Characteristics of Phenolic Compounds in Peucedanum japonicum According to Various Stem and Seed Colors
Abstract
1. Introduction
2. Results and Discussion
3. Material and Methods
3.1. Plant Materials
3.2. Plant Identification
3.3. Phenological Status
3.4. Seed Collection and Processing
3.5. Instruments and Reagents
3.6. Sample Extraction and Preparation
3.7. Analysis of TPC
3.8. Analysis of TFC
3.9. HPLC Conditions
3.10. HPLC Calibration Curve
3.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sarkhail, P. Traditional Uses, Phytochemistry and Pharmacological Properties of the Genus Peucedanum: A Review. J. Ethnopharmacol. 2014, 156, 235–270. [Google Scholar] [CrossRef] [PubMed]
- Nukitrangsan, N.; Okabe, T.; Toda, T.; Inafuku, M.; Iwasaki, H.; Oku, H. Anti-Obesity Activity of Peucedanum japonicum Thunb Extract in Obese Diabetic Animal Model C57BL/6J Ham Slc-Ob/Ob Mice. Int. J. Life Sci. Med. Res. 2012, 2, 28–34. [Google Scholar] [CrossRef]
- Nam, J.Y.; Ryu, K.S. Pharmacognostical Studies on Korean “Bang Poong”. Korean J. Pharmacogn. 1975, 6, 151–159. [Google Scholar]
- Okabe, T.; Toda, T.; Nukitrangsan, N.; Inafuku, M.; Iwasaki, H.; Oku, H. Peucedanum japonicum Thunb Inhibits High-Fat Diet Induced Obesity in Mice. Phytother. Res. 2011, 25, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.Y.; Nam, S.J.; Ham, J.R.; Lee, H.I.; Yee, S.T.; Kang, K.Y.; Seo, K.I.; Lee, J.H.; Kim, M.J.; Lee, M.K. Anti-Adipogenic and Anti-Diabetic Effects of Cis-3′,4′-Diisovalerylkhellactone Isolated from Peucedanum japonicum Thunb Leaves in Vitro. Bioorg. Med. Chem. Lett. 2016, 26, 4655–4660. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jong, H.S.; Yoon, M.H.; Oh, S.H.; Jung, K.T. Antinociceptive Effect of Intrathecal Sec-O-Glucosylhamaudol on the Formalin-Induced Pain in Rats. Korean J. Pain 2017, 30, 98–103. [Google Scholar] [CrossRef]
- Kim, J.M.; Erkhembaatar, M.; Lee, G.S.; Lee, J.H.; Noh, E.M.; Lee, M.; Song, H.K.; Lee, C.H.; Kwon, K.B.; Kim, M.S.; et al. Peucedanum japonicum Thunb. Ethanol Extract Suppresses RANKL-Mediated Osteoclastogenesis. Exp. Ther. Med. 2017, 14, 410–416. [Google Scholar] [CrossRef]
