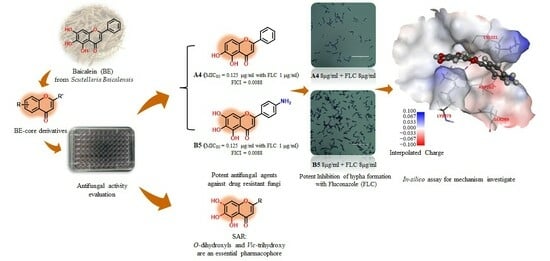
Exploration of Baicalein-Core Derivatives as Potent Antifungal Agents: SAR and Mechanism Insights
Abstract
Highlights
- Baicalein-Core Derivatives were designed and synthesized as Potent Anti-Fluconazole-resistant fungal Agents.
- O-dihydroxyls and vic-trihydroxy groups on either the A ring or B ring of flavones play a crucial role.
- MoA: Inhibit hypha formation; Little effect on ergosterol biosynthesis; Weak inhibitory effect on Eno1.
- Potential targets: 1,3-β-d-glucan synthase catalytic subunit, 1,3-β-d-glucan-UDP glucosyltransferase, and glycosyl-phosphatidylinositol protein by in-silico assay.
- Provide potential synergistic antifungals with new MoA.
Abstract
1. Introduction
2. Results
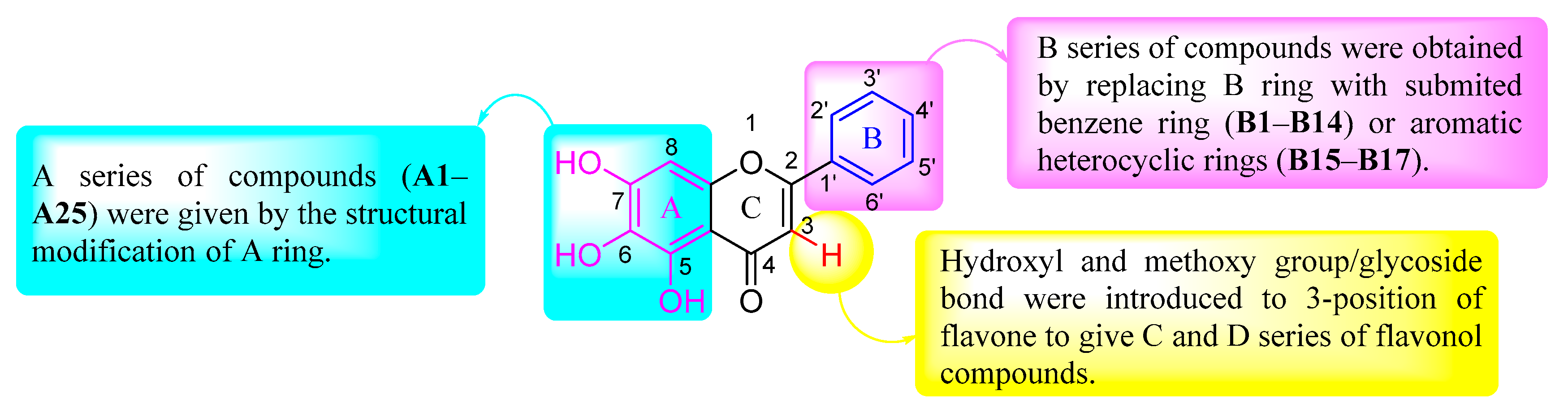
2.1. Molecular Design of BE-Core Derivatives
2.2. Chemistry
2.3. In Vitro Synergistic Antifungal Activities and SAR
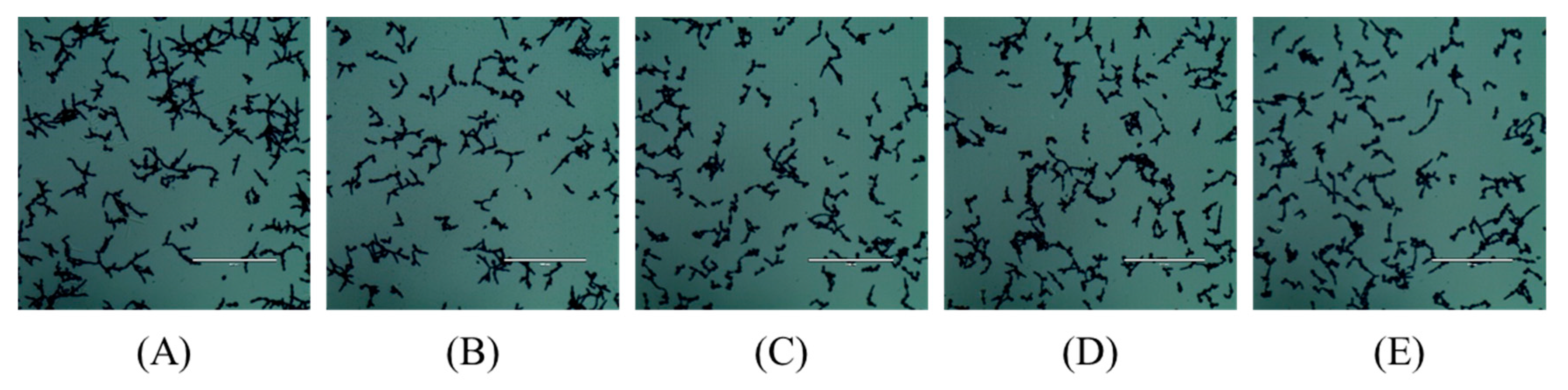
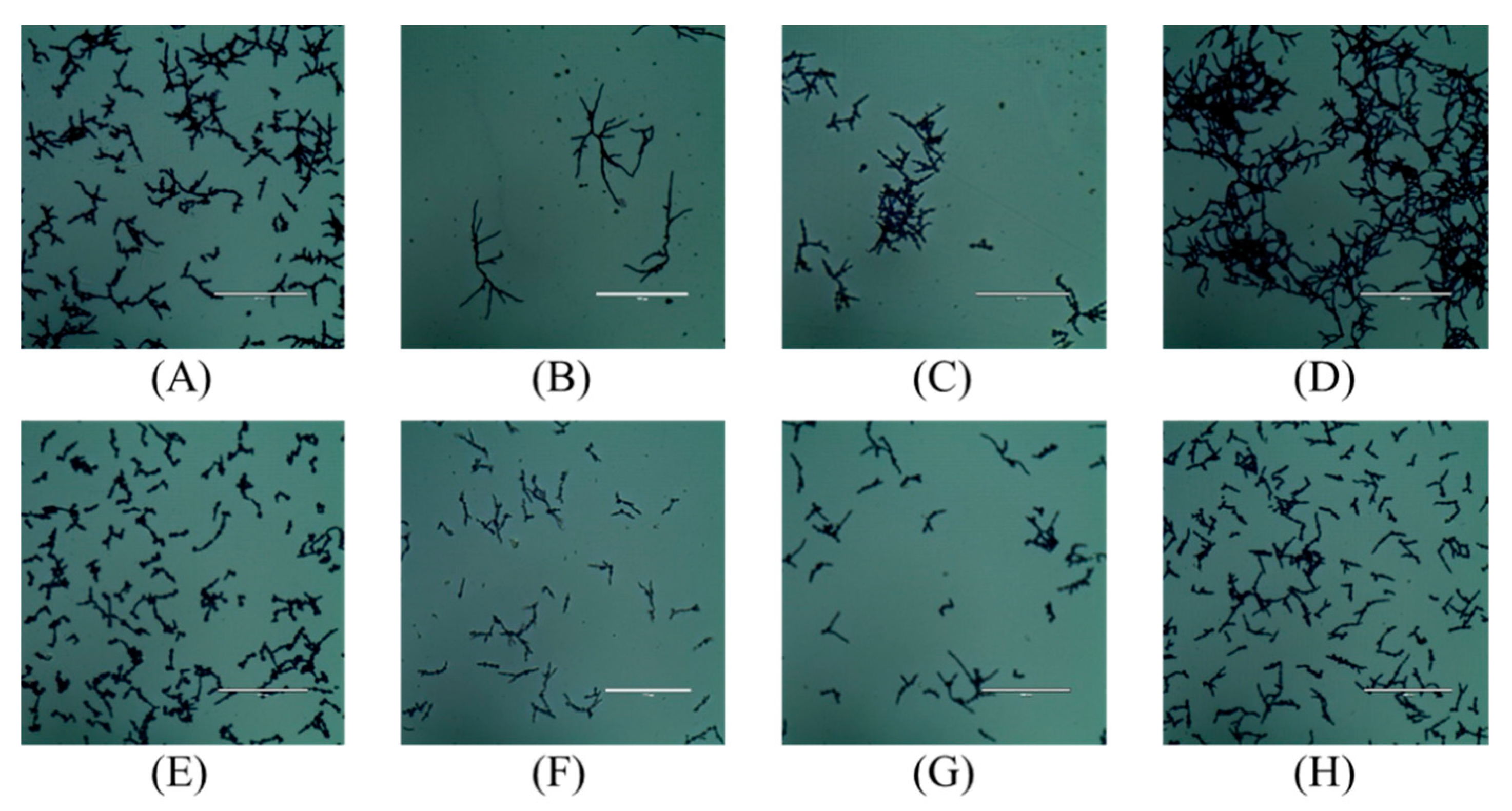
2.4. In Vitro Hyphal Formation Assay
2.5. In Vitro Sterol Composition Assay
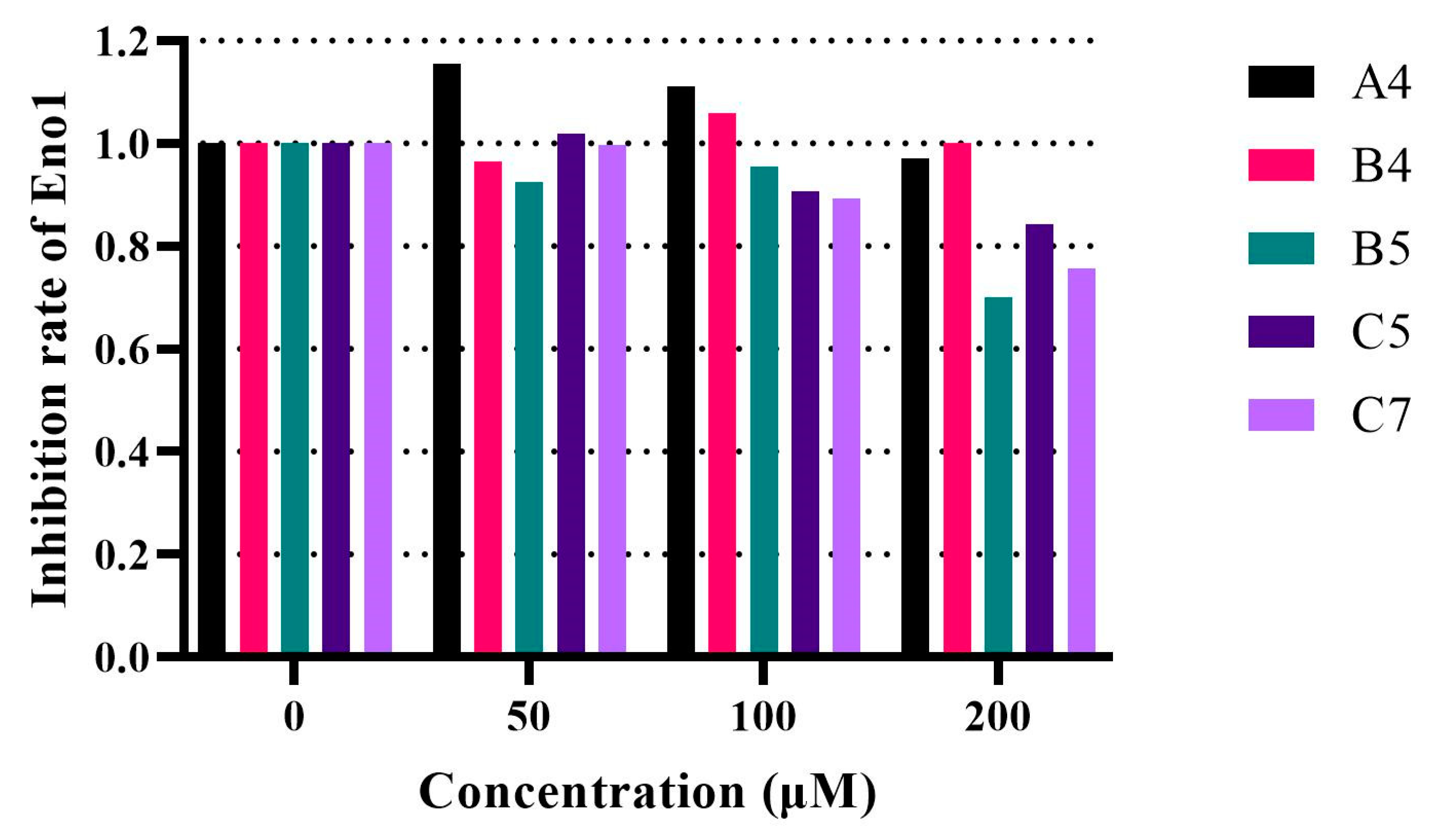
2.6. In Vitro Eno1 Enzymatic Assay
2.7. In Silico Studies
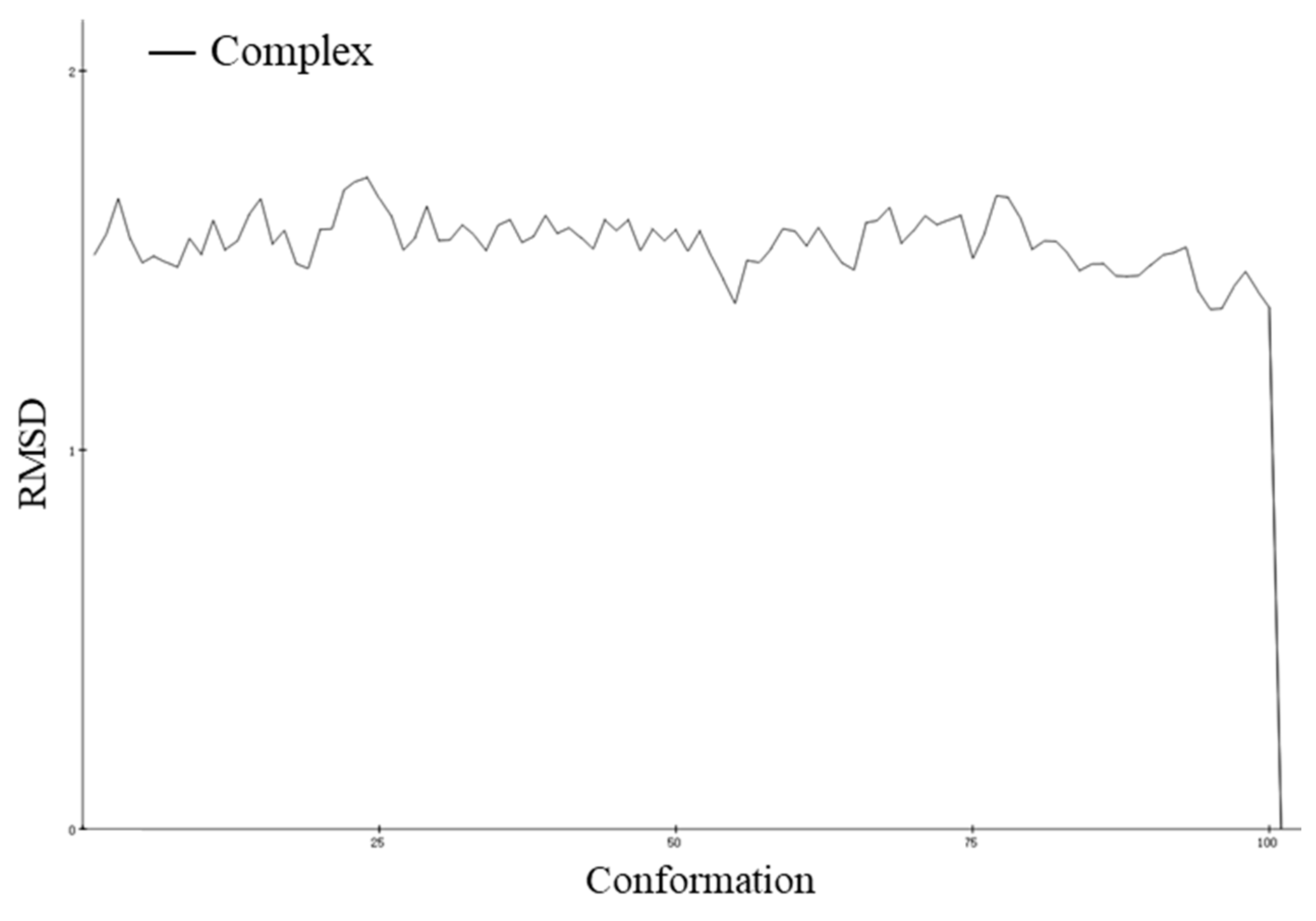
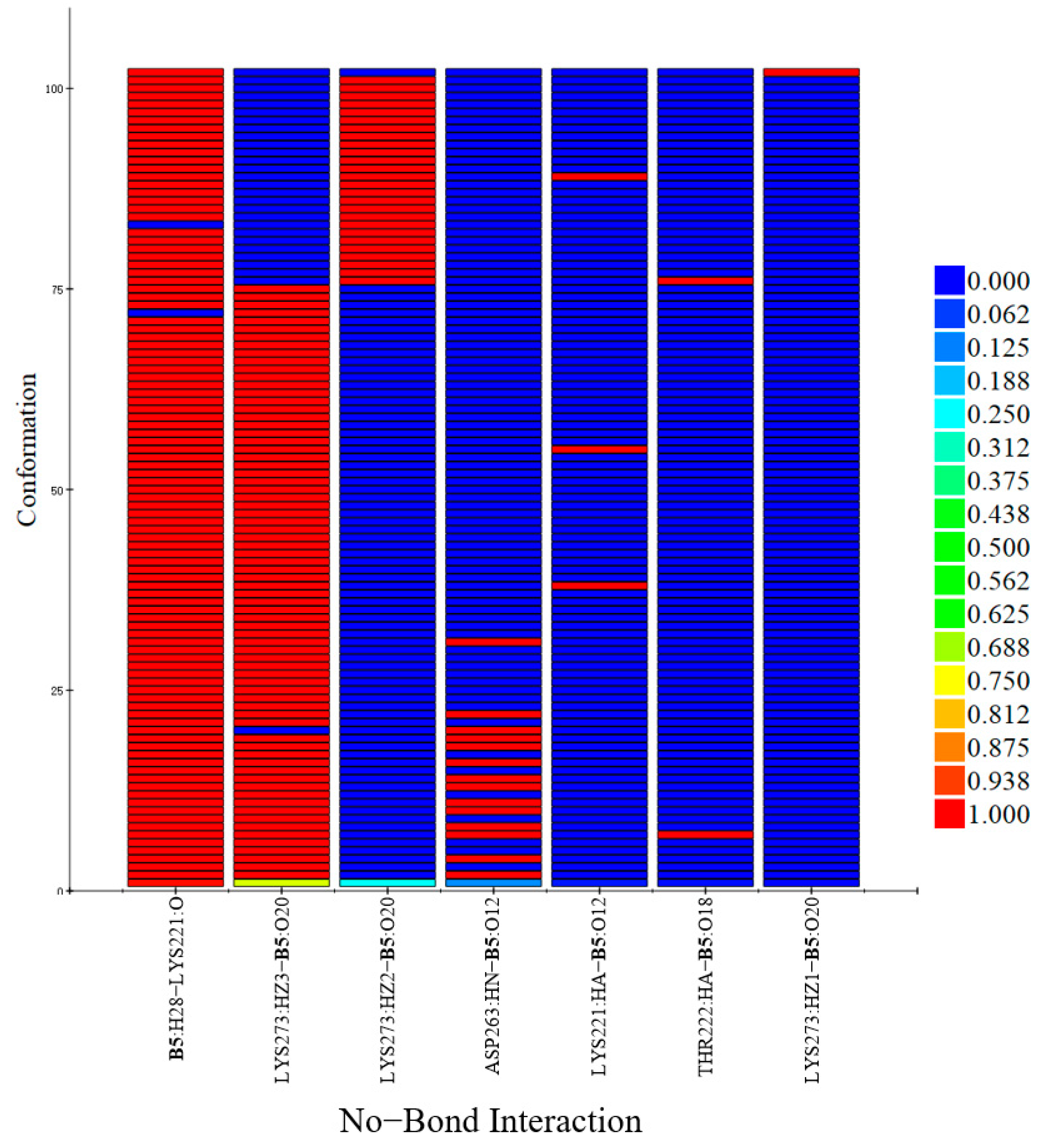
2.7.1. Molecular Dynamic Simulation
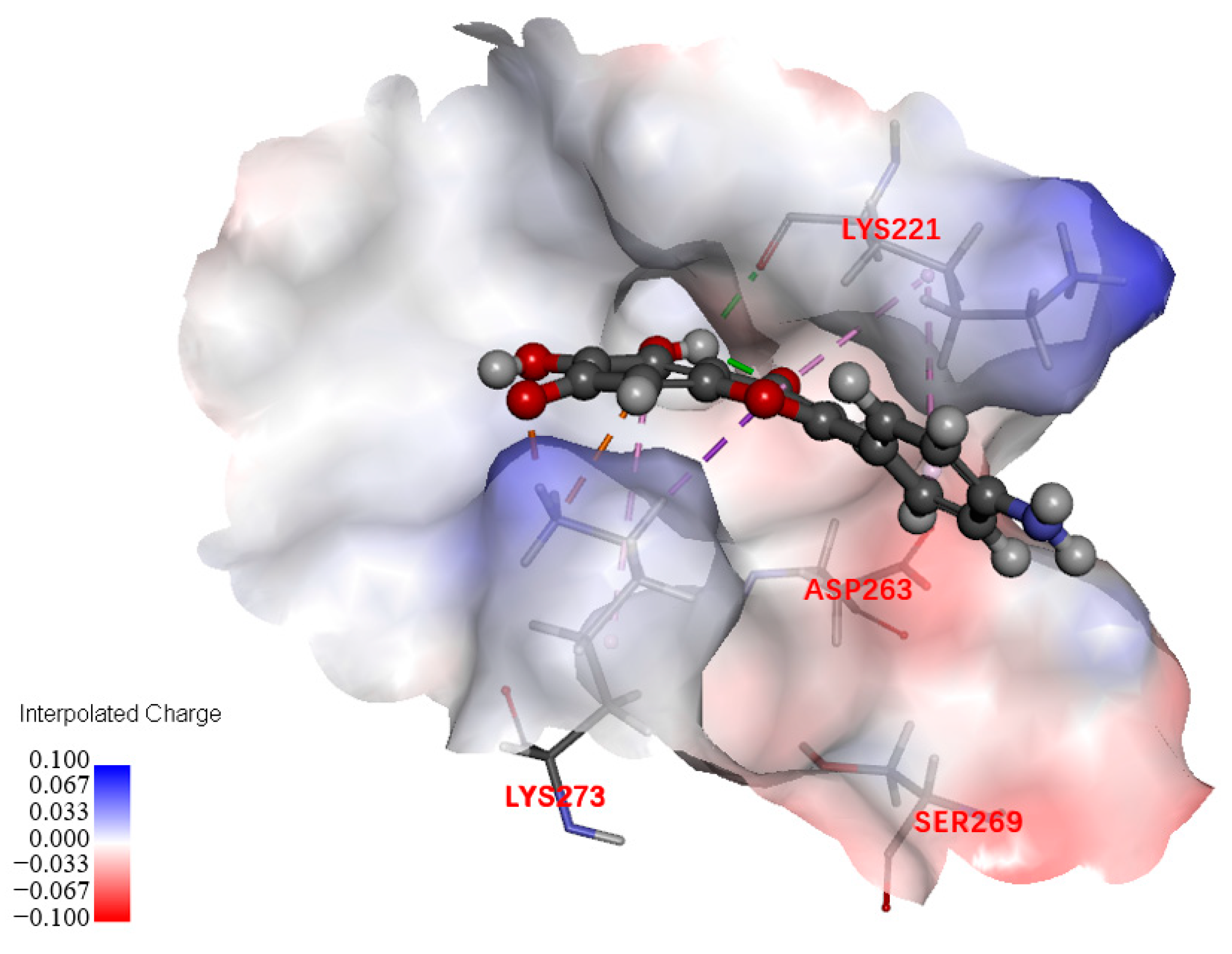
2.7.2. Exploration of Possible Targets
3. Discussion
4. Materials and Methods
4.1. Chemistry
Synthetic Methods for Title Compounds
- 6-hydroxy-2-phenyl-4H-chromen-4-one (A2)To a solution of MeOH (20 mL), 2′-hydroxy-4′-methoxyacetophenone (185 mg, 1.11 mmol) and benzaldehyde (141.2 mg, 1.33 mmol) were added. Then, 40% NaOH aqueous solution was added dropwise. The mixture was kept at room temperature along with stirring for 24 h. Upon completion of the reaction (monitored by TLC), 50 mL of water were added into