Comparison of Reactive Sites in 2(1H)-Quinolone Derivatives for the Detection of Biologically Important Sulfur Compounds
Abstract
1. Introduction
2. Results
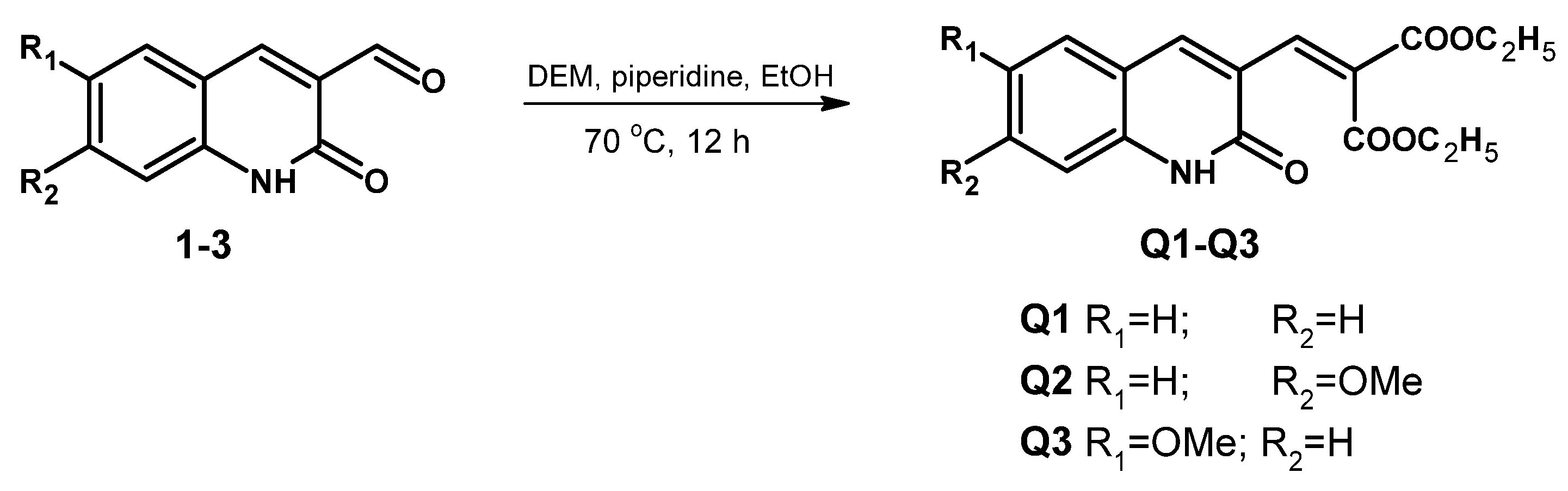
2.1. Synthesis and Characterization of Q1–Q3
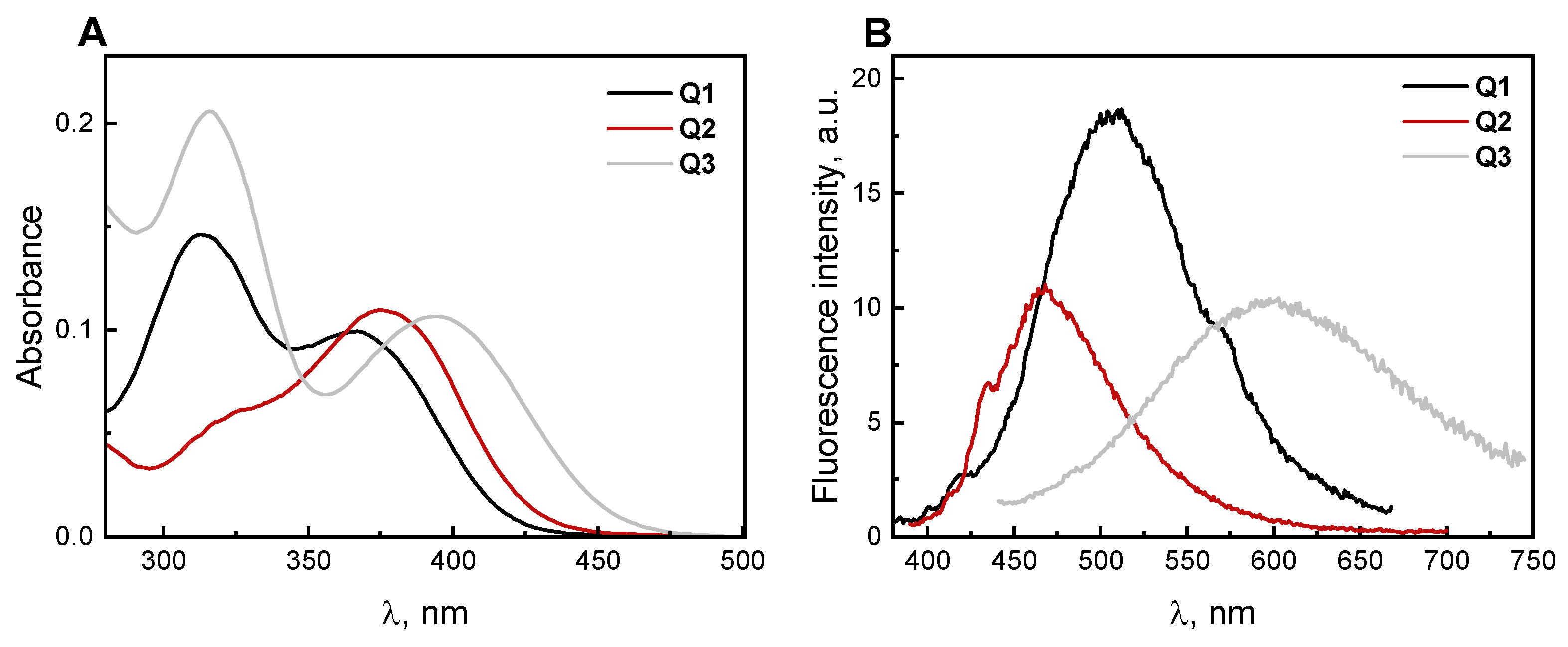
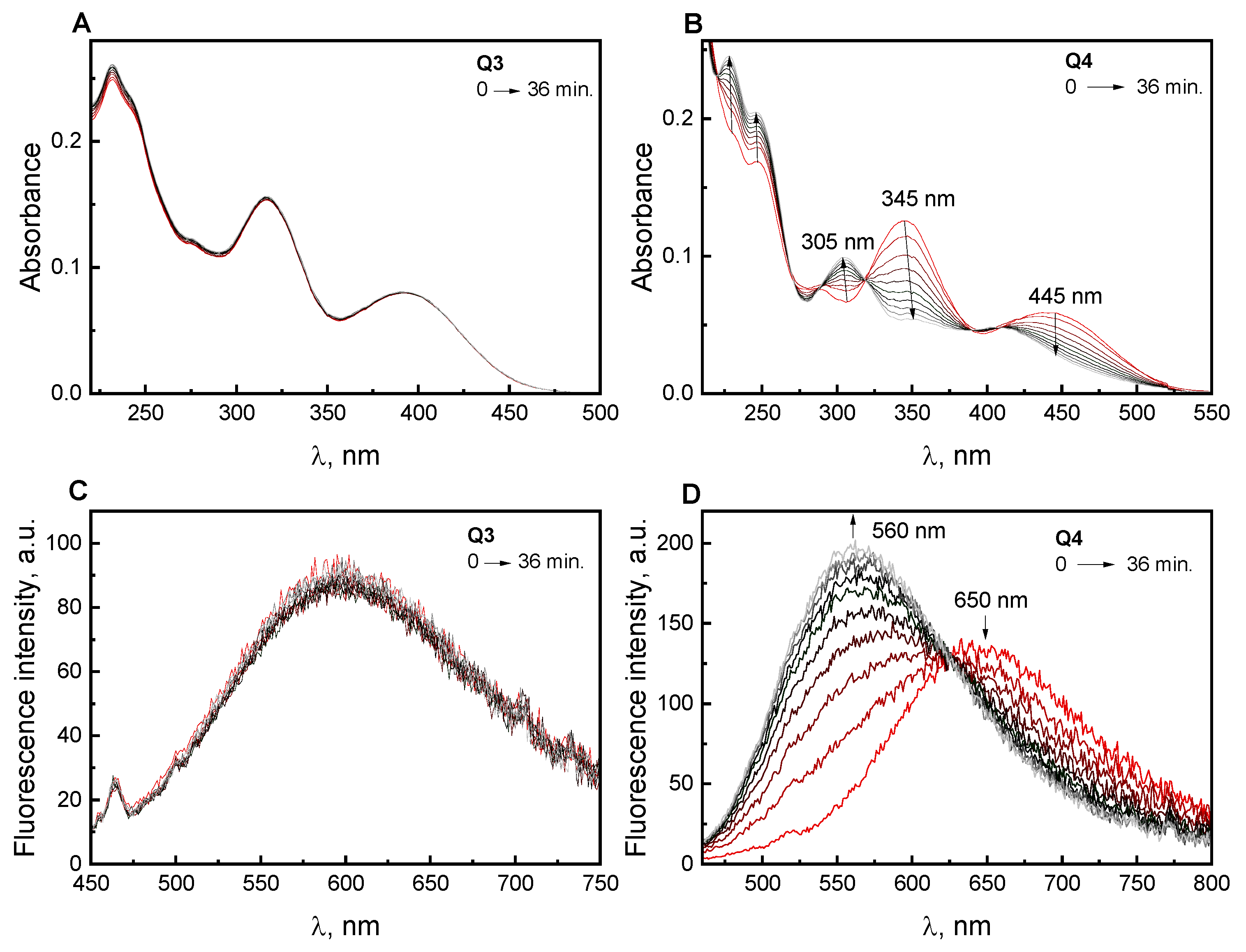
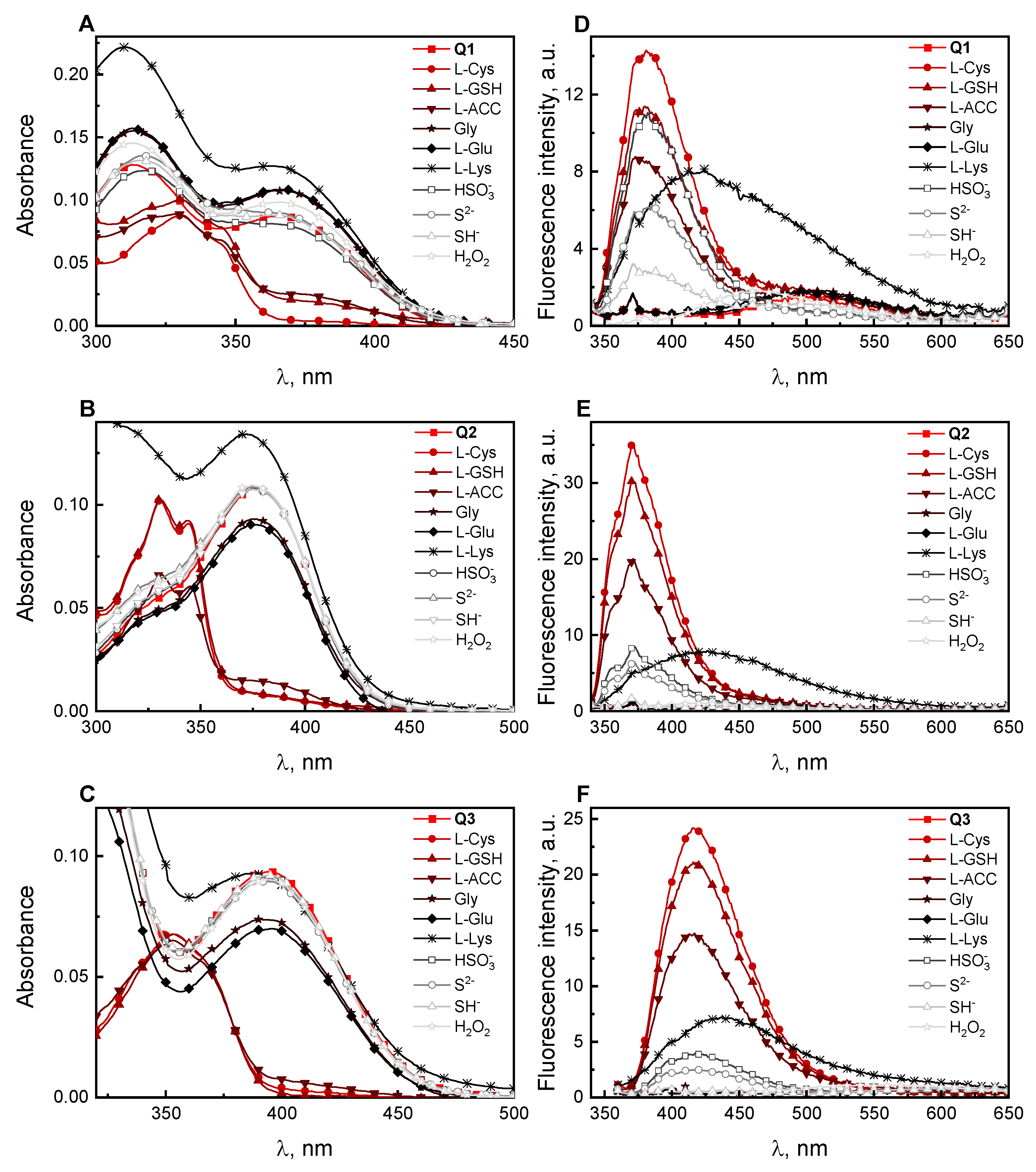
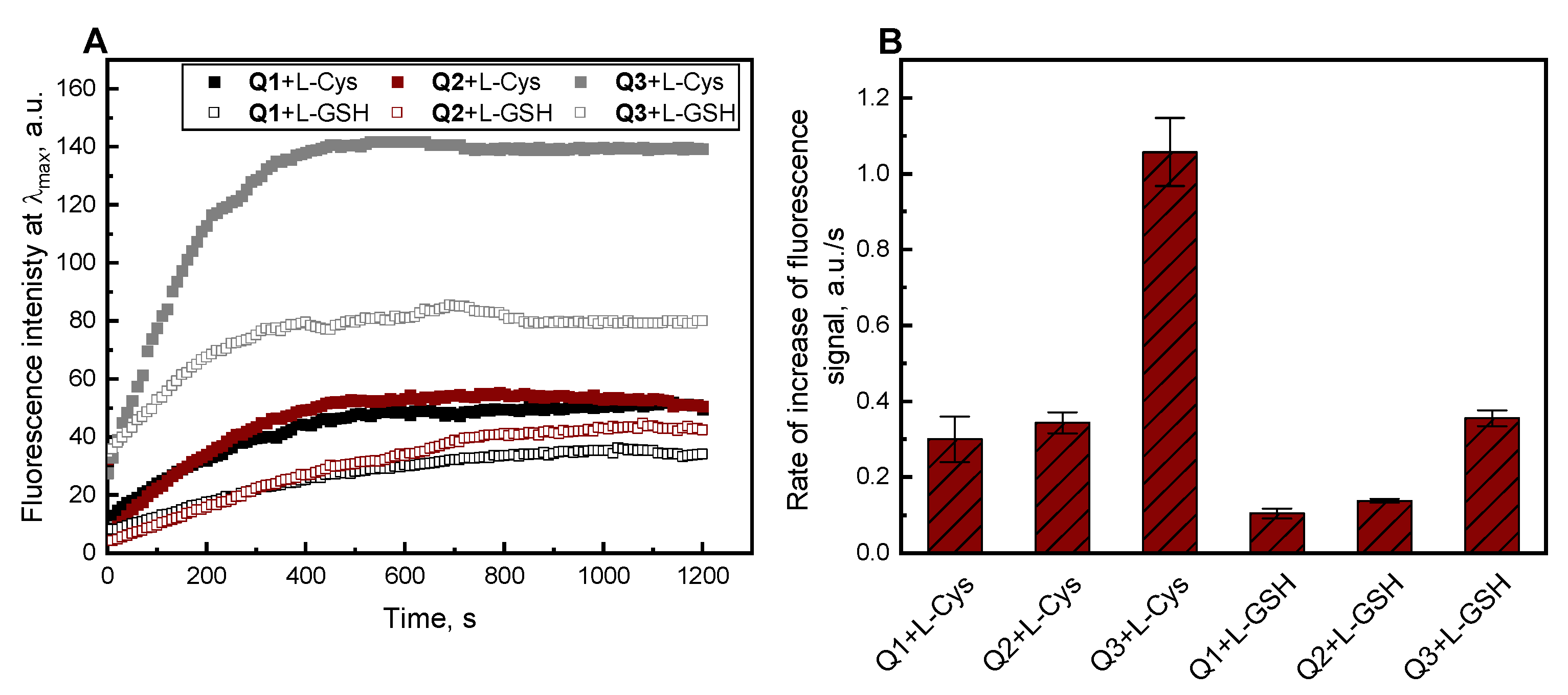
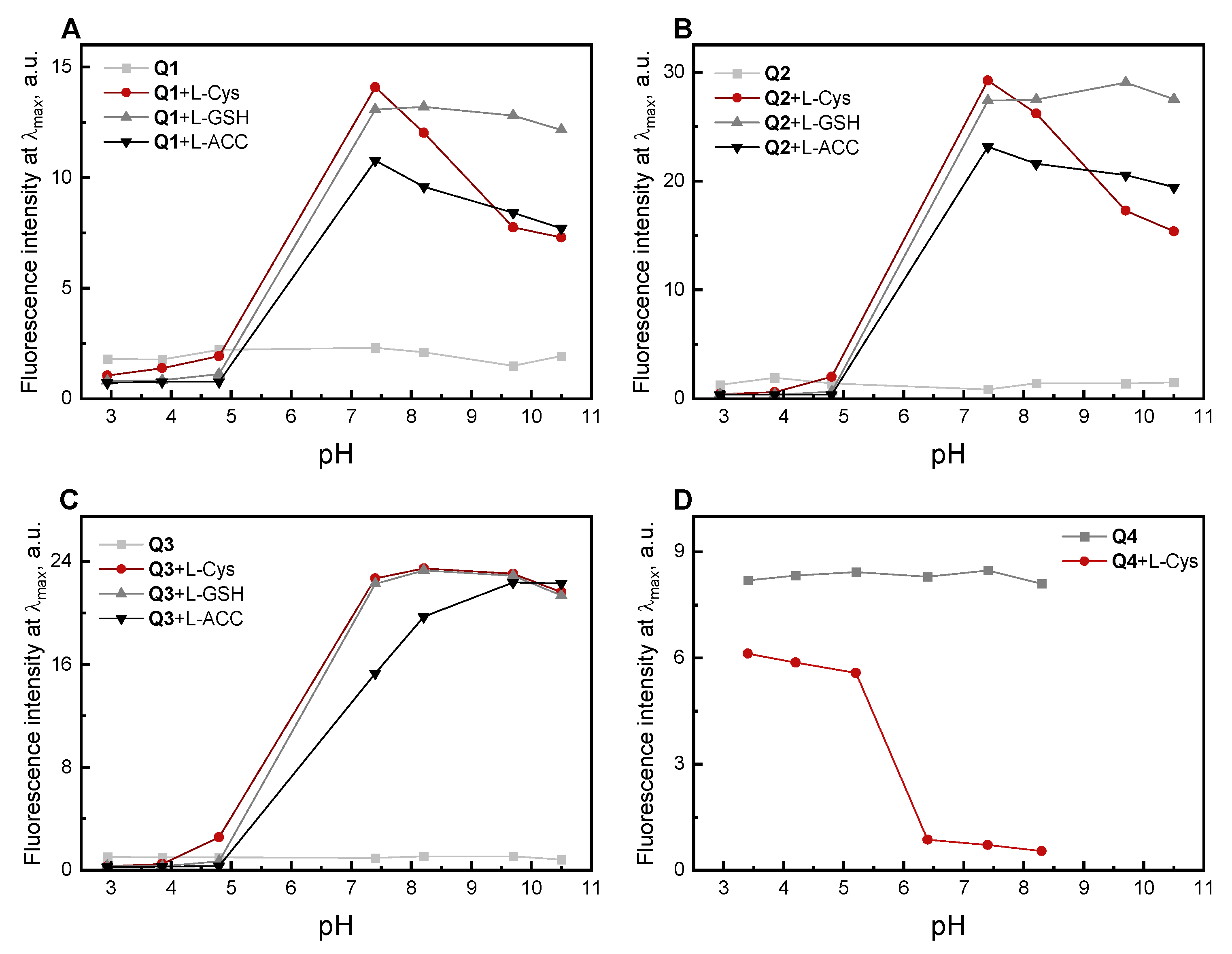
2.2. Absorption and Fluorescence Responses of Q1–Q3 toward the Thiols
3. Materials and Methods
3.1. General
3.2. Spectroscopic Measurements
3.3. General Procedure Synthesis of Compound Q1–Q3
3.4. General Procedure Spectroscopic Experiments of Compounds Q1–Q4
3.5. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gruhlke, M.C.H.; Slusarenko, A.J. The Biology of Reactive Sulfur Species (RSS). Plant Physiol. Biochem. 2012, 59, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.S.; Chen, W.; Xian, M.; Chang, C.J. Chemical Probes for Molecular Imaging and Detection of Hydrogen Sulfide and Reactive Sulfur Species in Biological Systems. Chem. Soc. Rev. 2015, 44, 4596–4618. [Google Scholar] [CrossRef] [PubMed]
- Giles, G.I.; Nasim, M.J.; Ali, W.; Jacob, C. The Reactive Sulfur Species Concept: 15 Years On. Antioxidants 2017, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.; Pluth, M.D. Reactive Sulfur Species (RSS): Persulfides, Polysulfides, Potential, and Problems. Curr. Opin. Chem. Biol. 2019, 49, 1–8. [Google Scholar] [CrossRef]
