Development of a Colorimetric Paper Sensor for Hg2+ Detection in Water Using Cyanuric Acid-Conjugated Gold Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
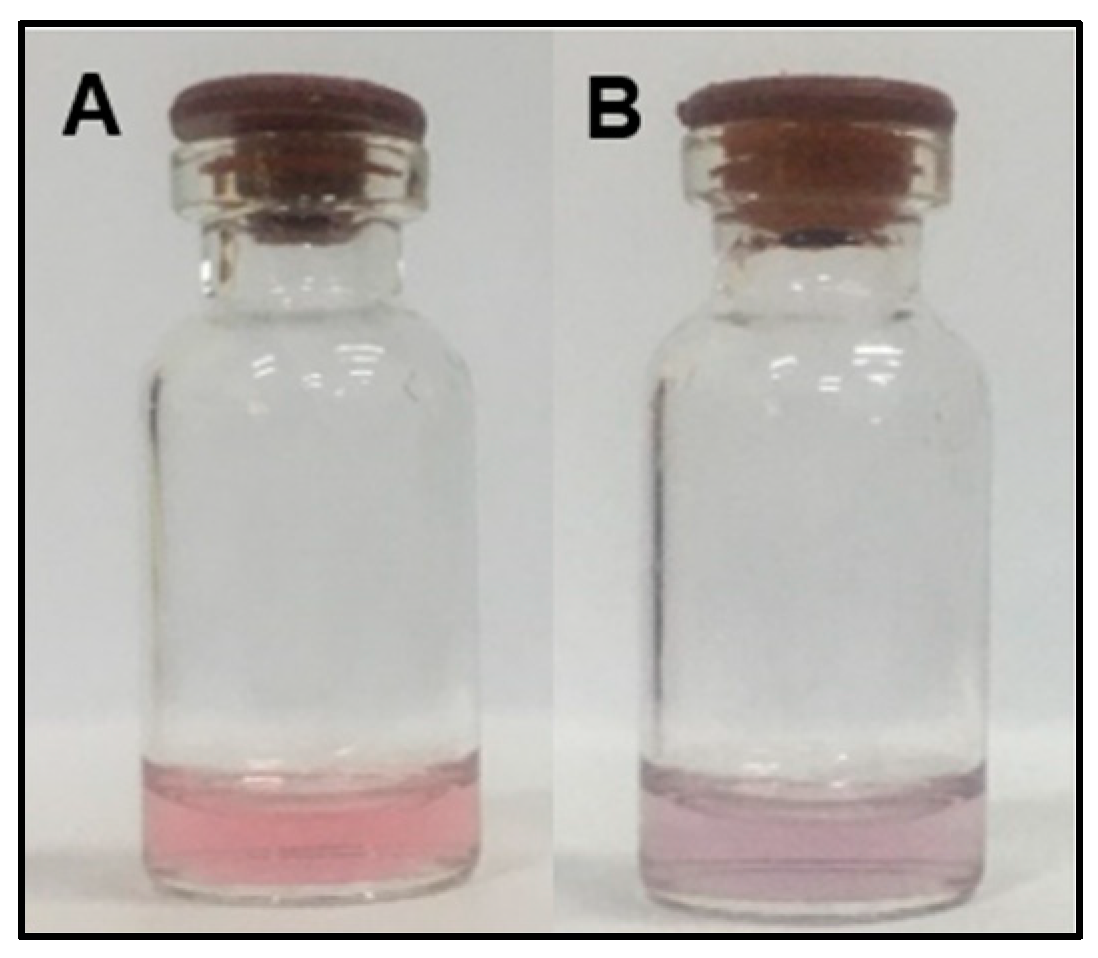
2.1. Orientation and Characterization of CA-AuNPs and Detection of Hg2+
2.2. Optimization of Colorimetric Paper Sensor
2.2.1. Optimization of Paper Types
2.2.2. Optimization of CA-AuNPs Paper Soaking Time
2.2.3. Optimization of CA-AuNPs Paper Drying Temperature
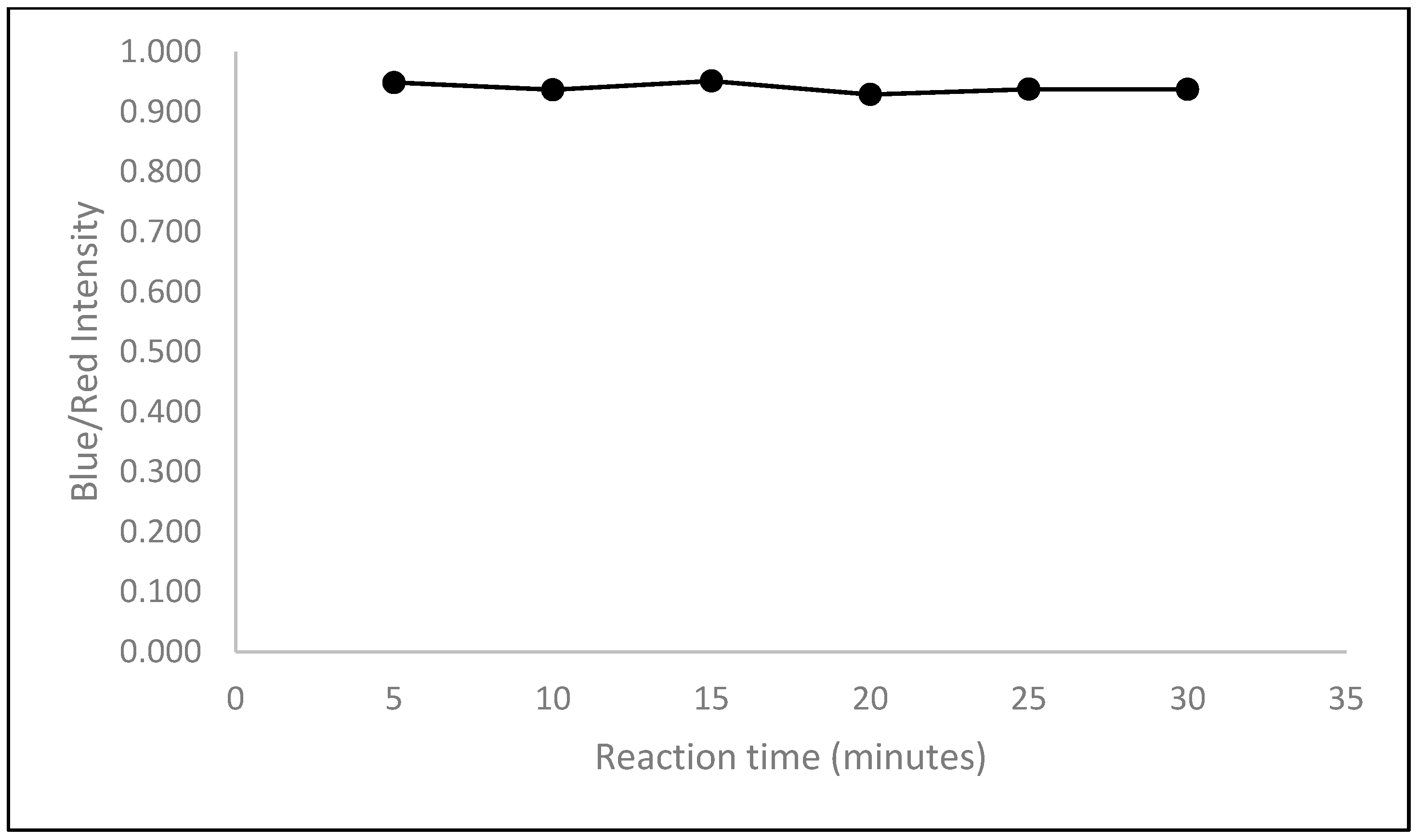
2.2.4. Optimization of Reaction Time
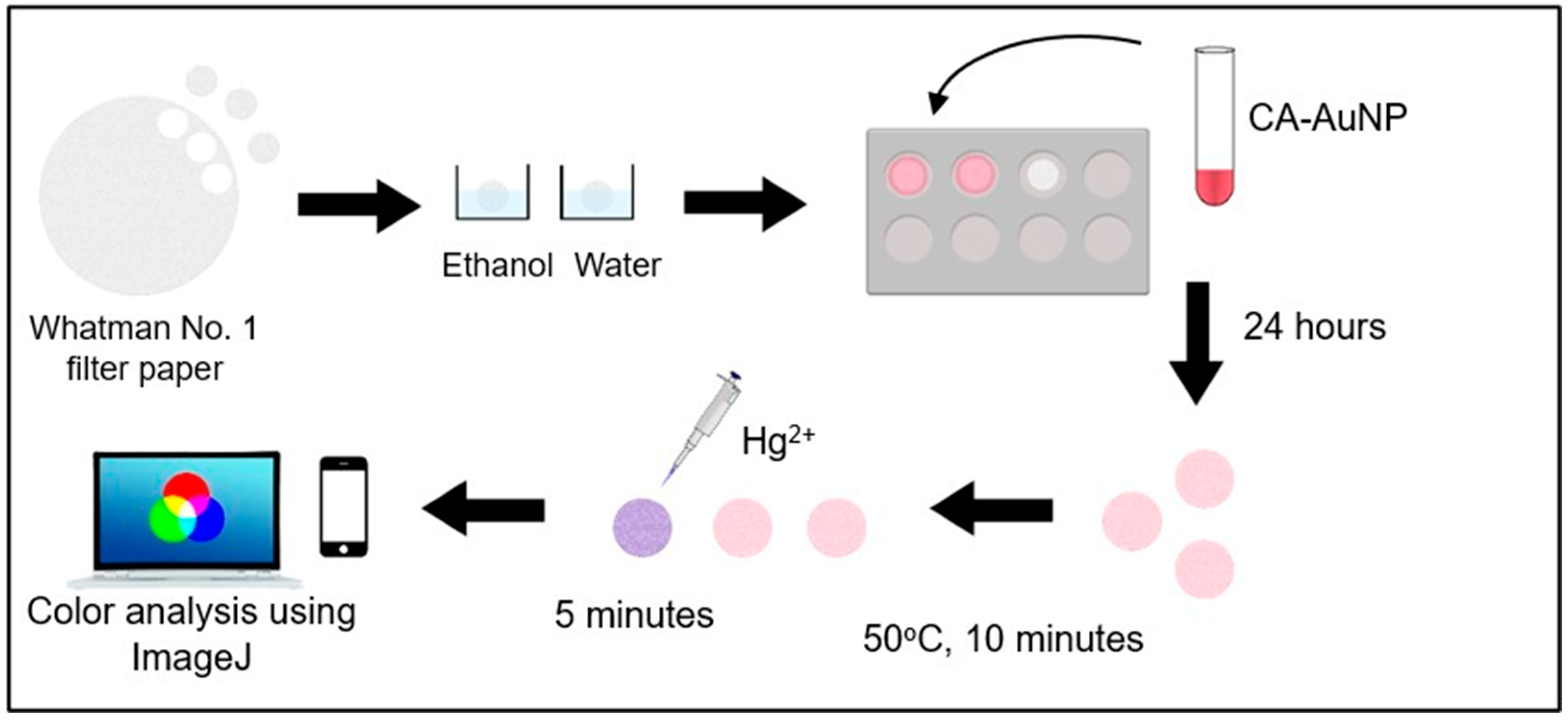
2.3. Preparation of Colorimetric Paper Sensor
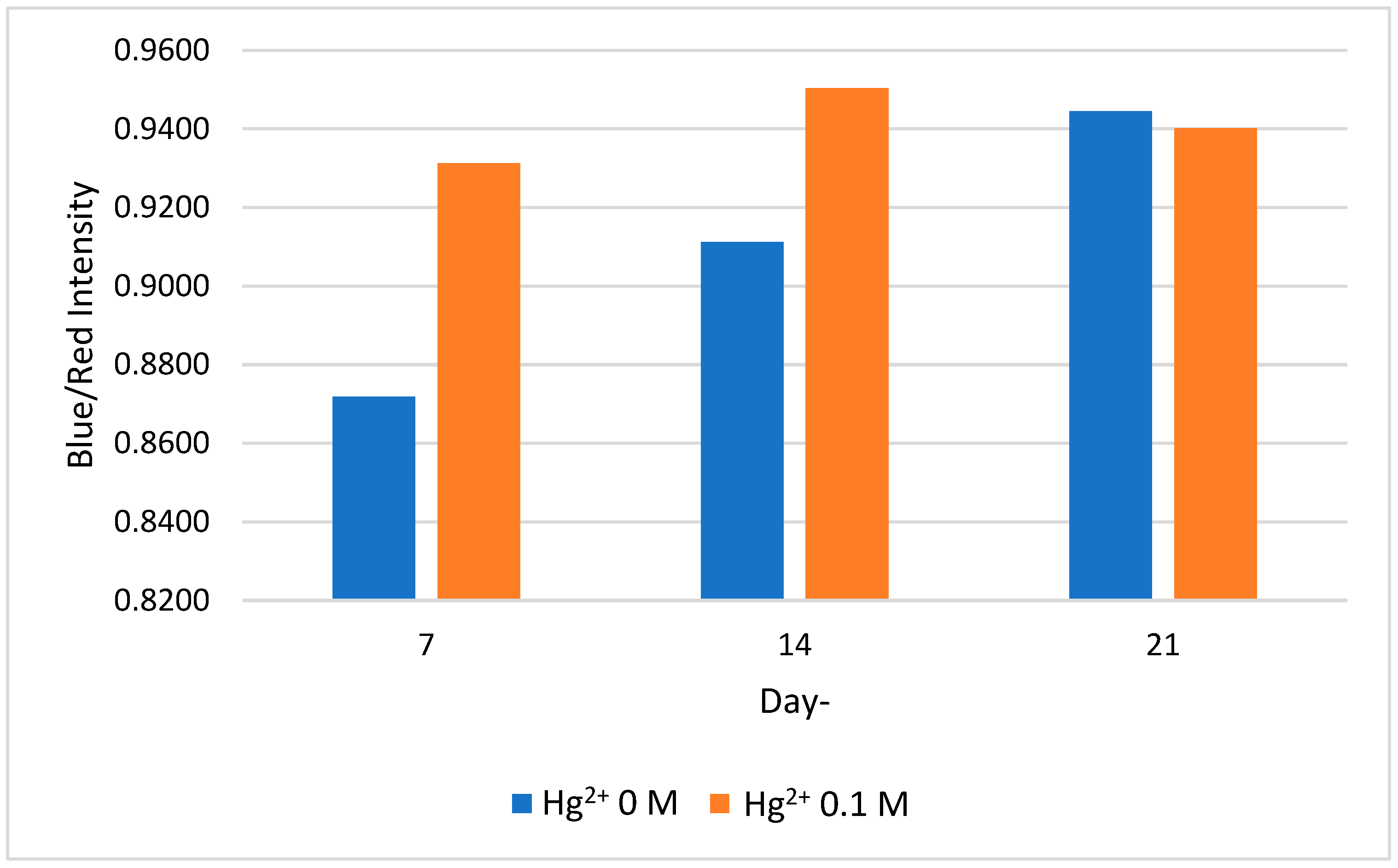
2.4. Stability of Colorimetric Paper Sensor
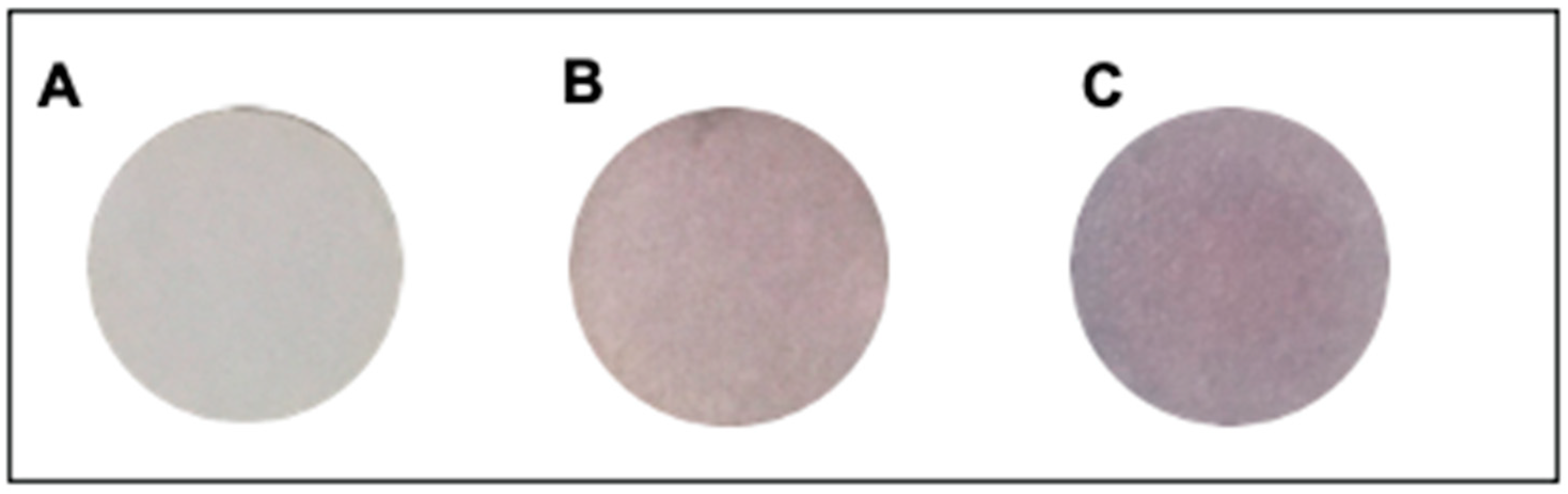
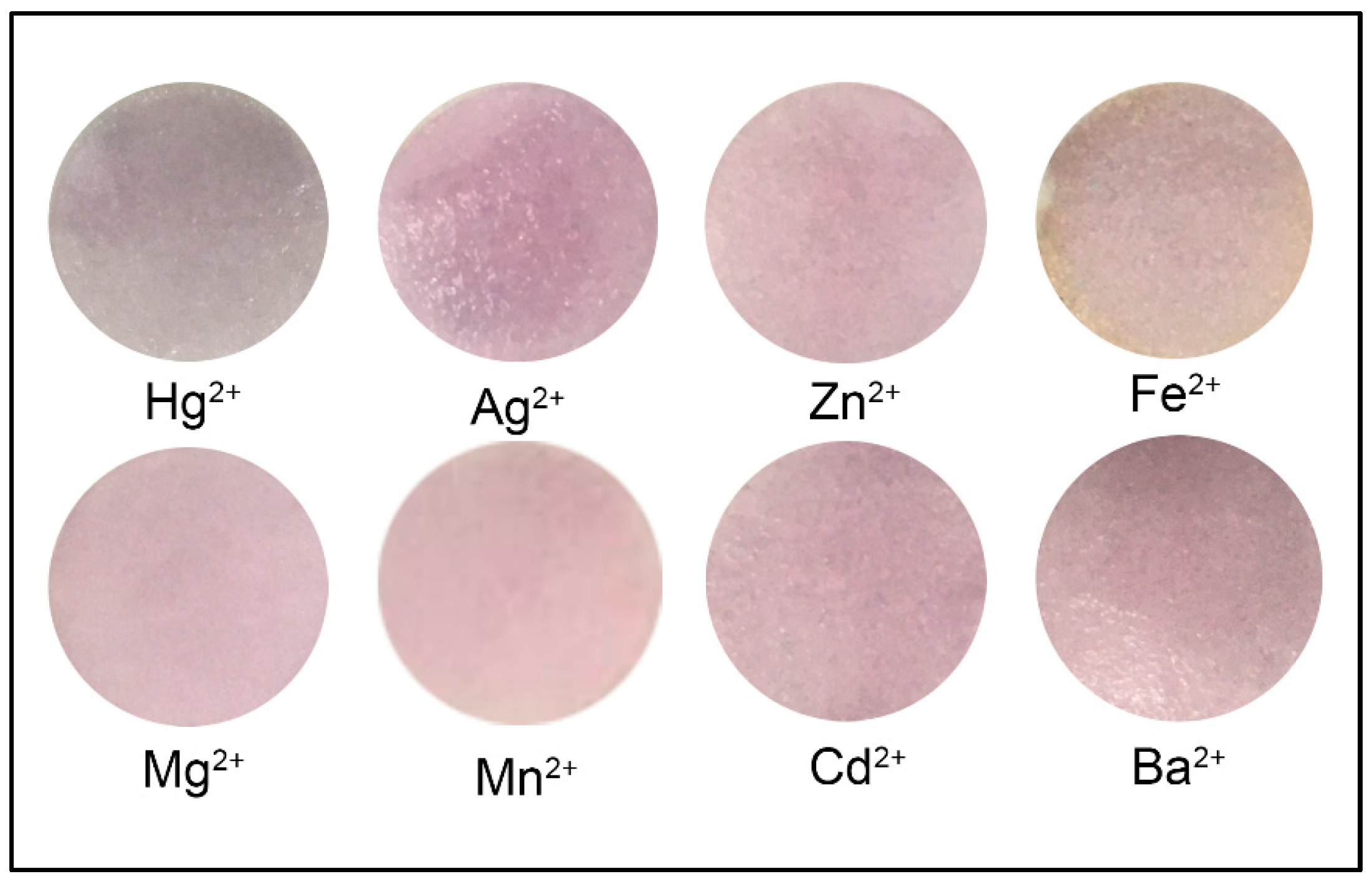
2.5. Selectivity of Colorimetric Paper Sensor
2.6. Sensitivity of Colorimetric Paper Sensor
2.7. Application of Colorimetric Paper Sensor in Water Samples
2.8. Advantages and Shortcoming of the Method
3. Materials and Methods
3.1. Reagents and Apparatus
3.2. Orientation and Characterization of CA-AuNPs and Detection of Hg2+
3.3. Optimization of Colorimetric Paper Sensor
3.3.1. Optimization of Paper Types
3.3.2. Optimization of CA-AuNPs Paper Soaking Time
3.3.3. Optimization of CA-AuNPs Paper Drying Temperature
3.3.4. Optimization of Reaction Time
3.4. Preparation of Colorimetric Paper Sensor
3.5. Stability of Colorimetric Paper Sensor
