J. Org. Chem. 1995, 60, 6499–6510. [Google Scholar] [CrossRef]
- Khan, M.S.Y.; Mueed, M.A. Scope of mercuric acetate oxidation of chalcones and the antibacterial activity of resulting aurones. Ind. J. Chem. 2004, 43, 1794–1797. [Google Scholar] [CrossRef]
- Thanigaimalai, P.; Yang, H.M.; Sharma, V.K.; Kim, Y.; Jung, S.H. The scope of thallium nitrate oxidative cyclization of chalcones; synthesis and evaluation of isoflavone and aurone analogs for their inhibitory activity against interleukin-5. Bioorg. Med. Chem. 2010, 18, 4441–4445. [Google Scholar] [CrossRef] [PubMed]
- Meguellati, A.; Ahmed-Belkacem, A.; Yi, W.; Haudecoeur, R.; Crouillère, M.; Brillet, R.; Pawlotsky, J.-M.; Boumendjel, A.; Peuchmaur, M. B-ring modified aurones as promising allosteric inhibitors of hepatitis C virus RNA-dependent RNA polymerase. Eur. J. Med. Chem. 2014, 80, 579–592. [Google Scholar] [CrossRef]
- Li, Y.; Qiang, X.; Luo, L.; Yang, X.; Xiao, G.; Liu, Q.; Ai, J.; Tan, Z.; Deng, Y. Aurone Mannich base derivatives as promising multifunctional agents with acetylcholinesterase inhibition, anti-β-amyloid aggragation and neuroprotective properties for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017, 126, 762–775. [Google Scholar] [CrossRef]
- Sutton, C.L.; Taylor, Z.E.; Farone, M.B.; Handy, S.T. Antifungal activity of substituted aurones. Bioorg. Med. Chem. Lett. 2017, 27, 901–903. [Google Scholar] [CrossRef]
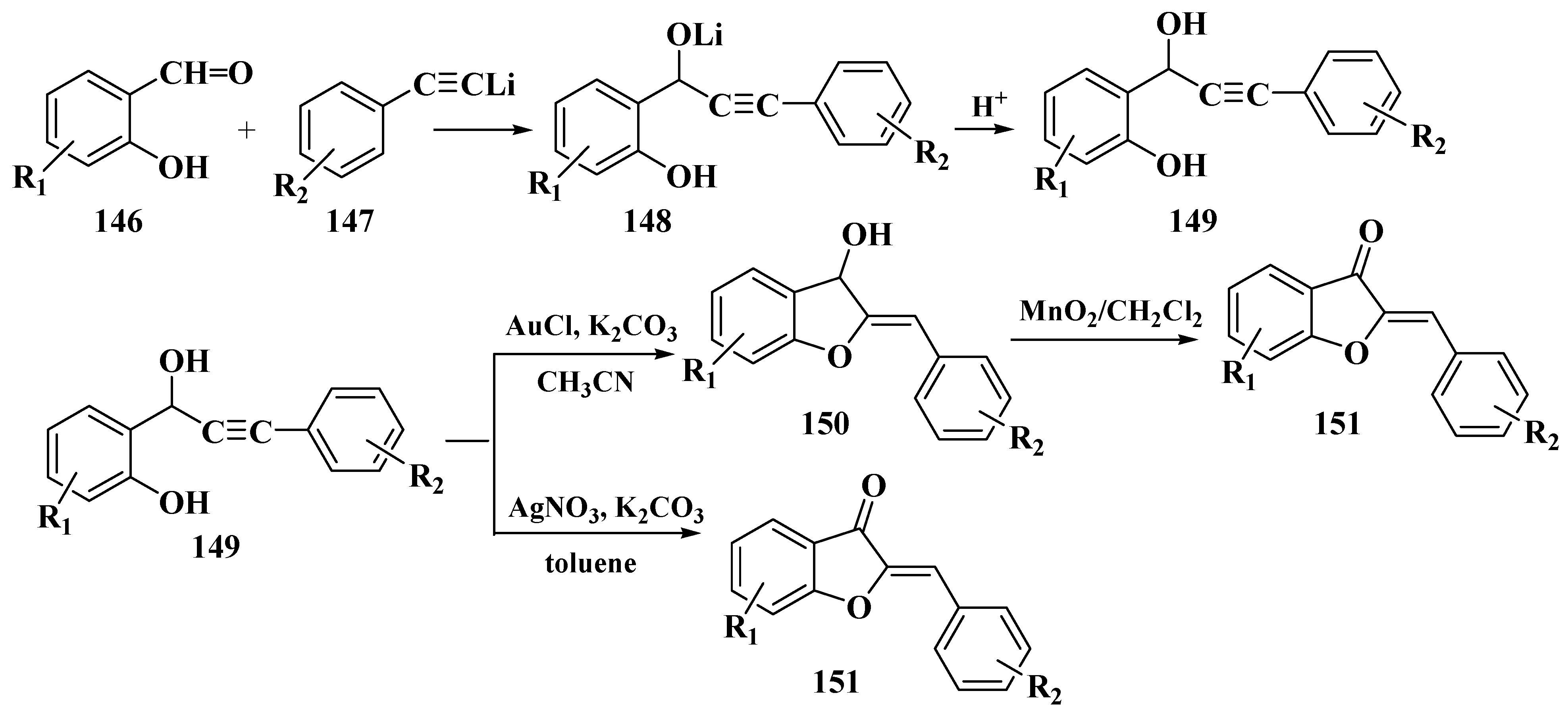
- Harkat, H.; Blanc, A.; Weibel, J.-M.; Pale, P. Versatile and Expeditious Synthesis of Aurones via Au I -Catalyzed Cyclization. J. Org. Chem. 2008, 73, 1620–1623. [Google Scholar] [CrossRef]
- Li, S.; Jin, F.; Viji, M.; Jo, H.; Sim, J.; Kim, H.S.; Lee, H.; Jung, J.-K. A novel cyclization/oxidation strategy for a two-step synthesis of (Z)-aurone. Tetrahedron Lett. 2017, 58, 1417–1420. [Google Scholar] [CrossRef]