- Włodek, L. Beneficial and Harmful Effects of Thiols. Pol. J. Pharmacol. 2002, 54, 215–223. [Google Scholar]
- Giles, G.I.; Jacob, C. Reactive Sulfur Species: An Emerging Concept in Oxidative Stress. Biol. Chem. 2002, 383, 375–388. [Google Scholar] [CrossRef]
- Giles, G.I.; Tasker, K.M.; Jacob, C. Hypothesis: The Role of Reactive Sulfur Species in Oxidative Stress. Free Radic. Biol. Med. 2001, 31, 1279–1283. [Google Scholar] [CrossRef]
- Münzel, T.; Hahad, O.; Sørensen, M.; Lelieveld, J.; Duerr, G.D.; Nieuwenhuijsen, M.; Daiber, A. Environmental Risk Factors and Cardiovascular Diseases: A Comprehensive Expert Review. Cardiovasc. Res. 2022, 118, 2880–2902. [Google Scholar] [CrossRef]
- Manivannan, Y.; Manivannan, B.; Beach, T.G.; Halden, R.U. Role of Environmental Contaminants in the Etiology of Alzheimer’s Disease: A Review. Curr. Alzheimer Res. 2015, 12, 116–146. [Google Scholar] [CrossRef]
- Ulrich, K.; Jakob, U. The Role of Thiols in Antioxidant Systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef]
- Pean, A.R.; Parsons, R.B.; Waring, R.H.; Williams, A.C.; Ramsden, D.B. Toxicity of Sulphur-Containing Compounds to Neuronal Cell Lines. J. Neurol. Sci. 1995, 129, 107–108. [Google Scholar] [CrossRef]
- Janáky, R.; Varga, V.; Hermann, A.; Saransaari, P.; Oja, S.S. Mechanisms of L-Cysteine Neurotoxicity. Neurochem. Res. 2000, 25, 1397–1405. [Google Scholar] [CrossRef]
- Yin, J.; Ren, W.; Yang, G.; Duan, J.; Huang, X.; Fang, R.; Li, C.; Li, T.; Yin, Y.; Hou, Y.; et al. L-Cysteine Metabolism and Its Nutritional Implications. Mol. Nutr. Food Res. 2016, 60, 134–146. [Google Scholar] [CrossRef]