3.6. Selectivity of Colorimetric Paper Sensor
3.7. Sensitivity of Colorimetric Paper Sensor
3.8. Application of Colorimetric Paper Sensor in Water Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Li, Y.-L.; Leng, Y.-M.; Zhang, Y.-J.; Li, T.-H.; Shen, Z.-Y.; Wu, A.-G. A New Simple and Reliable Hg2+ Detection System Based on Anti-Aggregation of Unmodified Gold Nanoparticles in the Presence of O-Phenylenediamine. Sens. Actuators B Chem. 2014, 200, 140–146. [Google Scholar] [CrossRef]
- Wen, G.; Liang, A.; Jiang, Z.; Liao, X.; Li, J.; Jiang, H. Resonance Scattering Spectral Detection of Ultratrace Hg(II) Using Herring Sperm DNA Modified Nanogold Probe as Catalyst. Luminescence 2010, 25, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Karunasagar, D.; Arunachalam, J.; Gangadharan, S. Development of a ‘Collect and Punch’ Cold Vapour Inductively Coupled Plasma Mass Spectrometric Method for the Direct Determination of Mercury at Nanograms per Litre Levels. J. Anal. At. Spectrom. 1998, 13, 679–682. [Google Scholar] [CrossRef]
- KopyŚĆ, E.; PyrzyŃslka, K.; GarboŚ, S.; Buska, E. Determination of Mercury by Cold-Vapor Atomic Absorption Spectrometry with Preconcentration on a Gold-Trap. Anal. Sci. 2000, 16, 1309–1312. [Google Scholar] [CrossRef]
- Clevenger, W.L.; Smith, B.W.; Winefordner, J.D. Trace Determination of Mercury: A Review. Crit. Rev. Anal. Chem. 1997, 27, 1–26. [Google Scholar] [CrossRef]
