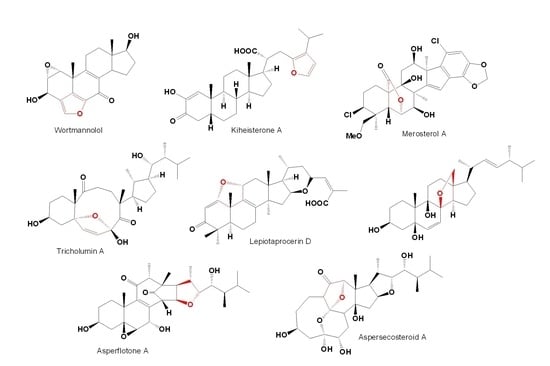
Fascinating Furanosteroids and Their Pharmacological Profile
Abstract
1. Introduction
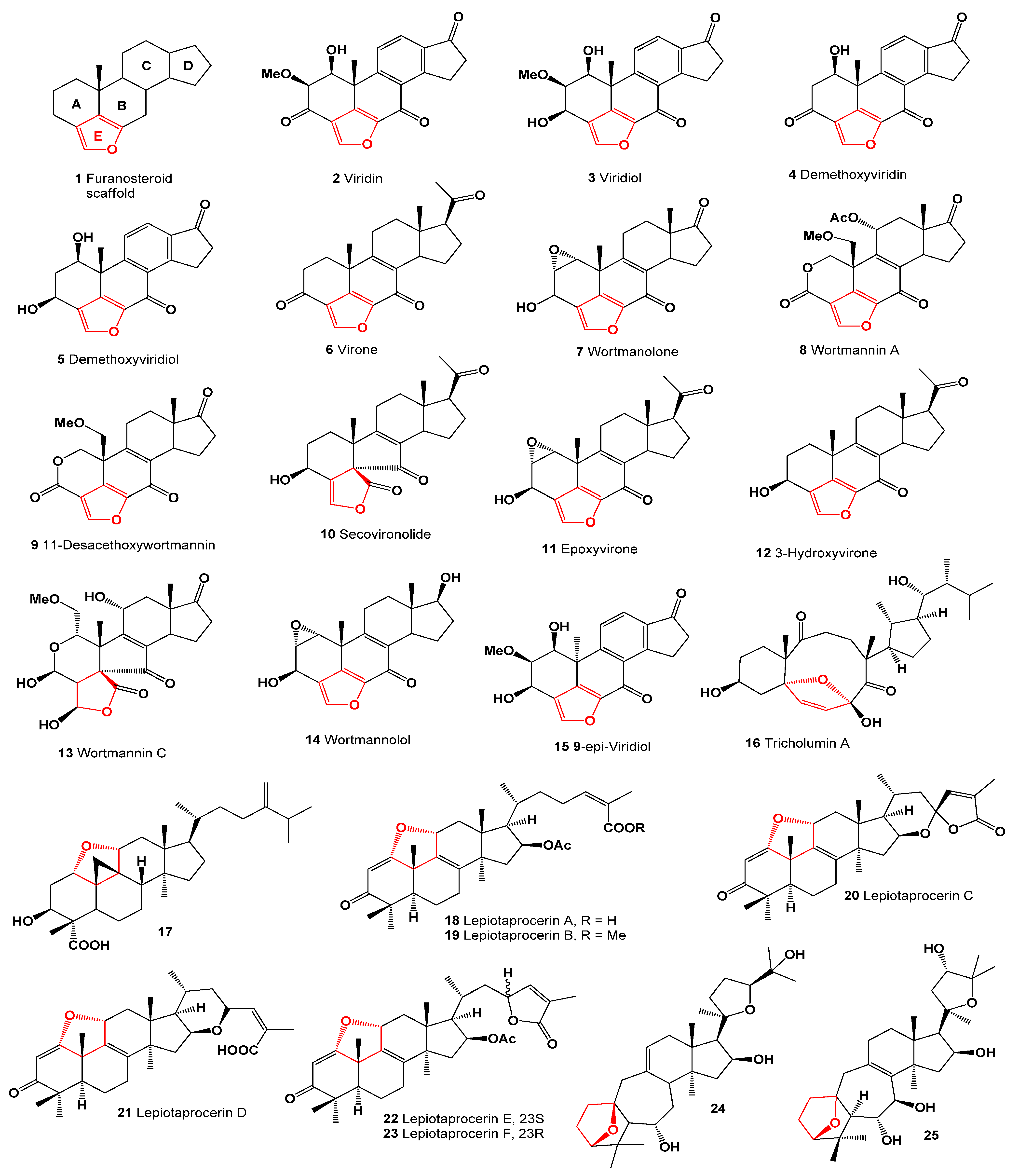
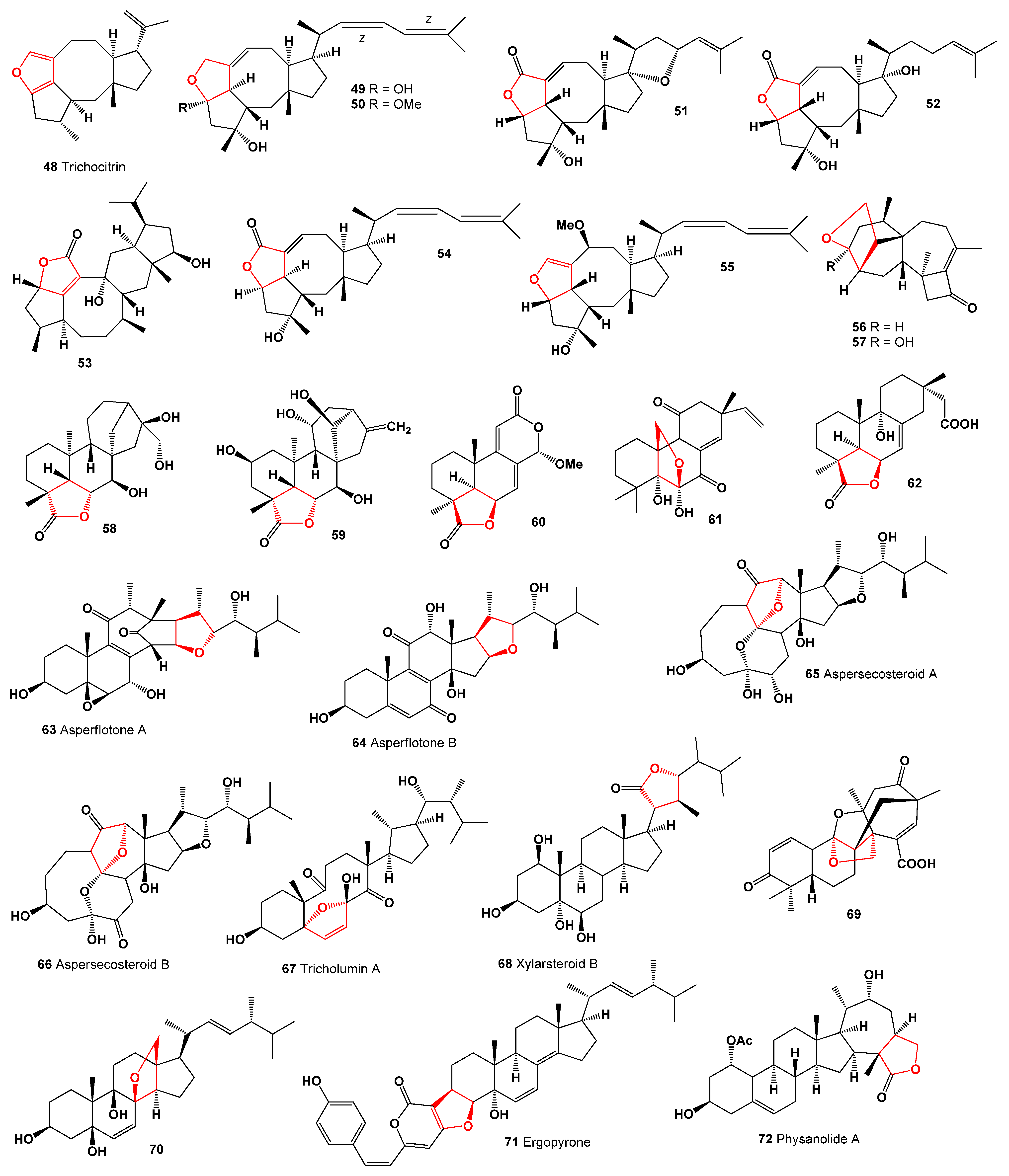
2. Furanosteroids Produced by Fungi and Fungal Endophytes


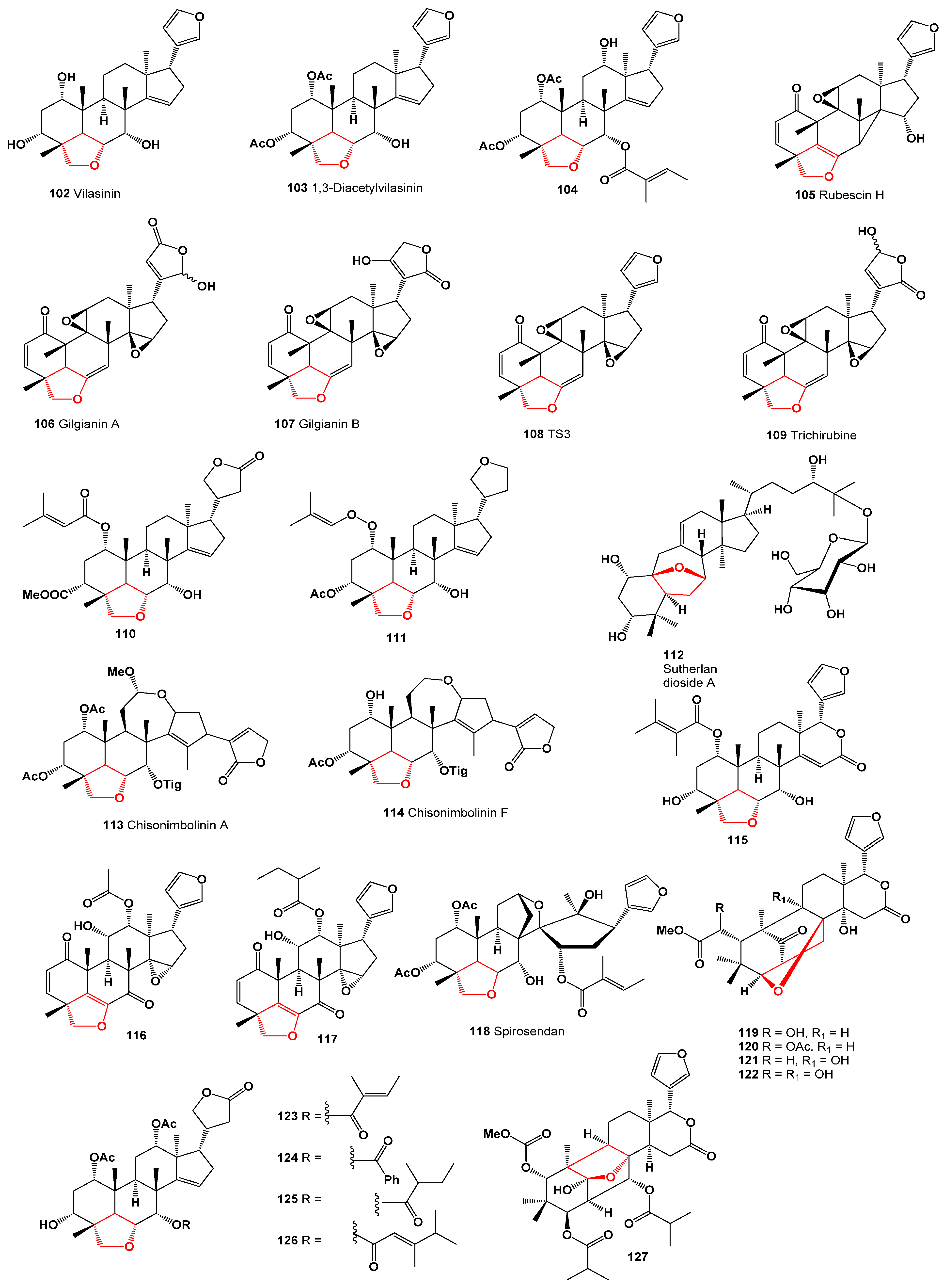
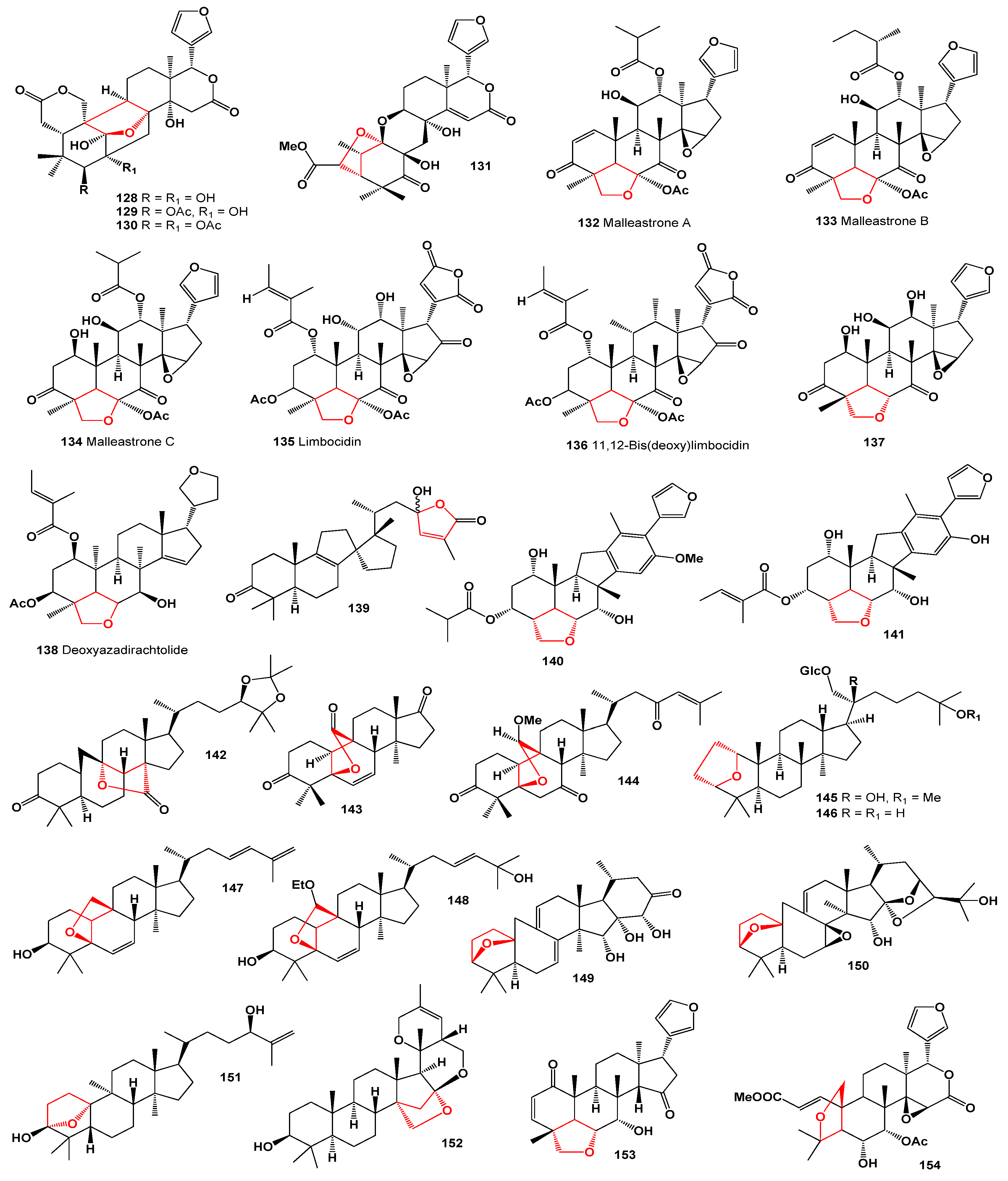
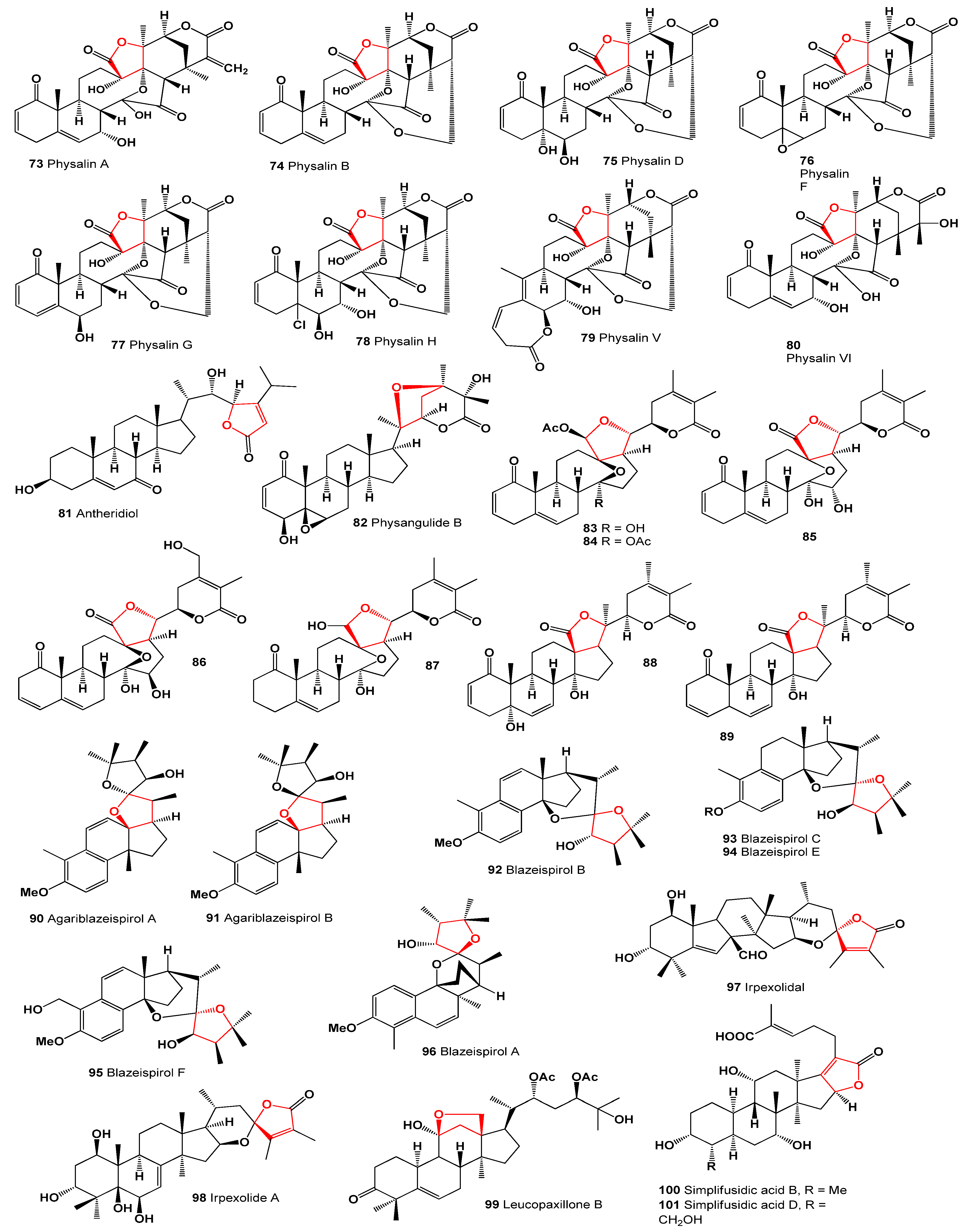
3. Furanosteroids Derived from Plant Species


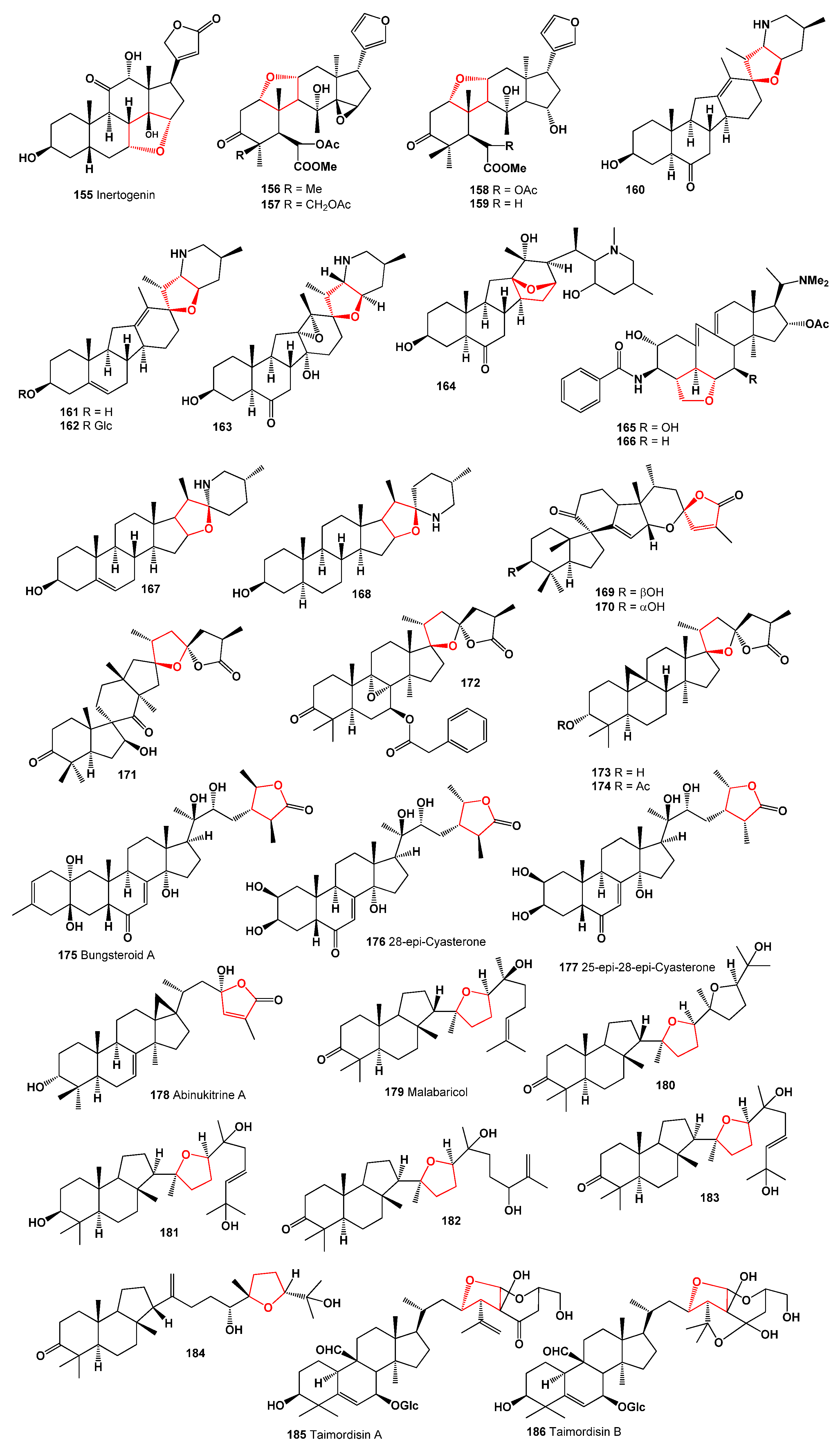


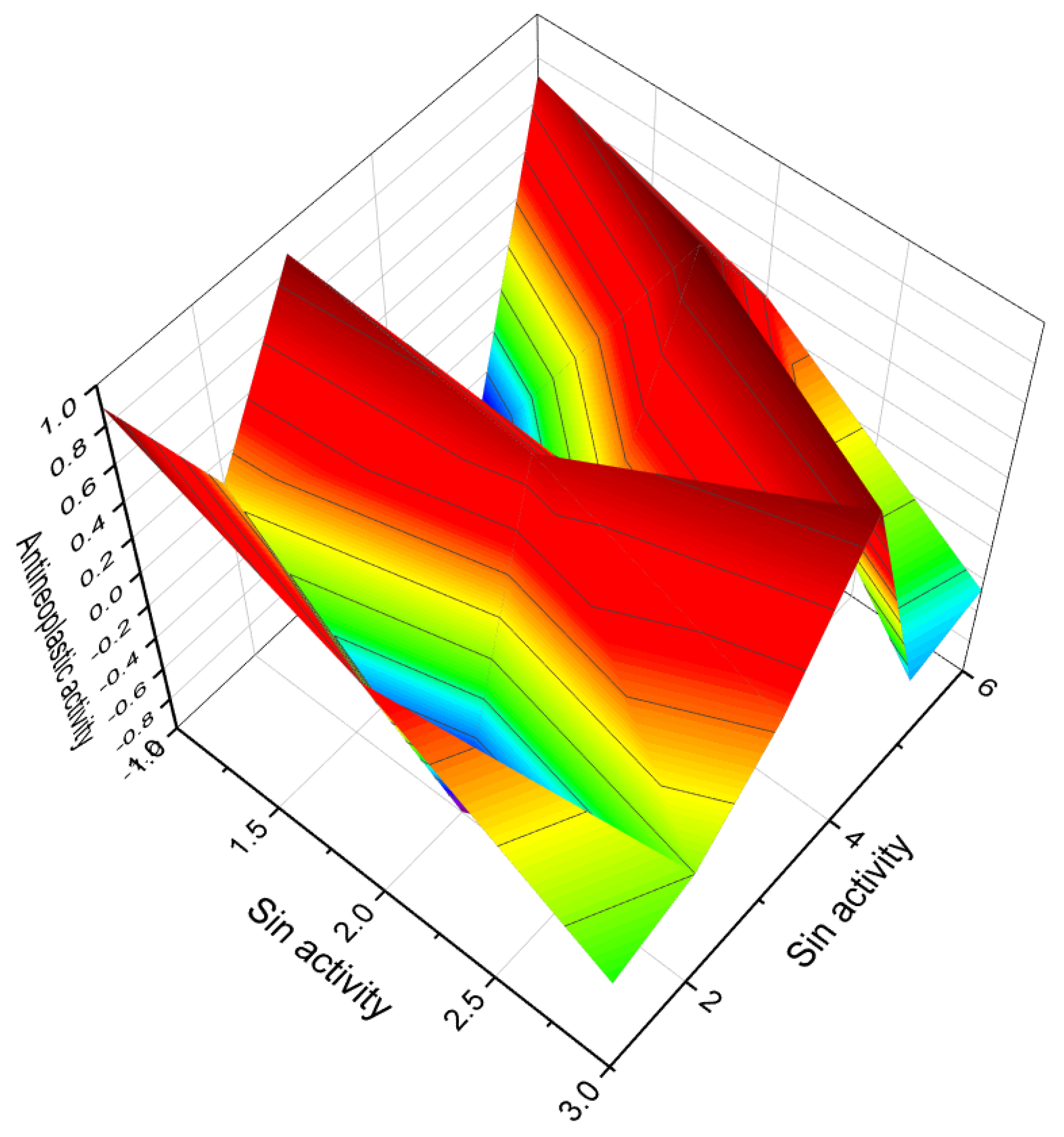
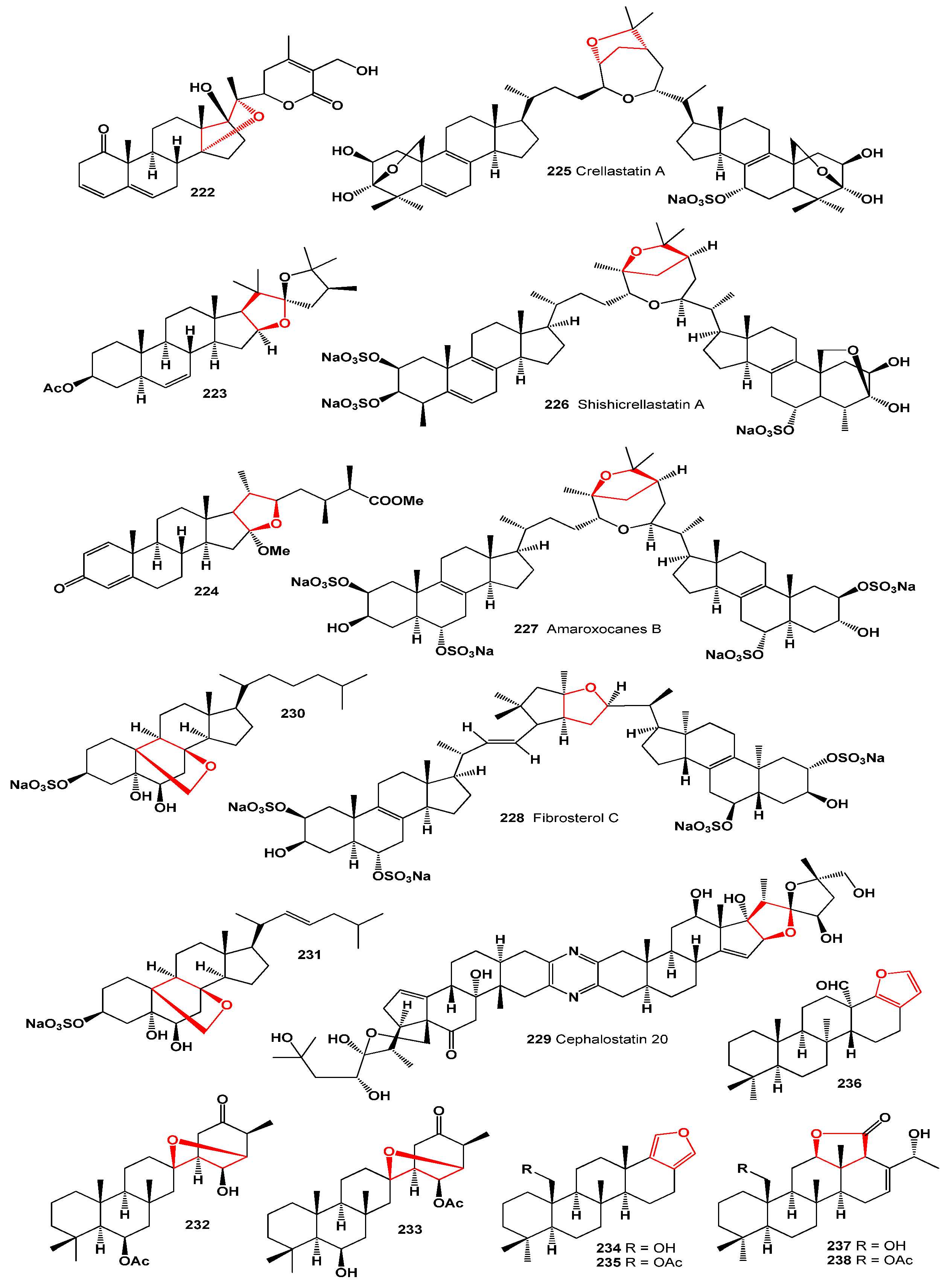
4. Furanosteroids Derived from Marine Sources
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peterson, L.A. Reactive metabolites in the biotransformation of molecules containing a furan ring. Chem. Res. Toxicol. 2013, 26, 6–25. [Google Scholar] [CrossRef] [PubMed]
- Saeid, H.; Al Sayed, H.; Bader, M. A review on biological and medicinal significance of furan. Alqalam J. Med. App. Sci. 2023, 6, 44–58. [Google Scholar]
- Tian, M.; Peng, Y.; Zheng, J. Metabolic activation, and hepatotoxicity of furan-containing compounds. Drug Metabol. Disposit. 2022, 50, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Kumar, H.K.S.; Banerjee, M. Medicinal significance of furan derivatives: A Review. Int. J. Rev. Life Sci. 2012, 2, 7–16. [Google Scholar]
- Alizadeh, M.; Jalal, M.; Hamed, K.; Saber, A. Recent updates on anti-inflammatory and antimicrobial effects of furan natural derivatives. J. Inflammat. Res. 2020, 13, 451–463. [Google Scholar] [CrossRef]
- Mawlong, I.; Sujith Kumar, M.S.; Singh, D. Furan fatty acids: Their role in plant systems. Phytochem. Rev. 2016, 15, 121–127. [Google Scholar] [CrossRef]
- Buckingham, J. Dictionary of Natural Products; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Senapati, B.K. Recent progress in the synthesis of the furanosteroid family of natural products. Org. Chem. Front. 2021, 8, 2608–2642. [Google Scholar] [CrossRef]
- Xue, D.; He, H.; Gao, S. Strategies for the total synthesis of the furanosteroids: Wortmannin and viridin. Chem. Lett. 2021, 50, 497–502. [Google Scholar] [CrossRef]
- Hu, D.; Gao, Y.H.; Yao, X.S.; Gao, H. Recent advances in dissecting the demethylation reactions in natural product biosynthesis. Curr. Opin. Chem. Biol. 2020, 59, 47–53. [Google Scholar] [CrossRef]
- Zhungietu, G.I.; Dorofeenko, G.N. Progress in the field of the chemistry of steroidal heterocycles. Russ. Chem. Rev. 1967, 36, 24–36. [Google Scholar] [CrossRef]
- Hatti, K.S.; Muralitharan, R.L.; Kush, H.; Kush, A. Convenient database for neem secondary metabolites. Bioinformation 2014, 10, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Ui, M.; Okada, T.; Hazeki, K.; Hazeki, O. Wortmannin as a unique probe for an intracellular signalling protein, phosphoinositide 3-kinase. Trends Biochem. Sci. 1995, 20, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.R. The viridin family of steroidal antibiotics. Nat. Prod. Rep. 1995, 12, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Brian, P.W.; Curtis, P.J.; Hemming, H.G. The production of viridin by pigment-forming strains of Trichoderma viride. Ann. Appl. Biol. 1946, 33, 190–200. [Google Scholar] [CrossRef]
- Brian, P.W.; McGowan, J.C. Viridin: A highly fungistatic substance produced by Trichoderma viride. Nature 1945, 156, 144–145. [Google Scholar] [CrossRef]
- Brian, P.W.; Curtis, P.J.; Hemming, H.G.; Norris, G.L.F. Wortmannin, an antibiotic produced by Penicillium wortmanni. Transact. Br. Mycol. Soc. 1957, 40, 365–368. [Google Scholar] [CrossRef]
- Viswanathan, K.; Ononye, S.N.; Cooper, H.D.; Kyle Hadden, M.; Anderson, A.C.; Wright, D.L. Viridin analogs derived from steroidal building blocks. Bioorg. Med. Chem. Lett. 2012, 22, 6919–6922. [Google Scholar] [CrossRef]
- Jones, R.W.; Hancock, J.G. Conversion of viridin to viridiol by viridin-producing fungi. Can. J. Microbiol. 1987, 33, 963–966. [Google Scholar] [CrossRef]
- Aldridge, D.C.; Turner, W.B.; Geddes, A.J.; Sheldrick, B. Demethoxyviridin and demethoxyviridiol: New fungal metabolites. J. Chem. Soc. Perkin Trans. 1 1975, 10, 943–945. [Google Scholar] [CrossRef]
- Andersson, P.F.; Bengtsson, S.; Cleary, M.; Stenlid, J.; Broberg, A. Viridin-like steroids from Hymenoscyphus pseudoalbidus. Phytochemistry 2013, 86, 195–200. [Google Scholar] [CrossRef]
- Zhai, M.M.; Li, J.; Jiang, C.X.; Shi, J.Y.P.; Di, D.L.; Crews, P. The bioactive secondary metabolites from Talaromyces species. Nat. Prod. Bioprospect. 2016, 6, 1. [Google Scholar] [CrossRef]
- Noinart, J. Bioactive Secondary Metabolites from the Culture of the Marine Sponge-Associated Fungus Talaromyces stipitatus KUFA. Ph.D Thesis, University of Porto, Porto, Portugal, 2017. [Google Scholar]
- Ding, H.-E.; Yang, Z.-D.; Sheng, L.; Zhou, S.-Y.; Li, S.; Yao, X.-J.; Zhi, K.-K.; Wang, Y.-G.; Zhang, F. Secovironolide, a novel furanosteroid scaffold with a fivemembered B ring from the endophytic fungus Talaromyces wortmannii LGT-4. Tetrahedron Lett. 2015, 56, 6754–6757. [Google Scholar] [CrossRef]
- Phuwapraisirisan, P.; Rangsan, J.; Siripong, P.; Tip-Pyang, S. 9- epi -Viridiol, a novel cytotoxic furanosteroid from soil fungus Trichoderma virens. Nat. Prod. Res. 2006, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Mirocha, C.J. Isolation and purification of a hemorrhagic factor (wortmannin) from Fusarium oxysporum (N17B). Appl. Environ. Microbiol. 1988, 54, 1268–1274. [Google Scholar] [CrossRef]
- Singh, V.; Praveen, V.; Tripathi, D.; Haque, S.; Somvanshi, P. Isolation, characterization and antifungal docking studies of wortmannin isolated from Penicillium radicum. Sci. Rep. 2015, 5, 11948. [Google Scholar] [CrossRef]
- Wipf, P.; Halter, R.J. Chemistry and biology of wortmannin. Org. Biomol. Chem. 2005, 3, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Yuan, H.; Weissleder, R.; Cantley, L.C.; Josephson, L. A wortmannin-cetuximab as a double drug. Bioconjug. Chem. 2009, 20, 2185–2189. [Google Scholar] [CrossRef]
- Kong, D.; Yamori, T. Phosphatidylinositol 3-kinase inhibitors: Promising drug candidates for cancer therapy. Cancer Sci. 2008, 99, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Morales-Oyervidesa, L.; Ruiz-Sánchez, J.P.; Oliveira, J.C. Biotechnological approaches for the production of natural colorants by Talaromyces/Penicillium: A review. Biotechnol. Adv. 2020, 43, 107601. [Google Scholar] [CrossRef] [PubMed]
- Nagwa, E.; Kassem, A.H.; Hamed, M.A.; El-Fekya, A.M.; Elnaggard, M.A.A.; Mahmouda, K.; Alie, M.A. Isolation and characterization of the bioactive metabolites from the soil derived fungus Trichoderma viride. Mycology 2018, 9, 70–80. [Google Scholar]
- Ghisalberti, E.L.; Sivasithamparam, K. Antifungal antibiotics produced by Trichoderma spp. Soil Biol. Biochem. 1991, 23, 1011–1020. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Horwitz, B.A.; Kenerley, C.M. Secondary metabolism in Trichoderma—A genomic perspective. Microbiology 2012, 158, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-P.; Shi, Z.-Z.; Miao, F.-P.; Fang, S.-T.; Yin, X.-L.; Ji, N.-Y. Tricholumin A, a highly transformed ergosterol derivative from the alga endophytic fungus Trichoderma asperellum. Org. Lett. 2018, 20, 6306–6309. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Makin, H.L.J.; Kirk, D.N.; Murphy, G.M. Dictionary of Steroids, 1st ed.; Chapman and Hall: Boca Raton, FL, USA, 1991. [Google Scholar]
- Chen, H.P.; Zhao, Z.Z.; Li, Z.H.; Huang, Y.; Zhang, S.B.; Tang, Y.; Yao, J.N.; Chen, L.; Isaka, M.; Feng, T.; et al. Anti-proliferative and anti-Inflammatory lanostane triterpenoids from the Polish edible mushroom Macrolepiota procera. J. Agric. Food Chem. 2018, 66, 3146–3154. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Kumar, A.; de los Santos-Villalobos, S.; Parra-Cota, F.I.; del Orozco-Mosqueda, C.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma species: Our best fungal allies in the biocontrol of plant diseases—A Review. Plants 2023, 12, 432. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Gomdola, D.; Bhunjun, C.S.; Hyde, K.D.; Jeewon, R.; Pem, D.; Jayawardena, R.S. Ten important forest fungal pathogens: A review on their emergence and biology. Mycosphere 2022, 13, 612–671. [Google Scholar] [CrossRef]
- Lorenc, F.; Samek, M. Pathogens threatening Czech Republic Forest ecosystems—A review. Sylwan 2021, 165, 853–871. [Google Scholar]
- Benjamin, C.R. Ascocarps of Aspergillus and Penicillium. Mycologia 1955, 47, 669–687. [Google Scholar] [CrossRef]
- Frisvad, J.C. Taxonomy, chemodiversity, and chemoconsistency of Aspergillus, Penicillium, and Talaromyces species. Front. Microbiol. 2015, 5, 773. [Google Scholar] [CrossRef]
- Houbraken, J.; de Vries, R.P.; Samson, R.A. Modern taxonomy of biotechnologically important Aspergillus and Penicillium species. Adv. Appl. Microbiol. 2014, 86, 199–249. [Google Scholar] [PubMed]
- Ramani, G.; Meera, B.; Vanitha, C.; Rao, M. Production, purification, and characterization of a β-glucosidase of Penicillium funiculosum NCL1. Appl. Biochem. Biotechnol. 2012, 167, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Fravel, D.; Olivain, C.; Alabouvette, C. Fusarium oxysporum and its biocontrol. New Phytol. 2003, 157, 493–502. [Google Scholar] [CrossRef]