the reaction mixture. Then, 2 mol/L diluted hydrochloric acid were added to the mixture to adjust the pH value of the solution to pH 5–6, and the yellow solid precipitate was collected by filtration. After recrystallization with ethyl alcohol, 193 mg of pure white products of 2′-hydroxy-4′-methoxychalcone were obtained with a yield of 60%.To a solution of DMSO (20 mL), 2′-hydroxy-4′-methoxychalcone (127 mg, 0.5 mmol) and I2 (253 mg, 0.1 mmol) were added, then the mixture was heated to 120–140 °C and kept for 4–8 h. Upon completion of the reaction (monitored by TLC), the reaction mixture was cooled. After 100 mL of water and 100 mg Na2S2O3 were added into the reaction mixture, the yellow solid precipitate was collected by filtration. After recrystallization with ethyl alcohol, 73 mg of pure yellow products of 6-methoxyflavone (A23) were obtained with a yield of 59%.To a solution of anhydrous DCM (10 mL), A23 (71 mg, 0.28 mmol) and 1 M of BBr3/DCM solution (2.8 mL, 1.4 mmol) were added at a temperature of −15 °C under argon atmosphere condition. Then the mixture was kept, along with stirring at room temperature, for another 12 h. After quenching the reaction by adding 10 mL of water at the temperature of −15 °C, the mixture was poured into a separatory funnel and separated. The aqueous layer was extracted with DCM (30 mL) three times. The combined organic layers were washed with saturated NaCl solution and dried over anhydrous Na2SO4 and evaporated to dryness to give crude products. After recrystallization with ethyl alcohol, 50 mg of pure yellow products of 6-hydroxyflavone (A2) were obtained with a yield of 42%.1H NMR (300 MHz, DMSO-d6) δ 10.04 (s, 1H), 8.12–8.03 (m, 2H), 7.66 (d, J = 9.0 Hz, 1H), 7.66–7.51 (m, 3H), 7.33 (d, J = 2.9 Hz, 1H), 7.26 (dd, J = 9.0, 3.0 Hz, 1H), 6.96 (s, 1H). 13C NMR (75 MHz, DMSO-d6) δ 177.00, 162.18, 154.90, 149.39, 131.62, 131.38, 129.09 (×2), 126.24 (×2), 124.24, 123.10, 119.85, 107.49, 105.93.