- Chun, J.M.; Lee, A.R.; Kim, H.S.; Lee, A.Y.; Gu, G.J.; Moon, B.C.; Kwon, B.I. Peucedanum japonicum Extract Attenuates Allergic Airway Inflammation by Inhibiting Th2 Cell Activation and Production of Pro-Inflammatory Mediators. J. Ethnopharmacol. 2018, 211, 78–88. [Google Scholar] [CrossRef]
- Kim, K.N.; Choi, M.J.; Lee, Y.; Cho, S.H. The Protective and Recovery Effects of Peucedanum japonicum Thunberg for Vascular Dementia. J. Orient. Neuropsychiatry 2013, 24, 123–130. [Google Scholar] [CrossRef][Green Version]
- Hisamoto, M.; Kikuzaki, H.; Ohigashi, H.; Nakatani, N. Antioxidant Compounds from the Leaves of Peucedanum japonicum Thunb. J. Agric. Food Chem. 2003, 51, 5255–5261. [Google Scholar] [CrossRef]
- Hisamoto, M.; Kikuzaki, H.; Nakatani, N. Constituents of the Leaves of Peucedanum japonicum Thunb. and Their Biological Activity. J. Agric. Food Chem. 2004, 52, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Han, C.S.; Kim, G.E.; Kim, J.H.; Kim, S.G.; Kim, H.K.; Oh, O.J.; Whang, W.K. Biological Activities of Isolated Compounds from Peucedani Radix. Yakhak Hoeji 2009, 53, 130–137. [Google Scholar]
- Bielli, A.; Scioli, M.G.; Mazzaglia, D.; Doldo, E.; Orlandi, A. Antioxidants and Vascular Health. Life Sci. 2015, 143, 209–216. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse Association between Habitual Polyphenol Intake and Incidence of Cardiovascular Events in the PREDIMED Study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Lee, Y.G.; Kim, H.G.; Yoon, D.; Jeong, J.T.; Lee, D.Y.; Baek, N.I. New Dibenzocyclooctadiene Lignan from Schisandra chinensis (Turcz.) Baill. Fruits. Appl. Biol. Chem. 2021, 64, 46. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Bang, S.I.; Shin, H.N.; Cho, E.J.; Lee, S. Antioxidant Activity of Edible Sprouts and Phytosterol Contents by HPLC/UV Analysis. Hortic. Environ. Biotechnol. 2022, 63, 769–778. [Google Scholar] [CrossRef]
