Exploration of Binding Affinities of a 3β,6β-Diacetoxy-5α-cholestan-5-ol with Human Serum Albumin: Insights from Synthesis, Characterization, Crystal Structure, Antioxidant and Molecular Docking
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. X-ray Crystal Structure and Molecular Geometry of the Synthesized 3β,6β-Diacetoxy-5-hydroxy-5α-cholestane (3)
2.3. Reduced Density Gradients and Frontier Molecular Orbitals (FMOs)
2.4. Hirshfeld Surface Analysis
2.5. Human Serum Albumin (HSA)–Steroid Binding Studies
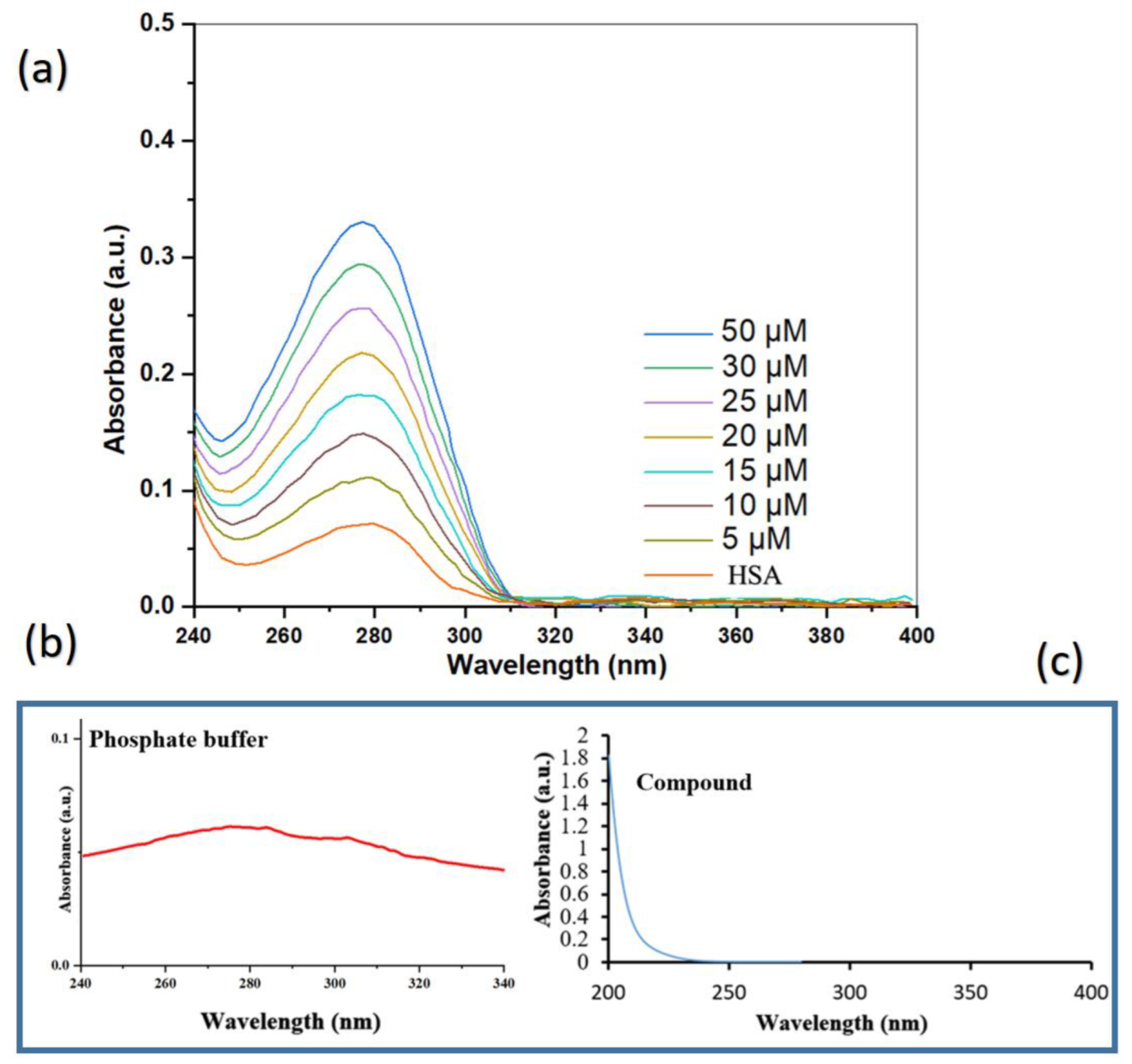
2.6. UV–Vis Absorption Analysis
2.7. Fluorescence Studies
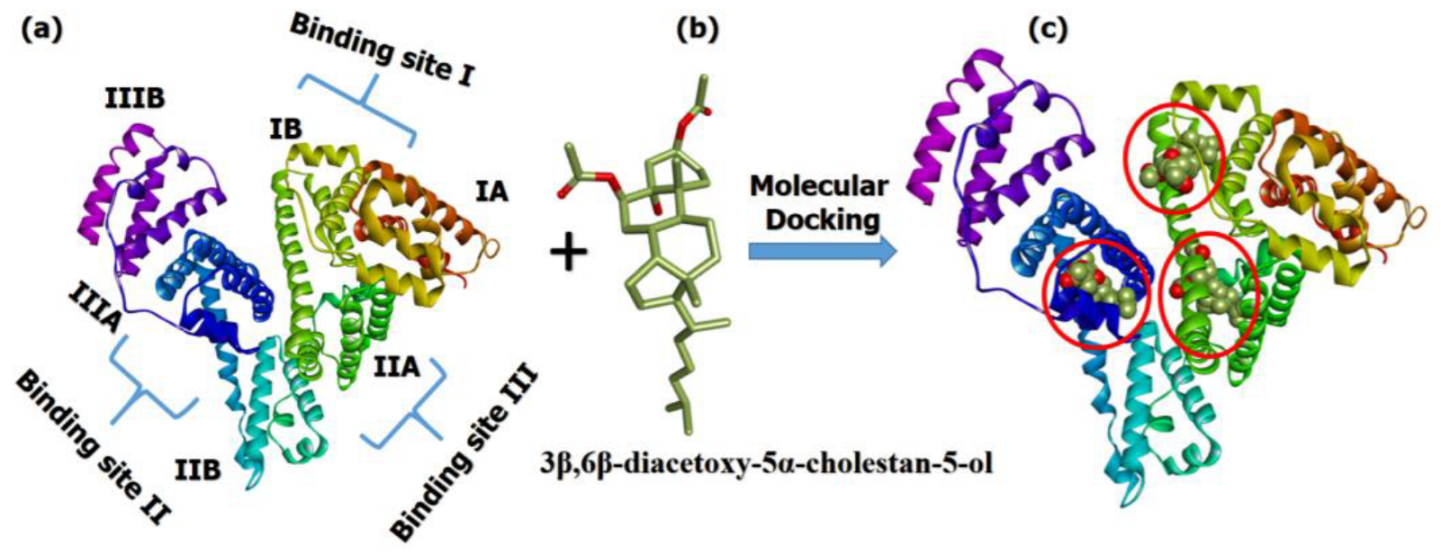
2.8. Molecular Docking Analysis
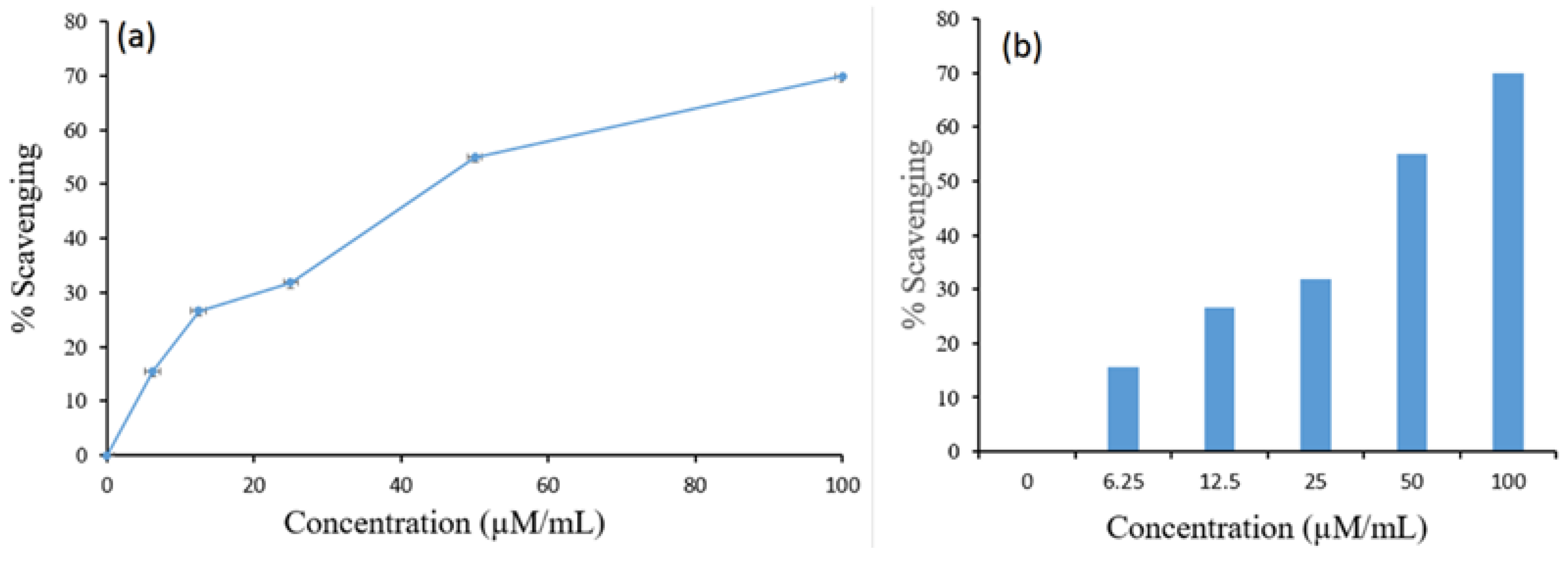
2.9. Antioxidant Potential Analysis
3. Materials and Method
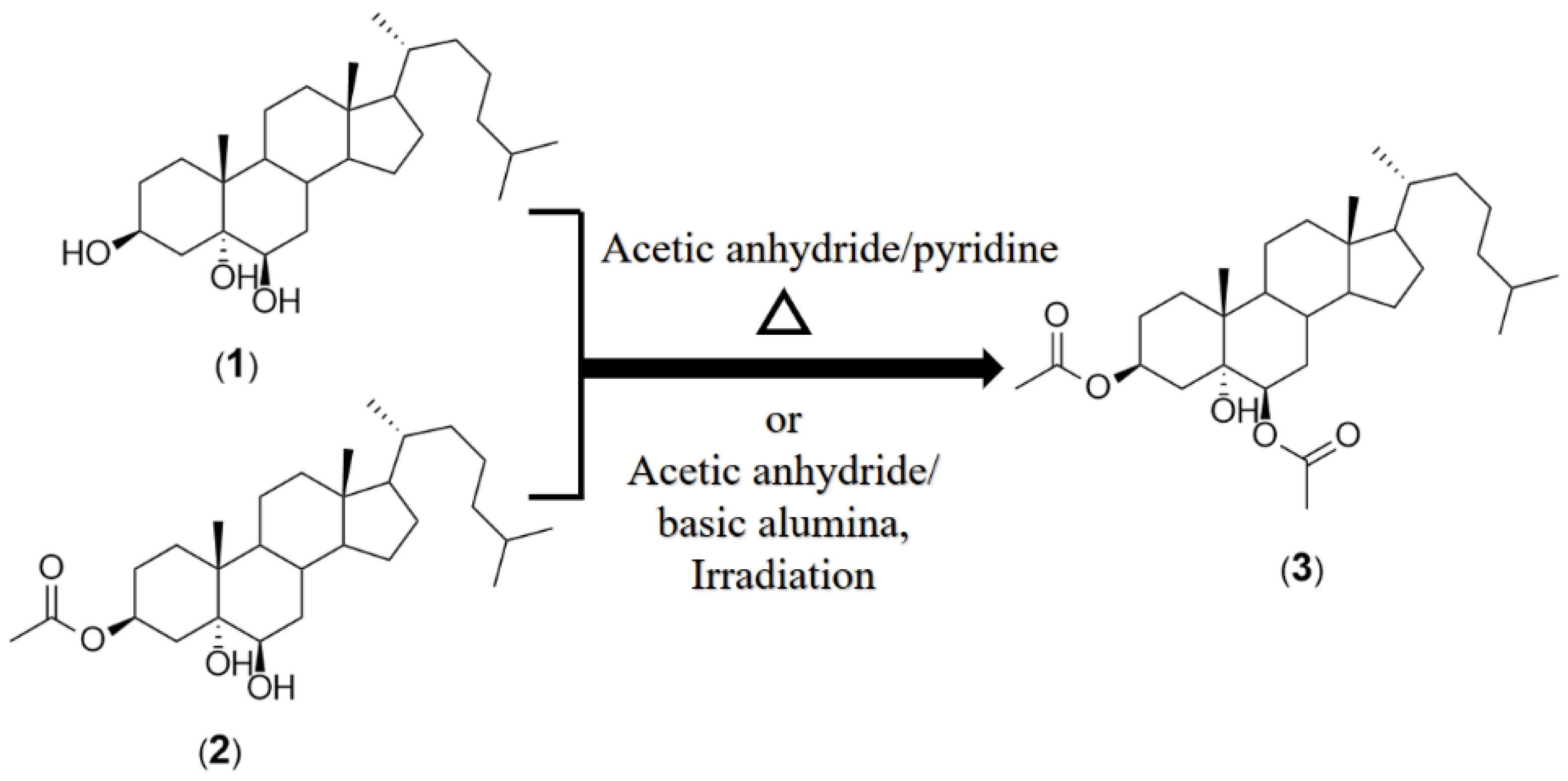
3.1. Preparation of 3β,6β-Diacetoxy-5α-cholestan-5-ol
3.2. Single X-ray Crystallography and Computational Details
3.3. Computational and Molecular Docking Studies
3.4. HSA-Binding Experiments
3.4.1. HSA Sample Preparation
3.4.2. UV–Vis Absorption Titration
3.4.3. Fluorescence Quenching Measurement
3.5. In Vitro Measurement of Antioxidant Properties Using the DPPH Radical Scavenging Assay
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xie, J.; Zhang, S.-J.; Yue, G.G.-L.; Yu, L.-L.; Liu, H.; Ma, W.-Y.; Yan, H.; Ni, W.; Bik-San Lau, C.; Liu, H.-Y. Isolation, structural elucidation, and bioactivity of cholestane derivatives from Ypsilandra thibetica. New J. Chem. 2023, 47, 324–332. [Google Scholar] [CrossRef]
- Ure, E.M.; Harris, L.D.; Cameron, S.A.; Weymouth-Wilson, A.; Furneaux, R.H.; Pitman, J.L.; Hinkley, S.F.; Luxenburger, A. Synthesis of 12β-methyl-18-nor-avicholic acid analogues as potential TGR5 agonists. Org. Biomol. Chem. 2022, 20, 3511–3527. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, G.; Hong, C.; Zheng, X.; Yu, H.; Zhang, Y. Potential of Steroidal Alkaloids in Cancer: Perspective Insight into Structure–Activity Relationships. Front. Oncol. 2021, 11, 733369. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Huo, C.; Kuo, L.-K.; Hiipakka, R.A.; Jones, R.B.; Lin, H.-P.; Hung, Y.; Su, L.-C.; Tseng, J.-C.; Kuo, Y.-Y. Cholestane-3β, 5α, 6β-triol suppresses proliferation, migration, and invasion of human prostate cancer cells. PLoS ONE 2013, 8, e65734. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Wang, Y.; Lu, K.; Hong, Y.; Xu, M.; Han, X.; Liu, Y. Modeling maternal cholesterol exposure reveals a reduction of neural progenitor proliferation using human cerebral organoids. Life Med. 2023, 2, lnac034. [Google Scholar] [CrossRef]
- German, C.A.; Liao, J.K. Understanding the molecular mechanisms of statin pleiotropic effects. Arch. Toxicol. 2023, 97, 1529–1545. [Google Scholar] [CrossRef]
- Dolivo, D.M.; Reed, C.R.; Gargiulo, K.A.; Rodrigues, A.E.; Galiano, R.D.; Mustoe, T.A.; Hong, S.J. Anti-fibrotic effects of statin drugs: A review of evidence and mechanisms. Biochem. Pharmacol. 2023, 214, 115644. [Google Scholar] [CrossRef]
- Andelova, K.; Bacova, B.S.; Sykora, M.; Hlivak, P.; Barancik, M.; Tribulova, N. Mechanisms underlying antiarrhythmic properties of cardioprotective agents impacting inflammation and oxidative stress. Int. J. Mol. Sci. 2022, 23, 1416. [Google Scholar] [CrossRef]
- Wiggs, A.G.; Chandler, J.K.; Aktas, A.; Sumner, S.J.; Stewart, D.A. The effects of diet and exercise on endogenous estrogens and subsequent breast cancer risk in postmenopausal women. Front. Endocrinol. 2021, 12, 732255. [Google Scholar] [CrossRef]