- 5,6-dihydroxy-2-phenyl-4H-chromen-4-one (A4)A2 was oxidized by iodo-benzene diacetate (IBD) according to the reference method to give A14 [20], then demethylated by BBr3 to A4.1H NMR (300 MHz, DMSO-d6) δ 10.04 (s, 1H), 8.12–8.03 (m, 2H), 7.66 (d, J = 9.0 Hz, 1H), 7.66–7.51 (m, 3H), 7.33 (d, J = 2.9 Hz, 1H), 7.26 (dd, J = 9.0, 3.0 Hz, 1H), 6.96 (s, 1H).13C NMR (75 MHz, DMSO) δ 177.00, 162.18, 154.90, 149.39, 131.62, 131.38, 129.09 (×2), 126.24 (×2), 124.24, 123.10, 119.85, 107.49, 105.93.
- 6,7-dihydroxy-2-phenyl-4H-chromen-4-one (A6)2′-hydroxy-3′,4′-methyleneacetophenone was reacted with benzaldehyde in a similar way as described for A2 to give the product A6.1H NMR (600 MHz, DMSO-d6) δ 10.45 (s, 1H), 9.79 (s, 1H), 8.05–8.00 (m, 2H), 7.61–7.51 (m, 3H), 7.31 (s, 1H), 7.04 (s, 1H), 6.83 (s, 1H). 13C NMR (151 MHz, DMSO-d6) δ 176.22, 161.43, 152.41, 150.86, 144.69, 131.60, 131.29, 129.05 (×2), 126.02 (×2), 116.16, 107.62, 105.97, 103.20.
- 6,7-dimethoxy-2-phenyl-4H-chromen-4-one (A11)Dissolve 1 equivalent of A6 in THF, add 2 equivalents of K2CO3 and CH3I, reflux for 8 h at 70 °C, stop heating after monitoring the reaction completely by TLC, and cool to room temperature. The mixture was poured into 100mL of water and stirred for 30 min and filtered by extraction. The filter cake was oven dried and then recrystallized in methanol to obtain A11.1H NMR (300 MHz, Chloroform-d) δ 7.95–7.85 (m, 2H), 7.55 (s, 1H), 7.50 (dd, J = 5.2, 1.9 Hz, 3H), 6.99 (d, J = 1.5 Hz, 1H), 6.83 (d, J = 1.5 Hz, 1H), 4.01 (s, 3H), 3.97 (d, J = 1.7 Hz, 3H).13C NMR (75 MHz, CDCl3) δ 177.84, 162.91, 154.60, 152.38, 147.74, 131.88, 131.37, 129.02 (×2), 126.10 (×2), 117.24, 106.98, 104.37, 99.77, 56.50, 56.37.
