Green Ultrasound-Assisted Synthesis of Rare-Earth-Based MOFs
Abstract
:1. Introduction
2. Results
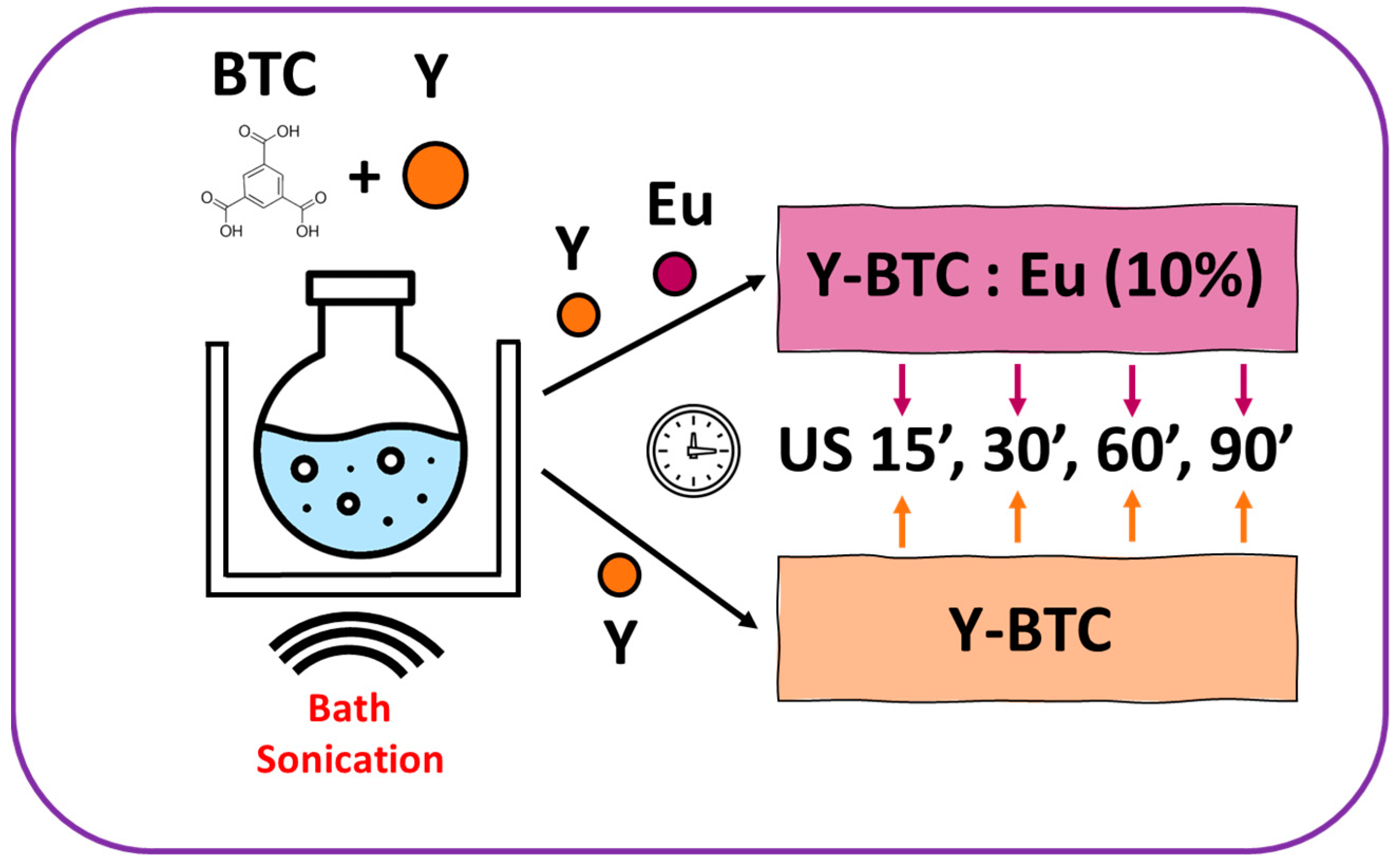
2.1. MOF Synthesis
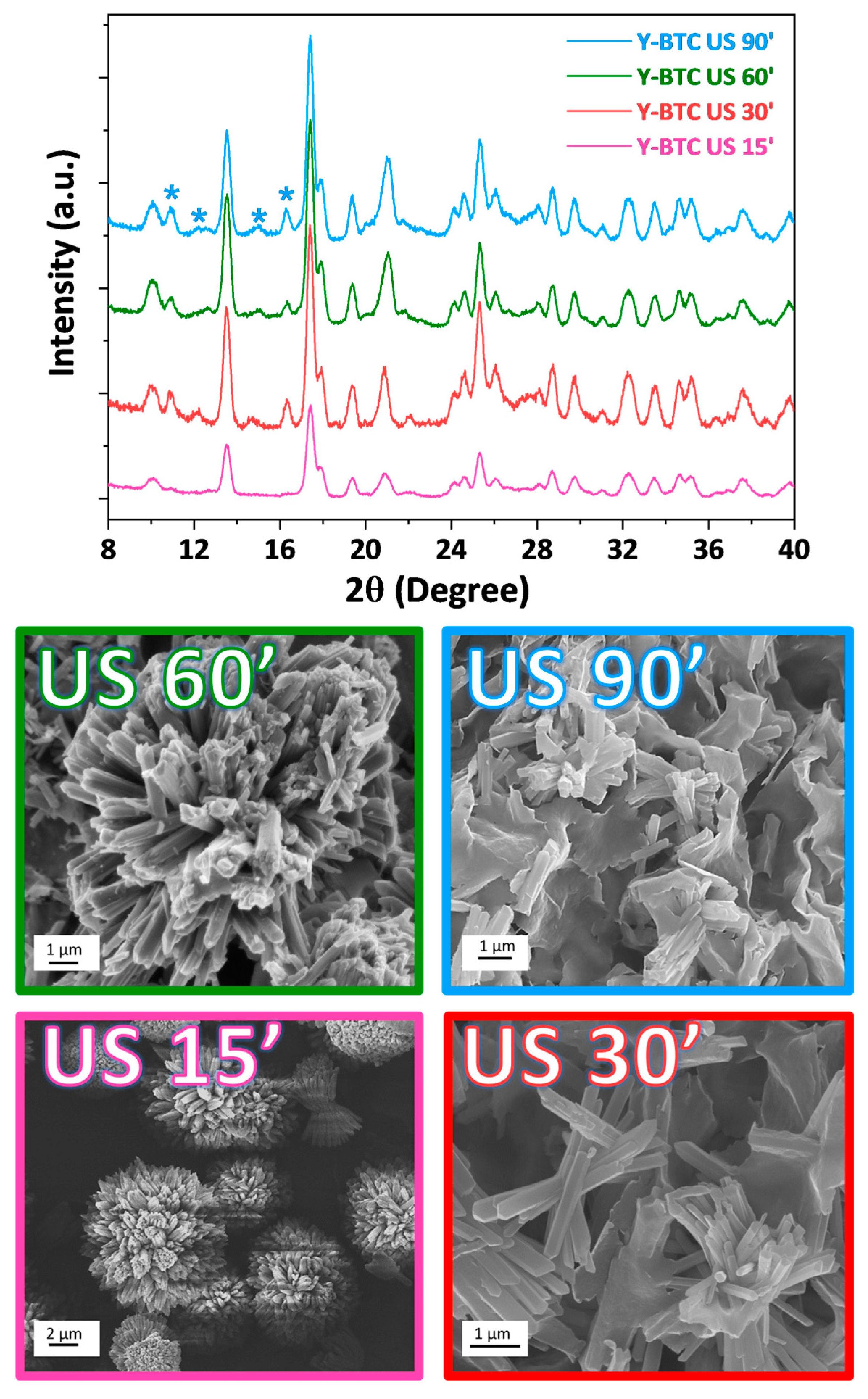
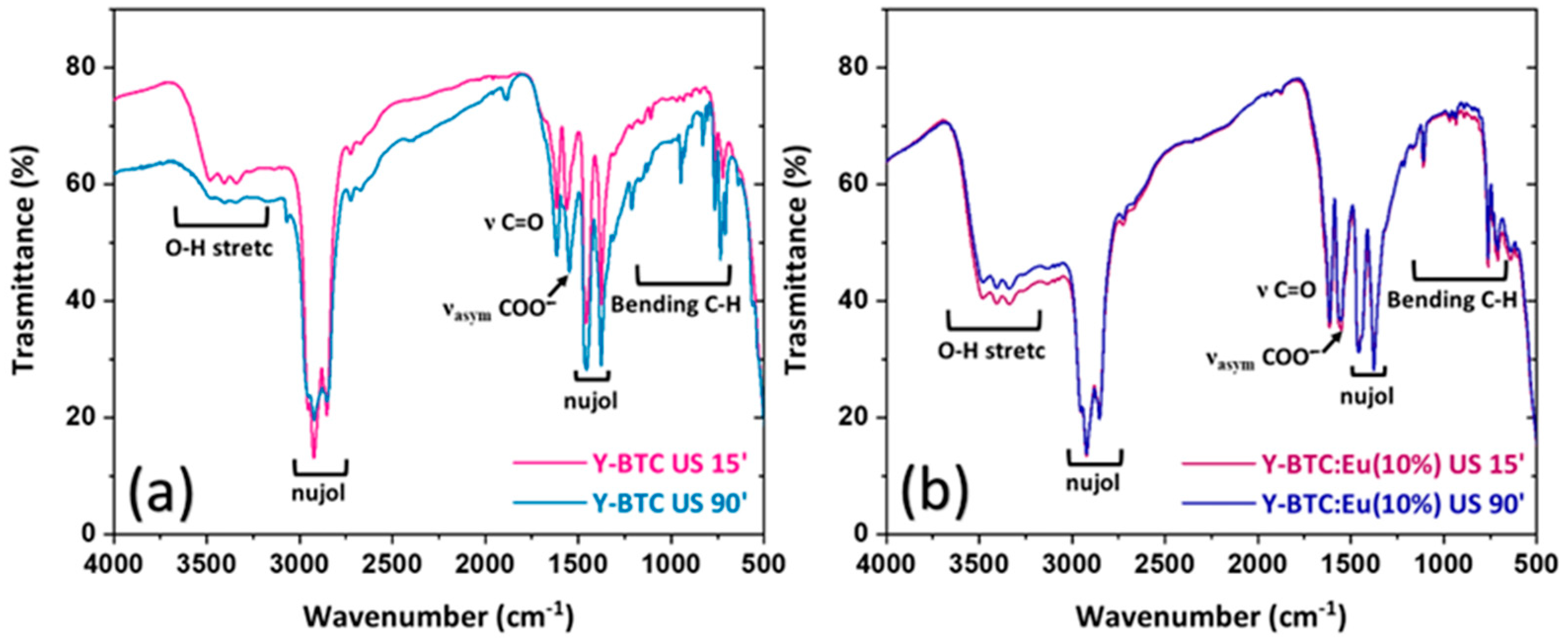
2.2. Structural and Morphological Characterisation
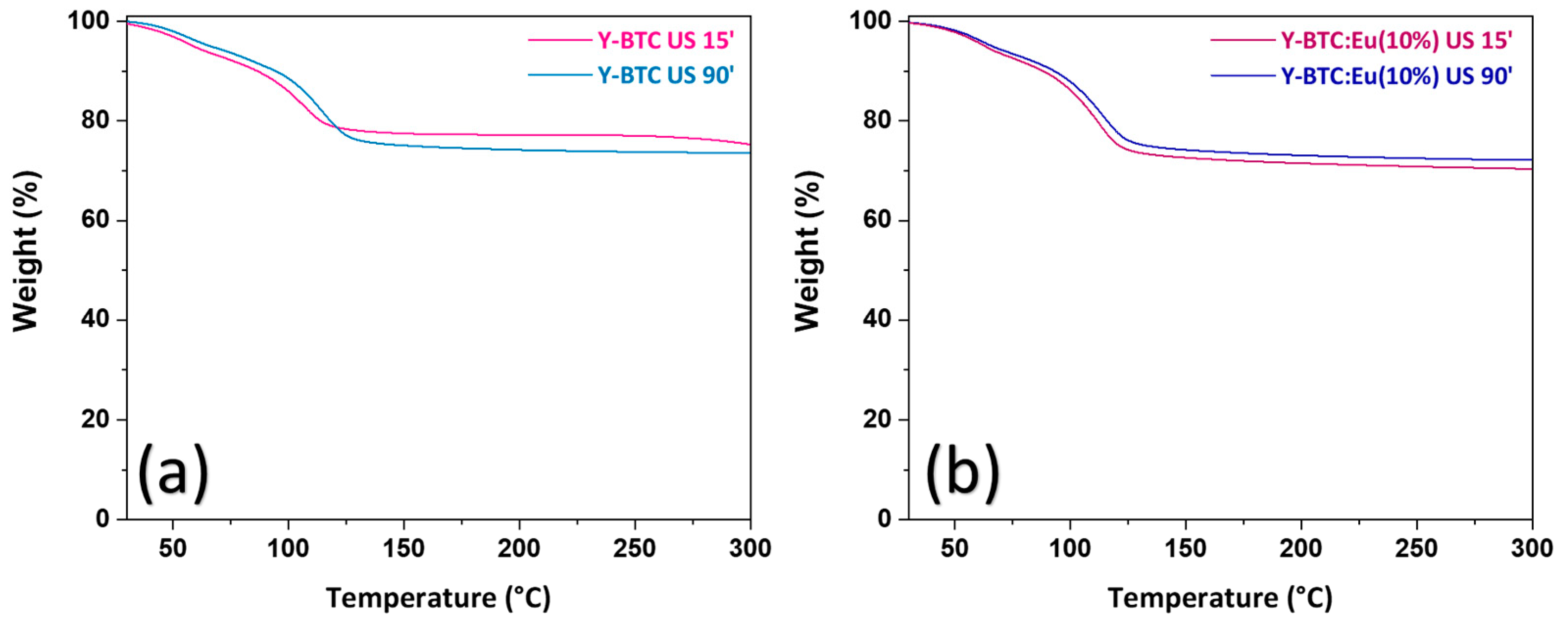
2.3. Thermal Properties
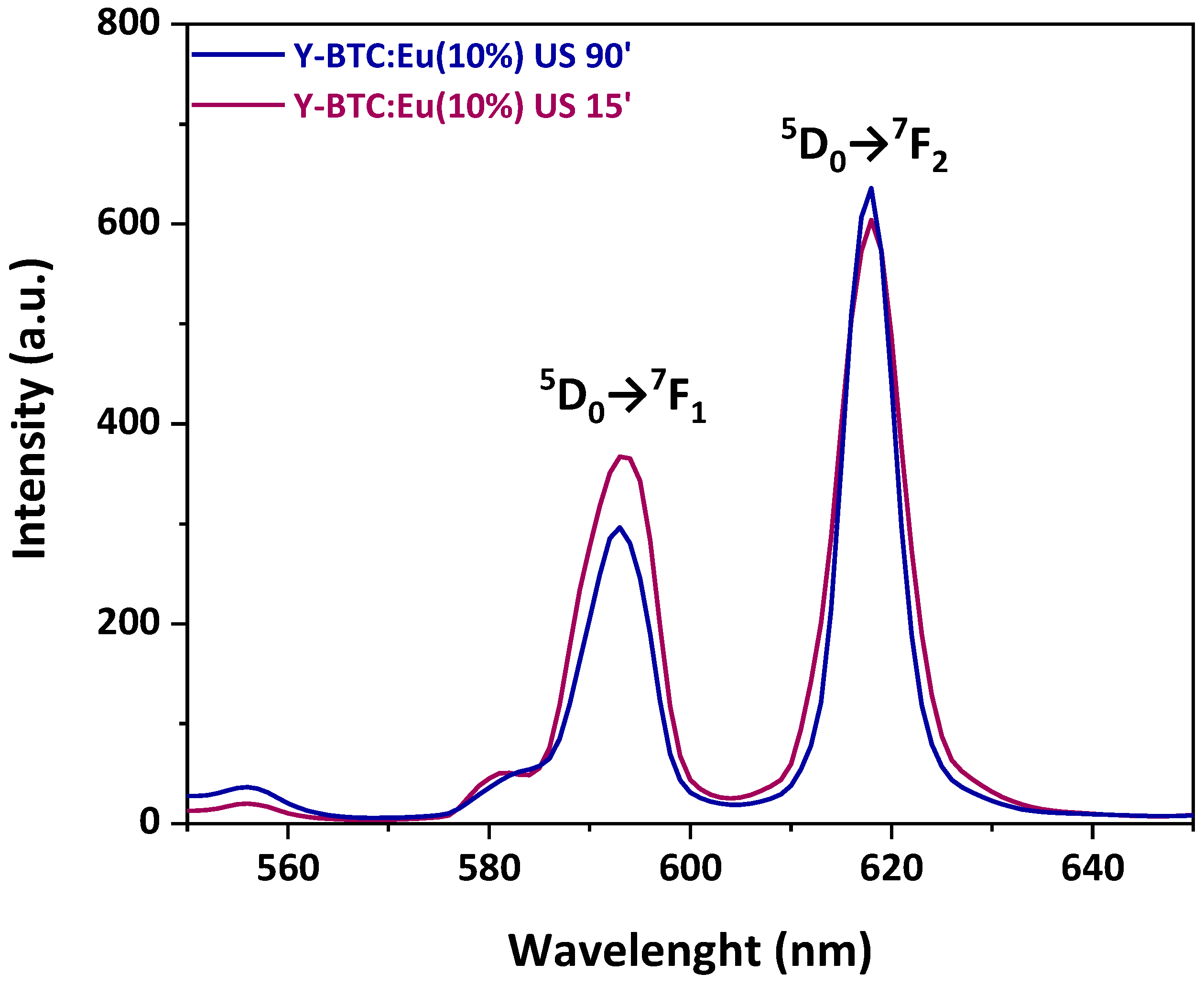
2.4. Luminescence Characterisation
2.5. Sensing of Nitro Derivatives
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis under Ultrasound
4.3. Characterisations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal–Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef]
- Hao, Z.; Song, X.; Zhu, M.; Meng, X.; Zhao, S.; Su, S.; Yang, W.; Songa, S.; Zhang, H. One-dimensional channel-structured Eu-MOF for sensing small organic molecules and Cu2+ ion. J. Mater. Chem. A 2013, 1, 11043–11050. [Google Scholar] [CrossRef]
- Barroso, N.; Andreo, J.; Beobide, G.; Castillo, O.; Luque, A.; Pérez-Yáñez, S.; Wuttke, S. Magnetic sustentation as an adsorption characterization technique for paramagnetic metal-organic frameworks. Commun. Chem. 2023, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Karbalaee Hosseini, A.; Tadjarodi, A. Luminescent Cd coordination polymer based on thiazole as a dual-responsive chemosensor for 4-nitroaniline and CrO42− in water. Sci. Rep. 2023, 13, 269. [Google Scholar] [CrossRef]
- Ma, D.; Li, Z.; Zhu, J.; Zhou, Y.; Chen, L.; Mai, X.; Liufu, M.; Wu, Y.; Li, Y. Inverse and highly selective separation of CO2/C2H2 on a thulium–organic framework. J. Mater. Chem. A 2020, 8, 11933–11937. [Google Scholar] [CrossRef]
- Stillman, Z.S.; Decker, G.E.; Dworzak, M.R.; Bloch, E.D.; Fromen, C.A. Aluminum-based metal–organic framework nanoparticles as pulmonary vaccine adjuvants. J. Nanobiotechnol. 2023, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Chen, H.; Xie, X.; Wan, Y.; Li, Q.; Wu, F.; Yang, R.; Wang, W.; Kong, X. Bright Tm3+-based downshifting luminescence nanoprobe operating around 1800 nm for NIR-IIb and c bioimaging. Nat. Commun. 2023, 14, 1079. [Google Scholar] [CrossRef]
- Zhong, Y.; Peng, Z.; Peng, Y.; Li, B.; Pan, Y.; Ouyang, Q.; Sakiyama, H.; Muddassir, M.; Liu, J. Construction of Fe-doped ZIF-8/DOX nanocomposites for ferroptosis strategy in the treatment of breast cancer. J. Mater. Chem. B 2023, 11, 6335–6345. [Google Scholar] [CrossRef]