- McIver, L.A.; Siddique, M.S. Atorvastatin; Statpearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Kim, T.-H.; Yu, G.-R.; Kim, H.; Kim, J.-E.; Lim, D.-W.; Park, W.-H. Network Pharmacological Analysis of a New Herbal Combination Targeting Hyperlipidemia and Efficacy Validation In Vitro. Curr. Issues Mol. Biol. 2023, 45, 1314–1332. [Google Scholar] [CrossRef]
- Lewek, J.; Surma, S.; Banach, M. Statins and COVID-19 (Mechanism of Action, Effect on Prognosis). In Cardiovascular Complications of COVID-19: Acute and Long-Term Impacts; Springer: Berlin/Heidelberg, Germany, 2023; pp. 285–302. [Google Scholar]
- Yu, D.; Liao, J.K. Emerging views of statin pleiotropy and cholesterol lowering. Cardiovasc. Res. 2022, 118, 413–423. [Google Scholar] [CrossRef]
- Hariri, L.; Rehman, A. Estradiol; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Geraci, A.; Calvani, R.; Ferri, E.; Marzetti, E.; Arosio, B.; Cesari, M. Sarcopenia and menopause: The role of estradiol. Front. Endocrinol. 2021, 12, 682012. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Heath, R.J. Structural and biochemical features of human serum albumin essential for eukaryotic cell culture. Int. J. Mol. Sci. 2021, 22, 8411. [Google Scholar] [CrossRef]
- Ma, J.; Yang, B.; Hu, X.; Gao, Y.; Qin, C. The binding mechanism of benzophenone-type UV filters and human serum albumin: The role of site, number, and type of functional group substitutions. Environ. Pollut. 2023, 324, 121342. [Google Scholar] [CrossRef]
- Gallagher, A.; Kar, S.; Sepúlveda, M.S. Computational Modeling of Human Serum Albumin Binding of Per-and Polyfluoroalkyl Substances Employing QSAR, Read-Across, and Docking. Molecules 2023, 28, 5375. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, W.; Liu, Z.; Zhai, Z.; Hou, X.; Wang, P.; Ge, G.; Wang, F. Lysine reactivity profiling reveals molecular insights into human serum albumin–small-molecule drug interactions. Anal. Bioanal. Chem. 2021, 413, 7431–7440. [Google Scholar] [CrossRef]
- Mariño-Ocampo, N.; Rodríguez, D.F.; Guerra Díaz, D.; Zúñiga-Núñez, D.; Duarte, Y.; Fuentealba, D.; Zacconi, F.C. Direct Oral FXa Inhibitors Binding to Human Serum Albumin: Spectroscopic, Calorimetric, and Computational Studies. Int. J. Mol. Sci. 2023, 24, 4900. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, Y.; Liang, H. Interactive association of drugs binding to human serum albumin. Int. J. Mol. Sci. 2014, 15, 3580–3595. [Google Scholar] [CrossRef]
- Abdollahpour, N.; Soheili, V.; Saberi, M.R.; Chamani, J. Investigation of the interaction between human serum albumin and two drugs as binary and ternary systems. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 705–721. [Google Scholar] [CrossRef]
- di Masi, A. Human serum albumin: From molecular aspects to biotechnological applications. Int. J. Mol. Sci. 2023, 24, 4081. [Google Scholar] [CrossRef]
- Chadha, N.; Singh, D.; Milton, M.D.; Mishra, G.; Daniel, J.; Mishra, A.K.; Tiwari, A.K. Computational prediction of interaction and pharmacokinetics profile study for polyamino-polycarboxylic ligands on binding with human serum albumin. New J. Chem. 2020, 44, 2907–2918. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, J.-J.; Cheng, J.-H.; Liang, W. Characterization of the interactions of human serum albumin with carmine and amaranth using multi-spectroscopic techniques and molecular docking. J. Food Meas. Charact. 2022, 16, 4345–4354. [Google Scholar] [CrossRef]