- Gordon, T.R.; Martyn, R.D. The evolutionary biology of Fusarium oxysporum. Annu. Rev. Phytopathol. 1997, 35, 111–128. [Google Scholar] [CrossRef]
- Ekiz, G.; Yılmaz, S.; Yusufoglu, H.; Kırmızıbayrak, P.B.; Bedir, E. Microbial transformation of cycloastragenol and astragenol by endophytic fungi isolated from Astragalus species. J. Nat. Prod. 2019, 82, 2979–2985. [Google Scholar] [CrossRef]
- Küçüksolak, M.; Üner, G.; Kırmızıbayrak, P.B.; Bedir, E. Neuroprotective metabolites via fungal biotransformation of a novel sapogenin, cyclocephagenol. Sci. Rep. 2022, 12, 18481. [Google Scholar] [CrossRef] [PubMed]
- Galappaththi, M.C.A.; Patabendige, N.M.; Premarathne, B.M.; Hapuarachchi, K.K.; Tibpromma, S.; Dai, D.-Q.; Suwannarach, N.; Rapior, S.; Karunarathna, S.C. A Review of Ganoderma triterpenoids and their bioactivities. Biomolecules 2023, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Su, H.-G.; Wang, Q.; Zhou, L.; Peng, X.-R.; Xiong, W.-Y.; Qiu, M.-H. Functional triterpenoids from medicinal fungi Ganoderma applanatum: A continuous search for antiadipogenic agents. Bioorg. Chem. 2021, 112, 104977. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Peng, X.R.; Dong, J.R.; Lu, S.Y.; Li, X.N.; Zhou, L. Rearranged lanostane-type triterpenoids with anti-hepatic fibrosis activities from Ganoderma applanatum. RSC Adv. 2018, 8, 31287–31295. [Google Scholar] [CrossRef]
- Peng, X.-R.; Liu, J.-Q.; Wang, C.-F.; Li, X.-Y.; Shu, Y.; Zhou, L.; Qiu, M.-H. Hepatoprotective effects of triterpenoids from Ganoderma cochlear. J. Nat. Prod. 2014, 77, 737–743. [Google Scholar] [CrossRef]
- Niu, X.; Qiu, M.; Li, Z.; Lu, Y.; Cao, P.; Zheng, Q. Two novel 3,4-seco-trinorlanostane triterpenoids isolated from Ganoderma fornicatum. Tetrahedron Lett. 2004, 45, 2989–2993. [Google Scholar] [CrossRef]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2020, 37, 962. [Google Scholar] [CrossRef]
- Ma, K.; Li, L.; Bao, L.; He, L.; Sun, C.; Zhou, B.; Si, S.; Liu, H. Six new 3, 4-seco-27-norlanostane triterpenes from the medicinal mushroom Ganoderma boninense and their antiplasmodial activity and agonistic activity to LXRβ. Tetrahedron 2015, 71, 1808–1814. [Google Scholar] [CrossRef]
- Zhang, S.-S.; Wang, Y.-G.; Ma, Q.-Y.; Huang, S.-Z.; Hu, L.-L.; Dai, H.-F.; Yu, Z.-F.; Zhao, Y.-X. Three new lanostanoids from the mushroom Ganoderma tropicum. Molecules 2015, 20, 3281–3289. [Google Scholar] [CrossRef]
- Peng, X.; Liu, J.; Xia, J.; Wang, C.; Li, X.; Deng, Y.; Bao, N.; Zhang, Z.; Qiu, M. Lanostane triterpenoids from Ganoderma hainanense. Phytochemistry 2015, 114, 137–145. [Google Scholar] [CrossRef]
- Li, Q.; Chen, C.; Cheng, L. Emeridones A–F, a series of 3,5-demethylorsellinic acid-based meroterpenoids with rearranged skeletons from an endophytic fungus Emericella sp. TJ29. J. Org. Chem. 2019, 84, 1534–1541. [Google Scholar] [CrossRef]
- Liang, X.-R.; Miao, F.-P.; Song, Y.-P.; Guo, Z.-Y.; Ji, N.-Y. Trichocitrin, a new fusicoccane diterpene from the marine brown alga-endophytic fungus Trichoderma citrinoviride cf-27. Nat. Prod. Res. 2016, 30, 1605–1610. [Google Scholar] [CrossRef]
- Liu, H.B.; Edrada-Ebel, R.A.; Ebel, R.; Wang, Y.; Schulz, B.; Draeger, S. Ophiobolin sesterterpenoids and pyrrolidine alkaloids from the sponge derived fungus Aspergillus ustus. Helvetica Chim. Acta 2011, 94, 623–631. [Google Scholar] [CrossRef]
- Li, Y.L.; Xue, L.J.; Li, J.; Chen, L.M.; Wu, J.J.; Xu, Z.N.; Chen, Y.; Tiana, Y.P.; Yang, X.W. Abinukitrine A, a unique 17,18-cyclolanostane triterpenoid from Abies nukiangensis. Org. Biomol. Chem. 2019, 17, 2107–2109. [Google Scholar]
- Sugawara, F.; Strobel, G.; Strange, R.N.; Siedow, J.N.; Van Duyne, G.D.; Clardy, J. Phytotoxins from the pathogenic fungi Drechslera maydis and Drechslera sorghicola. Proc. Natl. Acad. Sci. USA 1987, 84, 3081–3085. [Google Scholar] [CrossRef]
- Li, E.; Clark, A.M.; Rotella, D.P.; Hufford, C.D. Microbial metabolites of ophiobolin A and antimicrobial evaluation of ophiobolins. J. Nat. Prod. 1995, 58, 74–81. [Google Scholar] [CrossRef]
- Ebel, R. Terpenes from marine-derived fungi. Mar. Drugs 2010, 8, 2340–2368. [Google Scholar] [CrossRef]
- Elissawy, A.M.; El-Shazly, M.; Ebada, S.S.; Singab, A.B.; Proksch, P. Bioactive terpenes from marine-derived fungi. Mar. Drugs 2015, 13, 1966–1992. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Chen, R.; Zhao, J.; Xie, K.; Chen, D.; Feng, K.; Dai, J. Two furanharzianones with 4/7/5/6/5 ring system from microbial transformation of harzianone. Org. Lett. 2017, 19, 1168–1171. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Chen, R.; Zhao, J.; Xie, K.; Chen, D.; Feng, K.; Dai, J. Microbial oxidation of harzianone by Bacillus sp. IMM-006. Tetrahedron 2017, 73, 7195–7199. [Google Scholar] [CrossRef]
- Dong, H.; Zhou, C.; Li, X.; Gu, H.; Zhang, H.E.; Zhou, F.; Zhao, Z.; Fan, T. Ultraperformance liquid chromatography-quadrupole time-of-flight mass spectrometry based untargeted metabolomics to reveal the characteristics of Dictyophora rubrovolvata from different drying methods. Front. Nutr. 2022, 9, 1056598. [Google Scholar] [CrossRef]
- Sato, M.; Kakisawa, H. Structures of three new C16 terpenoids from an Acrostalagmus fungus. J. Chem. Soc. Perkin Trans. I 1976, 22, 2407–2413. [Google Scholar] [CrossRef]
- Kakisawa, H.; Sato, M.; Ruo, T.I.; Hayashi, T. Biosynthesis of a C16-terpenoid lactone, a plant growth regulator. J. Chem. Soc. Chem. Commun. 1973, 9, 802–803. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, J.; Zhang, Y.; Wei, F.; Liu, X. Pimarane diterpenes from the fungus Epicoccum sp. HS-1 associated with Apostichopus japonicus. Bioorg. Med. Chem. Lett. 2012, 22, 3017–3019. [Google Scholar] [CrossRef]
- Li, Y.; Lu, C.; Hu, Z.; Huang, Y.; Shen, Y. Secondary metabolites of Tubercularia sp. TF5, an endophytic fungal strain of Taxus mairei. Nat. Prod. Res. 2009, 23, 70–76. [Google Scholar]
- Shiono, Y.; Motoki, S.; Koseki, T.; Murayama, T.; Tojima, M.; Kimura, K.I. Isopimarane diterpene glycosides, apoptosis inducers, obtained from fruiting bodies of the ascomycete Xylaria polymorpha. Phytochemistry 2009, 70, 935–939. [Google Scholar] [CrossRef]
- Li, Y.Y.; Hu, Z.Y.; Lu, C.H.; Shen, Y.M. Four new terpenoids from Xylaria sp. 101. Helv. Chim. Acta 2010, 93, 796–802. [Google Scholar] [CrossRef]
- Gu, B.B.; Wu, W.; Jiao, F.R.; Jiao, W.J.; Li, L.; Sun, F.; Wang, S.P.; Yang, F.; Lin, H.W. Asperflotone, an 8(14→15)-abeo-ergostane from the sponge derived fungus Aspergillus flocculosus 16D-1. J. Org. Chem. 2019, 84, 300–306. [Google Scholar] [CrossRef]
- Gu, B.-B.; Wu, W.; Jiao, F.-R.; Jiao, W.-h.; Li, L.; Sun, F.; Wang, S.-P.; Yang, F.; Lin, H.-W. Aspersecosteroids A and B, two 11(9→10)-abeo-5,10-secosteroids with a dioxatetraheterocyclic ring system from Aspergillus flocculosus 16D-1. Org. Lett. 2018, 20, 7957–7960. [Google Scholar] [CrossRef]
- Dong, G.A.N.; Li, C.; Shu, Y.; Wu, J. Steroids and dihydroisocoumarin glycosides from Xylaria sp. by the one strain many compounds strategy and their bioactivities. Chin. J. Nat. Med. 2023, 21, 154–160. [Google Scholar]
- Liangsakul, J.; Srisurichan, S.; Pornpakakul, S. Anthraquinone–steroids, evanthrasterol A and B, and a meroterpenoid, emericellic acid, from endophytic fungus, Emericella variecolor. Steroids 2016, 106, 78–85. [Google Scholar] [CrossRef]
- Wu, J.; Suzuki, T.; Choi, J.H.; Yasuda, N.; Noguchi, K.; Hirai, H.; Kawagishi, H. An unusual sterol from the mushroom Stropharia rugosoannulata. Tetrahedron Lett. 2013, 54, 4900–4902. [Google Scholar] [CrossRef]
- Lee, S.; Kim, C.S.; Yu, J.S.; Kang, H.; Yoo, M.J.; Youn, U.J.; Ryoo, R.; Bae, H.K.; Kim, K.H. Ergopyrone, a styrylpyrone-fused steroid with a hexacyclic 6/5/6/6/6/5 skeleton from a mushroom Gymnopilus orientispectabilis. Org. Lett. 2021, 23, 3315–3319. [Google Scholar] [CrossRef]
- Kuo, P.C.; Kuo, T.H.; Damu, A.G.; Su, C.R.; Lee, E.J.; Wu, T.S.; Shu, R.; Chen, C.M.; Kenneth, F.; Bastow, T.-H.; et al. Physanolide A, a novel skeleton steroid, and other cytotoxic principles from Physalis angulate. Org. Lett. 2006, 8, 2953–2956. [Google Scholar] [CrossRef]
- Sun, C.P.; Qiu, C.Y.; Zhao, F. Physalins V-IX, 16,24-cyclo-13,14-seco withanolides from Physalis angulata and their antiproliferative and anti-inflammatory activities. Sci. Rep. 2017, 7, 4057. [Google Scholar] [CrossRef]
- Arsenault, G.P.; Biemann, K.; Barksdale, A.W.; McMorris, T.C. The structure of antheridiol, a sex hormone in Achyla bisexualis. J. Am. Chem. Soc. 1968, 90, 5635–5636. [Google Scholar] [CrossRef]
- Maldonado, E.; Hurtado, N.E.; Pérez-Castorena, A.L.; Martínez, M. Cytotoxic 20,24-epoxy-withanolides from Physalis angulata. Steroids 2015, 104, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Fan, J.; Liu, Z.; Li, X.; Zhang, F.; Zhang, Y.; Sun, Y.; Li, L.; Xia Liu, X.; Hua, E. Cytotoxic withanolides from the whole herb of Physalis angulata L. Molecules 2019, 24, 1608. [Google Scholar] [CrossRef]