- Sarkar, B.; Mondal, P. Colorimetric Sensors of Hg2+ Ion Based on Functionalized Gold and Silver Nanoparticles. J. Water Environ. Nanotechnol. 2021, 6, 22–40. [Google Scholar] [CrossRef]
- Lee, J.-S.; Han, M.S.; Mirkin, C.A. Colorimetric Detection of Mercuric Ion (Hg2+) in Aqueous Media Using DNA-Functionalized Gold Nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 4093–4096. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, J.; Tong, S.; Cao, Q.; Yuan, H. Colorimetric Detection of Hg2+ Ions in Aqueous Media Using CA–Au NPs. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 97, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.C.; DeGregory, P.R.; Crooks, R.M. New Functionalities for Paper-Based Sensors Lead to Simplified User Operation, Lower Limits of Detection, and New Applications. Annu. Rev. Anal. Chem. 2016, 9, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gritsenko, D.; Feng, S.; Teh, Y.C.; Lu, X.; Xu, J. Detection of Heavy Metal by Paper-Based Microfluidics. Biosens. Bioelectron. 2016, 83, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, C.M.; Hatamie, A.; Simchi, A.; Willander, M.; Malhotra, B.D. Nanomaterial-Modified Conducting Paper: Fabrication, Properties, and Emerging Biomedical Applications. Glob. Chall. 2019, 3, 1900041. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, F.; Fahimi-Kashani, N.; Bigdeli, A.; Alshatteri, A.H.; Abbasi-Moayed, S.; Al-Jaf, S.H.; Merry, M.Y.; Omer, K.M.; Hormozi-Nezhad, M.R. Paper-Based Optical Nanosensors—A Review. Anal. Chim. Acta 2023, 1238, 340640. