Antimicrobial Synergistic Effects of Linezolid and Vancomycin with a Small Synthesized 2-Mercaptobenzothiazole Derivative: A Challenge for MRSA Solving
Abstract
:1. Introduction
1.1. MRSA and MRSE Infections: State of the Art
1.2. MRSA and MRSE Infections: A Future Perspective
2. Results and Discussion
2.1. Susceptibility Testing of Planktonic Cells to Antibiotics
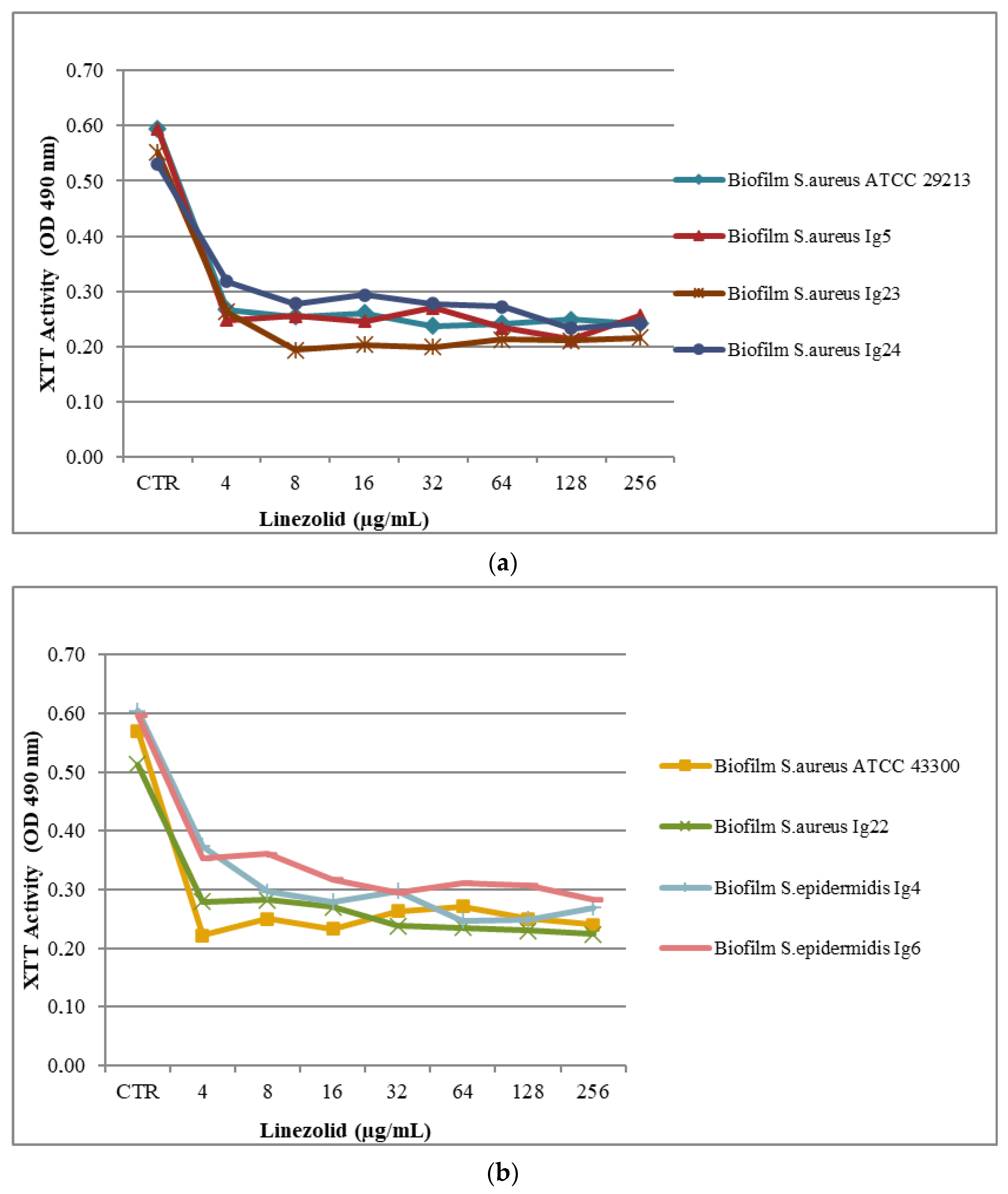
