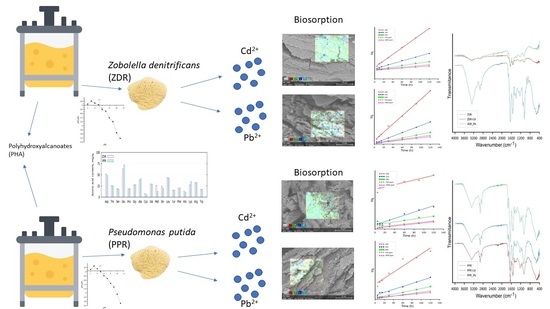
Upcycling Waste Streams from a Biorefinery Process—A Case Study on Cadmium and Lead Biosorption by Two Types of Biopolymer Post-Extraction Biomass
Abstract
:1. Introduction
2. Results and Discussion
2.1. Spent Biomass Characterisation
2.2. Metal Sorption Studies
2.3. Point of Zero Charge (PZC)
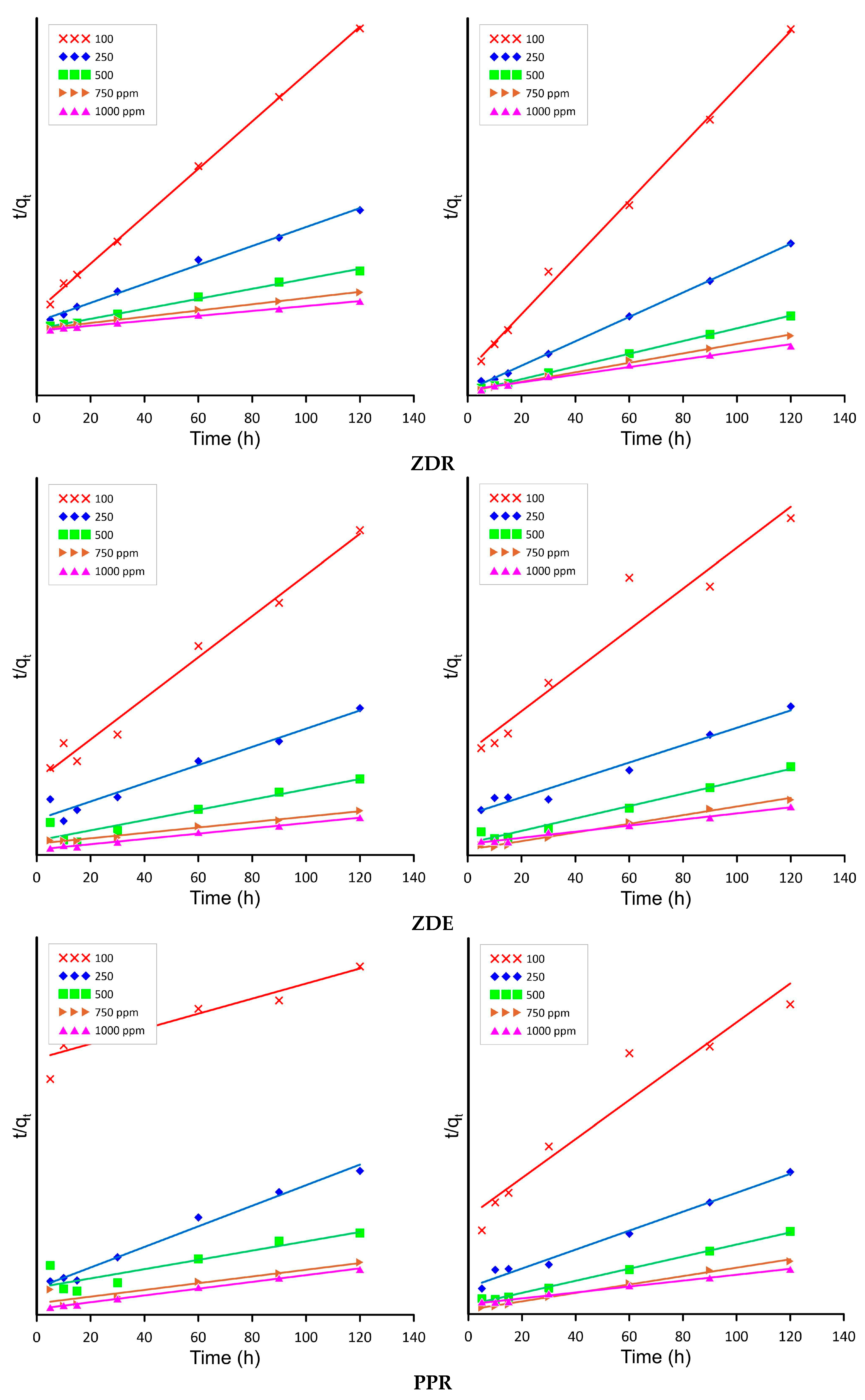
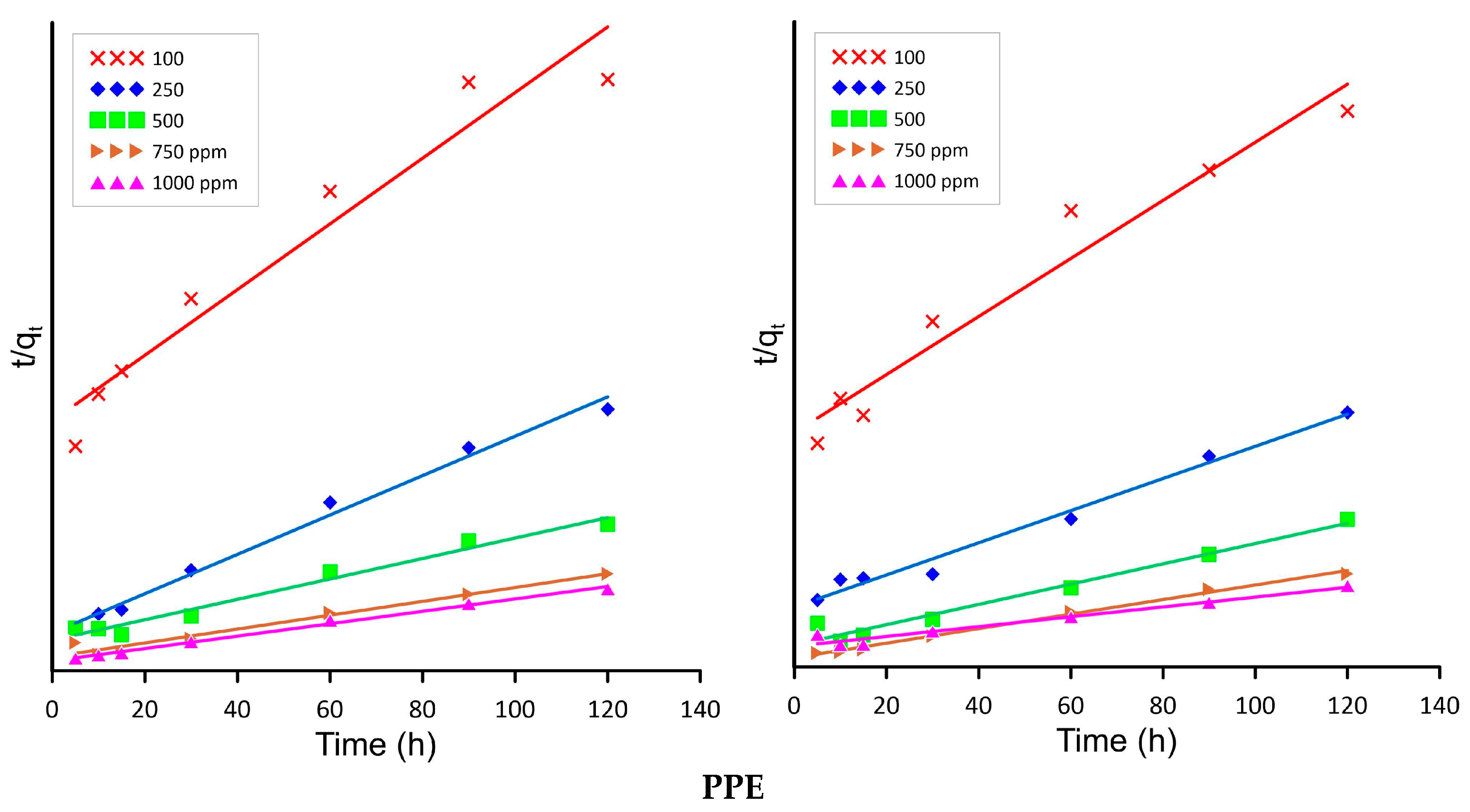
2.4. Isotherm and Kinetics
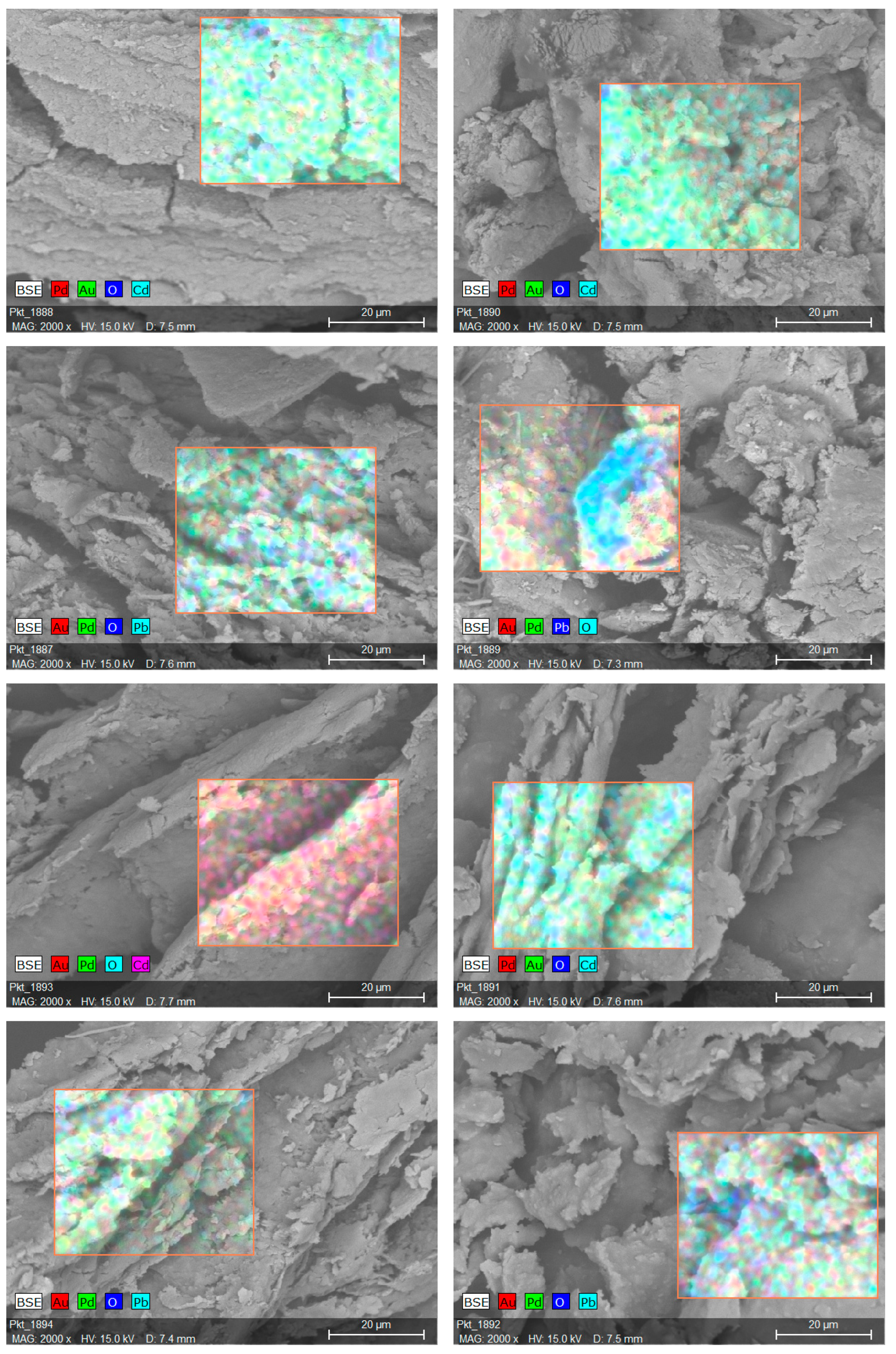
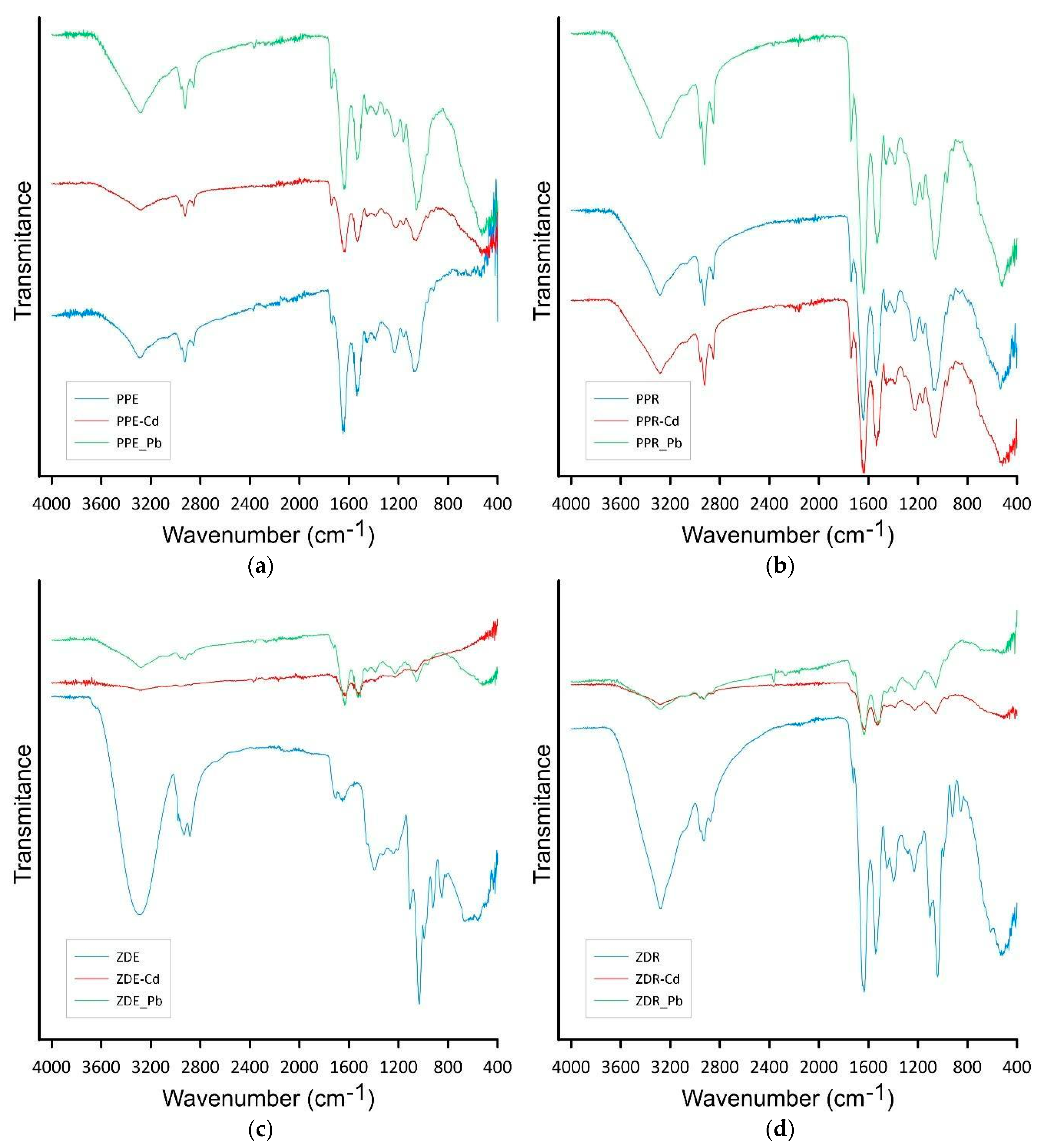
2.5. Sorption Mechanism
2.6. Desorption Studies
3. Materials and Methods
3.1. Chemicals
3.2. General Methods
3.3. Spent Biomass
3.4. Biomass Characterization
3.5. Sorption Process
3.6. Sorption Equilibrium
3.7. Sorption Kinetic Modelling
3.8. Desorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, S.; Xing, Y.; Liu, S.; Luo, X.; Chen, W.; Huang, Q. Co-effect of minerals and Cd(II) promoted the formation of bacterial biofilm and consequently enhanced the sorption of Cd(II). Environ. Pollut. 2019, 258, 113774. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C., Jr.; Bricka, M.; Smith, F.; Yancey, B.; Mohammad, J.; Steele, P.; Alexandre-Franco, M.; Gomez-Serrano, V.; Gong, H. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J. Colloid Interface Sci. 2007, 310, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.A.; Imran, M.; Amjad, M.; Abbas, G.; Tahir, M.; Murtaza, B.; Zakir, A.; Shahid, M.; Bulgariu, L.; Ahmad, I. Batch and column scale removal of cadmium from water using raw and acid activated wheat straw biochar. Water 2019, 11, 1438. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Bajda, T.; Tomczyk, A.; Kuśmierz, M.; Komaniecka, I. Immobilization mechanism of Cd2+/HCrO4−/CrO42− ions and carboxin on montmorillonite modified with Rhizobium leguminosarum bv. trifolii exopolysaccharide. J. Hazard. Mater. 2022, 428, 128228. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, H.N.; Safa, Y.; Yakout, S.M.; Shair, O.H.; Iqbal, M.; Nazir, A. Efficient removal of dyes using carboxymethyl cellulose/alginate/polyvinyl alcohol/rice husk composite: Adsorption/desorption, kinetics and recycling studies. Int. J. Biol. Macromol. 2020, 150, 861–870. [Google Scholar] [CrossRef]
- Ding, Z.; Hu, X.; Wan, Y.; Wang, S.; Gao, B. Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: Batch and column tests. J. Ind. Eng. Chem. 2016, 33, 239–245. [Google Scholar] [CrossRef]
- Montazer-Rahmati, M.M.; Rabbani, P.; Abdolali, A.; Keshtkar, A.R. Kinetics and equilibrium studies on biosorption of cadmium, lead, and nickel ions from aqueous solutions by intact and chemically modified brown algae. J. Hazard. Mater. 2011, 185, 401–407. [Google Scholar] [CrossRef]
- Nicola, R.; Costişor, O.; Ciopec, M.; Negrea, A.; Lazău, R.; Ianăşi, C.; Picioruş, E.; Len, A.; Almásy, L.; Szerb, E.; et al. Silica-Coated Magnetic Nanocomposites for Pb2+ Removal from Aqueous Solution. Appl. Sci. 2020, 10, 2726. [Google Scholar] [CrossRef]
