Analysis of Pork in Beef Sausages Using LC-Orbitrap HRMS Untargeted Metabolomics Combined with Chemometrics for Halal Authentication Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Untargeted Metabolomics Analysis
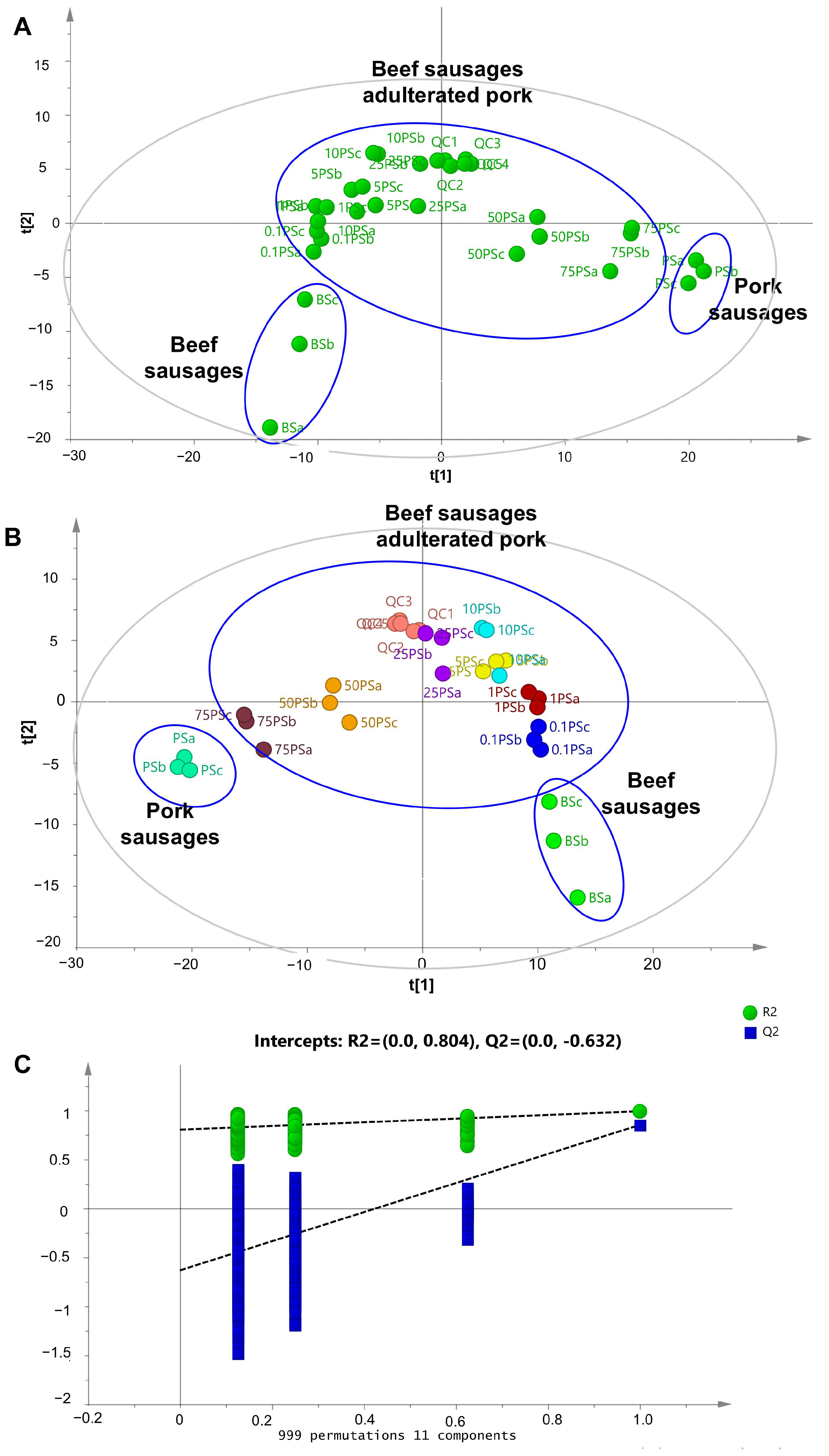
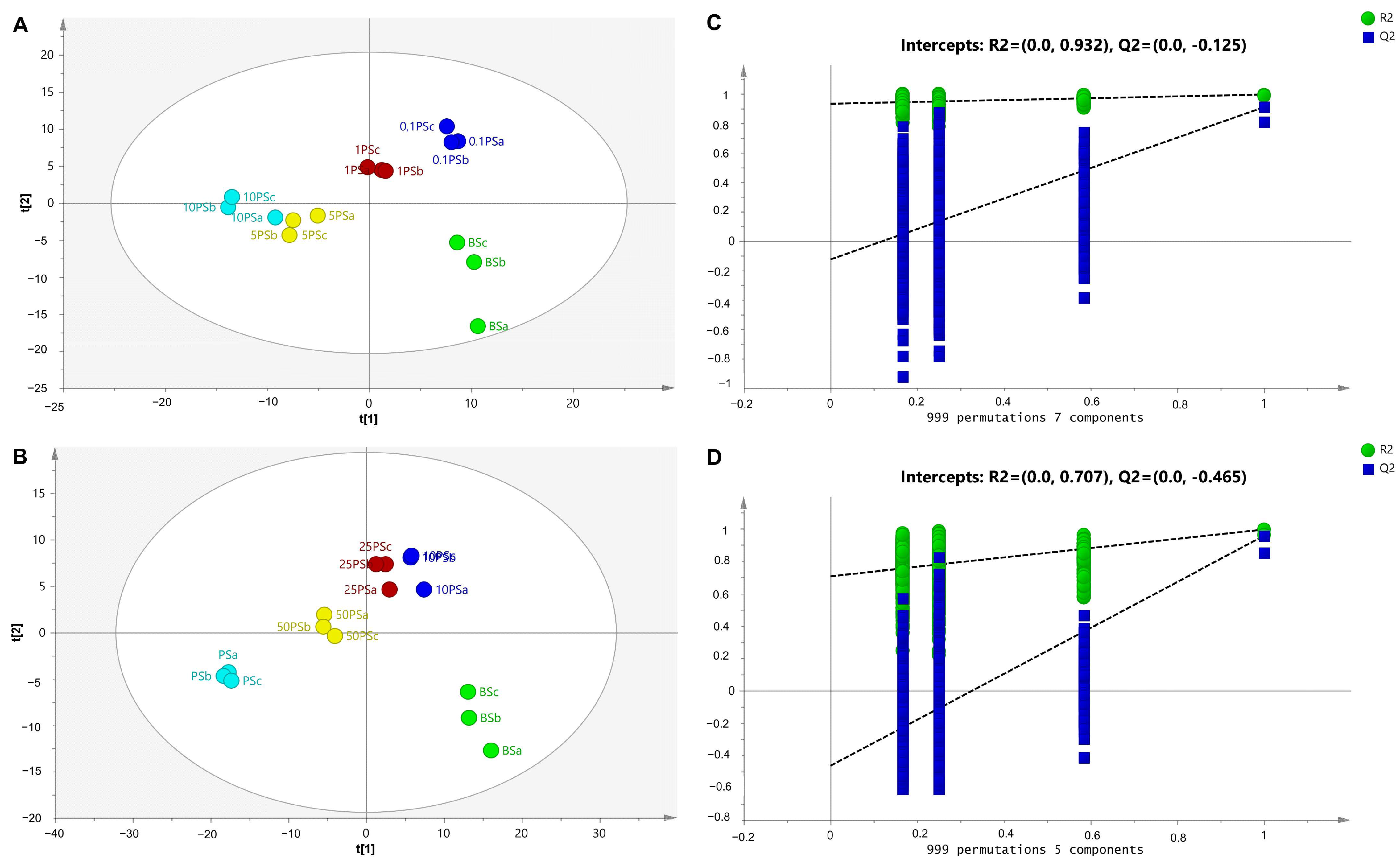
2.2. Chemometrics Analysis: Supervised and Unsupervised Pattern Recognition
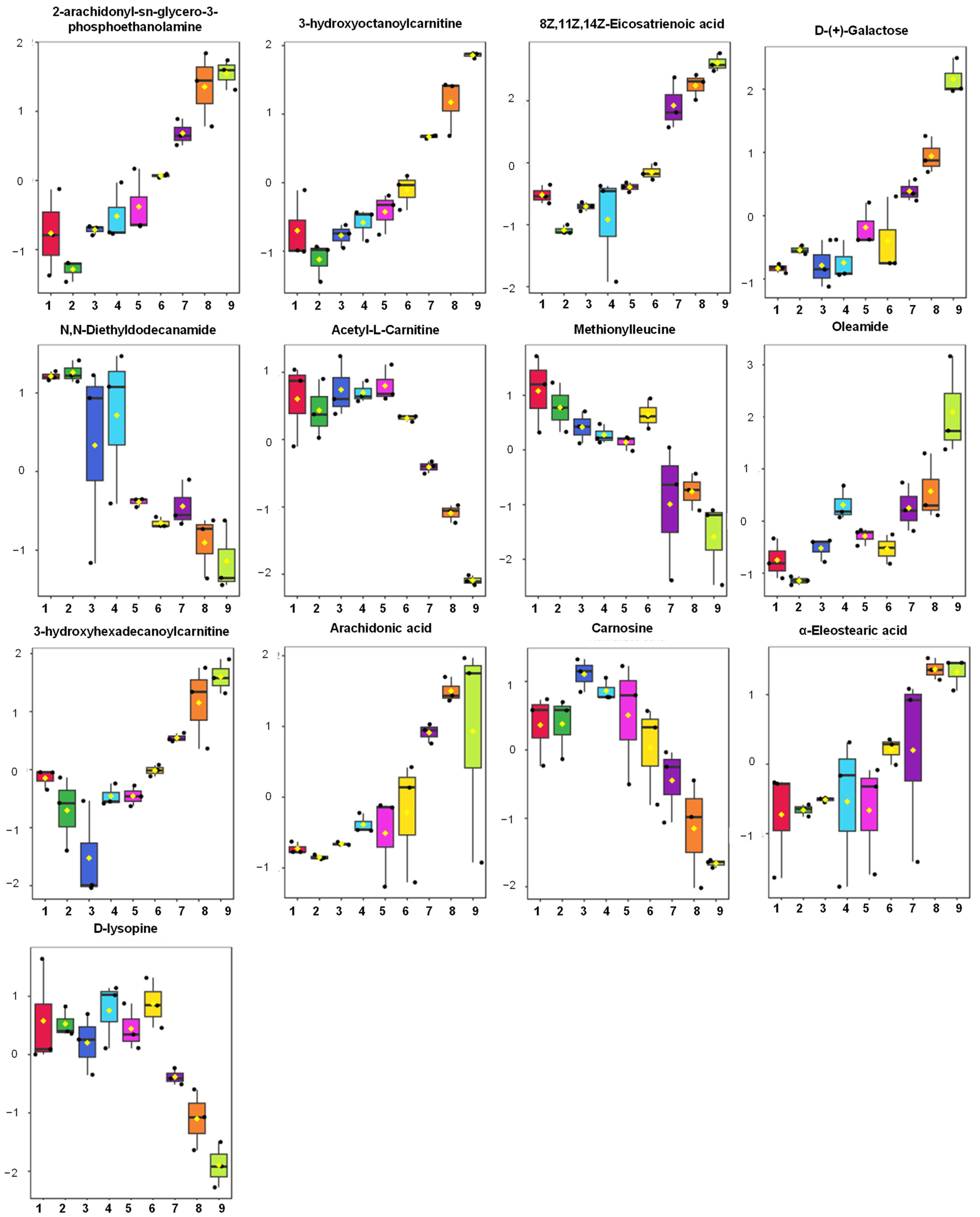
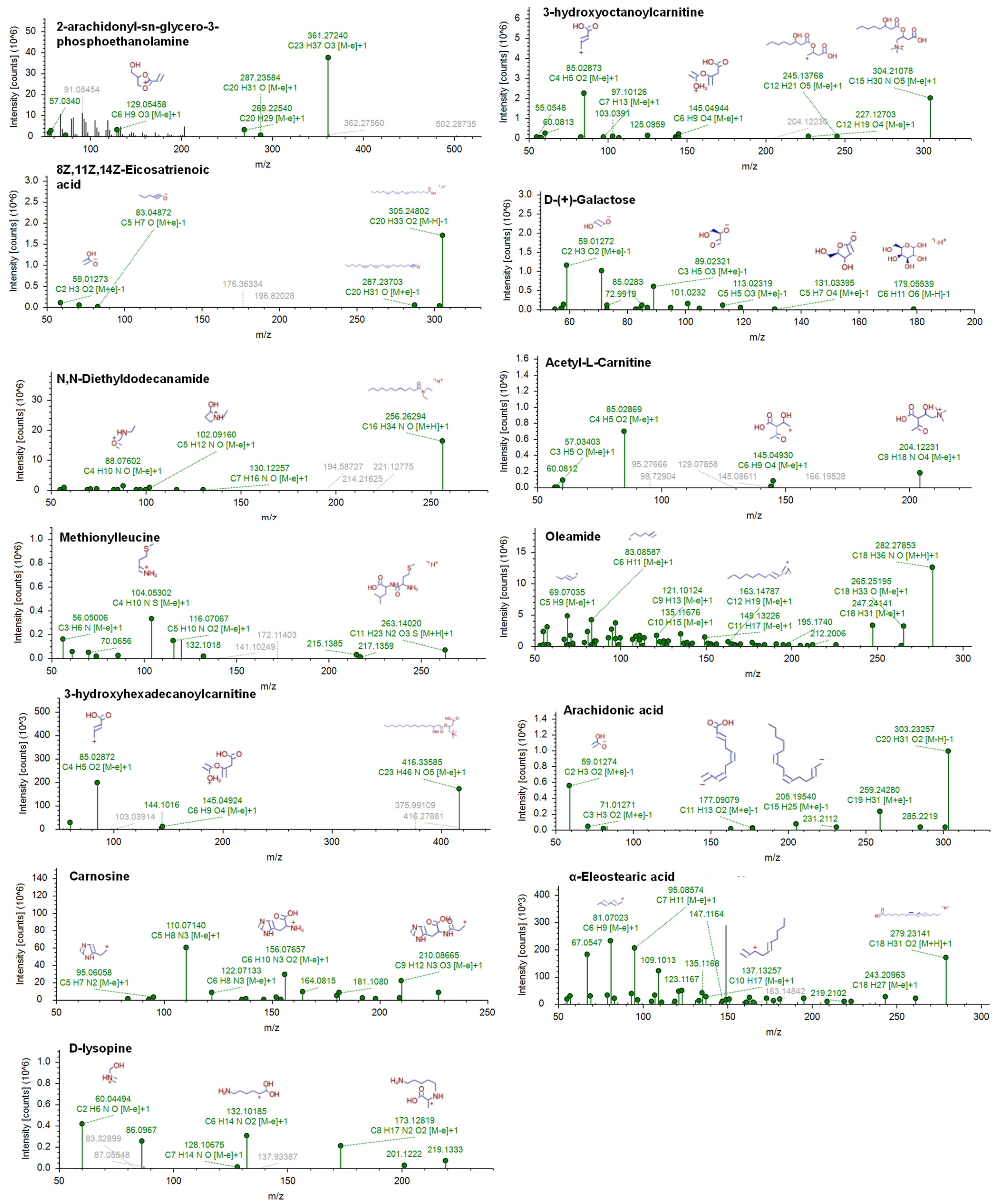
2.3. Identification of Discriminating Metabolites
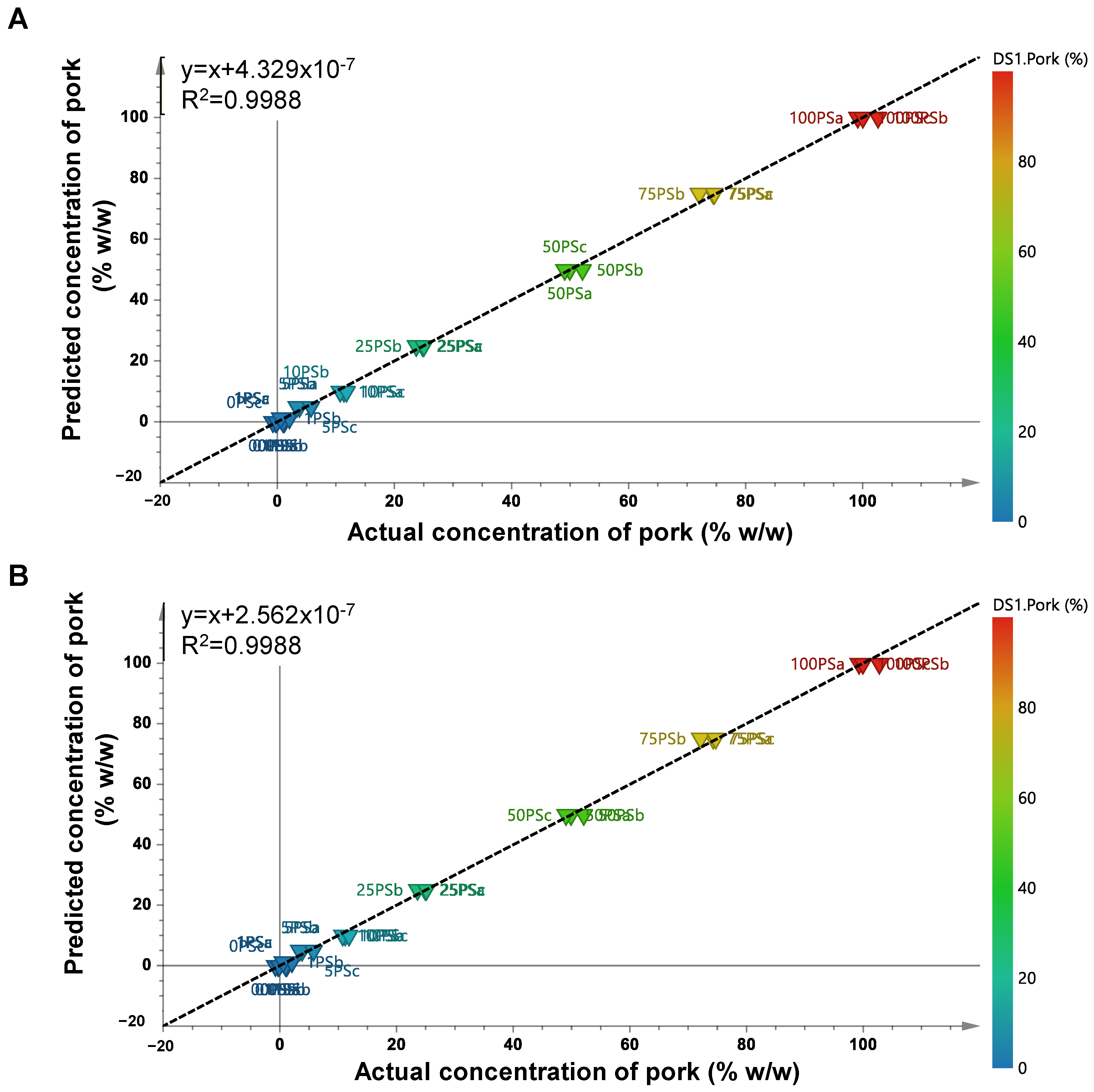
2.4. Chemometrics Analysis: Multivariate Calibration
3. Materials and Methods
3.1. Chemicals
3.2. Meat Collection
3.3. Sausage Formulation
3.4. Extraction of Metabolites from Sausages
3.5. Metabolomics Analysis
3.6. Data Processing
3.7. Chemometrics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ng, P.C.; Ahmad Ruslan, N.A.S.; Chin, L.X.; Ahmad, M.; Abu Hanifah, S.; Abdullah, Z.; Khor, S.M. Recent Advances in Halal Food Authentication: Challenges and Strategies. J. Food Sci. 2022, 87, 8–35. [Google Scholar] [CrossRef] [PubMed]
- Owolabi, I.O.; Olayinka, J.A. Incidence of Fraud and Adulterations in ASEAN Food/Feed Exports: A 20-Year Analysis of RASFF’s Notifications. PLoS ONE 2021, 16, e0259298. [Google Scholar] [CrossRef]
- Rohman, A.; Windarsih, A. The Application of Molecular Spectroscopy in Combination with Chemometrics for Halal Authentication Analysis: A Review. Int. J. Mol. Sci. 2020, 21, 5155. [Google Scholar] [CrossRef]
- Martuscelli, M.; Serio, A.; Capezio, O.; Mastrocola, D. Safety, Quality and Analytical Authentication of Halal Meat Products, with Particular Emphasis on Salami: A Review. Foods 2020, 9, 1111. [Google Scholar] [CrossRef]