- 9-hydroxy-6-phenyl-8H-[1,3]dioxolo[4,5-g]chromen-8-one (A13)A suspension of baicalein (100 mg, 0.37 mmol), BrCH2Cl (52.1 mg, 0.41 mmol) in ethanol (15 mL) and Cs2CO3 (100 mg) was heated to reflux with stirring at Ar2 atmosphere for 12 h. Then the mixture was poured into ice water. The solid precipitate was collected by filtration. The crude product was purified by silica gel chromatography eluted with PE: EtOAc = 25:1 to give product (80 mg, 77%) as yellow solid. M+ = 283.0, M + Na+ = 305.0.1H NMR (300 MHz, Chloroform-d) δ 12.70 (s, 1H), 7.86 (dt, J = 7.6, 1.4 Hz, 2H), 7.61–7.45 (m, 3H), 6.67 (s, 1H), 6.59 (s, 1H), 6.10 (s, 2H).13C NMR (75 MHz, CDCl3) δ 183.15, 164.16, 154.24, 153.43, 142.36, 132.01, 131.29, 130.26, 129.25 (×2), 126.37 (×2), 107.91, 105.66, 102.82, 89.62.
- 6-hydroxy-5-methoxy-2-phenyl-4H-chromen-4-one (A14)A suspension of 6-hydroxyflavone (100 mg, 0.66 mmol), IBD (278 mg, 0.77 mmol) in methanol (15 mL), was kept at room temperature along with stirring for 2 h, then heated to reflux for 1 h. After removal of solvent by under reduced pressure, the residues were purified by silica gel chromatography eluted with PE: EtOAc = 20:1 to give a pure yellow solid (96 mg, 54%). M+ = 269.3.1H NMR (300 MHz, DMSO-d6) δ 8.02 (dd, J = 8.3, 1.5 Hz, 2H), 7.69–7.49 (m, 4H), 6.60 (s, 1H), 6.52 (dd, J = 10.3, 2.0 Hz, 1H), 3.43 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 184.93, 181.04, 165.44, 144.04, 140.40, 132.58, 131.25, 130.21, 129.07 (×2), 126.83, 126.71 (×2), 103.07, 95.62, 52.02.
- 5-methoxy-2-phenyl-4H-chromen-4-one (A17)2′-hydroxy-6′-methoxyacetophenone was reacted with benzaldehyde in a similar way as described for A2 to give the product A17.1H NMR (300 MHz, Chloroform-d) δ 7.87 (dd, J = 6.9, 2.9 Hz, 2H), 7.55 (t, J = 8.4 Hz, 1H), 7.48 (dd, J = 5.2, 1.9 Hz, 3H), 7.11 (d, J = 8.4 Hz, 1H), 6.81 (d, J = 8.3 Hz, 1H), 6.72 (s, 1H), 3.98 (s, 3H). 13C NMR (75 MHz, Chloroform-d) δ 178.38, 161.13, 159.83, 158.35, 133.82, 131.50, 131.41, 129.02, 126.11, 114.66, 110.22, 109.14, 106.53, 56.57.
- 6,8-dibromo-5,7-dihydroxy-2-phenyl-4H-chromen-4-one (A18)To a suspension of AcOH (15 mL) and chrysin (A5, 254 mg, 1 mmol), Br2 (3 mmol) was added, along with stirring at room temperature. Then the mixture was kept for 3 h. Upon completion of the reaction (monitored by TLC), 100 mL of water and 100 mg Na2S2O3 were added into the reaction mixture, and the yellow solid precipitate was collected by filtration. After recrystallization with EtOH, pure product was obtained (336 mg, 82%). M+ = 411.1, 413.2.1H NMR (300 MHz, DMSO-d6) δ 13.73 (s, 1H), 8.17–8.09 (m, 2H), 7.61 (d, J = 7.3 Hz, 3H), 7.19 (s, 1H). 13C NMR (75 MHz, DMSO-d6) δ 181.56, 163.48, 157.50, 157.03, 152.30, 132.48, 130.24, 129.26 (×2), 126.47 (×2), 105.13, 105.04, 94.58, 88.48.
- 5,7-dihydroxy-8-nitro-2-phenyl-4H-chromen-4-one (A19)A19 was synthesized according to the references with 62% yield [21]. 1H NMR (300 MHz, DMSO-d6) δ 6.37 (s, 1H), 7.19 (s, 1H), 7.61 (t, J = 6.6 Hz, 3H), 7.91–8.06 (m, 2H), 13.23 (s, 1H).1H NMR (300 MHz, DMSO-d6) δ 13.24 (s, 1H), 8.03–7.94 (m, 2H), 7.70–7.54 (m, 3H), 7.20 (s, 1H), 6.38 (s, 1H).
- 5,7-dimethoxy-8-nitro-2-phenyl-4H-chromen-4-one (A20)A19 was reacted with CH3I in a similar way as described for A11 to give the product A20.1H NMR (300 MHz, Chloroform-d) δ 7.84–7.75 (m, 2H), 7.49 (d, J = 7.1 Hz, 3H), 6.72 (s, 1H), 6.43 (s, 1H), 4.07 (s, 3H), 4.06 (s, 3H). 13C NMR (75 MHz, Chloroform-d) δ 176.14, 162.52, 161.01, 156.16, 151.16, 132.03, 130.38, 129.30 (×2), 126.31 (×2), 109.03, 108.33, 91.36, 77.36, 57.04 (×2).
- 5,6,7-trimethoxy-2-phenyl-4H-chromen-4-one (A21)BE was reacted with CH3I in a similar way as described for A11 to give the product A21.1H NMR (300 MHz, Chloroform-d) δ 7.93–7.82 (m, 2H), 7.50 (dd, J = 5.1, 1.9 Hz, 3H), 6.81 (s, 1H), 6.67 (s, 1H), 3.98 (s, 3H), 3.98 (s, 3H), 3.91 (s, 3H). 13C NMR (75 MHz, Chloroform-d) δ 177.32, 161.25, 157.93, 154.70, 152.69, 140.55, 131.71, 131.39, 129.09 (×2), 126.09 (×2), 113.08, 108.52, 96.41, 62.31, 61.66, 56.43.
- 8-amino-5-hydroxy-7-methoxy-2-phenyl-4H-chromen-4-one (A22)A19 was reacted with equimolar CH3I in a similar way as described for A11 to give the 5-hydroxy-7-methoxy-8-nitroflavone. 5-hydroxy-7-methoxy-8-nitroflavone was reduced in a similar way as described for A19 to give the A22.1H NMR (300 MHz, Chloroform-d) δ 12.10 (s, 1H), 7.92–7.80 (m, 2H), 7.53 (q, J = 4.1 Hz, 3H), 6.60 (s, 1H), 6.42 (s, 1H), 3.93 (s, 3H). 13C NMR (75 MHz, Chloroform-d) δ 183.04, 163.75, 153.73, 153.02, 143.86, 131.89, 131.80, 129.23 (×2), 126.40 (×2), 116.05, 105.66, 104.99, 95.32, 56.29.
- 6-methoxy-2-phenyl-4H-chromen-4-one (A23)1-(2-hydroxy-5-methoxyphenyl) ethan-1-one was reacted with benzaldehyde in a similar way as described for A2 to give the product A23.1H NMR (300 MHz, Chloroform-d) δ 7.98–7.88 (m, 2H), 7.64–7.45 (m, 3H), 7.44 (d, J = 2.3 Hz, 1H), 7.34 (d, J = 2.7 Hz, 1H), 7.13–7.02 (m, 1H), 6.83 (s, 1H), 3.91 (s, 3H).13C NMR (75 MHz, CDCl3) δ 178.20, 166.62, 157.41, 151.16, 131.63, 130.49, 129.17 (×2), 126.39 (×2), 125.52, 123.95, 119.93, 105.19, 105.02, 90.58, 56.11.
- 6-phenyl-8H-[1,3]dioxolo[4,5-g] chromen-8-one (A24)2′-hydroxy-3′,4′-methyleneacetophenone was reacted with benzaldehyde in a similar way as described for A2 to give the product A24.1H NMR (600 MHz, DMSO-d6) δ 8.07–8.02 (m, 2H), 7.61–7.50 (m, 3H), 7.37 (d, J = 0.8 Hz, 1H), 7.32 (d, J = 0.7 Hz, 1H), 6.95 (d, J = 0.7 Hz, 1H), 6.21 (s, 2H). 13C NMR (151 MHz, DMSO) δ 175.97, 161.79, 152.93, 152.60, 146.05, 131.53, 131.09, 129.04 (×2), 126.05 (×2), 118.18, 106.22, 102.80, 100.82, 98.48.
- 5,6,7-trimethoxy-2-(4-nitrophenyl)-4H-chromen-4-one (B1)1-(6-hydroxy-2,3,4-trimethoxyphenyl) ethan-1-one was reacted with 4-nitrobenzaldehyde in a similar way as described for A2 to give the product B1.1H NMR (300 MHz, DMSO-d6) δ 8.36 (s, 4H), 7.27 (s, 1H), 7.03 (s, 1H), 3.96 (s, 3H), 3.81 (s, 3H), 3.78 (s, 3H).
- 5,6,7-trihydroxy-2-(4-nitrophenyl)-4H-chromen-4-one (B2)B1 was reacted in a similar way as described for A2 to give the product B2.1H NMR (300 MHz, DMSO-d6) δ 12.53 (s, 1H), 10.68 (s, 1H), 8.33 (t, J = 3.3 Hz, 4H), 7.11 (s, 1H), 6.64 (s, 1H). 13C NMR (75 MHz, DMSO-d6) δ 181.91, 160.32, 153.99, 149.83, 148.99, 146.87, 136.87, 129.58, 127.68 (×2), 124.05 (×2), 106.81, 104.51, 94.15.
- 2-(4-bromophenyl)-5,6,7-trimethoxy-4H-chromen-4-one (B3)1-(6-hydroxy-2,3,4-trimethoxyphenyl) ethan-1-one was reacted with 4-bromobenzaldehyde in a similar way as described for A2 to give the product B3.1H NMR (300 MHz, Chloroform-d) δ 7.74 (d, J = 8.4 Hz, 2H), 7.64 (d, J = 8.3 Hz, 2H), 6.80 (s, 1H), 6.67 (d, J = 1.2 Hz, 1H), 3.98 (d, J = 1.2 Hz, 6H), 3.92 (s, 3H).
- 2-(4-bromophenyl)-5,6,7-trihydroxy-4H-chromen-4-one (B4)B3 was reacted in a similar way as described for A2 to give the product B4.1H NMR (600 MHz, DMSO-d6) δ 12.58 (s, 1H), 10.59 (s, 1H), 7.99 (d, J = 8.4 Hz, 2H), 7.75 (d, J = 8.3 Hz, 2H), 6.95 (s, 1H), 6.61 (s, 1H).