- Zemestani, M.; Rafraf, M.; Asghari-Jafarabadi, M. Chamomile Tea Improves Glycemic Indices and Antioxidants Status in Patients with Type 2 Diabetes Mellitus. Nutrition 2016, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How Do Nutritional Antioxidants Really Work: Nucleophilic Tone and Para-Hormesis versus Free Radical Scavenging in Vivo. Free. Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef]
- Uleberg, E.; Rohloff, J.; Jaakola, L.; Trôst, K.; Junttila, O.; Häggman, H.; Martinussen, I. Effects of Temperature and Photoperiod on Yield and Chemical Composition of Northern and Southern Clones of Bilberry (Vaccinium myrtillus L.). J. Agric. Food Chem. 2012, 60, 10406–10414. [Google Scholar] [CrossRef]
- Hamed, Y.S.; Abdin, M.; Chen, G.; Akhtar, H.M.S.; Zeng, X. Effects of Impregnate Temperature on Extraction of Caffeoylquinic Acid Derivatives from Moringa oleifera Leaves and Evaluation of Inhibitory Activity on Digestive Enzyme, Antioxidant, Anti-Proliferative and Antibacterial Activities of the Extract. Int. J. Food Sci. Technol. 2020, 55, 3082–3090. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Angeloni, S.; Navarini, L.; Angeloni, C.; Freschi, M.; Hrelia, S.; Vitali, L.A.; Sagratini, G.; Vittori, S.; Caprioli, G. Coffee Silverskin Extracts: Quantification of 30 Bioactive Compounds by a New HPLC-MS/MS Method and Evaluation of Their Antioxidant and Antibacterial Activities. Food Res. Int. 2020, 133, 109128. [Google Scholar] [CrossRef]
- Trendafilova, A.; Ivanova, V.; Rangelov, M.; Todorova, M.; Ozek, G.; Yur, S.; Ozek, T.; Aneva, I.; Veleva, R. Caffeoylquinic Acids, Cytotoxic, Antioxidant, Acetylcholinesterase and Tyrosinase Enzyme Inhibitory Activities of Six Inula Species from Bulgaria. Chem. Biodivers. 2020, 17, e2000051. [Google Scholar] [CrossRef]
- Bourgou, S.; Bettaieb Rebey, I.; Mkadmini, K.; Isoda, H.; Ksouri, R.; Ksouri, W.M. LC-ESI-TOF-MS and GC-MS Profiling of Artemisia herba-alba and Evaluation of Its Bioactive Properties. Food Res. Int. 2017, 99, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Bulgakov, V.P.; Vereshchagina, Y.V.; Veremeichik, G.N. Anticancer Polyphenols from Cultured Plant Cells: Production and New Bioengineering Strategies. Curr. Med. Chem. 2018, 25, 4671–4692. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, C.; Mena, P.; Del Rio, D.; Brighenti, F.; Barocelli, E.; Hassan-Mohamed, I.; Callegari, D.; Lodola, A.; Tognolini, M. The Ellagitannin Colonic Metabolite Urolithin D Selectively Inhibits EphA2 Phosphorylation in Prostate Cancer Cells. Mol. Nutr. Food Res. 2015, 59, 2155–2167. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef]
- Murad, L.D.; Soares, N.D.C.P.; Brand, C.; Monteiro, M.C.; Teodoro, A.J. Effects of Caffeic and 5-Caffeoylquinic Acids on Cell Viability and Cellular Uptake in Human Colon Adenocarcinoma Cells. Nutr. Cancer 2015, 67, 532–542. [Google Scholar] [CrossRef]
- Taira, J.; Uehara, M.; Tsuchida, E.; Ohmine, W. Inhibition of the β-Catenin/Tcf Signaling by Caffeoylquinic Acids in Sweet Potato Leaf through Down Regulation of the Tcf-4 Transcription. J. Agric. Food Chem. 2014, 62, 167–172. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Gevrenova, R.; Zaharieva, M.M.; Najdenski, H.; Ruseva, S.; Lozanov, V.; Balabanova, V.; Yagi, S.; Momekov, G.; Mitev, V. HPLC-UV and LC–MS Analyses of Acylquinic Acids in Geigeria alata (DC) Oliv. & Hiern. and Their Contribution to Antioxidant and Antimicrobial Capacity. Phytochem. Anal. 2016, 28, 176–184. [Google Scholar] [CrossRef]
- Gray, N.E.; Morré, J.; Kelley, J.; Maier, C.S.; Stevens, J.F.; Quinn, J.F.; Soumyanath, A. Caffeoylquinic Acids in Centella asiatica Protect against Amyloid-β Toxicity. J. Alzheimer’s Dis. 2014, 40, 359–373. [Google Scholar] [CrossRef]
- Metwally, D.M.; Alajmi, R.A.; El-Khadragy, M.F.; Yehia, H.M.; AL-Megrin, W.A.; Akabawy, A.M.A.; Amin, H.K.; Abdel Moneim, A.E. Chlorogenic Acid Confers Robust Neuroprotection against Arsenite Toxicity in Mice by Reversing Oxidative Stress, Inflammation, and Apoptosis. J. Funct. Foods 2020, 75, 104202. [Google Scholar] [CrossRef]
- Sasaki, K.; Davies, J.; Doldán, N.G. 3,4,5-Tricaffeoylquinic Acid Induces Adult Neurogenesis and Improves Deficit of Learning and Memory in Aging Model Senescence-Accelerated Prone 8 Mice. Aging 2019, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Adriana, F.; Paula, L.J. Consumption of Chlorogenic Acids through Coffee and Health Implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef]