- Hossain, M.A.M.; Uddin, S.M.K.; Sultana, S.; Wahab, Y.A.; Sagadevan, S.; Johan, M.R.; Ali, M.E. Authentication of Halal and Kosher Meat and Meat Products: Analytical Approaches, Current Progresses and Future Prospects. Crit. Rev. Food Sci. Nutr. 2022, 62, 285–310. [Google Scholar] [CrossRef]
- Siswara, H.N.; Erwanto, Y.; Suryanto, E. Study of Meat Species Adulteration in Indonesian Commercial Beef Meatballs Related to Halal Law Implementation. Front. Sustain. Food Syst. 2022, 6, 271–280. [Google Scholar] [CrossRef]
- Perestam; Fujisaki, K.K.; Nava, O.; Hellberg, R.S. Comparison of Real-Time PCR and ELISA-Based Methods for the Detection of Beef and Pork in Processed Meat Products. Food Control 2017, 71, 346–352. [Google Scholar] [CrossRef]
- Pranata, A.W.; Yuliana, N.D.; Amalia, L.; Darmawan, N. Volatilomics for Halal and Non-Halal Meatball Authentication Using Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry. Arab. J. Chem. 2021, 14, 103146. [Google Scholar] [CrossRef]
- Nurani, L.H.; Riswanto, F.D.O.; Windarsih, A.; Edityaningrum, C.A.; Guntarti, A.; Rohman, A. Use of Chromatographic-Based Techniques and Chemometrics for Halal Authentication of Food Products: A Review. Int. J. Food Prop. 2022, 25, 1399–1416. [Google Scholar] [CrossRef]
- Teixeira, J.L.d.P.; Caramês, E.T.d.S.; Baptista, D.P.; Gigante, M.L.; Pallone, J.A.L. Vibrational Spectroscopy and Chemometrics Tools for Authenticity and Improvement the Safety Control in Goat Milk. Food Control 2020, 112, 107105. [Google Scholar] [CrossRef]
