Abstract
Surfactants are amphiphilic molecules and one of the most versatile products of the chemical industry. They can be absorbed at the air–water interface and can align themselves so that the hydrophobic part is in the air while the hydrophilic part is in water. This alignment lowers the surface or interfacial tension. Gemini surfactants are a modern variety of surfactants with unique properties and a very wide range of potential applications. Hexamethylene-1,6-bis(N-dodecyl-N,N-dimethylammonium bromide) is one such representative compound that is a better alternative to a single analogue. It shows excellent surface, antimicrobial, and anticorrosion properties. With a highly efficient synthetic method and a good ecological profile, it is a potential candidate for numerous applications, including biomedical applications.
1. Introduction
Interactions at the interface are of fundamental importance in chemistry, physics, and biology. The thermodynamic parameters of these interactions can be modulated using surfactants. Owing to their amphiphilic structure, which comprises a hydrophilic and hydrophobic part, surfactants decrease the surface tension or interfacial tension between two liquids, a liquid and gas, or a liquid and solid. Hence, they can act as wetting, dispersing, and emulsifying agents. These properties enable the production of a large number of products required in household chemistry and cosmetic, pharmaceutical, agrochemical, petrochemical, textile, and paper industries.
The dynamic development of surfactant chemistry is a continuation of what was initiated by nature, which created the biosurfactants necessary for the functioning of living organisms. Biosurfactants usually refer to surfactants of microbial origin and comprise lecithin, rhamnolipids, sophorolipids, and emulsan [1,2]. Biosurfactants, in addition to their intrinsic role, have a wide range of technical applications. For instance, they can solubilise hydrocarbon contaminants and can be used in enhanced oil recovery [3,4]. Owing to their low toxicity and biodegradability, biosurfactants are extremely valuable products from the viewpoint of environmental protection. The global market size for biosurfactants reached a value of more than USD 2.33 billion in 2021 and is expected to grow at a compound annual growth rate (CAGR) of 5.8% between 2023 and 2028, reaching a projected value of USD 3.27 billion by 2027 [5,6]. Unfortunately, the number of available biosurfactants as well as the range of their applications does not meet the requirements expected from surfactants.
Currently, the widest groups of surfactants that meet the application requirements are nonionic, anionic, cationic, and amphoteric synthetic surfactants. The global demand for these surfactants, including soaps, exceeds 20 million tons per year [6]. The global surfactant market stood at a value of approximately USD 41.84 billion in 2022. The market is further expected to grow in the forecast period 2023–2030 at a CAGR of 4.6% to reach a value of USD 59.95 billion by 2030 [6]. However, the large volume of surfactants disposed into the environment, despite the wastewater treatment processes, is a serious burden and threat to the environment. Thus, owing to the increasing demand for surfactants, the development of new and more effective surfactants is extremely important.
Cationic gemini surfactants have emerged as a result of the research conducted in this field over the last several years. Gemini surfactants are compounds that are composed of two hydrophilic head groups and two hydrophobic tails linked by a spacer at the head groups or close to them (Figure 1). The spacers can be flexible (methylenes) or rigid (aromatic structures). The type of spacer (short or long, hydrophilic or hydrophobic) influences the shape of the micelles. The neutral charge of the molecule is retained in the presence of organic or inorganic counterions [7,8,9].
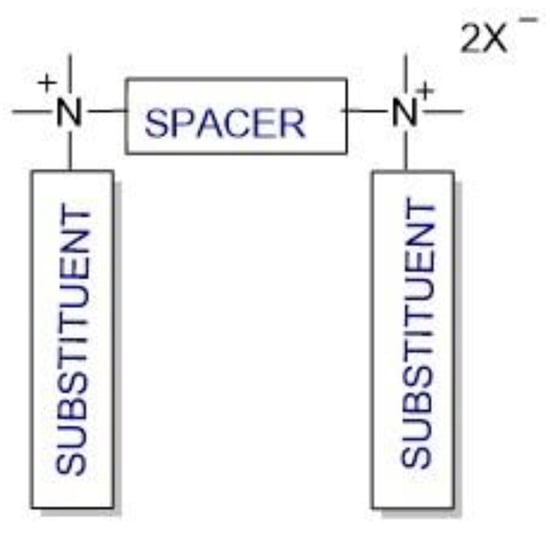
Figure 1.
Structure of gemini surfactants.
The critical micelle concentration (CMC), surface tension (γ), and minimal inhibitory concentration (MIC) of gemini surfactants are a dozen times lower than those of monomeric surfactants. The unique properties of gemini surfactants with a wide range of hydrophilic–lipophilic balance (HLB) render them a very useful and innovative material in chemistry (e.g., for corrosion inhibition, micellar catalysis, nanoparticle synthesis, preparation of supramolecular solvents, nanoemulsion preparation, and synthesis of precisely defined polymers) [10,11,12,13,14], medicine (as biocides, drug carriers, and capping agents for metal nanoparticles with biocidal properties or for preparing nonviral gene delivery systems and inducing protein conformational changes) [15,16,17,18,19,20], and optoelectronics (through a spatial network of well-dispersed molecules) [7]. Gemini surfactants are a modern solution for all areas that need surfactants, including households, detergents, personal care, institutional and industrial cleaning, food processing, plastics, paints and coatings, oilfield chemicals, petrochemistry, agricultural chemicals, adhesives, and textiles [20].
There is an increasing interest in gemini surfactants (Figure 2). Among the large number of gemini surfactants reported in recent years, hexamethylene-1,6-bis(N-dodecyl-N,N-dimethylammonium bromide) (12-6-12) deserves a special mention from the viewpoint of application. This review systematically presents the current studies devoted to the structure elucidation, synthesis, properties, and applications of 12-6-12, which can be a safer alternative to the surfactants used so far.
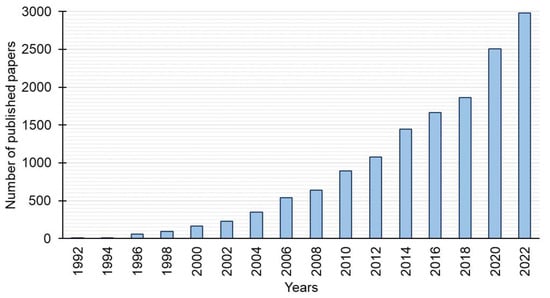
Figure 2.
Number of papers published on gemini surfactants versus the year of publication (based on Scopus).
2. Structure and Synthesis
12-6-12 consists of two N-dodecyl-N,N-dimethylammonium units connected with a chain of six methylene groups as a spacer. Bromine ions are present as counterions (Figure 3). This compound has been classified under chemical abstracts service (CAS) number 18507-15-8 and is a dimeric analogue of N-dodecyl-N,N,N-trimethylammonium bromide (DTAB), which is commonly used as a microbiocide.
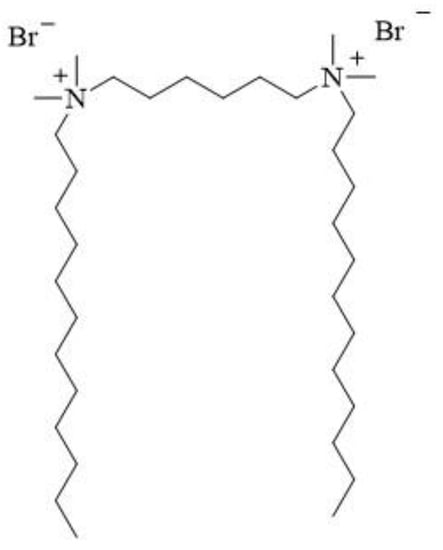
Figure 3.
Chemical structure of 12-6-12.