- Al-Saida, B.; Sandouqa, A.; Shawabkeh, R.; Hussein, I. Synthesis of Nanosilica for the Removal of Multicomponent Cd2+ and Cu2+ from Synthetic Water: An Experimental and Theoretical Study. Molecules 2022, 27, 7536. [Google Scholar] [CrossRef]
- Lucaci, A.R.; Bulgariu, D.; Ahmad, I.; Lisa, G.; Mocanu, A.M.; Bulgariu, L. Potential use of biochar from variouswaste biomass as biosorbent in Co(II) removal processes. Water 2019, 11, 1565. [Google Scholar] [CrossRef]
- Tsai, W.T.; Liu, S.C.; Hsieh, C.H. Preparation and fuel properties of biochars from the pyrolysis of exhausted coffee residue. J. Anal. Appl. Pyrolysis 2012, 93, 63–67. [Google Scholar] [CrossRef]
- Nitkiewicz, T.; Wojnarowska, M.; Sołtysik, M.; Kaczmarski, A.; Witko, T.; Ingrao, C.; Guzik, M. How sustainable are biopolymers? Findings from a life cycle assessment of polyhydroxyalkanoate production from rapeseed-oil derivatives. Sci. Total Environ. 2020, 749, 141279. [Google Scholar] [CrossRef] [PubMed]
- Lawford, H.G.; Rousseau, J.D. Studies on Nutrient Requirements and Cost-Effective Supplements for Ethanol Production by Recombinant, E. coli. Appl. Biochem. Biotechnol. 1996, 57, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Crickmore, N.; Berry, C.; Panneerselvam, S.; Mishra, R.; Connor, T.; Bonning, B. A structure-based nomenclature for Bacillus thuringiensis and other bacteria-derived pesticidal proteins. J. Invertebr. Pathol. 2021, 186, 107438. [Google Scholar] [CrossRef]