- Giles, N.M.; Watts, A.B.; Giles, G.I.; Fry, F.H.; Littlechild, J.A.; Jacob, C. Metal and Redox Modulation of Cysteine Protein Function. Chem. Biol. 2003, 10, 677–693. [Google Scholar] [CrossRef]
- Meyer, A.J.; Hell, R. Glutathione Homeostasis and Redox-Regulation by Sulfhydryl Groups. Photosynth. Res. 2005, 86, 435–457. [Google Scholar] [CrossRef]
- Kaplowitz, N.; Fernändez-Checa, J.C.; Kannan, R.; Garcia-Ruiz, C.; Ookhtens, M.; Yi, J.-R. GSH Transporters: Molecular Characterization and Role in GSH Homeostasis. Biol. Chem. Hoppe Seyler. 1996, 377, 267–274. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef]
- Sies, H. Glutathione and Its Role in Cellular Functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef]
- Kosower, N.S.; Kosower, E.M. The Glutathione Status of Cells. In International Review of Cytology; Bourne, G.H., Danielli, J.F., Jeon, K.W., Eds.; Academic Press: Cambridge, MA, USA, 1978; Volume 54, pp. 109–160. [Google Scholar]
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione Dysregulation and the Etiology and Progression of Human Diseases. Biol. Chem. 2009, 390, 191–214. [Google Scholar] [CrossRef]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The Chemistry and Biological Activities of N-Acetylcysteine. Biochim. Biophys. Acta 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Millea, P.J. N-Acetylcysteine: Multiple Clinical Applications. Am. Fam. Phys. 2009, 80, 265–269. [Google Scholar]