- Chen, X.; Li, M.; Lin, M.; Lu, C.; Kumar, A.; Pan, Y.; Liu, J.; Peng, Y. Current and promising applications of Hf(IV)-based MOFs in clinical cancer therapy. J. Mater. Chem. B 2023, 11, 5693–5714. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Z.; Huang, S.; Ye, K.; Jiang, Y.; Liu, J.; Liu, J.; Lu, X.; Li, B. A metal-organic framework-based immunomodulatory nanoplatform for anti-atherosclerosis treatment. J. Control. Release 2023, 354, 615–625. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhaktaa, R.K.; Houka, R.J.T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Soares-Santos, P.C.R.; Cunha-Silva, L.; Paz, F.A.A.; Ferreira, R.A.S.; Rocha, J.; Trindade, T.; Carlos, L.D.; Nogueira, H.I.S. Hydro-Ionothermal Synthesis of Lanthanide-Organic Frameworks with 1,4-Phenylenebis(methylene)diphosphonate. Cryst. Growth Des. 2008, 8, 2505–2516. [Google Scholar] [CrossRef]
- Hu, S.; Liu, J.; Wang, Y.; Liang, Z.; Hu, B.; Xie, J.; Wong, W.-L.; Wong, K.-Y.; Qiu, B.; Peng, W. A new fluorescent biosensor based on inner filter effect and competitive coordination with the europium ion of non-luminescent Eu-MOF nanosheets for the determination of alkaline phosphatase activity in human serum. Sens. Actuators B Chem. 2023, 380, 133379. [Google Scholar] [CrossRef]
- Hooda, A.; Singh, D.; Dalal, A.; Nehra, K.; Kumar, S.; Singh Malik, R.; Kumarc, R.; Kumarc, P. Optical, electrochemical and photophysical analyses of heteroleptic luminescent Ln(III) complexes for lighting applications. RSC Adv. 2023, 13, 9033–9045. [Google Scholar] [CrossRef]
- Main, R.M.; Vornholt, S.M.; Rice, C.M.; Elliott, C.; Russell, S.E.; Kerr, P.J.; Warren, M.R.; Morris, R.E. In situ single-crystal synchrotron X-ray diffraction studies of biologically active gases in metal-organic frameworks. Commun. Chem. 2023, 6, 44. [Google Scholar] [CrossRef]
- Liu, M.; Shang, C.; Zhao, T.; Yu, H.; Kou, Y.; Lv, Z.; Hou, M.; Zhang, F.; Li, Q.; Zhao, D.; et al. Site-specific anisotropic assembly of amorphous mesoporous subunits on crystalline metal–organic framework. Nat. Commun. 2023, 14, 1211. [Google Scholar] [CrossRef]
- Shah, A.H.; Ul Abideen, Z.; Maqsood, S.; Rashid, F.; Ullah, R.; Ur Rehman, A.; Dildar, M.; Ahmad, M.; Ullah, K.; Rafi, M.N.; et al. Porous Cu-based metal organic framework (Cu-MOF) for highly selective adsorption of organic pollutants. J. Solid State Chem. 2023, 322, 123935. [Google Scholar] [CrossRef]