- Jannathulla, R.; Sravanthi, O.; Moomeen, S.; Gopikrishna, G.; Jagabattula, S. Microbial products in terms of isolates, whole-cell biomass, and live organisms as aquafeed ingredients: Production, nutritional values, and market potential—A review. Aquac. Int. 2021, 29, 623–650. [Google Scholar] [CrossRef]
- Ke, Y.; Zhao, J.; Verkerk, U.; Hopkinson, A.; Siu, K. Histidine, Lysine, and Arginine Radical Cations: Isomer Control via the Choice of Auxiliary Ligand (L) in the Dissociation of [CuII(L)(amino acid)]•2+ Complexes. J. Phys. Chem. B. 2007, 111, 14318–14328. [Google Scholar] [CrossRef]
- Pouran, H.M.; Banwart, S.A.; Romero-Gonzalez, M. Characterizing the Cell Surface Properties of Hydrocarbon-Degrading Bacterial Strains, a Case Study. In Handbook of Environmental Materials Management; Springer: Cham, Switzerland, 2018; pp. 1–28. [Google Scholar] [CrossRef]
- Ramrakhiani, L.; Ghosh, S.; Majumdar, S. Surface Modification of Naturally Available Biomass for Enhancement of Heavy Metal Removal Efficiency, Upscaling Prospects, and Management Aspects of Spent Biosorbents: A Review. Appl. Biochem. Biotechnol. 2010, 180, 41–78. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, Y.; Feng, C.; Zhang, Z.; Lei, Z.; Shimizu, K. Predicting equilibrium time by adsorption kinetic equations and modifying Langmuir isotherm by fractal-like approach. J. Mol. Liq. 2018, 268, 728–733. [Google Scholar] [CrossRef]