- 2-(4-aminophenyl)-5,6,7-trihydroxy-4H-chromen-4-one (B5)B2 was reduced in a similar way as described for A19 to give the B5.1H NMR (300 MHz, DMSO-d6) δ 12.97 (s, 1H), 10.38 (s, 1H), 8.67 (s, 1H), 7.74 (d, J = 8.5 Hz, 2H), 6.65 (d, J = 8.5 Hz, 2H), 6.59 (s, 1H), 6.53 (s, 1H), 6.04 (s, 2H). 13C NMR (75 MHz, DMSO-d6) δ 181.78, 164.37, 152.95, 152.75, 149.51, 147.15, 128.94, 128.01 (×2), 116.82, 113.46 (×2), 103.83, 100.25, 93.69.
- 5,6,7-trimethoxy-2-(3-nitrophenyl)-4H-chromen-4-one (B6)1-(6-hydroxy-2,3,4-trimethoxyphenyl) ethan-1-one was reacted with 3-nitrobenzaldehyde in a similar way as described for A2 to give the product B6.1H NMR (300 MHz, DMSO-d6) δ 8.81 (s, 1H), 8.52 (s, 1H), 8.41 (s, 1H), 7.83 (d, J = 7.2 Hz, 1H), 7.31 (s, 1H), 7.03 (s, 1H), 3.97 (s, 3H), 3.81 (s, 3H), 3.77 (s, 3H).
- 5,6,7-trihydroxy-2-(3-nitrophenyl)-4H-chromen-4-one (B7)B6 was reacted in a similar way as described for A2 to give the product B7.1H NMR (300 MHz, DMSO-d6) δ 12.51 (s, 1H), 10.65 (s, 1H), 8.75 (t, J = 2.0 Hz, 1H), 8.53–8.44 (m, 1H), 8.40 (dd, J = 8.1, 2.3 Hz, 1H), 7.84 (t, J = 8.1 Hz, 1H), 7.13 (s, 1H), 6.66 (s, 1H). 13C NMR (75 MHz, DMSO-d6) δ 182.01, 160.37, 153.90, 149.79, 148.38, 146.90, 132.71, 132.55, 130.78, 129.53, 126.05, 120.84, 106.06, 104.42, 94.19.
- 2-(3-aminophenyl)-5,6,7-trihydroxy-4H-chromen-4-one (B8)B7 was reduced in a similar way as described for A19 to give the B8.1H NMR (300 MHz, DMSO-d6) δ 12.65 (s, 1H), 7.16 (dt, J = 9.6, 4.8 Hz, 3H), 6.76 (d, J = 7.7 Hz, 1H), 6.67 (s, 1H), 6.55 (s, 1H), 5.41 (s, 2H). 13C NMR (75 MHz, DMSO-d6) δ 181.95, 163.82, 149.88, 149.32, 146.79, 131.54, 129.65 (×2), 129.40, 117.21, 113.73, 110.79, 104.05, 103.94 (×2), 93.77.
- 2-(3,4-dihydroxyphenyl)-5-hydroxy-4H-chromen-4-one (B10)1-(2-hydroxy-6-methoxyphenyl) ethan-1-one was reacted with 3,4-dimethoxybenzaldehyde in a similar way as described for A2 to give the product B10.1H NMR (300 MHz, DMSO-d6) δ 12.84 (s, 1H), 9.77 (s, 2H), 7.65 (t, J = 8.3 Hz, 1H), 7.53–7.42 (m, 2H), 7.13 (dd, J = 8.4, 0.9 Hz, 1H), 6.91 (d, J = 8.2 Hz, 1H), 6.83 (s, 1H), 6.82–6.76 (m, 1H). 13C NMR (75 MHz, DMSO-d6) δ 182.85, 164.85, 159.90, 155.79, 150.08, 145.81, 135.66, 121.30, 119.35, 116.04, 113.59, 110.84, 109.93, 107.26, 103.40.
- 2-(benzo[d][1,3]dioxol-5-yl)-5-methoxy-4H-chromen-4-one (B14)2′-hydroxy-6′-methoxyacetophenone was reacted with benzo[d][1,3]dioxole-5-carbaldehyde in a similar way as described for A2 to give the product B14.1H NMR (300 MHz, DMSO-d6) δ 7.68 (t, J = 8.4 Hz, 1H), 7.63 (dt, J = 4.4, 2.2 Hz, 2H), 7.27 (dd, J = 8.4, 0.9 Hz, 1H), 7.09 (d, J = 8.7 Hz, 1H), 6.98 (d, J = 8.3 Hz, 1H), 6.78 (s, 1H), 6.15 (s, 2H), 3.86 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 176.42, 159.74, 159.03, 157.48, 150.13, 148.15, 134.14, 124.68, 121.17, 113.64, 110.00, 108.68, 107.37, 107.20, 106.09, 101.95, 56.09.
- 5-methoxy-2-(thiophen-2-yl)-4H-chromen-4-one (B15)2′-hydroxy-6′-methoxyacetophenone was reacted with thiophene-2-carbaldehyde in a similar way as described for A2 to give the product B15.1H NMR (300 MHz, DMSO-d6) δ 7.95 (dd, J = 3.8, 1.2 Hz, 1H), 7.93 (dd, J = 5.0, 1.2 Hz, 1H), 7.66 (t, J = 8.4 Hz, 1H), 7.26 (dd, J = 5.0, 3.8 Hz, 1H), 7.15 (dd, J = 8.4, 0.9 Hz, 1H), 6.97 (dd, J = 8.5, 1.0 Hz, 1H), 6.71 (s, 1H), 3.85 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 175.88, 159.10, 157.20, 156.24, 134.29, 133.83, 131.29, 129.08, 128.89, 113.69, 109.72, 107.44, 106.80, 56.11.
- 2-(furan-2-yl)-5-methoxy-4H-chromen-4-one (B16)2′-hydroxy-6′-methoxyacetophenone was reacted with furan-2-carbaldehyde in a similar way as described for A2 to give the product B16.1H NMR (300 MHz, Chloroform-d) δ 7.56 (dd, J = 7.3, 1.1 Hz, 1H), 7.51 (dd, J = 8.4, 1.0 Hz, 1H), 7.08–6.99 (m, 2H), 6.79 (d, J = 8.3 Hz, 1H), 6.61 (s, 1H), 6.56 (dd, J = 3.5, 1.6 Hz, 1H), 3.96 (s, 3H). 13C NMR (75 MHz, Chloroform-d) δ 177.83, 159.86, 157.92, 153.26, 146.21, 145.62, 133.82, 114.84, 112.65, 112.45, 110.09, 107.16, 106.66, 56.57.
- 5-hydroxy-2-(thiophen-2-yl)-4H-chromen-4-one (B17)The 2′-hydroxy-6′-methoxyacetophenone was reacted with thiophene-2-carbaldehyde in a similar way as described for A2 to give the product B17.1H NMR (300 MHz, Methanol-d4) δ 7.79 (d, J = 3.8 Hz, 1H), 7.67 (d, J = 4.9 Hz, 1H), 7.61–7.45 (m, 2H), 7.20 (t, J = 4.4 Hz, 1H), 6.99 (d, J = 8.4 Hz, 1H), 6.77 (d, J = 8.2 Hz, 1H), 6.61 (s, 1H). 13C NMR (75 MHz, Methanol-d4) δ 183.87, 161.25, 160.74, 156.64, 136.12, 134.70, 131.98, 130.08, 129.22, 111.98, 111.02, 107.75, 104.55.