- Bennett, R.; Wallsgrove, R. Secondary Metabolites in Plant Defence Mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.; Paiva, N. Stress-Induced Phenylpropanoid Metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Assefa, A.D.; Jeong, Y.J.; Kim, D.J.; Jeon, Y.A.; Lee, J.R.; Ko, H.C.; Baek, H.J.; Sung, J.S. Assessing Phenolic Content and Antioxidant Potential Diversity in Allium Plants Using Multivariate Data Analysis. Hortic. Environ. Biotechnol. 2018, 59, 759–773. [Google Scholar] [CrossRef]
- Mayer, A.M.; Harel, E. Polyphenol Oxidases in Plants. Phytochemistry 1979, 18, 193–215. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Moridani, M.Y.; Scobie, H. Caffeic Acid, Chlorogenic Acid, and Dihydrocaffeic Acid Metabolism: Glutathione Conjugate Formation. Drug Metab. Dispos. 2001, 21, 1432–1439. [Google Scholar]
- Clifford, M.N. Chlorogenic Acids and Other Cinnamates-Nature, Occurrence and Dietary Burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Zang, L.Y.; Cosma, G.; Gardner, H.; Castranova, V.; Vallyathan, V. Effect of Chlorogenic Acid on Hydroxyl Radical. Mol. Cell. Biochem. 2003, 247, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Kobayashi, K.; Tagawa, S.; Adachi, K. Antioxidant Activity of Polyphenolics in Diets: Rate Constants of Reactions of Chlorogenic Acid and Caffeic Acid with Reactive Species of Oxygen and Nitrogen. Biochim. Biophys. Acta BBA-Gen. Subj. 1997, 1335, 335–342. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Yahara, S.; Okuno, S.; Shahidul Islam, M.; Ishiguro, K.; Yamakawa, O. Antimutagenicity of Mono-, Di-, and Tricaffeoylquinic Acid Derivatives Isolated from Sweetpotato (Ipomoea batatas L.) Leaf. Biosci. Biotechnol. Biochem. 2002, 66, 2336–2341. [Google Scholar] [CrossRef]
- Chiang, L.C.; Ng, L.T.; Chiang, W. Immunomodulatory Activities of Flavonoids, Monoterpenoids, Triterpenoids, Iridoid Glycosides and Phenolic Compounds of Plantago Species. Planta Med. 2003, 69, 600–604. [Google Scholar] [CrossRef]