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, Y.; Deng, Y.; Yao, L.; Adeloju, S.B.; Pan, D.; Xue, F.; Wu, Y.; Zheng, L.; Chen, W. Ultrasensitive Detection of Mercury with a Novel One-Step Signal Amplified Lateral Flow Strip Based on Gold Nanoparticle-Labeled SsDNA Recognition and Enhancement Probes. Biosens. Bioelectron. 2014, 61, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-H.; Chen, W.-Y.; Yen, Y.-C.; Wang, C.-W.; Chang, H.-T.; Chen, C.-F. Detection of Mercury(II) Ions Using Colorimetric Gold Nanoparticles on Paper-Based Analytical Devices. Anal. Chem. 2014, 86, 6843–6849. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, X.; Zeng, K.; Zhang, S.; Baloda, M.; Gurung, A.S.; Liu, G. Visual Detection of Hg2+ in Aqueous Solution Using Gold Nanoparticles and Thymine-Rich Hairpin DNA Probes. Biosens. Bioelectron. 2011, 26, 4464–4470. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, H.; Zhang, X.; Li, Z.; Yang, Y.; Zheng, R.; Qu, Y. Colorimetric Detection of Hg2+ Using Gold Nanoparticles Synthesized by Trichosporon Montevideense WIN. Biotechnol. Lett. 2020, 42, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Christau, S.; Moeller, T.; Genzer, J.; Koehler, R.; von Klitzing, R. Salt-Induced Aggregation of Negatively Charged Gold Nanoparticles Confined in a Polymer Brush Matrix. Macromolecules 2017, 50, 7333–7343. [Google Scholar] [CrossRef]