12-6-12 is one of the first compound to be classified as a double quaternary ammonium salt and defined as a gemini surfactant. This compound is synthesised via the quaternisation of amines, a process referred to as the Menschutkin reaction:
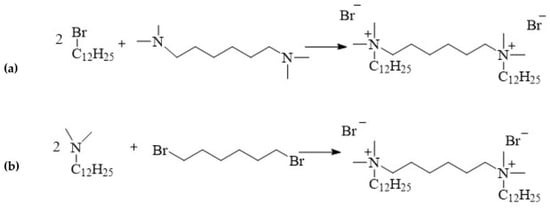 Figure 4. Synthesis of 12-6-12.
Figure 4. Synthesis of 12-6-12.
The synthesis of 12-6-12 was first reported in 1968 by Sindenko et al. [21]. Stoichiometric amounts of dodecylbromide and hexamethylene-bis(N,N-dimethylamine) were reacted in ethanol for the synthesis (yield 70%) [21]. Regardless of the synthetic pathway, the quaternisation reaction always follows the SN2 nucleophilic substitution mechanism. The rate of the reaction depends on the concentrations of both reagents, although there are reports of the use of excess amine [22,23,24,25] or bromide [26,27,28]. Typically, polar solvents such as alcohols (methanol [27,29], ethanol [30,31], isopropanol [32]), acetone [19,23,33], or acetonitrile [22,24,34] are used in the synthesis of 12-6-12. These reactions occur at the boiling point of the solvent. The type of solvent used determines the reaction time because SN2 reactions are the fastest in polar aprotic solvents such as acetonitrile. Replacing ethanol with acetonitrile reduces the reaction time from 24 to 5 h [25,35]. 12-6-12 can also be synthesised in solvent mixtures such as the acetonitrile/toluene mixture [36]. The reaction time can be shortened using microwave radiation [37]. However, the most economical and ecological approach is to synthesise 12-6-12 under stoichiometric conditions at room temperature without a solvent [38]. In this case, good yields of over 90% can be achieved using small amounts of reagents over a reaction time of 0.5 h [38]. Solvent-free synthesis can also be easily carried out on a large scale.
To obtain pure 12-6-12, the crude product can be crystallised from acetonitrile [35] or from dichloromethane–diethyl ether [39], acetone–methanol [40,41], ethanol–ethyl acetate [23], ethanol–diethyl ether [24], acetone–ethanol [21], and acetone–ethyl acetate [25] mixtures.
3. Analysis
12-6-12 is a white water-soluble solid [26]. It melts with decomposition at 225–226 °C [28,30]. The structure of a compound is usually confirmed using proton and carbon nuclear magnetic resonance (1H and 13C NMR). The structure and numbering of 12-6-12 are shown in Figure 5.
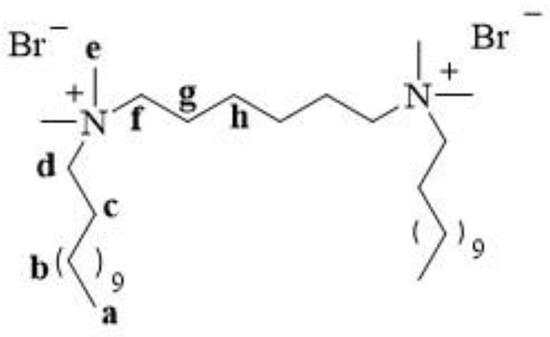
Figure 5.
Structure and numbering of 12-6-12.
In the 1H NMR spectra, signals from the protons of the terminal methyl groups of the long alkyl chains (a) were observed at the lowest ppm values. The protons of the methyl (e) and methylene groups (in spacer (f) and alkyl chains (d)) next to the quaternary nitrogen atom exhibited signals at the highest ppm values. Table 1 shows the 1H NMR chemical shifts for 12-6-12.

Table 1.
1H NMR chemical shifts (δ, ppm) of 12-6-12 in different solvents.
In the 13C NMR spectra, signals from the carbons of the terminal methyl groups of the long alkyl chains were observed at the lowest ppm values, similar to that in the 1H NMR spectra. Signals from the methylene groups in the alkyl chains, spacers, and methyl groups adjacent to the quaternary ammonium nitrogen appeared at the highest ppm values. Table 2 shows the 13C NMR chemical shifts of 12-6-12.

Table 2.
13C NMR chemical shifts (δ, ppm) of 12-6-12 in different solvents.
In the FTIR spectrum of 12-6-12, broad intense absorption bands corresponding to asymmetric stretching (νas) and symmetric stretching (νs) vibrations of the methyl and methylene groups were observed at 2980–2850 cm−1, while typical bands corresponding to deformation vibrations (δ) of the methyl and methylene groups appeared at 1490–1370 cm−1. At 720 cm−1, there was a typical band corresponding to the rocking vibrations (ρ) of the methylene groups derived from the long alkyl hydrocarbon chains. No stretching vibration bands for the N-H and O-H bonds were observed, which confirmed the purity of the compound [38].
Mass spectrometry is another analytical method for confirming the structure and purity of synthesised compounds. Currently, soft ionisation techniques, such as electrospray ionisation (ESI), are used for diagnostic purposes. Although methods leading to many decays and fragmentation ions for 12-6-12 have been published [43], they are currently of little diagnostic importance. Buse et al. published the electrospray ionisation quadrupole time-of-flight hybrid tandem mass spectrometry of a homologous series of gemini surfactants [44]. The fragments and their corresponding m/z values for 12-6-12 are listed in Table 3.

Table 3.
Molecular and fragmentation ions and their corresponding m/z value for 12-6-12.
4. Properties
4.1. Surface Activity in Aqueous Solutions
Surfactants are molecules that lower the surface tension between two materials: a gas and liquid, a liquid and liquid, or a liquid and solid. Double quaternary ammonium salts, such as 12-6-12, are cationic surfactants. They possess long hydrocarbon chains (surfactant tails) and hydrophilic nitrogen groups (surfactant heads). The broad structural diversity of gemini surfactants renders it possible to obtain compounds with desirable HLB values and expected physicochemical properties. Gemini surfactants can interact very effectively with oppositely charged surfaces. Surfactant molecules in solution can form aggregates called micelles. The structure of micelles depends on many factors, including the solvent polarity. However, in every case, monomeric analogues require a higher number of molecules to form micelles than gemini surfactants [45,46,47]. 12-6-12 has much better surface properties than its single-chain analogue. The CMC, micelle ionisation degree (α), Gibbs free energy of micellisation (ΔG°mic), surface tension at CMC point (γCMC), area per molecule (Å2), and number of molecules per nm2 for 12-6-12 and DTAB are listed in Table 4. The CMCs of 12-6-12 were two orders of magnitude lower than those of DTAB, regardless of the determination method. The ΔG°mic value is more negative for 12-6-12 than for the monomer, suggesting a higher spontaneity of micellisation for the former. Generally, CMC values are highly dependent on temperature—the CMC values decrease with increasing temperature (Figure 6) [48]. A linear dependence with a high linear regression coefficient (r2 = 0.99) is observed.

Table 4.
Various micellisation and thermodynamic parameters of DTAB and 12-6-12.
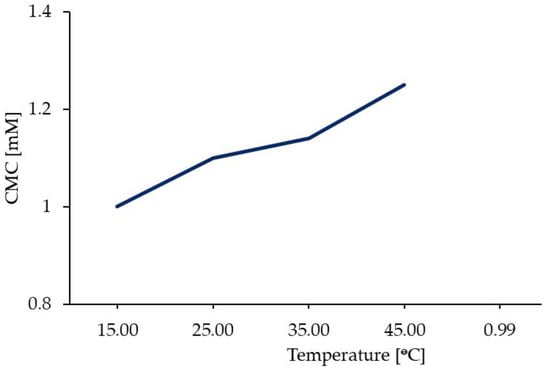
Figure 6.
Plot of CMC versus temperature for 12-6-12.
The CMC values depend on the measurement method. The CMC values of 12-6-12 obtained by conductivity, fluorescence, and microcalorimetric measurements were 1.01, 1.09, and 0.89 mM, respectively [52]. Notably, the main unit responsible for the surface properties of 12-6-12 is the ammonium dication; the counterion is only of secondary importance (Table 5) [23]. Sulphates and nitrates have the lowest CMC values in this case, while bromide is the most active among the halides. Bromides can also be easily obtained via the SN2 reaction, without the need for ion-exchange columns [52].