- Sahmoune, M.N. Performance of Streptomyces rimosus biomass in biosorption of heavy metals from aqueous solutions. Microchem. J. 2018, 141, 87–95. [Google Scholar] [CrossRef]
- Yue, Z.B.; Li, Q.; Li, C.; Chen, T.; Wang, J. Component analysis and heavy metal adsorption ability of extracellular polymeric substances (EPS) from sulfate reducing bacteria. Bioresour. Technol. 2015, 194, 399–402. [Google Scholar] [CrossRef]
- Carballo-Meilan, A.; Hernández-Francisco, E.; Sosa-Loyde, G.; Bonilla-Cruz, J.; Russell, P.; Ali, A.; García-García, A.; Arizpe-Zapata, A.; Longoria-Rodríguez, F.; Lara-Ceniceros, E.; et al. Biosorption of copper using nopal fibres: Moolooite formation and magnesium role in the reactive crystallization mechanism. Cellulose 2020, 27, 10259–10276. [Google Scholar] [CrossRef]
- Kamnev, A.; Sadovnikova, J.; Tarantilis, P.; Polissiou, M.; Antonyuk, L. Responses of Azospirillum brasilense to Nitrogen Deficiency and to Wheat Lectin: A Diffuse Reflectance Infrared Fourier Transform (DRIFT) Spectroscopic Study. Microb. Ecol. 2008, 56, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Nessim, R.; Bassiouny, A.; Zaki, H.; Moawad, M.; Kandeel, K. Biosorption of lead and cadmium using marine algae. Chem. Ecol. 2011, 27, 579–594. [Google Scholar] [CrossRef]
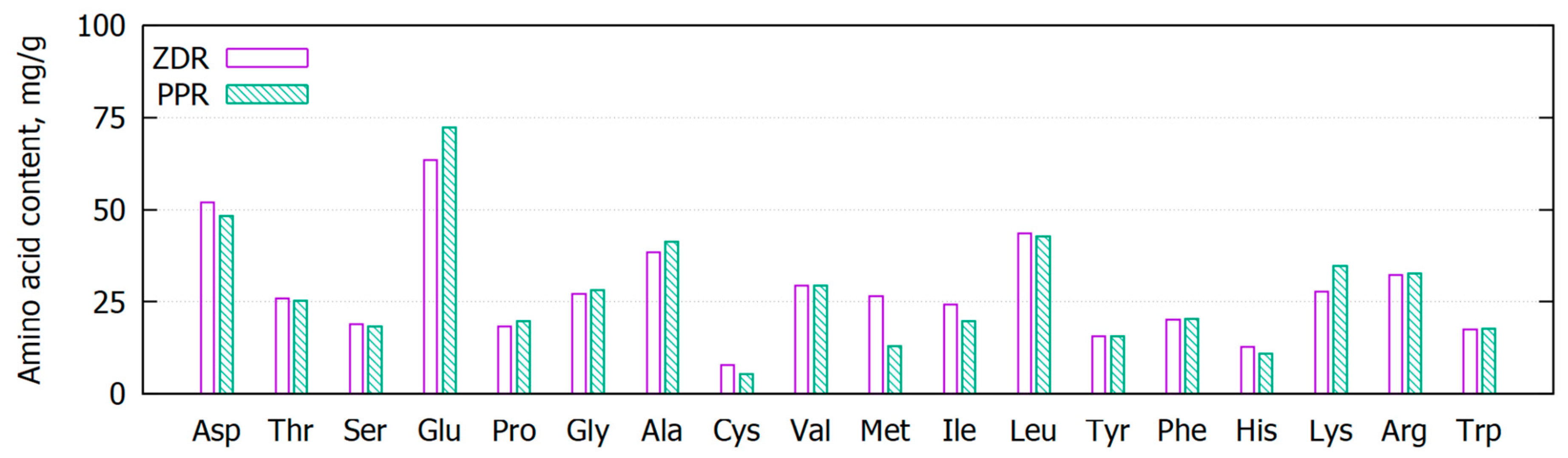


| Spent Biomass | Dry Weight, % | Mineral Content, % | Total Nitrogen, % d.w. | Total Phosphorus, % d.w. | Water Soluble Fraction, % | Acetone Soluble Fraction, % |
