Natural Rubber/Hexagonal Mesoporous Silica Nanocomposites as Efficient Adsorbents for the Selective Adsorption of (−)-Epigallocatechin Gallate and Caffeine from Green Tea
Abstract
1. Introduction
2. Results and Discussion
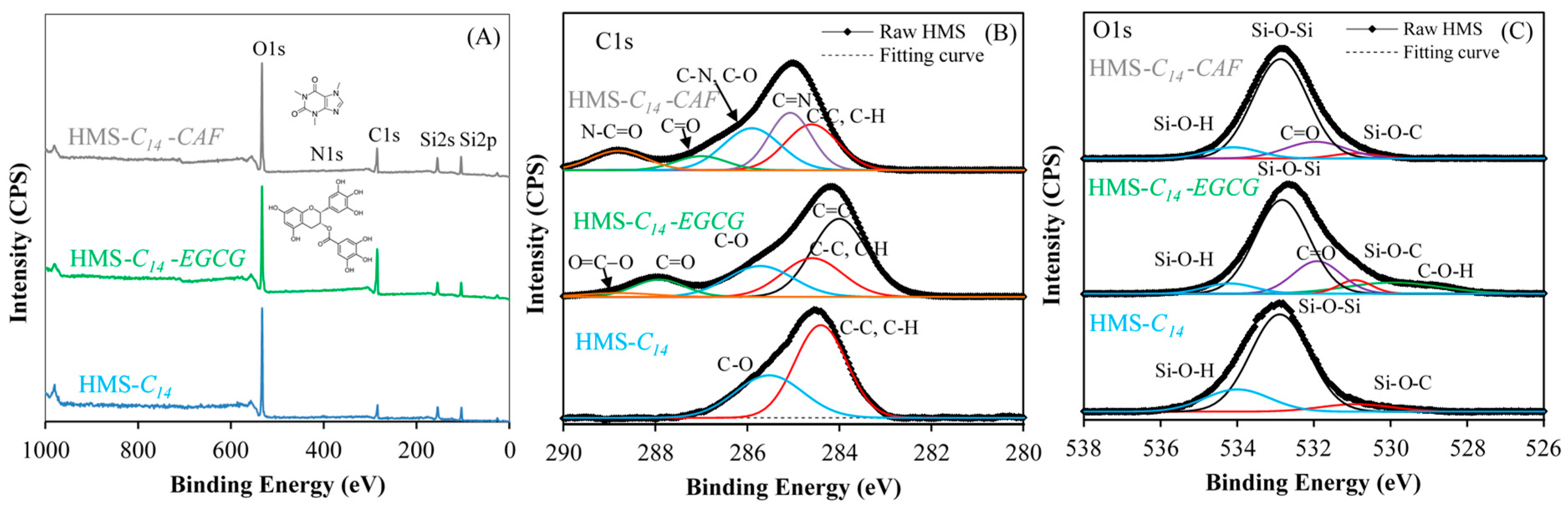
2.1. Physicochemical Properties of Synthesized Adsorbents
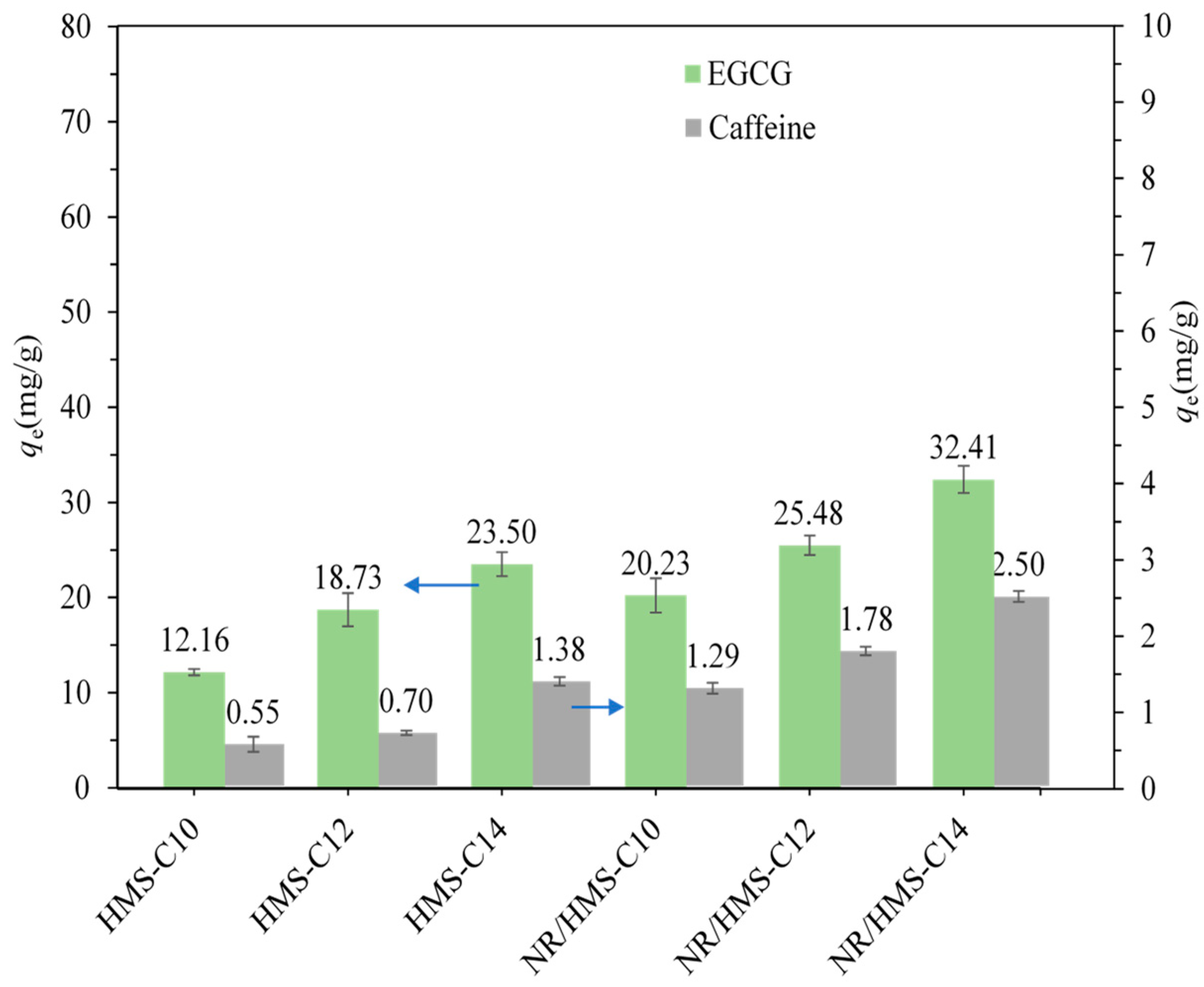
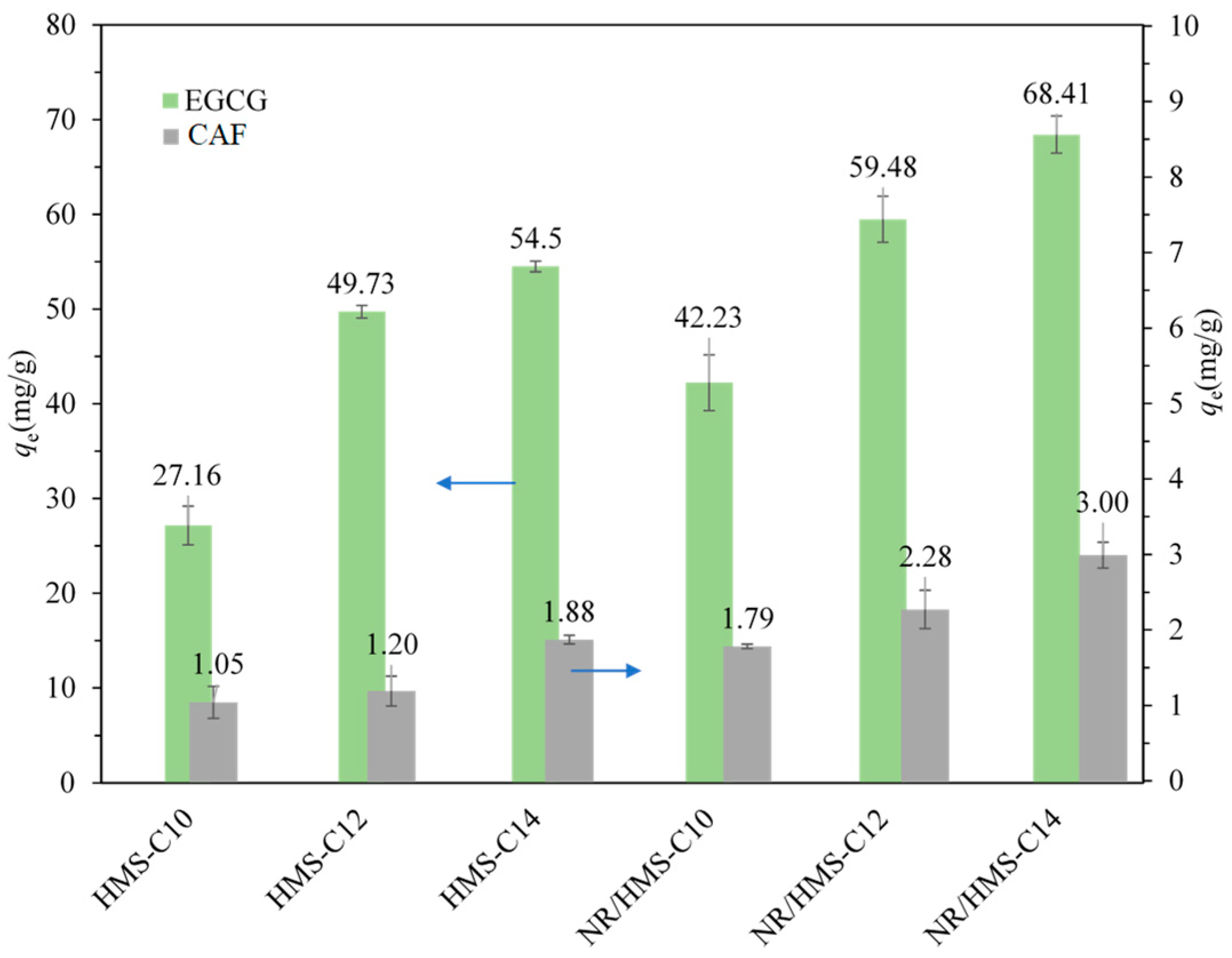
2.2. Adsorption of EGCG and CAF on Pure-Silica HMS and NR/HMS Materials
2.3. Adsorption Behavior and Mechanism
2.3.1. Adsorption Kinetics
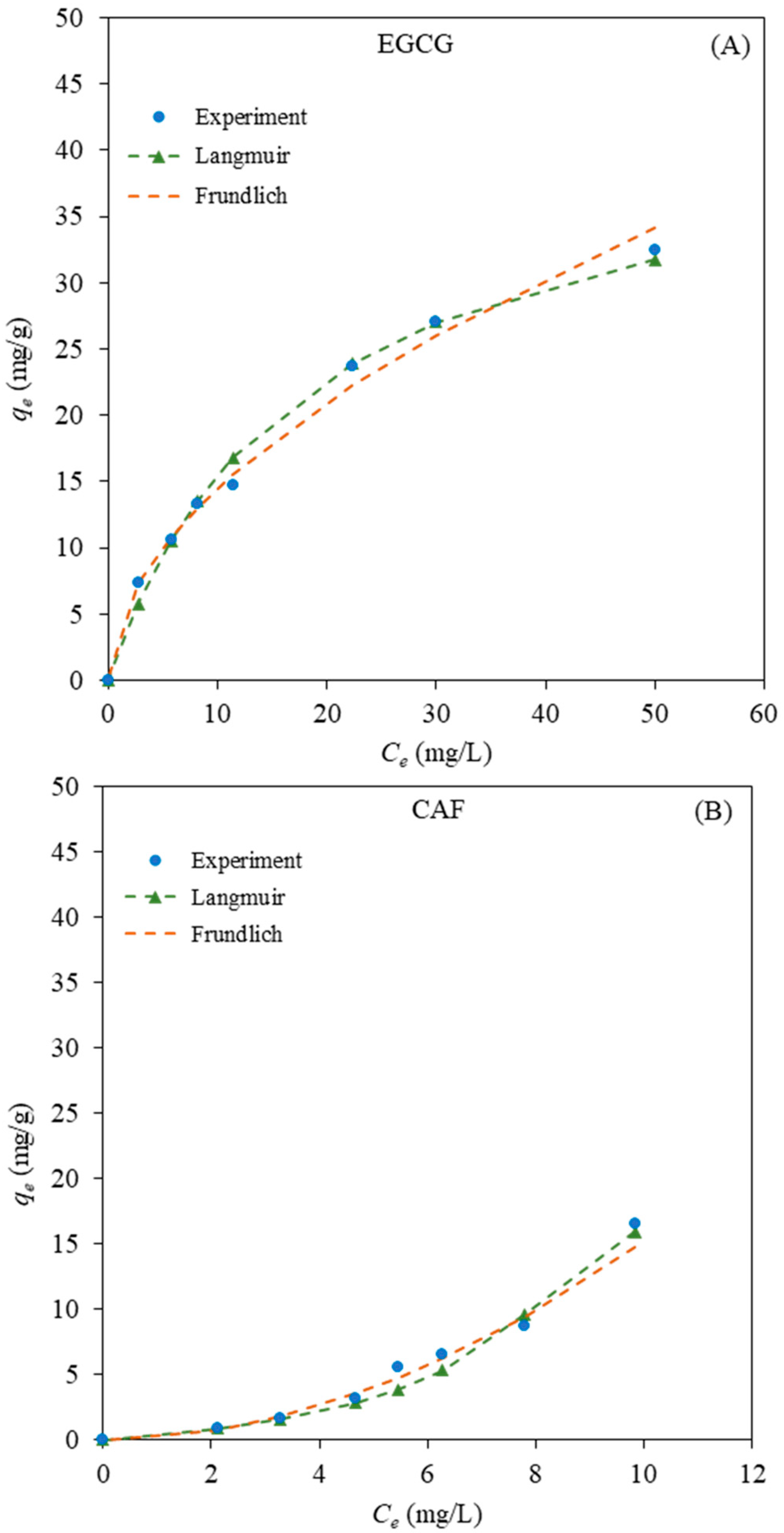
2.3.2. Adsorption Isotherms
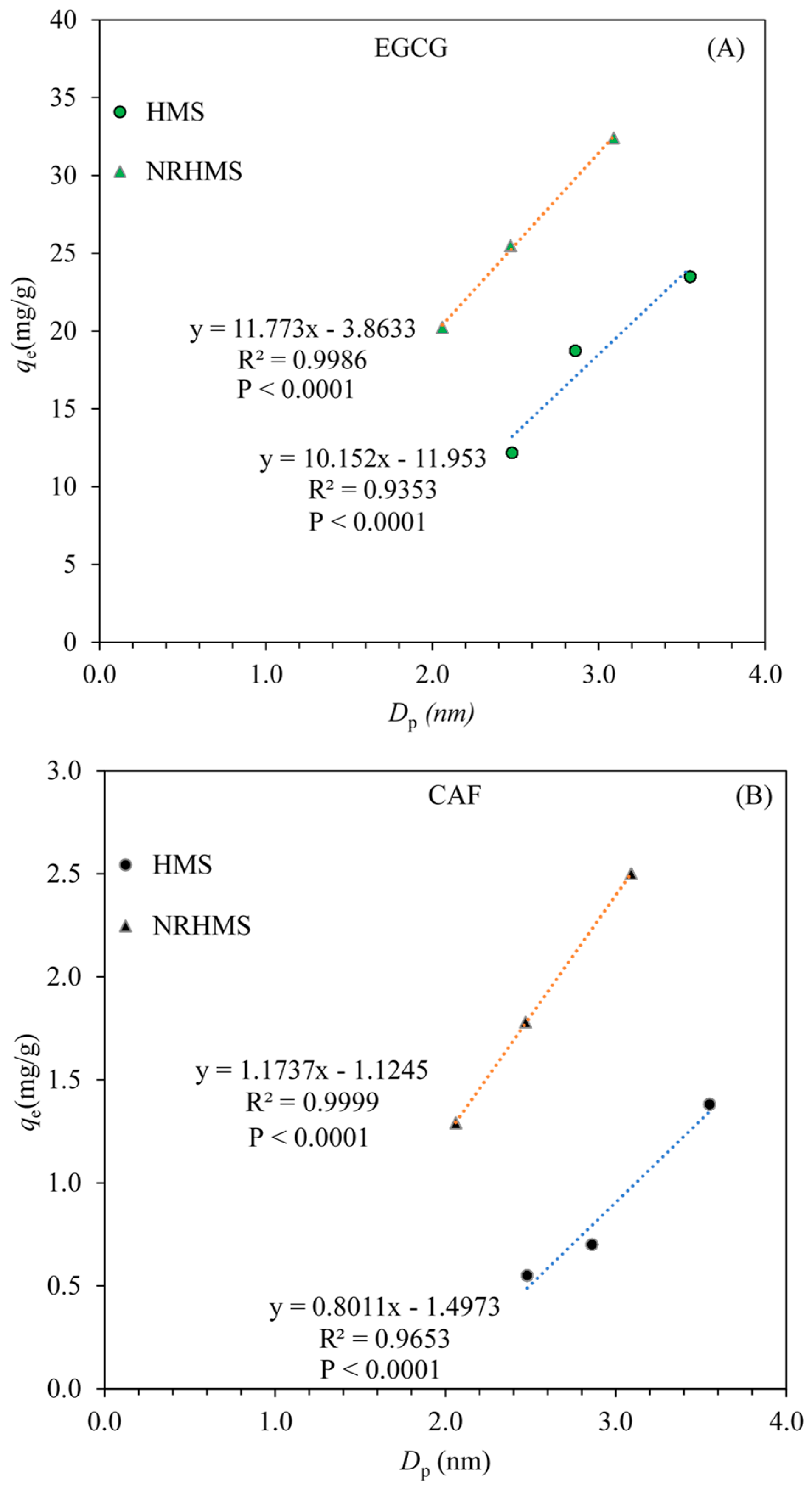
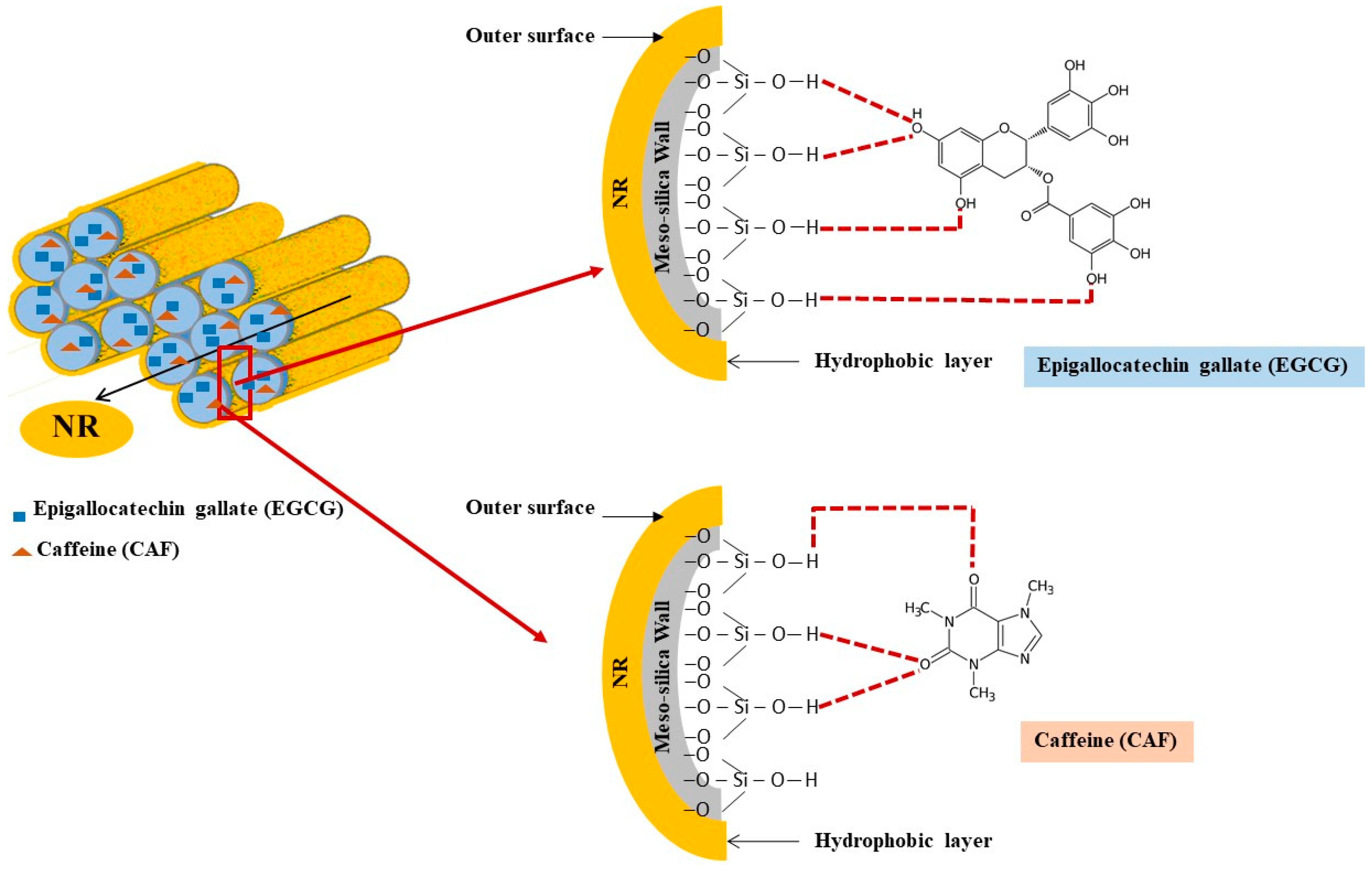
2.3.3. Adsorption Mechanism of EGCG and CAF on Mesoporous Silica Surface
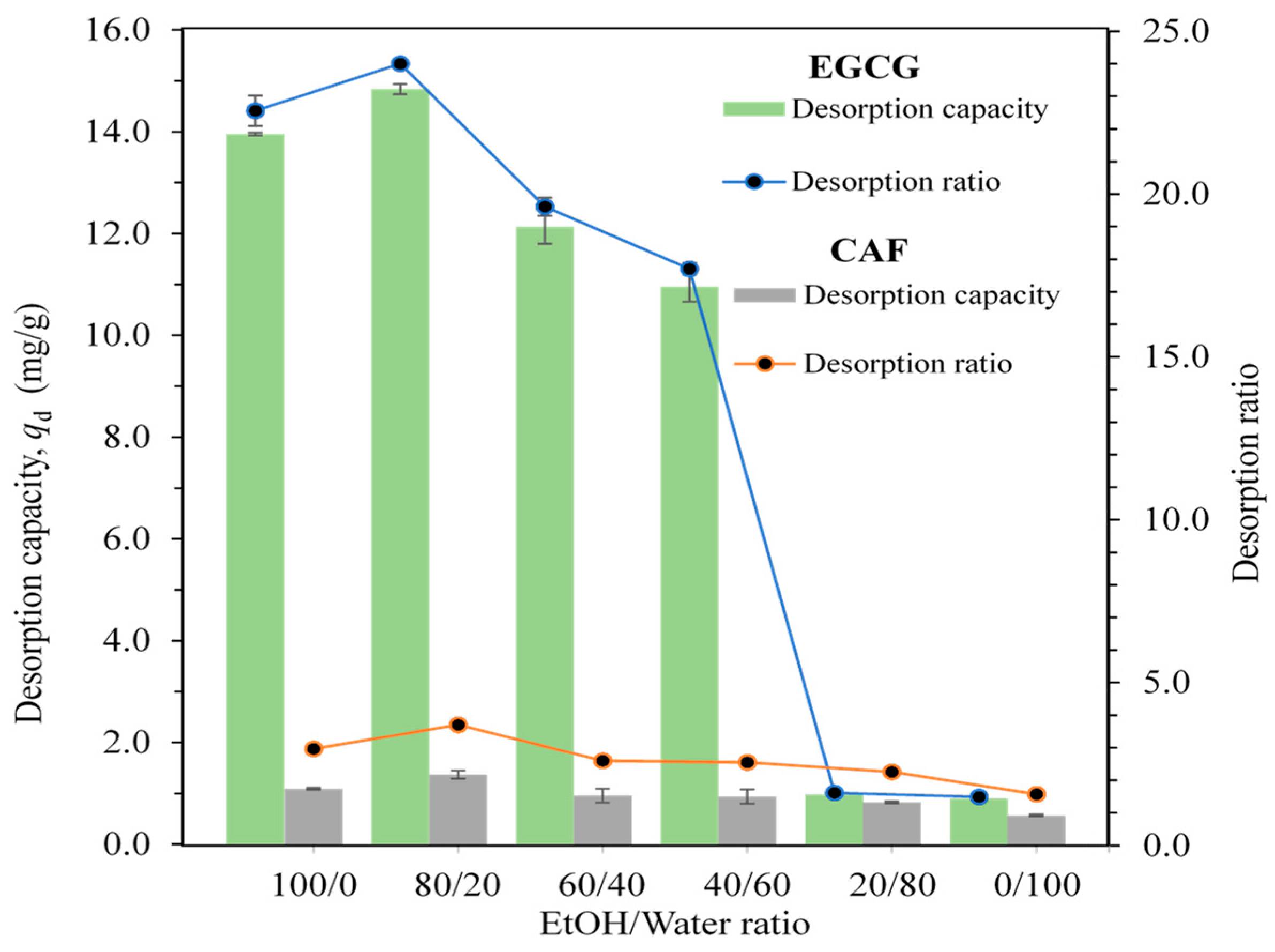
2.4. Desorption Capacity and Desorption Ratio
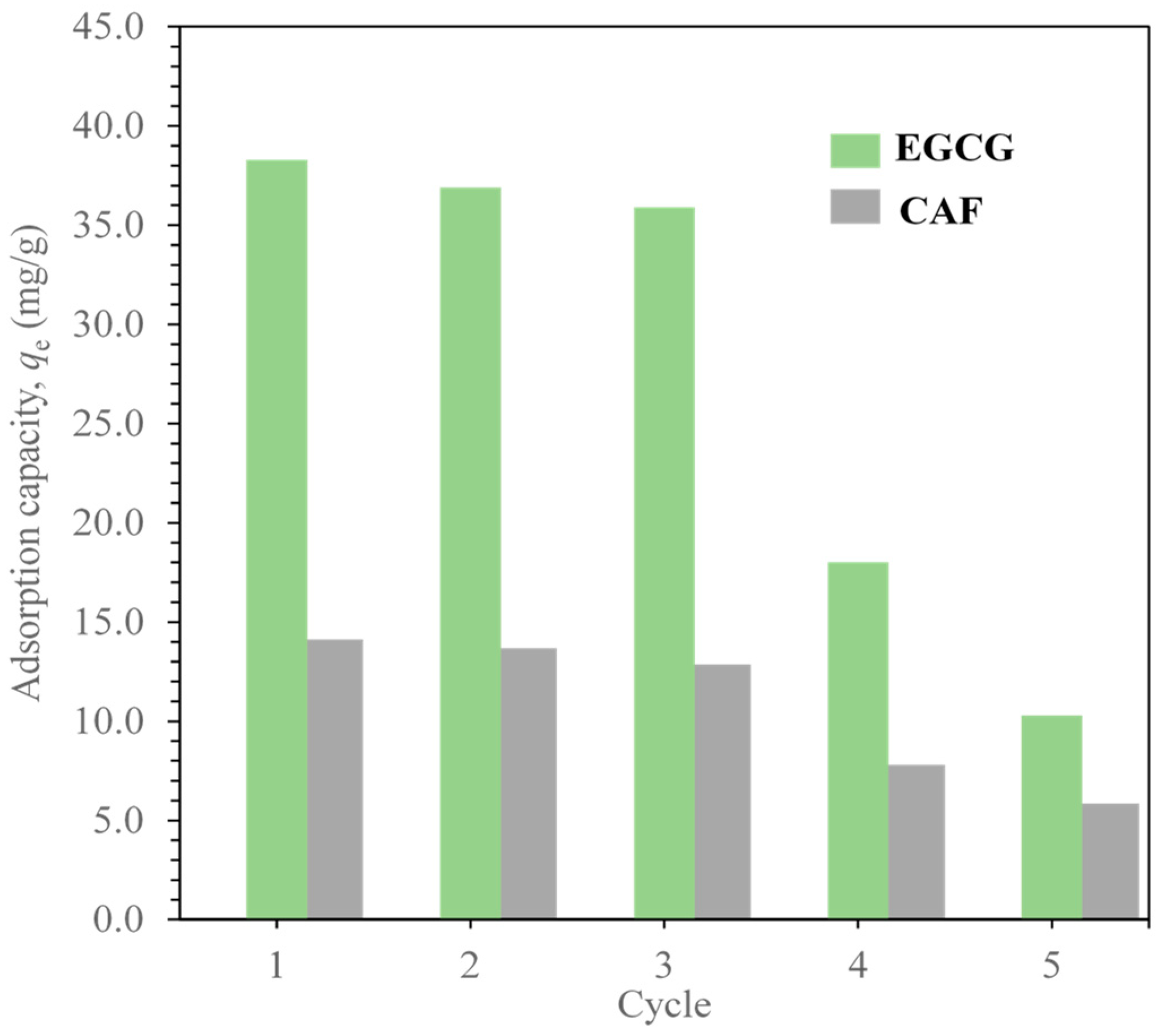
2.5. Reusability
2.6. Application to Reduce the CAF in Green Tea
3. Experimental
3.1. Materials and Chemical Reagents
3.2. Preparation of Adsorbents
3.2.1. Synthesis of Pure-Silica HMS Materials
3.2.2. Synthesis of NR/HMS Nanocomposites
3.3. Characterization of Pure-Silica HMS and NR/HMS Materials
3.4. Preparation of EGCG, CAF, and Green Tea Extract Solutions
3.5. Determination of EGCG and CAF Concentrations
3.6. Adsorption Procedure
3.7. Adsorption Kinetic Study
3.8. Adsorption Isotherm Study