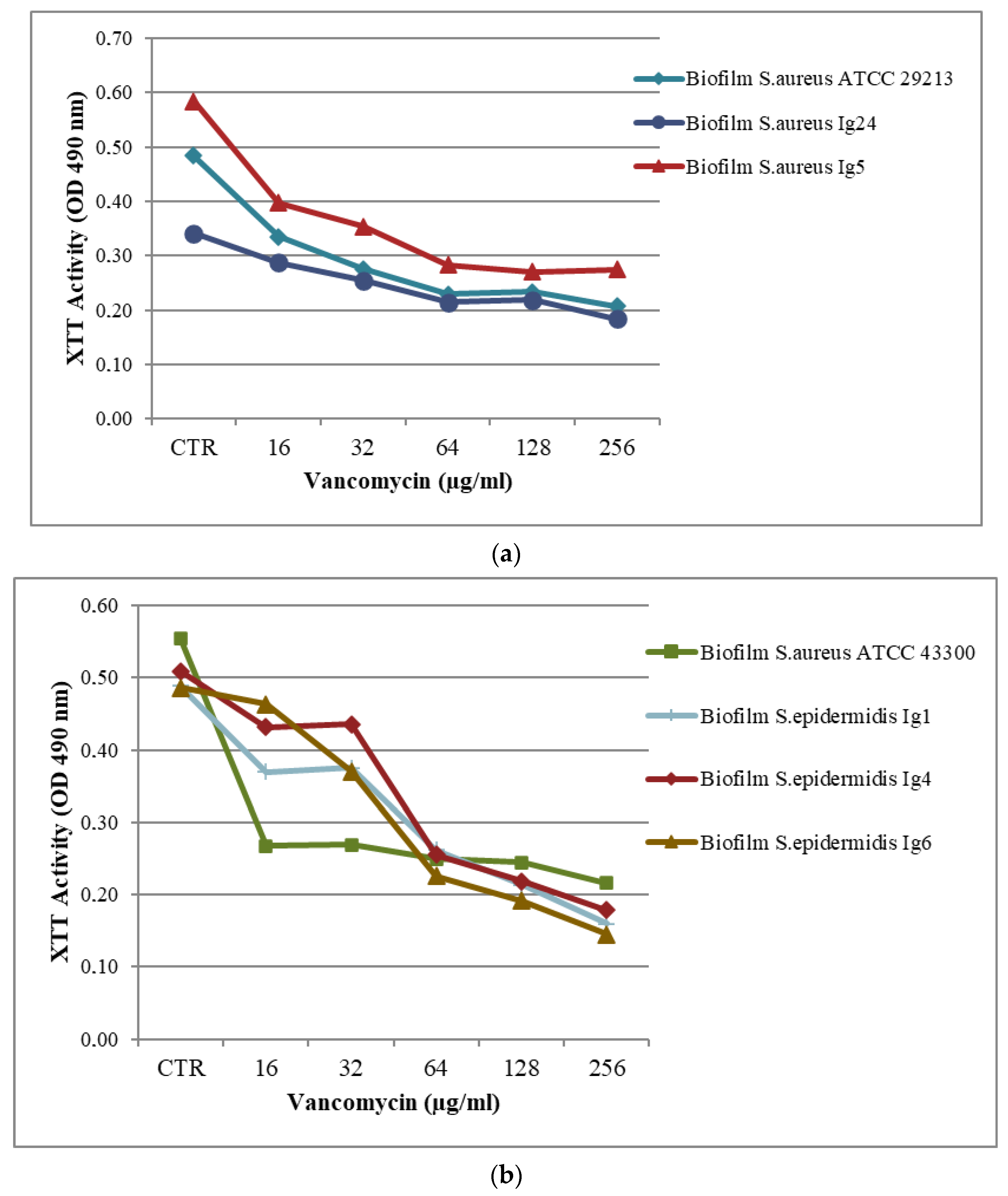
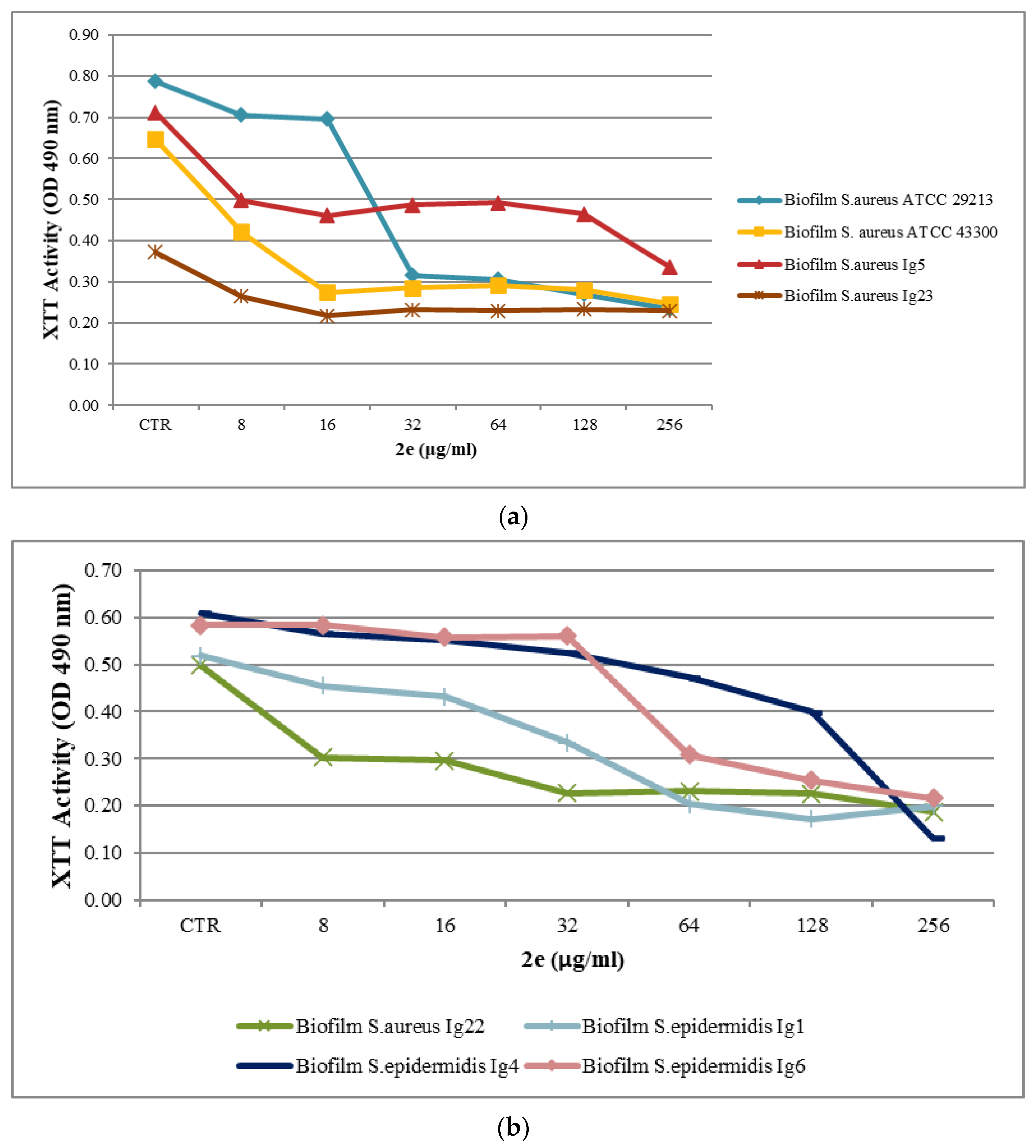
2.2. Biofilm Assay
2.3. Checkerboard Assay Results
3. Materials and Methods
3.1. Bacterial Strains and Culture Conditions
3.2. Test Compounds
3.3. Planktonic Susceptibility Testing
3.4. Biomass Assay
3.5. Culture of Biofilms and Susceptibility Testing to Antimicrobial Compounds
3.6. Microdilution Checkerboard Technique
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Available online: https://www.who.int/news/item/09-12-2022-report-signals-increasing-resistance-to-antibiotics-in-bacterial-infections-in-humans-and-need-for-better-data (accessed on 2 July 2023).
- (EARS-Net) 2017. Available online: https://www.ecdc.europa.eu/en/antimicrobial-resistance/surveillance-and-disease-data/report (accessed on 2 July 2023).