4.2. Antifungal Activity (Broth Microdilution Method)
4.3. In Vitro Hyphal Formation Assay
4.4. Analysis of Fungal Sterol Composition Assay
4.5. Enolase Activity Analysis
4.6. Docking Studies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Perfect, J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Prim. 2018, 4, 18026. [Google Scholar] [CrossRef]
- Sanguinetti, M.; Posteraro, B.; Lass-Flörl, C. Antifungal drug resistance among Candida species: Mechanisms and clinical impact. Mycoses 2015, 58, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; He, J.-B.; Bai, X.; Li, X.-N.; Lu, L.-F.; Liu, Y.-C.; Zhang, K.-Q.; Li, S.-H.; Niu, X.-M. Unexpected Biosynthesis of Fluorescein-Like Arthrocolins against Resistant Strains in an Engineered Escherichia coli. Org. Lett. 2019, 21, 6499–6503. [Google Scholar] [CrossRef]
- Xie, F.; Hao, Y.; Liu, J.; Bao, J.; Ni, T.; Liu, Y.; Chi, X.; Wang, T.; Yu, S.; Jin, Y.; et al. Discovery of Novel Thiosemicarbazides Containing 1,3,5-Triazines Derivatives as Potential Synergists against Fluconazole-Resistant Candida albicans. Pharmaceutics 2022, 14, 2334. [Google Scholar] [CrossRef]
- Garcia-Gomes, A.S.; Curvelo, J.A.; Soares, R.M.; Ferreira-Pereira, A. Curcumin acts synergistically with fluconazole to sensitize a clinical isolate of Candida albicans showing a MDR phenotype. Med. Mycol. 2012, 50, 26–32. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.P.; Zhang, J.D.; Li, Q.; Shen, H.; Chen, S.M.; He, L.J.; Yan, L.; Xu, G.T.; An, M.M.; et al. Synergistic Antifungal Effect of Glabridin and Fluconazole. PLoS ONE 2014, 9, e103442. [Google Scholar] [CrossRef]
- Zhao, F.; Dong, H.-H.; Wang, Y.-H.; Wang, T.-Y.; Yan, Z.-H.; Yan, F.; Zhang, D.-Z.; Cao, Y.-Y.; Jin, Y.-S. Synthesis and synergistic antifungal effects of monoketone derivatives of curcumin against fluconazole-resistant Candida spp. MedChemComm 2017, 8, 1093–1102. [Google Scholar] [CrossRef]
- Al-Maharik, N.; Jaradat, N. Phytochemical Profile, Antimicrobial, Cytotoxic, and Antioxidant Activities of Fresh and Air-Dried Satureja nabateorum Essential Oils. Molecules 2021, 27, 125. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, W.; Huang, X.; Hao, L.; Li, Y.; Sun, S. Antifungal Activity and Potential Mechanism of N-Butylphthalide Alone and in Combination with Fluconazole Against Candida albicans. Front. Microbiol. 2019, 10, 1461. [Google Scholar] [CrossRef]
- Nobile, C.J.; Andes, D.R.; Nett, J.E.; Smith, F.J.; Yue, F.; Phan, Q.-T.; Edwards, J.E., Jr.; Filler, S.G.; Mitchell, A.P. Critical Role of Bcr1-Dependent Adhesins in C. albicans Biofilm Formation In Vitro and In Vivo. PLoS Pathog. 2006, 2, e63. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Luan, X.; Xie, F.; Chang, W.; Lou, H. Erg6 Acts as a Downstream Effector of the Transcription Factor Flo8 to Regulate Biofilm Formation in Candida albicans. Microbiol. Spectr. 2023, 11, e0039323. [Google Scholar] [CrossRef]
- Wu, H.; Du, C.; Xu, Y.; Liu, L.; Zhou, X.; Ji, Q. Design, synthesis, and biological evaluation of novel spiro[pyrrolidine-2,3′-quinolin]-2′-one derivatives as potential chitin synthase inhibitors and antifungal agents. Eur. J. Med. Chem. 2022, 233, 114208. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.-J.; Zhou, H.-Y.; Liu, Y.; Dong, J.-X.; Tang, W.-D.; Zhao, P.; Tang, H.-L.; Jin, Y.-S. Synthesis of baicalein derivatives and evaluation of their antiviral activity against arboviruses. Bioorg. Med. Chem. Lett. 2022, 72, 128863. [Google Scholar] [CrossRef]
- Cao, Y.; Dai, B.; Wang, Y.; Huang, S.; Xu, Y.; Cao, Y.; Gao, P.; Zhu, Z.; Jiang, Y. In vitro activity of baicalein against Candida albicans biofilms. Int. J. Antimicrob. Agents 2008, 32, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Cao, Y.Y.; Di Dai, B.; Sun, X.R.; Zhu, Z.Y.; Cao, Y.B.; Wang, Y.; Gao, P.H.; Jiang, Y.Y. In Vitro Synergism of Fluconazole and Baicalein against Clinical Isolates of Candida albicans Resistant to Fluconazole. Biol. Pharm. Bull. 2008, 31, 2234–2236. [Google Scholar] [CrossRef]
- Li, L.; Lu, H.; Zhang, X.; Whiteway, M.; Wu, H.; Tan, S.; Zang, J.; Tian, S.; Zhen, C.; Meng, X.; et al. Baicalein Acts against Candida albicans by Targeting Eno1 and Inhibiting Glycolysis. Microbiol. Spectr. 2022, 10, e0208522. [Google Scholar] [CrossRef]
- Wang, J.-F.; Ding, N.; Zhang, W.; Wang, P.; Li, Y.-X. Synthesis of Ring A-Modified Baicalein Derivatives. Helvetica Chim. Acta 2011, 94, 2221–2230. [Google Scholar] [CrossRef]
- Prakash, O.; Kaur, H.; Sharma, V.; Bhardwaj, V.; Pundeer, R. A novel and facile iodine(III)-mediated approach for C(5)-acetoxylation of 6-hydroxyflavone and 6-hydroxyflavanones. Tetrahedron Lett. 2004, 45, 9065–9067. [Google Scholar] [CrossRef]
- Gao, H.; Kawabata, J. Alpha-Glucosidase inhibition of 6-hydroxyflavones. Part 3: Synthesis and evaluation of 2,3,4-trihydroxybenzoyl-containing flavonoid analogs and 6-aminoflavones as alpha-glucosidase inhibitors. Bioorg. Med. Chem. 2005, 13, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; Approved Standard M27-A3; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Dong, H.-H.; Zhao, F.; Wang, J.; Yan, F.; Jiang, Y.-Y.; Jin, Y.-S. The synthesis and synergistic antifungal effects of chalcones against drug resistant Candida albicans. Bioorg. Med. Chem. Lett. 2016, 26, 3098–3102. [Google Scholar] [CrossRef]
- Singh, S.B.; Herath, K.; Kahn, J.N.; Mann, P.; Abruzzo, G.; Motyl, M. Synthesis and antifungal evaluation of pentyloxyl-diphenylisoxazoloyl pneumocandins and echinocandins. Bioorg. Med. Chem. Lett. 2013, 23, 3253–3256. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.M.; Abe, T.; Komiyama, T.; Wang, W.; Hattori, M.; Daneshtalab, M. Synthesis, anti-fungal and 1,3-β-d-glucan synthase inhibitory activities of caffeic and quinic acid derivatives. Bioorg. Med. Chem. 2010, 18, 7009–7014. [Google Scholar] [CrossRef] [PubMed]
- Masubuchi, K.; Okada, T.; Kohchi, M.; Sakaitani, M.; Mizuguchi, E.; Shirai, H.; Aoki, M.; Watanabe, T.; Kondoh, O.; Yamazaki, T.; et al. Synthesis and antifungal activities of novel 1,3-beta-d-glucan synthase inhibitors. Part 1. Bioorg. Med. Chem. Lett. 2001, 11, 395–398. [Google Scholar] [CrossRef]
- Masubuchi, K.; Okada, T.; Kohchi, M.; Murata, T.; Tsukazaki, M.; Kondoh, O.; Yamazaki, T.; Satoh, Y.; Ono, Y.; Tsukaguchi, T.; et al. Synthesis and antifungal activities of novel 1,3-beta-d-glucan synthase inhibitors. Part 2. Bioorg. Med. Chem. Lett. 2001, 11, 1273–1276. [Google Scholar] [CrossRef]
- Castelli, M.V.; Kouznetsov, V.V.; López, S.N.; Sortino, M.; Enriz, R.D.; Ribas, J.C.; Zacchino, S. In vitro antifungal activity of new series of homoallylamines and related compounds with inhibitory properties of the synthesis of fungal cell wall polymers. Bioorg. Med. Chem. 2003, 11, 1531–1550. [Google Scholar] [CrossRef]
- López, S.N.; Castelli, M.V.; Zacchino, S.A.; Domínguez, J.N.; Lobo, G.; Charris-Charris, J.; Cortés, J.C.; Ribas, J.C.; Devia, C.; Rodríguez, A.M.; et al. In vitro antifungal evaluation and structure-activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorg. Med. Chem. 2001, 9, 1999–2013. [Google Scholar] [CrossRef] [PubMed]
- Urbina, J.M.; Cortés, J.C.; Palma, A.; López, S.N.; Zacchino, S.A.; Enriz, R.D.; Ribas, J.C.; Kouznetzov, V.V. Inhibitors of the fungal cell wall. Synthesis of 4-aryl-4-N-arylamine-1-butenes and related compounds with inhibitory activities on beta(1-3) glucan and chitin synthases. Bioorg. Med. Chem. 2000, 8, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Masubuchi, K.; Taniguchi, M.; Umeda, I.; Hattori, K.; Suda, H.; Kohchi, Y.; Isshiki, Y.; Sakai, T.; Kohchi, M.; Shirai, M.; et al. Synthesis and structure–activity relationships of novel fungal chitin synthase inhibitors. Bioorg. Med. Chem. Lett. 2000, 10, 1459–1462. [Google Scholar] [CrossRef]