- Kelly, G.S. Clinical Applications of N-Acetylcysteine. Altern. Med. Rev. 1998, 3, 114–127. [Google Scholar] [PubMed]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butler, J. The Antioxidant Action of N-Acetylcysteine: Its Reaction with Hydrogen Peroxide, Hydroxyl Radical, Superoxide, and Hypochlorous Acid. Free Radic. Biol. Med. 1989, 6, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Mlejnek, P.; Dolezel, P.; Kriegova, E.; Pastvova, N. N-Acetylcysteine Can Induce Massive Oxidative Stress, Resulting in Cell Death with Apoptotic Features in Human Leukemia Cells. Int. J. Mol. Sci. 2021, 22, 12635. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Ma, C.; Zhang, P.; Fu, Y.; Shen, B. Recent Progress in the Development of Fluorescent Probes for Detection of Biothiols. Dyes Pigment. 2020, 177, 108321. [Google Scholar] [CrossRef]
- Ding, S.; Liu, M.; Hong, Y. Biothiol-Specific Fluorescent Probes with Aggregation-Induced Emission Characteristics. Sci. China Chem. 2018, 61, 882–891. [Google Scholar] [CrossRef]
- Liu, W.; Chen, J.; Xu, Z. Fluorescent Probes for Biothiols Based on Metal Complex. Coord. Chem. Rev. 2021, 429, 213638. [Google Scholar] [CrossRef]
- Dong, J.; Lu, G.; Tu, Y.; Fan, C. Recent Research Progress of Red-Emitting/near-Infrared Fluorescent Probes for Biothiols. New J. Chem. 2022, 46, 10995–11020. [Google Scholar] [CrossRef]
- Kowalska, A.; Kolińska, J.; Podsiadły, R.; Sokołowska, J. Dyes Derived from 3-Formyl-2(1H)-Quinolone—Synthesis, Spectroscopic Characterisation, and Their Behaviour in the Presence of Sulfhydryl and Non-Sulfhydryl Amino Acids. Color. Technol. 2015, 131, 157–164. [Google Scholar] [CrossRef]