- Howden, B.P.; Davies, J.K.; Johnson, P.D.R.; Stinear, T.P.; Grayson, M.L. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: Resistance mechanisms, labora-tory detection, and clinical implications. Clin. Microbiol. Rev. 2010, 23, 99–139. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations the Review on Antimicrobial Resistance; Wellcome Trust and UK Government: London, UK, 2016; pp. 1–81. [Google Scholar]
- Cascioferro, S.; Carbone, D.; Parrino, B.; Pecoraro, C.; Giovannetti, E.; Cirrincione, G.; Diana, P. Therapeutic strategies to counteract antibiotic resistance in MRSA biofilm-associated infections. ChemMedChem 2021, 16, 65–80. [Google Scholar] [CrossRef] [PubMed]
- King, M.D.; Humphrey, B.J.; Wang, Y.F.; Kourbatova, E.V.; Ray, S.M.; Blumberg, H.M. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann. Intern. Med. 2006, 144, 309–317. [Google Scholar] [CrossRef]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: Executive summary. Clin. Infect. Dis. 2011, 52, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, P.; Kumar, P.; Mickymaray, S.; Alothaim, A.S.; Somasundaram, J.; Rajan, M. Recent Developments in Methicillin-Resistant Staphylococcus aureus (MRSA) Treatment: A. Review. Antibiotics 2022, 11, 606. [Google Scholar] [CrossRef] [PubMed]
- Altowayan, W.M.; Mobark, M.A.; Alharbi, A.; Alduhami, A.A.; Rabbani, S.I. The influence of vancomycin on renal functions, the predictors and associated factors for nephrotoxicity. PLoS ONE 2023 18, e0284223. [CrossRef]
- Jian, Y.; Lv, H.; Liu, J.; Huang, Q.; Liu, Y.; Liu, Q.; Li, M. Dynamic Changes of Staphylococcus aureus Susceptibility to Vancomycin, Teicoplanin, and Linezolid in a Central Teaching Hospital in Shanghai, China, 2008-2018. Front Microbiol. 2020, 11, 908. [Google Scholar] [CrossRef]
- Wilcox, M.H.; Kit, P.; Mills, K.; Sudgen, S. In situ measurement of linezolid and vancomycin concentrations in intravascular catheter-associated biofilm. J. Antimicrob. Chemother. 2001, 47, 171–175. [Google Scholar] [CrossRef]
- El-Azizi, M.; Rao, S.; Kanchanapoom, T.; Khardori, N. In vitro activity of vancomycin, quinupristin/dalfopristin, and linezolid against intact and disrupted biofilms of staphylococci. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 2. [Google Scholar] [CrossRef]
- Oehadian, A.; Santoso, P.; Menzies, D.; Ruslami, R. Concise Clinical Review of Hematologic Toxicity of Linezolid in Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis: Role of Mitochondria. Tuberc. Respir Dis. 2022, 85, 111–121. [Google Scholar] [CrossRef]
- Kawasuji, H.; Nagaoka, K.; Tsuji, Y.; Kimoto, K.; Takegoshi, Y.; Kaneda, M.; Murai, Y.; Karaushi, H.; Mitsutake, K.; Yamamoto, Y. Effectiveness and Safety of Linezolid Versus Vancomycin, Teicoplanin, or Daptomycin against Methicillin-Resistant Staphylococcus aureus Bacteremia: A Systematic Review and Meta-Analysis. Antibiotics 2023, 12, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, M.-J.; Alfaro, M.; Rodríguez-Avial, I.; Baos, E.; Rodríguez-Avial, C.; Culebras, E. Synergy of Linezolid with Several Antimicrobial Agents against Linezolid-Methicillin-Resistant Staphylococcal Strains. Antibiotics 2020, 9, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.C.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.V.; Levine, D.P.; Murray, B.A.; et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, L.; Liu, Y.; Wu, M.; Xu, S.; Zhang, G.; Qi, C.; Du, Y.; Wang, M.; Li, J.; et al. In vitro activity and post-antibiotic effects of linezolid in combination with fosfomycin against clinical isolates of Staphylococcus aureus. Infect. Drug Resist. 2018, 11, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Chen, P.Y.; Wang, J.T.; Chan, S.D. A study on combination of daptomycin with selected antimicrobial agents: In vitro synergistic effect of MIC value of 1mg/L against MRSA strains. BMC Pharmacol. Toxicol. 2019, 20, 25. [Google Scholar] [CrossRef]
- Armenise, D.; Carocci, A.; Catalano, A.; Muraglia, M.; Defrenza, I.; De Laurentis, N.; Rosato, A.; Corbo, F.; Franchini, C. Synthesis and Antimicrobial Evaluation of a New Series of N-1, 3-Benzothiazol-2-ylbenzamides. J. Chem. 2013, 2013, 181758. [Google Scholar] [CrossRef]
- Defrenza, I.; Catalano, A.; Carocci, A.; Carrieri, A.; Muraglia, M.; Rosato, A.; Corbo, F.; Franchini, C. 1, 3-Benzothiazoles as Antimicrobial Agents. J. Heterocycl. Chem. 2015, 52, 1705–1712. [Google Scholar] [CrossRef]
- Franchini, C.; Muraglia, M.; Corbo, F.; Florio, M.A.; Di Mola, A.; Rosato, A.; Matucci, R.; Nesi, M.; Van Bambeke, F.; Vitali, C. Synthesis and Biological Evaluation of 2-Mercapto-1,3-benzothiazole Derivatives with Potential Antimicrobial Activity. Arch. Pharm. 2009, 342, 605–613. [Google Scholar] [CrossRef]
- NCCLS. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 6th ed.; Approved Standard M7-A6, National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2003. [Google Scholar]
- CLSI 2021. MS-100; Performance Standards for Antimicrobial Susceptibility Testing, 31st ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021.