|---|---|---|---|---|---|---|
| ZDR | 92.0 | 9.55 | 8.6 | 1.2 | 31 | 6 |
| PPR | 93.3 | 9.55 | 10.1 | 2.0 | 22 | 4 |
| Cd2+ | Pb2+ | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | ZDR | ZDE | PPR | PPE | ZDR | ZDE | PPR | PPE |
| Freundlich | ||||||||
| R2 | 0.9961 | 0.9784 | 0.9856 | 0.9942 | 0.9967 | 0.9794 | 0.9822 | 0.9808 |
| 1/n | 0.9977 | 0.9280 | 0.8094 | 0.7408 | 0.2595 | 0.6436 | 0.5791 | 0.6197 |
| KF(mg1−(1/n)(dm3)1/ng−1) | 0.3166 | 0.3212 | 0.7314 | 1.1972 | 38.587 | 2.5852 | 4.1795 | 3.0740 |
| parameter | ZDE | ZDR | PPE | PPR | ||||||||||||||||
| Cd2+ Concentration (mg/dm3) | ||||||||||||||||||||
| 100 | 250 | 500 | 750 | 1000 | 100 | 250 | 500 | 750 | 1000 | 100 | 250 | 500 | 750 | 1000 | 100 | 250 | 500 | 750 | 1000 | |
| II Order | ||||||||||||||||||||
| qe | 14.779 | 34.571 | 72.875 | 115.323 | 111.936 | 12.921 | 29.020 | 58.236 | 93.899 | 117.836 | 16.374 | 31.291 | 65.540 | 104.629 | 101.982 | 38.901 | 31.089 | 108.334 | 125.343 | 101.789 |
| k2 | 0.0019 | 0.0007 | 0.0004 | 0.0002 | 0.0005 | 0.0093 | 0.0046 | 0.0032 | 0.0012 | 0.0014 | 0.0012 | 0.0024 | 0.0006 | 0.0005 | 0.0009 | 0.0001 | 0.0017 | 0.0001 | 0.0002 | 0.0007 |
| R2 | 0.983 | 0.957 | 0.890 | 0.985 | 0.992 | 0.999 | 0.995 | 0.995 | 0.998 | 0.998 | 0.940 | 0.989 | 0.973 | 0.969 | 0.994 | 0.971 | 0.984 | 0.796 | 0.853 | 0.996 |
| parameter | ZDE | ZDR | PPE | PPR | ||||||||||||||||
| Pb2+ Concentration (mg/dm3) | ||||||||||||||||||||