- Grzelakowska, A.; Kolińska, J.; Sokołowska, J. Synthesis, Spectroscopic Characterisation, and Potential Application of Dyes Containing a Carbostyril Skeleton as Sensors for Thiols. Color. Technol. 2016, 132, 121–129. [Google Scholar] [CrossRef]
- Kolińska, J.; Grzelakowska, A. Novel Styrylbenzimidazolium-Based Fluorescent Probe for Reactive Sulfur Species: Selectively Distinguishing between Bisulfite and Thiol Amino Acids. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 262, 120151. [Google Scholar] [CrossRef]
- Kolińska, J.; Grzelakowska, A.; Sokołowska, J. Dyes Based on the 2(1H)-Quinolone Skeleton as Potential Colorimetric and Fluorescent Sensors for Cyanide Anions. Color. Technol. 2019, 135, 501–509. [Google Scholar] [CrossRef]
- Zuo, Q.-P.; Li, B.; Pei, Q.; Li, Z.; Liu, S.-K. A Highly Selective Fluorescent Probe for Detection of Biological Samples Thiol and Its Application in Living Cells. J. Fluoresc. 2010, 20, 1307–1313. [Google Scholar] [CrossRef]
- Dai, C.-G.; Liu, X.-L.; Du, X.-J.; Zhang, Y.; Song, Q.-H. Two-Input Fluorescent Probe for Thiols and Hydrogen Sulfide Chemosensing and Live Cell Imaging. ACS Sens. 2016, 1, 888–895. [Google Scholar] [CrossRef]
- Wu, Q.-Q.; Xiao, Z.-F.; Du, X.-J.; Song, Q.-H. A Novel Ratiometric Two-Photon Fluorescent Probe for the Detection of Biothiols in Solution and Imaging of Living Cells. Chem. Asian J. 2013, 8, 2564–2568. [Google Scholar] [CrossRef]