- Sun, C.P.; Qiu, C.Y.; Yuan, T.; Nie, X.F.; Sun, H.X.; Zhang, Q.; Li, H.X.; Ding, L.Q.; Zhao, F.; Chen, L.X.; et al. Antiproliferative and anti-inflammatory withanolides from Physalis angulata. J. Nat. Prod. 2016, 79, 1586–1597. [Google Scholar] [CrossRef]
- Hirotani, M.; Sai, K.; Hirotani, S.; Yoshikawa, T. Blazeispirols B, C, E and F, des-A-ergostane-type compounds, from the cultured mycelia of the fungus Agaricus blazei. Phytochemistry 2002, 59, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Hirotani, M.; Hirotani, S.; Takayanagi, H.; Komiyama, K.; Yoshikawa, T. Agariblazeispirols A and B, an unprecedented skeleton from the cultured mycelia of the fungus, Agaricus blazei. Tetrahedron Lett. 2003, 44, 7975–7979. [Google Scholar] [CrossRef]
- Hirotani, M.; Hirotani, S.; Takayanagi, H.; Yoshikawa, T. Blazeispirol A, an unprecedented skeleton from the cultured mycelia of the fungus Agaricus blazei. Tetrahedron Lett. 1999, 40, 329–332. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, Z.Z.; Hu, K.; Feng, T.; Li, Z.H.; Chen, H.P.; Liu, J.K. Irpexolidal Represents a class of triterpenoid from the fruiting bodies of the medicinal fungus Irpex lacteus. J. Org. Chem. 2019, 84, 1845–1852. [Google Scholar] [CrossRef]
- Clericuzio, M.; Tabasso, S.; Bianco, M.A.; Pratesi, G.; Beretta, G.; Tinelli, S.; Zunino, F.; Vidari, G. Cucurbitane triterpenes from the fruiting bodies and cultivated mycelia of Leucopaxillus gentianeus. J. Nat. Prod. 2006, 69, 1796–1799. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, X.; Yao, F.H.; Liu, X.B.; Qi, S.H. Fusidane-type antibiotics from the marine-derived fungus Simplicillium sp. SCSIO 41513. J. Nat. Prod. 2021, 84, 2945–2952. [Google Scholar] [CrossRef]
- Macías-Rubalcava, M.L.; Sánchez-Fernández, R.E. Secondary metabolites of endophytic Xylaria species with potential applications in medicine and agriculture. World J. Microbiol. Biotechnol. 2017, 33, 15. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; El-Seedi, H.R.; Xu, B. Critical review on chemical compositions and health-promoting effects of mushroom Agaricus blazei Murill. Curr. Res. Food Sci. 2022, 5, 2190–2203. [Google Scholar] [CrossRef] [PubMed]
- Bertollo, A.G.; Mingoti, M.E.D.; Plissari, M.E.; Betti, G.; Junior, W.A.R.; Luzardo, A.R.; Ignácio, Z.M. Agaricus blazei Murrill mushroom: A review on the prevention and treatment of cancer. Pharmacol. Res. Mod. Chin. Med. 2022, 2, 100032. [Google Scholar] [CrossRef]
- Dong, X.M.; Song, X.H.; Liu, K.B.; Dong, C.H. Prospect and current research status of medicinal fungus Irpex lacteus. Mycosystema 2017, 36, 28–34. [Google Scholar]
- Mezule, L.; Civzele, A. Bioprospecting white-rot basidiomycete Irpex lacteus for improved extraction of lignocellulose-degrading enzymes and their further application. J. Fungi 2020, 6, 256. [Google Scholar] [CrossRef]
- Pachapurkar, R.V.; Kornule, P.M.; Narayana, C.R. A new hexacyclic tetranortriterpenoid. Chem. Lett. 1974, 3, 357–358. [Google Scholar] [CrossRef]
- Rogers, L.L.; Zeng, L.; Kozlowski, J.F.; Shimada, H.; Alali, F.Q.; Johnson, H.A.; McLaughlin, J.L. New bioactive triterpenoids from Melia volkensii. J. Nat. Prod. 1998, 61, 64. [Google Scholar] [CrossRef]
- Connolly, J.D.; Labbé, C.; Rycroft, D.S.; Taylor, D.A.H. Tetranortriterpenoids and related compounds. Part 22. New apotirucailol derivatives and tetranortriterpenoids from the wood and seeds of Chisocheton paniculatus (Meliaceae). J. Chem. Soc. Perkin Trans. 1979, 21, 2959–2964. [Google Scholar] [CrossRef]
- Kraus, W.; Cramer, R.; Sawitzki, G. Tetranortriterpenoids from the seeds of Azadirachta indica. Phytochemistry 1981, 20, 117–120. [Google Scholar] [CrossRef]
- Mulholland, D.A.; Monkhe, T.V.; Coombes, P.H.; Rajab, M.S. Limonoids from Turraea holstii and Turraea floribunda. Phytochemistry 1998, 49, 2585. [Google Scholar] [CrossRef]
- Kumar, C.S.S.R.; Srinivas, M.; Yakkundi, S. Limonoids from the seeds of Azadirachta indica. Phytochemistry 1996, 43, 451. [Google Scholar] [CrossRef]
- Coombesa, P.H.; Mulhollanda, D.A.; Randrianarivelojosia, M. Vilasinin limonoids from Malleastrum antsingyense J.F. Leroy (Meliodeae: Meliaceae). Biochem. Syst. Ecol. 2008, 36, 74–76. [Google Scholar] [CrossRef]
- Kowa, T.K.; Jansen, O.; Ledoux, A.; Mamede, L.; Wabo, H.K.; Tchinda, A.T.; Genta-Jouve, G.; Frédérich, M. Bioassay-guided isolation of vilasinin–type limonoids and phenyl alkene from the leaves of Trichilia gilgiana and their antiplasmodial activities. Nat. Prod. Res. 2022, 36, 5039–5047. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Pednekar, A.; Avalaskar, A.; Rathi, M. A comprehensive review on Meliaceae family. World J. Pharm. Sci. 2015, 3, 1572–1577. [Google Scholar]
- Islas, J.F.; Acosta, E.; Buentello, Z.G.; Delgado-Gallegos, J.L.; Moreno-Treviño, M.G.; Escalante, B.; Moreno-Cueva, J.E. An overview of Neem (Azadirachta indica) and its potential impact on health. J. Function. Foods 2020, 74, 104171. [Google Scholar] [CrossRef]
- Ma, C.; Chen, Y.; Chen, J.; Li, X.; Chen, Y. A Review on Annona squamosa L.: Phytochemicals and biological activities. Am. J. Chin. Med. 2017, 45, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; Nacpil, Z.D.; Natividad, G.M.; Tada, M.; Coll, J.C.; Rideout, J.A. Tetranortriterpenoids from Azadirachta indica. Phytochemistry 1997, 46, 555–558. [Google Scholar] [CrossRef]
- Fu, X.; Li, X.C.; Smillie, T.J.; Carvalho, P.; Mabusela, W.; Syce, J.; Johnson, Q.; Folk, W.; Avery, M.A.; Khan, I.A. Cycloartane glycosides from Sutherlandia frutescens. J. Nat. Prod. 2008, 71, 1749–1753. [Google Scholar] [CrossRef]
- Yang, M.H.; Wang, J.S.; Luo, J.G.; Wang, X.B.; Kong, L.Y. Tetranortriterpenoids from Chisocheton paniculatus. J. Nat. Prod. 2009, 72, 2014–2018. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Afshana, F.; Ghiasuddina, F.; Faizi, S.; Naqvi, S.N.H.; Tariq, R.M. Two insecticidal tetranortriterpenoids from Azadirachta indica. Phytochemistry 2000, 53, 371–376. [Google Scholar] [CrossRef]
- Trinh, B.T.D.; Nguyen, H.D.; Nguyen, H.T.; Pham, P.D.; Ngo, N.T.N. Cytotoxic limonoids from the bark of Walsura cochinchinensis. Fitoterapia 2019, 133, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Munehiro, N.; Bo, Z.J.; Kenjiro, T.; Hideo, N. Spirosendan, a novel spiro limonoid from Melia toosendan. Chem. Lett. 1998, 27, 1279–1280. [Google Scholar]
- Veitch, N.C.; Wright, G.A.; Stevenson, P.C. Four new tetranortriterpenoids from Cedrela odorata associated with leaf rejection by Exopthalmus jekelianus. J. Nat. Prod. 1999, 62, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Kernan, M.R.; Jolad, S.D.; Stoddart, C.A.; Bogan, M.; Cooper, R. Dysoxylins A–D, tetranortriterpenoids with potent anti-RSV activity from Dysoxylum gaudichaudianum. J. Nat. Prod. 2007, 70, 312–315. [Google Scholar] [CrossRef]
- Waratchareeyakul, W.; Hellemann, E.; Gil, R.R.; Chantrapromma, K.; Langat, M.K.; Mulholland, D.A. Application of residual dipolar couplings and selective quantitative NOE to establish the structures of tetranortriterpenoids from Xylocarpus rumphii. J. Nat. Prod. 2017, 80, 391–402. [Google Scholar] [CrossRef]
- Tchimene, M.K.; Tane, P.; Ngamga, D.; Connolly, J.D.; Farrugia, L.J. Four tetranortriterpenoids from the stem bark of Khaya anthotheca. Phytochemistry 2005, 66, 1088–1093. [Google Scholar] [CrossRef]
- Fauzi, F.M.; Meilanie, S.R.; Zulfikar; Farabi, K.; Herlina, T.; Al Anshori, J.; Mayanti, T. Kokosanolide D: A New tetranortriterpenoid from fruit peels of Lansium domesticum Corr. cv Kokossan. Molbank 2021, 2021, M1232. [Google Scholar] [CrossRef]
- Murphy, B.T. Isolation, and Structure Elucidation of Antiproliferative Natural Products from Madagascar. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2007. [Google Scholar]
- Murphy, B.T.; Brodie, P.; Slebodnick, C.; Miller, J.S.; Birkinshaw, C.; Randrianjanaka, L.M.; Andriantsiferana, R.; Rasamison, V.E.; Dyke, K.T.; Suh, E.M.; et al. Antiproliferative limonoids of a Malleastrum sp. from the Madagascar rainforest. J. Nat. Prod. 2008, 71, 325–329. [Google Scholar] [CrossRef][Green Version]
- Krief, S.; Martin, M.T.; Grellier, P.; Kasenene, J.; Sevenet, T. Novel antimalarial compounds isolated in a survey of self-medicative behavior of wild chimpanzees in Uganda. Antimicrob. Agents Chemother. 2004, 48, 3196–3199. [Google Scholar] [CrossRef]
- Batista, R.; de Jesus Silva Júnior, A.; Braga de Oliveira, A. Plant-derived antimalarial agents: New leads and efficient phytomedicines. Part II. Non-alkaloidal natural products. Molecules 2009, 14, 3037–3072. [Google Scholar] [CrossRef]
- Curcino Vieira, I.J.; da Silva Terra, W.; dos Santos Gonçalves, M.; Braz-Filho, R. Secondary metabolites of the genus Trichilia: Contribution to the chemistry of Meliaceae family. Am. J. Anal. Chem. 2014, 5, 42542. [Google Scholar] [CrossRef][Green Version]
- Siddiqui, S.; Ghiasuddin, S.; Siddiqui, B.S.; Faizi, S. Tetranortriterpenoids and steroidal glycosides from the seeds of Azadirachta indica A. Juss. Pak. J. Sci. Indust. Res. 1989, 32, 435. [Google Scholar]
- Schwikkard, S.L. Extractives from the Meliaceae and Simaroubaceae of Madagascar. Ph.D Thesis, University of Natal, Durban, South Africa, 1997. [Google Scholar]