- Miyake, Y.; Togashi, H.; Tashiro, M.; Yamaguchi, H.; Oda, S.; Kudo, M.; Tanaka, Y.; Kondo, Y.; Sawa, R.; Fujimoto, T.; et al. MercuryII-Mediated Formation of Thymine-HgII-Thymine Base Pairs in DNA Duplexes. J. Am. Chem. Soc. 2006, 128, 2172–2173. [Google Scholar] [CrossRef]
- Zhdanov, V.P. Nanoparticles without and with Protein Corona: Van Der Waals and Hydration Interaction. J. Biol. Phys. 2019, 45, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Pradela-Filho, L.A.; Veloso, W.B.; Arantes, I.V.S.; Gongoni, J.L.M.; de Farias, D.M.; Araujo, D.A.G.; Paixão, T.R.L.C. Paper-Based Analytical Devices for Point-of-Need Applications. Microchim. Acta 2023, 190, 179. [Google Scholar] [CrossRef]
- Liana, D.D.; Raguse, B.; Gooding, J.J.; Chow, E. Recent Advances in Paper-Based Sensors. Sensors 2012, 12, 11505–11526. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, R.; Septyani, R.N.; Febriany, R.; Saputri, F.A.; Nuwarda, R.F. Design and Optimization of Colorimetric Paper-Based Analytical Device for Rapid Detection of Allopurinol in Herbal Medicine. Int. J. Anal. Chem. 2019, 2019, 4682839. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Wang, S.; Bai, L.; Deng, Y.; Tao, J. Effect of Pore Size Distribution and Amination on Adsorption Capacities of Polymeric Adsorbents. Molecules 2021, 26, 5267. [Google Scholar] [CrossRef] [PubMed]