- Feng, J.; Yang, X.; Gao, S.; Shi, J.; Cao, R. Facile and Rapid Growth of Nanostructured Ln-BTC Metal–Organic Framework Films by Electrophoretic Deposition for Explosives sensing in Gas and Cr3+ Detection in Solution. Langmuir 2017, 33, 14238–14243. [Google Scholar] [CrossRef]
- Yang, X.P.; Jones, R.A.; Riversa, J.H.; Lai, R.P. Syntheses, structures and luminescent properties of new lanthanide-based coordination polymers based on 1,4-benzenedicarboxylate (bdc). Dalton Trans. 2007, 15, 3936–3942. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Ma, H.; Zhang, L.; Zhang, W. An RGH–MOF as a naked eye colorimetric fluorescent sensor for picric acid recognition. J. Mater. Chem. C 2017, 5, 4661–4669. [Google Scholar] [CrossRef]
- Alammar, T.; Hlova, I.Z.; Gupta, S.; Balema, V.; Pecharskya, V.K.; Mudring, A.V. Luminescence properties of mechanochemically synthesized lanthanide containing MIL-78 MOFs. Dalton Trans. 2018, 47, 7594–7601. [Google Scholar] [CrossRef] [PubMed]
- Almáši, M.; Zeleňák, V.; Kuchár, J.; Bourrelly, S.; Llewellyn, P.L. New members of MOF-76 family containing Ho(III) and Tm(III) ions: Characterization, stability and gas adsorption properties. Colloids Surf. 2016, 496, 114–124. [Google Scholar] [CrossRef]
- Yang, X.; Lin, X.; Zhao, Y.; Zhao, Y.S.; Yan, D. Lanthanide Metal–Organic Framework Microrods: Colored Optical Waveguides and Chiral Polarized Emission. Angew. Chem. Int. Ed. 2017, 129, 7961–7965. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, L. Multiple fluorescent sensing responses of a 2D framework structure towards explosive: Synthesis, characterization and sensing performance. J. Mol. Struct. 2021, 1238, 130418. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, L.; Sun, M.; Wei, P.; Li, G.; Li, Y. Highly sensitive luminescent detection toward polytypic antibiotics by a water-stable and white-light-emitting MOF-76 derivative. Dye. Pigment. 2020, 180, 108444. [Google Scholar] [CrossRef]
- Dong, X.; He, Q.; Li, M.; Wang, X.; Wang, Y.; Zhang, W. Fluorescence and electrochemical detection of iodine vapor in the presence of high humidity using Ln-based MOFs. Dalton Trans. 2021, 50, 15567–15575. [Google Scholar] [CrossRef]
- Wu, R.Z.; Yang, X.; Zhang, L.W.; Zhou, P.P. Luminescent lanthanide metal–organic frameworks for chemical sensing and toxic anion detection. Dalton Trans. 2017, 46, 9859–9867. [Google Scholar] [CrossRef]