3.9. Desorption of Adsorbed EGCG and CAF and Reusability of Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rady, I.; Mohamed, H.; Rady, M.; Siddiqui, I.A.; Mukhtar, H. Cancer preventive and therapeutic effects of EGCG, the major polyphenol in green tea. Egypt. J. Basic Appl. Sci. 2018, 5, 1–23. [Google Scholar] [CrossRef]
- Lambert, J.D.; Sang, S.; Hong, J.; Yang, C.S. Anticancer and Anti-inflammatory Effects of Cysteine Metabolites of the Green Tea Polyphenol, (−)-Epigallocatechin-3-gallate. J. Agric. Food Chem. 2010, 58, 10016–10019. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y. Novel uses of catechins in foods. Trends Food Sci. Technol. 2006, 17, 64–71. [Google Scholar] [CrossRef]
- Ho, H.-Y.; Cheng, M.-L.; Weng, S.-F.; Leu, Y.-L.; Chiu, D.T.-Y. Antiviral Effect of Epigallocatechin Gallate on Enterovirus 71. J. Agric. Food Chem. 2009, 57, 6140–6147. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, Y.; Ma, R.; Zhang, X. Separation of tea polyphenol from Green Tea Leaves by a combined CATUFM-adsorption resin process. J. Food Eng. 2005, 67, 253–260. [Google Scholar] [CrossRef]
- Jin, X.; Liu, M.; Chen, Z.; Mao, R.; Xiao, Q.; Gao, H.; Wei, M. Separation and purification of epigallocatechin-3-gallate (EGCG) from green tea using combined macroporous resin and polyamide column chromatography. J. Chromatogr. B 2015, 1002, 113–122. [Google Scholar] [CrossRef]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef]
- Smith, A. Effects of caffeine on human behavior. Food Chem. Toxicol. 2002, 40, 1243–1255. [Google Scholar] [CrossRef]