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Ćirkovic, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Schiavone, B.I.; Rosato, A.; Muraglia, M.; Gibbons, S.; Bombardelli, E.; Verotta, L.; Franchini, C.; Corbo, F. Biological evaluation of hyperforin and its hydrogenated analogue on bacterial growth and biofilm production. J. Nat. Prod. 2013, 76, 1819–1823. [Google Scholar] [CrossRef]
- CLSI 2021. M100-S17; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2007.
- Kuhn, D.M.; Balkis, M.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Uses and limitation of the XTT assay in studies of Candida growth and metabolism. J. Clin. Microbiol. 2003, 41, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Sblano, S.; Salvagno, L.; Carocci, A.; Clodoveo, M.L.; Corbo, F.; Fracchiolla, G. Anti-Biofilm Inhibitory Synergistic Effects of Combinations of Essential Oils and Antibiotics. Antibiotics 2020, 9, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Baoping, L.B.J.; Jinhua, Y.; Guizhi, Z.; Yang, C.; Yangchao, L. Antioxidant and antimicrobial activities of consecutive extracts from Galla chinesis: The polarity affects the bioactivities. Food Chem. 2009, 113, 173–179. [Google Scholar] [CrossRef]
- Hübner, N.-O.; Matthes, R.; Koban, I.; Rändler, C.; Müller, G.; Bender, C.; Kindel, E.; Kocher, T.; Kramer, A. Efficacy of Chlorhexidine, Polihexanide and Tissue-Tolerable Plasma against Pseudomonas aeruginosa Biofilms Grown on Polystyrene and Silicone Materials. Skin Pharmacol. Physiol. 2010, 23, 28–34. [Google Scholar] [CrossRef]
- Cocchietto, M.; Skert, N.; Nimis, P.L.; Sava, G. A review on usnic acid, an interesting natural compound. Naturwissenschaften 2002, 89, 137–146. [Google Scholar] [CrossRef]
- Kim, S.; Greenleaf, R.; Miller, M.C.; Satish, L.; Kathju, S.; Ehrlich, G.; Post, J.C.; Sotereanos, N.G.; Stoodley, P. Mechanical effects, antimicrobial efficacy and cytotoxicity of usnic acid as a biofilm prophylaxis in PMMA. J. Mater. Sci. Mater. Med. 2011, 22, 2773–2780. [Google Scholar] [CrossRef]
- Tunney, M.M.; Ramage, G.; Field, T.R.; Moriarty, T.F.; Storey, D.G. Rapid colorimetric assay for antimicrobial susceptibility testing of Pseudomonas aeruginosa. Antimicrob. Agent Chemother. 2004, 48, 1879–1881. [Google Scholar] [CrossRef]
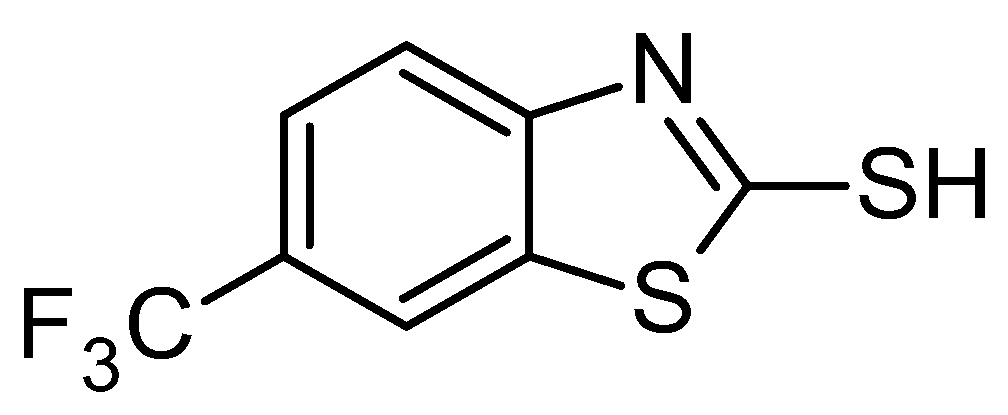
| MIC (μg/mL) | |||
|---|---|---|---|
| Bacterial Strain | VAN | LNZ | BTZ2e |
| S. aureus ATCC 29213 | 1 | 4 | 3.12 |
| S. aureus ATCC 43300 | 1 | 2.5 | 12.5 |