- Akhtar, M.T.; Samar, M.; Shami, A.A.; Mumtaz, M.W.; Mukhtar, H.; Tahir, A.; Shahzad-Ul-hussan, S.; Chaudhary, S.U.; Kaka, U. H-NMR-Based Metabolomics: An Integrated Approach for the Detection of the Adulteration in Chicken, Chevon, Beef and Donkey Meat. Molecules 2021, 26, 4643. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.M.; Zainal Abidin, S.A.S.; Bujang, A.; Taib, M.N.; Sagadevan, S.; Johan, M.R.; Ahmad Nizar, N.N. TaqMan Multiplex QPCR for Detecting Animal Species in Meat and Meat Products: Development, Recent Advances and Future Prospects. Food Control 2023, 150, 109761. [Google Scholar] [CrossRef]
- Capozzi, F.; Trimigno, A.; Ferranti, P. Proteomics and Metabolomics in Relation to Meat Quality; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081007631. [Google Scholar]
- Li, Y.C.; Liu, S.Y.; Meng, F.B.; Liu, D.Y.; Zhang, Y.; Wang, W.; Zhang, J.M. Comparative Review and the Recent Progress in Detection Technologies of Meat Product Adulteration. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2256–2296. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, Y.; Nunome, M.; Inoue, K. A Review on the Foodomics Based on Liquid Chromatography Mass Spectrometry. Chem. Pharm. Bull. 2022, 70, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Harrieder, E.M.; Kretschmer, F.; Böcker, S.; Witting, M. Current State-of-the-Art of Separation Methods Used in LC-MS Based Metabolomics and Lipidomics. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1188, 123069. [Google Scholar] [CrossRef]
- Hermanto, S.; Rudiana, T.; Ihda, M.; Zein, H.L.; Wisudawati, A.W. Methods Validation of Pork Authentication in Processed Meat Products (Sausages) Through Densitometry Analysis. Indones. J. Halal Res. 2022, 4, 35–44. [Google Scholar] [CrossRef]