- Puglisi, R.; Pellegrino, A.L.; Fiorenza, R.; Scirè, S.; Malandrino, G. A Facile One-Pot Approach to the Synthesis of Gd-Eu Based Metal-Organic Frameworks and Applications to Sensing of Fe3+ and Cr2O72− Ions. Sensors 2021, 21, 1679. [Google Scholar] [CrossRef]
- Jamali, A.; Tehrani, A.A.; Shemirani, F.; Morsali, A. Lanthanide metal–organic frameworks as selective microporous materials for adsorption of heavy metal ions. Dalton Trans. 2016, 45, 9193–9200. [Google Scholar] [CrossRef]
- Lian, X.; Yan, B. A lanthanide metal–organic framework (MOF-76) for adsorbing dyes and fluorescence detecting aromatic pollutants. RSC Adv. 2016, 6, 11570–11576. [Google Scholar] [CrossRef]
- Wang, L.; He, J.; Chen, X.; Lv, Y. A lanthanide MOF catalyst with an excellent thermal stability for the synthesis of polycarbonate diol. J. Iran. Chem. Soc. 2020, 17, 2335–2343. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Li, X.; Wu, J.; Han, Y.; Zhang, X.; Xu, Y. Dual-Emissive CsPbBr3@Eu-BTC Composite for Self-Calibrating Temperature Sensing Application. Cryst. Growth Des. 2020, 20, 454–459. [Google Scholar] [CrossRef]
- Zhou, X.; Li, S.; Zhou, X.; Chen, L.; Wu, J.; Yu, T.; Li, L.; Jiang, S.; Tang, X.; Xiang, G.; et al. Luminescent properties and ratiometric optical thermometry of Ln-BDC-F4 compounds. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 224, 117418. [Google Scholar] [CrossRef]
- Zaręba, J.K.; Nyk, M.; Janczak, J.; Samoć, M. Three-Photon Absorption of Coordination Polymer Transforms UV-to-VIS Thermometry into NIR-to-VIS Thermometry. ACS Appl. Mater. Interfaces 2019, 11, 10435–10441. [Google Scholar] [CrossRef]
- Chen, L.; Yu, H.; Li, Y.; Zhang, X.; Du, Y. Fabrication of a microporous Dy(III)-organic framework with polar channels for 5-Fu (fluorouracil) delivery and inhibiting human brain tumor cells. Struct. Chem. 2018, 29, 1885–1891. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Ma, H.; Zhang, L.; Jiang, H.; Song, H.; Zhang, L.; Luo, Y. A nanoscaled lanthanide metal–organic framework as a colorimetric fluorescence sensor for dipicolinic acid based on modulating energy transfer. J. Mater. Chem. C 2016, 4, 7294. [Google Scholar] [CrossRef]
- Shu, Y.; Meng, Y.; Chen, M.-L.; Wang, J.-H. Isolation of hemoglobin with metal–organic frameworks Y(BTC)(H2O)6. Chin. Chem. Lett. 2015, 26, 1460–1464. [Google Scholar] [CrossRef]