- Rosenfeld, L.S.; Mihalov, J.J.; Carlson, S.J.; Mattia, A. Regulatory status of caffeine in the United States. Nutr. Rev. 2014, 72, 23–33. [Google Scholar] [CrossRef]
- Copeland, E.; Clifford, M.; Williams, C. Preparation of (–)-epigallocatechin gallate from commercial green tea by caffeine precipitation and solvent partition. Food Chem. 1998, 61, 81–87. [Google Scholar] [CrossRef]
- Monsanto, M.; Hooshyar, N.; Meuldijk, J.; Zondervan, E. Modeling and optimization of green tea precipitation for the recovery of catechins. Sep. Purif. Technol. 2014, 129, 129–136. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Heidari, E. Extraction of epigallocatechin gallate from green tea via modified supercritical CO2: Experimental, modeling and optimization. J. Supercrit. Fluid. 2012, 72, 36–45. [Google Scholar] [CrossRef]
- Bermejo, D.V.; Ibáñez, E.; Reglero, G.; Fornari, T. Effect of cosolvents (ethyl lactate, ethyl acetate and ethanol) on the supercritical CO2 extraction of caffeine from green tea. J. Supercrit. Fluids. 2016, 107, 507–512. [Google Scholar] [CrossRef]
- Wang, S.; Liang, Y.; Zheng, S. Separation of epigallocatechin gallate from tea polyphenol by simulated moving bed chromatography. J. Chromatogr. A 2012, 1265, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Hong, S.; Liu, S. Simultaneous preparation of naturally abundant and rare catechins by tannase-mediated biotransformation combining high speed counter current chromatography. Food Chem. 2014, 151, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Ma, Y.; Xu, Z.; Liao, X.; Chen, A.; Yang, S. Isolation of strawberry anthocyanins using high-speed counter-current chromatography and the copigmentation with catechin or epicatechin by high pressure processing. Food Chem. 2018, 247, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.; Woonton, B.; Gee, M.L.; O’Connor, A.J. Hierarchical mesoporous silica materials for separation of functional food ingredients. Innov. Food Sci. Emerg. Technol. 2008, 9, 243–248. [Google Scholar] [CrossRef]
- Cotea, V.; Luchian, C.; Bilba, N.; Niculaua, M. Mesoporous silica SBA-15, a new adsorbent for bioactive polyphenols from red wine. Anal. Chim. Acta 2012, 732, 180–185. [Google Scholar] [CrossRef]
- Fan, J.-P.; Yuan, T.-T.; Xu, X.-K.; Zhang, X.-H.; Cheng, Y.-T.; Luo, J.-J. Preparation and characterization of mock strawberry-like aminopropyl-modified mesoporous silica for column chromatographic purification of paclitaxel in Taxus × Media. Chem. Eng. J. 2019, 359, 1509–1517. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwak, S.-Y. Functional mesoporous silica with controlled pore size for selective adsorption of free fatty acid and chlorophyll. Microporous Mesoporous Mater. 2020, 306, 110410. [Google Scholar] [CrossRef]
- Peter, J.; Nechikkattu, R.; Mohan, A.; Thomas, A.M.; Ha, C.-S. Stimuli-responsive organic-inorganic mesoporous silica hybrids: A comprehensive review on synthesis and recent advances. Mater. Sci. Eng. B 2021, 270, 115232. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, J.; Chen, L.; Liu, T.; Yu, L.; Liu, X.; Lu, C. Amino-functionalized ordered mesoporous silica SBA-15, a rapid and efficient adsorbent for the adsorption of (−)-epigallocatechin gallate from green tea extract. RSC Adv. 2014, 4, 41341–41347. [Google Scholar] [CrossRef]
- Nuntang, S.; Poompradub, S.; Butnark, S.; Yokoi, T.; Tatsumi, T.; Ngamcharussrivichai, C. Novel mesoporous composites based on natural rubber and hexagonal mesoporous silica: Synthesis and characterization. Mater. Chem. Phys. 2014, 143, 1199–1208. [Google Scholar] [CrossRef]
- Krueyai, Y.; Punyapalakul, P.; Wongrueng, A. Removal of haloacetonitrile by adsorption on thiol-functionalized meso-porous composites based on natural rubber and hexagonal mesoporous silica. Environ. Eng. Res. 2015, 20, 342–346. [Google Scholar] [CrossRef]
- Nuntang, S.; Yousatit, S.; Chaowamalee, S.; Yokoi, T.; Tatsumi, T.; Ngamcharussrivichai, C. Mesostructured natural rubber/in situ formed silica nanocomposites: A simple way to prepare mesoporous silica with hydrophobic properties. Microporous Mesoporous Mater. 2018, 259, 79–88. [Google Scholar] [CrossRef]
- Nuntang, S.; Yousatit, S.; Yokoi, T.; Ngamcharussrivichai, C. Tunable mesoporosity and hydrophobicity of natural rubber/hexagonal mesoporous silica nanocomposites. Microporous Mesoporous Mater. 2019, 275, 235–243. [Google Scholar] [CrossRef]