| 100 | 250 | 500 | 750 | 1000 | 100 | 250 | 500 | 750 | 1000 | 100 | 250 | 500 | 750 | 1000 | 100 | 250 | 500 | 750 | 1000 | |
| II Order | ||||||||||||||||||||
| qe | 14.710 | 76.371 | 91.155 | 103.421 | 147.170 | 21.9483 | 51.6364 | 98.5165 | 116.8087 | 140.1165 | 19.3292 | 61.1734 | 95.8474 | 103.6422 | 179.2039 | 15.0507 | 54.9285 | 83.0469 | 103.5272 | 145.8427 |
| k2 | 0.0027 | 0.0002 | 0.0004 | 0.0009 | 0.0002 | 0.0053 | 0.0042 | 0.0018 | 0.0015 | 0.0008 | 0.0010 | 0.0003 | 0.0003 | 0.0009 | 0.0001 | 0.0028 | 0.0005 | 0.0007 | 0.0012 | 0.0002 |
| R2 | 0.9332 | 0.9702 | 0.9772 | 0.9897 | 0.9855 | 0.9972 | 0.9992 | 0.9996 | 0.9960 | 0.9905 | 0.9530 | 0.9840 | 0.9683 | 0.9943 | 0.9541 | 0.9291 | 0.9849 | 0.9965 | 0.9953 | 0.9914 |
| Elution (%) | |||
|---|---|---|---|
| Eluent | Cycle 1 | Cycle 2 | Cycle 3 |
| ZDR | |||
| Acetic acid | 74 | 68 | 77 |
| Citric acid | 85 | 91 | 86 |
| Potassium chloride | 24 | 31 | 19 |
| Sodium chloride | 28 | 25 | 27 |
| Deionized water | 0.4 | 0.2 | 0.7 |
| ZDE | |||
| Acetic acid | 69 | 71 | 63 |
| Citric acid | 79 | 88 | 76 |
| Potassium chloride | 19 | 16 | 18 |
| Sodium chloride | 22 | 25 | 27 |
| Deionized water | 0.8 | 0.2 | 0.7 |
| PPR | |||
| Acetic acid | 81 | 78 | 74 |
| Citric acid | 92 | 87 | 89 |
| Potassium chloride | 17 | 24 | 21 |
| Sodium chloride | 22 | 25 | 17 |
| Deionized water | 0.8 | 0.5 | 0.1 |
| PPE | |||
| Acetic acid | 78 | 75 | 72 |
| Citric acid | 95 | 91 | 93 |