- Xiong, L.; Yu, L.; Li, S.; Feng, L.; Xiao, Y. Multifunctional lanthanide metal-organic framework based ratiometric fluorescence visual detection platform for alkaline phosphatase activity. Microchim. Acta 2021, 188, 236. [Google Scholar] [CrossRef]
- Santos, P.F.; Ribeiro, J.; Luz, P.P. Physicochemical and electrochemical characterization of Ce/carbonaceous matrices-based composites. Solid State Sci. 2020, 110, 106479. [Google Scholar] [CrossRef]
- Hu, M.; Shu, Y.; Kirillov, A.; Liu, W.; Yang, L.; Dou, W. Epoxy Functional Composites Based on Lanthanide Metal−Organic Frameworks for Luminescent Polymer Materials. ACS Appl. Mater. Interfaces 2021, 13, 7625–7634. [Google Scholar] [CrossRef]
- Tong, Y.J.; Yu, L.D.; Zheng, J.; Liu, G.; Ye, Y.; Huang, S.; Chen, G.; Yang, H.; Wen, C.; Wei, S.; et al. Graphene Oxide-Supported Lanthanide Metal−Organic Frameworks with Boosted Stabilities and Detection Sensitivities. Anal. Chem. 2020, 92, 15550–15557. [Google Scholar] [CrossRef] [PubMed]
- Moscoso, F.G.; Almeida, J.; Sousaraei, A.; Lopes-Costa, T.; Silva, A.M.G.; Cabanillas-Gonzalez, J.; Cunha-Silva, L.; Pedrosa, J.M. Luminescent MOF crystals embedded in PMMA/PDMS transparent films as effective NO2 gas sensors. Mol. Syst. Des. Eng. 2020, 5, 1048–1056. [Google Scholar] [CrossRef]
- Brunckova, H.; Mudra, E.; Rocha, L.; Nassar, E.; Nascimento, W.; Kolev, H.; Kovalcikova, A.; Molcanova, Z.; Podobova, M.; Medvecky, L. Preparation and characterization of isostructural lanthanide Eu/Gd/Tb metal-organic framework thin films for luminescent applications. Appl. Surf. Sci. 2021, 542, 148731. [Google Scholar] [CrossRef]
- Gao, W.; Huang, H.; Zhou, A.-M.; Wei, H.; Liu, J.-P.; Zhang, X.-M. Three 3D LnIII-MOFs based on a nitro-functionalized biphenyltricarboxylate ligand: Syntheses, structures, and magnetic properties. CrystEngComm 2020, 22, 267–274. [Google Scholar] [CrossRef]
- Dong, B.X.; Gua, X.J.; Xu, Q. Solvent effect on the construction of two microporous yttrium–organic frameworks with high thermostability viain situ ligand hydrolysis. Dalton Trans. 2010, 39, 5683–5687. [Google Scholar] [CrossRef]
- Luo, J.; Xu, H.; Liu, Y.; Zhao, Y.; Daemen, L.L.; Brown, C.; Timofeeva, T.V.; Ma, S.; Zhou, H.-C. Hydrogen adsorption in a highly stable porous rare-earth metal-organic framework: Sorption properties and neutron diffraction studies. J. Am. Chem. Soc. 2008, 130, 9626–9627. [Google Scholar] [CrossRef] [PubMed]