- Tan, Q.G.; Luo, X.D. Meliaceous limonoids: Chemistry and biological activities. Chem. Rev. 2011, 111, 7437–7522. [Google Scholar] [CrossRef]
- Banerji, R.; Misra, G.; Nigam, S.K. On the triterpenes of Azadirachta indica (Melia azadirachta). Fitoterapia 1977, 48, 166. [Google Scholar]
- Banerji, B.; Nigam, S.K. Wood constituents of Meliaceae: A review. Fitoterapia 1984, 55, 3. [Google Scholar]
- Akhila, A.; Rani, K. Chemistry of the neem tree (Azadirachta indica A. Juss.). In Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Herz, W., Falk, H., Kirby, G.W., Moore, R.E., Tamm, C., Eds.; Springer: Vienna, Austria, 1999. [Google Scholar]
- Gao, Q.; Sun, J.; Xun, H.; Yao, X.; Wang, J.; Tang, F. A new azadirachta from the crude extracts of neem (Azadirachta indica A. Juss) seeds. Nat. Prod. Res. 2017, 31, 1739–1746. [Google Scholar] [CrossRef]
- Ou-Yang, D.-W.; Wu, L.; Li, Y.-L.; Yang, P.-M.; Kong, D.-Y.; Yang, X.-W.; Zhang, W.-D. Miscellaneous terpenoid constituents of Abies nephrolepis and their moderate cytotoxic activities. Phytochemistry 2011, 72, 2197–2204. [Google Scholar] [CrossRef]
- Yang, S.P.; Yue, J.M. Discovery of structurally diverse and bioactive compounds from plant resources in China. Acta Pharmacol. Sin. 2012, 33, 1147–1158. [Google Scholar] [CrossRef][Green Version]
- Son, N.T. The genus Walsura: A rich resource of bioactive limonoids, triterpenoids, and other types of compounds. In Progress in the Chemistry of Organic Natural Products 118; Kinghorn, A.D., Falk, H., Gibbons, S., Asakawa, Y., Liu, J.K., Dirsch, V.M., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Li, Y.; Lin, W.; Huang, J. Anti-cancer effects of Gynostemma pentaphyllum (Thunb.) Makino (Jiaogulan). Chin. Med. 2016, 11, 43. [Google Scholar] [CrossRef]
- Razmovski-Naumovski, V.; Huang, T.H.W.; Tran, V.H.; Li, G.Q.; Duke, C.C.; Roufogalis, B.D. Chemistry and pharmacology of Gynostemma pentaphyllum. Phytochem. Rev. 2005, 4, 197–219. [Google Scholar] [CrossRef]
- Grover, J.K.; Yadav, S.P. Pharmacological actions and potential uses of Momordica charantia: A review. J. Ethnopharmacol. 2004, 93, 123–132. [Google Scholar] [CrossRef]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent Advances in Momordica charantia: Functional components and biological activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef]
- Han, M.-L.; Zhang, H.; Yang, S.-P.; Yue, J.-M. Walsucochinoids A and B: New rearranged limonoids from Walsura cochinchinensis. Org. Lett. 2012, 14, 486–489. [Google Scholar] [CrossRef]
- Simo Mpetga, J.D.; He, H.-P.; Hao, X.-J.; Leng, Y.; Tane, P. Further cycloartane and friedelane triterpenoids from the leaves of Caloncoba glauca. Phytochem. Lett. 2014, 7, 52–56. [Google Scholar] [CrossRef]
- Chen, J.-C.; Liu, W.-Q.; Lu, L.; Qiu, M.H.; Zheng, Y.T.; Yang, L.M. Kuguacins F-S, cucurbitane triterpenoids from Momordica charantia. Phytochemistry 2009, 70, 133–140. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Cao, J.-Q.; Zhao, C.; Wang, X.-d.; Wu, X.-j.; Zhao, Y.-Q. Novel dammarane-type triterpenes isolated from hydrolyzate of total Gynostemma pentaphyllum saponins Bioorg. Med. Chem. Lett. 2015, 25, 3095–3099. [Google Scholar] [CrossRef]
- Cao, J.-Q.; Zhang, Y.; Cui, J.-M.; Zhao, Y.-Q. Two new cucurbitane triterpenoids from Momordica charantia L. Chin. Chem. Lett. 2011, 22, 583–586. [Google Scholar] [CrossRef]
- Wang, W.-H.; Nian, Y.; He, Y.-J.; Wan, L.-S.; Bao, N.-M.; Zhu, G.-L.; Wang, F.; Qiu, M.-H. New cycloartane triterpenes from the aerial parts of Cimicifuga heracleifolia. Tetrahedron 2015, 71, 8018–8025. [Google Scholar] [CrossRef]
- Huang, D.; Qing, S.; Zeng, G.; Wang, Y.; Guo, H.; Tan, J.; Zhou, Y. Lipophilic components from fructus Viticis Negundo and their antitumor activities. Fitoterapia 2013, 86, 144–148. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, J.; Peng, L.; Li, Y.; Chen, W. Two novel saponins of 20, 26-epoxy derivatives of pseudojujubogenin from the seeds of Hovenia trichocarpa. Fitoterapia 2013, 87, 65–68. [Google Scholar] [CrossRef]
- Wong, C.P.; Shimada, M.; Nugroho, A.E.; Hirasawa, Y.; Kaneda, T.; Hadi, A.H.A.; Osamu, S.; Morita, H. Ceramicines J–L, new limonoids from Chisocheton ceramicus. J. Nat. Med. 2012, 66, 566–570. [Google Scholar] [CrossRef]
- Renkonen, O.; Schindler, O.; Reichstein, T. Die Konstitution von Sinogenin. Glykoside und Aglykone. 181. Mitteilung. Croatica Chem. Acta 1957, 29, 239–245. [Google Scholar]
- Liu, J.-Q.; Wang, C.-F.; Li, Y.; Chen, J.-C.; Zhou, L.; Qiu, M.-H. Limonoids from the leaves of Toona ciliata var. yunnanensis. Phytochemistry 2012, 76, 141–149. [Google Scholar] [CrossRef]
- Xu, D.-M.; Huang, E.-X.; Wang, S.-Q.; Wen, X.-G.; Wu, X.-Y. Studies on the chemical constituents of Fritillaria pallidiflora Schrenk. J. Integr. Plant Biol. 1990, 32, 789–793. [Google Scholar]
- Li, Y.; Yili, A.; Li, J.; Muhamat, A.; Aisa, H.A. New isosteroidal alkaloids with tracheal relaxant effect from the bulbs of Fritillaria pallidiflora Schrenk. Bioorg. Med. Chem. Lett. 2016, 26, 1983–1987. [Google Scholar] [CrossRef]
- Li, H.J.; Jiang, Y.; Ping Li, W.-C. Puqienine F, a novel veratramine alkaloid from the bulbs of Fritillaria puqiensis. Chem. Pharm. Bull. 2006, 54, 722–724. [Google Scholar] [CrossRef]
- Babar, Z.U.; Ata, A.; Meshkatalsadat, M.H. New bioactive steroidal alkaloids from Buxus hyrcana. Steroids 2006, 71, 1045–1051. [Google Scholar] [CrossRef]
- Niño, J.; Correa, Y.M.; Mosquera, O.M. Biological activities of steroidal alkaloids isolated from Solanum leucocarpum. Pharm. Biol. 2009, 47, 255–259. [Google Scholar] [CrossRef]
- Zhao, Q.-Q.; Song, Q.-Y.; Jiang, K.; Li, G.-D.; Wei, W.-J.; Li, Y.; Gao, K. Spirochensilides A and B, two new rearranged triterpenoids from Abies chensiensis. Org. Lett. 2015, 17, 2760–2763. [Google Scholar] [CrossRef]
- Wang, G.-W.; Lv, C.; Fang, X.; Tian, X.-H.; Ye, J.; Li, H.-L.; Shan, L.; Shen, Y.-H.; Zhang, W.-D. Eight pairs of epimeric triterpenoids involving a characteristic spiro-E/F ring from Abies faxoniana. J. Nat. Prod. 2015, 78, 50–60. [Google Scholar] [CrossRef]
- Meng, X.H.; Chai, T.; Shi, Y.P.; Yang, J.L. Bungsteroid A: One Unusual C34 Pentacyclic Steroid Analogue from Zanthoxylum bungeanum Maxim. J. Org. Chem. 2020, 85, 10806–10812. [Google Scholar] [CrossRef]
- Okuzumi, K.; Hara, N.; Uekusa, H.; Fujimoto, Y. Structure elucidation of cyasterone stereoisomers isolated from Cyathula officinalis. Org. Biomol. Chem. 2005, 3, 1227–1232. [Google Scholar] [CrossRef]
- Yi, J.; Luo, Y.; Li, B.; Zhang, G. Phytoecdysteroids and glycoceramides from Eriophyton wallchii. Steroids 2004, 69, 809–815. [Google Scholar] [CrossRef]
- Snogan, E.; Vahirua-Lechat, I.; Ho, R.; Bertho, G.; Girault, J.P.; Ortiga, S.; Maria, A.; Lafont, R. Ecdysteroids from the medicinal fern Microsorum scolopendria (Burm. f.). Phytochem. Anal. 2007, 18, 441–450. [Google Scholar] [CrossRef]
- Hao, D.-C.; Gu, X.-J.; Xiao, P.-G.; Peng, Y. Phytochemical, and biological research of Fritillaria medicinal resources. Chin. J. Nat. Med. 2013, 11, 0330–0344. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, H.; Ren, Q. Natural drug sources for respiratory diseases from Fritillaria: Chemical and biological analyses. Chin. Med. 2021, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Rushforth, K.D. Notes on Chinese silver firs 2. Notes R. Bot. Gard. Edinb. 1984, 41, 535–540. [Google Scholar]
- Dai, J.; Han, R.; Xu, Y.; Li, N.; Wang, J.; Dan, W. Recent progress of antibacterial natural products: Future antibiotics candidates. Bioorg. Chem. 2020, 101, 103922. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Zhu, L.; Li, T.; Jiang, W.; Zhou, J.; Peng, W.; Wu, C. Zanthoxylum bungeanum Maxim. (Rutaceae): A systematic review of its traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics, and toxicology. Int. J. Mol. Sci. 2017, 18, 2172. [Google Scholar] [CrossRef]
- Bao, Y.; Yang, L.; Fu, Q.; Fu, Y.; Tian, Q.; Wang, C.; Huang, Q. The current situation of Zanthoxylum bungeanum industry and the research and application prospect. A review. Fitoterapia 2023, 164, 105380. [Google Scholar] [CrossRef]
- Chawla, A.; Dev, S. A new class of triterpenoids from Ailanthus malabarica DC derivatives of malabaricane. Tetrahedron Lett. 1967, 48, 4837–4843. [Google Scholar] [CrossRef]
- Srinivas, P.V.; Rao, R.R.; Rao, J.M. Two new tetracyclic triterpenes from the heartwood of Ailanthus excelsa Roxb. Chem. Biodivers. 2006, 3, 930–934. [Google Scholar] [CrossRef]
- Ebada, S.S.; Lin, W.; Proksc, P. Bioactive sesterterpenes and triterpenes from marine sponges: Occurrence and pharmacological significance. Mar. Drugs 2010, 8, 313–346. [Google Scholar] [CrossRef]
- Khare, C.P. Indian Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-0-387-70637-5. [Google Scholar]
- Thongnest, S.; Boonsombat, J.; Prawat, H.; Mahidol, C.; Ruchirawat, S. Ailanthusins A-G and nor-lupane triterpenoids from Ailanthus triphysa. Phytochemistry 2017, 134, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, H.L.; Christensen, J.; Olsen, C.E.; Sittie, A.A.; Jaroszewski, J.W. New dammarane and malabaricane triterpenes from Caloncoba echinata. J. Nat. Prod. 2002, 65, 1764–1768. [Google Scholar] [CrossRef]
