Exploring the Potential of Lapatinib, Fulvestrant, and Paclitaxel Conjugated with Glycidylated PAMAM G4 Dendrimers for Cancer and Parasite Treatment
Abstract
1. Introduction
2. Results and Discussion
2.1. Syntheses and Characterization of Dendrimer Conjugates
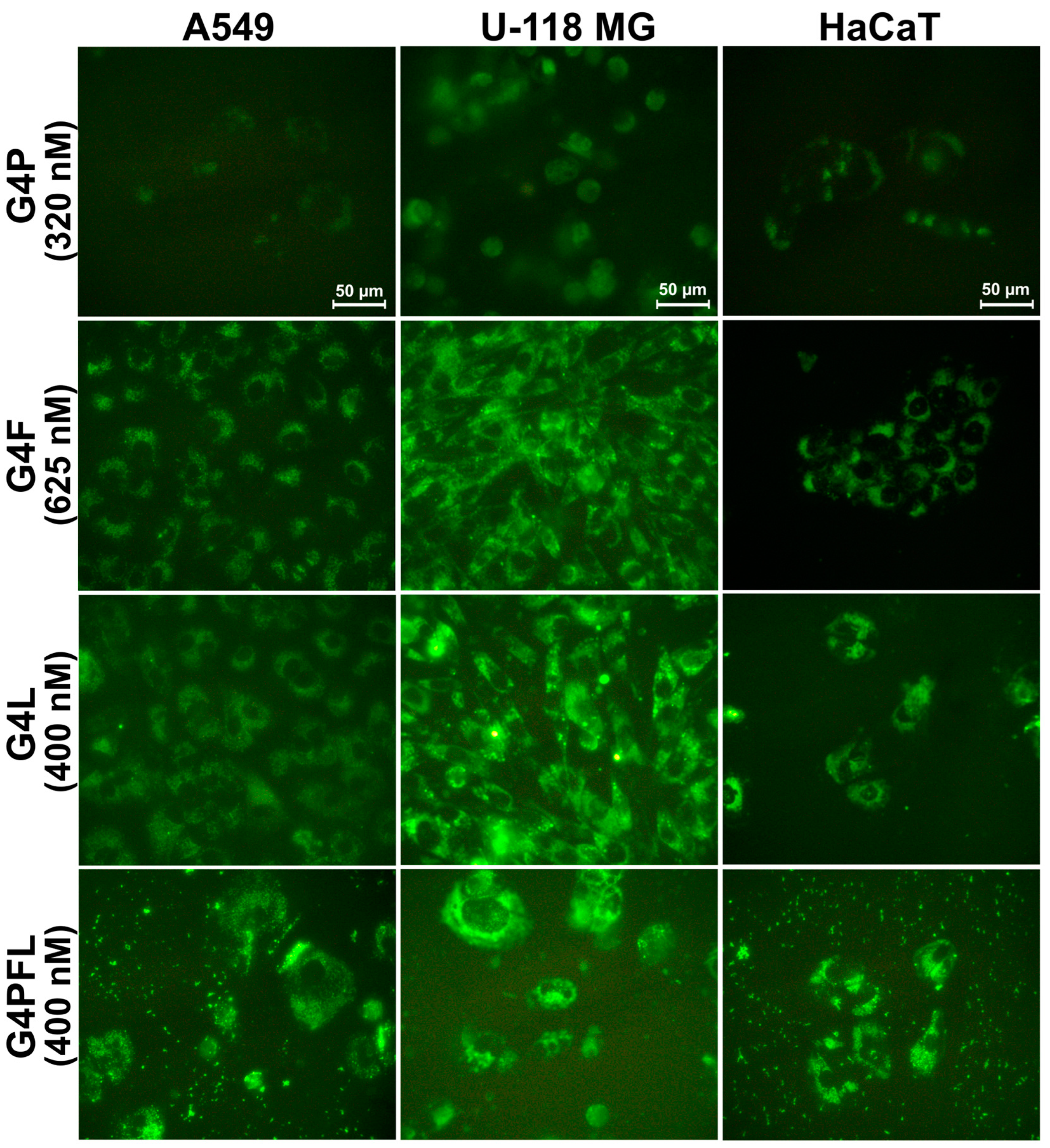
2.2. Cellular Accumulation
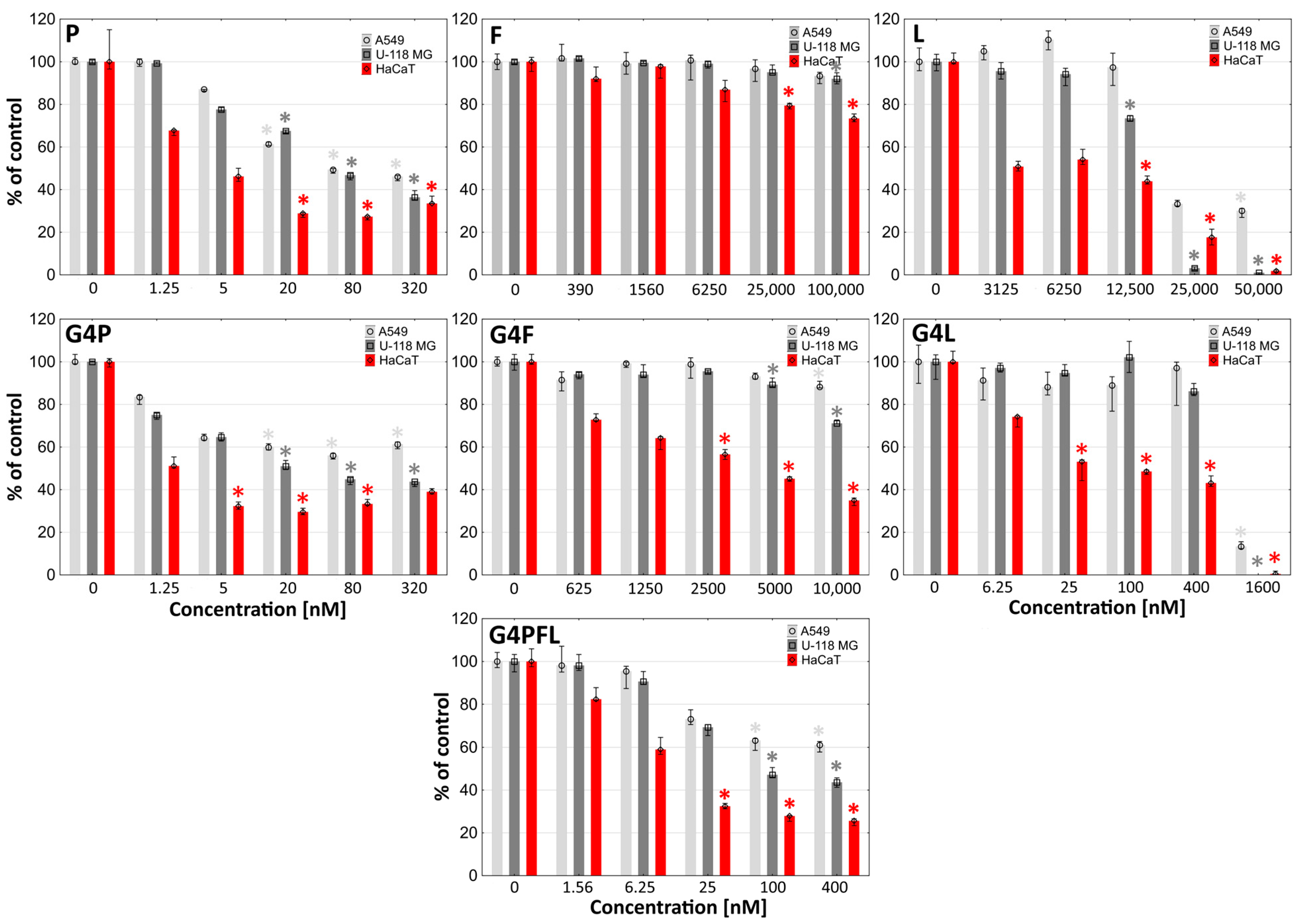
2.3. Cytotoxicity
2.4. Proliferation
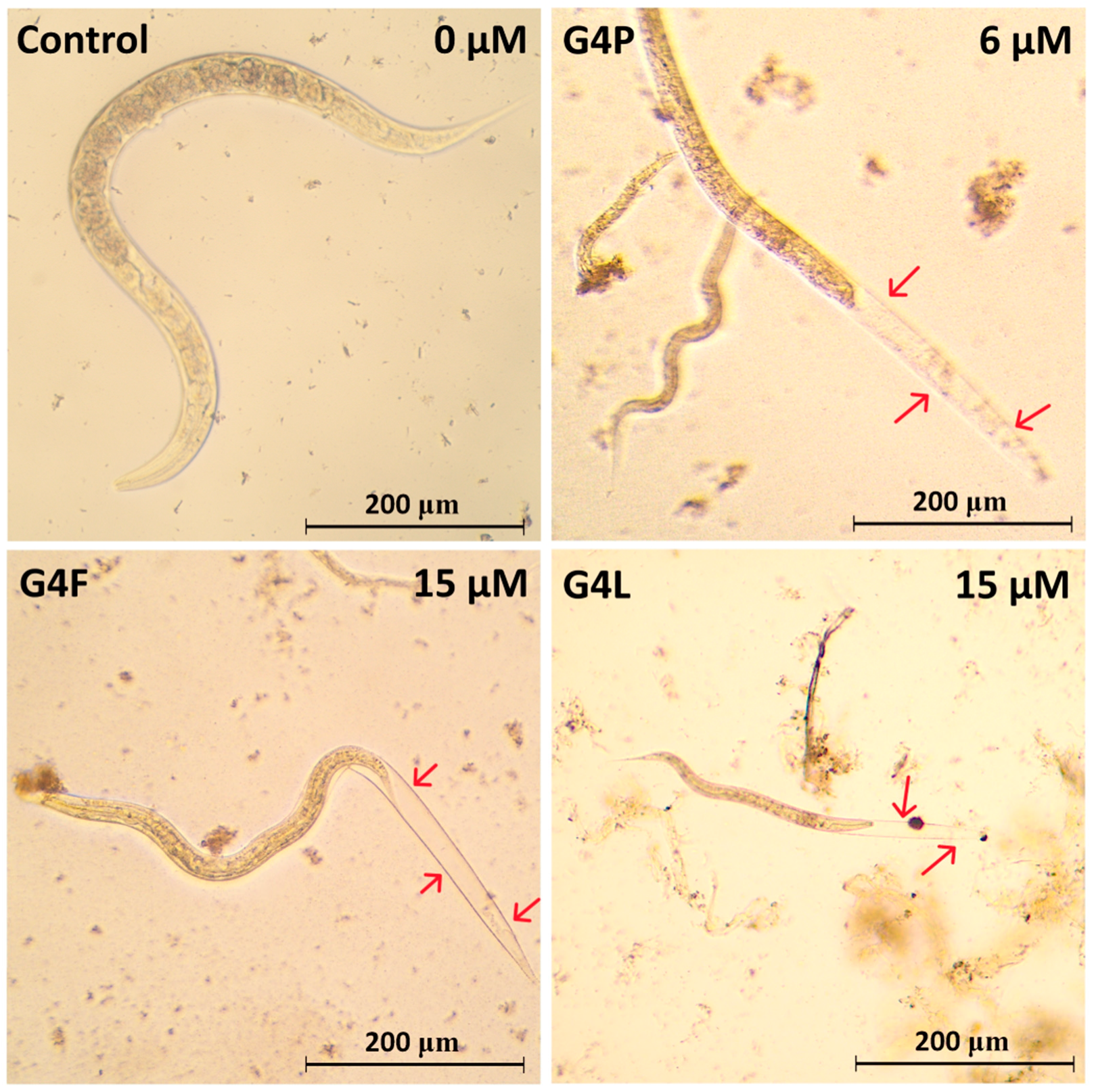
2.5. Effect on the Worm Survival
3. Materials and Methods
3.1. Dendrimer Synthesis and Characterization
3.1.1. Spectroscopy
3.1.2. Conjugate Size and ζ Potential
3.2. Syntheses of Conjugates
3.2.1. Conjugates of Paclitaxel (P), Lapatinib (L), and Fulvestrant (F) (Primary Conjugates)
3.2.2. Binary and Ternary Conjugates of G4* with Paclitaxel, Lapatinib and Fulvestrant
3.3. Biological Studies
3.3.1. Biochemical Reagents, Cell Lines and Materials
3.3.2. Cell Cultures
3.3.3. Toxicity Assays
3.3.4. Proliferation Assay
3.3.5. Cellular Accumulation of Labeled Conjugates
3.3.6. Toxicity to Caenorhabditis Elegans
3.3.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cancer Facts & Figures 2023. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html (accessed on 12 July 2023).