Table 5.
CMCs of different anions of 12-6-12.
12-6-12 forms micelles with a relatively small number of molecules. Wang et al. reported that 12-6-12 formed spherical micelles with 22 molecules [52]. This is in good agreement with the literature value [53]. Using small-angle neutron scattering (SANS) measurements, Burrows et al. confirmed that the radius of the spherical aggregates of 12-6-12 ranged from 1.74 ± 0.04 to 1.86 ± 0.04 nm [54].
Gemini surfactants undergo morphological transitions with increasing concentration, with the micelles changing from a spherical to an elongated shape. The concentration at which this morphological transition occurs is referred to as the second CMC and was reported to be 0.028 M for 12-6-12 by Graciani et al. [55]. At concentrations above the CMC, gemini surfactants tend to self-associate in water to form micelles whose characteristics depend on the nature of the surfactant as well as on the temperature. Additives affect the self-aggregation process and the features of the aggregates thus formed. This occurs owing to the variation in the chemical potential of the surfactant molecules in the bulk phase as well as in the micelles. The magnitude of an effect depends on the nature of the additive [55]. Currently, mixed micelles have become increasingly popular. These binary systems have been described for 12-6-12 with nonionic and zwitterionic surfactants [22,48,56].
4.2. Antimicrobial Properties
Gemini surfactants possess a broad spectrum of biocidal activity. The biocidal activity is initiated with the adsorption of quaternary ammonium cations on the negatively charged cell surface. Subsequently, long hydrocarbon chains diffuse through the bilayer of the cell, which increases the hydrophobicity of the bacterial cell membrane and triggers the disruption of the cytoplasmic membrane. Because of the damaged membrane, potassium ions and other low-molecular-weight cytoplasmic constituents are released, finally leading to the death of the microorganism cell. The biocidal activity of a microbiocide is usually determined by its MIC, that is, the minimal microbiocide concentration that inhibits the growth of microorganisms. MIC values are affected by several factors, such as the structure and concentration of microbiocide, time of contact, pH, temperature, and the presence of organic matter or other compounds [7,57,58,59,60]. The antimicrobial activity of gemini surfactants depends primarily on the length of the hydrocarbon substituent. Compounds with a dodecyl substituent are found to be the most effective microbicides [61,62]. The biocidal effectiveness of gemini surfactants depends on the type of microorganism. Gram-positive bacteria are more sensitive than Gram-negative bacteria. In general, the sensitivity of microorganisms to gemini surfactants decreases in the following order: Gram-positive bacteria > fungi > Gram-negative bacteria [7].
Sidenko et al. were the first to demonstrate the antimicrobial activity of 12-6-12 [21]. They found that 12-6-12 showed antibacterial effects at concentrations of 0.025% at 10 and 15 min and 0.01% at 20 and 25 min for S. aureus and E. coli, respectively [21]. Devinsky et al. studied the activity of gemini surfactants with different hydrocarbon chain lengths and found that the most effective microbicide was the one with a dodecyl substituent [63]. Ciganekowa et al. found 12-6-12 to be as effective as the commercially used disinfectants against different strains of Clostridium [64]. Many studies show that gemini surfactants have better antimicrobial activity than their monomeric analogues (Table 6) [32,65,66]. It is also worth noting that 12-6-12 is effective against planktonic forms at a very low concentration of 1.0145 mM, while it is effective at eradicating biofilms at a concentration of 0.29 mM [67]. Using scanning electron microscopy, Zhang et al. showed that this microbiome interacted with the bacterial cell membrane, disrupting the membrane integrity and ultimately killing the bacteria [32].

Table 6.
Antibacterial activity of DTAB and 12-6-12.
Gemini surfactants are also known to exhibit very high antifungal activity against yeasts and moulds in conidia and vegetative cells (Table 7) [7]. The MIC of 12-6-12 was 30 times lower than that of the single-chain analogue [65]. The mechanism of the antifungal action of 12-6-12 was studied by Koziróg et al. [68].

Table 7.
Antifungal activity of 12-6-12.
The antimicrobial activity of gemini surfactants against algae [70] and protozoa [39,71] has also been studied. Calas et al. showed that 12-6-12 inhibited the phospholipid metabolism of Plasmodium falciparum and exhibited good antimalarial activity. They reported the IC50 values of 12-6-12 and DTAB to be 0.22 and 0.5 µM, respectively [39].
Recent studies suggest that gemini surfactants exhibit antiviral activity. Khodsiani et al. reported that 12-6-12 and other gemini surfactants with long hydrocarbon chains showed the highest antiviral activity against influenza virus H1N1. This kind of compound may physically interact with hemagglutinin, a glycoprotein on the virus surface, at any dilution, indicating the ability of the compounds to inhibit viral attachment to the cell and the subsequent viral propagation. Apoptotic evaluation of the gemini surfactants highlighted their anti-apoptotic potential, especially for hydrophobic compounds [72].
4.3. Anticorrosion Properties
Recently, considerable attention has been paid to the use of gemini surfactants as corrosion inhibitors for metals and alloys. The mechanism of corrosion inhibition is based on the adsorption of the surfactant molecules onto the metal surface by displacing water molecules and the subsequent formation of a protective film. The mechanism can involve physical adsorption (electrostatic interaction), chemical adsorption (donor–acceptor interaction), or mixed adsorption [10]. The adsorption mechanism of 12-6-12 on a steel surface in an acidic medium depends on the surfactant concentration. The first phase for monolayer adsorption is formed below CMC, and the second phase for multilayer adsorption is formed at concentrations greater than CMC (Figure 7) [73].
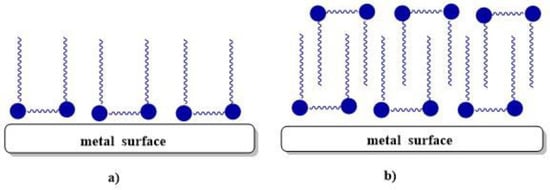
Figure 7.
Adsorption model of 12-6-12 onto metal surface in acidic medium at concentrations (a) below CMC and (b) above CMC [73].
12-6-12 shows excellent anticorrosion activity in acidic and salty media (Table 8). The anticorrosion activity is the highest and most effective at a concentration close to CMC [42,74].

Table 8.
Parameters in the absence and presence of different concentrations of 12-6-12 after immersion for 24 h (corrosion rate = CR; inhibition efficiency = IE).
The strong ability of 12-6-12 to adsorb onto a steel surface is responsible for its high anticorrosion activity. Analogous relationships have been demonstrated for metallic surfaces such as zinc surfaces [75].
12-6-12 exhibits antibacterial activity against D. salexigens and can act as a biocorrosion inhibitor even at low concentrations. After 12 days, the corrosion resistance was remarkably greater than that without an inhibitor, and no significant increase in sulfate-reducing bacteria was observed. The MIC against D. salexigens was 0.018 mM. An open-circuit potential experiment showed that this compound is an efficient biocide and corrosion inhibitor [74].
4.4. Interaction with Macromolecules
Gemini surfactants, as compounds containing ammonium cations, can interact with oppositely charged compounds and surfaces. Studies have been conducted on the adsorption of 12-6-12 on many non-organic surfaces, such as the silica–aqueous solution interface [53,76]; hydrophobised, hydrophilised, and untreated gold [24]; and a large group of aluminosilicate minerals [77,78]. The gemini surfactant can be used to tune the textual properties of zeolites or to endow biological properties to a material [78].