- Guntarti, A.; Ahda, M.; Kusbandari, A.; Prihandoko, S. Analysis of Lard in Sausage Using Fourier Transform Infrared Spectrophotometer Combined with Chemometrics. J. Pharm. Bioallied Sci. 2019, 11, S594–S600. [Google Scholar] [CrossRef]
- Böhme, K.; Calo-Mata, P.; Barros-Velázquez, J.; Ortea, I. Recent Applications of Omics-Based Technologies to Main Topics in Food Authentication. TrAC Trends Anal. Chem. 2019, 110, 221–232. [Google Scholar] [CrossRef]
- Abbas, N.; Ali, A.; Kumari, S.; Iqbal, A.; Husain, A.; Saeed, T.; AbdulAmer Al-Ballam, Z.; Ahmed, N.; El-Seedi, H.R.; Musharraf, S.G. Untargeted-Metabolomics Differentiation between Poultry Samples Slaughtered with and without Detaching Spinal Cord. Arab. J. Chem. 2020, 13, 9081–9089. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Y.; Zhou, R.; Ma, M. Comprehensive Identification of Molecular Profiles Related to Sensory and Nutritional Changes in Mongolian Cheese during Storage by Untargeted Metabolomics Coupled with Quantification of Free Amino Acids. Food Chem. 2022, 386, 132740. [Google Scholar] [CrossRef]
- Trivedi, D.K.; Hollywood, K.A.; Rattray, N.J.W.; Ward, H.; Trivedi, D.K.; Greenwood, J.; Ellis, D.I.; Goodacre, R. Meat, the Metabolites: An Integrated Metabolite Profiling and Lipidomics Approach for the Detection of the Adulteration of Beef with Pork. Analyst 2016, 141, 2155–2164. [Google Scholar] [CrossRef]