- Pisters, K.M.; Evans, W.K.; Azzoli, C.G.; Kris, M.G.; Smith, C.A.; Desch, C.E.; Somerfield, M.R.; Brouwers, M.C.; Darling, G.; Ellis, P.M.; et al. Cancer Care Ontario and American Society of Clinical Oncology Adjuvant Chemotherapy and Adjuvant Radiation Therapy for Stages I-IIIA Resectable Non Small-Cell Lung Cancer Guideline. J. Clin. Oncol. 2007, 25, 5506–5518. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.-P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.-Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung Adjuvant Cisplatin Evaluation: A Pooled Analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Tonn, J.-C.; Brada, M.; Pentheroudakis, G.; ESMO Guidelines Working Group. High-Grade Malignant Glioma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2010, 21 (Suppl. S5), v190–v193. [Google Scholar] [CrossRef]
- Oronsky, B.; Reid, T.R.; Oronsky, A.; Sandhu, N.; Knox, S.J. A Review of Newly Diagnosed Glioblastoma. Front. Oncol. 2021, 10, 574012. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y. Temozolomide Resistance in Glioblastoma Multiforme. Genes. Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Bello-Alvarez, C.; Camacho-Arroyo, I. Impact of Sex in the Prevalence and Progression of Glioblastomas: The Role of Gonadal Steroid Hormones. Biol. Sex. Differ. 2021, 12, 28. [Google Scholar] [CrossRef]
- González-Mora, A.M.; Garcia-Lopez, P. Estrogen Receptors as Molecular Targets of Endocrine Therapy for Glioblastoma. Int. J. Mol. Sci. 2021, 22, 12404. [Google Scholar] [CrossRef]
- González-Arenas, A.; Hansberg-Pastor, V.; Hernández-Hernández, O.T.; González-García, T.K.; Henderson-Villalpando, J.; Lemus-Hernández, D.; Cruz-Barrios, A.; Rivas-Suárez, M.; Camacho-Arroyo, I. Estradiol Increases Cell Growth in Human Astrocytoma Cell Lines through ERα Activation and Its Interaction with SRC-1 and SRC-3 Coactivators. Biochim. Biophys. Acta 2012, 1823, 379–386. [Google Scholar] [CrossRef]
- Wan, S.; Jiang, J.; Zheng, C.; Wang, N.; Zhai, X.; Fei, X.; Wu, R.; Jiang, X. Estrogen Nuclear Receptors Affect Cell Migration by Altering Sublocalization of AQP2 in Glioma Cell Lines. Cell Death Discov. 2018, 4, 49. [Google Scholar] [CrossRef]
- Peña-Gutiérrez, K.M.; Hernández-Ortega, K.; Bello-Alvarez, C.; Camacho-Arroyo, I. Expression and Estrogen Regulation of G Protein-coupled Estrogen Receptor in Human Glioblastoma Cells. Oncol. Lett. 2022, 24, 397. [Google Scholar] [CrossRef]
- Tam, A.; Morrish, D.; Wadsworth, S.; Dorscheid, D.; Man, S.P.; Sin, D.D. The Role of Female Hormones on Lung Function in Chronic Lung Diseases. BMC Women’s Health 2011, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lara, V.; Hernandez-Martinez, J.-M.; Arrieta, O. Influence of Estrogen in Non-Small Cell Lung Cancer and Its Clinical Implications. J. Thorac. Dis. 2018, 10, 482–497. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, C.; Li, M.; An, J.; Zhou, W.; Guo, J.; Xiao, Y. Estrogen Receptor Beta Promotes Lung Cancer Invasion via Increasing CXCR4 Expression. Cell Death Dis. 2022, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Nathan, M.R.; Schmid, P. A Review of Fulvestrant in Breast Cancer. Oncol. Ther. 2017, 5, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Movérare-Skrtic, S.; Börjesson, A.E.; Farman, H.H.; Sjögren, K.; Windahl, S.H.; Lagerquist, M.K.; Andersson, A.; Stubelius, A.; Carlsten, H.; Gustafsson, J.-Å.; et al. The Estrogen Receptor Antagonist ICI 182,780 Can Act Both as an Agonist and an Inverse Agonist When Estrogen Receptor α AF-2 Is Modified. Proc. Natl. Acad. Sci. USA 2014, 111, 1180–1185. [Google Scholar] [CrossRef]
- Osborne, C.K.; Wakeling, A.; Nicholson, R.I. Fulvestrant: An Oestrogen Receptor Antagonist with a Novel Mechanism of Action. Br. J. Cancer 2004, 90 (Suppl. S1), S2–S6. [Google Scholar] [CrossRef]
- Hamilton, D.H.; Griner, L.M.; Keller, J.M.; Hu, X.; Southall, N.; Marugan, J.; David, J.M.; Ferrer, M.; Palena, C. Targeting Estrogen Receptor Signaling with Fulvestrant Enhances Immune and Chemotherapy-Mediated Cytotoxicity of Human Lung Cancer. Clin. Cancer Res. 2016, 22, 6204–6216. [Google Scholar] [CrossRef]
- Sabbah, D.A.; Hajjo, R.; Sweidan, K. Review on Epidermal Growth Factor Receptor (EGFR) Structure, Signaling Pathways, Interactions, and Recent Updates of EGFR Inhibitors. Curr. Top. Med. Chem. 2020, 20, 815–834. [Google Scholar] [CrossRef]
- Ayati, A.; Moghimi, S.; Salarinejad, S.; Safavi, M.; Pouramiri, B.; Foroumadi, A. A Review on Progression of Epidermal Growth Factor Receptor (EGFR) Inhibitors as an Efficient Approach in Cancer Targeted Therapy. Bioorganic Chem. 2020, 99, 103811. [Google Scholar] [CrossRef]