- Goldstein, A.S.; Frye, L.L. Synthesis and Bioevaluation of Δ7-5-Desaturase Inhibitors, an Enzyme Late in the Biosynthesis of the Fungal Sterol Ergosterol. J. Med. Chem. 1997, 40, 3706. [Google Scholar] [CrossRef][Green Version]
- Tafi, A.; Costi, R.; Botta, M.; Di Santo, R.; Corelli, F.; Massa, S.; Ciacci, A.; Manetti, F.; Artico, M. Antifungal agents. 10. New derivatives of 1-[(aryl)[4-aryl-1H-pyrrol-3-yl]methyl]-1H-imidazole, synthesis, anti-candida activity, and quantitative structure-analysis relationship studies. J. Med. Chem. 2002, 45, 2720–2732. [Google Scholar] [CrossRef]
- Zhen, C.; Lu, H.; Jiang, Y. Novel Promising Antifungal Target Proteins for Conquering Invasive Fungal Infections. Front. Microbiol. 2022, 13, 911322. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.; Khan, M.A. Targeting the GPI biosynthetic pathway. Pathog. Glob. Health 2018, 112, 115–122. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Wang, Y.; Zhou, X.; Zhang, L.; Yang, G.; Gao, X.-D.; Murakami, Y.; Fujita, M.; Kinoshita, T. Accumulated precursors of specific GPI-anchored proteins upregulate GPI biosynthesis with ARV1. J. Cell Biol. 2023, 222, e202208159. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Oh, S.-H.; Jones, R.; Garnett, J.A.; Salgado, P.S.; Rusnakova, S.; Matthews, S.J.; Hoyer, L.L.; Cota, E. The Peptide-binding Cavity Is Essential for Als3-mediated Adhesion of Candida albicans to Human Cells. J. Biol. Chem. 2014, 289, 18401–18412. [Google Scholar] [CrossRef] [PubMed]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Arastehfar, A.; Gabaldón, T.; Garcia-Rubio, R.; Jenks, J.D.; Hoenigl, M.; Salzer, H.J.F.; Ilkit, M.; Lass-Flörl, C.; Perlin, D.S. Drug-Resistant Fungi: An Emerging Challenge Threatening Our Limited Antifungal Armamentarium. Antibiotics 2020, 9, 877. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Dasilva, D.; Haranahalli, K.; McCarthy, J.B.; Mallamo, J.; Ojima, I.; Del Poeta, M. The Future of Antifungal Drug Therapy: Novel Compounds and Targets. Antimicrob. Agents Chemother. 2021, 65, 10–1128. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Jin, Y.-S. Recent advances in natural antifungal flavonoids and their derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 126589. [Google Scholar] [CrossRef]
- O’meara, T.R.; Veri, A.O.; Ketela, T.; Jiang, B.; Roemer, T.; Cowen, L.E. Global analysis of fungal morphology exposes mechanisms of host cell escape. Nat. Commun. 2015, 6, 6741. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.; Usui, K.; Hiraki, T. Fluconazole modulates membrane rigidity, heterogeneity, and water penetration into the plasma membrane in Saccharomyces cerevisiae. Biochemistry 2009, 48, 8494–8504. [Google Scholar] [CrossRef]
- Robbins, N.; Wright, G.D.; Cowen, L.E. Antifungal Drugs: The Current Armamentarium and Development of New Agents. Microbiol. Spectr. 2016, 4, 903–922. [Google Scholar] [CrossRef]
- Cantelli, B.A.M.; Bitencourt, T.A.; Komoto, T.T.; Beleboni, R.O.; Marins, M.; Fachin, A.L. Caffeic acid and licochalcone A interfere with the glyoxylate cycle of Trichophyton rubrum. Biomed. Pharmacother. 2017, 96, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Mangoyi, R.; Midiwo, J.; Mukanganyama, S. Isolation and characterization of an antifungal compound 5-hydroxy-7,4′-dimethoxyflavone from Combretum zeyheri. BMC Complement. Altern. Med. 2015, 15, 405. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.; Carvalho, C.; Andrade, F.; Fernandes, O.; Arruda, W.; Silva, M. Fisetin as a promising antifungal agent against Cryptocococcus neoformans species complex. J. Appl. Microbiol. 2016, 121, 373–379. [Google Scholar] [CrossRef]
- Patel, M.; Srivastava, V.; Ahmad, A. Dodonaea viscosa var angustifolia derived 5,6,8-trihydroxy-7,4′ dimethoxy flavone inhibits ergosterol synthesis and the production of hyphae and biofilm in Candida albicans. J. Ethnopharmacol. 2020, 259, 112965. [Google Scholar] [CrossRef]
- Ko, H.-C.; Hsiao, T.-Y.; Chen, C.-T.; Yang, Y.-L. Candida albicans ENO1 null mutants exhibit altered drug susceptibility, hyphal formation, and virulence. J. Microbiol. 2013, 51, 345–351. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Chen, H.-F.; Kuo, T.-J.; Lin, C.-Y. Mutations on CaENO1 in Candida albicans inhibit cell growth in the presence of glucose. J. Biomed. Sci. 2006, 13, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Shrivastava, M.; Whiteway, M.; Jiang, Y. Candida albicans targets that potentially synergize with fluconazole. Crit. Rev. Microbiol. 2021, 47, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Marín, E.; Parra-Giraldo, C.M.; Hernández-Haro, C.; Hernáez, M.L.; Nombela, C.; Monteoliva, L.; Gil, C. Candida albicans Shaving to Profile Human Serum Proteins on Hyphal Surface. Front. Microbiol. 2015, 6, 1343. [Google Scholar] [CrossRef]
- Vaz, C.; Pitarch, A.; Gómez-Molero, E.; Amador-García, A.; Weig, M.; Bader, O.; Monteoliva, L.; Gil, C. Mass Spectrometry-Based Proteomic and Immunoproteomic Analyses of the Candida albicans Hyphal Secretome Reveal Diagnostic Biomarker Candidates for Invasive Candidiasis. J. Fungi 2021, 7, 501. [Google Scholar] [CrossRef]
- Dror, R.O.; Dirks, R.M.; Grossman, J.; Xu, H.; Shaw, D.E. Biomolecular Simulation: A Computational Microscope for Molecular Biology. Annu. Rev. Biophys. 2012, 41, 429–452. [Google Scholar] [CrossRef]
- Milardi, D.; Pappalardo, M. Molecular dynamics: New advances in drug discovery. Eur. J. Med. Chem. 2015, 91, 1–3. [Google Scholar] [CrossRef]
- Nam, K.H. Molecular Dynamics—From Small Molecules to Macromolecules. Int. J. Mol. Sci. 2021, 22, 3761. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhu, K.; Beautrait, A.; Vendome, J.; Borrelli, K.W.; Abel, R.; Friesner, R.A.; Miller, E.B. Induced-Fit Docking Enables Accurate Free Energy Perturbation Calculations in Homology Models. J. Chem. Theory Comput. 2022, 18, 5710–5724. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.Z.; Zhang, X.L.; Gao, L.; Yan, L.; Jiang, Y.Y. iTRAQ-based proteomics revealed baicalein enhanced oxidative stress of Candida albicans by upregulating CPD2 expression. Med. Mycol. 2022, 60, myac053. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, X.; Li, C.; Tu, J.; Liu, N.; Xu, D.; Sheng, C. Lanosterol 14α-demethylase (CYP51)/histone deacetylase (HDAC) dual inhibitors for treatment of Candida tropicalis and Cryptococcus neoformans infections. Eur. J. Med. Chem. 2021, 221, 113524. [Google Scholar] [CrossRef]
- Fukano, K.; Kimura, K. Measurement of Enolase Activity in Cell Lysates. Methods Enzymol. 2014, 542, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Best, R.B.; Zhu, X.; Shim, J.; Lopes, P.E.; Mittal, J.; Feig, M.; Mackerell, A.D., Jr. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone φ, ψ and side-chain χ(1) and χ(2) dihedral angles. J. Chem. Theory Comput. 2012, 8, 3257–3273. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal Drug Resistance: Molecular Mechanisms in Candida albicans and Beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef]
- Spitzer, M.; Robbins, N.; Wright, G.D. Combinatorial strategies for combating invasive fungal infections. Virulence 2017, 8, 169–185. [Google Scholar] [CrossRef]
- Onyewu, C.; Blankenship, J.R.; Del Poeta, M.; Heitman, J. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 2003, 47, 956–964. [Google Scholar] [CrossRef] [PubMed]