- Liaw, C.C.; Lo, I.W.; Lin, Y.C.; Huang, H.T.; Zhang, L.J.; Hsiao, P.C. Four cucurbitane glycosides taimordisins A–D with novel furopyranone skeletons isolated from the fruits of Momordica charantia. Food Chem. 2022, 14, 100286. [Google Scholar] [CrossRef] [PubMed]
- Rashid, I.; Yaqoob, U. Traditional uses, phytochemistry and pharmacology of genus Fritillaria—A review. Bull. Natl. Res. Cent. 2021, 45, 124. [Google Scholar] [CrossRef]
- Xu, Y.-H.; Dai, G.-M.; Tang, S.H.; Yang, H.-J.; Sun, Z.-G. Changes of anti-tuberculosis herbs formula during past three decades in contrast to ancient ones. Chin. J. Integr. Med. 2021, 27, 388–393. [Google Scholar] [CrossRef]
- Moosmann, P.; Ueoka, R.; Grauso, L.; Mangoni, A.; Morinaka, B.I.; Gugger, M.; Piel, J. Cyanobacterial ent-sterol-like natural products from a deviated ubiquinone pathway. Angew. Chem. Int. Ed. 2017, 56, 4987–4990. [Google Scholar] [CrossRef]
- Govindam, S.V.S.; Choi, B.-K.; Yoshioka, Y.; Kanamoto, A.; Fujiwara, T.; Okamoto, T.; Ojika, M. Novel cytotoxic polyoxygenated steroids from an Okinawan sponge Dysidea sp. Biosci. Biotechnol. Biochem. 2012, 76, 999–1002. [Google Scholar] [CrossRef]
- Braekman, J.C.; Daloze, D.; Moussiaux, B.; Vandervyver, G.; Riccio, R. Cholest-6-EN-11β,19-epoxy-3β,5α,8α,9α-tetrol, a novel polyoxygenated steroid from the sponge Dysidea tupha. Bull. Soc. Chim. Belg. 1988, 97, 293. [Google Scholar] [CrossRef]
- Corgiat, J.M.; Scheuer, P.J.; Rios Steiner, J.L.; Clardy, J. Three pregnane-10, 2-carbolactones from a sponge, Strongylophora sp. Tetrahedron 1993, 49, 1557. [Google Scholar] [CrossRef]
- Teruya, T.; Nakagawa, S.; Koyama, T.; Suenaga, K.; Kita, M.; Uemura, D. Nakiterpiosin, a novel cytotoxic C-nor-D-homosteroid from the Okinawan sponge Terpios hoshinota. Tetrahedron Lett. 2003, 44, 5171. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.h.; Kalinovsky, A.I.; Antonov, A.S.; Ponomarenko, L.P.; Dmitrenok, P.S.; Aminin, D.L.; Krasokhin, V.B.; Nosova, V.M.; Kisin, A.V. Isolation and structures of erylosides from the Carribean sponge Erylus goffrilleri. J. Nat. Prod. 2007, 70, 1871–1877. [Google Scholar] [CrossRef]
- Kolesnikova, S.A.; Lyakhova, E.G.; Kalinovsky, A.I.; Pushilin, M.A.; Afiyatullov, S.S.; Yurchenko, E.A.; Dyshlovoy, S.A.; Minh, C.V.; Stonik, V.A. Isolation, structures, and biological activities of triterpenoids from a marine sponge Penares sp. J. Nat. Prod. 2013, 76, 1746–1752. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Rao, V.L.; Sastry, V.G. A new spiroketal steroid from Gorgonella umbraculum. Nat. Prod. Res. 2003, 17, 149. [Google Scholar] [CrossRef]
- Tomono, Y.; Hirota, H.; Fusetani, N. Isogosterones A–D, antifouling 13,17-secosteroids from an octocoral Dendronephthya sp. J. Org. Chem. 1999, 64, 2272. [Google Scholar] [CrossRef]
- Rao, C.B.; Ramana, K.V.; Rao, D.V.; Fahy, E.; Faulkner, D.J. Metabolites of the gorgonian Isis hippuris from India. J. Nat. Prod. 1988, 51, 954. [Google Scholar] [CrossRef]
- Rao, C.B.; Kalidindi, R.S.H.S.N.; Trimurtulu, G.; Rao, D.V. Metabolites of porifera, part III. New 24-methylscalaranes from Phyllospongia dendyi of the Indian ocean. J. Nat. Prod. 1991, 54, 364. [Google Scholar] [CrossRef]
- Alvi, K.A.; Crews, P. Homoscalarane sesterterpenes from Lendenfeldia frondosa. J. Nat. Prod. 1992, 55, 859. [Google Scholar] [CrossRef]
- Crews, P.; Bescansa, P. Sesterterpenes from a common marine sponge, Hyrtios erecta. J. Nat. Prod. 1986, 49, 1041. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Fu, X.; Su, J.; Pordesimo, E.O.; Traeger, S.C.; Schmitz, F.J. Novel bishomoscalarane sesterterpenes from the sponge Phyllospongia foliascens. J. Nat. Prod. 1991, 54, 421. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Tseng, S.-W.; Liu, L.-L.; Chou, Y.; Ho, Y.-S.; Lu, M.-C.; Su, J.-H. Cytotoxic sesterterpenoids from a sponge Hippospongia sp. Mar. Drugs 2012, 10, 987–997. [Google Scholar] [CrossRef]
- Youssef, D.T.; Yamaki, R.K.; Kelly, M.; Scheuer, P.J. Salmahyrtisol A, a novel cytotoxic sesterterpene from the Red Sea sponge Hyrtios erecta. J. Nat. Prod. 2002, 65, 2–6. [Google Scholar] [CrossRef]
- Aoki, S.; Watanabe, Y.; Sanagawa, M.; Setiawan, A.; Kotoku, N.; Kobayashi, M. Cortistatins A, B, C, and D, anti-angiogenic steroidal alkaloids, from the marine sponge Corticium simplex. J. Am. Chem. Soc. 2006, 128, 3148–3149. [Google Scholar] [CrossRef] [PubMed]
- Roll, D.M.; Scheuer, P.J.; Matsumoto, G.K.; Clardy, J. Halenaquinone, a pentacyclic polyketide from a marine sponge. J. Am. Chem. Soc. 1983, 105, 6177–6178. [Google Scholar] [CrossRef]
- Cimino, G.; Madaio, A.; Trivellone, E. Minor triterpenoids from the Mediterranean sponge, Raspaciona aculeata. J. Nat. Prod. 1994, 57, 784–790. [Google Scholar] [CrossRef]
- Cimino, G.; Crispino, A.; Madaio, A.; Trivellone, E. Raspacionin B, a further triterpenoid from the mediterranean sponge Raspaciona aculeata. J. Nat. Prod. 1993, 56, 534–538. [Google Scholar] [CrossRef]
- Cimino, G.; Epifanio, R.D.A.; Madaio, A.; Puliti, R.; Trivellone, E. Absolute stereochemistry of raspacionin, the main triterpenoid from the marine sponge Raspaciona aculeata. J. Nat. Prod. 1993, 56, 1622–1626. [Google Scholar] [CrossRef]
- Chao, C.H.; Huang, L.F.; Yang, Y.L.; Su, J.H.; Wang, G.H.; Chiang, M.Y.; Wu, Y.C.; Dai, C.F.; Sheu, J.H. Polyoxygenated steroids from the Gorgonian Isis hippuris. J. Nat. Prod. 2005, 68, 880–885. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Krishna Murthy, M.V.R.; Gowri, P.M. Novel epoxy steroids from the Indian ocean soft coral Sarcophyton crassocaule. J. Nat. Prod. 2000, 63, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Su, J.S.; Liaw, C.C.; Sung, P.J.; Chiang, P.L.; Hwang, T.L.; Chang-Feng Dai, J.-H.; Sheu, H. Bioactive steroids with methyl ester group in the side chain from a reef soft coral Sinularia brassica cultured in a tank. Mar. Drugs 2017, 15, 280. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, M.V.; Giannini, C.; Zampella, A.; Minale, L.; Debitus, C.; Roussakis, C. Crellastatin A: A cytotoxic bis-steroid sulfate from the Vanuatu marine sponge Crella sp. J. Org. Chem. 1998, 63, 7382–7388. [Google Scholar] [CrossRef] [PubMed]
- Murayama, S.; Imae, Y.; Takada, K.; Kikuchi, J.; Nakao, Y.; van Soest, R.W.M.; Okada, S.; Matsunaga, S. Shishicrellastatins, inhibitors of cathepsin B, from the marine sponge Crella (Yvesia) spinulata. Bioorg. Med. Chem. 2011, 19, 6594–6598. [Google Scholar] [CrossRef] [PubMed]
- Morinaka, B.I.; Pawlik, J.R.; Molinski, T.F. Amaroxocanes A and B: Sulfated dimeric sterols defend the Caribbean coral reef sponge Phorbas amaranthus from fish predators. J. Nat. Prod. 2009, 72, 259–264. [Google Scholar] [CrossRef]
- Cheng, J.F.; Lee, J.S.; Sun, F.; Jares-Erijman, E.A.; Cross, S.; Rinehart, K.L. Hamigerols A and B, unprecedented polysulfate sterol dimers from the Mediterranean sponge Hamigera hamigera. J. Nat. Prod. 2007, 70, 1195–1199. [Google Scholar] [CrossRef]
- Whitson, E.L.; Bugni, T.S.; Chockalingam, P.S.; Conception, G.P.; Feng, X.; Jin, G. Fibrosterol sulfates from the Philippine sponge Lissodendoryx (Acanthodoryx) fibrosa: Sterol dimers that inhibit PKC. J. Org. Chem. 2009, 74, 5902–5908. [Google Scholar] [CrossRef]
- Pettit, G.R.; Xu, J.-P.; Chapuis, J.-C.; Melody, N. The cephalostatins. Isolation, structure, and cancer cell growth inhibition of cephalostatin 20. J. Nat. Prod. 2015, 78, 1446–1450. [Google Scholar] [CrossRef]
- Boonlarppradab, C.; Faulkner, D.J. Eurysterols A and B, cytotoxic and antifungal steroidal sulfates from a marine sponge of the genus Euryspongia. J. Nat. Prod. 2007, 70, 846–848. [Google Scholar] [CrossRef]
- Solanki, H.; Angulo-Preckler, C.; Calabro, K.; Kaur, N.; Lasserre, P.; Cautain, B.; de la Cruz, M.; Reyes, F.; Avila, C.; Thomas, O.P. Suberitane sesterterpenoids from the Antarctic sponge Phorbas areolatus (Thiele, 1905). Tetrahedron Lett. 2018, 59, 3353–3356. [Google Scholar] [CrossRef]
- Song, J.; Jeong, W.; Wang, N.; Lee, H.-S.; Sim, C.J.; Oh, K.-B.; Shin, J. Scalarane sesterterpenes from the sponge Smenospongia sp. J. Nat. Prod. 2008, 71, 1866–1871. [Google Scholar] [CrossRef] [PubMed]
- Kimura, J.; Hyosu, M. Two new sesterterpenes from the marine sponge, Coscinoderma mathewsi. Chem. Lett. 1999, 52, 61–62. [Google Scholar] [CrossRef]
- Hochlowski, J.E.; Faulkner, D.J.; Bass, L.S.; Clardy, J. Metabolites of the dorid nudibranch Chromodoris sedna. J. Org. Chem. 1983, 48, 1738–1740. [Google Scholar] [CrossRef]
- Keyzers, R.A.; Daoust, J.; Davies-Coleman, M.T.; Van Soest, R.; Balgi, A.; Donohue, E.; Roberge, M.; Andersen, R.J. Autophagy-modulating aminosteroids isolated from the sponge Cliona celata. Org. Lett. 2008, 10, 259–262. [Google Scholar] [CrossRef]
- Li, J.; Zhu, H.; Ren, J.; Deng, Z.; de Voogd, N.J.; Proksch, P.; Lin, W. Globostelletins JeS, isomalabaricanes with unusual cyclopentane sidechains from the marine sponge Rhabdastrella globostellata. Tetrahedron 2012, 68, 559–565. [Google Scholar] [CrossRef]