- Medina-Velazquez, D.Y.; Alejandre-Zuniga, B.Y.; Loera-Serna, S.; Ortiz, E.M.; Morales-Ramirez, A.D.J.; Garfias-Garcia, E.; Garcia-Murillo, A.; Falcony, C. An alkaline one-pot reaction to synthesize luminescent Eu-BTC MOF nanorods, highly pure and water-insoluble, under room conditions. J. Nanopart. Res. 2016, 18, 352. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, K.; Sun, X.; Guan, R.; Su, H.; You, H.; Qi, C. A series of nano/micro-sized metal–organic frameworks with tunable photoluminescence properties. Cryst. Eng. Comm. 2015, 17, 2321–2326. [Google Scholar] [CrossRef]
- Lo Presti, F.; Borzì, A.; Pellegrino, A.L.; Rossi, P.; Paoli, P.; Malandrino, G. Morphology controlled synthesis of yttrium metal–organic frameworks with a tritopic ligand. Results Chem. 2022, 4, 100640. [Google Scholar] [CrossRef]
- Nikseresht, A.; Ghasemi, S.; Parak, S. [Cu3(BTC)2]: A metal–organic framework as an environment-friendly and economically catalyst for the synthesis of tacrine analogues by Friedländer reaction under conventional and ultrasound irradiation. Polyhedron 2018, 151, 112–117. [Google Scholar] [CrossRef]
- Tehrani, A.A.; Safarifard, V.; Morsali, A.; Bruno, G.; Rudbari, H.A. Ultrasound-assisted synthesis of metal–organic framework nanorods of Zn-HKUST-1 and their templating effects for facile fabrication of zinc oxide nanorods via solid-state transformation. Inorg. Chem. Commun. 2015, 59, 41–45. [Google Scholar] [CrossRef]
- Khan, N.A.; Jhung, S.H. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord. Chem. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Kumari, P.; Kareem, A.; Jhariat, P.; Senthilkumar, S.; Panda, T. Phase Purity Regulated by Mechano-Chemical Synthesis of Metal−Organic Frameworks for the Electrocatalytic Oxygen Evolution Reaction. Inorg. Chem. 2023, 62, 3457–3463. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Han, Y.; Wu, J.; Zhang, X.; Wang, Z.; Xu, Y. Synergetic Effect of Tetraethylammonium Bromide Addition on the Morphology Evolution and Enhanced Photoluminescence of Rare-Earth Metal–Organic Frameworks. Inorg. Chem. 2020, 59, 14318–14325. [Google Scholar] [CrossRef]
- Xia, F.; Kang, S.; Xue, F.; Bu, X. Simple reductive synthesis of a novel mixed-lanthanide metal–organic framework with excellent cycling ability as a binder-free supercapacitor electrode. Mater. Lett. 2021, 282, 128715. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, B.; Zhou, Q.; Zhang, T.; Wu, W. Morphology effect of metal-organic framework HKUST-1 as a catalyst on benzene oxidation. Chem. Res. Chin. Univ. 2017, 33, 971–978. [Google Scholar] [CrossRef]