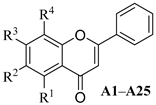 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compd. | R1 | R2 | R3 | R4 | Alone | with FLC [a] | FICI | Mode of Interaction |
| A1 | OH | H | H | H | >64 | 4 | 0.094 | Synergism |
| A2 | H | OH | H | H | >64 | >64 | 2.000 | Indifferent |
| A3 | H | H | OH | H | >64 | >64 | 2.000 | Indifferent |
| A4 | OH | OH | H | H | >64 | 1 | 0.070 | Synergism |
| A5 | OH | H | OH | H | >64 | 64 | 0.563 | Addition |
| A6 | H | OH | OH | H | >64 | 8 | 0.125 | Synergism |
| A7 | H | H | OH | OH | >64 | 4 | 0.094 | Synergism |
| A8 | OH | OH | OCH3 | H | >64 | 4 | 0.094 | Synergism |
| A9 | OH | OCH3 | OH | H | >64 | 4 | 0.094 | Synergism |
| A10 | OH | H | OCH3 | H | >64 | 16 | 0.188 | Synergism |
| A11 | H | OCH3 | OCH3 | H | >64 | >64 | 2.000 | Indifferent |
| A12 | OH | OCH3 | OCH3 | H | >64 | 8 | 0.125 | Synergism |
| A13 | OH | -OCH2O- | H | >64 | 4 | 0.094 | Synergism | |
| A14 | OCH3 | OH | H | H | >64 | 32 | 0.313 | Synergism |
| A15 | OCH3 | H | OCH3 | H | >64 | 64 | 0.563 | Addition |
| A16 | OH | H | OH | OCH3 | >64 | 8 | 0.125 | Synergism |
| A17 | OCH3 | H | H | H | >64 | 64 | 0.563 | Addition |
| A18 | OH | Br | OH | Br | >64 | 4 | 0.094 | Synergism |
| A19 | OH | H | OH | NO2 | >64 | 16 | 0.188 | Synergism |
| A20 | OCH3 | H | OCH3 | NO2 | >64 | 32 | 0.313 | Synergism |
| A21 | OCH3 | OCH3 | OCH3 | H | >64 | >64 | 2.000 | Indifferent |
| A22 | OH | H | OCH3 | NH2 | >64 | >64 | 2.000 | Indifferent |
| A23 | H | OCH3 | H | H | >64 | >64 | 2.000 | Indifferent |
| A24 | H | -OCH2O- | H | >64 | 4 | 0.094 | Synergism | |
| A25 | OH | OH | O-(1-glucuronide) | H | >64 | 16 | 0.188 | Synergism |
| BE | OH | OH | OH | H | 32 | 8 | 0.313 | Synergism |
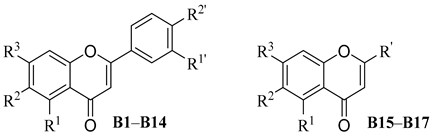 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compd. | R1 | R2 | R3 | R1′ | R2′ | R’ | Alone | with FLC [a] | FICI | Mode of Interaction |
| B1 | OCH3 | OCH3 | OCH3 | H | NO2 | – | >64 | >64 | 2.000 | Indifferent |
| B2 | OH | OH | OH | H | NO2 | – | >64 | 2 | 0.078 | Synergism |
| B3 | OCH3 | OCH3 | OCH3 | H | Br | – | >64 | >64 | 2.000 | Indifferent |
| B4 | OH | OH | OH | H | Br | – | 32 | 4 | 0.188 | Synergism |
| B5 | OH | OH | OH | H | NH2 | – | 16 | 2 | 0.188 | Synergism |
| B6 | OCH3 | OCH3 | OCH3 | NO2 | H | – | >64 | >64 | 2.000 | Indifferent |
| B7 | OH | OH | OH | NO2 | H | – | >64 | 4 | 0.094 | Synergism |
| B8 | OH | OH | OH | NH2 | H | – | 16 | 4 | 0.313 | Synergism |
| B9 | OH | OH | OH | H | OH | – | >64 | 16 | 0.188 | Synergism |
| B10 | OH | H | H | OH | OH | – | >64 | 32 | 0.313 | Synergism |
| B11 | OH | H | OH | OH | OH | – | >64 | 32 | 0.313 | Synergism |
| B12 | OH | H | OH | OH | OCH3 | – | >64 | 64 | 0.563 | Addition |
| B13 | OH | H | OH | H | OH | – | >64 | 64 | 0.563 | Addition |
| B14 | OCH3 | H | H | -OCH2O- | – | >64 | >64 | 2.000 | Indifferent | |
| B15 | OCH3 | H | H | – | – |  | >64 | 64 | 0.563 | Addition |
| B16 | OCH3 | H | H | – | – |  | >64 | 64 | 0.563 | Addition |
| B17 | OH | H | H | – | – | 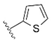 | >64 | 8 | 0.125 | Synergism |
| BE | OH | OH | OH | H | H | – | 64.0 | 4 | 0.125 | Synergism |
 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
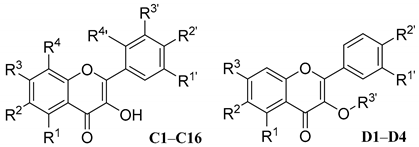
| Compd. | R1 | R2 | R3 | R4 | R1′ | R2′ | R3′ | R4′ | Alone | with FLC [a] | FICI | Mode of Interaction |
| C1 | OH | H | OH | H | H | H | H | H | >64 | 4 | 0.094 | Synergism |
| C2 | OH | H | OH | H | OH | OH | H | H | >64 | 4 | 0.094 | Synergism |
| C3 | OH | H | OH | H | H | OH | H | H | >64 | 8 | 0.125 | Synergism |
| C4 | OH | H | OH | OH | H | OH | H | H | >64 | 16 | 0.188 | Synergism |
| C5 | H | H | OH | H | OH | OH | OH | H | >64 | 2 | 0.078 | Synergism |
| C6 | OH | H | OH | H | H | OH | H | OH | >64 | 32 | 0.313 | Synergism |
| C7 | OH | H | OH | H | OH | OH | OH | H | >64 | 2 | 0.078 | Synergism |
| C8 | OH | H | OCH3 | H | OCH3 | OCH3 | H | H | >64 | 64 | 0.563 | Addition |
| C9 | OH | H | OH | Isopentenyl | H | OCH3 | H | H | >64 | 64 | 0.563 | Addition |
| C10 | OCH3 | H | OCH3 | H | OCH3 | OCH3 | H | H | >64 | 64 | 0.563 | Addition |
| C11 | H | OH | H | H | H | H | H | H | >64 | 32 | 0.313 | Synergism |
| C12 | H | H | OH | H | OH | OH | H | H | >64 | 32 | 0.313 | Synergism |
| C13 | H | H | H | H | OCH3 | OCH3 | H | H | >64 | >64 | 2.000 | Indifferent |
| C14 | H | H | H | H | OH | OH | H | H | >64 | 4 | 0.094 | Synergism |
| C15 | OH | H | OH | H | OCH3 | OH | H | H | >64 | 32 | 0.313 | Synergism |
| C16 | OH | H | Rha-O | H | OH | OH | H | H | >64 | 4 | 0.094 | Synergism |
| D1 | OCH3 | H | OCH3 | – | OCH3 | OCH3 | CH3 | NA | >64 | 64 | 0.563 | Addition |
| D2 | OH | H | OH | – | OH | OH | Rha | NA | >64 | 64 | 0.563 | Addition |
| D3 | OH | H | OH | – | OH | OH | Glu | NA | >64 | 64 | 0.563 | Addition |
| D4 | OH | H | OH | – | OH | OH | Gal | NA | >64 | >64 | 2.000 | Indifferent |
| BE | OH | OH | OH | H | H | H | H | H | 64 | 4 | 0.125 | Synergism |
| Compd. | Alone | Combination | FICI | Interaction | |
|---|---|---|---|---|---|
| FLC | Compd. | ||||
| A4 | >64 | 1 | 0.125 | 0.0088 | Syn |
| B4 | 16 | 1 | 1 | 0.0703 | Syn |
| B5 | >64 | 1 | 0.125 | 0.0088 | Syn |
| C5 | >64 | 0.25 | 4 | 0.0332 | Syn |
| C14 | >64 | 0.25 | 8 | 0.0645 | Syn |
| BE | 64 | 0.25 | 4 | 0.0645 | Syn |
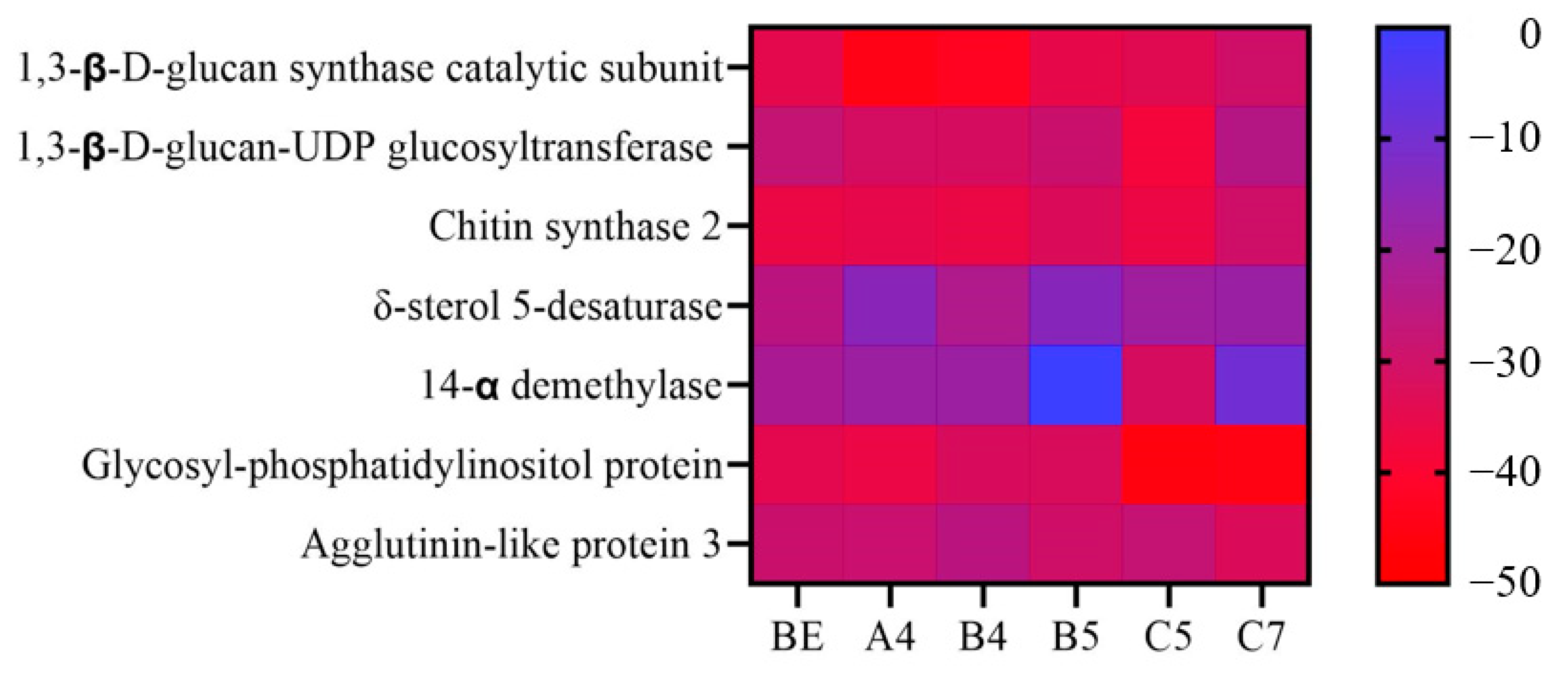
| Target Name | BE | A4 | B4 | B5 | C5 | C7 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Docking Score | Energy | Docking Score | Energy | Docking Score | Energy | Docking Score | Energy | Docking Score | Energy | Docking Score | Energy | |
| 1,3-β-d-glucan synthase catalytic subunit | −7.33 | −37.47 | −7.46 | −45.47 | −6.72 | −44.47 | −6.35 | −38.00 | −6.38 | −36.81 | −7.35 | −32.99 |
| 1,3-β-d-glucan-UDP glucosyltransferase | −8.30 | −31.06 | −7.52 | −34.49 | −8.28 | −34.35 | −7.51 | −32.49 | −8.32 | −40.55 | −6.33 | −28.13 |
| Chitin synthase 2 | −6.81 | −38.68 | −5.70 | −37.76 | −7.27 | −38.68 | −7.46 | −35.59 | −6.98 | −38.92 | −7.22 | −33.34 |
| δ-sterol 5-desaturase | −4.83 | −29.12 | −4.14 | −18.05 | −4.17 | −26.52 | −4.84 | −17.60 | −4.83 | −23.20 | −6.43 | −22.12 |
| 14-α demethylase | −11.57 | −25.30 | −8.38 | −22.48 | −9.50 | −22.47 | NA * | NA * | −10.26 | −34.70 | −8.25 | −12.21 |
| Glycosyl-phosphatidylinositol protein | −6.05 | −37.28 | −6.44 | −39.00 | −6.28 | −35.09 | −5.65 | −35.19 | −7.81 | −46.74 | −7.08 | −46.33 |
| Agglutinin-like protein 3 | −5.87 | −32.40 | −5.28 | −31.78 | −6.02 | −28.93 | −6.28 | −33.38 | −7.11 | −31.20 | −7.63 | −35.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Yang, N.; Li, W.; Peng, X.; Dong, J.; Jiang, Y.; Yan, L.; Zhang, D.; Jin, Y. Exploration of Baicalein-Core Derivatives as Potent Antifungal Agents: SAR and Mechanism Insights. Molecules 2023, 28, 6340. https://doi.org/10.3390/molecules28176340
Zhou H, Yang N, Li W, Peng X, Dong J, Jiang Y, Yan L, Zhang D, Jin Y. Exploration of Baicalein-Core Derivatives as Potent Antifungal Agents: SAR and Mechanism Insights. Molecules. 2023; 28(17):6340. https://doi.org/10.3390/molecules28176340
Chicago/Turabian StyleZhou, Heyang, Niao Yang, Wei Li, Xuemi Peng, Jiaxiao Dong, Yuanying Jiang, Lan Yan, Dazhi Zhang, and Yongsheng Jin. 2023. "Exploration of Baicalein-Core Derivatives as Potent Antifungal Agents: SAR and Mechanism Insights" Molecules 28, no. 17: 6340. https://doi.org/10.3390/molecules28176340
APA StyleZhou, H., Yang, N., Li, W., Peng, X., Dong, J., Jiang, Y., Yan, L., Zhang, D., & Jin, Y. (2023). Exploration of Baicalein-Core Derivatives as Potent Antifungal Agents: SAR and Mechanism Insights. Molecules, 28(17), 6340. https://doi.org/10.3390/molecules28176340