| S. aureus Ig5 | 2 | 4 | 6.25 |
| S. aureus Ig22 | 1 | 4 | 3.12 |
| S. aureus Ig23 | 2 | 2 | 3.12 |
| S. aureus Ig24 | 1 | 2 | 3.12 |
| S. epidermidis Ig1 | 2 | 2 | 50 |
| S. epidermidis Ig4 | 2 | 2 | 50 |
| S. epidermidis Ig6 | 2 | 2 | 50 |
| Bacterial Strain | OD (570 nm) | Description | Categories |
|---|---|---|---|
| S. aureus ATCC 29213 | 2.30 | strong biofilm producer | 3 |
| S. aureus ATCC 43300 | 1.43 | moderate biofilm producer | 2 |
| S. aureus Ig5 | 1.82 | moderate biofilm producer | 2 |
| S. aureus Ig22 | 2.40 | strong biofilm producer | 3 |
| S. aureus Ig23 | 1.65 | moderate biofilm producer | 2 |
| S. aureus Ig24 | 1.64 | moderate biofilm producer | 2 |
| S. epidermidis Ig1 | 2.35 | strong biofilm producer | 3 |
| S. epidermidis Ig4 | 2.26 | strong biofilm producer | 3 |
| S. epidermidis Ig6 | 1.44 | moderate biofilm producer | 2 |
| Negative control | 0.49 |
| a | ||
|---|---|---|
| Bacterial Strain | VAN | |
| MBIC50 (μg/mL) | % | |
| S. aureus ATCC 29213 | 64 | 52 |
| S. aureus ATCC 43300 | 16 | 51 |
| S. aureus Ig5 | 64 | 51 |
| S. aureus Ig22 | R | 23 |
| S. aureus Ig23 | R | 10 |
| S. aureus Ig24 | 256 | 46 |
| S. epidermidis Ig1 | 128 | 56 |
| S. epidermidis Ig4 | 64 | 50 |
| S. epidermidis Ig6 | 64 | 53 |
| b | ||
| Bacterial Strain | LNZ | |
| MBIC50 (μg/mL) | % | |
| S. aureus ATCC 29213 | 4 | 55 |
| S. aureus ATCC 43300 | 4 | 64 |
| S. aureus Ig5 | 4 | 58 |
| S. aureus Ig22 | 4 | 53 |
| S. aureus Ig23 | 4 | 52 |
| S. aureus Ig24 | 64 | 48 |
| S. epidermidis Ig1 | R | 30 |
| S. epidermidis Ig4 | 8 | 51 |
| S. epidermidis Ig6 | 32 | 50 |
| c | ||
| Bacterial Strain | BTZ2e | |
| MBIC50 (μg/mL) | % | |
| S. aureus ATCC 29213 | 32 | 60 |
| S. aureus ATCC 43300 | 16 | 58 |
| S. aureus Ig5 | 256 | 53 |
| S. aureus Ig22 | 32 | 54 |
| S. aureus Ig23 | 16 | 43 |
| S. aureus Ig24 | R | 38 |
| S. epidermidis Ig1 | 64 | 60 |
| S. epidermidis Ig4 | 256 | 78 |
| S. epidermidis Ig6 | 128 | 56 |
| LNZ | VAN | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | MICo | MICc | FIC | FICI | MICo | MICc | FIC | FICI |
| S. aureus ATCC 29213 | ||||||||
| BTZ2e | 3.12 | 0.39 | 0.12 | 0.22 | 3.12 | 0.39 | 0.12 | 0.17 |
| Antibiotic | 4 | 1.60 | 0.10 | 1 | 0.05 | 0.05 | ||
| S. aureus ATCC 43300 | ||||||||
| BTZ2e | 12.50 | 1.56 | 0.12 | 0.17 | NS | |||
| Antibiotic | 2.20 | 0.12 | 0.05 | |||||
| S. aureus Ig5 | ||||||||
| BTZ2e | 6.25 | 0.39 | 0.06 | 0.26 | 6.25 | 0.78 | 0.12 | 0.22 |
| Antibiotic | 4 | 0.80 | 0.20 | 1 | 0.10 | 0.10 | ||