- Song, Q.-H.; Wu, Q.-Q.; Liu, C.-H.; Du, X.-J.; Guo, Q.-X. A Novel Fluorescent Probe for Selective Detection of Thiols in Acidic Solutions and Labeling of Acidic Organelles in Live Cells. J. Mater. Chem. B 2013, 1, 438–442. [Google Scholar] [CrossRef]
- Fabian, W.M.; Niederreiter, K.S.; Uray, G.; Stadlbauer, W. Substituent effects on absorption and fluorescence spectra of carbostyrils. J. Mol. Struct. 1999, 477, 209–220. [Google Scholar] [CrossRef]
- Chu, X.-M.; Wang, C.; Liu, W.; Liang, L.-L.; Gong, K.-K.; Zhao, C.-Y.; Sun, K.-L. Quinoline and quinolone dimers and their biological activities: An overview. Eur. J. Med. Chem. 2019, 161, 101–117. [Google Scholar] [CrossRef]
- Li, X.-H.; Yan, J.-L.; Wu, W.-N.; Zhao, X.-L.; Wang, Y.; Fan, Y.-C.; Xu, Z.-H. A dual-response fluorescent probe for SO2 and viscosity and imaging application in lysosomes and zebrafish. Microchem. J. 2022, 181, 107653. [Google Scholar] [CrossRef]
- Li, X.-H.; Han, X.-F.; Wu, W.-N.; Zhao, X.-L.; Wang, Y.; Fan, Y.-C.; Xu, Z.-H. A quinoline-based probe for the ratiometric fluorescent detection of sulfite in lysosomes of living cells. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2022, 275, 121160. [Google Scholar] [CrossRef]
- Li, X.-H.; Han, X.-F.; Wu, W.-N.; Zhao, X.-L.; Wang, Y.; Fan, Y.-C.; Xu, Z.-H. Simultaneous detection of lysosomal SO2 and viscosity using a hemicyanine-based fluorescent probe. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 280, 121519. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.; Hofkens, J.; Enderlein, J. Handbook of Fluorescence Spectroscopy and Imaging: From Single Molecules to Ensembles; Wiley-VCH: Weinheim, Germany, 2011; ISBN 978-3-527-31669-4. [Google Scholar]