| Potassium chloride | 28 | 24 | 18 |
| Sodium chloride | 28 | 22 | 26 |
| Deionized water | 0.2 | 0.4 | 0.3 |
| Model | Equation |
|---|---|
| Langmuir | |
| Freundlich | |
| Temkin | = B ln + B ln B = |
| Model | Equation |
|---|---|
| PFO | log (− |
| PSO | |
| W-M | = |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chwastowski, J.; Guzik, M.; Bednarz, S.; Staroń, P. Upcycling Waste Streams from a Biorefinery Process—A Case Study on Cadmium and Lead Biosorption by Two Types of Biopolymer Post-Extraction Biomass. Molecules 2023, 28, 6345. https://doi.org/10.3390/molecules28176345
Chwastowski J, Guzik M, Bednarz S, Staroń P. Upcycling Waste Streams from a Biorefinery Process—A Case Study on Cadmium and Lead Biosorption by Two Types of Biopolymer Post-Extraction Biomass. Molecules. 2023; 28(17):6345. https://doi.org/10.3390/molecules28176345
Chicago/Turabian StyleChwastowski, Jarosław, Maciej Guzik, Szczepan Bednarz, and Paweł Staroń. 2023. "Upcycling Waste Streams from a Biorefinery Process—A Case Study on Cadmium and Lead Biosorption by Two Types of Biopolymer Post-Extraction Biomass" Molecules 28, no. 17: 6345. https://doi.org/10.3390/molecules28176345
APA StyleChwastowski, J., Guzik, M., Bednarz, S., & Staroń, P. (2023). Upcycling Waste Streams from a Biorefinery Process—A Case Study on Cadmium and Lead Biosorption by Two Types of Biopolymer Post-Extraction Biomass. Molecules, 28(17), 6345. https://doi.org/10.3390/molecules28176345