- Yousatit, S.; Pitayachinchot, H.; Wijitrat, A.; Chaowamalee, S.; Nuntang, S.; Soontaranon, S.; Rugmai, S.; Yokoi, T.; Ngamcharussrivichai, C. Natural rubber as a renewable carbon source for mesoporous carbon/silica nanocomposites. Sci. Rep. 2020, 10, 12977. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, X.; Alula, Y.; Yao, S. Bionic multi-tentacled ionic liquid-modified silica gel for adsorption and separation of polyphenols from green tea (Camellia sinensis) leaves. Food Chem. 2017, 230, 637–648. [Google Scholar] [CrossRef]
- Zhao, R.; Yan, Y.; Li, M.; Yan, H. Selective adsorption of tea polyphenols from aqueous solution of the mixture with caffeine on macroporous crosslinked poly(N-vinyl-2-pyrrolidinone). React. Funct. Polym. 2008, 68, 768–774. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Huang, D.; Liu, Y.; Wang, H.; Di, D. Comparison of adsorption selectivity for (–)-epigallocatechin gallate and caffeine by porous materials modified with different amino groups. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 520, 166–172. [Google Scholar] [CrossRef]
- Cazzola, M.; Corazzari, I.; Prenesti, E.; Bertone, E.; Vernè, E. Bioactive glass coupling with natural polyphenols: Surface modification, bioactivity and anti-oxidant ability. Appl. Surf. Sci. 2016, 367, 237–248. [Google Scholar] [CrossRef]
- Riccucci, G.; Cazzola, M.; Ferraris, S.; Gobbo, V.; Guaita, M.; Spriano, S. Surface functionalization of Ti6Al4V with an extract of polyphenols from red grape pomace. Mater. Des. 2021, 206, 109776. [Google Scholar] [CrossRef]