Mixtures of surfactants and polymers can be used to endow improved properties or novel functions that cannot be achieved using surfactants or polymers alone. Consequently, such mixtures have many practical applications, such as in paint and coating products, food processing, personal care formulations, enhanced oil recovery, and pharmaceutical formulations [79,80,81,82,83]. According to Han et al., the co-assembly of the poly(ethylene glycol)-b-poly-(glutamate sodium) copolymer with 12-6-12 leads to the formation of ordered nanosheets with a sandwich-like packing and an average size of 68 nm, exhibiting properties like those of superamphiphiles. The gemini molecules associate through hydrophobic interactions and constitute the middle part of the nanosheets, whereas the top and bottom of the nanosheets comprise hydrophilic polymer chains [79].
Gemini surfactants can interact effectively with biological macromolecules such as DNA [15]. This interaction must be strong enough to overcome the biological membrane barrier and weak enough to release DNA at the right location in the cell. The gemini surfactant is shown to bind and compact DNA efficiently and form a “lipoplex”. Lipoplexes can penetrate the outer membranes of many cell types to enter into the cytoplasm encapsulated within endosomes. The escape from the endosome may be controlled by changes in the aggregation behaviour of the lipoplex as the pH decreases. DNAs may be released from lipoplexes before their entry into the nucleus, where a new gene can be expressed with high efficiency [7,23]. Pisárčik and Devínsky studied the binding of gemini surfactants to DNA and found that the adsorption of DNA on 12-6-12 was the weakest, with only 7% of the surfactant molecules adsorbed [40,84]. Gemini surfactants can also interact strongly with proteins such as bovine serum albumin via electrostatic and hydrophobic forces [85,86].
5. Toxicity and Environmental Impact
Generally, amphiphiles are known to influence the organisation of lipid membranes, and surfactants have been extensively studied in systems involving interactions with lipid membranes [87]. Above a certain concentration, these compounds may exhibit undesirable properties, including toxicity. Therefore, it is very important to study the effects of substances on cells and living organisms before introducing them for use. Toxicity and CMC have been linked to gemini surfactants. Above a certain concentration, the toxicity of these compounds increases. It has also been shown that gemini surfactants are less toxic than their single-chain analogues [88].
Almeida et al. studied the cytotoxicity of gemini surfactants and suggested that the toxicity increases with increasing spacer length and that surfactants with longer tails are less toxic than those with shorter tails [87]. They chose the NCTC 2544 cell line, a human skin keratinocyte cell line, as a model of skin irritation. After 24 h of exposure to low concentrations of 12-6-12 (up to 10 mM), no significant cytotoxicity was observed in the cell line. However, at a 12-6-12 concentration of 50 mM, strong toxicity was observed [87]. The cytotoxicity and skin irritation profiles of 12-6-12 were also studied by Silva et al. [89]. These studies were performed in cultured human epidermal keratinocytes and human dermal fibroblasts. The skin integrity evaluation studies did not indicate relevant changes in the skin structure after the use of 12-6-12, while cytotoxicity studies established a relative cytotoxicity [89].
Koziróg and Brycki tested the haemolytic activity of 12-6-12 and DTAB in terms of the MIC against the morphotic elements of sheep blood [65]. 12-6-12 did not exhibit haemolytic activity at the highest MIC (0.029 μM/mL). A two-fold increase in the concentration lysed the erythrocytes slightly. Considerable disintegration of the erythrocyte membranes was observed at a 12-6-12 concentration of 0.58 μM/mL. DTAB, at the highest MIC of 1.01 μM/mL, caused slight haemolysis. In samples with 2.02 μM/mL of DTAB, a high degree (60%) of haemolysis was observed [65]. Thus, 12-6-12 shows lower haemotoxicity at the used concentration than its monomeric analogue. However, considering the reduced cytotoxicity of 12-6-12 compared to DTAB and that its required concentrations are many times lower than those of DTAB in order to obtain the same effect, its environmental impact will be much less than that of monomeric cationic surfactants.
Zhang et al. studied the cytotoxicity of gemini surfactants against a rat glioma cell line (C6) and human kidney cell line (HEK293) [32]. They presented the cytotoxic effect in terms of the IC50 values, which were 5.1 and 3.3 µM for C6 and HEK293, respectively [32]. Thus, 12-6-12 has potential applications in the medical field.
Research on the ecotoxicity of 12-6-12 and other gemini surfactants is sparse. Generally, these compounds are not readily biodegradable [90]. However, their degree of biodegradation can be increased using bacteria immobilised on alginate [91]. The degree of biodegradation of 12-6-12 determined by the CO2 headspace test was 0% [88]. Garcia et al. studied the aquatoxicity of 12-6-12 and DTAB against D. magna, and the IC50 values were 0.65 and 0.38 mg/L, respectively [88]. This confirms the previous conclusion that gemini surfactants are less toxic to aquatic organisms than their monomeric analogues.
6. Applications
Surfactants are ubiquitous, being key components in a diverse range of complex industrial processes and utilitarian products such as dispersants, solubilisers, emulsifiers, demulsifiers, foaming agents, wetting agents, disinfectants, corrosion inhibitors, antistatic agents, and viscosity modifiers. In the last few decades, significant efforts have been made for the synthesis of new gemini surfactants, fuelled by their remarkably improved physicochemical properties that can be achieved by the modification of structural factors [92]. Cationic gemini surfactants have wide applications due to their excellent surface activity [7,20]:
- in foaming, agrichemical spreading aids, and cleaning;
- from industrial to personal care applications;
- phase transfer catalyst;
- bleach activator;
- as hair conditioners and fabric softeners.
12-6-12 was first described 55 years ago, but the increased application interest of this compound can be defined in the 21st century. 12-6-12 can be used in several practical applications (Figure 8).
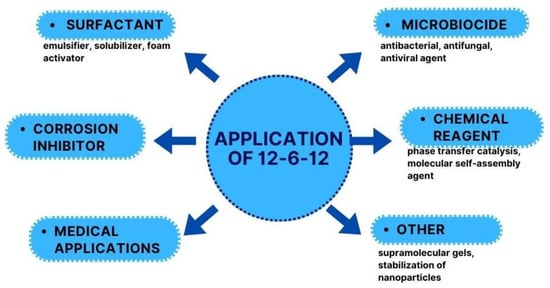
Figure 8.
Applications of 12-6-12.
12-6-12 is characterised by excellent surface, antimicrobial, and anticorrosion properties. As a compound that can be obtained economically and ecologically, it is an ideal product that has many industrial applications, including bioapplications (Table 9).

Table 9.
Potential bioapplications of 12-6-12.
Moreover, 12-6-12 can be used in chemical synthesis for the preparation of supramolecular gels [101] and as a nanoparticle stabiliser [28,41,102], an interfacial transfer catalyst [30,31,103,104,105], and a molecular self-assembly agent [106].
In conclusion, quaternary ammonium gemini surfactants are known for their multifunctional utility properties such as antimicrobial, surfactant, and anticorrosion properties. 12-6-12 has all the above mentioned properties of gemini surfactants and can be obtained in a one-step reaction, which is an indisputable and economically justified advantage over other gemini surfactants. Compared to its monochain counterpart, it shows better surface, antimicrobial, and anticorrosion activity and is characterised by a lower toxicity. The use of this compound in concentrations much lower than DTAB while providing the same utility effect makes 12-6-12 a candidate in many applications. Initially, this compound was used in applications as typical quaternary ammonium salts as an interfacial transfer catalyst. Currently, this compound finds potential applications in many different areas of life. Particularly noteworthy is the testing of this compound in biomedical applications. Due to its anticorrosive, biocidal, dispersing, and detergent properties, 12-6-12 can be used as an additive to car fuels and as an anticorrosion and antimicrobial agent in oil pipelines, smart anticorrosion coatings, etc. Hence, the possibility of producing it in large quantities in a one-step, waste-free synthesis is a huge advantage over other gemini surfactants.
Author Contributions
Conceptualization, B.B. and A.S.; methodology, I.K. and J.B.; writing—original draft preparation, A.S.; writing—review and editing, B.B., I.K. and J.B.; supervision, B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
A sample of the compound is not available from the authors.