- Guzii, A.G.; Makarieva, T.N.; Denisenko, V.A.; Dmitrenok, P.S.; Burtseva, Y.V.; Krasokhin, V.B.; Stonik, V.A. Topsentiasterol sulfates with novel iodinated and chlorinated side chains from the marine sponge Topsentia sp. Tetrahedron Lett. 2008, 49, 7191–7193. [Google Scholar] [CrossRef]
- Fusetani, N.; Takahashi, M.; Matsunaga, S. Topsentiasterol sulfates, antimicrobial sterol sulfates possessing novel side chains, from a marine sponge, Topsentia sp. Tetrahedron 1994, 50, 7765–7770. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Liaw, C.-C.; Chen, B.-W.; Chen, P.-C.; Su, J.-H. Withanolide-based steroids from the cultured soft coral Sinularia brassica. J. Nat. Prod. 2013, 76, 1902–1908. [Google Scholar] [CrossRef]
- Lal, A.R.; Cambie, R.C.; Rickard, C.E.F.; Bergquist, P.R. Sesterterpene lactones from a sponge species of the genus Dactylospongia. Tetrahedron Lett. 1994, 35, 2603–2606. [Google Scholar] [CrossRef]
- Cambie, C.R.; Lal, A.R.; Rickard, C.E.F. A sesterterpene lactone from Petrosaspongia nigra sp. nov. Acta Cryst. 1996, C52, 709–711. [Google Scholar] [CrossRef]
- Randazzo, A.; Debitus, C.; Minale, L.; Pastor, P.G.; Alcaraz, M.J.; Payá, M.; Gomez-Paloma, L. Petrosaspongiolodes M–R: New potent and selective phosphoplipase A2 inhibitors from the New Caledonian marine sponge Petrosaspongia nigra. J. Nat. Prod. 1998, 61, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.R.; Yoshida, W.Y.; Scheuer, P.J. Kiheisterones, new cytotoxic steroids from a Maui sponge. J. Org. Chem. 1992, 57, 6637–6640. [Google Scholar] [CrossRef]
- Miyamoto, T.; Sakamoto, K.; Amano, H.; Higuchi, R.; Komori, T.; Sasaki, T. Three new cytotoxic sesterterpenoids, inorolide A, B, and C from the nudibranch Chromodoris inornata. Tetrahedron Lett. 1992, 33, 5811–5814. [Google Scholar] [CrossRef]
- D’Auria, M.V.; Paloma, L.G.; Minale, L.; Riccio, R.; Zampella, A. Isolation, structure characterization and conformational analysis of a unique 4α,9α-epoxysteroid sulphate from the okinawan ophiuroid Ophiomastix annulosa. Tetrahedron Lett. 1992, 33, 4641–4644. [Google Scholar] [CrossRef]
- Jiménez, J.I.; Yoshida, W.Y.; Scheuer, P.J.; Lobkovsky, E.; Clardy, J.; Kelly, M. Honulactones: New bishomoscalarane sesterterpenes from the Indonesian sponge Strepsichordaia aliena. J. Org. Chem. 2000, 65, 6837–6840. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M. Marine terpenes and terpenoids. IV. Isolation of new cembranoid and secocembranoid lactones from the soft coral Sinularia mayi. Chem. Pharm. Bull. 1988, 36, 488–494. [Google Scholar] [CrossRef]
- Carmely, S.; Kashman, Y. Isolation and structure elucidation of lobophytosterol, depresosterol and three other closely related sterols: Five new C28 polyoxygenated sterols from the red sea soft coral Lobophytum depressum. Tetrahedron 1981, 37, 2397–2403. [Google Scholar] [CrossRef]
- Kashman, V.; Carmely, S. Four novel C28 sterols from Lobophytum depressum. Tetrahedron Lett. 1980, 21, 4939–4942. [Google Scholar] [CrossRef]
- Kim, S.K.; van Ta, Q. Bioactive sterols from marine resources and their potential benefits for human health. Adv. Food Nutrit. Res. 2012, 65, 261–268. [Google Scholar]
- Donets, M.M.; Tsygankov, V.Y. Trace elements in commercial marine organisms from the Russian part of the Northwest Pacific (2010–2018). Environ. Chem. Lett. 2019, 17, 1727–1740. [Google Scholar] [CrossRef]










| No. | Dominated Biological Activity (Pa) * | Additional Predicted Activities (Pa) * |
|---|---|---|
| 2 | Antifungal (0.912) Anti-inflammatory (0.912) Antiprotozoal (Plasmodium) (0.794) Antibacterial (0.783) | Antineoplastic (0.909) Apoptosis agonist (0.828) Prostate disorders treatment (0.600) Proliferative diseases treatment (0.578) |
| 3 | Antineoplastic (0.802) Apoptosis agonist (0.647) Chemopreventive (0.573) | Anti-inflammatory (0.629) Antibacterial (0.547) Cytochrome P450 inhibitor (0.503) |
| 4 | Antineoplastic (0.779) Apoptosis agonist (0.766) | Antibacterial (0.718) Antifungal (0.709) |
| 5 | Antineoplastic (0.837) Apoptosis agonist (0.704) | Antibacterial (0.745) |
| 6 | Anti-infertility, female (0.789) Lipid metabolism regulator (0.789) | Antineoplastic (0.756) Apoptosis agonist (0.749) |
| 7 | Antineoplastic (0.913) Apoptosis agonist (0.907) Inositol-3-kinase inhibitor (0.898) | Lipid metabolism regulator (0.700) Hypolipemic (0.693) Antibacterial (0.642) |
| 8 | Inositol-4-kinase inhibitor (0.945) Apoptosis agonist (0.865) Inositol-3-kinase inhibitor (0.848) | Antifungal (0.876) Antibacterial (0.733) |
| 9 | Antineoplastic (0.934) Apoptosis agonist (0.865) | Antifungal (0.881) Antibacterial (0.766) |
| 10 | Inositol-4-kinase inhibitor (0.949) Inositol-3-kinase inhibitor (0.843) Monoamine oxidase inhibitor (0.837) | Apoptosis agonist (0.899) Antineoplastic (0.879) |
| 11 | Antineoplastic (0.924) Apoptosis agonist (0.833) | Antifungal (0.876) Antibacterial (0.733) |
| 12 | Antineoplastic (0.903) Apoptosis agonist (0.807) Prostate disorders treatment (0.800) | Lipid metabolism regulator (0.700) Hypolipemic (0.693) |
| 13 | Antineoplastic (0.934) Apoptosis agonist (0.907) Prostate disorders treatment (0.866) | Lipid metabolism regulator (0.897) Antifungal (0.881) Hypolipemic (0.793) |
| 14 | Antiproliferative (0.938) Antineoplastic (0.902) Apoptosis agonist (0.889) | Prostate disorders treatment (0.900) Proliferative diseases treatment (0.878) |
| 15 | Antiproliferative (0.929) Antineoplastic (0.922) | Prostate disorders treatment (0.876) Proliferative diseases treatment (0.844) |
| 16 | Antiproliferative (0.915) Apoptosis agonist (0.882) | Prostate disorders treatment (0.854) Proliferative diseases treatment (0.823) |
| 17 | Antineoplastic (0.877) Apoptosis agonist (0.856) | Ovulation inhibitor (0.698) Anti-inflammatory (0.682) |
| 18 | Antineoplastic (0.879) Apoptosis agonist (0.876) | Ovulation inhibitor (0.728) Anti-inflammatory (0.717) |
| 19 | Antineoplastic (0.865) Antiprotozoal (Plasmodium) (0.621) | Antifungal (0.779) Antibacterial (0.747) |
| 20 | Antineoplastic (0.888) | Antifungal (0.843) |
| 21 | Antineoplastic (0.910) | Apoptosis agonist (0.877) |
| 22 | Nitric oxide inhibitor (0.921) | Apoptosis agonist (0.879) |
| 23 | Nitric oxide inhibitor (0.914) Antifungal (0.862) | Apoptosis agonist (0.867) Antineoplastic (0.844) |
| 24 | Antineoplastic (0.937) | Apoptosis agonist (0.892) |
| 25 | Antineoplastic (0.929) | Apoptosis agonist (0.887) |
| No. | Dominated Biological Activity (Pa) * | Additional Predicted Activities (Pa) * |
|---|---|---|
| 26 | Antineoplastic (0.922) Apoptosis agonist (0.844) | Antifungal (0.887) Antibacterial (0.754) |
| 27 | Antineoplastic (0.929) Apoptosis agonist (0.839) | Antifungal (0.832) Antibacterial (0.782) |
| 28 | Antineoplastic (0.921) Apoptosis agonist (0.841) | Antifungal (0.833) Antibacterial (0.755) |
| 29 | Antineoplastic (0.889) | Antibacterial (0.766) |
| 30 | Antineoplastic (0.833) | Antibacterial (0.733) |
| 31 | Hepatoprotectant (0.860) Kidney function stimulant (0.719) | Muscle relaxant (0.599) Spasmolytic (0.587) |
| 32 | Hepatoprotectant (0.875) Kidney function stimulant (0.686) | Muscle relaxant (0.612) Spasmolytic (0.595) |
| 33 | Hepatoprotectant (0.839) Kidney function stimulant (0.719) | Muscle relaxant (0.782) |
| 34 | Antiprotozoal (Plasmodium) (0.718) | Anti-inflammatory (0.778) |
| 35 | Antiprotozoal (0.657) | Anti-inflammatory (0.766) |
| 36 | Antiprotozoal (Plasmodium) (0.947) Antiprotozoal (Leishmania) (0.888) Antiprotozoal (0.879) | Antineoplastic (0.866) Apoptosis agonist (0.795) Chemopreventive (0.722) |
| 37 | Antiprotozoal (Plasmodium) (0.949) Antiprotozoal (Leishmania) (0.868) Antiprotozoal (0.823) | Antineoplastic (0.859) Apoptosis agonist (0.791) Chemopreventive (0.717) |
| 38 | Antiprotozoal (Plasmodium) (0.927) Antiprotozoal (Leishmania) (0.914) Antiprotozoal (0.884) | Antineoplastic (0.843) Apoptosis agonist (0.747) Chemopreventive (0.698) |
| 39 | Antiprotozoal (Plasmodium) (0.912) Antiprotozoal (Leishmania) (0.886) Antiprotozoal (0.778) | Antineoplastic (0.876) Chemopreventive (0.773) |
| 40 | Antiprotozoal (Plasmodium) (0.955) Antiprotozoal (Leishmania) (0.948) Antiprotozoal (0.903) | Chemopreventive (0.903) Antineoplastic (0.822) Apoptosis agonist (0.747) |
| 41 | Antiprotozoal (0.822) | Anti-inflammatory (0.712) |
| 42 | Antibacterial (0.883) Antifungal (0.876) | Laxative (0.735) Anti-eczematic (0.717) |
| 43 | Antibacterial (0.907) Antifungal (0.865) | Laxative (0.722) Anti-eczematic (0.686) |
| 44 | Antibacterial (0.885) Antifungal (0.876) | Anti-inflammatory (0.712) |
| 45 | Antibacterial (0.912) Antifungal (0.875) | Anti-eczematic (0.638) Anti-inflammatory (0.612) |
| 46 | Antibacterial (0.901) Antifungal (0.867) | Anti-eczematic (0.689) |
| 47 | Antibacterial (0.889) Antifungal (0.882) | Anti-eczematic (0.654) |
| No. | Dominated Biological Activity (Pa) * | Additional Predicted Activities (Pa) * |
|---|---|---|
| 48 | Antibacterial (0.856) Antifungal (0.811) | Antiviral (0.578) |
| 49 | Nitric oxide inhibitor (0.906) Apoptosis agonist (0.667) | Antineoplastic (0.764) |
| 50 | Nitric oxide inhibitor (0.874) Apoptosis agonist (0.685) | Antineoplastic (0.785) |
| 51 | Plant growth inhibitor (0.876) | Antifungal (0.732) |