- Rosell, R.; Moran, T.; Queralt, C.; Porta, R.; Cardenal, F.; Camps, C.; Majem, M.; Lopez-Vivanco, G.; Isla, D.; Provencio, M.; et al. Screening for Epidermal Growth Factor Receptor Mutations in Lung Cancer. N. Engl. J. Med. 2009, 361, 958–967. [Google Scholar] [CrossRef]
- Xu, H.; Zong, H.; Ma, C.; Ming, X.; Shang, M.; Li, K.; He, X.; Du, H.; Cao, L. Epidermal Growth Factor Receptor in Glioblastoma (Review). Oncol. Lett. 2017, 14, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Bethune, G.; Bethune, D.; Ridgway, N.; Xu, Z. Epidermal Growth Factor Receptor (EGFR) in Lung Cancer: An Overview and Update. J. Thorac. Dis. 2010, 2, 48–51. [Google Scholar] [PubMed]
- Lau, S.C.; Chooback, N.; Ho, C.; Melosky, B. Outcome Differences between First- and Second-Generation EGFR Inhibitors in Advanced EGFR Mutated NSCLC in a Large Population-Based Cohort. Clin. Lung Cancer 2019, 20, e576–e583. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.-W.; Weiss, W.A. Epidermal Growth Factor Receptor and EGFRvIII in Glioblastoma: Signaling Pathways and Targeted Therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.W.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The Somatic Genomic Landscape of Glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef]
- Watanabe, K.; Tachibana, O.; Sato, K.; Yonekawa, Y.; Kleihues, P.; Ohgaki, H. Overexpression of the EGF Receptor and P53 Mutations Are Mutually Exclusive in the Evolution of Primary and Secondary Glioblastomas. Brain Pathol. 1996, 6, 217–223. [Google Scholar] [CrossRef]
- Tevaarwerk, A.J.; Kolesar, J.M. Lapatinib: A Small-Molecule Inhibitor of Epidermal Growth Factor Receptor and Human Epidermal Growth Factor Receptor-2 Tyrosine Kinases Used in the Treatment of Breast Cancer. Clin. Ther. 2009, 31 Pt 2, 2332–2348. [Google Scholar] [CrossRef]
- Mansi, M.; Howley, R.; Chandratre, S.; Chen, B. Inhibition of ABCG2 Transporter by Lapatinib Enhances 5-Aminolevulinic Acid-Mediated Protoporphyrin IX Fluorescence and Photodynamic Therapy Response in Human Glioma Cell Lines. Biochem. Pharmacol. 2022, 200, 115031. [Google Scholar] [CrossRef]
- Yu, A.; Faiq, N.; Green, S.; Lai, A.; Green, R.; Hu, J.; Cloughesy, T.F.; Mellinghoff, I.; Nghiemphu, P.L. Report of Safety of Pulse Dosing of Lapatinib with Temozolomide and Radiation Therapy for Newly-Diagnosed Glioblastoma in a Pilot Phase II Study. J. Neurooncol. 2017, 134, 357–362. [Google Scholar] [CrossRef]
- Gao, H.; Yang, Z.; Cao, S.; Xi, Z.; Zhang, S.; Pang, Z.; Jiang, X. Behavior and Anti-Glioma Effect of Lapatinib-Incorporated Lipoprotein-like Nanoparticles. Nanotechnology 2012, 23, 435101. [Google Scholar] [CrossRef]
- Thiessen, B.; Stewart, C.; Tsao, M.; Kamel-Reid, S.; Schaiquevich, P.; Mason, W.; Easaw, J.; Belanger, K.; Forsyth, P.; McIntosh, L.; et al. A Phase I/II Trial of GW572016 (Lapatinib) in Recurrent Glioblastoma Multiforme: Clinical Outcomes, Pharmacokinetics and Molecular Correlation. Cancer Chemother. Pharmacol. 2010, 65, 353–361. [Google Scholar] [CrossRef]
- Uy, N.F.; Merkhofer, C.M.; Baik, C.S. HER2 in Non-Small Cell Lung Cancer: A Review of Emerging Therapies. Cancers 2022, 14, 4155. [Google Scholar] [CrossRef] [PubMed]
- Ross, H.J.; Blumenschein, G.R.; Aisner, J.; Damjanov, N.; Dowlati, A.; Garst, J.; Rigas, J.R.; Smylie, M.; Hassani, H.; Allen, K.E.; et al. Randomized Phase II Multicenter Trial of Two Schedules of Lapatinib as First- or Second-Line Monotherapy in Patients with Advanced or Metastatic Non-Small Cell Lung Cancer. Clin. Cancer Res. 2010, 16, 1938–1949. [Google Scholar] [CrossRef]
- Ramlau, R.; Thomas, M.; Novello, S.; Plummer, R.; Reck, M.; Kaneko, T.; Lau, M.R.; Margetts, J.; Lunec, J.; Nutt, J.; et al. Phase I Study of Lapatinib and Pemetrexed in the Second-Line Treatment of Advanced or Metastatic Non-Small-Cell Lung Cancer with Assessment of Circulating Cell Free Thymidylate Synthase RNA as a Potential Biomarker. Clin. Lung Cancer 2015, 16, 348–357. [Google Scholar] [CrossRef]
- Nose, N.; Sugio, K.; Oyama, T.; Nozoe, T.; Uramoto, H.; Iwata, T.; Onitsuka, T.; Yasumoto, K. Association between Estrogen Receptor-β Expression and Epidermal Growth Factor Receptor Mutation in the Postoperative Prognosis of Adenocarcinoma of the Lung. J. Clin. Oncol. 2009, 27, 411–417. [Google Scholar] [CrossRef]
- Márquez-Garbán, D.C.; Chen, H.-W.; Fishbein, M.C.; Goodglick, L.; Pietras, R.J. Estrogen Receptor Signaling Pathways in Human Non-Small Cell Lung Cancer. Steroids 2007, 72, 135–143. [Google Scholar] [CrossRef]
- Stabile, L.P.; Lyker, J.S.; Gubish, C.T.; Zhang, W.; Grandis, J.R.; Siegfried, J.M. Combined Targeting of the Estrogen Receptor and the Epidermal Growth Factor Receptor in Non-Small Cell Lung Cancer Shows Enhanced Antiproliferative Effects. Cancer Res. 2005, 65, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Pietras, R.J.; Márquez, D.C.; Chen, H.-W.; Tsai, E.; Weinberg, O.; Fishbein, M. Estrogen and Growth Factor Receptor Interactions in Human Breast and Non-Small Cell Lung Cancer Cells. Steroids 2005, 70, 372–381. [Google Scholar] [CrossRef]