- Budlayan, M.L.; Dalagan, J.; Lagare-Oracion, J.P.; Patricio, J.; Arco, S.; Latayada, F.; Vales, T.; Baje, B.; Alguno, A.; Capangpangan, R. Detecting Mercury Ions in Water Using a Low-Cost Colorimetric Sensor Derived from Immobilized Silver Nanoparticles on a Paper Substrate. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100736. [Google Scholar] [CrossRef]
- Yuan, H.; Sun, G.; Peng, W.; Ji, W.; Chu, S.; Liu, Q.; Liang, Y. Thymine-Functionalized Gold Nanoparticles (Au NPs) for a Highly Sensitive Fiber-Optic Surface Plasmon Resonance Mercury Ion Nanosensor. Nanomaterials 2021, 11, 397. [Google Scholar] [CrossRef]
- Hao, Y.; Yu, L.; Li, T.; Chen, L.; Han, X.; Chai, F. The Synthesis of Carbon Dots by Folic Acid and Utilized as Sustainable Probe and Paper Sensor for Hg2+ Sensing and Cellular Imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 285, 121865. [Google Scholar] [CrossRef] [PubMed]


| Paper Type | Hg2+ Concentration | Grayscale Intensity | Absorbance |
|---|---|---|---|
| Whatman No. 1 | 0 M | 103.231 | 0.3927 |
| 0.1 M | 105.800 | 0.3821 | |
| Whatman No. 41 | 0 M | 123.203 | 0.3159 |
| 0.1 M | 136.244 | 0.2722 | |
| Whatman No. 91 | 0 M | 107.775 | 0.3740 |
| 0.1 M | 108.605 | 0.3707 | |
| Whatman Chromatography No. 1 | 0 M | 122.709 | 0.3177 |
| 0.1 M | 119.341 | 0.3298 |
| Hours | Hg2+ Concentration | Grayscale Intensity | Absorbance |
|---|---|---|---|
| 3 h | 0 M | 133.500 | 0.2811 |
| 0.1 M | 120.974 | 0.3238 | |
| 6 h | 0 M | 132.767 | 0.2835 |
| 0.1 M | 121.400 | 0.3223 | |
| 12 h | 0 M | 125.072 | 0.3094 |