- Xiao, J.D.; Qiu, L.G.; Ke, F.; Yuan, Y.P.; Xu, G.S.; Wang, Y.M.; Jiang, X. Rapid synthesis of nanoscale terbium-based metal–organic frameworks by a combined ultrasound-vapour phase diffusion method for highly selective sensing of picric acid. J. Mater. Chem. A 2013, 1, 8745–8752. [Google Scholar] [CrossRef]
- Hu, S.M.; Niu, H.L.; Qiu, L.G.; Yuan, Y.P.; Jiang, X.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Facile synthesis of highly luminescent nanowires of a terbium-based metal–organic framework by an ultrasonic-assisted method and their application as a luminescent probe for selective sensing of organoamines. Inorg. Chem. Commun. 2012, 17, 147–150. [Google Scholar] [CrossRef]
- Khan, N.A.; Haque, M.M.; Jhung, S.H. Accelerated Syntheses of Porous Isostructural Lanthanide–Benzenetricarboxylates (Ln–BTC) under Ultrasound at Room Temperature. Eur. J. Inorg. Chem. 2010, 2010, 4975–4981. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Rizvandi, M.; Azadbakht, A.; Rostamnia, S. Controlled uptake and release of imatinib from ultrasound nanoparticles Cu3(BTC)2 metal–organic framework in comparison with bulk structure. J. Colloid Interface Sci. 2016, 471, 112–117. [Google Scholar] [CrossRef]
- Xu, W.; Li, G.; Lia, W.; Zhang, H. Facile room temperature synthesis of metal–organic frameworks from newly synthesized copper/zinc hydroxide and their application in adsorptive desulfurization. RSC Adv. 2016, 6, 37530–37534. [Google Scholar] [CrossRef]
- Ley, S.V.; Low, C.M.R. Ultrasound in synthesis. In Reactivity and Structure Concepts in Organic Chemistry; Hafner, K., Lehn, J.-M., Rees, C.W., von Rague Schleyer, P., Trost, B.M., Zahradnik, R., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 1989; Volume 27, pp. 1–129. [Google Scholar]
- Daiguebonne, C.; Guilloa, O.; Gérault, Y.; Lecerf, A.; Boubekeur, K. Synthesis and crystal structure of two new rare earth trimesate complexes: ErTMA(H2O)5⋅3.5H2O and YTMA(H2O)6. Inorg. Chim. Acta 1999, 284, 139–145. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Turro, N.J. Modern Molecular Photochemistry; University Science Books: Sausalito, CA, USA, 1991. [Google Scholar]
- Liu, L.; Ding, R.; Mao, Y.; Sun, B. Theoretical investigations on the nitro-explosive sensing process of a MOF sensor: Roles of hydrogen bond and π-π stacking. Chem. Phys. Lett. 2022, 793, 139393. [Google Scholar] [CrossRef]