- Ina, H.; Yamada, K. Effects of Benzyl Glucoside and Chlorogenic Acid from Prunus mume on Adrenocorticotropic Hormone (ACTH) and Catecholamine Levels in Plasma of Experimental. Biol. Pharm. Bull. 2004, 27, 136–137. [Google Scholar] [CrossRef][Green Version]
- Choi, J.; Kim, J.; Lee, H.D.; Cho, H.; Paje, L.A.; Shin, H.; Lee, S. Development of an Analytical Approach for the Utilization of Edible Tree Sprouts. Nat. Prod. Sci. 2022, 28, 27–32. [Google Scholar] [CrossRef]
- Özkök, A.; Keskin, M.; Tanuğur Samancı, A.E.; Yorulmaz Önder, E.; Takma, Ç. Determination of Antioxidant Activity and Phenolic Compounds for Basic Standardization of Turkish Propolis. Appl. Biol. Chem. 2021, 64, 37. [Google Scholar] [CrossRef]
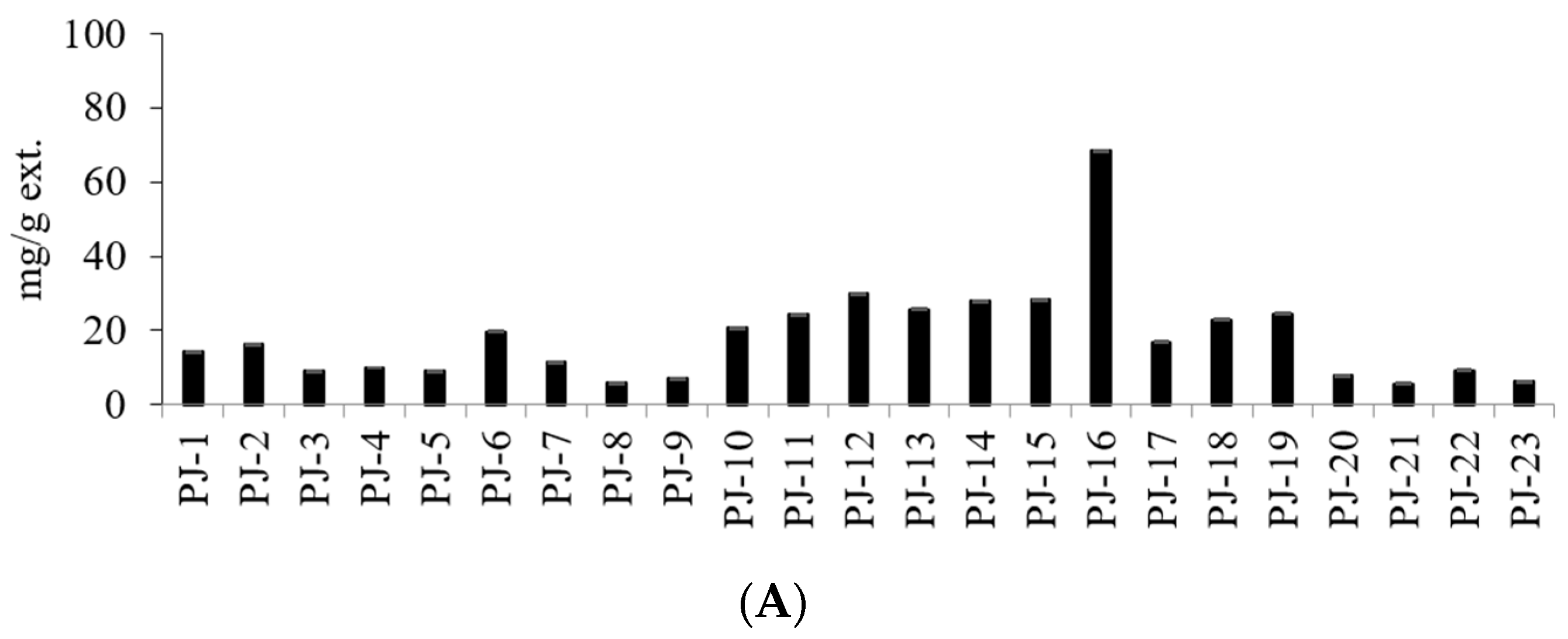
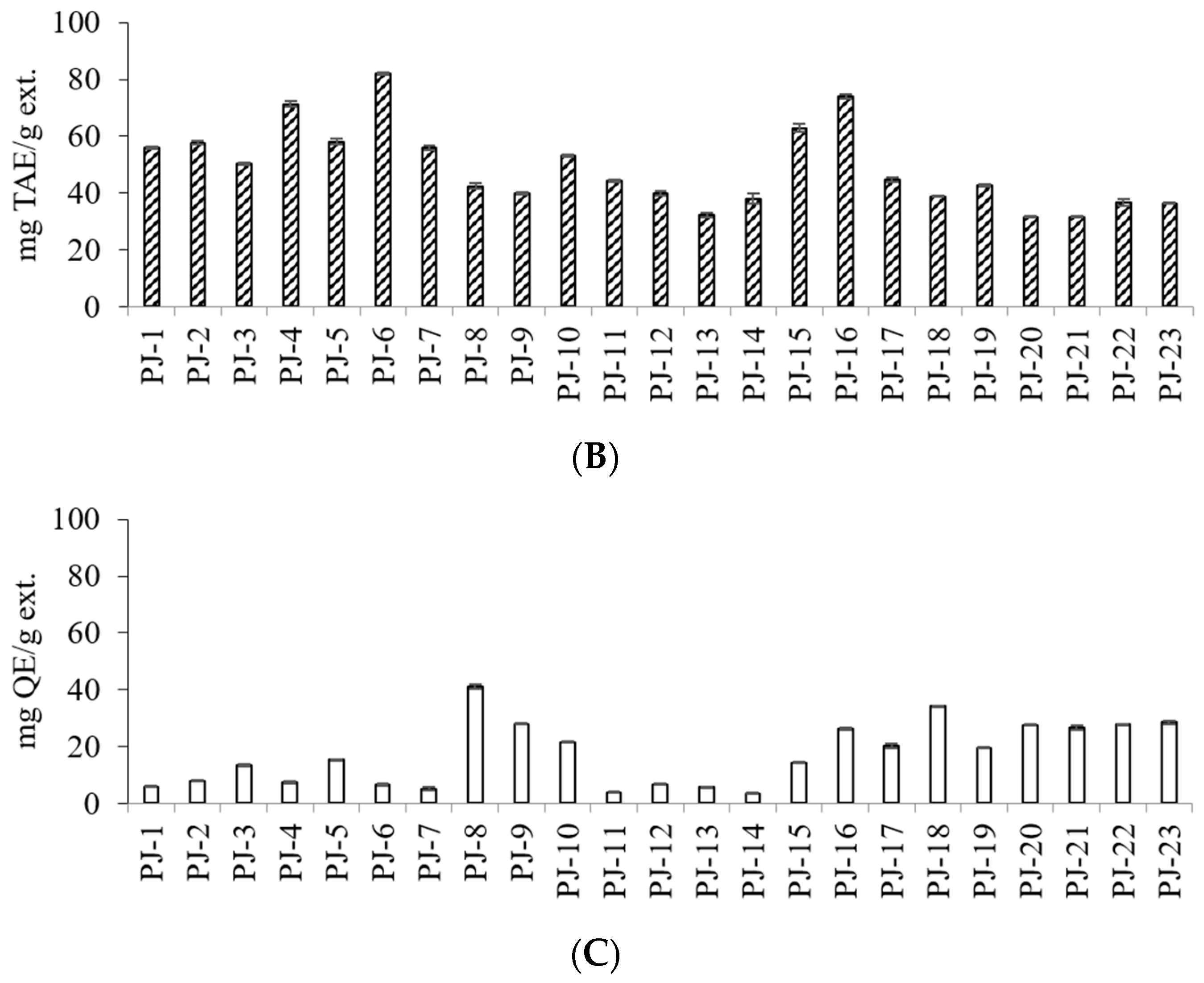


| Sample No. | Stem Color z | Seed Color y | TPC (mg TAE/g ext.) | TFC (mg QE/g ext.) | ||
|---|---|---|---|---|---|---|
| PJ-1 | G | DB | 56.01 ± 0.37 | F | 5.93 ± 0.22 | P |
| PJ-2 | G | LB | 57.60 ± 0.55 | E | 7.97 ± 0.11 | M |
| PJ-3 | G | B | 50.20 ± 0.44 | H | 13.35 ± 0.25 | L |
| PJ-4 | LR | B | 71.30 ± 1.11 | C | 7.31 ± 0.28 | N |
| PJ-5 | LR | DB | 58.00 ± 0.94 | E | 15.37 ± 0.14 | J |
| PJ-6 | R | B | 81.91 ± 0.32 | A | 6.59 ± 0.31 | O |
| PJ-7 | LR | B | 55.99 ± 0.69 | F | 5.16 ± 0.42 | Q |
| PJ-8 | LR | LB | 42.48 ± 1.19 | J | 40.98 ± 0.89 | A |
| PJ-9 | LR | B | 39.99 ± 0.47 | K | 28.04 ± 0.04 | DE |
| PJ-10 | LR | LB | 53.15 ± 0.47 | G | 21.61 ± 0.28 | G |
| PJ-11 | LR | DB | 44.38 ± 0.38 | I | 3.80 ± 0.16 | R |
| PJ-12 | LR | B | 40.04 ± 0.82 | K | 6.71 ± 0.14 | O |
| PJ-13 | LR | LB | 32.21 ± 0.87 | O | 5.78 ± 0.04 | O |
| PJ-14 | LR | LB | 38.00 ± 1.79 | L | 3.51 ± 0.19 | P |
| PJ-15 | LR | LB | 62.94 ± 1.52 | D | 14.25 ± 0.22 | K |
| PJ-16 | R | DB | 73.88 ± 0.81 | B | 26.34 ± 0.42 | F |
| PJ-17 | LR | B | 44.70 ± 0.77 | I | 20.29 ± 0.73 | H |
| PJ-18 | LR | LB | 38.86 ± 0.05 | N | 34.16 ± 0.14 | B |
| PJ-19 | LR | B | 42.77 ± 0.43 | J | 19.61 ± 0.26 | I |