- Xie, X.; Shao, X.; Ma, W.; Zhao, D.; Shi, S.; Li, Q.; Lin, Y. Overcoming Drug-Resistant Lung Cancer by Paclitaxel Loaded Tetrahedral DNA Nanostructures. Nanoscale 2018, 10, 5457–5465. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Belani, C.P. Paclitaxel for Non-Small Cell Lung Cancer. Expert Opin. Pharmacother. 2004, 5, 1771–1780. [Google Scholar] [CrossRef]
- Socinski, M.A. Single-Agent Paclitaxel in the Treatment of Advanced Non-Small Cell Lung Cancer. Oncologist 1999, 4, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Von Eiff, D.; Bozorgmehr, F.; Chung, I.; Bernhardt, D.; Rieken, S.; Liersch, S.; Muley, T.; Kobinger, S.; Thomas, M.; Christopoulos, P.; et al. Paclitaxel for Treatment of Advanced Small Cell Lung Cancer (SCLC): A Retrospective Study of 185 Patients. J. Thorac. Dis. 2020, 12, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Camps, C.; Caballero, C.; Blasco, A.; Safont, M.J.; Berrocal, A.; Garde, J.; Juarez, A.; Sirera, R.; Bremnes, R.M. Weekly Paclitaxel as Second/Third-Line Treatment in Advanced Non-Small Cell Lung Cancer Patients: Efficacy and Tolerability. Anticancer. Res. 2005, 25, 4611–4614. [Google Scholar] [PubMed]
- Mohiuddin, M.; Kasahara, K. Paclitaxel Impedes EGFR-Mutated PC9 Cell Growth via Reactive Oxygen Species-Mediated DNA Damage and EGFR/PI3K/AKT/MTOR Signaling Pathway Suppression. Cancer Genom. Proteom. 2021, 18, 645–659. [Google Scholar] [CrossRef]
- Chamberlain, M.C.; Kormanik, P. Salvage Chemotherapy with Paclitaxel for Recurrent Primary Brain Tumors. J. Clin. Oncol. 1995, 13, 2066–2071. [Google Scholar] [CrossRef]
- Prados, M.D.; Schold, S.C.; Spence, A.M.; Berger, M.S.; McAllister, L.D.; Mehta, M.P.; Gilbert, M.R.; Fulton, D.; Kuhn, J.; Lamborn, K.; et al. Phase II Study of Paclitaxel in Patients with Recurrent Malignant Glioma. J. Clin. Oncol. 1996, 14, 2316–2321. [Google Scholar] [CrossRef]
- Fetell, M.R.; Grossman, S.A.; Fisher, J.D.; Erlanger, B.; Rowinsky, E.; Stockel, J.; Piantadosi, S. Preirradiation Paclitaxel in Glioblastoma Multiforme: Efficacy, Pharmacology, and Drug Interactions. New Approaches to Brain Tumor Therapy Central Nervous System Consortium. J. Clin. Oncol. 1997, 15, 3121–3128. [Google Scholar] [CrossRef]
- Chang, S.M.; Kuhn, J.G.; Rizzo, J.; Robins, H.I.; Schold, S.C.; Spence, A.M.; Berger, M.S.; Mehta, M.P.; Bozik, M.E.; Pollack, I.; et al. Phase I Study of Paclitaxel in Patients with Recurrent Malignant Glioma: A North American Brain Tumor Consortium Report. J. Clin. Oncol. 1998, 16, 2188–2194. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Dmello, C.; Chen, L.; Arrieta, V.A.; Gonzalez-Buendia, E.; Kane, J.R.; Magnusson, L.P.; Baran, A.; James, C.D.; Horbinski, C.; et al. Ultrasound-Mediated Delivery of Paclitaxel for Glioma: A Comparative Study of Distribution, Toxicity, and Efficacy of Albumin-Bound Versus Cremophor Formulations. Clin. Cancer Res. 2020, 26, 477–486. [Google Scholar] [CrossRef]
- Surekha, B.; Kommana, N.S.; Dubey, S.K.; Kumar, A.V.P.; Shukla, R.; Kesharwani, P. PAMAM Dendrimer as a Talented Multifunctional Biomimetic Nanocarrier for Cancer Diagnosis and Therapy. Colloids Surf. B Biointerfaces 2021, 204, 111837. [Google Scholar] [CrossRef]
- Mishra, V.; Kesharwani, P. Dendrimer Technologies for Brain Tumor. Drug Discov. Today 2016, 21, 766–778. [Google Scholar] [CrossRef]
- Malinga-Drozd, M.; Uram, Ł.; Wróbel, K.; Wołowiec, S. Chiral Recognition of Homochiral Poly (Amidoamine) Dendrimers Substituted with R- and S-Glycidol by Keratinocyte (HaCaT) and Squamous Carcinoma (SCC-15) Cells In Vitro. Polymers 2021, 13, 1049. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Mohd Amin, M.C.I.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM Dendrimers: Enhancing Efficacy and Mitigating Toxicity for Effective Anticancer Drug and Gene Delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.R. The C. elegans Model in Toxicity Testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.B.; Hamza, A.; Singh, T.; Flibotte, S.; Hieter, P.; O’Neil, N.J. A Multimodal Genotoxic Anticancer Drug Characterized by Pharmacogenetic Analysis in Caenorhabditis elegans. Genetics 2020, 215, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.-K.; Sung, J.Y.; Kim, Y.-N.; Kim, S.; Hong, K.M.; Kim, H.T.; Choi, M.S.; Kwon, J.Y.; Shim, J. An In Vivo C. elegans Model System for Screening EGFR-Inhibiting Anti-Cancer Drugs. PLoS ONE 2012, 7, e42441. [Google Scholar] [CrossRef]