- Sun, B.; Tao, T.; Liu, L.; Ding, R.; Mao, Y. Electron Transfer Facilitated by π−π Stacking during the Nitrobenzene Recognition Process of an MOF Sensor. J. Phys. Chem. C 2021, 125, 12433–12440. [Google Scholar] [CrossRef]
- Du, Y.; Yang, H.; Liu, R.; Shao, C.; Yang, L. A multi-responsive chemosensor for highly sensitive and selective detection of Fe3+, Cu2+, Cr2O72− and nitrobenzene based on a luminescent lanthanide metal–organic framework. Dalton Trans. 2020, 49, 13003–13016. [Google Scholar] [CrossRef]
- Chen, L.; Cheng, Z.; Peng, X.; Qiu, G.; Wang, L. Eu-Doped MOF-based high-efficiency fluorescent sensor for detecting 2,4-dinitrophenol and 2,4,6-trinitrophenol simultaneously. Anal. Methods 2022, 14, 44–51. [Google Scholar] [CrossRef]
- Jornet-Mollá, V.; Dreessen, C.; Romero, F.M. Robust Lanthanoid Picolinate-Based Coordination Polymers for Luminescence and Sensing Applications. Inorg. Chem. 2021, 60, 10572–10584. [Google Scholar] [CrossRef]
- Cheng, X.; Hu, J.; Li, J.; Zhang, M. Tunable emission and selective luminescence sensing for nitro- pollutants and metal ions based on bifunctional lanthanide metal-organic frameworks. J. Lumin. 2020, 221, 117100. [Google Scholar] [CrossRef]
- Yang, X.; Liang, Y.; Shi, J.; Wang, Y.; Feng, W. Dual-lanthanide hollow luminescence metal-organic frameworks and their sensing performance for trace water and nitrobenzene. Opt. Mater. 2023, 135, 113343. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Presti, F.; Pellegrino, A.L.; Consoli, N.; Malandrino, G. Green Ultrasound-Assisted Synthesis of Rare-Earth-Based MOFs. Molecules 2023, 28, 6088. https://doi.org/10.3390/molecules28166088
Lo Presti F, Pellegrino AL, Consoli N, Malandrino G. Green Ultrasound-Assisted Synthesis of Rare-Earth-Based MOFs. Molecules. 2023; 28(16):6088. https://doi.org/10.3390/molecules28166088
Chicago/Turabian StyleLo Presti, Francesca, Anna L. Pellegrino, Nancy Consoli, and Graziella Malandrino. 2023. "Green Ultrasound-Assisted Synthesis of Rare-Earth-Based MOFs" Molecules 28, no. 16: 6088. https://doi.org/10.3390/molecules28166088
APA StyleLo Presti, F., Pellegrino, A. L., Consoli, N., & Malandrino, G. (2023). Green Ultrasound-Assisted Synthesis of Rare-Earth-Based MOFs. Molecules, 28(16), 6088. https://doi.org/10.3390/molecules28166088