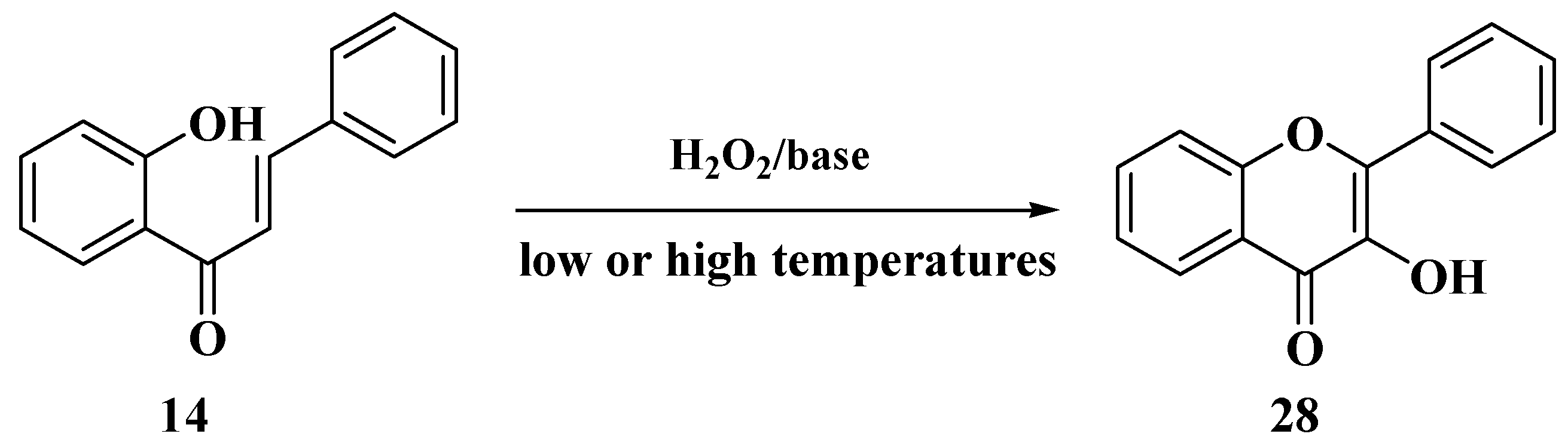

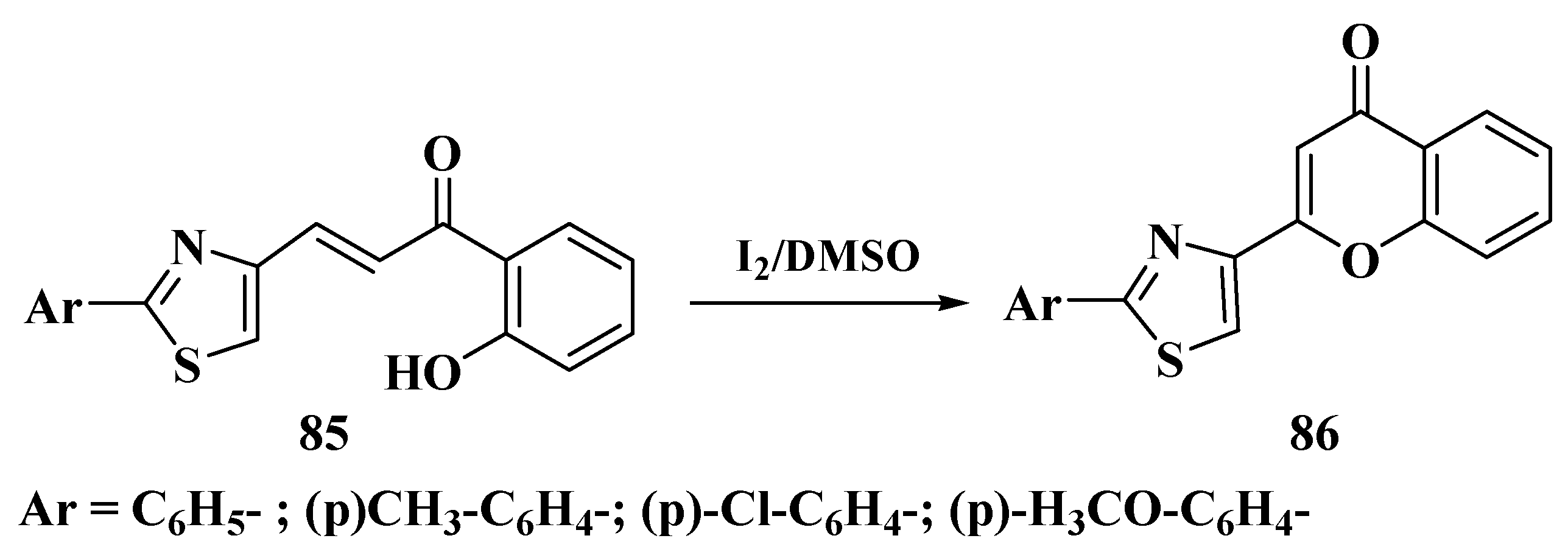







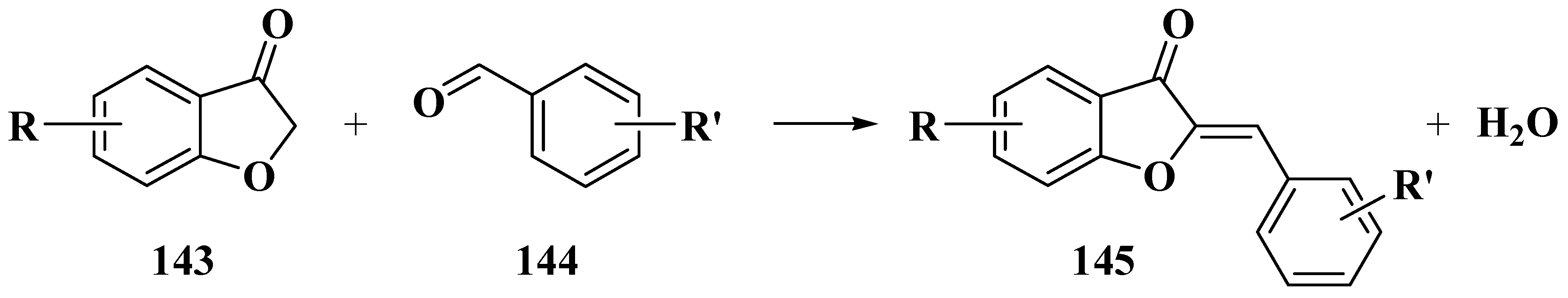
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonte, D.; Ungureanu, D.; Zaharia, V. Flavones and Related Compounds: Synthesis and Biological Activity. Molecules 2023, 28, 6528. https://doi.org/10.3390/molecules28186528
Leonte D, Ungureanu D, Zaharia V. Flavones and Related Compounds: Synthesis and Biological Activity. Molecules. 2023; 28(18):6528. https://doi.org/10.3390/molecules28186528
Chicago/Turabian StyleLeonte, Denisa, Daniel Ungureanu, and Valentin Zaharia. 2023. "Flavones and Related Compounds: Synthesis and Biological Activity" Molecules 28, no. 18: 6528. https://doi.org/10.3390/molecules28186528
APA StyleLeonte, D., Ungureanu, D., & Zaharia, V. (2023). Flavones and Related Compounds: Synthesis and Biological Activity. Molecules, 28(18), 6528. https://doi.org/10.3390/molecules28186528