- Diot, C.; García-González, A.P.; Vieira, A.F.; Walker, M.; Honeywell, M.; Doyle, H.; Ponomarova, O.; Rivera, Y.; Na, H.; Zhang, H.; et al. Bacterial Diet Modulates Tamoxifen-Induced Death via Host Fatty Acid Metabolism. Nat. Commun. 2022, 13, 5595. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Mizukami, M.; Hiroka, Y.; Miyasaka, K.; Niwa, K.; Arizono, K.; Ichikawa, N. Evaluation of Neurotoxicity of Anticancer Drugs Using Nematode Caenorhabditis elegans as a Model Organism. J. Toxicol. Sci. 2023, 48, 311–321. [Google Scholar] [CrossRef]
- Wittkowski, P.; Marx-Stoelting, P.; Violet, N.; Fetz, V.; Schwarz, F.; Oelgeschläger, M.; Schönfelder, G.; Vogl, S. Caenorhabditis elegans As a Promising Alternative Model for Environmental Chemical Mixture Effect Assessment—A Comparative Study. Environ. Sci. Technol. 2019, 53, 12725–12733. [Google Scholar] [CrossRef]
- Ma, P.; Zhang, X.; Ni, L.; Li, J.; Zhang, F.; Wang, Z.; Lian, S.; Sun, K. Targeted Delivery of Polyamidoamine-Paclitaxel Conjugate Functionalized with Anti-Human Epidermal Growth Factor Receptor 2 Trastuzumab. Int. J. Nanomed. 2015, 10, 2173–2190. [Google Scholar] [CrossRef]
- Lewińska, A.; Wróbel, K.; Błoniarz, D.; Adamczyk-Grochala, J.; Wołowiec, S.; Wnuk, M. Lapatinib- and Fulvestrant-PAMAM Dendrimer Conjugates Promote Apoptosis in Chemotherapy-Induced Senescent Breast Cancer Cells with Different Receptor Status. Biomater. Adv. 2022, 140, 213047. [Google Scholar] [CrossRef] [PubMed]
- Peržeľová, V.; Sabol, F.; Vasilenko, T.; Novotný, M.; Kováč, I.; Slezák, M.; Ďurkáč, J.; Hollý, M.; Pilátová, M.; Szabo, P.; et al. Pharmacological Activation of Estrogen Receptors-α and -β Differentially Modulates Keratinocyte Differentiation with Functional Impact on Wound Healing. Int. J. Mol. Med. 2016, 37, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, M.M.; Mazhawidza, W.; Dougherty, S.M.; Klinge, C.M. Sex Differences in Estrogen Receptor Subcellular Location and Activity in Lung Adenocarcinoma Cells. Am. J. Respir. Cell Mol. Biol. 2010, 42, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Hu, J.; Wang, X.; Wang, J.; Zhang, Y.; Wang, C. Epidermal Growth Factor Receptor Expression Affects Proliferation and Apoptosis in Non-Small Cell Lung Cancer Cells via the Extracellular Signal-Regulated Kinase/MicroRNA 200a Signaling Pathway. Oncol. Lett. 2018, 15, 5201–5207. [Google Scholar] [CrossRef]
- Cichocki, M.; Szaefer, H.; Krajka-Kuźniak, V.; Baer-Dubowska, W. The Effect of Resveratrol and Its Methylthio-Derivatives on EGFR and Stat3 Activation in Human HaCaT and A431 Cells. Mol. Cell Biochem. 2014, 396, 221–228. [Google Scholar] [CrossRef]
- Leslie, K.K.; Keefe, D.; Powlll, S.; Naftolin, F. Estrogen Receptors Are Identified in the Glioblastoma Cell Line UI38MG. Reprod. Sci. 1994, 1, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Zitron, I.M.; Thakur, A.; Norkina, O.; Barger, G.R.; Lum, L.G.; Mittal, S. Targeting and Killing of Glioblastoma with Activated T Cells Armed with Bispecific Antibodies. BMC Cancer 2013, 13, 83. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar] [CrossRef]
- Martinho, N.; Florindo, H.; Silva, L.; Brocchini, S.; Zloh, M.; Barata, T. Molecular Modeling to Study Dendrimers for Biomedical Applications. Molecules 2014, 19, 20424–20467. [Google Scholar] [CrossRef]
- De Trizio, I.; Errede, M.; D’Amati, A.; Girolamo, F.; Virgintino, D. Expression of P-Gp in Glioblastoma: What We Can Learn from Brain Development. Curr. Pharm. Des. 2020, 26, 1428–1437. [Google Scholar] [CrossRef]
- Heming, C.P.; Muriithi, W.; Macharia, L.W.; Filho, P.N.; Moura-Neto, V.; Aran, V. P-Glycoprotein and Cancer: What Do We Currently Know? Heliyon 2022, 8, e11171. [Google Scholar] [CrossRef]
- Mamnoon, B.; Feng, L.; Froberg, J.; Choi, Y.; Sathish, V.; Taratula, O.; Taratula, O.; Mallik, S. Targeting Estrogen Receptor-Positive Breast Microtumors with Endoxifen-Conjugated, Hypoxia-Sensitive Polymersomes. ACS Omega 2021, 6, 27654–27667. [Google Scholar] [CrossRef]
- Santos, E.d.S.; Nogueira, K.A.B.; Fernandes, L.C.C.; Martins, J.R.P.; Reis, A.V.F.; Neto, J.d.B.V.; da Silva Júnior, I.J.; Pessoa, C.; Petrilli, R.; Eloy, J.O. EGFR Targeting for Cancer Therapy: Pharmacology and Immunoconjugates with Drugs and Nanoparticles. Int. J. Pharm. 2021, 592, 120082. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, S.; Argenzio, E.; Tosoni, D.; Cavallaro, E.; Polo, S.; Di Fiore, P.P. Clathrin-Mediated Internalization Is Essential for Sustained EGFR Signaling but Dispensable for Degradation. Dev. Cell 2008, 15, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Higa, S.; Furuichi, Y.; Naka, R.; Yumoto, R. Suppression of P-Glycoprotein by Cigarette Smoke Extract in Human Lung-Derived A549/P-Gp Cells. Drug Metab. Pharmacokinet. 2020, 35, 214–219. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, M.S.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding Nanoparticle Endocytosis to Improve Targeting Strategies in Nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef]