- Fan, J.; Gilmartin, K.; Octaviano, S.; Villar, F.; Remache, B.; Regan, J. Using human serum albumin binding affinities as a proactive strategy to affect the pharmacodynamics and pharmacokinetics of preclinical drug candidates. ACS Pharmacol. Transl. Sci. 2022, 5, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Alavi, F.S.; Ghadari, R.; Zahedi, M. Exploration of the binding properties of the human serum albumin sites with neurology drugs by docking and molecular dynamics simulation. J. Iran. Chem. Soc. 2017, 14, 19–35. [Google Scholar] [CrossRef]
- Bratty, M.A. Spectroscopic and molecular docking studies for characterizing binding mechanism and conformational changes of human serum albumin upon interaction with Telmisartan. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2020, 28, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Hu, X.; Hong, X.; Zheng, J.; Liu, X.; Gong, D.; Zhang, G. Interaction characterization of 5-hydroxymethyl-2-furaldehyde with human serum albumin: Binding characteristics, conformational change and mechanism. J. Mol. Liq. 2020, 297, 111835. [Google Scholar] [CrossRef]
- Pontremoli, C.; Barbero, N.; Viscardi, G.; Visentin, S. Insight into the interaction of inhaled corticosteroids with human serum albumin: A spectroscopic-based study. J. Pharm. Anal. 2018, 8, 37–44. [Google Scholar] [CrossRef]
- Nda-Umar, U.I.; Ramli, I.B.; Muhamad, E.N.; Azri, N.; Amadi, U.F.; Taufiq-Yap, Y.H. Influence of heterogeneous catalysts and reaction parameters on the acetylation of glycerol to acetin: A review. Appl. Sci. 2020, 10, 7155. [Google Scholar] [CrossRef]
- Castro, A.; Andrade, I.M.G.; Coelho, M.C.; da Costa, D.P.; Moreira, D.d.N.; Maia, R.A.; Lima, G.d.S.; dos Santos, G.F.; Vaz, B.G.; Militão, G.C.G. Multicomponent synthesis of spiro 1, 3, 4-thiadiazolines with anticancer activity by using deep eutectic solvent under microwave irradiation. J. Heterocycl. Chem. 2023, 60, 392–405. [Google Scholar] [CrossRef]
- Pasricha, S.; Rangarajan, T. Green Acetylation of Primary Aromatic Amines. Resonance 2023, 28, 325–331. [Google Scholar] [CrossRef]
- Palma, V.; Barba, D.; Cortese, M.; Martino, M.; Renda, S.; Meloni, E. Microwaves and heterogeneous catalysis: A review on selected catalytic processes. Catalysts 2020, 10, 246. [Google Scholar] [CrossRef]
- Rajnikant, V.; Jasrotia, D.; Chand, B. Comparative crystallographic and hydrogen-bonding analysis of pregnane derivatives. J. Chem. Crystallogr. 2008, 38, 211–230. [Google Scholar] [CrossRef]
- Pinto, R.; Ramos Silva, M.; Matos Beja, A.; Salvador, J.; Paixao, J. 6β-Acetamido-5α-hydroxycholestan-3β-yl acetate. Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, o2303. [Google Scholar] [CrossRef] [PubMed]
- Cremer, D.t.; Pople, J. General definition of ring puckering coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- Huang, Y.; Rong, C.; Zhang, R.; Liu, S. Evaluating frontier orbital energy and HOMO/LUMO gap with descriptors from density functional reactivity theory. J. Mol. Model. 2017, 23, 3. [Google Scholar] [CrossRef]
- Padrón, J.; Carrasco, R.; Pellon, R. Molecular descriptor based on a molar refractivity partition using Randic-type graph-theoretical invariant. J. Pharm. Pharmaceut. Sci 2002, 5, 258–266. [Google Scholar]