| 52 | Plant growth inhibitor (0.855) | Antifungal (0.689) |
| 53 | Antibacterial (0.769) Antifungal (0.716) | Antiviral (0.577) |
| 54 | Nitric oxide inhibitor (0.779) | Antibacterial (0.686) |
| 55 | Nitric oxide inhibitor (0.812) | Antibacterial (0.677) |
| 56 | Antineoplastic (0.755) | Anti-inflammatory (0.612) |
| 57 | Antineoplastic (0.734) | Antifungal (0.521) |
| 58 | Antibacterial (0.772) Antifungal (0.631) | Antiviral (0.589) |
| 59 | Antibacterial (0.856) | Antiviral (0.618) |
| 60 | Antifungal (0.789) Antibacterial (0.634) | Antiviral (0.611) |
| 61 | Antineoplastic (0.785) | Anti-inflammatory (0.622) |
| 62 | Cytoprotectant (0.758) | |
| 63 | Antibacterial (0.882) | Antifungal (0.668) |
| 64 | Antibacterial (0.872) | Antifungal (0.675) |
| 65 | Antibacterial (0.868) | Antifungal (0.712) |
| 66 | Antibacterial (0.856) | Antifungal (0.682) |
| 67 | Antimicrobial (0.902) Antibacterial (0.856) | Antifungal (0.754) |
| 68 | Antibacterial (0.911) | Antimicrobial (0.773) |
| 69 | Antiprotozoal (0.886) Antibacterial (0.847) | Antimicrobial (0.638) |
| 70 | Antineoplastic (0.772) | Antifungal (0.722) |
| 71 | Antineoplastic (0.882) Chemopreventive (0.791) | Anti-inflammatory (0.634) |
| 72 | Antineoplastic (0.913) Chemopreventive (0.882) Apoptosis agonist (0.798) | Antiviral (0.832) Anti-inflammatory (0.711) |
| No. | Dominated Biological Activity (Pa) * | Additional Predicted Activities (Pa) * |
|---|---|---|
| 73 | Antineoplastic (0.884) | Antifungal (0.621) |
| 74 | Antineoplastic (0.975) Apoptosis agonist (0.889) Chemopreventive (0.887) | Lipid metabolism regulator (0.858) Hypolipemic (0.677) |
| 75 | Antineoplastic (0.968) Chemopreventive (0.882) Apoptosis agonist (0.798) | Lipid metabolism regulator (0.897) Hypolipemic (0.693) |
| 76 | Antineoplastic (0.973) Apoptosis agonist (0.798) Chemopreventive (0.882) | Lipid metabolism regulator (0.872) Hypolipemic (0.654) |
| 77 | Antineoplastic (0.865) | Antifungal (0.633) |
| 78 | Antineoplastic (0.834) | Antifungal (0.611) |
| 79 | Antineoplastic (0.844) | Anti-inflammatory (0.666) |
| 80 | Antineoplastic (0.851) | Antifungal (0.597) |
| 81 | Antiproliferative (0.834) | Cytotoxic (0.658) |
| 82 | Antiproliferative (0.812) | Anti-inflammatory (0.645) |
| 83 | Antiproliferative (0.876) Antineoplastic (0.875) | Cytotoxic (0.745) Antifungal (0.677) |
| 84 | Antiproliferative (0.876) | Anti-inflammatory (0.668) |
| 85 | Antiproliferative (0.924) Cytotoxic (0.892) | Cytotoxic (0.821) Antifungal (0.734) |
| 86 | Antiproliferative (0.902) Cytotoxic (0.883) | Cytotoxic (0.833) Antifungal (0.680) |
| 87 | Antineoplastic (0.823) | Anti-inflammatory (0.619) |
| 88 | Antiproliferative (0.831) | Anti-inflammatory (0.644) |
| 89 | Antiproliferative (0.849) | Hypolipemic (0.623) |
| 90 | Antineoplastic (0.736) | Antifungal (0.567) |
| 91 | Antineoplastic (0.729) | Antifungal (0.564) |
| 92 | Antineoplastic (0.751) | Antifungal (0.592) |
| 93 | Antineoplastic (0.722) | Antimicrobial (0.612) |
| 94 | Antineoplastic (0.733) | Antimicrobial (0.622) |
| 95 | Antineoplastic (0.728) | Antimicrobial (0.641) |
| 96 | Antineoplastic (0.802) | Antimicrobial (0.632) |
| 97 | Allergic conjunctivitis treatment (0.687) | Antifungal (0.566) |
| 98 | Allergic conjunctivitis treatment (0.705) | Antifungal (0.587) |
| 99 | Antineoplastic (0.902) Antiproliferative (0.883) Apoptosis agonist (0.870) | Cytotoxic (0.844) Anti-inflammatory (0.634) |
| 100 | Antibacterial (0.903) | Antimicrobial (0.734) |
| 101 | Antibacterial (0.868) | Antimicrobial (0.698) |
| No. | Dominated Biological Activity (Pa) * | Additional Predicted Activities (Pa) * |
|---|---|---|
| 102 | Antineoplastic (0.879) Chemopreventive (0.683) Apoptosis agonist (0.641) | Anti-inflammatory (0.840) Antifungal (0.671) |
| 103 | Antineoplastic (0.851) Chemopreventive (0.662) Apoptosis agonist (0.637) | Anti-inflammatory (0.790) Antifungal (0.653) |
| 104 | Analgesic (0.761) Antitussive (0.652) | Respiratory analeptic (0.744) Oxygen scavenger (0.651) |
| 105 | Antiprotozoal (Plasmodium) (0.869) Antiprotozoal (0.832) | Antineoplastic (0.798) Apoptosis agonist (0.795) |
| 106 | Antiprotozoal (Plasmodium) (0.939) Antiprotozoal (Leishmania) (0.891) Antiprotozoal (0.883) | Antineoplastic (0.874) Apoptosis agonist (0.765) Chemopreventive (0.703) |
| 107 | Antiprotozoal (Plasmodium) (0.954) Antiprotozoal (Leishmania) (0.912) Antiprotozoal (0.889) | Antineoplastic (0.911) Apoptosis agonist (0.893) Chemopreventive (0.791) |
| 108 | Antiprotozoal (Plasmodium) (0.941) Antiprotozoal (Leishmania) (0.866) Antiprotozoal (0.836) | Antineoplastic (0.876) Apoptosis agonist (0.795) Chemopreventive (0.722) |
| 109 | Antiprotozoal (Plasmodium) (0.940) Antiprotozoal (Leishmania) (0.848) Antiprotozoal (0.842) | Antineoplastic (0.868) Apoptosis agonist (0.794) Chemopreventive (0.713) |
| 110 | Antineoplastic (0.955) Apoptosis agonist (0.802) | Antiviral (0.818) Anti-inflammatory (0.721) |
| 111 | Antineoplastic (0.946) Apoptosis agonist (0.766) | Antiviral (0.832) Anti-inflammatory (0.734) |
| 112 | Antineoplastic (0.932) Apoptosis agonist (0.778) | Antiviral (0.822) |
| 113 | Antineoplastic (0.829) Cytochrome P450 inhibitor (0.691) | Anti-inflammatory (0.790) Antimitotic (0.592) |
| 114 | Antineoplastic (0.867) Cytochrome P450 inhibitor (0.748) | Anti-inflammatory (0.801) Antimitotic (0.622) |
| 115 | Antineoplastic (0.793) | Anti-inflammatory (0.715) |
| 116 | Antiprotozoal (Plasmodium) (0.707) Antiprotozoal (0.697) | Antineoplastic (0.707) Apoptosis agonist (0.570) |
| 117 | Antineoplastic (0.872) Apoptosis agonist (0.712) | Antiprotozoal (Plasmodium) (0.746) Antiprotozoal (0.703) |
| 118 | Antineoplastic (0.854) Apoptosis agonist (0.743) | Antiprotozoal (Plasmodium) (0.721) Antiprotozoal (0.693) |
| 119 | Antineoplastic (0.850) Apoptosis agonist (0.691) Cytostatic (0.683) | Hepatoprotectant (0.767) Immunosuppressant (0.712) Anti-hypercholesterolemic (0.566) |
| 120 | Antineoplastic (0.855) Apoptosis agonist (0.697) | Hepatoprotectant (0.768) Immunosuppressant (0.718) |
| 121 | Antineoplastic (0.858) Apoptosis agonist (0.690) | Hepatoprotectant (0.753) Anti-hypercholesterolemic (0.584) |
| 122 | Antineoplastic (0.860) Apoptosis agonist (0.699) | Hepatoprotectant (0.755) Anti-hypercholesterolemic (0.616) |
| 123 | Antiviral (0.832) | Anti-inflammatory (0.721) |
| 124 | Antiviral (0.856) | Anti-inflammatory (0.734) |
| 125 | Antiviral (0.887) Antiviral (Arbovirus) (0.790) | Antiprotozoal (0.693) |
| 126 | Antiviral (0.877) | Anti-inflammatory (0.715) |
| 127 | Antiviral (0.839) | Anti-inflammatory (0.698) |
| No. | Dominated Biological Activity (Pa) * | Additional Predicted Activities (Pa) * |
|---|---|---|
| 128 | Hepatoprotectant (0.784) | Immunosuppressant (0.734) |
| 129 | Hepatoprotectant (0.772) | Immunosuppressant (0.728) |
| 130 | Hepatoprotectant (0.777) | Immunosuppressant (0.729) |
| 131 | Antimicrobial (0.841) | Antibacterial (0.823) |
| 132 | Antineoplastic (0.823) | Antiprotozoal (0.821) |
| 133 | Antineoplastic (0.894) Chemopreventive (0.763) | Antiprotozoal (0.865) |
| 134 | Antineoplastic (0.902) Chemopreventive (0.883) | Antiprotozoal (0.872) |
| 135 | Antifungal (0.833) | Anti-inflammatory (0.790) |
| 136 | Antifungal (0.797) | Antimicrobial (0.671) |
| 137 | Antifungal (0.803) | Antimicrobial (0.666) |
| 138 | Antimicrobial (0.854) | Antifungal (0.687) |
| 139 | Antimicrobial (0.798) | Antifungal (0.693) |
| 140 | Cortisone reductase inhibitor (0.875) | Antineoplastic (0.825) |
| 141 | Cortisone reductase inhibitor (0.902) | Antineoplastic (0.887) |
| 142 | Cortisone reductase inhibitor (0.898) | Antineoplastic (0.879) |
| 143 | Anti-HIV-1 (0.894) Antiviral (0.839) | Anti-inflammatory (0.768) |
| 144 | Anti-HIV-1 (0.904) Antiviral (0.885) | Anti-inflammatory (0.754) |
| 145 | Prostate cancer treatment (0.928) Antineoplastic (0.911) | Antimicrobial (0.723) |
| 146 | Prostate cancer treatment (0.914) Antineoplastic (0.896) | Antimicrobial (0.741) |
| 147 | Antineoplastic (0.907) Prostate cancer treatment (0.894) | Antimicrobial (0.654) |
| 148 | Antineoplastic (0.897) Prostate cancer treatment (0.887) | Antimicrobial (0.678) |
| 149 | Antineoplastic (0.838) | Antimicrobial (0.611) |
| 150 | Antineoplastic (0.864) Apoptosis agonist (0.723) | Antileukemic (0.822) |
| 151 | Antineoplastic (0.843) Apoptosis agonist (0.711) | Antifungal (0.733) |
| 152 | Antineoplastic (0.929) Antimetastatic (0.834) | Antileukemic (0.814) |
| 153 | Antineoplastic (0.924) Antimetastatic (0.876) | Antileukemic (0.754) Antimicrobial (0.629) |
| 154 | Antineoplastic (0.932) Antimetastatic (0.839) | Antileukemic (0.724) Antimicrobial (0.623) |
| No. | Dominated Biological Activity (Pa) * | Additional Predicted Activities (Pa) * |
|---|---|---|
| 155 | Antifungal (0.778) | Anti-inflammatory (0.709) |
| 156 | Antineoplastic (0.918) Prostate cancer treatment (0.915) Antimetastatic (0.734) | Antifungal (0.734) Antimicrobial (0.652) |
| 157 | Antineoplastic (0.923) Prostate cancer treatment (0.922) Antimetastatic (0.834) | Antifungal (0.736) Antimicrobial (0.655) |