- Garon, E.B.; Pietras, R.J.; Finn, R.S.; Kamranpour, N.; Pitts, S.; Márquez-Garbán, D.C.; Desai, A.J.; Dering, J.; Hosmer, W.; von Euw, E.M.; et al. Antiestrogen Fulvestrant Enhances the Antiproliferative Effects of Epidermal Growth Factor Receptor Inhibitors in Human Non-Small-Cell Lung Cancer. J. Thorac. Oncol. 2013, 8, 270–278. [Google Scholar] [CrossRef]
- Sebaugh, J.L. Guidelines for Accurate EC50/IC50 Estimation. Pharmaceut. Statist. 2011, 10, 128–134. [Google Scholar] [CrossRef]
- Kyakulaga, A.H.; Aqil, F.; Munagala, R.; Gupta, R.C. Synergistic Combinations of Paclitaxel and Withaferin A against Human Non-Small Cell Lung Cancer Cells. Oncotarget 2020, 11, 1399–1416. [Google Scholar] [CrossRef]
- Koziara, J.M.; Lockman, P.R.; Allen, D.D.; Mumper, R.J. Paclitaxel Nanoparticles for the Potential Treatment of Brain Tumors. J. Control. Release 2004, 99, 259–269. [Google Scholar] [CrossRef]
- Olaussen, K.A.; Commo, F.; Tailler, M.; Lacroix, L.; Vitale, I.; Raza, S.Q.; Richon, C.; Dessen, P.; Lazar, V.; Soria, J.-C.; et al. Synergistic Proapoptotic Effects of the Two Tyrosine Kinase Inhibitors Pazopanib and Lapatinib on Multiple Carcinoma Cell Lines. Oncogene 2009, 28, 4249–4260. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Song, S.; Zhu, Z.; Sun, W.; Gifts, J.E.; Sun, S.; Li, Q.S.; Yu, Y.; Li, K.K. LncRNAs GIHCG and SPINT1-AS1 Are Crucial Factors for Pan-Cancer Cells Sensitivity to Lapatinib. Front. Genet. 2019, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; IIZUKA, M.; Watanabe, M.; Narita, T.; Kato, C.; Kakibuchi, D.; Kitano, F.; Ouchi, Y.; Sakaguchi, K.; Taguchi, T. Oxidative Stress Induces EGFR Inhibition-Related Skin Cell Death. J. Clin. Biochem. Nutr. 2021, 68, 235–242. [Google Scholar] [CrossRef]
- Liebmann, J.E.; Cook, J.A.; Lipschultz, C.; Teague, D.; Fisher, J.; Mitchell, J.B. Cytotoxic Studies of Paclitaxel (Taxol) in Human Tumour Cell Lines. Br. J. Cancer 1993, 68, 1104–1109. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Uram, Ł.; Markowicz, J.; Misiorek, M.; Filipowicz-Rachwał, A.; Wołowiec, S.; Wałajtys-Rode, E. Celecoxib Substituted Biotinylated Poly(Amidoamine) G3 Dendrimer as Potential Treatment for Temozolomide Resistant Glioma Therapy and Anti-Nematode Agent. Eur. J. Pharm. Sci. 2020, 152, 105439. [Google Scholar] [CrossRef]
- Nsairat, H.; Al-Sulaibi, M.; Alshaer, W. PEGylated Nanoassemblies Composed of Edelfosine and Fulvestrant Drugs: In Vitro Antiproliferative Effect against Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2023, 85, 104612. [Google Scholar] [CrossRef]
- Agrawal, A.; Robertson, J.F.R.; Cheung, K.L.; Gutteridge, E.; Ellis, I.O.; Nicholson, R.I.; Gee, J.M.W. Biological Effects of Fulvestrant on Estrogen Receptor Positive Human Breast Cancer: Short, Medium and Long-Term Effects Based on Sequential Biopsies. Int. J. Cancer 2016, 138, 146–159. [Google Scholar] [CrossRef]
- Claus, J.; Patel, G.; Autore, F.; Colomba, A.; Weitsman, G.; Soliman, T.N.; Roberts, S.; Zanetti-Domingues, L.C.; Hirsch, M.; Collu, F.; et al. Inhibitor-Induced HER2-HER3 Heterodimerisation Promotes Proliferation through a Novel Dimer Interface. eLife 2018, 7, e32271. [Google Scholar] [CrossRef]
- Khachigian, L.M. Emerging Insights on Functions of the Anthelmintic Flubendazole as a Repurposed Anticancer Agent. Cancer Lett. 2021, 522, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Mimoto, A.; Fujii, M.; Usami, M.; Shimamura, M.; Hirabayashi, N.; Kaneko, T.; Sasagawa, N.; Ishiura, S. Identification of an Estrogenic Hormone Receptor in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2007, 364, 883–888. [Google Scholar] [CrossRef]
- Tominaga, N.; Ura, K.; Kawakami, M.; Kawaguchi, T.; Kohra, S.; Mitsui, Y.; Iguchi, T.; Arizono, K. Caenorhabditis elegans Responses to Specific Steroid Hormones. J. Health Sci. 2003, 49, 28–33. [Google Scholar] [CrossRef]
- Konietzka, J.; Fritz, M.; Spiri, S.; McWhirter, R.; Leha, A.; Palumbos, S.; Costa, W.S.; Oranth, A.; Gottschalk, A.; Miller, D.M.; et al. Epidermal Growth Factor Signaling Promotes Sleep through a Combined Series and Parallel Neural Circuit. Curr. Biol. 2020, 30, 1–16.e13. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, C.; Greenwald, I. EGFR Signal Transduction Is Downregulated in C. elegans Vulval Precursor Cells during Dauer Diapause. Development 2022, 149, dev201094. [Google Scholar] [CrossRef]
- Kamal, M.; Tokmakjian, L.; Knox, J.; Mastrangelo, P.; Ji, J.; Cai, H.; Wojciechowski, J.W.; Hughes, M.P.; Takács, K.; Chu, X.; et al. A Spatiotemporal Reconstruction of the C. elegans Pharyngeal Cuticle Reveals a Structure Rich in Phase-Separating Proteins. eLife 2022, 11, e79396. [Google Scholar] [CrossRef]