References
- Roy, A. A Review on the Biosurfactants: Properties, Types and its Applications. J. Fundam. Renew. Energy Appl. 2018, 8, 248. [Google Scholar] [CrossRef]
- Gayathiri, E.; Prakash, P.; Karmegam, N.; Varjani, S.; Awasthi, M.K.; Ravindran, B. Biosurfactants: Potential and Eco-Friendly Material for Sustainable Agriculture and Environmental Safety—A Review. Agronomy 2022, 12, 662. [Google Scholar] [CrossRef]
- Karnwal, A.; Shrivastava, S.; Al-Tawaha, A.R.M.S.; Kumar, G.; Singh, R.; Kumar, A.; Mohan, A.; Yogita; Malik, T. Microbial Biosurfactant as an Alternate to Chemical Surfactants for Application in Cosmetics Industries in Personal and Skin Care Products: A Critical Review. BioMed Res. Int. 2023, 2023, 2375223. [Google Scholar] [CrossRef]
- Eras-Muñoz, E.; Farré, A.; Sánchez, A.; Font, X.; Gea, T. Microbial biosurfactants: A review of recent environmental applications. Bioengineered 2022, 13, 12365–12391. [Google Scholar] [CrossRef]
- Global Biosurfactants Market: By Type: Glycolipids, Lipopeptides, Fatty Acids, Polymerics, Others; By Application: Emulsifiers, Humectants, Preserving Agents, By End Use: Detergents, Personal Care, Food Processing, Agrochemicals; Regional Analysis; Competitive Landscape; Key Trends and Developments in the Market; 2023–2028. Available online: https://www.expertmarketresearch.com/reports/biosurfactants-market (accessed on 29 August 2023).
- Surfactants Market—Global Industry Assessment & Forecast. Available online: https://www.vantagemarketresearch.com/industry-report/surfactants-market-1671 (accessed on 29 August 2023).
- Brycki, B.E.; Kowalczyk, I.H.; Szulc, A.; Kaczerewska, O.; Pakiet, M. Multifunctional Gemini Surfactants: Structure, Synthesis, Properties and Applications. In Application and Characterization of Surfactants; Najjar, R., Ed.; InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-3325-4. [Google Scholar]
- Menger, F.M.; Littau, C.A. Gemini-surfactants: Synthesis and properties. J. Am. Chem. Soc. 1991, 113, 1451–1452. [Google Scholar] [CrossRef]
- Menger, F.M.; Keiper, J.S. Gemini Surfactants. Angew. Chem. Int. Ed. 2000, 39, 1906–1920. [Google Scholar] [CrossRef]
- Brycki, B.; Szulc, A. Gemini surfactants as corrosion inhibitors. A review. J. Mol. Liq. 2021, 344, 117686. [Google Scholar] [CrossRef]
- Li, D.; Fang, W.; Feng, Y.; Geng, Q.; Song, M. Stability properties of water-based gold and silver nanofluids stabilized by cationic gemini surfactants. J. Taiwan Inst. Chem. Eng. 2019, 97, 458–465. [Google Scholar] [CrossRef]
- Feizi, N.; Yamini, Y.; Moradi, M.; Karimi, M.; Salamat, Q.; Amanzadeh, H. A new generation of nano-structured supramolecular solvents based on propanol/gemini surfactant for liquid phase microextraction. Anal. Chim. Acta 2017, 953, 1–9. [Google Scholar] [CrossRef]
- Yadav, S.K.; Parikh, K.; Kumar, S. Solubilization potentials of single and mixed oppositely charged gemini surfactants: A case of polycyclic aromatic hydrocarbons. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 514, 47–55. [Google Scholar] [CrossRef]
- Ghosh, K.K.; Kolay, S.; Bal, S.; Satnami, M.L.; Quagliotto, P.; Dafonte, P.R. Effect of cationic gemini surfactants on the hydrolysis of carboxylate and phosphate esters using hydroxamate ions. Colloid Polym. Sci. 2008, 286, 293–303. [Google Scholar] [CrossRef]
- Kirby, A.J.; Camilleri, P.; Engberts, J.B.F.N.; Feiters, M.C.; Nolte, R.J.M.; Söderman, O.; Bergsma, M.; Bell, P.C.; Fielden, M.L.; García Rodríguez, C.L.; et al. Gemini Surfactants: New Synthetic Vectors for Gene Transfection. Angew. Chem. Int. Ed. 2003, 42, 1448–1457. [Google Scholar] [CrossRef]
- Akram, M.; Ansari, F.; Bhat, I.A.; Din, K.U. Probing interaction of bovine serum albumin (BSA) with the biodegradable version of cationic gemini surfactants. J. Mol. Liq. 2019, 276, 519–528. [Google Scholar] [CrossRef]
- Akram, M.; Ansari, F.; Bhat, I.A.; Chaturvedi, S.K.; Khan, R.H.; Din, K.U. Analyzing the interaction between porcine serum albumin (PSA) and ester-functionalized cationic gemini surfactants. Process Biochem. 2017, 63, 145–153. [Google Scholar] [CrossRef]
- Ahmady, A.R.; Hosseinzadeh, P.; Solouk, A.; Akbari, S.; Szulc, A.M.; Brycki, B.E. Cationic gemini surfactant properties, its potential as a promising bioapplication candidate, and strategies for improving its biocompatibility: A review. Adv. Colloid Interface Sci. 2022, 299, 102581. [Google Scholar] [CrossRef] [PubMed]
- Mirgorodskaya, A.B.; Ya Zakharova, L.; Khairutdinova, E.I.; Lukashenko, S.S.; Sinyashin, O.G. Supramolecular systems based on gemini surfactants for enhancing solubility of spectral probes and drugs in aqueous solution. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 510, 33–42. [Google Scholar] [CrossRef]
- Kumar, N.; Tyagi, R. Industrial Applications of Dimeric Surfactants: A Review. J. Dispers. Sci. Technol. 2014, 35, 205–214. [Google Scholar] [CrossRef]
- Sidenko, Z.S.; Limanov, V.E.; Skvortsova, E.K.; Dziomko, V.M. Synthesis and antibacterial activity of several bisammonium compounds. Pharm. Chem. J. 1968, 2, 247–250. [Google Scholar] [CrossRef]
- McLachlan, A.; Singh, K.; Piggott, E.; McAlduff, M.; MacLennan, S.; Sandre, V.; Reid, T.; Marangoni, D.G. m-s-m Cationic Gemini and Zwitterionic Surfactants–Spacer-Dependent Synergistic Interactions. J. Phys. Chem. B 2019, 123, 1855–1868. [Google Scholar] [CrossRef]
- Jiang, N.; Li, P.; Wang, Y.; Wang, J.; Yan, H.; Thomas, R.K. Micellization of Cationic Gemini Surfactants with Various Counterions and Their Interaction with DNA in Aqueous Solution. J. Phys. Chem. B 2004, 108, 15385–15391. [Google Scholar] [CrossRef]
- Mivehi, L.; Bordes, R.; Holmberg, K. Adsorption of Cationic Gemini Surfactants at Solid Surfaces Studied by QCM-D and SPR: Effect of the Rigidity of the Spacer. Langmuir 2011, 27, 7549–7557. [Google Scholar] [CrossRef]
- Zana, R.; Benrraou, M.; Rueff, R. Alkanediyl-.alpha.,.omega.-bis(dimethylalkylammonium bromide) surfactants. 1. Effect of the spacer chain length on the critical micelle concentration and micelle ionization degree. Langmuir 1991, 7, 1072–1075. [Google Scholar] [CrossRef]
- Szymaniak, D.; Maćkowiak, A.; Ciarka, K.; Praczyk, T.; Marcinkowska, K.; Pernak, J. Synthesis and Characterization of Double-Salt Herbicidal Ionic Liquids Comprising both 4-Chloro-2-methylphenoxyacetate and trans-Cinnamate Anions. ChemPlusChem 2020, 85, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Junior, P.B.S.; Tiera, V.A.O.; Tiera, M.J. A fluorescence probe study of gemini surfactants in aqueous solution: A comparison between n-2-n and n-6-n series of the alkanediyl-α,ω-bis (dimethylalkylammonium bromides). Eclet. Quím. 2007, 32, 47–54. [Google Scholar] [CrossRef]