- Alam, M.J.; Ahmad, S. Molecular structure, anharmonic vibrational analysis and electronic spectra of o-, m-, p-iodonitrobenzene using DFT calculations. J. Mol. Struct. 2014, 1059, 239–254. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, L.; Sun, X.; Han, X.; Guo, C.; Kang, P. Spectroscopic studies on the interaction between sodium ozagrel and bovine serum albumin. J. Mol. Struct. 2009, 928, 114–120. [Google Scholar] [CrossRef]
- Gowda, J.I.; Nandibewoor, S.T. Binding and conformational changes of human serum albumin upon interaction with 4-aminoantipyrine studied by spectroscopic methods and cyclic voltammetry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 124, 397–403. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, X.; Huang, J.; Wang, H. Probing the interaction between human serum albumin and 9-hydroxyphenanthrene: A spectroscopic and molecular docking study. ACS Omega 2020, 5, 16833–16840. [Google Scholar] [CrossRef]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, L.; Hansen, J.; Egelhaaf, S.U.; Platten, F. The crystallization enthalpy and entropy of protein solutions: Microcalorimetry, van’t Hoff determination and linearized Poisson–Boltzmann model of tetragonal lysozyme crystals. Phys. Chem. Chem. Phys. 2021, 23, 2686–2696. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, A.; Schwanz, H.A.; Spencer, D.J.; Bhasin, S.; Hamilton, J.A.; Jayaram, B.; Goldman, A.L.; Krishna, M.; Krishnan, M.; Shah, A. Allosterically coupled multisite binding of testosterone to human serum albumin. Endocrinology 2021, 162, bqaa199. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Liu, Y.-X.; Li, S.-M.; Jiang, C.-K.; Wang, B.-F.; Xiong, Y.-H.; Mao, Z.-W.; Le, X.-Y. Synthesis, crystal structures, molecular docking and in vitro cytotoxicity studies of two new copper (II) complexes: Special emphasis on their binding to HSA. New J. Chem. 2017, 41, 12429–12441. [Google Scholar] [CrossRef]
- Veeralakshmi, S.; Sabapathi, G.; Nehru, S.; Venuvanalingam, P.; Arunachalam, S. Surfactant–cobalt (III) complexes: The impact of hydrophobicity on interaction with HSA and DNA–insights from experimental and theoretical approach. Colloids Surf. B Biointerfaces 2017, 153, 85–94. [Google Scholar] [CrossRef]
- Brighente, I.; Dias, M.; Verdi, L.; Pizzolatti, M. Antioxidant activity and total phenolic content of some Brazilian species. Pharm. Biol. 2007, 45, 156–161. [Google Scholar] [CrossRef]
- Mahadevan, V.; Lundberg, W. Preparation of cholesterol esters of long-chain fatty acids and characterization of cholesteryl arachidonate. J. Lipid Res. 1962, 3, 106–110. [Google Scholar] [CrossRef]
- Petersen, Q.R. Reductive Preparation of Oximes and the Selective Hydrolysis of their Acetates on Alumina. Indiana Acad. Sci. 1963, 73, 127–131. [Google Scholar]
- Tsui, P.; Just, G. Some Reactions of 3 β-Mesyloxycholestane-5α, 6β-diol and Cholest-2-ene-5α, 6β-diol Acetates. Can. J. Chem. 1973, 51, 3502–3507. [Google Scholar] [CrossRef]
- Lieberman, S.; Fukushima, D.K. ▵ 5-Cholestene-3β, 4β, 7α-triol and the Inhibition of the Oxidation of Hydroxyl Groups by Vicinal Substituents. J. Am. Chem. Soc. 1950, 72, 5211–5218. [Google Scholar] [CrossRef]
- Rowland, A.T. An Attempted Westphalen Rearrangement of a 5β-Hydroxy Steroid1. J. Org. Chem. 1964, 29, 222–224. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision, D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009; Available online: http://www.gaussian.com (accessed on 1 January 2014).