- Kand, D.; Kalle, A.M.; Talukdar, P. Chromenoquinoline-Based Thiol Probes: A Study on the Quencher Position for Controlling Fluorescent Off–On Characteristics. Org. Biomol. Chem. 2013, 11, 1691. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.; Rouser, G. Spectrophotometric Assay for Reaction of N-Ethylmaleimide with Sulfhydryl Groups. Anal. Chem. 1958, 30, 1291–1292. [Google Scholar] [CrossRef]
- Hansen, R.E.; Winther, J.R. An Introduction to Methods for Analyzing Thiols and Disulfides: Reactions, Reagents, and Practical Considerations. Anal. Biochem. 2009, 394, 147–158. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Shi, J.; Izquierdo, M.A.; Oh, S.; Park, S.Y.; Milián-Medina, B.; Roca-Sanjuán, D.; Gierschner, J. Inverted Energy Gap Law for the Nonradiative Decay in Fluorescent Floppy Molecules: Larger Fluorescence Quantum Yields for Smaller Energy Gaps. Org. Chem. Front. 2019, 6, 1948–1954. [Google Scholar] [CrossRef]
- Umberger, J.Q.; LaMer, V.K. The Kinetics of Diffusion Controlled Molecular and Ionic Reactions in Solution as Determined by Measurements of the Quenching of Fluorescence. J. Am. Chem. Soc. 1945, 67, 1099–1109. [Google Scholar] [CrossRef]
- Gaussian 09 Citation|Gaussian.com. Available online: https://gaussian.com/g09citation/ (accessed on 13 September 2022).