| PJ-20 | G | B | 31.53 ± 0.13 | O | 27.58 ± 0.19 | E |
| PJ-21 | G | B | 31.48 ± 0.18 | O | 30.91 ± 0.37 | C |
| PJ-22 | LR | DB | 36.67 ± 1.12 | M | 27.73 ± 0.22 | E |
| PJ-23 | LR | DB | 36.30 ± 0.20 | M | 28.51 ± 0.59 | D |
| One-way ANOVA x | Significance | |||||
| Assay | F | p | ||||
| TPC | 1035.22 | <0.0001 | ||||
| TFC | 3088.67 | <0.0001 | ||||
| Sample No. | Stem Color z | Seed Color y | Contents (mg/g ext.) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Total | ||||||
| PJ-1 | G | DB | 0.53 ± 0.01 | U | 13.39 ± 0.01 | I | 0.21 ± 0.01 | V | 14.13 ± 0.03 |
| PJ-2 | G | LB | 1.24 ± 0.01 | P | 12.45 ± 0.16 | J | 2.63 ± 0.01 | L | 16.32 ± 0.18 |
| PJ-3 | G | B | 0.78 ± 0.01 | T | 6.92 ± 0.00 | M | 1.26 ± 0.01 | Q | 8.96 ± 0.02 |
| PJ-4 | LR | B | 1.49 ± 0.00 | N | 6.65 ± 0.00 | N | 1.71 ± 0.00 | O | 9.85 ± 0.00 |
| PJ-5 | LR | DB | 1.29 ± 0.01 | O | 5.64 ± 0.01 | P | 2.02 ± 0.01 | N | 8.95 ± 0.03 |
| PJ-6 | R | B | 4.40 ± 0.03 | D | 10.44 ± 0.02 | L | 4.81 ± 0.04 | D | 19.65 ± 0.09 |
| PJ-7 | LR | B | 2.50 ± 0.01 | K | 6.47 ± 0.01 | O | 2.35 ± 0.00 | M | 11.32 ± 0.02 |
| PJ-8 | LR | LB | 1.13 ± 0.00 | Q | 4.09 ± 0.01 | S | 0.61 ± 0.01 | T | 5.83 ± 0.02 |
| PJ-9 | LR | B | 1.04 ± 0.00 | R | 5.36 ± 0.01 | Q | 0.81 ± 0.01 | S | 7.21 ± 0.02 |
| PJ-10 | LR | LB | 3.40 ± 0.02 | H | 13.85 ± 0.05 | H | 3.39 ± 0.01 | I | 20.64 ± 0.08 |
| PJ-11 | LR | DB | 3.20 ± 0.01 | I | 18.17 ± 0.04 | D | 3.10 ± 0.01 | J | 24.47 ± 0.06 |
| PJ-12 | LR | B | 5.29 ± 0.02 | B | 19.65 ± 0.05 | B | 5.01 ± 0.01 | C | 29.95 ± 0.08 |
| PJ-13 | LR | LB | 3.53 ± 0.02 | F | 17.71 ± 0.03 | E | 4.47 ± 0.05 | G | 25.71 ± 0.1 |
| PJ-14 | LR | LB | 4.25 ± 0.02 | E | 19.17 ± 0.08 | C | 4.59 ± 0.01 | F | 28.01 ± 0.11 |
| PJ-15 | LR | LB | 6.18 ± 0.01 | A | 16.73 ± 0.06 | F | 5.29 ± 0.01 | B | 28.2 ± 0.08 |
| PJ-16 | R | DB | 2.40 ± 0.01 | L | 61.73 ± 0.05 | A | 4.24 ± 0.01 | H | 68.37 ± 0.07 |
| PJ-17 | LR | B | 2.76 ± 0.01 | J | 11.15 ± 0.05 | K | 2.97 ± 0.01 | K | 16.88 ± 0.07 |
| PJ-18 | LR | LB | 3.44 ± 0.02 | G | 14.70 ± 0.06 | G | 4.70 ± 0.02 | E | 22.84 ± 0.1 |