- Mialon, N.; Roig, B.; Capodanno, E.; Cadiere, A. Untargeted Metabolomic Approaches in Food Authenticity: A Review That Showcases Biomarkers. Food Chem. 2023, 398, 133856. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Carpena, M.; Garcia-Oliveira, P.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Analytical Metabolomics and Applications in Health, Environmental and Food Science. Crit. Rev. Anal. Chem. 2020, 52, 712–734. [Google Scholar] [CrossRef]
- Nurani, L.H.; Rohman, A.; Windarsih, A.; Guntarti, A.; Riswanto, F.D.O.; Lukitaningsih, E.; Fadzillah, N.A.; Rafi, M. Metabolite Fingerprinting Using 1H-NMR Spectroscopy and Chemometrics for Classification of Three Curcuma Species from Different Origins. Molecules 2021, 26, 7626. [Google Scholar] [CrossRef]
- Aydoğan, C. Recent Advances and Applications in LC-HRMS for Food and Plant Natural Products: A Critical Review. Anal. Bioanal. Chem. 2020, 412, 1973–1991. [Google Scholar] [CrossRef]
- Paul, A.; De, P.; Harrington, B. Chemometric Applications in Metabolomic Studies Using Chromatography-Mass Spectrometry. TrAC Trends Anal. Chem. 2021, 135, 116165. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metabolomics 2013, 1, 92–107. [Google Scholar] [CrossRef]
- Feizi, N.; Hashemi-Nasab, F.S.; Golpelichi, F.; Sabouruh, N.; Parastar, H. Recent Trends in Application of Chemometric Methods for GC-MS and GC×GC-MS-Based Metabolomic Studies. TrAC Trends Anal. Chem. 2021, 138, 116239. [Google Scholar] [CrossRef]
- Liu, H.; Wei, B.; Tang, Q.; Chen, C.; Li, Y.; Yang, Q.; Wang, J.; Li, J.; Qi, J.; Xi, Y.; et al. Non-Target Metabolomics Reveals the Changes of Small Molecular Substances in Duck Breast Meat under Different Preservation Time. Food Res. Int. 2022, 161, 111859. [Google Scholar] [CrossRef]