| 0.1 M | 117.704 | 0.3357 | |
| 24 h | 0 M | 103.231 | 0.3927 |
| 0.1 M | 105.800 | 0.3821 |
| Temperature | Hg2+ Concentration | Intensity | ||||
|---|---|---|---|---|---|---|
| Red | Green | Blue | Blue/Red | Blue/Red (0.1 M/0 M) | ||
| 30° | 0 M | 113.100 | 97.081 | 100.203 | 0.8860 | 1.0690 |
| 0.1 M | 111.343 | 100.402 | 105.454 | 0.9471 | ||
| 50° | 0 M | 174.457 | 154.375 | 157.355 | 0.9020 | 1.0837 |
| 0.1 M | 157.472 | 145.819 | 153.921 | 0.9774 | ||
| 75° | 0 M | 113.963 | 101.226 | 104.007 | 0.9126 | 1.0063 |
| 0.1 M | 133.145 | 115.635 | 122.273 | 0.9183 | ||
| 100° | 0 M | 123.709 | 110.631 | 112.694 | 0.9110 | 1.0180 |
| 0.1 M | 126.754 | 113.248 | 117.549 | 0.9274 | ||
| Metal Ions | Intensity | |||
|---|---|---|---|---|
| Red | Green | Blue | Blue/Red | |
| Hg2+ | 173.321 | 161.452 | 161.614 | 0.9325 * |
| Mg2+ | 167.572 | 147.820 | 148.230 | 0.8846 |
| Fe2+ | 198.262 | 177.329 | 167.888 | 0.8468 |
| Cd2+ | 194.926 | 167.481 | 173.015 | 0.8876 |
| Zn2+ | 201.482 | 176.317 | 180.412 | 0.8954 |
| Ag2+ | 190.155 | 160.861 | 170.877 | 0.8986 |
| Ba2+ | 175.092 | 150.728 | 150.532 | 0.8597 |
| Mn2+ | 210.09 | 182.610 | 183.489 | 0.8734 |
| No | Lake Water Sample | Hg2+ Addition | Intensity | |||
|---|---|---|---|---|---|---|
| Red | Green | Blue | Blue/Red | |||
| 1. | Kenanga | 0 M | 147.418 | 127.108 | 128.371 | 0.8708 |
| 0.01 M | 169.476 | 157.839 | 161.568 | 0.9533 | ||
| 2. | FMIPA | 0 M | 143.432 | 128.982 | 128.546 | 0.8962 |