| S. aureus Ig22 | ||||||||
| BTZ2e | 3.12 | 0.09 | 0.03 | 0.13 | NS | |||
| Antibiotic | 4 | 0.40 | 0.10 | |||||
| S. aureus Ig23 | ||||||||
| BTZ2e | 3.12 | 0.09 | 0.03 | 0.43 | NS | |||
| Antibiotic | 2 | 0.80 | 0.40 | |||||
| S. aureus Ig24 | ||||||||
| BTZ2e | 6.25 | 0.19 | 0.03 | 0.23 | 6.25 | 1.56 | 0.25 | 0.30 |
| Antibiotic | 2 | 0.40 | 0.20 | 2 | 0.10 | 0.05 | ||
| LNZ | VAN | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample (μg/mL) | MICo | MICc | FIC | FICI | MICo | MICc | FIC | FICI |
| S. aureus ATCC 29213 | ||||||||
| BTZ2e | 3.12 | 0.31 | 0.10 | 0.22 | NS | |||
| Antibiotic | 4 | 0.50 | 0.12 | |||||
| S. aureus ATCC 43300 | ||||||||
| BTZ2e | 12.50 | 1.25 | 0.20 | 0.22 | NS | |||
| Antibiotic | 2.50 | 1.25 | 0.12 | |||||
| S. aureus Ig5 | ||||||||
| BTZ2e | 6.25 | 0.31 | 0.05 | 0.30 | NS | |||
| Antibiotic | 4 | 1 | 0.25 | |||||
| S. aureus Ig22 | ||||||||
| BTZ2e | 3.12 | 0.31 | 0.10 | 0.13 | NS | |||
| Antibiotic | 4 | 0.12 | 0.03 | |||||
| S. aureus Ig23 | ||||||||
| BTZ2e | 3.12 | 1.25 | 0.40 | 0.65 | NS | |||
| Antibiotic | 2 | 0.50 | 0.25 | |||||
| S. aureus Ig24 | ||||||||
| BTZ2e | 6.25 | 0.31 | 0.05 | 0.08 | 6.25 | 2.50 | 0.40 | 0.43 |
| Antibiotic | 2 | 0.06 | 0.03 | 1 | 0.03 | 0.03 | ||
| S. epidermidis Ig4 | ||||||||
| BTZ2e | 50 | 10 | 0.20 | 0.22 | 50 | 20 | 0.40 | 0.43 |
| Antibiotic | 2 | 0.25 | 0.12 | 2 | 0.06 | 0.03 | ||
| LNZ | VAN | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample(μg/mL) | MBICo | MBICc | FIC | FICI | MBICo | MBICc | FIC | FICI |
| S. aureus ATCC 29213 | ||||||||
| BTZ2e | 32 | 4 | 0.25 | 0.65 | NS | |||
| Antibiotic | 4 | 0.80 | 0.40 | |||||
| S. aureus ATCC 43300 | ||||||||
| BTZ2e | NS | 16 | 0.50 | 0.03 | 0.08 | |||
| Antibiotic | 16 | 0.60 | 0.05 | |||||
| S. aureus Ig5 | ||||||||
| BTZ2e | 256 | 8 | 0.03 | 0.43 | NS | |||
| Antibiotic | 4 | 1.60 | 0.40 | |||||
| S. aureus Ig22 | ||||||||
| BTZ2e | 32 | 8 | 0.25 | 0.45 | 32 | 8 | 0.25 | 0.45 |
| Antibiotic | 32 | 6.40 | 0.20 | 256 | 51.20 | 0.20 | ||
| S. aureus Ig24 | ||||||||
| BTZ2e | NE | 512 | 128 | 0.25 | 0.35 | |||
| Antibiotic | 256 | 25.60 | 0.10 | |||||
| S. epidermidis Ig1 | ||||||||
| BTZ2e | NE | 64 | 2 | 0.03 | 0.43 | |||
| Antibiotic | 128 | 51.20 | 0.40 | |||||
| S. epidermidis Ig4 | ||||||||
| BTZ2e | NS | 256 | 64 | 0.25 | 0.65 | |||
| Antibiotic | 64 | 25.60 | 0.40 | |||||
| S. epidermidis Ig6 | ||||||||
| BTZ2e | NS | 128 | 8 | 0.06 | 0.26 | |||
| Antibiotic | 64 | 12.80 | 0.20 | |||||