- Han, H.; Li, M.; Liu, Y.; Yu, H.; Cao, X.; Zhao, H.; Wang, B.; Yue, X.; Zheng, Y. Non-Volatile Metabolite Changes in Low-Temperature Sausage Stored at Room Temperature. Food Packag. Shelf Life 2022, 31, 100805. [Google Scholar] [CrossRef]
- Jia, W.; Wu, X.; Zhang, R.; Shi, L. UHPLC-Q-Orbitrap-Based Lipidomics Reveals Molecular Mechanism of Lipid Changes during Preservatives Treatment of Hengshan Goat Meat Sausages. Food Chem. 2022, 369, 130948. [Google Scholar] [CrossRef]
- Suratno; Windarsih, A.; Warmiko, H.D.; Khasanah, Y.; Indrianingsih, A.W.; Rohman, A. Metabolomics and Proteomics Approach Using LC-Orbitrap HRMS for the Detection of Pork in Tuna Meat for Halal Authentication. Food Anal. Methods 2023, 16, 867–877. [Google Scholar] [CrossRef]
- Windarsih, A.; Riswanto, F.D.O.; Bakar, N.K.A.; Yuliana, N.D.; Dachriyanus; Rohman, A. Detection of Pork in Beef Meatballs Using LC-HRMS Based Untargeted Metabolomics and Chemometrics for Halal Authentication. Molecules 2022, 27, 8325. [Google Scholar] [CrossRef]
- Cao, M.; Han, Q.; Zhang, J.; Zhang, R.; Wang, J.; Gu, W.; Kang, W.; Lian, K.; Ai, L. An Untargeted and Pseudotargeted Metabolomic Combination Approach to Identify Differential Markers to Distinguish Live from Dead Pork Meat by Liquid Chromatography–Mass Spectrometry. J. Chromatogr. A 2020, 1610, 460553. [Google Scholar] [CrossRef]
- Wen, D.; Liu, Y.; Yu, Q. Metabolomic Approach to Measuring Quality of Chilled Chicken Meat during Storage. Poult. Sci. 2020, 99, 2543–2554. [Google Scholar] [CrossRef]
- Panseri, S.; Arioli, F.; Pavlovic, R.; Di Cesare, F.; Nobile, M.; Mosconi, G.; Villa, R.; Chiesa, L.M.; Bonerba, E. Impact of Irradiation on Metabolomics Profile of Ground Meat and Its Implications toward Food Safety. LWT-Food Sci. Technol. 2022, 161, 113305. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. PCA as a Practical Indicator of OPLS-DA Model Reliability. Curr. Metabolomics 2016, 4, 97–103. [Google Scholar] [CrossRef]