- Takács, D.; Péter, T.; Vargáné Árok, Z.; Katana, B.; Papović, S.; Gadzuric, S.; Vraneš, M.; Szilágyi, I. Structure–Stability Relationship in Aqueous Colloids of Latex Particles and Gemini Surfactants. J. Phys. Chem. B 2022, 126, 9095–9104. [Google Scholar] [CrossRef] [PubMed]
- Imam, T.; Devinsky, F.; Lacko, I.; Mlynarčík, D.; Krasnec, L. Preparation and antimicrobial activity of some new bisquaternary ammonium salts. Pharmazie 1983, 38, 308–310. [Google Scholar] [CrossRef]
- Esen, I.; Yolacan, C.; Aydogan, F. Long Chain Dicationic Phase Transfer Catalysts in the Condensation Reactions of Aromatic Aldehydes in Water Under Ultrasonic Effect. Bull. Korean Chem. Soc. 2010, 31, 2289–2292. [Google Scholar] [CrossRef]
- Öge, A.; MaviŞ, M.E.; Yolaçan, Ç.; Aydoğan, F. Solvent-free Michael addition of 2-cyclohexenone under ultrasonic irradiation in the presence of long chain dicationic ammonium salts. Turk. J. Chem. 2012, 36, 137–146. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, S.; Yu, J.; Chen, X.; Lei, Q.; Fang, W. Antibacterial Activity, in Vitro Cytotoxicity, and Cell Cycle Arrest of Gemini Quaternary Ammonium Surfactants. Langmuir 2015, 31, 12161–12169. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.-C.; Zhang, J.-Z.; Chai, S.-G. Resonance light scattering method for the determination of DNA with cationic methacrylate based polymer nanoparticle probes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 82, 437–443. [Google Scholar] [CrossRef]
- Lee, H.I.; Pak, C.; Yi, S.H.; Shon, J.K.; Kim, S.S.; So, B.G.; Chang, H.; Yie, J.E.; Kwon, Y.-U.; Kim, J.M. Systematic phase control of periodic mesoporous organosilicas using Gemini surfactants. J. Mater. Chem. 2005, 15, 4711. [Google Scholar] [CrossRef]
- Brycki, B.; Kowalczyk, I.; Kozirog, A. Synthesis, Molecular Structure, Spectral Properties and Antifungal Activity of Polymethylene-α,ω-bis(N,N-dimethyl-N-dodecyloammonium Bromides). Molecules 2011, 16, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, W.; Zhou, L.; Wang, J.; Liu, L.; Wang, S.; Wang, Y. Soft Particles of Gemini Surfactant/Conjugated Polymer for Enhanced Anticancer Activity of Chemotherapeutics. ACS Appl. Mater. Interfaces 2018, 10, 37–41. [Google Scholar] [CrossRef]
- Singer, O.M.; Campbell, J.W.; Hoare, J.G.; Masuda, J.D.; Marangoni, G.; Singer, R.D. Improved Green Synthesis and Crystal Structures of Symmetrical Cationic Gemini Surfactants. ACS Omega 2022, 7, 35326–35330. [Google Scholar] [CrossRef] [PubMed]
- Brycki, B.; Drgas, M.; Bielawska, M.; Zdziennicka, A.; Jańczuk, B. Synthesis, spectroscopic studies, aggregation and surface behavior of hexamethylene-1,6-bis(N,N-dimethyl-N-dodecylammonium bromide). J. Mol. Liq. 2016, 221, 1086–1096. [Google Scholar] [CrossRef]
- Calas, M.; Ancelin, M.L.; Cordina, G.; Portefaix, P.; Piquet, G.; Vidal-Sailhan, V.; Vial, H. Antimalarial Activity of Compounds Interfering with Plasmodium falciparum Phospholipid Metabolism: Comparison between Mono- and Bisquaternary Ammonium Salts. J. Med. Chem. 2000, 43, 505–516. [Google Scholar] [CrossRef]
- Devinsky, F.; Pisarcik, M.; Lacko, I. Hydrodynamic size of DNA/cationic gemini surfactant complex as a function of surfactant structure. Gen. Physiol. Biophys. 2009, 28, 160–167. [Google Scholar] [CrossRef]
- Pisárčik, M.; Jampílek, J.; Lukáč, M.; Horáková, R.; Devínsky, F.; Bukovský, M.; Kalina, M.; Tkacz, J.; Opravil, T. Silver Nanoparticles Stabilised by Cationic Gemini Surfactants with Variable Spacer Length. Molecules 2017, 22, 1794. [Google Scholar] [CrossRef]
- Kaczerewska, O.; Leiva-Garcia, R.; Akid, R.; Brycki, B.; Kowalczyk, I.; Pospieszny, T. Heteroatoms and π electrons as favorable factors for efficient corrosion protection. Mater. Corros. 2019, 70, 1099–1110. [Google Scholar] [CrossRef]
- Aubagnac, J.-L.; Gilles, I.; Calas, M.; Cordina, G.; Piquet, G.; Portefaix, P.; Giral, L. Fast atom bombardment, frit fast atom bombardment and electrospray ionization mass spectrometric study of organic salts C2+ 2X−: Matrix and anion effects. J. Mass Spectrom. 1995, 30, 985–992. [Google Scholar] [CrossRef]
- Buse, J.; Badea, I.; Verrall, R.E.; El-Aneed, A. Tandem Mass Spectrometric Analysis of the Novel Gemini Surfactant Nanoparticle Families G12-s and G18:1-s. Spectrosc. Lett. 2010, 43, 447–457. [Google Scholar] [CrossRef]
- Woch, J.; Iłowska, J.; Hordyjewicz-Baran, Z.; Arabasz, S.; Kaczmarczyk, B.; Grabowski, R.; Libera, M.; Dworak, A.; Trzebicka, B. Aqueous solution behaviour and solubilisation properties of octadecyl cationic gemini surfactants and their comparison with their amide gemini analogues. Soft Matter 2018, 14, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Akbaş, H.; Elemenli, A.; Boz, M. Aggregation and Thermodynamic Properties of Some Cationic Gemini Surfactants. J. Surfactants Deterg. 2012, 15, 33–40. [Google Scholar] [CrossRef]
- Alami, E.; Beinert, G.; Marie, P.; Zana, R. Alkanediyl-α,ω-bis(dimethylalkylammonium bromide) surfactants. 3. Behavior at the air-water interface. Langmuir 1993, 9, 1465–1467. [Google Scholar] [CrossRef]
- Bakshi, M.S.; Kaur, G.; Yoshimura, T.; Esumi, K. Azeotropic mixing of C16peLac with different surfactants of monomeric and dimeric nature under the effect of temperature. Colloids Surf. A Physicochem. Eng. Asp. 2006, 281, 163–170. [Google Scholar] [CrossRef]
- Kuperkar, K.; Modi, J.; Patel, K. Surface-Active Properties and Antimicrobial Study of Conventional Cationic and Synthesized Symmetrical Gemini Surfactants. J. Surfactants Deterg. 2012, 15, 107–115. [Google Scholar] [CrossRef]
- Garcia, M.T.; Kaczerewska, O.; Ribosa, I.; Brycki, B.; Materna, P.; Drgas, M. Hydrophilicity and flexibility of the spacer as critical parameters on the aggregation behavior of long alkyl chain cationic gemini surfactants in aqueous solution. J. Mol. Liq. 2017, 230, 453–460. [Google Scholar] [CrossRef]
- Chavda, S.; Kuperkar, K.; Bahadur, P. Formation and Growth of Gemini Surfactant (12- s -12) Micelles as a Modulate by Spacers: A Thermodynamic and Small-Angle Neutron Scattering (SANS) Study. J. Chem. Eng. Data 2011, 56, 2647–2654. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Wang, Y.; Ye, J.; Yan, H.; Thomas, R.K. Micellization of a Series of Dissymmetric Gemini Surfactants in Aqueous Solution. J. Phys. Chem. B 2003, 107, 11428–11432. [Google Scholar] [CrossRef]