| 0.01 M | 180.410 | 164.549 | 168.933 | 0.9364 | ||
| 3. | Mahoni | 0 M | 155.028 | 136.602 | 136.415 | 0.8799 |
| 0.01 M | 184.737 | 168.058 | 174.036 | 0.9421 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saputri, F.A.; Zubaidah, E.U.; Kenanga, A.W.P.; Jatmika, C.; Pratiwi, R.; Dhumale, V.A. Development of a Colorimetric Paper Sensor for Hg2+ Detection in Water Using Cyanuric Acid-Conjugated Gold Nanoparticles. Molecules 2023, 28, 6527. https://doi.org/10.3390/molecules28186527
Saputri FA, Zubaidah EU, Kenanga AWP, Jatmika C, Pratiwi R, Dhumale VA. Development of a Colorimetric Paper Sensor for Hg2+ Detection in Water Using Cyanuric Acid-Conjugated Gold Nanoparticles. Molecules. 2023; 28(18):6527. https://doi.org/10.3390/molecules28186527
Chicago/Turabian StyleSaputri, Febrina Amelia, Eka Ulya Zubaidah, Amaranggani Wikan Puspita Kenanga, Catur Jatmika, Rimadani Pratiwi, and Vinayak A. Dhumale. 2023. "Development of a Colorimetric Paper Sensor for Hg2+ Detection in Water Using Cyanuric Acid-Conjugated Gold Nanoparticles" Molecules 28, no. 18: 6527. https://doi.org/10.3390/molecules28186527
APA StyleSaputri, F. A., Zubaidah, E. U., Kenanga, A. W. P., Jatmika, C., Pratiwi, R., & Dhumale, V. A. (2023). Development of a Colorimetric Paper Sensor for Hg2+ Detection in Water Using Cyanuric Acid-Conjugated Gold Nanoparticles. Molecules, 28(18), 6527. https://doi.org/10.3390/molecules28186527