- Becke, A.D. Densityfunctional thermochemistry. III. the role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- Anand, U.; Jash, C.; Mukherjee, S. Spectroscopic probing of the microenvironment in a protein− surfactant assembly. J. Phys. Chem. B 2010, 114, 15839–15845. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhao, X.; Wang, P.; Dai, Z.; Zou, X. Electrochemical site marker competitive method for probing the binding site and binding mode between bovine serum albumin and alizarin red S. Electrochim. Acta 2011, 56, 4181–4187. [Google Scholar] [CrossRef]
- Neamtu, S.; Mic, M.; Bogdan, M.; Turcu, I. The artifactual nature of stavudine binding to human serum albumin. A fluorescence quenching and isothermal titration calorimetry study. J. Pharm. Biomed. Anal. 2013, 72, 134–138. [Google Scholar] [CrossRef]
- Katsube, T.; Tabata, H.; Ohta, Y.; Yamasaki, Y.; Anuurad, E.; Shiwaku, K.; Yamane, Y. Screening for antioxidant activity in edible plant products: Comparison of low-density lipoprotein oxidation assay, DPPH radical scavenging assay, and Folin− Ciocalteu assay. J. Agric. Food Chem. 2004, 52, 2391–2396. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Takamura, C.; Sugita, F.; Masuoka, C.; Yoshimitsu, H.; Ikeda, T.; Nohara, T. Two new steroid glycosides and a new sesquiterpenoid glycoside from the underground parts of Trillium kamtschaticum. Chem. Pharm. Bull. 2007, 55, 551–556. [Google Scholar] [CrossRef]
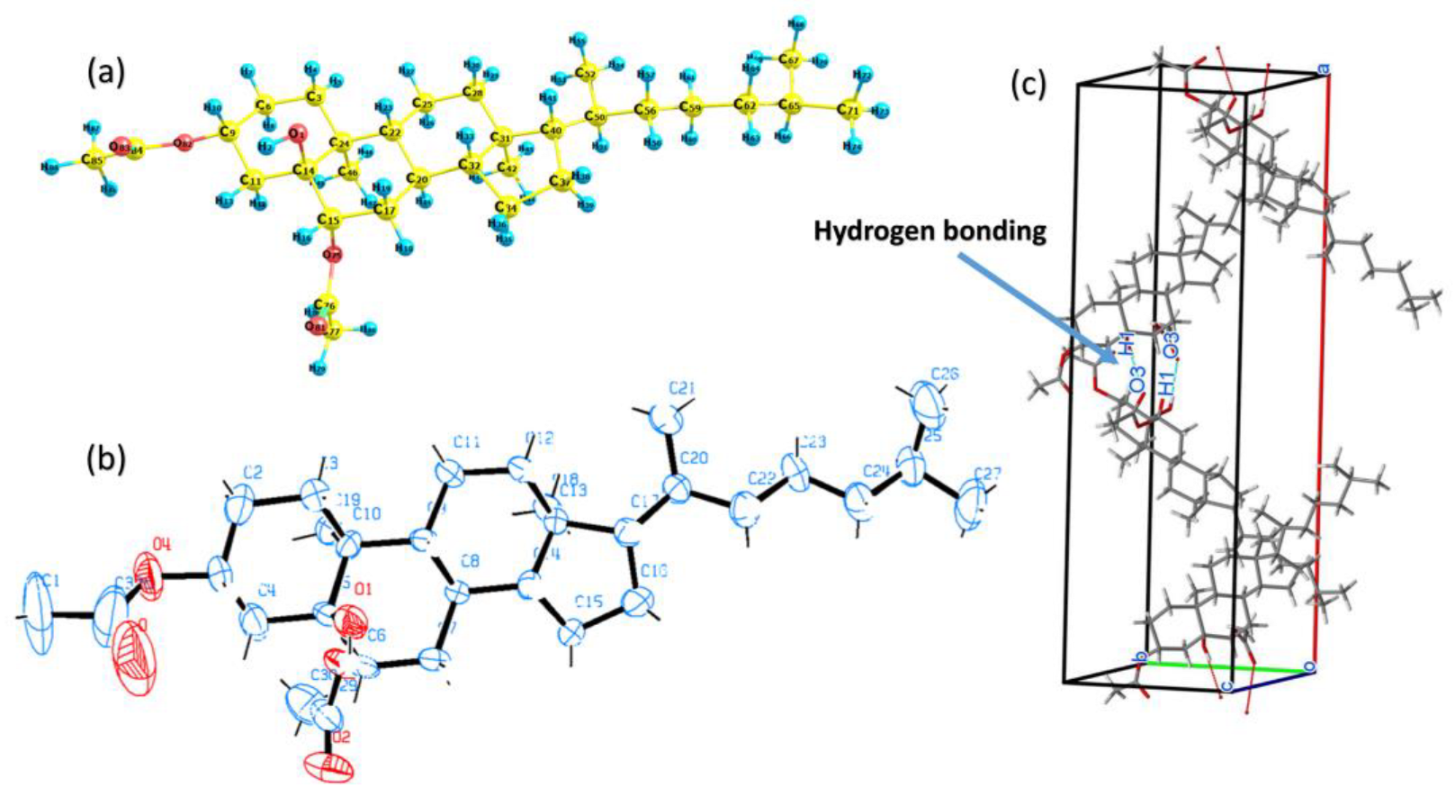
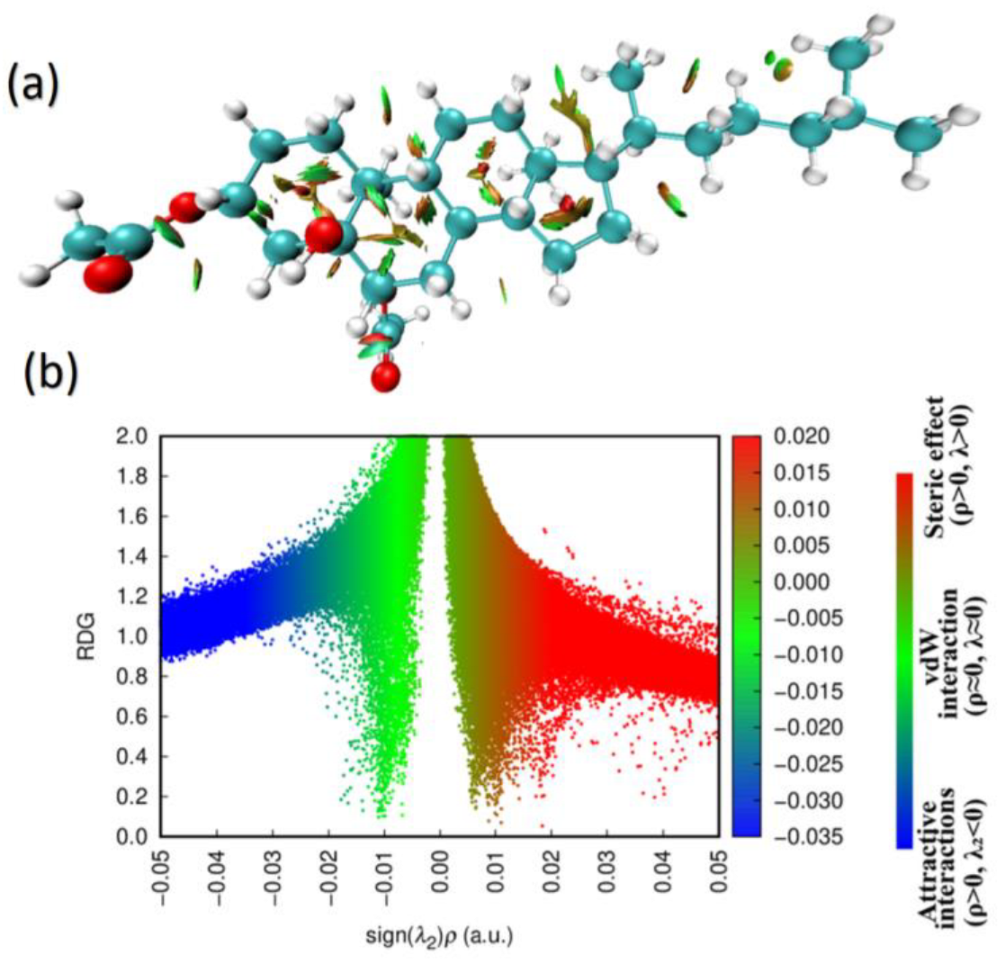
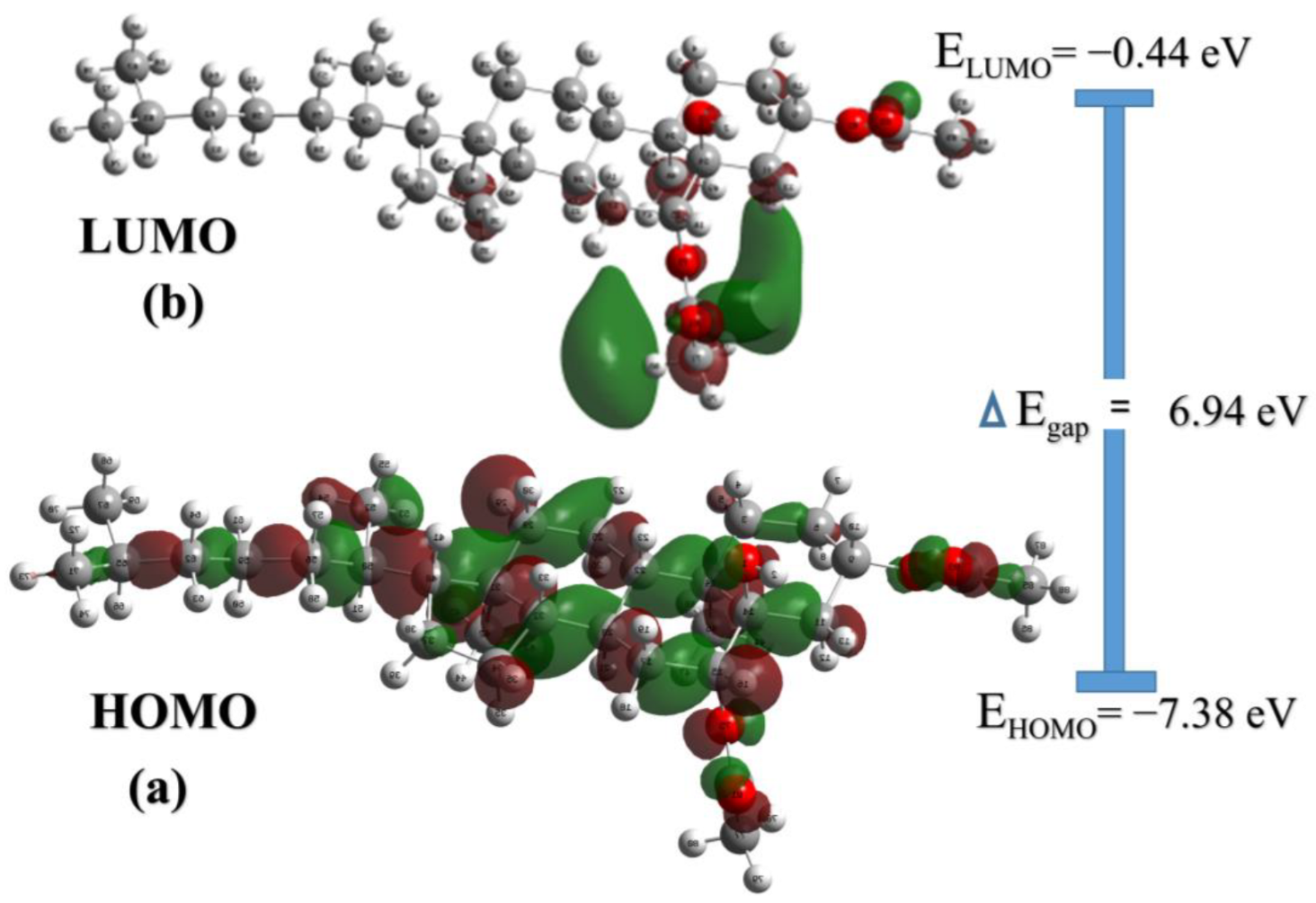
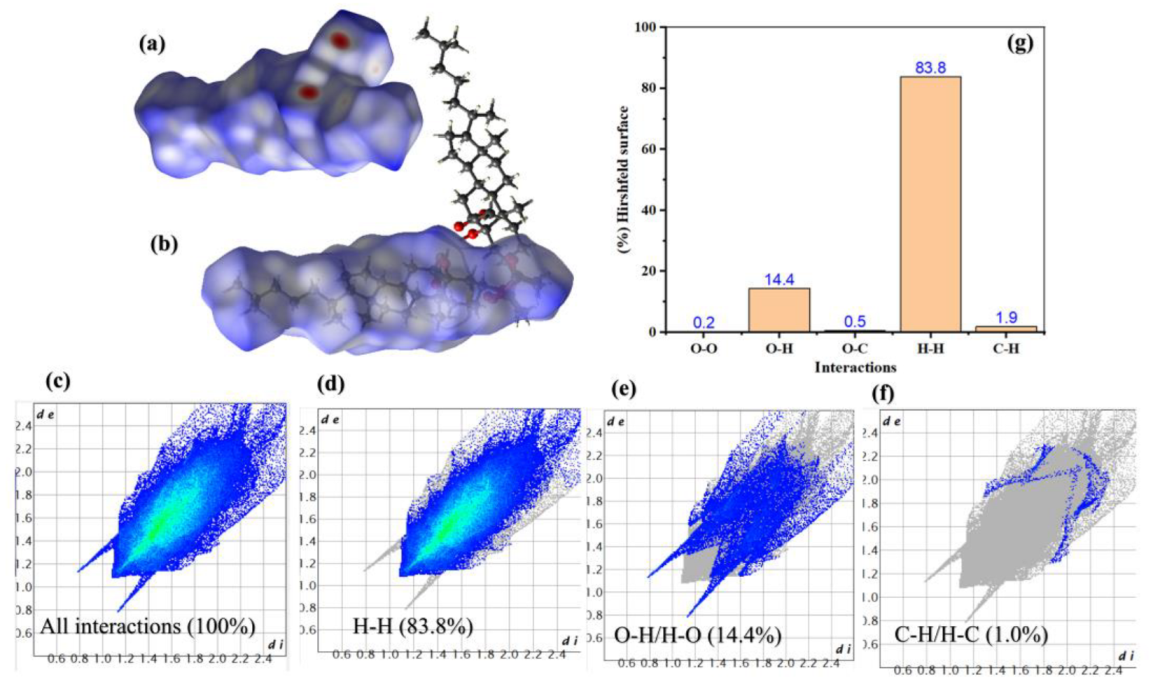


| Bond Length (Å) | DFT | XRD | Bond Angle (°) | DFT | XRD | Bond Dihedral (°) | DFT | XRD |
|---|---|---|---|---|---|---|---|---|
| C9-O82 | 1.458 | 1.434 | C11C9O82 | 109.7313 | 115.22 | C85C84O82C9 | 178.52 | −168.28 |
| O82-C84 | 1.350 | 1.430 | C6C9O82 | 107.2475 | 96.2 | C9O82C84O83 | −112.4 | −46.56 |
| C84-O82 | 1.208 | 1.51 | C9O82C84 | 117.6730 | 102.24 | C6C3C24C22 | −173.09 | −172.70 |
| C84-C85 | 1.508 | 1.665 | O82C84C85 | 110.9093 | 97.0 | C6C3C24C46 | 65.46 | 66.37 |
| C14-O1 | 1.449 | 1.425 | C3C24C22 | 111.0458 | 109.35 | C45C24C22C25 | 61.91 | 58.17 |
| O1-H2 | 0.964 | 0.821 | C3C24C46 | 108.2078 | 109.77 | C11C14O1H2 | 48.470 | 73.57 |
| C9-H10 | 1.089 | 0.980 | C46C24C22 | 110.5004 | 110.14 | C11C14C15C17 | 179.67 | 178.89 |