- Atkin, R.; Craig, V.S.J.; Wanless, E.J.; Biggs, S. Adsorption of 12- s -12 Gemini Surfactants at the Silica−Aqueous Solution Interface. J. Phys. Chem. B 2003, 107, 2978–2985. [Google Scholar] [CrossRef]
- Burrows, H.D.; Tapia, M.J.; Silva, C.L.; Pais, A.A.C.C.; Fonseca, S.M.; Pina, J.; Seixas de Melo, J.; Wang, Y.; Marques, E.F.; Knaapila, M.; et al. Interplay of Electrostatic and Hydrophobic Effects with Binding of Cationic Gemini Surfactants and a Conjugated Polyanion: Experimental and Molecular Modeling Studies. J. Phys. Chem. B 2007, 111, 4401–4410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Graciani, M.M.; Rodríguez, A.; Martín, V.I.; Fernández, G.; Moyá, M.L. Concentration and Medium Micellar Kinetic Effects Caused by Morphological Transitions. Langmuir 2010, 26, 18659–18668. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, A.; Singh, K.; McAlduff, M.; Marangoni, D.G.; Shortall, S.; Wettig, S.D. m-s-m cationic gemini and zwitterionic surfactants—A thermodynamic analysis of their mixed micelle formation. RSC Adv. 2020, 10, 3221–3232. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Yuan, H.; Yin, J.; Hu, M. Antibacterial Mechanism of Octamethylene-1,8-Bis(Dodecyldimethylammonium Bromide) Against E. coli. J. Surfactants Deterg. 2017, 20, 717–723. [Google Scholar] [CrossRef]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary Ammonium Compounds: An Antimicrobial Mainstay and Platform for Innovation to Address Bacterial Resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.A.; Guastavino, J.F.; Nicollier, R.A.; Lancelle, M.V.; Russell-White, K.; Murguía, M.C. Synthesis and Properties of Cleavable Quaternary Ammonium Compounds. J. Oleo Sci. 2021, 70, 59–65. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y. Structure–activity relationship of cationic surfactants as antimicrobial agents. Curr. Opin. Colloid Interface Sci. 2020, 45, 28–43. [Google Scholar] [CrossRef]
- Balgavý, P.; Devinsky, F. Cut-off effects in biological activities of surfactants. Adv. Colloid Interface Sci. 1996, 66, 23–63. [Google Scholar] [CrossRef]
- Devinsky, F.; Lacko, I.; Mlynarčík, D.; Racansky, V.; Krasnec, L. Relationship between critical micelle concentration and minimum inhibitory concentration for some non-aromatic quaternary ammonium salts and amine oxides. Tenside Surfactants Deterg. 1985, 22, 10–15. [Google Scholar] [CrossRef]
- Ciganekova, V.; Kallova, J.; Devinsky, F.; Lacko, I. Effect of N,N’-Bis(alkyldimethyl)- -α,ω-alkanediammonium Dibromides on Bacteria of the Genus Clostridium. Folia Microbiol. 1989, 34, 202–208. [Google Scholar] [CrossRef]
- Koziróg, A.; Brycki, B. Monomeric and gemini surfactants as antimicrobial agents—Influence on environmental and reference strains. Acta Biochim. Pol. 2015, 62, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, A.; Kręgiel, D.; Brycki, B. Action of Monomeric/Gemini Surfactants on Free Cells and Biofilm of Asaia lannensis. Molecules 2017, 22, 2036. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, A.; Otlewska, A.; Brycki, B. Viability, Enzymatic and Protein Profiles of Pseudomonas aeruginosa Biofilm and Planktonic Cells after Monomeric/Gemini Surfactant Treatment. Molecules 2018, 23, 1294. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, A.; Otlewska, A.; Gapińska, M.; Michlewska, S. Influence of Gemini Surfactants on Biochemical Profile and Ultrastructure of Aspergillus brasiliensis. Appl. Sci. 2019, 9, 245. [Google Scholar] [CrossRef]
- Koziróg, A.; Brycki, B.; Pielech-Przybylska, K. Impact of Cationic and Neutral Gemini Surfactants on Conidia and Hyphal Forms of Aspergillus brasiliensis. IJMS 2018, 19, 873. [Google Scholar] [CrossRef] [PubMed]
- Winnicki, K.; Łudzik, K.; Żabka, A.; Polit, J.T.; Zawisza, A.; Maszewski, J. Anti-algal activity of the 12-5-12 gemini surfactant results from its impact on the photosynthetic apparatus. Sci. Rep. 2021, 11, 2360. [Google Scholar] [CrossRef] [PubMed]
- Ancelin, M.L.; Calas, M.; Bonhoure, A.; Herbute, S.; Vial, H.J. In Vivo Antimalarial Activities of Mono- and Bis Quaternary Ammonium Salts Interfering with Plasmodium Phospholipid Metabolism. Antimicrob. Agents Chemother. 2003, 47, 2598–2605. [Google Scholar] [CrossRef] [PubMed]
- Khodsiani, M.; Kianmehr, Z.; Brycki, B.; Szulc, A.; Mehrbod, P. Evaluation of the antiviral potential of gemini surfactants against influenza virus H1N1. Arch. Microbiol. 2023, 205, 184. [Google Scholar] [CrossRef]
- Qiu, L.-G.; Xie, A.-J.; Shen, Y.-H. Understanding the effect of the spacer length on adsorption of gemini surfactants onto steel surface in acid medium. Appl. Surf. Sci. 2005, 246, 1–5. [Google Scholar] [CrossRef]
- Pakiet, M.; Kowalczyk, I.; Leiva Garcia, R.; Moorcroft, R.; Nichol, T.; Smith, T.; Akid, R.; Brycki, B. Gemini surfactant as multifunctional corrosion and biocorrosion inhibitors for mild steel. Bioelectrochemistry 2019, 128, 252–262. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, J. Adsorption of quaternary ammonium gemini surfactants on zinc and the inhibitive effect on zinc corrosion in vitriolic solution. Colloids Surf. A 2006, 278, 246–251. [Google Scholar] [CrossRef]
- Chorro, C.; Chorro, M.; Dolladille, O.; Partyka, S.; Zana, R. Adsorption of Dimeric (Gemini) Surfactants at the Aqueous Solution/Silica Interface. J. Colloid Interface Sci. 1998, 199, 169–176. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Z.; He, B.; Sun, C.; Li, X.; Wu, X.; An, X.; Xie, X. Gemini quaternary ammonium salt cationic surfactant-assisted hydrothermal synthesis: An effective way to tune the textural properties of zeolite and the acidity of Beta molecular sieves. Appl. Organometal. Chem. 2018, 32, e4145. [Google Scholar] [CrossRef]
- Majchrzycka, K.; Okrasa, M.; Szulc, J.; Brycki, B.; Gutarowska, B. Time-Dependent Antimicrobial Activity of Filtering Nonwovens with Gemini Surfactant-Based Biocides. Molecules 2017, 22, 1620. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, W.; Tang, Y.; Zhang, S.; Li, Z.; Wang, Y. Coassembly of Poly(ethylene glycol)- block -Poly(glutamate sodium) and Gemini Surfactants with Different Spacer Lengths. Langmuir 2013, 29, 9316–9323. [Google Scholar] [CrossRef]
- Pi, Y.; Shang, Y.; Peng, C.; Liu, H.; Hu, Y.; Jiang, J. Phase behavior of gemini surfactant hexylene-1,6-bis(dodecyldimethylammonium bromide) and polyelectrolyte NaPAA. J. Colloid Interface Sci. 2006, 299, 410–415. [Google Scholar] [CrossRef]