- Kim, H.J.; Bae, I.-S.; Cho, S.-J.; Boo, J.-H.; Lee, B.-C.; Heo, J.; Chung, I.; Hong, B. Synthesis and characteristics of NH2-functionalized polymer films to align and immobilize DNA molecules. Nanoscale Res. Lett. 2012, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Zhang, S.; Lee, Y.R.; Kang, K.K.; Kim, J.M.; Ahn, J.W.; Ahn, W.S. EDTA-functionalized KCC-1 and KIT-6 mesoporous silicas for Nd3+ ion recovery from aqueous solutions. J. Ind. Eng. Chem. 2018, 67, 210–218. [Google Scholar] [CrossRef]
- Fan, F.-Y.; Gan, Q.; Dong, Z.-B.; Song, K.-J.; Zheng, X.-Q.; Liang, Y.-R.; Lu, J.-L. Selective elution of tea catechins and caffeine from polyvinylpolypyrrolidone. Int. J. Food Sci. Technol. 2014, 49, 1626–1634. [Google Scholar] [CrossRef]


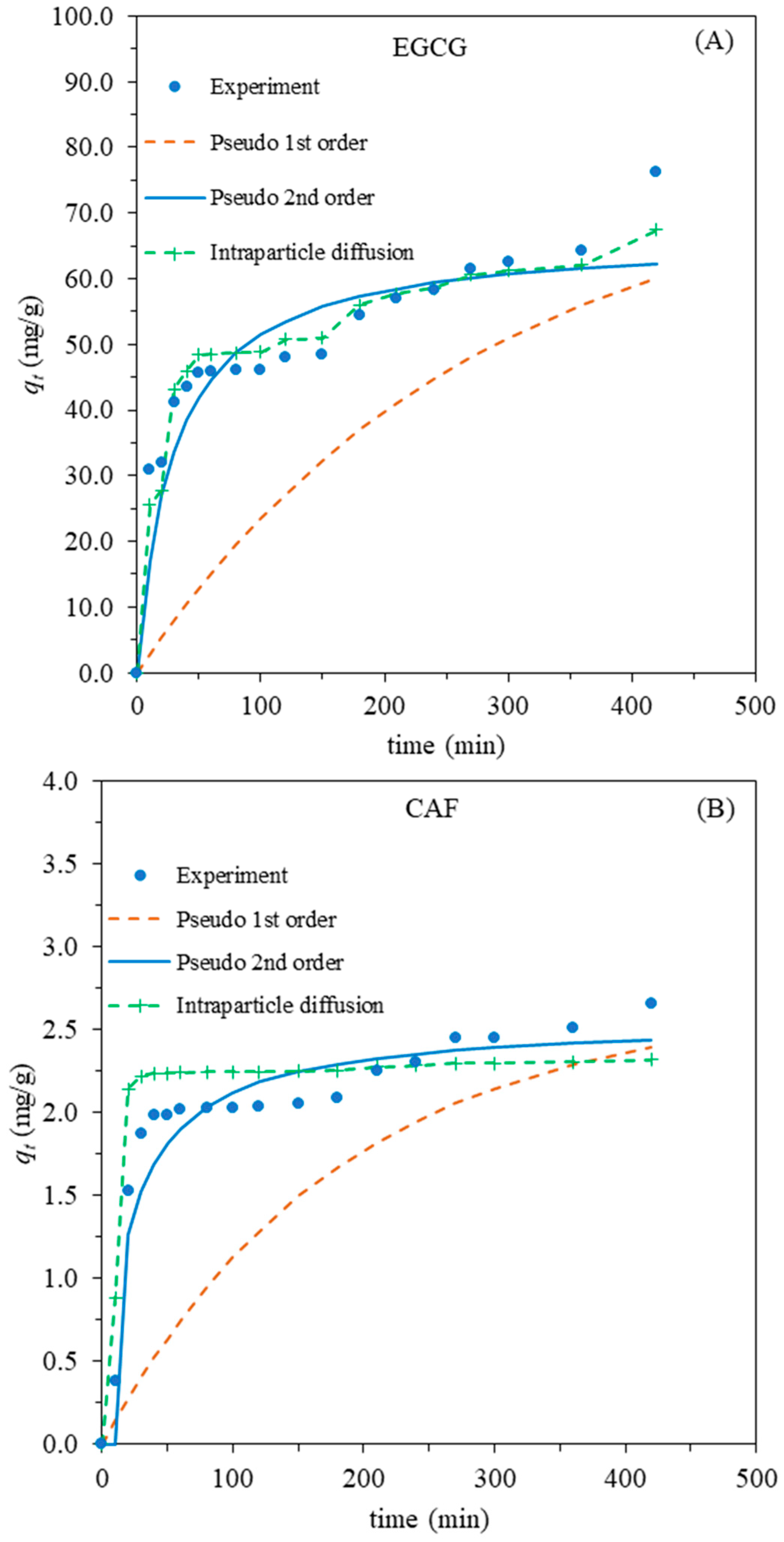

| Sample | qe | Pseudo-First-Order Model | Pseudo-Second-Order Model | Intraparticle Diffusion | |||||
|---|---|---|---|---|---|---|---|---|---|
| K1 | qe | R2 | K2 | qe | R2 | KP | R2 | ||
| (exp) (mg/g) | (min−1) | (mg/g) | (g/mg min) | (cal) (mg/g) | (mg/g min1/2) | ||||
| EGCG | 76.2 | 0.00369 | 41.6 | 0.9769 | 0.00051 | 66.7 | 0.9903 | 1.11 | 0.9151 |
| CAF | 2.7 | 0.00553 | 1.2 | 0.9418 | 0.01920 | 2.6 | 0.9911 | 0.01 | 0.9123 |
| Sample | Freundlich | Langmuir | ||||
|---|---|---|---|---|---|---|
| KF | n | R2 | KL | qm | R2 | |
| ((mg/g)(mL/mg)1/n) | (mg/mL) | (mg/g) | ||||
| EGCG | 4.2384 | 1.8741 | 0.9929 | 0.05589 | 43.1 | 0.9992 |