| C15-H16 | 1.092 | 0.979 | C28C31C40 | 116.4791 | 118.42 | C1C14C15O75 | 173.30 | 177.62 |
| C15-O75 | 1.457 | 1.456 | C28C31C42 | 110.6404 | 109.22 | C15C14C15O75 | −178.32 | 178.60 |
| O75-C76 | 1.354 | 1.314 | C42C31C40 | 110.1862 | 109.14 | C15O75C76O81 | 1.046 | 0.81 |
| C76-O81 | 1.206 | 1.234 | C37C40C50 | 112.5386 | 112.93 | C25C28C31C40 | −165.88 | −164.94 |
| C76-C77 | 1.508 | 1.506 | C40C50C52 | 113.2645 | 113.19 | C25C28C31C42 | 67.276 | 69.37 |
| C77-H80 | 1.093 | 0.959 | C40C50C56 | 110.1896 | 111.27 | C42C31C40C58 | 46.779 | 48.17 |
| C24-C45 | 1.548 | 1.563 | C52C50C56 | 110.3467 | 110.83 | C42C31C40C37 | −79.231 | −79.66 |
| C31-C42 | 1.544 | 1.563 | C14O1H2 | 108.4482 | 109.44 | C37C40C50C52 | 177.46 | 176.77 |
| C40-C50 | 1.549 | 1.540 | C11C14C15 | 112.0757 | 113.42 | C40C50C56C59 | 168.60 | 175.86 |
| C50-C52 | 1.537 | 1.523 | C14C15O75 | 109.2216 | 109.22 | C52C50C56C59 | −65.573 | −55.65 |
| C40-H41 | 1.099 | 0.980 | C17C15O75 | 110.9283 | 109.83 | O1C14C24C46 | −178.66 | −179.90 |
| C50-H51 | 1.098 | 0.980 | C15O75C76 | 117.5999 | 119.15 | H33C32C31C42 | −177.83 | −179.52 |
| C65-C71 | 1.535 | 1.523 | O75C76C77 | 110.8554 | 111.16 | C59C62C65C71 | −171.31 | −169.70 |
| C65-C67 | 1.535 | 1.471 | O75C76O81 | 124.0454 | 122.62 | C59C62C65C67 | 64.6696 | 69.04 |
| C65-C66 | 1.099 | 0.980 | C67C75C71 | 110.4441 | 109.45 | C15C17C28C32 | 173.10 | 171.49 |
| D—H···A | D—H | H···A | D···A | D—H···A | Symmetry Code |
|---|---|---|---|---|---|
| O1—H1···O3 | 0.78(9) | 2.11(9) | 2.881(9) | 173(12) | x, y, 1−z |
Cholestane-Skeleton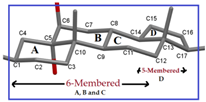 | Puckering Parameters | ||
|---|---|---|---|
| Q (Å) | Θ (°) | Φ (°) | |
| Ring A (Six-Membered) | 0.584 | 3.6 | 212 |
| Ring B (Six-Membered) | 0.549 | 104 | 173.6 |
| Ring C (Six-Membered) | 0.576 | 176.7 | 216 |
| Ring D (Five-Membered) | 0.452 | - | 13.6 |
| Temp | Ksv (×104 M−1) | Kq (×1012 M−1s−1) | R2 | Kb (×104 M−1) | n | ΔG° (Kcal/mol) | R2 |
|---|---|---|---|---|---|---|---|
| 298 K | 1.45 | 1.45 | 0.9846 | 3.18 | 1 | −9.86 | 0.9193 |
| 308 K | 1.49 | 1.49 | 0.9853 | 2.72 | 1 | −9.53 | 0.962 |
| 318 K | 1.5 | 1.5 | 0.9855 | 2.42 | 1 | −9.20 | 0.985 |
| Temperature (K) | ΔG° (kcal/mol) | ΔH° (kJ/mol) | ΔS° (J/mol/K) |
|---|---|---|---|
| 298 | −9.86 | −11.87 | 20.12 |
| 308 | −9.53 | −11.54 | 19.72 |
| 318 | −9.20 | −11.21 | 19.32 |