| LNZ | VAN | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample(μg/mL) | MBICo | MBICc | FIC | FICI | MBICo | MBICc | FIC | FICI |
| S. aureus ATCC 29213 | ||||||||
| BTZ2e | NS | 32 | 1.60 | 0.05 | 0.30 | |||
| Antibiotic | 64 | 16 | 0.25 | |||||
| S. aureus ATCC 43300 | ||||||||
| BTZ2e | NS | 16 | 0.80 | 0.05 | 0.08 | |||
| Antibiotic | 16 | 0.50 | 0.03 | |||||
| S. aureus Ig5 | ||||||||
| BTZ2e | 256 | 12,80 | 0.05 | 0.30 | 256 | 25.60 | 0.10 | 0.35 |
| Antibiotic | 4 | 1 | 0.25 | 64 | 16 | 0.25 | ||
| S. aureus Ig22 | ||||||||
| BTZ2e | NS | 32 | 3.20 | 0.10 | 0.35 | |||
| Antibiotic | 256 | 64 | 0.25 | |||||
| S. aureus Ig24 | ||||||||
| BTZ2e | ND | 512 | 51.20 | 0.10 | 0.16 | |||
| Antibiotic | 256 | 16 | 0.06 | |||||
| S. epidermidis Ig1 | ||||||||
| BTZ2e | ND | 64 | 3.20 | 0.05 | 0.17 | |||
| Antibiotic | 128 | 16 | 0.12 | |||||
| S. epidermidis Ig4 | ||||||||
| BTZ2e | 256 | 12.80 | 0.05 | 0.30 | 256 | 25.60 | 0.10 | 0.13 |
| Antibiotic | 8 | 2 | 0.25 | 64 | 2 | 0.03 | ||
| S. epidermidis Ig6 | ||||||||
| BTZ2e | 128 | 51.20 | 0.40 | 0.43 | 128 | 6.40 | 0.05 | 0.30 |
| Antibiotic | 32 | 1 | 0.03 | 64 | 16 | 0.25 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muraglia, M.; Schiavone, B.I.P.; Rosato, A.; Clodoveo, M.L.; Corbo, F. Antimicrobial Synergistic Effects of Linezolid and Vancomycin with a Small Synthesized 2-Mercaptobenzothiazole Derivative: A Challenge for MRSA Solving. Molecules 2023, 28, 6348. https://doi.org/10.3390/molecules28176348
Muraglia M, Schiavone BIP, Rosato A, Clodoveo ML, Corbo F. Antimicrobial Synergistic Effects of Linezolid and Vancomycin with a Small Synthesized 2-Mercaptobenzothiazole Derivative: A Challenge for MRSA Solving. Molecules. 2023; 28(17):6348. https://doi.org/10.3390/molecules28176348
Chicago/Turabian StyleMuraglia, Marilena, Brigida Immacolata Pia Schiavone, Antonio Rosato, Maria Lisa Clodoveo, and Filomena Corbo. 2023. "Antimicrobial Synergistic Effects of Linezolid and Vancomycin with a Small Synthesized 2-Mercaptobenzothiazole Derivative: A Challenge for MRSA Solving" Molecules 28, no. 17: 6348. https://doi.org/10.3390/molecules28176348
APA StyleMuraglia, M., Schiavone, B. I. P., Rosato, A., Clodoveo, M. L., & Corbo, F. (2023). Antimicrobial Synergistic Effects of Linezolid and Vancomycin with a Small Synthesized 2-Mercaptobenzothiazole Derivative: A Challenge for MRSA Solving. Molecules, 28(17), 6348. https://doi.org/10.3390/molecules28176348