| 158 | Antineoplastic (0.888) Apoptosis agonist (0.793) | Antifungal (0.713) Antimicrobial (0.654) |
| 159 | Antineoplastic (0.854) Apoptosis agonist (0.721) | Antifungal (0.743) Antimicrobial (0.664) |
| 160 | Anti-tuberculosis treatment (0.896) | Antibacterial (0.809) |
| 161 | Anti-tuberculosis treatment (0.903) Antimicrobial (0.745) | Antibacterial (0.811) |
| 162 | Anti-tuberculosis treatment (0.937) Antimicrobial (0.772) | Antibacterial (0.854) |
| 163 | Anti-tuberculosis treatment (0.875) | Antibacterial (0.754) |
| 164 | Anti-tuberculosis treatment (0.862) | Antibacterial (0.761) |
| 165 | Acetyl-cholinesterase inhibitor (0.931) | Antimetastatic (0.829) |
| 166 | Acetyl-cholinesterase inhibitor (0.942) | Antimetastatic (0.842) |
| 167 | DNA topoisomerase inhibitor (0.928) Antineoplastic (0.916) Antimetastatic (0.863) | Apoptosis agonist (0.834) |
| 168 | DNA topoisomerase inhibitor (0.917) Antineoplastic (0.922) Antimetastatic (0.821) | Apoptosis agonist (0.871) |
| 169 | Anti-inflammatory (0.909) Antiviral (0.844) | Antimicrobial (0.688) |
| 170 | Anti-inflammatory (0.902) Antiviral (0.829) | Antimicrobial (0.679) |
| 171 | Antineoplastic (0.886) | Apoptosis agonist (0.756) |
| 172 | Antineoplastic (0.874) | Apoptosis agonist (0.771) |
| 173 | Antineoplastic (0.859) | Apoptosis agonist (0.754) |
| 174 | Antineoplastic (0.851) | Apoptosis agonist (0.764) |
| 175 | Antiproliferative (0.889) | Anti-inflammatory (0.654) |
| 176 | Antiproliferative (0.882) | Anti-inflammatory (0.659) |
| 177 | Antiproliferative (0.895) | Anti-inflammatory (0.663) |
| 178 | Anti-hepatitis C virus (0.912) Antiviral (0.898) | Antimicrobial (0.687) |
| 179 | Antineoplastic (0.873) | Apoptosis agonist (0.775) |
| 180 | Antineoplastic (0.869) | Apoptosis agonist (0.768) |
| 181 | Hepatocellular carcinoma inhibitor (0.897) | Hepatoprotectant (0.860) |
| 182 | Hepatocellular carcinoma inhibitor (0.906) | Hepatoprotectant (0.873) |
| 183 | Hepatocellular carcinoma inhibitor (0.911) | Hepatoprotectant (0.869) |
| 184 | Hepatocellular carcinoma inhibitor (0.889) | Hepatoprotectant (0.856) |
| 185 | Anti-inflammatory (0.832) Antiviral (0.754) | Antibacterial (0.785) |
| 186 | Anti-inflammatory (0.834) Antiviral (0.765) | Antibacterial (0.744) |
| No. | Dominated Biological Activity (Pa) * | Additional Predicted Activities (Pa) * |
|---|---|---|
| 187 | Antineoplastic (0.913) Antimetastatic (0.786) | Apoptosis agonist (0.619) |
| 188 | Antineoplastic (0.908) Antimetastatic (0.804) | Apoptosis agonist (0.634) |
| 189 | Antineoplastic (0.902) Antimetastatic (0.798) | Apoptosis agonist (0.624) |
| 190 | Cytoprotectant (0.768) Antineoplastic (0.723) | Antimicrobial (0.776) |
| 191 | Cytoprotectant (0.787) | Antimicrobial (0.685) |
| 192 | Cytoprotectant (0.766) | Antimicrobial (0.692) |
| 193 | Antineoplastic (0.900) Apoptosis agonist (0.836) | Antimetastatic (0.698) |
| 194 | Antineoplastic (0.889) Apoptosis agonist (0.821) | Antimetastatic (0.682) |
| 195 | Antineoplastic (0.872) | Antiprotozoal (0.765) |
| 196 | Antineoplastic (0.868) | Antiprotozoal (0.756) |
| 197 | Antiviral (0.776) | Anti-inflammatory (0.682) |
| 198 | Antiviral (0.749) | Anti-inflammatory (0.678) |
| 199 | Antineoplastic (0.773) | Apoptosis agonist (0.653) |
| 200 | Antibacterial (0.747) | Antifungal (0.683) |
| 201 | Antibacterial (0.736) | Antifungal (0.651) |
| 202 | Apoptosis agonist (0.833) | Antineoplastic (0.711) |
| 203 | Apoptosis agonist (0.824) | Antineoplastic (0.683) |
| 204 | Antineoplastic (0.744) | Apoptosis agonist (0.621) |
| 205 | Antineoplastic (0.738) | Apoptosis agonist (0.619) |
| 206 | Antiallergic (0.728) | Anti-asthmatic (0.671) |
| 207 | Antiallergic (0.734) | Anti-asthmatic (0.698) |
| 208 | Antiallergic (0.742) | Anti-asthmatic (0.661) |
| 209 | Antiallergic (0.741) | Anti-asthmatic (0.676) |
| 210 | Antineoplastic (0.926) | Antimetastatic (0.855) |
| 211 | Antineoplastic (0.914) | Antimetastatic (0.836) |
| 212 | Antiproliferative (0.818) | Antineoplastic (0.654) |
| 213 | Antiproliferative (0.828) | Antineoplastic (0.662) |
| 214 | Antiproliferative (0.833) | Antineoplastic (0.659) |
| 215 | Antiproliferative (0.867) | Antineoplastic (0.678) |
| 216 | Apoptosis agonist (0.818) Antineoplastic (0.762) | Lipid metabolism regulator (0.638) Anti-inflammatory (0.691) |
| 217 | Antineoplastic (0.917) | Antimetastatic (0.743) |
| 218 | Antineoplastic (0.919) | Antimetastatic (0.767) |
| 219 | Antineoplastic (0.928) Apoptosis agonist (0.854) | Antimetastatic (0.711) |
| 220 | Antineoplastic (0.931) Apoptosis agonist (0.871) | Antimetastatic (0.721) |
| 221 | Antineoplastic (0.924) | Antimetastatic (0.734) |
| No. | Dominated Biological Activity (Pa) * | Additional Predicted Activities (Pa) * |
|---|---|---|
| 222 | Antiviral (0.788) Antiviral (Arbovirus) (0.750) | Anti-inflammatory (0.682) |
| 223 | Allergic conjunctivitis treatment (0.705) | Antifungal (0.637) |
| 224 | Antineoplastic (0.898) Apoptosis agonist (0.834) | Antimetastatic (0.723) |
| 225 | Antineoplastic (0.918) Apoptosis agonist (0.856) | Antimetastatic (0.743) |
| 226 | Cathepsin B inhibitor (0.889) | Apoptosis agonist (0.698) |
| 227 | Antifeedant (0.867) | Antifungal (0.703) |
| 228 | Protein kinase C inhibitor (0.921) Antineoplastic (0.834) Antimetastatic (0.748) | Antiviral (0.829) Autoimmune disorders treatment (0.619) |
| 229 | Antineoplastic (0.924) Apoptosis agonist (0.745) | Antifungal (0.698) |
| 230 | Antineoplastic (0.931) Apoptosis agonist (0.805) | Anti-inflammatory (0.654) |
| 231 | Antineoplastic (0.918) Apoptosis agonist (0.764) | Anti-inflammatory (0.662) |
| 232 | Antineoplastic (0.932) Chemopreventive (0.738) | Apoptosis agonist (0.768) |
| 233 | Antineoplastic (0.929) Chemopreventive (0.787) | Apoptosis agonist (0.729) |
| 234 | Antineoplastic (0.943) Chemopreventive (0.818) | Apoptosis agonist (0.815) |
| 235 | Antimicrobial (0.886) | Antifungal (0.687) |
| 236 | Antineoplastic (0.943) Antimicrobial (0.922) | Apoptosis agonist (0.898) Antifungal (0.847) |
| 237 | Antibacterial (0.921) | Antifungal (0.727) |
| 238 | Antibacterial (0.765) |
| No. | Dominated Biological Activity (Pa) * | Additional Predicted Activities (Pa) * |
|---|---|---|
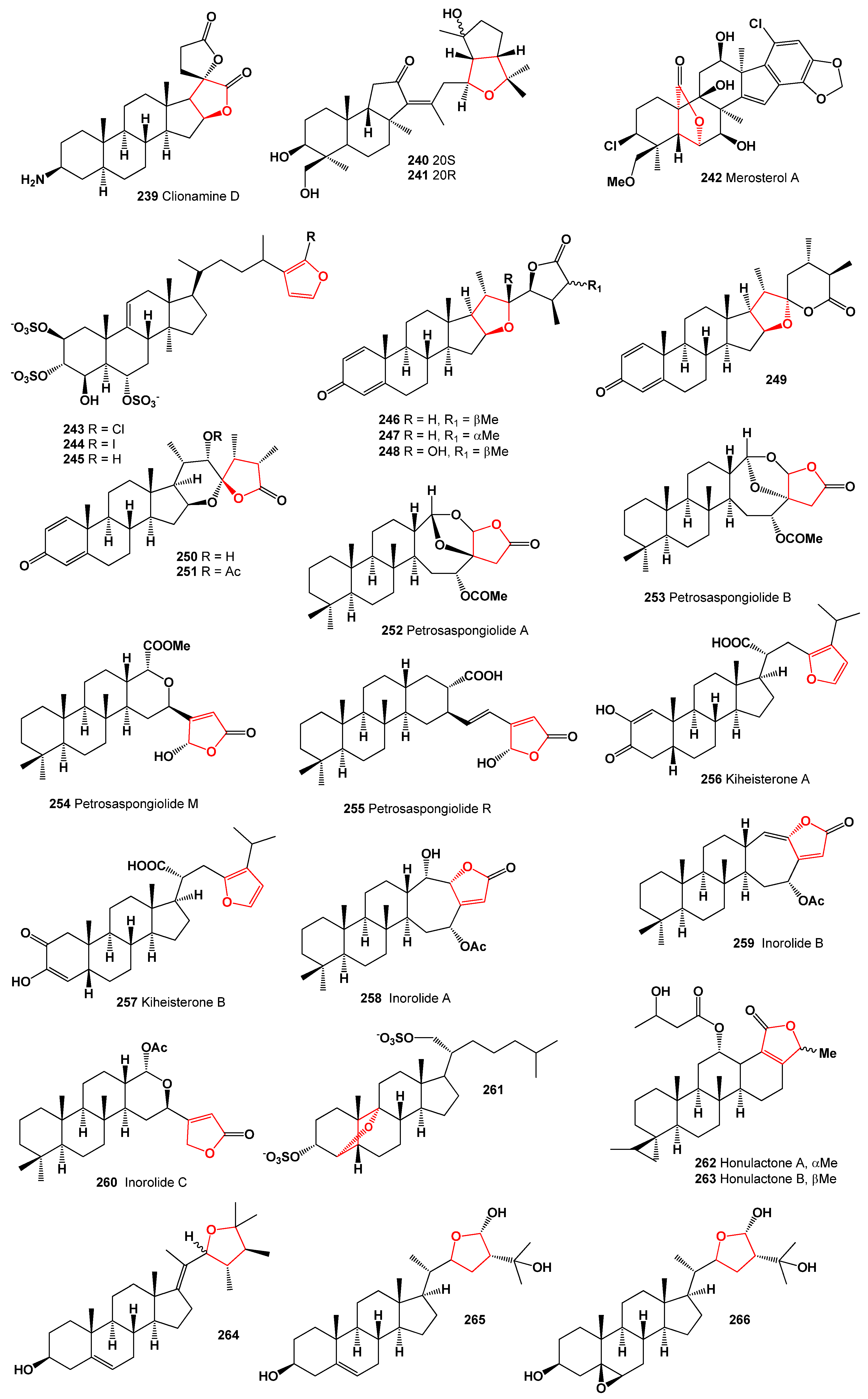
| 239 | Antineoplastic (0.913) Apoptosis agonist (0.886) | Antimetastatic (0.829) |
| 240 | Antineoplastic (0.922) Chemopreventive (0.731) Anticarcinogenic (0.698) | Apoptosis agonist (0.876) |
| 241 | Antineoplastic (0.929) Chemopreventive (0.720) Anticarcinogenic (0.678) | Apoptosis agonist (0.854) |
| 242 | Antineoplastic (0.889) Anticarcinogenic (0.718) | Chemopreventive (0.683) |
| 243 | Antibacterial (0.881) | Antifungal (0.683) |
| 244 | Antibacterial (0.879) | Antifungal (0.627) |
| 245 | Antibacterial (0.902) | Antifungal (0.638) |
| 246 | Antineoplastic (0.938) Apoptosis agonist (0.834) | Chemopreventive (0.712) |
| 247 | Antineoplastic (0.886) | Apoptosis agonist (0.726) |
| 248 | Antineoplastic (0.887) | Apoptosis agonist (0.734) |
| 249 | Antineoplastic (0.889) | Apoptosis agonist (0.722) |
| 250 | Antineoplastic (0.900) | Apoptosis agonist (0.783) |
| 251 | Antineoplastic (0.911) | Apoptosis agonist (0.803) |
| 252 | Antineoplastic (0.935) Apoptosis agonist (0.814) Chemopreventive (0.756) | Antimetastatic (0.820) |
| 253 | Antineoplastic (0.929) Apoptosis agonist (0.821) Chemopreventive (0.744) | Antimetastatic (0.822) |
| 254 | Phospholipase A2 inhibitor (0.923) Antibacterial (0.883) | Anti-inflammatory (0.689) |
| 255 | Phospholipase A2 inhibitor (0.827) Antibacterial (0.757) | Anti-inflammatory (0.683) |
| 256 | Antineoplastic (0.884) Apoptosis agonist (0.756) | Antimetastatic (0.688) |
| 257 | Antineoplastic (0.891) Apoptosis agonist (0.785) | Antimetastatic (0.679) |
| 258 | Antineoplastic (0.814) | Anti-inflammatory (0.661) |
| 259 | Antineoplastic (0.828) | Anti-inflammatory (0.658) |
| 260 | Antineoplastic (0.832) | Anti-inflammatory (0.650) |
| 261 | Antineoplastic (0.915) Antimetastatic (0.839) | Chemopreventive (0.672) |
| 262 | Antineoplastic (0.912) Antimetastatic (0.821) | Chemopreventive (0.669) |
| 263 | Antineoplastic (0.910) Apoptosis agonist (0.752) | Antimetastatic (0.712) |
| 264 | Antineoplastic (0.921) Apoptosis agonist (0.774) | Antimetastatic (0.729) |
| 265 | Antineoplastic (0.905) Apoptosis agonist (0.739) | Antimetastatic (0.704) |
| 266 | Antineoplastic (0.918) Apoptosis agonist (0.699) | Antimetastatic (0.687) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dembitsky, V.M. Fascinating Furanosteroids and Their Pharmacological Profile. Molecules 2023, 28, 5669. https://doi.org/10.3390/molecules28155669
Dembitsky VM. Fascinating Furanosteroids and Their Pharmacological Profile. Molecules. 2023; 28(15):5669. https://doi.org/10.3390/molecules28155669
Chicago/Turabian StyleDembitsky, Valery M. 2023. "Fascinating Furanosteroids and Their Pharmacological Profile" Molecules 28, no. 15: 5669. https://doi.org/10.3390/molecules28155669
APA StyleDembitsky, V. M. (2023). Fascinating Furanosteroids and Their Pharmacological Profile. Molecules, 28(15), 5669. https://doi.org/10.3390/molecules28155669