- Lottering, R.T.; Govender, M.; Peerbhay, K.; Lottering, S. Comparing Partial Least Squares (PLS) Discriminant Analysis and Sparse PLS Discriminant Analysis in Detecting and Mapping Solanum Mauritianum in Commercial Forest Plantations Using Image Texture. ISPRS J. Photogramm. Remote Sens. 2020, 159, 271–280. [Google Scholar] [CrossRef]
- Medina, S.; Perestrelo, R.; Silva, P.; Pereira, J.A.M.; Câmara, J.S. Current Trends and Recent Advances on Food Authenticity Technologies and Chemometric Approaches. Trends Food Sci. Technol. 2019, 85, 163–176. [Google Scholar] [CrossRef]
- Jalil, H.; Shahidhussain, S.; Siddiqi, A.F. An Empirical Study of Meat Supply Chain and PricesPattern in Lahore (Pakistan): A Case Study. J. Supply Chain Manag. Syst. 2013, 2, 44–52. [Google Scholar]
- Knüttel-Gustavsen, S.; Harmeyer, J. The Content of L-Carnitine in Meat after Different Methods of Heat Treatment. Br. Food J. 2011, 113, 1114–1126. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and Pathophysiology of Carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Triki, M.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Quality Assessment of Fresh Meat from Several Species Based on Free Amino Acid and Biogenic Amine Contents during Chilled Storage. Foods 2018, 7, 132. [Google Scholar] [CrossRef]
- Galindo-Prieto, B.; Eriksson, L.; Trygg, J. Variable Influence on Projection (VIP) for OPLS Models and Its Applicability in Multivariate Time Series Analysis. Chemom. Intell. Lab. Syst. 2015, 146, 297–304. [Google Scholar] [CrossRef]