- Wang, K.; He, X.; Rong, C.; Zhong, A.; Liu, S.; Zhao, D. On the origin and nature of internal methyl rotation barriers: An information-theoretic approach study. Theor. Chem. Acc. 2022, 141, 68. [Google Scholar] [CrossRef]
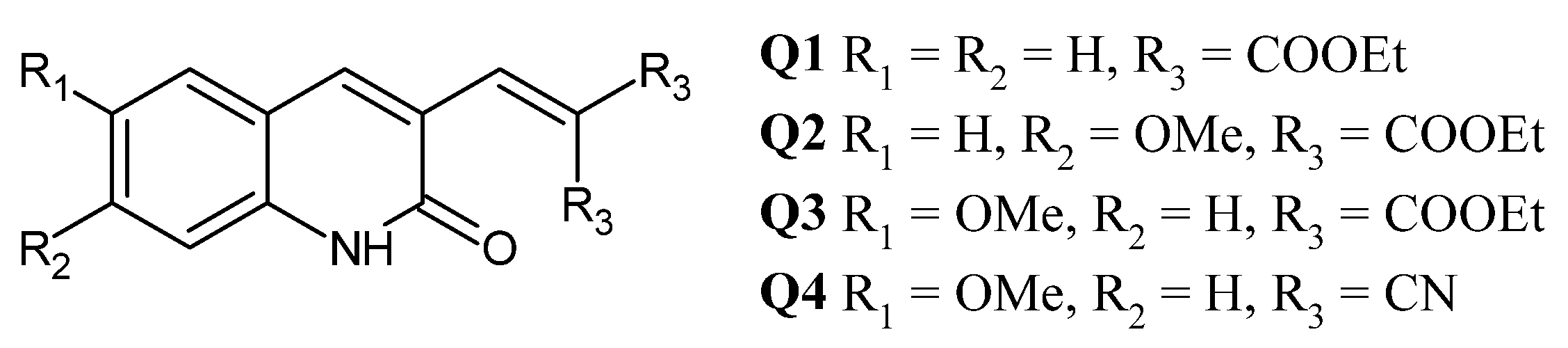




| Q1 | Q2 | Q3 | Q4 | |
|---|---|---|---|---|
| λabs (nm) | 372 a | 378 a | 402 a | 456 a [30] |
| 366 b | 376 b | 394 b | ||
| ε (M−1 × cm−1) | 9700 a | 14,300 a | 7100 a | 7900 a [30] |
| λem (nm) | 462 a | 460 a | 548 a | 625 a [30] |
| 508 b | 468 b | 600 b | ||
| Φem (%) | 0.20 a | 0.19 a | 2.42 a | 9.3 a [30] |
| SS (nm) | 90 a | 82 a | 146 a | 169 a [30] |
| 142 b | 92 b | 206 b | ||
| τ (ns) | 1.25 a | 1.86 a | 3.09 a | 3.12 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolińska, J.; Grzelakowska, A.; Szala, M.; Podsiadły, R. Comparison of Reactive Sites in 2(1H)-Quinolone Derivatives for the Detection of Biologically Important Sulfur Compounds. Molecules 2023, 28, 5965. https://doi.org/10.3390/molecules28165965
Kolińska J, Grzelakowska A, Szala M, Podsiadły R. Comparison of Reactive Sites in 2(1H)-Quinolone Derivatives for the Detection of Biologically Important Sulfur Compounds. Molecules. 2023; 28(16):5965. https://doi.org/10.3390/molecules28165965
Chicago/Turabian StyleKolińska, Jolanta, Aleksandra Grzelakowska, Marcin Szala, and Radosław Podsiadły. 2023. "Comparison of Reactive Sites in 2(1H)-Quinolone Derivatives for the Detection of Biologically Important Sulfur Compounds" Molecules 28, no. 16: 5965. https://doi.org/10.3390/molecules28165965
APA StyleKolińska, J., Grzelakowska, A., Szala, M., & Podsiadły, R. (2023). Comparison of Reactive Sites in 2(1H)-Quinolone Derivatives for the Detection of Biologically Important Sulfur Compounds. Molecules, 28(16), 5965. https://doi.org/10.3390/molecules28165965