| PJ-19 | LR | B | 5.16 ± 0.01 | C | 13.83 ± 0.04 | H | 5.47 ± 0.03 | A | 24.46 ± 0.08 |
| PJ-20 | G | B | 1.49 ± 0.01 | N | 5.61 ± 0.01 | P | 0.82 ± 0.03 | P | 7.92 ± 0.05 |
| PJ-21 | G | B | 1.05 ± 0.01 | R | 4.05 ± 0.02 | S | 0.56 ± 0.01 | U | 5.66 ± 0.04 |
| PJ-22 | LR | DB | 2.01 ± 0.03 | M | 5.59 ± 0.02 | P | 1.68 ± 0.02 | P | 9.28 ± 0.07 |
| PJ-23 | LR | DB | 1.00 ± 0.01 | S | 4.28 ± 0.00 | R | 0.87 ± 0.02 | R | 6.15 ± 0.03 |
| One-way ANOVA x | Significance | ||||||||
| Compound | F | p | |||||||
| 5-CQA | 41,949.0 | <0.0001 | |||||||
| 3-CQA | 183,315 | <0.0001 | |||||||
| 4-CQA | 27,746.1 | <0.0001 | |||||||
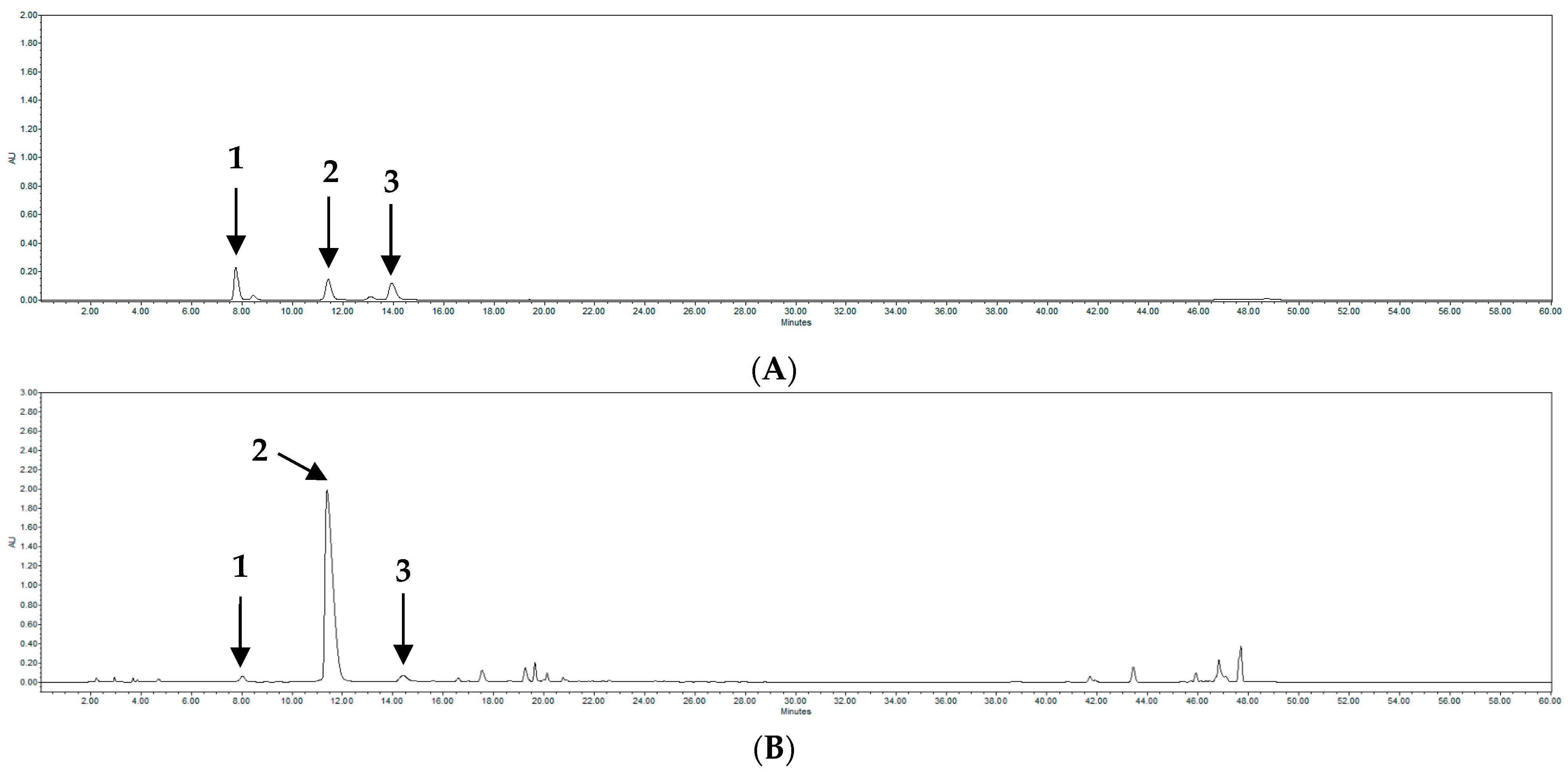
| Compound | tR | Calibration Equation z | Correlation Factor, r2 y |
|---|---|---|---|
| 1 | 7.9 | Y = 9894.8 X − 63,398 | 0.9992 |
| 2 | 11.6 | Y = 23,864 X − 1,000,000 | 0.9997 |
| 3 | 14.4 | Y = 7430.8 X − 120,529 | 0.9992 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-D.; Cho, H.; Shim, J.; Tran, G.H.; Lee, H.-D.; Ahn, K.H.; Yoo, E.; Chung, M.J.; Lee, S. Characteristics of Phenolic Compounds in Peucedanum japonicum According to Various Stem and Seed Colors. Molecules 2023, 28, 6266. https://doi.org/10.3390/molecules28176266
Lee C-D, Cho H, Shim J, Tran GH, Lee H-D, Ahn KH, Yoo E, Chung MJ, Lee S. Characteristics of Phenolic Compounds in Peucedanum japonicum According to Various Stem and Seed Colors. Molecules. 2023; 28(17):6266. https://doi.org/10.3390/molecules28176266
Chicago/Turabian StyleLee, Chang-Dae, Hyejin Cho, Jeehyoung Shim, Gia Han Tran, Hak-Dong Lee, Kwang Hoon Ahn, Eunae Yoo, Mi Ja Chung, and Sanghyun Lee. 2023. "Characteristics of Phenolic Compounds in Peucedanum japonicum According to Various Stem and Seed Colors" Molecules 28, no. 17: 6266. https://doi.org/10.3390/molecules28176266
APA StyleLee, C.-D., Cho, H., Shim, J., Tran, G. H., Lee, H.-D., Ahn, K. H., Yoo, E., Chung, M. J., & Lee, S. (2023). Characteristics of Phenolic Compounds in Peucedanum japonicum According to Various Stem and Seed Colors. Molecules, 28(17), 6266. https://doi.org/10.3390/molecules28176266