- Shawky, E.; Abu El-Khair, R.A.; Selim, D.A. NIR Spectroscopy-Multivariate Analysis for Rapid Authentication, Detection and Quantification of Common Plant Adulterants in Saffron (Crocus sativus L.) Stigmas. LWT-Food Sci. Technol. 2020, 122, 109032. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Windarsih, A.; Bakar, N.K.A.; Dachriyanus; Yuliana, N.D.; Riswanto, F.D.O.; Rohman, A. Analysis of Pork in Beef Sausages Using LC-Orbitrap HRMS Untargeted Metabolomics Combined with Chemometrics for Halal Authentication Study. Molecules 2023, 28, 5964. https://doi.org/10.3390/molecules28165964
Windarsih A, Bakar NKA, Dachriyanus, Yuliana ND, Riswanto FDO, Rohman A. Analysis of Pork in Beef Sausages Using LC-Orbitrap HRMS Untargeted Metabolomics Combined with Chemometrics for Halal Authentication Study. Molecules. 2023; 28(16):5964. https://doi.org/10.3390/molecules28165964
Chicago/Turabian StyleWindarsih, Anjar, Nor Kartini Abu Bakar, Dachriyanus, Nancy Dewi Yuliana, Florentinus Dika Octa Riswanto, and Abdul Rohman. 2023. "Analysis of Pork in Beef Sausages Using LC-Orbitrap HRMS Untargeted Metabolomics Combined with Chemometrics for Halal Authentication Study" Molecules 28, no. 16: 5964. https://doi.org/10.3390/molecules28165964
APA StyleWindarsih, A., Bakar, N. K. A., Dachriyanus, Yuliana, N. D., Riswanto, F. D. O., & Rohman, A. (2023). Analysis of Pork in Beef Sausages Using LC-Orbitrap HRMS Untargeted Metabolomics Combined with Chemometrics for Halal Authentication Study. Molecules, 28(16), 5964. https://doi.org/10.3390/molecules28165964