- Pi, Y.; Shang, Y.; Liu, H.; Hu, Y.; Jiang, J. Salt effect on the interactions between gemini surfactant and oppositely charged polyelectrolyte in aqueous solution. J. Colloid Interface Sci. 2007, 306, 405–410. [Google Scholar] [CrossRef]
- Wang, R.; Yan, H.; Ma, W.; Li, Y. Complex formation between cationic gemini surfactant and sodium carboxymethylcellulose in the absence and presence of organic salt. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 293–300. [Google Scholar] [CrossRef]
- Wettig, S.D.; Verrall, R.E. Studies of the Interaction of Cationic Gemini Surfactants with Polymers and Triblock Copolymers in Aqueous Solution. J. Colloid Interface Sci. 2001, 244, 377–385. [Google Scholar] [CrossRef]
- Pisárčik, M.; Devínsky, F. Surface tension study of cationic gemini surfactants binding to DNA. Cent. Eur. J. Chem. 2014, 12, 577–585. [Google Scholar] [CrossRef]
- Hu, M.; Wang, X.; Li, L.; He, Y.; Song, G. Mechanism studies on the interaction of Gemini surfactant 12-6-12 with bovine serum albumin by fluorescence method. Anal. Chem. 2011, 10, 817–824. [Google Scholar]
- Kumari, S.; Halder, S.; Aggrawal, R.; Aswal, V.K.; Sundar, G.; Saha, S.K. Refolding of protein unfolded by gemini surfactants using β-cyclodextrin and sodium dodecyl sulfate in aqueous medium: Study on role of spacer chain of surfactants. J. Mol. Liq. 2020, 300, 112238. [Google Scholar] [CrossRef]
- Almeida, J.A.S.; Faneca, H.; Carvalho, R.A.; Marques, E.F.; Pais, A.A.C.C. Dicationic Alkylammonium Bromide Gemini Surfactants. Membrane Perturbation and Skin Irritation. PLoS ONE 2011, 6, e26965. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.T.; Kaczerewska, O.; Ribosa, I.; Brycki, B.; Materna, P.; Drgas, M. Biodegradability and aquatic toxicity of quaternary ammonium-based gemini surfactants: Effect of the spacer on their ecological properties. Chemosphere 2016, 154, 155–160. [Google Scholar] [CrossRef]
- Silva, S.M.C.; Sousa, J.J.S.; Marques, E.F.; Pais, A.A.C.C.; Michniak-Kohn, B.B. Structure Activity Relationships in Alkylammonium C12-Gemini Surfactants Used as Dermal Permeation Enhancers. AAPS J. 2013, 15, 1119–1127. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Holmberg, K.; van Ginkel, C.G.; Kean, M. Cationic gemini surfactants with cleavable spacer: Chemical hydrolysis, biodegradation, and toxicity. J. Colloid Interface Sci. 2015, 449, 72–79. [Google Scholar] [CrossRef]
- Bergero, M.F.; Liffourrena, A.S.; Opizzo, B.A.; Fochesatto, A.S.; Lucchesi, G.I. Immobilization of a microbial consortium on Ca-alginate enhances degradation of cationic surfactants in flasks and bioreactor. Int. Biodeterior. Biodegrad. 2017, 117, 39–44. [Google Scholar] [CrossRef]
- Sharma, R.; Kamal, A.; Abdinejad, M.; Mahajan, R.K.; Kraatz, H.-B. Advances in the synthesis, molecular architectures and potential applications of gemini surfactants. Adv. Colloid Interface Sci. 2017, 248, 35–68. [Google Scholar] [CrossRef]
- Yang, J.; Yun, L.; Zhao, G.; Zhang, F.; Chen, Y.; Wang, C. Fabrication of pH-responsive system based on cationic gemini surfactant/sodium octanedioate and its application on controlled release of paclitaxel. Colloids Surf. A 2018, 539, 101–108. [Google Scholar] [CrossRef]
- Wang, M.; Wu, C.; Tang, Y.; Fan, Y.; Han, Y.; Wang, Y. Interactions of cationic trimeric, gemini and monomeric surfactants with trianionic curcumin in aqueous solution. Soft Matter 2014, 10, 3432. [Google Scholar] [CrossRef]
- Wettig, S.D.; Deubry, R.; Akbar, J.; Kaur, T.; Wang, H.; Sheinin, T.; Joseph, J.W.; Slavcev, R.A. Thermodynamic investigation of the binding of dissymmetric pyrenyl-gemini surfactants to DNA. Phys. Chem. Chem. Phys. 2010, 12, 4821–4826. [Google Scholar] [CrossRef]
- Falsini, S.; Di Cola, E.; In, M.; Giordani, M.; Borocci, S.; Ristori, S. Complexation of short ds RNA/DNA oligonucleotides with Gemini micelles: A time resolved SAXS and computational study. Phys. Chem. Chem. Phys. 2017, 19, 3046–3055. [Google Scholar] [CrossRef]
- Acosta-Martínez, D.R.; Rodríguez-Velázquez, E.; Araiza-Verduzco, F.; Taboada, P.; Prieto, G.; Rivero, I.A.; Pina-Luis, G.; Alatorre-Meda, M. Bis-quaternary ammonium gemini surfactants for gene therapy: Effects of the spacer hydrophobicity on the DNA complexation and biological activity. Colloids Surf. B Biointerfaces 2020, 189, 110817. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, A.; Brycki, B.; Olejnik, K.; Wysocka-Robak, A.; Dębska-Winkler, P. Cellulose products modified with monomeric and gemini surfactants: Antimicrobial aspects. Cellulose 2019, 26, 5559–5570. [Google Scholar] [CrossRef]
- Wietecha, J.; Kopania, E.; Ciechańska, D.; Wiśniewska-Wrona, M.; Gruchała, B.; Data, M.; Brycki, B.; Kowalczyk, I. Kompozycja sanityzująca do wyrobów papierniczych. Patent PL 232698B1, 1 August 2016. [Google Scholar]
- Akbari, S.; Pakpour, S.; Shabanloo, R.; Mirsalehi, M.; Ko, F.; Sadeghzadeh Milani, A.; Brycki, B.; Pyrzyński, K. Active electrospun layer for DNA filteration. Patent WO2022157547A1, 23 January 2021. [Google Scholar]
- Wang, H.; He, L.; Brycki, B.E.; Kowalczyk, I.H.; Kuliszewska, E.; Yang, Y. Electrochemical characterization of the hydrophobic microenvironment within gemini surfactant micellar-hybridized supramolecular gels. Electrochim. Acta 2013, 90, 326–331. [Google Scholar] [CrossRef]
- Gustafsson, H.; Isaksson, S.; Altskär, A.; Holmberg, K. Mesoporous silica nanoparticles with controllable morphology prepared from oil-in-water emulsions. J. Colloid Interface Sci. 2016, 467, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Mirgorodskaya, A.B.; Kudryavtseva, L.A.; Pankratov, V.A.; Lukashenko, S.S.; Rizvanova, L.Z.; Konovalov, A.I. Geminal alkylammonium surfactants: Aggregation properties and catalytic activity. Russ. J. Gen. Chem. 2006, 76, 1625–1631. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Kudryavtseva, L.A. Aqueous solutions of geminal alkylammonium surfactants as a medium for reactions of long-chain amines. Russ. J. Gen. Chem. 2009, 79, 42–48. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Yackevich, E.I.; Valeeva, F.G.; Pankratov, V.A.; Zakharova, L.Y. Solubilizing and catalytic properties of supramolecular systems based on gemini surfactants. Russ. Chem. Bull. 2014, 63, 82–87. [Google Scholar] [CrossRef]
- Deng, S.; Zhao, J.; Wen, Z. Self-Assembly of Quaternary Ammonium Gemini Surfactants in Cyclohexane upon Reinforcement by Simple Counterions. RSC Adv. 2018, 8, 18880–18888. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).