| Binding Site I | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hydrophobic Interactions | |||||||||
| Index | Residue | Amino Acid | Distance | Ligand Atom | Protein Atom | Binding Energy (kcal/mol) | |||
| 1 | 115A | LEU | 3.93 | 9098 | 1736 | −8.2 | |||
| 2 | 115A | LEU | 3.82 | 9103 | 1739 | ||||
| 3 | 115A | LEU | 3.64 | 9119 | 1737 | ||||
| 4 | 138A | TYR | 3.76 | 9124 | 2106 | ||||
| 5 | 138A | TYR | 3.71 | 9125 | 2107 | ||||
| 6 | 142A | ILE | 3.30 | 9103 | 2183 | ||||
| 7 | 161A | TYR | 3.70 | 9123 | 2527 | ||||
| 8 | 165A | PHE | 3.71 | 9125 | 2591 | ||||
| 9 | 186A | ARG | 3.70 | 9109 | 2896 | ||||
| Hydrogen Bonds | |||||||||
| Index | Residue | Amino Acid | Distance H-A | Distance D-A | Donor Angle | Protein Donor? | Side Chain | Donor Atom | Acceptor Atom |
| 1 | 115A | LEU | 2.35 | 2.98 | 116.15 | √ | Χ | 1080 [Nam] | 5609 [O3] |
| 2 | 115A | LEU | 2.19 | 2.79 | 118.50 | Χ | Χ | 5609 [O3] | 1083 [O2] |
| Salt Bridges | |||||||||
| Index | Residue | Amino Acid | Distance Protein | Protein Positive | Ligand Group | Ligand Atoms | |||
| 1 | 146A | HIS | 5.36 | √ | Carboxylate | 9126, 9128 | |||
| 2 | 186A | ARG | 5.00 | √ | Carboxylate | 9130, 9132 | |||
| 3 | 190A | LYS | 4.21 | √ | Carboxylate | 9130, 9132 | |||
| Binding site II | |||||||||
| Hydrophobic Interactions | |||||||||
| Index | Residue | Amino Acid | Distance | Ligand Atom | Protein Atom | Binding Energy (kcal/mol) | |||
| 1 | 214A | TRP | 3.28 | 9108 | 3327 | −8.5 | |||
| 2 | 218A | ARG | 3.52 | 9108 | 3380 | ||||
| 3 | 219A | LEU | 3.45 | 9125 | 3405 | ||||
| 4 | 223A | PHE | 3.73 | 9122 | 3480 | ||||
| 5 | 223A | PHE | 3.78 | 9125 | 3479 | ||||
| 6 | 234A | LEU | 3.53 | 9125 | 3651 | ||||
| 7 | 238A | LEU | 3.50 | 9123 | 3712 | ||||
| 8 | 238A | LEU | 3.71 | 9124 | 3713 | ||||
| 9 | 260A | LEU | 3.44 | 9124 | 4020 | ||||
| 10 | 264A | ILE | 3.34 | 9124 | 4092 | ||||
| 11 | 290A | ILE | 3.58 | 9122 | 4487 | ||||
| 12 | 291A | ALA | 3.38 | 9119 | 4504 | ||||
| Hydrogen Bonds | |||||||||
| Index | Residue | Amino Acid | Distance H-A | Distance D-A | Donor Angle | Protein Donor? | Side Chain | Donor Atom | Acceptor Atom |
| 1 | 222A | ARG | 3.45 | 4.03 | 115.90 | √ | √ | 3456 [Ng+] | 5646 [O3] |
| Salt Bridges | |||||||||
| Index | Residue | Amino Acid | Distance Protein | Protein Positive | Ligand Group | Ligand Atoms | |||