- So, S.; Miyahara, K.; Ohshima, Y. Control of Body Size in C. elegans Dependent on Food and Insulin/IGF-1 Signal. Genes. Cells 2011, 16, 639–651. [Google Scholar] [CrossRef]
- Soete, G.; Betist, M.C.; Korswagen, H.C. Regulation of Caenorhabditis elegans Body Size and Male Tail Development by the Novel Gene Lon-8. BMC Dev. Biol. 2007, 7, 20. [Google Scholar] [CrossRef]
- Flemming, A.J.; Shen, Z.-Z.; Cunha, A.; Emmons, S.W.; Leroi, A.M. Somatic Polyploidization and Cellular Proliferation Drive Body Size Evolution in Nematodes. Proc. Natl. Acad. Sci. USA 2000, 97, 5285–5290. [Google Scholar] [CrossRef]
- Page, A.P.; Stepek, G.; Winter, A.D.; Pertab, D. Enzymology of the Nematode Cuticle: A Potential Drug Target? Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 133–141. [Google Scholar] [CrossRef]
- Madaan, U.; Yzeiraj, E.; Meade, M.; Rushlow, C.A.; Savage-Dunn, C. Caenorhabditis elegans BMP Transcriptional Program Implicates Collagen Genes in Body Size Regulation. bioRxiv 2017, 108225. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Uram, Ł.; Szuster, M.; Filipowicz, A.; Zaręba, M.; Wałajtys-Rode, E.; Wołowiec, S. Cellular Uptake of Glucoheptoamidated Poly(Amidoamine) PAMAM G3 Dendrimer with Amide-Conjugated Biotin, a Potential Carrier of Anticancer Drugs. Bioorg. Med. Chem. 2017, 25, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A.; Fleming, J.T. Basic Culture Methods. Methods Cell Biol. 1995, 48, 3–29. [Google Scholar] [PubMed]
- Scanlan, L.D.; Lund, S.P.; Coskun, S.H.; Hanna, S.K.; Johnson, M.E.; Sims, C.M.; Brignoni, K.; Lapasset, P.; Petersen, E.J.; Elliott, J.T.; et al. Counting Caenorhabditis elegans: Protocol Optimization and Applications for Population Growth and Toxicity Studies in Liquid Medium. Sci. Rep. 2018, 8, 904. [Google Scholar] [CrossRef] [PubMed]
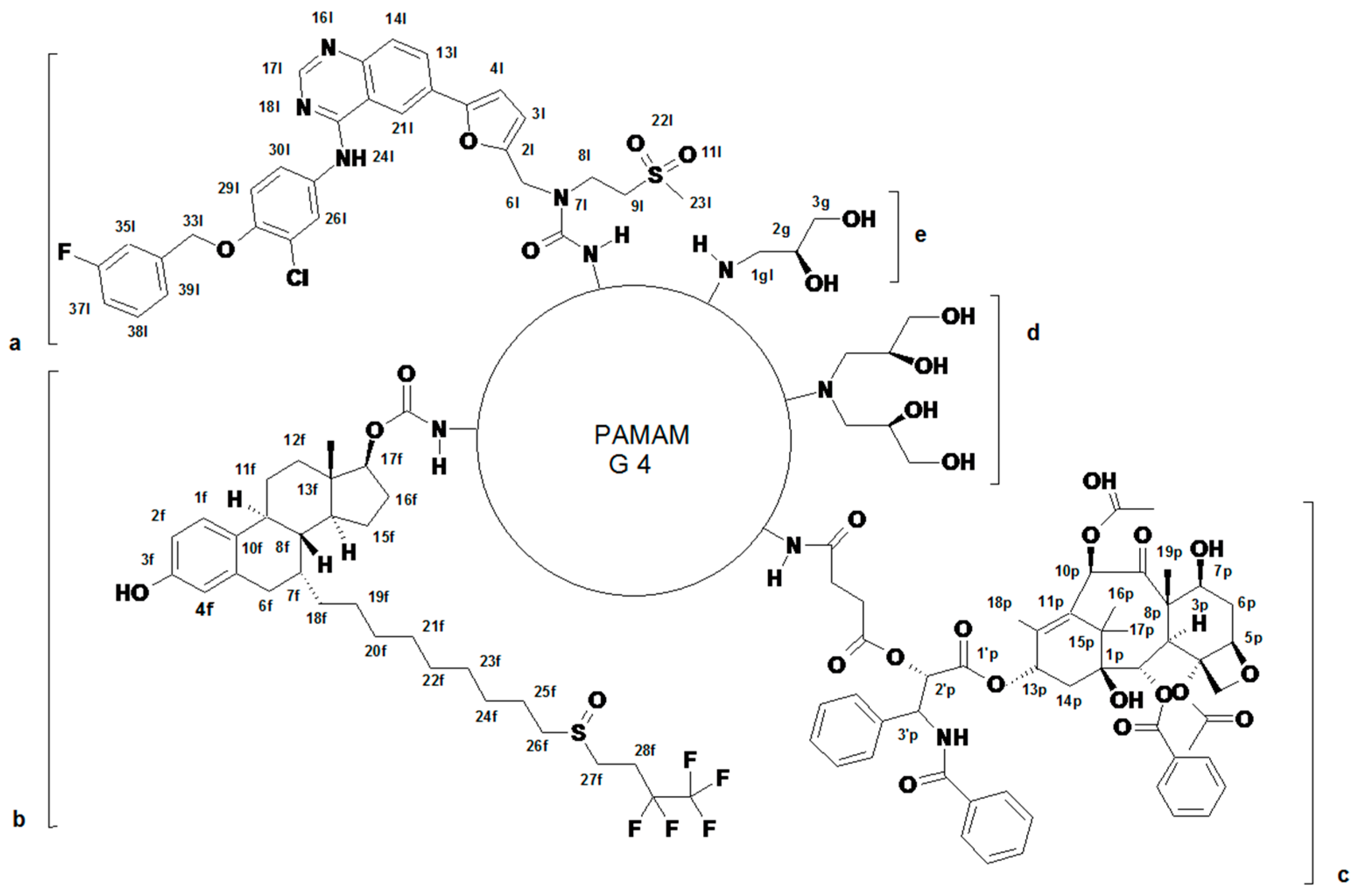





| Conjugate | MW [kDa] | Size (SD) [nm] | Polydispersity Index PDI (SD) | ζ (SD) [mV] |
|---|---|---|---|---|
| G4*126gl G4 | 19.1 | 5.12 (0.25) | 0.080 (0.002) | 4.35 (0.28) |
| G4*4P109gl G4P | 26.4 | 99.20 (7.28) | 0.134 (0.016) | 13.57 (0.43) |
| G4*13F64gl G4F | 27.6 | 138.23 (6.24) | 0.155 (0.022) | 38.02 (0.54) |
| G4*13L85gl G4L | 28.8 | 113.13 (4.64) | 0.112 (0.014) | 40.29 (0.74) |
| G4*4P10L98gl G4PL | 31.7 | 95.20 (4.46) | 0.125 (0.010) | 19.92 (0.53) |
| G4*4P10F98gl G4PF | 32.0 | 113.41 (5.48) | 0.128 (0.015) | 29.29 (0.72) |
| G4*4P11F11L74gl G4PFL | 37.5 | 105.47 (5.39) | 0.159 (0.018) | 34.33 (0.65) |
| IC50 [nM] NR Assay | |||
|---|---|---|---|
| A549 | U-118 MG | HaCaT | |
| Paclitaxel | 50.35 | 55.56 | 2.53 |
| Fulvestrant | ≫100,00 * | ≫100,000 * | ≫100,000 * |
| Lapatinib | 16,701.61 | 14,878.63 | 6150.48 |
| G4P | ≫320 * ≫1280 * p | 25.28 101.12 p | 1.63 6.52 p |
| G4F | ≫1000 * ≫130,000 * f | ≫10,000 * ≫130,000 * f | 2927.15 38,052.97 f |
| G4L | 1324.05 17,212.75 l | 54,395 6527.4 l | 57.87 765.31 l |
| G4PFL | ≫400 * ≫400 * p ≫4400 * fl | 78.70 314.80 p 865.70 fl | 9.53 38.12 p 104.83 fl |
| IC50 [nM] XTT Assay | |||
| G4P | ≫320 ≫1280 * p | 3.14 12.56 p | 0.99 3.96 |
| G4F | >20,000 >260,000 f | 6062.64 78,814.32 f | 1282.15 16,667.95 f |
| G4L | 3885.91 50,516.83 l | 1147.86 14,922.18 l | 76.90 999.70 l |
| G4PFL | >1600 >6400 p >17,600 fl | 9.45 37.80 p 103.95 fl | 2.00 8.00 p 22.00 fl |
| Compound | LC50 [µM] |
|---|---|
| Mebendazole | 4.00 |
| G4P | >24.00 |
| G4F | 12.50 |
| G4L | 14.80 |
| G4PFL | 6.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uram, Ł.; Wróbel, K.; Walczak, M.; Szymaszek, Ż.; Twardowska, M.; Wołowiec, S. Exploring the Potential of Lapatinib, Fulvestrant, and Paclitaxel Conjugated with Glycidylated PAMAM G4 Dendrimers for Cancer and Parasite Treatment. Molecules 2023, 28, 6334. https://doi.org/10.3390/molecules28176334
Uram Ł, Wróbel K, Walczak M, Szymaszek Ż, Twardowska M, Wołowiec S. Exploring the Potential of Lapatinib, Fulvestrant, and Paclitaxel Conjugated with Glycidylated PAMAM G4 Dendrimers for Cancer and Parasite Treatment. Molecules. 2023; 28(17):6334. https://doi.org/10.3390/molecules28176334
Chicago/Turabian StyleUram, Łukasz, Konrad Wróbel, Małgorzata Walczak, Żaneta Szymaszek, Magdalena Twardowska, and Stanisław Wołowiec. 2023. "Exploring the Potential of Lapatinib, Fulvestrant, and Paclitaxel Conjugated with Glycidylated PAMAM G4 Dendrimers for Cancer and Parasite Treatment" Molecules 28, no. 17: 6334. https://doi.org/10.3390/molecules28176334
APA StyleUram, Ł., Wróbel, K., Walczak, M., Szymaszek, Ż., Twardowska, M., & Wołowiec, S. (2023). Exploring the Potential of Lapatinib, Fulvestrant, and Paclitaxel Conjugated with Glycidylated PAMAM G4 Dendrimers for Cancer and Parasite Treatment. Molecules, 28(17), 6334. https://doi.org/10.3390/molecules28176334