| CAF | 0.1834 | 0.5211 | 0.9907 | 0.09696 | 3.4 | 0.9929 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jermjun, K.; Khumho, R.; Thongoiam, M.; Yousatit, S.; Yokoi, T.; Ngamcharussrivichai, C.; Nuntang, S. Natural Rubber/Hexagonal Mesoporous Silica Nanocomposites as Efficient Adsorbents for the Selective Adsorption of (−)-Epigallocatechin Gallate and Caffeine from Green Tea. Molecules 2023, 28, 6019. https://doi.org/10.3390/molecules28166019
Jermjun K, Khumho R, Thongoiam M, Yousatit S, Yokoi T, Ngamcharussrivichai C, Nuntang S. Natural Rubber/Hexagonal Mesoporous Silica Nanocomposites as Efficient Adsorbents for the Selective Adsorption of (−)-Epigallocatechin Gallate and Caffeine from Green Tea. Molecules. 2023; 28(16):6019. https://doi.org/10.3390/molecules28166019
Chicago/Turabian StyleJermjun, Kamolwan, Rujeeluk Khumho, Mookarin Thongoiam, Satit Yousatit, Toshiyuki Yokoi, Chawalit Ngamcharussrivichai, and Sakdinun Nuntang. 2023. "Natural Rubber/Hexagonal Mesoporous Silica Nanocomposites as Efficient Adsorbents for the Selective Adsorption of (−)-Epigallocatechin Gallate and Caffeine from Green Tea" Molecules 28, no. 16: 6019. https://doi.org/10.3390/molecules28166019
APA StyleJermjun, K., Khumho, R., Thongoiam, M., Yousatit, S., Yokoi, T., Ngamcharussrivichai, C., & Nuntang, S. (2023). Natural Rubber/Hexagonal Mesoporous Silica Nanocomposites as Efficient Adsorbents for the Selective Adsorption of (−)-Epigallocatechin Gallate and Caffeine from Green Tea. Molecules, 28(16), 6019. https://doi.org/10.3390/molecules28166019