| 1 | 195A | LYS | 4.68 | √ | Carboxylate | 9130, 9132 | |||
| 2 | 195A | LYS | 4.38 | √ | Carboxylate | 9126, 9128 | |||
| Binding Site III | |||||||||
| Hydrophobic Interactions | |||||||||
| Index | Residue | Amino Acid | Distance | Ligand Atom | Protein Atom | Binding Energy (kcal/mol) | |||
| 1 | 344A | VAL | 3.39 | 9124 | 5311 | −8.6 | |||
| 2 | 387A | LEU | 2.92 | 9105 | 5991 | ||||
| 3 | 387A | LEU | 3.14 | 9103 | 5993 | ||||
| 4 | 388A | ILE | 3.78 | 9105 | 6011 | ||||
| 5 | 407A | LEU | 3.63 | 9133 | 6325 | ||||
| 6 | 430A | LEU | 3.54 | 9133 | 6711 | ||||
| 7 | 430A | LEU | 3.81 | 9114 | 6712 | ||||
| 8 | 449A | ALA | 3.26 | 9107 | 7000 | ||||
| 9 | 450A | GLU | 3.73 | 9123 | 7011 | ||||
| 10 | 453A | LEU | 3.06 | 9106 | 7061 | ||||
| 11 | 453A | LEU | 3.39 | 9125 | 7058 | ||||
| 12 | 485A | ARG | 3.53 | 9124 | 7547 | ||||
| 13 | 488A | PHE | 3.82 | 9129 | 7597 | ||||
| 14 | 488A | PHE | 3.37 | 9097 | 7595 | ||||
| Salt Bridges | |||||||||
| Index | Residue | Amino Acid | Distance Protein | Protein Positive | Ligand Group | Ligand Atoms | |||
| 1 | 410A | ARG | 4.77 | √ | Carboxylate | 9130, 9132 | |||
| 2 | 414A | LYS | 5.26 | √ | Carboxylate | 9126, 9128 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M. Exploration of Binding Affinities of a 3β,6β-Diacetoxy-5α-cholestan-5-ol with Human Serum Albumin: Insights from Synthesis, Characterization, Crystal Structure, Antioxidant and Molecular Docking. Molecules 2023, 28, 5942. https://doi.org/10.3390/molecules28165942
Alam M. Exploration of Binding Affinities of a 3β,6β-Diacetoxy-5α-cholestan-5-ol with Human Serum Albumin: Insights from Synthesis, Characterization, Crystal Structure, Antioxidant and Molecular Docking. Molecules. 2023; 28(16):5942. https://doi.org/10.3390/molecules28165942
Chicago/Turabian StyleAlam, Mahboob. 2023. "Exploration of Binding Affinities of a 3β,6β-Diacetoxy-5α-cholestan-5-ol with Human Serum Albumin: Insights from Synthesis, Characterization, Crystal Structure, Antioxidant and Molecular Docking" Molecules 28, no. 16: 5942. https://doi.org/10.3390/molecules28165942
APA StyleAlam, M. (2023). Exploration of Binding Affinities of a 3β,6β-Diacetoxy-5α-cholestan-5-ol with Human Serum Albumin: Insights from Synthesis, Characterization, Crystal Structure, Antioxidant and Molecular Docking. Molecules, 28(16), 5942. https://doi.org/10.3390/molecules28165942









